Abstract
Target of rapamycin inhibition by rapamycin feeding has previously been shown to extend life in genetically heterogeneous mice. To examine whether it similarly affected mouse health, we fed encapsulated rapamycin or a control diet to C57BL/6Nia mice of both sexes starting at 19 months of age. We performed a range of health assessments 6 and 12 months later. Rapamycin feeding significantly reduced mTOR activity in most but not all tissues. It also reduced total and resting metabolic rate during the light (inactive) phase of the light:dark cycle in females only but had no effect on spontaneous activity or metabolism during the dark (active) phase of either sex. Males only had less fragmented sleep when fed rapamycin, whereas stride length and rotarod performance were improved in both sexes. Survival was also improved by this late-life rapamycin feeding, and some pathological lesions were delayed. We found no adverse health consequences associated with rapamycin treatment.
Key Words: Aging, Health span, Rapamycin, Sex differences.
RAPAMYCIN, a potent inhibitor of the target of rapamycin (TOR) kinase, robustly extends mouse life span in both sexes, in heterogeneous and inbred genetic backgrounds, whether administered in food or via injection and whether begun prior to complete physical maturity (2 months), in adulthood (9 months), or in later life (20 and 22–24 months) (1–4). Rapamycin treatment also increases replicative (5) and chronological (6) life span in yeast. Genetic inhibition of the TOR-signaling network also enhances longevity in worms, flies, and mice (reviewed in Kapahi et al.) (7) and has been implicated in dietary restriction’s impact on longevity (7,8). However, complete loss of TOR signaling is detrimental, causing serious defects in growth and/or development in worms, flies, and mice (9).
The TOR-signaling network generally promotes anabolic, but inhibits catabolic, processes. Thus, activation of TOR stimulates ribosome biogenesis, protein synthesis, and importation of nutrients into cells, which together result in increased cellular metabolism, growth, and proliferation (10). Activation of TOR also inhibits stress-responsive pathways and protein degradation via autophagy. By contrast, TOR inhibition by nutrient restriction, stress, or pharmacological agents such as rapamycin does the opposite—retards protein synthesis, cell growth, and proliferation; enhances stress responsive transcription; and stimulates autophagy.
The generality of the effects of TOR inhibition on life span combined with the fact that rapamycin is already approved for human use raises the tantalizing prospect that rapamycin may extend human life. However, increasing life span without simultaneously increasing health span is a fool’s errand, and whether the life-extending effects of rapamycin treatment are reflected in extended health has not yet been extensively investigated. Some evidence suggests that rapamycin might have protective effects against multiple degenerative processes. For instance, because of its antiproliferative effects, it is currently being investigated for therapeutic efficacy against several human cancers and vascular diseases (11,12). In mouse models, it has proven successful at delaying the onset of Alzheimer’s pathology (13,14), at reducing cancer incidence (3), at inhibiting the development of atherosclerotic plaques (15), at enhancing vaccine response in aged mice (4), and at delaying normal, age-related cognitive decline (16). On the other hand, rapamycin could conceivably have deleterious effects on some aspects of health. For instance, its use as part of immunosuppressive therapy after organ transplantation (17) raises concerns about the impact of rapamycin on immune function. Additionally, because it inhibits protein synthesis, cellular processes requiring de novo protein synthesis such as growth, tissue repair, and regeneration may be compromised by chronic rapamycin administration. Thus, some rodent studies have observed that mTOR (the mammalian version) inhibition retards recovery from skeletal (18) or cardiac muscle injury (19). Moreover, rapamycin has been reported to negatively affect neuronal long-term potentiation and memory consolidation (20,21). Furthermore, both human and rodent studies have associated inhibition of mTOR with insulin resistance (22).
Given the uncertainty of chronic rapamycin administration on health, we initiated a study of its impact on longevity and a wide range of health parameters of C57BL/6 mice of both sexes. We employed the same food-administered dose that had previously been shown to extend life in genetically heterogeneous mice (1,2) and assessed functional mTOR suppression in a variety of tissues. We initiated rapamycin feeding at either 4 or 19 months of age. In this article, we only present results of rapamycin fed from 19 months of age. Results from the mice fed rapamycin from 4 months of age will be covered in a subsequent article. The impact of chronic rapamycin in this mouse strain had not previously been evaluated except in a short-term study of older mice (4).
Methods
Animals and Husbandry
Care of animals employed UT Health Science Center Institutional Animal Care and Use Committees approved procedures. Animals were assessed for rapamycin concentration, autophagy, proteasome activity, mTOR signaling, and health at 25 months (after 6 months of treatment) and for health alone at 31 months of age (after 12 months of treatment). All behavioral and physiological assays were performed single blind, during the dark (active) phase of the 12:12 light cycle. Details of all experimental procedures may be found in the supplemental materials.
Animals
. —Specific pathogen-free C57BL/6Nia mice were purchased from the National Institutes of Health colony reared in the Charles River Laboratories at 17 months of age and fed on food-containing encapsulated rapamycin or empty capsules (eudragit = control) at 19 months of age. Mice were maintained under barrier conditions by the UTHSCSA Nathan Shock Center Aging Animal and Longevity Assessment Core.
Diet Preparation
Microencapsulated rapamycin or empty microcapsules (control) were incorporated into Purina 5LG6 diet. The rapamycin diet was prepared at 14 ppm using methods described by Harrison et al. (1).
Rapamycin Quantification
Rapamycin concentration was quantified in diet and uncoagulated whole-mouse blood by high-performance liquid chromatography with tandem mass spectrometry. Blood was collected from 25-month-old mice that had been on rapamycin for 6 months.
mTOR Signaling
Upon activation, the mTOR kinase phosphorylates the ribosomal protein S6, a substrate of S6K1. Therefore, we assessed the effect of rapamycin feeding on mTORC1 inhibition by the ratio of phosphorylated (at Ser 240 and Ser 244) to unphosphorylated ribosomal protein S6 in heart, kidney, liver, white adipose tissue, and brain. mTOR activation also inhibits autophagy. We employed a second measure of mTORC1 inhibition by quantifying autophagy, using LC3II/LC3I ratio. LC3I is a cytosolic protein, which after processing, converts to LC3II that then associates with the autophagosome membrane (23). Thus, the LC3II/LC3I ratio indicates the extent of autophagosome formation. As rapamycin is known to decrease protein synthesis and enhance protein degradation via autophagy, we also wished to assess P62 activity. We measured the protein level of p62 using Western blot analysis with p62-specific antibody (Sigma, St. Louis, MO) (24).
Proteasome Activity
As rapamycin is known to decrease protein synthesis and enhance protein degradation via autophagy, we wished to also observe what effect it might have on proteasome activity. Proteasome activity was measured as chymotrypsin-like enzyme activity of the 20S proteasome in heart, brain, liver, and kidney using a fluorescent substrate-based plate method as described elsewhere (25).
Food Consumption
Food consumption and body mass were measured as described elsewhere (26). Briefly, food consumption and body mass were measured weekly for 6 months in a subset of male and female control and rapamycin-fed mice (n = 15 per sex and treatment group), starting at 19 months of age (start of rapamycin feeding) until 25 months of age.
Body Composition
Lean mass, fat mass, and free water of each mouse were measured every month starting at 25 months of age (6 months of rapamycin treatment), using a quantitative nuclear magnetic resonance system (EchoMRI 3-in-1 System, Houston, TX).
Energetics and Spontaneous Activity
Resting metabolic rate, oxygen consumption, carbon dioxide production, and total metabolism were measured for a period of 24 hours using a MARS indirect calorimetry system (Sable Systems International, Las Vegas, NV). To assess total spontaneous activity and its temporal patterns, animals are individually housed for 36 hours in clear, plexiglass (40.6 × 22.9 × 14.0cm) cages surrounded by a 2.5-cm grid of infrared sensors in the x, y, and z plane with normal access to food, water, and bedding. Sensors record spontaneous activity through the monitoring period. Animals are acclimated for the first 12 hours, following which activity data are collected for 24 hours, one light and one dark phase.
Movement and Strength Parameters
Gait analysis.—
Gait analysis allows highly sensitive, noninvasive detection of many pathophysiological conditions including arthritis, neuromuscular, and skeletal muscle diseases. Mice were tested on the TreadScan (Clever Sys, Reston, VA) apparatus starting at 12cm/s. Treadmill speed is adjusted until the mouse can maintain a consistent walking speed for 5 minutes. This device uses a high-speed digital camera to record the reflected images of the footpads at 80 frames/s. The output is fed directly to a computer program that employs mouse-specific algorithms to analyze more than 40 gait parameters.
Rotarod.—
To assess balance and coordination, we trained mice to use the rotarod with an acceleration of 0.2 rotation/s starting from 4rpm during four sessions, spanning 2 weeks. The average of six trials during the final fifth session is used to compare rotarod performance (latency to fall).
Grip strength.—
We assessed fore- and hind-limb grip strength using a Grip Strength Meter with mesh grid pull bar (Columbus Instruments 1027 CSM) specifically designed for mice. Mice were allowed to grasp the pull bar with fore limbs and hind limbs and were then gradually pulled backward in a horizontal plane until they lost their grip. Maximum grip strength is recorded on the device. Mice are not trained prior to testing. The highest value from five consecutive trials is designated the mouse’s maximum grip strength.
Survival Analysis
Relative survival was assessed both by Kaplan–Meier analysis and by Cox proportional hazard model, with sex and rapamycin as additive predictor variables. A model with a term representing the interaction of sex and rapamycin was also fitted but was found to not have a significantly better fit than the simpler additive model reported here.
Pathology
Full gross and histopathology was performed. Pathological lesions were identified and graded for severity as previously described (1–4). When possible, cause of death was identified. In total, 111 distinct types of lesions were identified. To ensure reasonable statistical power, analyses were performed only when a disease was present in at least 11 mice (control and rapamycin groups combined). Only 14 lesions met this threshold in males, and only 9 lesions met the criterion in females. All but three mice had glomerulonephritis; therefore, its prevalence is not reported. Only disease prevalence is reported here. For definition of lesion categories, lesion incidence, and cause of death, see Supplementary Materials.
Statistical Methods
For each continuous outcome, we used a linear mixed-effect model to simultaneously assess the effects of the three-way analysis of variance model with the factors age group, sex, and treatment. We considered all two-way and three-way interactions. A random intercept was used to model the correlations induced by repeated measures. We considered both original scale and log transformations based upon the normality of the distribution of residuals assessed by visual inspection of histograms. We also evaluated the homogeneity of variances with respect to factors like group and age using the Breusch-Pagan test (27) against heteroscedasticity. The associations of factors and interactions were tested using Type II sums of squares. All statistical methods were implemented using the R Program v2.13 (Vienna, Austria) with the nlme (28) and the lme4 (29) packages for mixed-effect models.
Results
Rapamycin levels
Six months of rapamycin feeding to C57BL/6Nia mice beginning at 19 months of age resulted in whole-blood concentrations of ~3–4ng/mL rapamycin, as measured by high-performance liquid chromatography with tandem mass spectrometry (Supplementary Figure 1). There was no difference in rapamycin levels between rapamycin-fed males and females although rapamycin-fed females consumed significantly more food per gram body mass than rapamycin-fed males (repeated measures analysis of variance p = .004). These rapamycin levels are an order of magnitude lower than those previously reported in genetically heterogeneous mice fed the same diet initiated at roughly the same age (1) and about one-third the concentration found in those same heterogeneous mice with the diet initiated at 9 months of age (30). It is highly unlikely that differences in food consumption between the C57BL/6 used in this study and genetically heterogeneous mice used in previous studies could produce an order of magnitude difference in whole-blood levels of rapamycin; thus, mouse genetic background appears to have a significant effect on absorption or metabolism of rapamycin.
Organ-Specific mTOR Activity
To determine the effects of long-term rapamycin administration on mTOR signaling in vivo, we quantified the phosphorylation of ribosome subunit 6 (S6), a downstream target of the mTOR. Decreased phosphorylation of S6 indicates inhibition of mTOR signaling. We found a moderate (15%–30%) but significant reduction of phosphorylated S6 (pS6) in heart, liver, kidney, and white adipose tissue from rapamycin-fed animals compared with controls (Figure 1A and B). Surprisingly, rapamycin feeding did not similarly reduce S6 phosphorylation in homogenates of brain cortex, mid-brain, or hippocampus (data not shown), suggesting either an unequal distribution of rapamycin among tissues or a tissue-specific response to rapamycin in these areas of the brain.
Figure 1.
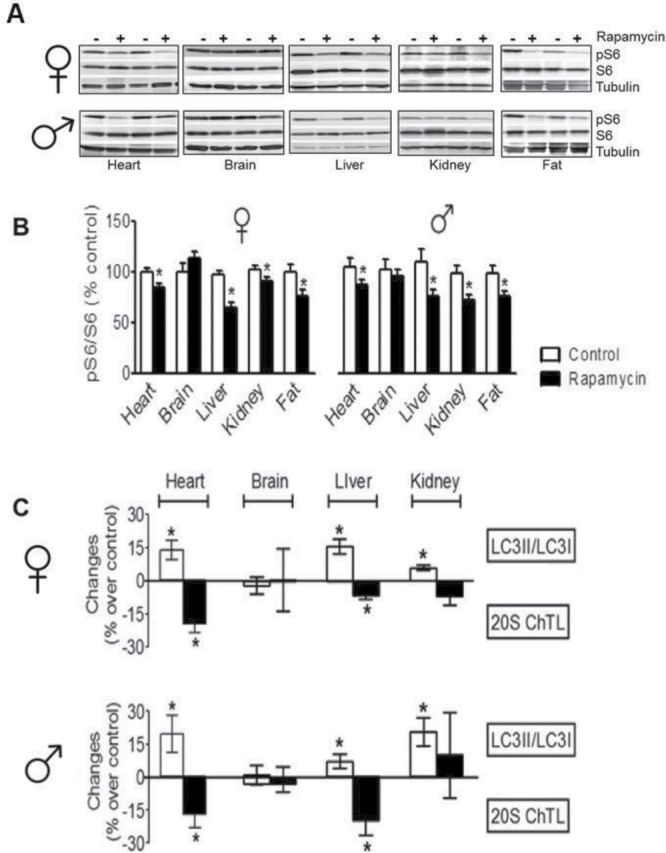
Inhibition of mTOR signaling and proteasome activity and enhancement of autophagy in multiple tissues of rapamycin-fed mice. (A and B) Effect of rapamycin on S6 and phosphorylated S6 (pS6). Cortex = brain cortex. (C) Effect of rapamycin on autophagy and proteasome activity. N > 12 in all cases. *p < .05.
Inhibition of mTOR is known to enhance autophagy, and increased autophagy has been reported to extend life in other organisms (31,32). We hypothesized that enhanced autophagy may be a major mechanism through which rapamycin extends life in mice. To examine relative autophagic activity, we measured the steady-state level of LC3 conversion (ie, the ratio of LC3II/LC3I), which indicates the volume of cellular autophagosomes (33). Rapamycin feeding increased LC3II/LC3I ratio significantly in liver, heart, and kidney tissues taken from rapamycin-fed mice compared with controls, indicating increased autophagy (Figure 1C, Supplementary Figure 5A). Rapamycin feeding also significantly increased autophagy in white adipose tissue, with a greater increase observed in females (37%) compared with males (14%; p = .049, data not shown). Consistent with the previous results showing no difference in pS6 activity in brain tissue, the LC3II/LC3I ratio in brain tissue did not differ between rapamycin-fed and control mice. We also measured the levels of p62 by Western blots as p62 has been reported to be degraded during autophagy (24). As the data in Supplementary Figure 5B show, we observed no significant change in p62 levels even though we observed changes in the LC3II/LC3I ratio in most tissues tested (Figure 1C, Supplementary Figure 5A). The utility of p62 as a marker of autophagy is somewhat controversial given that p62 is regulated both transcriptionally and posttranslationally (34), and several studies have found that p62 levels did not change during autophagy (35,36).
We also evaluated the impact of rapamycin feeding on proteasome activity, finding a significant reduction of 20S chymotrypsin-like activity in liver and heart tissues from rapamycin-fed males and females. There was no similar reduction in brain cortex or kidney (Figure 1C).
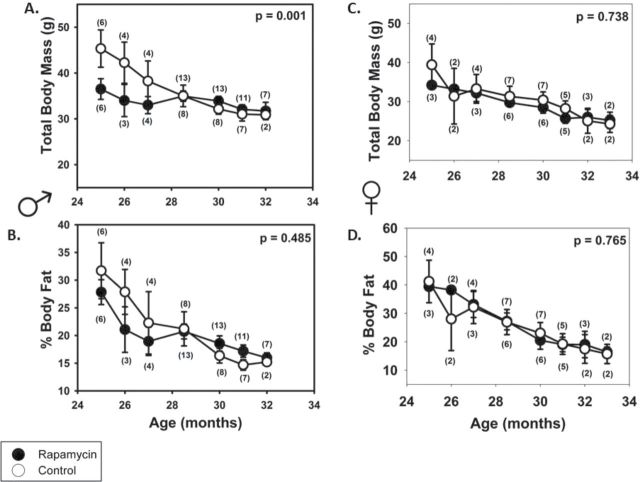
Body Composition
We observed a steady decline with age in total body mass and percent body fat from 25 months in all groups except rapamycin-fed males (Figure 2A and C), which maintained weight through 32 months of age. Neither rapamycin-fed females nor males differed in body mass from same-sex control-fed mice at the start of rapamycin feeding (age = 19 months, n = 15 per group, t test, p = .941 and p = .278, respectively); however, rapamycin-fed males were lighter than control males at 25 months. Given these results, it is surprising that food consumption per gram body mass did not differ between rapamycin-fed and control-fed males (p = .082, Supplementary Figure 4). In contrast, rapamycin-fed females consumed less food per gram body mass than controls, and that difference increased with increasing age (p = .005, Supplementary Figure 4). Given that control and rapamycin-fed females’ body mass did not differ at any age, it is highly unlikely that this difference in food consumption represents self-restriction. Regardless of treatment, females consumed more food per gram body mass than males at all ages (repeated measures analysis of variance p < .01). Previous work on genetically heterogeneous mice fed rapamycin from 20 months of age found no effect on weight gain (1), but when the same heterogeneous genotypes initiated rapamycin feeding at 9 months of age, there was a small but significant effect on weight gain (2). There were no differences among treatments in percent body fat at any age (Figure 2B and D).
Figure 2.
Body composition and rapamycin feeding. Total body mass is lower in (A) rapamycin-fed males at 25 mo of age (rapamycin treatment for 6 mo), but the mass difference gradually disappears with increasing age; however, rapamycin-fed males appear to maintain body mass at older ages compared with controls Treatment • Age • Sex p = .001. (B) Rapamycin-fed and control females do not differ in body mass at any age although both groups’ body mass declines with age. (C and D) Rapamycin feeding does not affect percent body fat in either sex at any age.
Metabolism and Spontaneous Activity
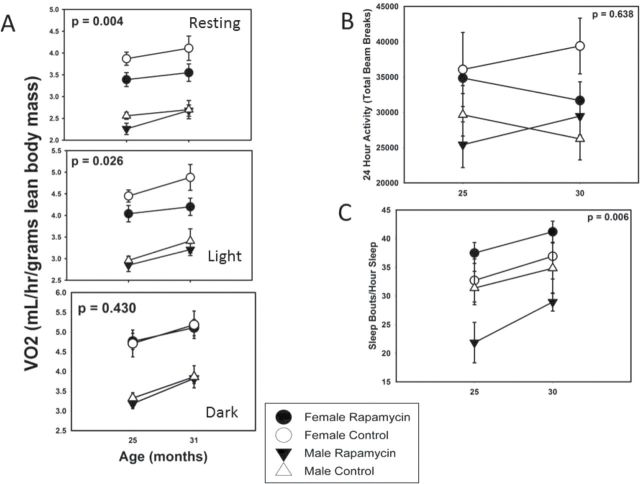
Surprisingly, metabolic rate per unit of lean body mass tended to increase with age in all groups between 25 and 31 months of age and was consistently lower for males relative to females (p << .001). Although there was no significant effect of rapamycin feeding during the dark (active) phase of the 24-hour light:dark cycle in either sex, there was a significant reduction in metabolism in rapamycin-fed females only during the light (inactive) phase and in resting metabolic rate (estimated using the 10 lowest metabolism values for an assessment period of 24 hours; Figure 3A). Note that this difference is not likely due to a difference in thermal insulation as neither body mass nor percent body fat was affected by rapamycin feeding in females (Figure 2C and D).
Figure 3.
Energetics and activity. (A) Metabolic rate was significantly reduced in females only by rapamycin feeding during the light (inactive) phase of 24-h light:dark cycle and when mice were resting; however, there was no effect during the dark (active) phase. Note that metabolic rate increased with age irrespective of treatment, and females always exhibited higher metabolic rate than males. (B) Total activity showed no clear pattern with age and was not affected by rapamycin feeding. (C) Sleep was increasingly fragmented with age (greater number of sleep bouts per hour of sleep), but rapamycin-fed males had less fragmented sleep than controls.
Variation in total 24-hour activity differed dramatically between individual animals, regardless of treatment. Given this variability, there was no effect of rapamycin feeding (p = .63) although there was a statistically significant effect of sex (Figure 3B, p = .009). Females were more active.
Temporal sleep patterns can be monitored in C57BL/6 mice by analyzing periods of inactivity of a critical length (37). Using this metric, sleep fragmentation, as assessed by number of sleep bouts per hour sleep, increased with age (Figure 3C, p = .028), as has been reported many times for both mice and humans. Overall, rapamycin feeding reduced sleep fragmentation (p = .006); however, the effect was strongly sex-specific. Upon analyzing the sexes separately, there was a nonsignificant trend for rapamycin-fed females to have more fragmented sleep than controls (p = .20), whereas rapamycin-fed males clearly had less fragmented sleep (p = .003). Rapamycin, in other words, maintained males—and only males—in a more youthful sleep pattern. This technique of high-throughput sleep assessment has only been validated in male C57BL/6 mice (33), so the implication of the female trend is unclear.
Movement and Strength Parameters
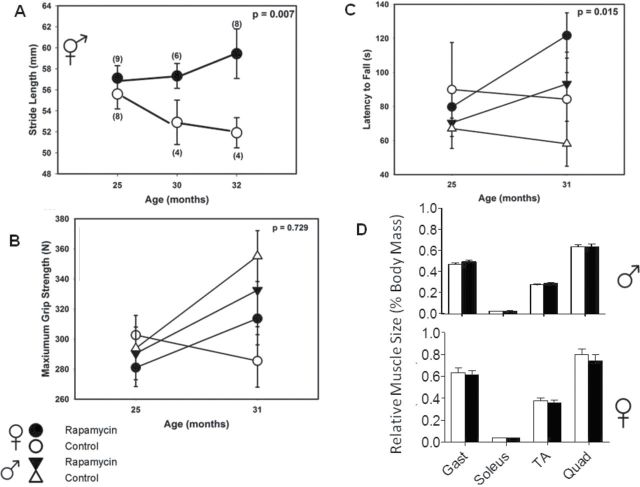
Rapamycin feeding affected numerous gait parameters in a non sex–specific manner. Therefore, we combined data for the two sexes. To simplify the analysis, we focused on one parameter with intuitive significance and human relevance—stride length—which is known to decline with age in humans (38). Stride length did indeed decline with age between 25 and 32 months in control mice but not in rapamycin-fed mice such that there was a statistically significant difference (p = .007) at 32 months of age (Figure 4A).
Figure 4.
Motor and muscular function. (A) Stride length declined with age in controls but showed no change in rapamycin-fed mice. (B) Grip strength was not affected by rapamycin feeding. (C) Rotarod performance improved with rapamycin feeding with similar patterns among the sexes. (D) There were no significant differences in hind-limb muscle mass between rapamycin-fed and control animals of either sex at 25 mo of age (6 mo of rapamycin treatment). Wet weight of each individual muscle normalized to whole-body weight (n = 18–23). Gast = gastrocnemius; TA = tibialis anterior; Quad = quadriceps.
Grip strength was not related to individual body mass (r 2 = .074, p = .10), so we analyzed grip strength without correcting for body size. We found no significant decline between 25 and 31 months of age and no statistically significant effect of rapamycin feeding (Figure 4B).
Rotarod performance (latency to fall) is known to be affected by body size, lighter individuals perform better. Indeed, we also found a weak but significant effect of body size (Supplementary Figure 2). However, as body size did not differ between rapamycin-fed mice and controls (Figure 2), we did not correct for body size in this analysis either. There were negligible differences between rapamycin-fed animals at 25 months of age (Figure 4C) but substantial and significant differences in both sexes by 31 months (p = .015) due to trends for increasing latency to fall in rapamycin-fed animals and decreasing latency in controls.
In sum, rapamycin feeding improved gait and rotarod performance and had no effect on grip strength.
Survival
Rapamycin feeding significantly diminished mortality in C57BL/6Nia mice even when initiated at 19 months of age according to a Cox Proportional Hazard model incorporating dietary treatment and sex; rapamycin-fed mice displayed lower mortality than controls, and males exhibited lower mortality than females (Table 1), with no interaction between sex and dietary treatment (p = .53, data not shown). A standard but less powerful Kaplan–Meier analysis on sexes individually indicated a significant improvement of survival in females and lack of a statistically significant effect in males (Figure 5).
Table 1.
Cox Proportional Hazard Model of Survival Data
| Coefficient | Exp (Coefficient) | SE | Z | p | |
|---|---|---|---|---|---|
| Sex (male vs female) | −0.3775 | 0.6856 | 0.1535 | −2.458 | .014 |
| Diet (rapa vs control) | −0.3584 | 0.6988 | 0.1547 | −2.317 | .021 |
Notes: Coefficients represent the log of the effect of the corresponding predictor variable (sex or treatment) on risk of death, and the exponentiated values in the following column are a direct estimate of risk of death for males relative to females and rapamycin-fed relative to control animals. SE and Z indicate the corresponding standard error and Z-score, respectively. The p value is the significance level for rejecting the null hypothesis that sex and diet have no effect. N = 180.
Figure 5.
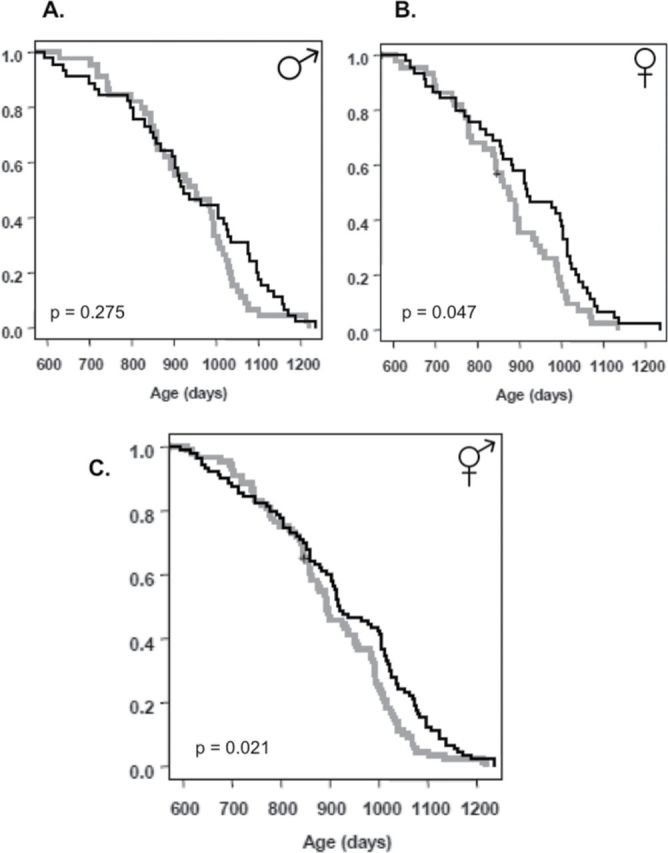
Survival. (A) Male control (n = 44) control and rapamycin-fed (n = 45), (B) Female control (n = 43) and rapamycin-fed (n = 45), and (C) Both sexes combined control (n = 87) and rapamycin fed (n = 90). Controls are shown in grey and rapamycin-fed mice in black. The survival curves were compared using the Cox-Mantel log-rank test, the p-values for males, females, and the sexes combined are shown; sex • treatment interaction was not significant (p = .532). A more powerful Cox Proportional Hazards Model found a significant effect of rapamycin feeding (p = .01) and sex (p = .02). See text for details.
Pathology
Rapamycin feeding begun in older C57BL/6 did not affect the prevalence of fatal pathological lesions (Table 2). Isolated effects on lesion prevalence and severity were found in males only and on disease burden in females only (Supplementary Tables 1 –3). Specifically, females fed rapamycin displayed lower numbers of neoplastic lesions (p = .008) and adenomas (p = .04) than controls (Figure 6). Given the false discovery rates associated with so many analyses, these statistically significant results should be interpreted with caution.
Table 2.
Pathological Lesions at Death (in Order From Most Common to Least Common)
| Control | Rapamycin | |
|---|---|---|
| Males | ||
| Lesion type | ||
| Seminal vesicle cysts | 0.91 (31/45) | 0.91 (39/43) |
| Lymphoma | 0.60 (27/45) | 0.56 (24/43) |
| Alveolar/bronchiolar adenoma | 0.40 (18/45) | 0.19 (8/43) |
| Kidney lymphocytic infiltrate | 0.33 (15/45) | 0.26 (11/43) |
| Salivary gland lymphocytic infiltrate | 0.30 (13/44) | 0.17 (7/42) |
| Kidney mineralization | 0.27 (12/45) | 0.26 (11/43) |
| Brain mineralization | 0.24 (11/45) | 0.33 (14/43) |
| Hepatic lymphocytic infiltrate | 0.24 (11/45) | 0.07 (3/43) |
| Subscapular adrenal hyperplasia | 0.21 (9/43) | 0.23 (9/40) |
| Hemangioma | 0.20 (9/45) | 0.05 (2/43) |
| Steatosis | 0.16 (7/45) | 0.12 (5/43) |
| Intestinal amyloid | 0.14 (6/44) | 0.26 (11/43) |
| Cardiac amyloid | 0.13 (6/45) | 0.16 (7/43) |
| Pulmonary lymphocytic infiltrate | 0.11 (5/45) | 0.14 (6/43) |
| Presumptive cause of death | ||
| All neoplasia | 0.62 (28/45) | 0.49 (21/43) |
| Nonneoplasia | 0.24 (11/45) | 0.26 (18/43) |
| Females | ||
| Lesion type | ||
| Lymphoma | 0.88 (36/41) | 0.91 (40/44) |
| Subscapular adrenal hyperplasia | 0.71 (29/41) | 0.73 (32/44) |
| Pituitary adenoma | 0.60 (21/35) | 0.49 (16/33) |
| Endometrial hyperplasia | 0.59 (24/41) | 0.65 (28/43) |
| All ovarian lesions | 0.26 (10/38) | 0.18 (7/39) |
| Thyroid adenoma | 0.24 (9/38) | 0.19 (7/37) |
| Thyroid hyperplasia | 0.21 (8/38) | 0.19 (7/37) |
| Brain mineralization | 0.17 (7/41) | 0.18 (8/44) |
| Kidney mineralization | 0.17 (7/41) | 0.21 (9/44) |
| Presumptive cause of death | ||
| All neoplasia | 0.73 (30/41) | 0.70 (31/44) |
| Nonneoplasia | 0.22 (9/41) | 0.16 (7/44) |
Figure 6.
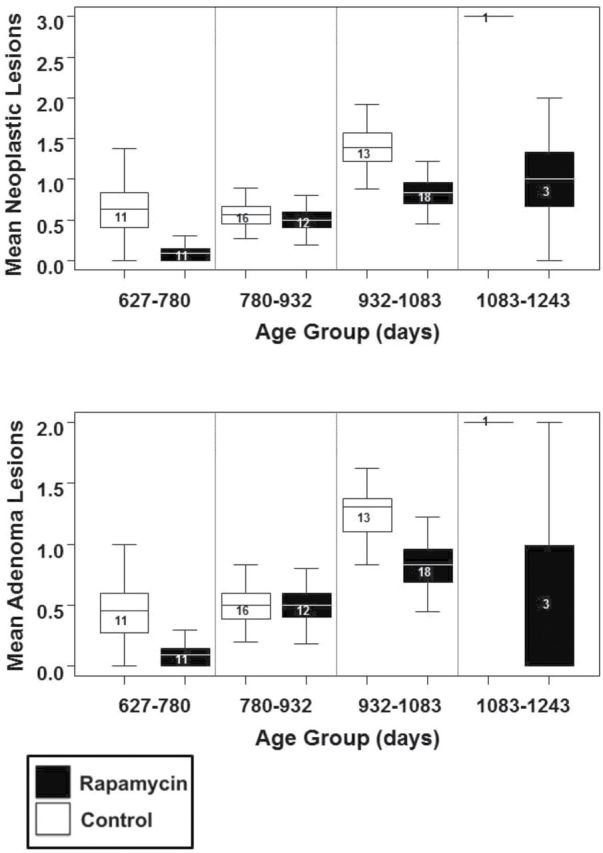
Effect of rapamycin on pathology burden in females. (A) Neoplastic lesion burden increases with age but is significantly lower in rapamycin-fed females than controls (p = .008, false discovery rate = 4.1%). (B) Adenoma burden also increases with age but is significantly lower in rapamycin-fed females than controls (p = .04, false discovery rate = 9.7%). Box plots mean and 75% CI, whiskers 95% CI sample size within box.
Discussion
We have shown that chronic administration of enteric rapamycin initiated at 19 months of age improves survival and enhances multiple measures of health in later life in C57BL/6Nia mice. This is not the first report of extended longevity due to rapamycin in C57BL/6 mice. Rapamycin treatment in 22- to 24-month-old males of the same genotype was previously reported to increase longevity and preserve hematopoetic stem cell function (39). We found a number of additional health benefits, however, including maintenance of more youthful late-life stride length and rotarod performance in both sexes, as well as preservation of body weight and reduced sleep fragmentation in males only. Only in females, we observed reduced resting and total metabolic rate during the light (inactive) phase of their 24-hour light:dark cycle. Because females in our two groups did not differ in body composition, this lower resting metabolic rate suggests that body temperature might possibly be reduced in this sex only. There was no impact in either sex on metabolic rate during the active (dark) phase of the 24-hour light:dark cycle or on total amount of spontaneous activity. Rapamycin-fed females also exhibited a greater survival enhancement than males, as has been previously reported in genetically heterogeneous mice (1,2). Sex differences were also present in end-of-life pathology, with males experiencing reduced prevalence and severity of some lesions and females displaying reduced neoplastic disease burden relative to age-matched controls (Supplementary Tables 1 – 4).
Sex specificity of treatments that retard aging and/or extend life has been seen repeatedly in mice (40–44) and flies (45,46), the two model systems in which both sexes are commonly studied. However, the sex specificity of effects in our study are particularly complex, with males seemingly more beneficially affected in terms of late-age body weight maintenance, sleep pattern, and some measures of pathology and females more beneficially affected in longevity and other measures of pathology. Sex differences in response to life-extending treatments appears to be a widespread phenomenon that deserves more attention, particularly because in humans, men and women age differently in similarly complex ways (47).
A completely unanticipated result was the improvement in sleep consolidation in male mice resulting from rapamycin feeding. Although rapamycin has been previously reported to have various psychotropic effects, improvement in sleep is not one of these effects although it is not clear whether it has ever been investigated. It is tempting to speculate that some of the rapamycin-induced cognitive and noncognitive behavioral benefits, such as reduced depression and anxiety (48,49), reported recently may be due to improved sleep. Sleep fragmentation is known to compromise cognitive function (50). Conversely, it is possible that the improved sleep consolidation is a consequence of reduced depression and anxiety, which may still benefit cognitive function.
Blood levels of rapamycin in these C57BL/6Nia mice were about an order of magnitude lower than that in genetically heterogeneous mice fed the same diet from about the same age (1), indicating a clear genetic background effect in either drug absorption or metabolism. However, decreased phosphorylation of S6K and increased autophagy in multiple tissues both indicate that rapamycin at this blood level effectively inhibits mTOR signaling in C57BL/6 mice as it does in low-dose rapamycin chemotherapy in humans (51).
Interestingly, in a number of the tissues in which autophagy was significantly increased, proteasome activity was simultaneously decreased (Figure 1C). As inhibition of mTOR is known to reduce the rate of protein synthesis and increase protein degradation via autophagy (10), it may be that, as yet undescribed, cellular homeostatic mechanisms compensatorily inhibit other protein degradation pathways such as the ubiquitin–proteasome system. Somewhat unexpectedly, we note that inhibition of mTOR in the brain was not detected in these experiments. This was unexpected because of the previously demonstrated cognitive benefits seen with rapamycin treatment (16,48) and the fact that rapamycin is known to cross the blood–brain barrier (52). However, the phosphorylation status of S6 has been reported to be a poor biomarker of mTOR inhibition in the brain (52). In addition, cognitive benefits have not been reported in mice fed rapamycin at such a late age as in this study.
There were a priori reasons to suspect that rapamycin feeding might have some unwanted side effects on health. For instance, mTORC1 activity has been shown to be essential for muscle repair and maintenance, and it has been shown that rapamycin can inhibit muscle hypertrophy and recovery from atrophy in vivo (53,54). Rapamycin has also been reported to inhibit recovery from injury and growth in the dorsal root ganglia of the peripheral nervous system (55). Moreover, a previous study on genetically heterogeneous mice fed rapamycin from a much younger age (9 months) showed accelerated cataract development and testicular degeneration by the time of sacrifice at 22 months (56). However, in our study in which rapamycin feeding was initiated, and health assessed, much later in life, far from damaging motor function, coordination, or balance in aging mice, rapamycin-fed mice had either enhanced function or no worse function than age-matched controls. We did not investigate cataract formation, and the age at which our mice were necropsied, testicular degeneration was considerably more advanced in both treated and control males.
Similarly, there have been previous reports that inhibition of mTOR harms long-term memory in mice (20,21). However, identical doses of enteric rapamycin to that used here have been reported to prevent normal age-related cognitive decline (16,48). Furthermore, rapamycin is used in combination with other drugs as an immunosuppressive agent (57); however, rapamycin alone has been reported to improve vaccine response in older mice (39) and to protect them from some respiratory pathogens (58). It is not clear at present why these potential side effects have not been seen in our studies. However, one possibility is that rapamycin enterically delivered in normal chow maintains a low-to-moderate continuous level of bioavailability compared with when the same drug is received as an injectable bolus as is commonly done in mice or orally, where circulating levels may spike to very high levels and then gradually taper off before spiking again at the next treatment.
It is important to emphasize that the results observed here are specific to C57BL/6Nia mice chronically fed rapamycin beginning relatively late in life (19 months of age). We did not observe some effects such as slower loss of spontaneous activity, which have been seen in mice treated with rapamycin earlier in life (2,30). Moreover, although we saw a delay is some pathologies, we did not see the robust delay in pathological lesions that have been reported when rapamycin feeding is begun earlier (30). Age of initiation and duration of rapamycin administration clearly affects its organismal impact. For instance, UM-HET3 mice when fed diets containing 14 ppm microencapsulated rapamycin from 9 to about 26 months of age achieved blood levels of 60–70ng/mL, whereas the same genotype fed the same diet from 3 to 4 months of age for the next 6 months only achieved a mean of 13.4ng/mL (1,30). By comparison, our C57BL/6Nia mice on the same rapamycin diet from 19 to 25 months of age exhibited blood levels of only 3–4ng/mL. So there is a genotype and an age effect.
In sum, we have documented that enteric rapamycin fed from 19 months of age has beneficial effects on both life span and health span of C57BL/6Nia mice and that a number of these effects are sex specific. Several potentially unwanted side effects have not been seen in these mice at this dose and this route of administration.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/.
Funding
This work was supported by National Institutes of Health grant RC2 AG036613 and the University of Texas Health Science Center at San Antonio Nathan Shock Center of Excellence in the Biology of Aging (P30 AG13319).
Acknowledgments
We thank John Ramos, Randy Sharp, and Elizabeth Fernandez for technical assistance. We also thank three anonymous reviewers and the editors for helpful comments.
References
- 1. Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66:191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Anisimov VN, Zabezhinski MA, Popovich IG, et al. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236 [DOI] [PubMed] [Google Scholar]
- 4. Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Medvedik O, Lamming DW, Kim KD, Sinclair DA. MSN2 and MSN4 link calorie restriction and TOR to sirtuin-mediated lifespan extension in Saccharomyces cerevisiae. PLoS Biol. 2007;5:e261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Powers RW, III, Kaeberlein M, Caldwell SD, Kennedy BK, Fields S. Extension of chronological life span in yeast by decreased TOR pathway signaling. Genes Dev. 2006;20:174–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kapahi P, Chen D, Rogers AN, et al. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 2010;11:453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kaeberlein M, Powers RW, III, Steffen KK, et al. Regulation of yeast replicative life span by TOR and Sch9 in response to nutrients. Science. 2005;310:1193–1196 [DOI] [PubMed] [Google Scholar]
- 9. Katewa SD, Kapahi P. Role of TOR signaling in aging and related biological processes in Drosophila melanogaster. Exp Gerontol. 2011;46:382–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484 [DOI] [PubMed] [Google Scholar]
- 11. Adelman SJ. Sirolimus and its analogs and its effects on vascular diseases. Curr Pharm Des. 2010;16:4002–4011 [DOI] [PubMed] [Google Scholar]
- 12. Heng DY. Combination therapy in metastatic renal cell carcinoma. Lancet Oncol. 2011;12:613–614 [DOI] [PubMed] [Google Scholar]
- 13. Caccamo A, Majumder S, Richardson A, Strong R, Oddo S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J Biol Chem. 2010;285:13107–13120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spilman P, Podlutskaya N, Hart MJ, et al. Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-beta levels in a mouse model of Alzheimer’s disease. PLoS One. 2010;5:e9979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pakala R, Stabile E, Jang GJ, Clavijo L, Waksman R. Rapamycin attenuates atherosclerotic plaque progression in apolipoprotein E knockout mice: inhibitory effect on monocyte chemotaxis. J Cardiovasc Pharmacol. 2005;46:481–486 [DOI] [PubMed] [Google Scholar]
- 16. Majumder S, Caccamo A, Medina DX, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith JM, Nemeth TL, McDonald RA. Current immunosuppressive agents: efficacy, side effects, and utilization. Pediatr Clin North Am. 2003;50:1283–1300 [DOI] [PubMed] [Google Scholar]
- 18. Dickinson JM, Fry CS, Drummond MJ, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aoyagi T, Kusakari Y, Xiao CY, et al. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2012;303:H75–H85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stoica L, Zhu PJ, Huang W, Zhou H, Kozma SC, Costa-Mattioli M. Selective pharmacogenetic inhibition of mammalian target of Rapamycin complex I (mTORC1) blocks long-term synaptic plasticity and memory storage. Proc Natl Acad Sci U S A. 2011;108:3791–3796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Parsons RG, Gafford GM, Helmstetter FJ. Translational control via the mammalian target of rapamycin pathway is critical for the formation and stability of long-term fear memory in amygdala neurons. J Neurosci. 2006;26:12977–12983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larsen JL, Bennett RG, Burkman T, et al. Tacrolimus and sirolimus cause insulin resistance in normal sprague dawley rats. Transplantation. 2006;82:466–470 [DOI] [PubMed] [Google Scholar]
- 23. Kabeya Y, Mizushima N, Ueno T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–5728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bjørkøy G, Lamark T, Johansen T. p62/SQSTM1: a missing link between protein aggregates and the autophagy machinery. Autophagy. 2006;2:138–139 [DOI] [PubMed] [Google Scholar]
- 25. Lima CF, Rattan SI. Determination of proteasomal activities. In: Bross P, Gregersen N, eds. Protein Misfolding and Cellular Stress in Disease and Aging. Newark, NJ: Springer; 2010:183–192 [Google Scholar]
- 26. Ikeno Y, Hubbard GB, Lee S, et al. Housing density does not influence the longevity effect of calorie restriction. J Gerontol A Biol Sci Med Sci. 2005;60:1510–1517 [DOI] [PubMed] [Google Scholar]
- 27. Zeileis A, Hothorn T. Diagnostic checking in regression relationships. R News. 2002;2:7–10 [Google Scholar]
- 28. Pinheiro J, Bates D, DebRoy S, Sarkar D. NLME: mixed effects models. R package version 3 2007. 1–112 http://cran.r-project.org/web/packages/nlme/index.html Accessed January 10, 2013.
- 29. Bates D, Maechler M, Bolker B. LME4: linear mixed-effects models using S4 classes. R package version 0.999375 -39 http://cran.r-project.org/web/packages/lme4/index.html Accessed January 10, 2013.
- 30. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat Cell Biol. 2010;12:842–846 [DOI] [PubMed] [Google Scholar]
- 32. Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Klionsky DJ, Abeliovich H, Agostinis P, et al. Guidelines for the use and interpretation of assays for monitoring autophagy in higher eukaryotes. Autophagy. 2008;4:151–175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Puissant A, Fenouille N, Auberger P. When autophagy meets cancer through p62/SQSTM1. Am J Cancer Res. 2012;2:397–413 [PMC free article] [PubMed] [Google Scholar]
- 35. Puissant A, Robert G, Fenouille N, et al. Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 2010;70:1042–1052 [DOI] [PubMed] [Google Scholar]
- 36. Colosetti P, Puissant A, Robert G, et al. Autophagy is an important event for megakaryocytic differentiation of the chronic myelogenous leukemia K562 cell line. Autophagy. 2009;5:1092–1098 [DOI] [PubMed] [Google Scholar]
- 37. Pack AI, Galante RJ, Maislin G, et al. Novel method for high-throughput phenotyping of sleep in mice. Physiol Genomics. 2007;28:232–238 [DOI] [PubMed] [Google Scholar]
- 38. Judge JO, Davis RB, III, Ounpuu S. Step length reductions in advanced age: the role of ankle and hip kinetics. J Gerontol A Biol Sci Med Sci. 1996;51:M303–M312 [DOI] [PubMed] [Google Scholar]
- 39. Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal. 2009;2:ra75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187 [DOI] [PubMed] [Google Scholar]
- 41. Bokov AF, Garg N, Ikeno Y, et al. Does reduced IGF-1R signaling in Igf1r+/- mice alter aging? PLoS One. 2011;6:e26891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Selman C, Tullet JM, Wieser D, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science. 2009;326:140–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7:641–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Selman C, Lingard S, Choudhury AI, et al. Evidence for lifespan extension and delayed age-related biomarkers in insulin receptor substrate 1 null mice. FASEB J. 2008;22:807–818 [DOI] [PubMed] [Google Scholar]
- 45. Tatar M, Kopelman A, Epstein D, Tu MP, Yin CM, Garofalo RS. A mutant Drosophila insulin receptor homolog that extends life-span and impairs neuroendocrine function. Science. 2001;292:107–110 [DOI] [PubMed] [Google Scholar]
- 46. Clancy DJ, Gems D, Harshman LG, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106 [DOI] [PubMed] [Google Scholar]
- 47. Austad SN. Sex differences in longevity and aging. In: Masoro EJ, Austad SN, eds. The Handbook of the Biology of Aging. San Diego, CA: Academic Press; 2011:479–496 [Google Scholar]
- 48. Halloran J, Hussong SA, Burbank R, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience. 2012;223:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Majumder S, Caccamo A, Medina DX, et al. Lifelong rapamycin administration ameliorates age-dependent cognitive deficits by reducing IL-1β and enhancing NMDA signaling. Aging Cell. 2012;11:326–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Durmer JS, Dinges DF. Neurocognitive consequences of sleep deprivation. Semin Neurol. 2005;25:117–129 [DOI] [PubMed] [Google Scholar]
- 51. Heuer M, Benkö T, Cicinnati VR, et al. Effect of low-dose rapamycin on tumor growth in two human hepatocellular cancer cell lines. Transplant Proc. 2009;41:359–365 [DOI] [PubMed] [Google Scholar]
- 52. Banerjee S, Gianino SM, Gao F, Christians U, Gutmann DH. Interpreting mammalian target of rapamycin and cell growth inhibition in a genetically engineered mouse model of Nf1-deficient astrocytes. Mol Cancer Ther. 2011;10:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bentzinger CF, Romanino K, Cloëtta D, et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008;8:411–424 [DOI] [PubMed] [Google Scholar]
- 54. Bodine SC, Stitt TN, Gonzalez M, et al. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019 [DOI] [PubMed] [Google Scholar]
- 55. Abe N, Borson SH, Gambello MJ, Wang F, Cavalli V. Mammalian target of rapamycin (mTOR) activation increases axonal growth capacity of injured peripheral nerves. J Biol Chem. 2010;285:28034–28043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wilkinson JE, Burmeister L, Brooks SV, et al. Rapamycin slows aging in mice. Aging Cell. 2012;11:675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Charbonnier LM, Le Moine A. Rapamycin as immunosuppressant in murine transplantation model. Methods Mol Biol. 2012;821:435–445 [DOI] [PubMed] [Google Scholar]
- 58. Hinojosa CA, Mgbemena V, Van Roekel S, et al. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol. 2012;47:958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]