‘The Emperor's New Clothes’ is the title of a famous fairy tale from the Danish writer Hans Christian Andersen (1805–75). Two weavers promised an Emperor a splendid suit of clothes, supposedly invisible to those unfit for their positions, stupid or incompetent. When the Emperor paraded before his subjects in his new clothes, a child said: ‘But he isn't wearing anything at all!’ This was whispered from person to person until everyone in the crowd was shouting that the emperor had nothing on. The emperor heard it, but held his head high and finished the procession.
FROM SYMPLICITY HTN-2 TO SYMPLICITY HTN-3: A PREDICTABLE FAILURE
The Symplicity studies [1–3] demonstrated the feasibility of catheter-based endovascular sympathetic renal denervation (RDN) in drug-resistant hypertension. In Symplicity HTN-2, the first randomized trial testing the efficacy and safety of RDN plus medical treatment versus medical treatment only, blood pressure (BP) decreased by 32/12 mmHg (P < 0.0001) in the RDN-treated group, whereas it remained unchanged in the control group (1/0 mmHg, P ≥ 0.77) [2]. No serious procedure-related or device-related complications occurred, and the incidence of adverse events was similar in both groups [2]. The Symplicity HTN-2 trial was small (∼50 patients/group), with short-term follow-up (6 months), and the primary endpoint was based on an unblinded assessment of office rather than 24-h ambulatory BP [2], which is the gold standard. In the accompanying editorial, Doumas and Doumas [4] emphasized additional limitations of the trial, including the lack of per-protocol exclusion of white-coat and secondary hypertension, the variable and unpredictable BP response and the low overall rate of BP control after RDN (39%) [4]. According to the Editorialists, RDN might evolve into a novel treatment modality of hypertension but ‘the jury was still out’ [4].
Despite this wise statement, RDN was rapidly and aggressively promoted as an established treatment of drug-resistant hypertension. Dozens of editorial and reviews were published, most of which were pure eulogies, without critical or balanced assessment of the existing evidence. The Symplicity investigators themselves questioned the interest of identifying responder profiles [3]. Allegedly, with an underlying 90% response rate in conjunction with the low observed morbidity [3], RDN was to be applied indiscriminately to all patients with resistant hypertension. Based on shaky evidence derived from a single short-term randomized trial with unblinded endpoint assessment [2], the speculation was that RDN ‘could potentially help alleviate some of the $500 billion impact that hypertension has on our health care systems by reducing or eliminating costly and lifelong medication use’ [5] and pharmaco-economic models were hastily elaborated to demonstrate the cost-effectiveness of the technique [6–7]. The hype generated by the Simplicity studies [1–3] prompted >10 companies to engage in the footsteps of Ardian–Medtronic® and to develop their own RDN system, five of which obtained the CE mark (http://en.wikipedia.org/wiki/Renal_sympathetic_denervation). While RDN remained an experimental procedure in the USA, pending the results of Symplicity HTN-3 [8] (NCT01418261), a large randomized controlled trial including a sham procedure, reimbursement of RDN was granted in Germany, Australia, the Netherlands, Switzerland and Sweden, leading to an outburst in the number of procedures performed (>5000 worldwide, of which 600 in a single German centre) (http://www.spiegel.de/spiegel/print/d-102241746.html). RDN was applied indiscriminately to patients with white-coat resistant hypertension [9], isolated systolic hypertension [10], stenotic [11] or stented [12] renal arteries and even in a patient with Münchausen syndrome [13]. Finally, based almost exclusively on observational, small studies, RDN was proposed as a potential treatment in a host of medical conditions, including chronic kidney disease, atrial fibrillation, heart failure, obstructive sleep apnoea, metabolic syndrome and polycystic ovary syndrome [14].
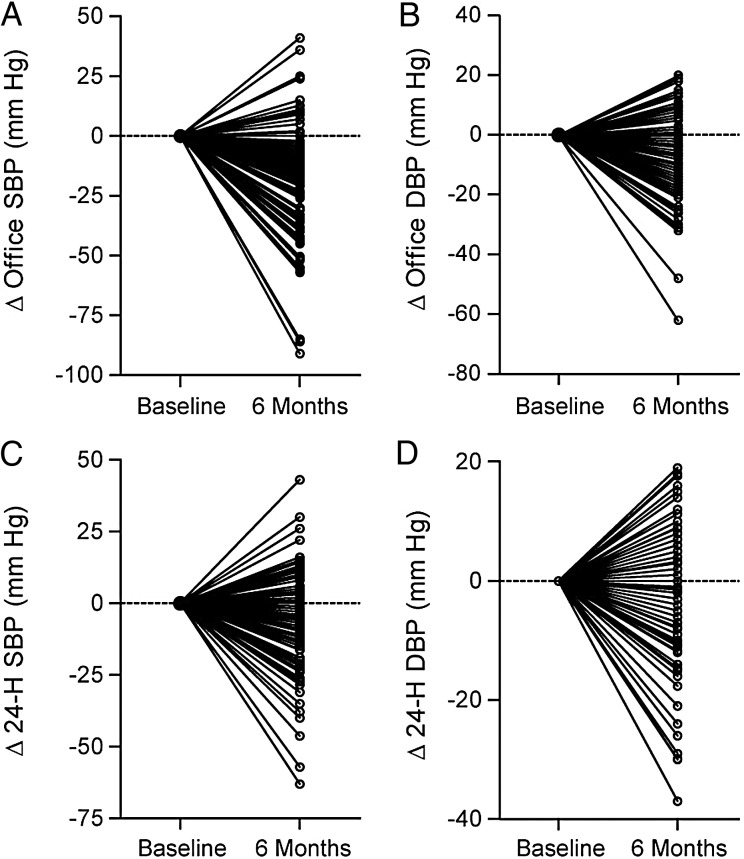
While dissenting voices [5, 15] remained almost unheard in the triumphal symphony of RDN, the first cautionary message came with the presentation and subsequent publication [16] of a patient-level meta-analysis, including BP results obtained in 109 patients with resistant hypertension who underwent RDN within the European Network COordinating research on Renal Denervation (ENCOReD). Six months after RDN, office BP decreased by 14.2/6.5 mmHg and 24-h BP by only 5.2/3.1 mmHg (baseline and centre-adjusted results, P < 0.001 for both). Pill burden remained virtually unchanged (number of antihypertensive drugs: 4.4 versus 4.7 at baseline, P = 0.001). Individual BP responses were highly variable, with more than one-third of patients showing BP increases after the procedure [16] (Figure 1). ‘Belgian Study rains on Renal Denervation Parade’ wrote an editorialist (http://www.medlatest.com/2013/06/18/belgian-study-rains-renal-denervation-parade/). Notwithstanding some other, more critical assessments of the efficacy and safety of the technique [17, 18], the parade went on apparently undisturbed until the debacle caused by the announcement that Symplicity HTN-3 [8] had failed to reach its primary efficacy endpoint (http://www.tctmd.com/show.aspx?Id=123265). This news had a devastating impact on the device industry, leading to definitive or temporary stopping of large ongoing research programmes (http://www.reuters.com/article/2014/01/24/us-covidien-divestment-idUSBREA0N18B20140124).
FIGURE 1:
Individual responses of systolic (A, C) and diastolic (B, D) blood pressure on office (A, B) and 24-h ambulatory (C, D) measurement after renal denervation in 109 patients from the ENCOReD network. These results highlight the crucial importance of identification of responder profiles before renal denervation is considered an established treatment of resistant hypertension (adapted from reference [16], with authorization).
To us, however, the failure of Symplicity HTN-3 [8] was not a major surprise. We had since long considered this as a reasonable working hypothesis in view of the multiple weaknesses and potential biases of current studies [5, 14, 19, 20]. In particular, the rationale, mechanism of action, efficacy and safety of the technique rest on weak and fragmentary evidence.
RENAL DENERVATION: CRITICAL APPRAISAL OF THE EVIDENCE
Pathophysiological basis of renal denervation
The development of catheter-based RDN was based on several assumptions [21]: (i) excessive activation of the sympathetic nervous system is a major player in the pathogenesis of resistant hypertension, (ii) disruption of fibres located within the adventitia of renal arteries leads to inhibition of the efferent pathway, with subsequent decrease in salt and water retention and renin release and increased renal plasma flow, (iii) part of the benefit of RDN is mediated by disruption of the afferent pathway, leading to decreased cardiac hypertrophy and vascular remodelling and (iv) current RDN systems allow efficient and reproducible inhibition of this bidirectional traffic, leading to predictable BP responses.
Unfortunately, despite performance of thousands of RDN procedures worldwide, most data establishing the role of the sympathetic nervous system in resistant hypertension are still limited to extrapolation of animal data, indirect evidence derived from hypothetical pathophysiological processes and data on file. Direct evidence suggesting an increased contribution of the sympathetic nervous system in resistant versus controlled or uncontrolled non-resistant hypertension is lacking. While in Symplicity HTN-1 [1], Krum et al. reported a 47% decrease (95% CI, 28–65%) in whole-body spill-over of norepinephrine from baseline to 15–30 days after the procedure, the effects of RDN on peripheral sympathetic activity assessed by microneurography and on baroreflex sensitivity are still controversial [22, 23]. The impact of RDN on salt and water reabsorption and renal plasma flow also remains undocumented. Furthermore, per-procedural tests allowing to check the completeness of RDN and to benchmark the reproducibility and efficacy of different renal ablation systems are currently unavailable. Finally, conditions associated with sympathetic overactivity, such as obesity or renal dysfunction, were not associated with an increased likelihood of BP improvement after RDN [5]. On the contrary, preliminary analysis of the ENCOReD registry [16] shows an inverse association of the BP response to RDN with serum creatinine and body mass index.
Efficacy of RDN
Given the fact that 24h-ABPM is an independent predictor of cardiovascular events in patients with resistant hypertension, while, after adjustment for traditional risk factors, office BP has little predictive value [24], evaluation of the efficacy of RDN should primarily be based on out-of-the-office BP. Unfortunately, 24-h ambulatory BP decrease after RDN was seldom reported and when available was not always significant [20]. In larger cohorts [2, 16], it was only one-third of office BP decrease, compared with the expected two-thirds observed in drug trials [5]. This discrepancy between office and ambulatory BP decrease after RDN likely reflects an overestimation of the true effect of RDN on office BP due to Hawthorne effect, regression to the mean and observer-related biases. Furthermore, individual BP responses to RDN are highly variable [16, 20], with more than one-third of patients experiencing an increase rather than a decrease in ambulatory BP [16]. Finally, in a small Norwegian cohort [25] including patients carefully selected after witnessed intake of drugs, both office and 24-h ambulatory BP remained unchanged after RDN, raising further concerns about the residual effect of RDN in drug-adherent, truly resistant hypertensive patients [20].
Safety of RDN
The Simplicity HTN-1 registry [3, 26] shows a substantial decrease of estimated glomerular filtration rate (eGFR) 2 and 3 years after RDN. In the absence of a control group and detailed medication history, whether this corresponds to the natural history of the disease, reflects changes in diuretics and/or antihypertensive drugs or is due to an effect of RDN per se remains unclear. Notably also, in the EnligHTN1 trial [27], eGFR decreased significantly from 87 to 82 mL/min/1.73 m2 6 months after RDN (P = 0.004). The safety of RDN for renal function remains thus to be demonstrated.
Potential damage to the renal arteries induced by RDN is another matter of concern. In seven swines euthanized 6 months after RDN [28], the renal arteries showed fibrosis from 10 to 25% of the total media and the underlying adventitia. In man, optical coherence tomography disclosed diffuse renal artery constriction and local tissue damage after RDN, with oedema and thrombus formation at the ablation site [29]. Furthermore, several cases of renal artery stenosis or stenosis progression have been reported with different catheters [14]. Unfortunately, evaluation of renal arteries in Symplicity HTN- 2 [2] was limited to renal duplex in most cases (37/43) and was also suboptimal in the Symplicity HTN-1 registry [3]. In the absence of systematic, state-of-the-art renal artery imaging in randomized studies, the incidence and prognosis of renal artery stenosis post-RDN remain to be established.
Rationale underlying widespread use of RDN
Besides efficacy and safety of the procedure, the rationale underlying a widespread use of RDN in drug-resistant hypertension rests on several assumptions: (i) drug-resistant hypertension is frequent, (ii) in most patients with resistant hypertension, BP control cannot be achieved by drugs and (iii) a large proportion of patients with resistant hypertension are eligible for the procedure.
A short review of the evidence shows that none of these claims is justified. Indeed, while the prevalence of apparently resistant hypertension is estimated to be 10–15% of the treated hypertensive population [5], exclusion of patients with white-coat resistant hypertension (∼30–40%) [30] and poor treatment adherence (up to 50%) would lead to a much lower proportion. Furthermore, as demonstrated in a multicentre cohort of 731 patients, a substantial proportion of patients with resistant hypertension can reach BP control after skilful treatment adjustment in expert centres, removing the indication of RDN [31]. Finally, many other patients do not qualify for RDN due to secondary hypertension, morbid obesity or unsuitable renal anatomy, dramatically reducing the proportion of patients eventually amenable to RDN [32–33].
Moving ahead
The story of RDN uncontrolled deployment in the absence of solid evidence of efficacy and safety raises concerns. It shows what happens when scientists and health authorities cannot assume their responsibility in a concerted way, leaving the way open for market-driven, short view strategies, and thus exposing thousands of patients to unnecessary and potentially harmful procedures. We now know that the giant had feet of clay, as the sole announcement of the failure of Symplicity HTN-3 [8] to reach its primary endpoint proved sufficient to put a stop to large and costly industry-driven research programmes, even before the results of the trial were in the public domain.
Does this mean the end of RDN? In no way. Rather, it points to the urgent need for more and better evidence [14] before further clinical application of the technique. RDN is not—and probably will never be—the standard treatment for all patients, whose BP remains uncontrolled on triple antihypertensive therapy. From a public health perspective, it may prove more cost-effective to promote education of both patients and physicians and improve drug treatment and drug adherence. Indeed, as previously discussed, in expert hands, most patients with difficult-to-treat or resistant hypertension can achieve BP control. Furthermore, a substantial proportion of those who remain uncontrolled will not qualify for RDN, due to irreversibly stiffened arteries, advanced renal failure or unsuitable renal anatomy. Still, the technique may prove efficient and safe in a subset of patients in whom all efforts to obtain BP control failed.
To identify potential responders to RDN, using state-of-the-art end points and methods to evaluate safety and efficacy, we designed the INSPiRED randomized controlled trial (NCT01505010) [34]. Compared with previous and ongoing trials, INSPiRED has unique features: (i) exclusion of patients with chronic kidney disease stage 3 (<60 mL/min/1.73 m2) and/or isolated systolic hypertension, (ii) age limit <70 years, (iii) drug treatment optimization with systematic assessment of adherence throughout study, use of single-pill combinations including up to three antihypertensive agents and long-acting ‘forgiving’ drugs, (iv) state-of-the-art renal artery imaging by magnetic resonance or preferably computerized tomographic angiography, (v) validation of urinary proteomic biomarkers to predict BP responses and changes in renal function, (vi) out-of-the-office BP for patient selection and follow-up, (vii) safety assessment based on glomerular filtration rate estimated according to both Chronic Kidney Disease Epidemiology Collaboration and Modification of Diet in Renal Disease formulas, (viii) extension of the follow-up beyond 6 months up to 3 years to assess the incidence of morbidity and mortality, (ix) use of RDN systems with a design different from the Symplicity catheter and (x) use of heart rate variability in all patients and renal nerve stimulation in selected centres to assess the completeness of RDN acutely and chronically. Along with other randomized trials, such as the Oslo RDN (NCT01673516) and DENER-HTN (NCT01570777) trials, INSPiRED will provide decisive information on safety, efficacy and cost-effectiveness of RDN, and by doing so inform guideline committees and health policy-makers. In the meantime, RDN should remain the ultima ratio in truly resistant hypertensive patients, preferably in a research context [5].
CONFLICT OF INTEREST
None declared. (See related article by Blankestijn et al. Sympathetic renal denervation in hypertension and in chronic kidney disease. Nephrol Dial Transplant 2014; 29: 1120–1123; See related article by Zoccali and Mallamaci. Renal denervation: The jury is still out and the verdict will be more complex than initially envisaged. Nephrol Dial Transplant 2014; 29: 1124–1126.)
REFERENCES
- 1.Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. doi: 10.1016/S0140-6736(09)60566-3. [DOI] [PubMed] [Google Scholar]
- 2.Esler MD, Krum H, Sobotka PA, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. doi: 10.1016/S0140-6736(10)62039-9. [DOI] [PubMed] [Google Scholar]
- 3.Symplicity HTN-1 investigators. Catheter-based renal sympathetic denervation for resistant hypertension: durability of blood pressure reduction out to 24 months. Hypertension. 2011;57:911–917. doi: 10.1161/HYPERTENSIONAHA.110.163014. [DOI] [PubMed] [Google Scholar]
- 4.Doumas M, Douma S. Renal sympathetic denervation: the jury is still out. Lancet. 2010;376:1878–1880. doi: 10.1016/S0140-6736(10)62111-3. [DOI] [PubMed] [Google Scholar]
- 5.Persu A, Renkin J, Thijs L, et al. Renal denervation: ultima ratio or standard in treatment-resistant hypertension. Hypertension. 2012;60:596–606. doi: 10.1161/HYPERTENSIONAHA.112.195263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Geisler BP, Egan BM, Cohen JT, et al. Cost-effectiveness and clinical effectiveness of catheter-based renal denervation for resistant hypertension. J Am Coll Cardiol. 2012;60:1271–1277. doi: 10.1016/j.jacc.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 7.Dorenkamp M, Bonaventura K, Leber AW, et al. Potential lifetime cost-effectiveness of catheter-based renal sympathetic denervation in patients with resistant hypertension. Eur Heart J. 2013;34:451–461. doi: 10.1093/eurheartj/ehs355. [DOI] [PubMed] [Google Scholar]
- 8.Kandzari DE, Bhatt DL, Sobotka PA, et al. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528–535. doi: 10.1002/clc.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mahfoud F, Ukena C, Schmieder RE, et al. Ambulatory blood pressure changes after renal sympathetic denervation in patients with resistant hypertension. Circulation. 2013;128:132–140. doi: 10.1161/CIRCULATIONAHA.112.000949. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler AK, Bertog S, Kaltenbach B, et al. Efficacy and safety of renal denervation in elderly patients with resistant hypertension. Catheter Cardiovasc Interv. 2013 doi: 10.1002/ccd.25166. [Epub ahead of print August 27, 2013 ] [DOI] [PubMed] [Google Scholar]
- 11.Giordano A, Polimeno M, Messina S, et al. Transcatheter renal sympathetic denervation despite angiographically significant proximal stenosis: proof of concept from a case report. Int J Cardiol. 2014;172:224–225. doi: 10.1016/j.ijcard.2013.12.272. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler AK, Franke J, Bertog SC. Renal denervation in a patient with prior renal artery stenting. Catheter Cardiovasc Interv. 2013;81:342–345. doi: 10.1002/ccd.24507. [DOI] [PubMed] [Google Scholar]
- 13.Pessina AC, Bisogni V, Fassina A, et al. Munchausen syndrome: a novel cause of drug-resistant hypertension. J Hypertens. 2013;31:1473–1476. doi: 10.1097/HJH.0b013e328360e9ae. [DOI] [PubMed] [Google Scholar]
- 14.Persu A, Renkin J, Asayama K, et al. Renal denervation in treatment-resistant hypertension: the need for restraint and more and better evidence. Expert Rev Cardiovasc Ther. 2013;11:739–749. doi: 10.1586/erc.13.52. [DOI] [PubMed] [Google Scholar]
- 15.Azizi M, Steichen O, Frank M, et al. Catheter-based radiofrequency renal-nerve ablation in patients with resistant hypertension. Eur J Vasc Endovasc Surg. 2012;43:293–299. doi: 10.1016/j.ejvs.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 16.Persu A, Jin Y, Azizi M, et al. Blood pressure changes after renal denervation at 10 European expert centers. J Hum Hypertens. 2013;28:150–156. doi: 10.1038/jhh.2013.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campese VM. Interventional hypertension: a new hope or a new hype? The need to redefine resistant hypertension. J Hypertens. 2013;31:2118–2121. doi: 10.1097/HJH.0b013e328364d3f1. [DOI] [PubMed] [Google Scholar]
- 18.Taddei S, Bruno RM. Renal denervation: still more questions than answers. J Hypertens. 2014;32:28–29. doi: 10.1097/HJH.0000000000000041. [DOI] [PubMed] [Google Scholar]
- 19.Jin Y, Persu A, Staessen JA. Renal denervation in the management of resistant hypertension: current evidence and perspectives. Curr Opin Nephrol Hypertens. 2013;22:511–518. doi: 10.1097/MNH.0b013e3283640024. [DOI] [PubMed] [Google Scholar]
- 20.Persu A, Azizi M, Burnier M, et al. Residual effect of renal denervation in patients with truly resistant hypertension. Hypertension. 2013;62:450–452. doi: 10.1161/HYPERTENSIONAHA.113.01632. [DOI] [PubMed] [Google Scholar]
- 21.Krum H, Sobotka P, Mahfoud F, et al. Device-based antihypertensive therapy: therapeutic modulation of the autonomic nervous system. Circulation. 2011;123:209–215. doi: 10.1161/CIRCULATIONAHA.110.971580. [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann J, Heusser K, Schmidt BM, et al. Catheter-based renal nerve ablation and centrally generated sympathetic activity in difficult-to-control hypertensive patients: prospective case series. Hypertension. 2012;60:1485–1490. doi: 10.1161/HYPERTENSIONAHA.112.201186. [DOI] [PubMed] [Google Scholar]
- 23.Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61:457–464. doi: 10.1161/HYPERTENSIONAHA.111.00194. [DOI] [PubMed] [Google Scholar]
- 24.Salles GF, Cardoso CR, Muxfeldt ES. Prognostic influence of office and ambulatory blood pressures in resistant hypertension. Arch Intern Med. 2008;168:2340–2346. doi: 10.1001/archinte.168.21.2340. [DOI] [PubMed] [Google Scholar]
- 25.Fadl Elmula FE, Hoffmann P, Fossum E, et al. Renal sympathetic denervation in patients with treatment-resistant hypertension after witnessed intake of medication before qualifying ambulatory blood pressure. Hypertension. 2013;62:526–532. doi: 10.1161/HYPERTENSIONAHA.113.01452. [DOI] [PubMed] [Google Scholar]
- 26.Krum H, Schlaich MP, Böhm M, et al. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2013;383:622–629. doi: 10.1016/S0140-6736(13)62192-3. [DOI] [PubMed] [Google Scholar]
- 27.Worthley SG, Tsioufis CP, Worthley MI, et al. Safety and efficacy of a multi-electrode renal sympathetic denervation system in resistant hypertension: the EnligHTN I trial. Eur Heart J. 2013;34:2132–2140. doi: 10.1093/eurheartj/eht197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rippy MK, Zarins D, Barman NC, et al. Catheter-based renal sympathetic denervation: chronic preclinical evidence for renal artery safety. Clin Res Cardiol. 2011;100:1095–1101. doi: 10.1007/s00392-011-0346-8. [DOI] [PubMed] [Google Scholar]
- 29.Templin C, Jaguszewski M, Ghadri JR, et al. Vascular lesions induced by renal nerve ablation as assessed by optical coherence tomography: pre- and post-procedural comparison with the Simplicity catheter system and the EnligHTN multi-electrode renal denervation catheter. 1. Eur Heart J. 2013;34:2141–2148. doi: 10.1093/eurheartj/eht141. 2148b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Sierra A, Segura J, Banegas JR, et al. Clinical features of 8295 patients with resistant hypertension classified on the basis of ambulatory blood pressure monitoring. Hypertension. 2011;57:898–902. doi: 10.1161/HYPERTENSIONAHA.110.168948. [DOI] [PubMed] [Google Scholar]
- 31.Persu A, Jin Y, Baelen M, et al. Eligibility for renal denervation: experience at 11 European expert centers. Hypertension. doi: 10.1161/HYPERTENSIONAHA.114.03194. 2014. [Epub ahead of print March 24, 2014] [DOI] [PubMed] [Google Scholar]
- 32.Savard S, Frank M, Bobrie G, et al. Eligibility for renal denervation in patients with resistant hypertension: when enthusiasm meets reality in real-life patients. J Am Coll Cardiol. 2012;60:2422–2424. doi: 10.1016/j.jacc.2012.08.1002. [DOI] [PubMed] [Google Scholar]
- 33.Hayek SS, Abdou MH, Demoss BD, et al. Prevalence of resistant hypertension and eligibility for catheter-based renal denervation in hypertensive outpatients. Am J Hypertens. 2013;26:1452–1458. doi: 10.1093/ajh/hpt132. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Jacobs L, Baelen M, et al. Rationale and design of the Investigator-Steered Project on intravascular Renal Denervation for Management of Drug-Resistant Hypertension (INSPiRED) trial. doi: 10.3109/08037051.2014.899297. (in press) Blood Press 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]