Abstract
Schistosomiasis caused by infection with parasitic helminths of Schistosoma spp. is a major global health problem due to inadequate treatment and lack of a vaccine. The immune response to schistosomes includes glycan antigens, which could be valuable diagnostic markers and vaccine targets. However, no precedent exists for how to design vaccines targeting eukaryotic glycoconjugates. The di- and tri-saccharide motifs LacdiNAc (GalNAcβ1,4GlcNAc; LDN) and fucosylated LacdiNAc (GalNAcβ1,4(Fucα1-3)GlcNAc; LDNF) are the basis for several important schistosome glycan antigens. They occur in monomeric form or as repeating units (poly-LDNF) and as part of a variety of different glycoconjugates. Because chemical synthesis and conjugation of such antigens is exceedingly difficult, we sought to develop a recombinant expression system for parasite glycans. We hypothesized that presentation of parasite glycans on the cell surface would induce glycan-specific antibodies. We generated Chinese hamster ovary (CHO) Lec8 cell lines expressing poly-LDN (L8-GT) and poly-LDNF (L8-GTFT) abundantly on their membrane glycoproteins. Sera from Schistosoma mansoni-infected mice were highly cross-reactive with the cells and with cell-surface N-glycans. Immunizing mice with L8-GT and L8-GTFT cells induced glycan-specific antibodies. The L8-GTFT cells induced a sustained booster response, with antibodies that bound to S. mansoni lysates and recapitulated the exquisite specificity of the anti-parasite response for particular presentations of LDNF antigen. In summary, this recombinant expression system promotes successful generation of antibodies to the glycans of S. mansoni, and it can be adapted to study the role of glycan antigens and anti-glycan immune responses in many other infections and pathologies.
Keywords: cellular engineering, glyco-conjugate, helminth, LDNF, schistosome
Introduction
Human helminthiases, infections caused by multicellular parasitic worms, affect 1–2 billion people and may account for up to 100 million disability-adjusted life years (The World Health Organization 2008, 2010; Hotez, Bethony et al. 2010). The most common are hookworm and schistosomiasis, with Schistosoma mansoni being one of the most widespread schistosomes (Gryseels et al. 2006; The World Health Organization 2010). With the poorest 20% of the world's population infected by helminths, treatment needs are far from being met (Hotez et al. 2009, Hotez, Engels et al. 2010). To date, no vaccines against helminths or any human parasites are in use. Attenuated or killed organisms are impractical as vaccine solutions because of complex helminth life cycles, and several recombinant protein candidates have been tried without success (Mcmanus and Loukas 2008; Diemert et al. 2012). Thus, we are in need of novel scientific approaches to developing vaccines for these complex pathogens.
Helminth glycans represent a major untapped reservoir of vaccine candidates. Larvae, adult worms and eggs produce an abundance of glycoconjugates on their surfaces and in secretions that are exposed to the host immune system. The S. mansoni cercarial glycocalyx is roughly 80% carbohydrate by weight (Samuelson and Caulfield 1985; Caulfield et al. 1987). Glycans are effective vaccine targets for many encapsulated bacterial pathogens (Lin et al. 2001; Weckx et al. 2012), however, development of such vaccines has been largely empirical and no precedent exists for how to design vaccines targeting eukaryotic glycoconjugates. Parasite glycans contain many structures and/or modifications which are foreign to the host's immune system (recently reviewed in Prasanphanich et al. 2013). In contrast to bacterial polysaccharides, however, they may have core structures or other structural features in common with their mammalian hosts. We currently lack a thorough understanding of the structural basis for antigenicity and immunogenicity of eukaryotic glycans.
In schistosomiasis, glycans behave both as immunomodulators and as antigens. The infection is initiated when water-born cercariae penetrate mammalian skin, transform into schistosomula (larvae) and migrate into the vasculature. Over the course of about 6 weeks, they grow into adult worms which lay eggs in the mesenteric venules. Schistosome egg glycoconjugates are instrumental in biasing the immune response towards a Th2 phenotype and inducing the eosinophilic granulomas which characterize the pathology of this disease (Okano et al. 1999, 2001; Faveeuw et al. 2003; Thomas and Harn 2004; Van de Vijver et al. 2004, 2006). In S. mansoni-infected humans, primates and mice, the majority of the antibody response is directed against glycans (Omer-Ali et al. 1986; Omer Ali et al. 1989; Eberl et al. 2001; Kariuki et al. 2008). Anti-glycan antibodies are able to kill helminths in vitro and/or protect against infection in several models of helminth infection when passively transferred (Grzych et al. 1982, 1985; Harn et al. 1984; Ko et al. 1990; Ellis et al. 1994; Nyame et al. 2003; Van Stijn, van den Broek et al. 2010). However, the protective ability of anti-glycan antibodies appears to be highly dependent on the particular molecular target and the isotype/subisotype of antibody (Grzych et al. 1984; Omer Ali et al. 1988; Ko et al. 1990). In order to exploit helminth glycans as vaccine targets, we must identify those associated with protection and learn how to mimic the parasite's immunogenic presentation of such glycans so as to elicit protective antibody isotypes.
The LacdiNAc (GalNAcβ1,4GlcNAc; LDN) family of glycans, especially LDNF (GalNAcβ1,4(Fucα1,3)GlcNAc) and several multiply-fucosylated variants, are highly targeted in anti-schistosomal responses (Nyame et al. 2000; Van Remoortere et al. 2001; Naus, Remoortere et al. 2003; Robijn et al. 2005; Van Diepen, Van der Velden et al. 2012). Antibodies to LDNF have been identified in the sera of S. mansoni-infected humans, mice and importantly, brown rats and rhesus monkeys, which are models of natural protective immunity (Nyame et al. 2000, 2003; Naus, Remoortere et al. 2003; Luyai et al. 2014). Although it is yet unknown whether they directly contribute to protection in vivo, a monoclonal antibody to LDN kills schistosomula in vitro, and antibodies to LDNF are found in high titer in rhesus and mouse antisera that are lethal to schistosomula (Nyame et al. 2003; Luyai et al. 2014). Several multi-fucosylated versions of LDN(F) appear to be completely unique to schistosomes (Van Remoortere et al. 2001; Kantelhardt et al. 2002; Naus, Booth et al. 2003). For these reasons, we believe that the LDN(F) family of antigens includes potential vaccine and diagnostic candidates.
Though the antigenicity of the LDNF is well established, it is yet unclear which particular glycoconjugates and/or structural variations of the epitope provoke this abundant antibody response. LDNF is expressed by intramammalian and intramolluscan schistosome stages and, importantly, is found on the surface of larvae, the most immunologically vulnerable stage, and adult worms, which are cleared in some animal models (Nyame et al. 2000, 2002, 2003, 2004; Van Remoortere et al. 2000; Robijn et al. 2005; Wilson et al. 2008). Both glycoproteins and glycolipids in adult worms and eggs express LDNF, and importantly, male worms express polymeric repeats of LDN and LDNF on their N-glycan antennae (Robijn et al. 2005; Wuhrer, Koeleman, Deelder et al. 2006; Wuhrer, Koeleman, Fitzpatrick et al. 2006; Frank et al. 2012). In contrast, the expression of LDNF in mammals is rare, occurring strictly as a monomer on N-glycans of only a few known proteins (Dell et al. 1995; Van den Eijnden et al. 1995; Van Den Nieuwenhof et al. 2000). Determining the optimal mode of presentation of parasite glycans in vaccines has been virtually impossible due the current limitations of synthetic technology to replicate certain aspects of natural glycan presentation. Additionally, many antigenic schistosome glycans, such as poly-LDNF, are the result of glycosyltransferase activities and target specificities unique to the parasite, which are ill-defined and cannot be replicated in vitro. Thus, if anti-glycan vaccines are to be useful, it will be necessary to develop a vaccine platform that allows “native-like” expression and presentation of pathogen glycoconjugates, where well-defined variations in glycan structure and presentation can be made for the purposes of studying glycoconjugate immunogenicity and glycan antigen discovery.
We hypothesized that expression of schistosome glycan epitopes in a mammalian expression system would recapitulate the immunogenicity and antigenicity of these epitopes generated by the parasite itself. Whole cells and cell membrane preparations have often been used as immunogens for the production of polyclonal and monoclonal antibodies to specific surface glycans (Brockhaus et al. 1981; Hirohashi et al. 1985; Lee et al. 2002; Iwamori et al. 2009). Whole-cell vaccination has more recently been pursued as a tumor vaccination strategy, with both autologous and allogeneic cancer cells as well as dendritic cells (DC) and malignant-DC fusions all under clinical investigation (reviewed in De Gruijl et al. 2008; Milani et al. 2013; Palucka and Banchereau 2013; Srivatsan et al. 2014). Chinese hamster ovary (CHO) cells are a model system for glycosylation pathways and can be readily manipulated to produce a variety of glycans that are very similar to the native parasite antigens, in a high-density, membrane-bound presentation. In previous work by our lab, Caenorhabditis elegans β1,4-N-acetylgalactosaminyltransferase (B4GALNT1) and human α1,3-fucosyltransferase 9 (FUT9) were transfected into the stable mutant CHO cell line, Lec8, to generate Lec8-GalNAcT (L8-GT) and Lec8-GalNAcT-FucT (L8-GTFT) cells (Kawar et al. 2002, 2005). These cell lines produce polymers of LDN and LDNF on N-glycans as terminal extensions on complex-type structures, which we will refer to as poly-LDN and poly-LDNF. In this study, we have shown that immunizing mice with these cells elicits abundant and highly specific antibodies to LDN and LDNF, the latter of which are sustained for at least 20 weeks after a booster immunization, and bind to parasite glycans. We have thus validated the approach of using recombinant cellular expression of parasite glycans for future use in vaccine development for this very important class of pathogens.
Results
Recombinantly engineered Lec8 cells express surface-bound poly-LDN and poly-LDNF
We developed a recombinant expression system for the LDN(F) family of schistosome glycan antigens using the Lec8 cell line, a stable glycosylation mutant of CHO cells, which have a nonfunctional UDP-galactose transporter. All of the N-glycan branches homogeneously truncate in N-acetylglucosamine (GlcNAc) and O-glycans truncate in N-acetylgalactosamine (GalNAc or Tn antigen) (Oelmann et al. 2001; Patnaik and Stanley 2006). We serially transfected Lec8 cells with C. elegans B4GALNT1 and human FUT9 (Kawar et al. 2005). The resulting stable, clonal cell lines are referred to as L8-GT and L8-GTFT. Activity of an endogenous mammalian β1,3-N-acetylglucosaminyltransferase, which normally allows extension of poly-N-acetyllactosamine (LN) and poly-LewisX (LeX) branches in wild-type cells, most likely catalyzed extension of the corresponding engineered antigens, poly-LDN and poly-LDNF, in our cell lines. MALDI-TOF mass spectrometry indicated that 2–6 linear repeats of LDN and 2–4 linear repeats of LDNF were present on each N-glycan branch of the L8-GT and L8-GTFT cells, respectively, depending on the number of branches, indicating that the activity of the enzymes was sufficient to alter the glycome (Kawar et al. 2005). The O-glycans and glycolipids of these cells do not contain the antigens as the B4GALNT1 can only use β-linked GlcNAc as acceptor (Stanley 1980; Kawar et al. 2002, 2005; Patnaik and Stanley 2006).
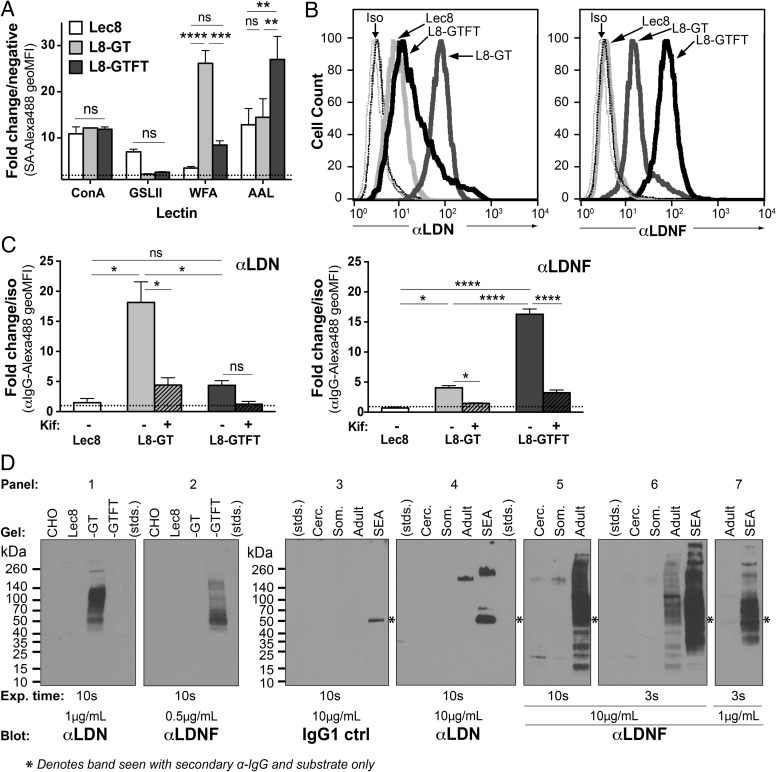
We first examined surface expression of the engineered glycans by performing flow cytometry on paraformaldehyde-fixed cells stained with lectins (Figure 1A) and monoclonal antibodies (Figure 1B and C). The lectins Concanavalin A (ConA), Griffonia simplicifolia lectin II (GSL-II), Wisteria floribunda agglutinin (WFA) and Aleuria aurantia lectin (AAL) bind tri-mannose, terminal GlcNAc, terminal GalNAc and fucose, respectively (Cummings and Etzler 2009). ConA binding indicated that the cell lines expressed similar amounts of N-glycans on their surface. WFA and AAL binding were significantly higher in the L8-GT and L8-GTFT cells, respectively, indicating a successful transformation from mostly GlcNAc-terminating glycans in Lec8, to mostly GalNAc-terminating glycans in L8-GT and fucosylated glycans in L8-GTFT (Figure 1A). We stained the cells with monoclonal antibodies to the LDN and LDNF epitopes, generated from hybridomas derived from spleen cells of S. mansoni-infected mice by methods described previously (Nyame et al. 2000; Mandalasi 2011; Mandalasi et al. 2013). Anti-LDN bound significantly higher to L8-GT cells compared with the other two lines (Figure 1B and C—left). Anti-LDN reactivity with L8-GTFT cells was reduced to a level not significantly different from Lec8, indicating a robust loss of LDN epitopes as they are converted to LDNF epitopes. Anti-LDNF bound significantly higher to L8-GTFT cells compared with the other two lines, and only slightly higher to L8-GT than to Lec8 (Figure 1B and C—right), which could indicate low-level cross-reactivity with LDN epitopes. The binding of both anti-LDN and -LDNF was significantly abrogated by growing cells in the presence of kifunensine (Elbein et al. 1990), an α-mannosidase inhibitor of complex N-glycan processing, verifying that the expression of LDN and LDNF occurs on N-glycans (Figure 1C).
Fig. 1.
Recombinantly engineered Lec8 cells express surface-bound LDN and LDNF. (A–C) Lec8 (white bars), Lec8-GalNAcT (L8-GT; gray bars) and Lec8-GalNAcT-FucT (L8-GTFT; black bars) were fixed and stained with biotinylated lectins (A) and mouse monoclonal antibodies (B and C) for flow cytometry analysis. (A) Data are expressed as fold change in geoMFI over the negative control, SA-488, represented on the graph as a dotted line. Mean ± SEM of two independent experiments are shown and two-way ANOVA with Tukey's multiple comparisons test was used to determine significance. (B) A representative shift with each monoclonal antibody is shown. (C) Cells were treated with (hashed bars) or without kifunensine for 8 days to disrupt complex N-glycan synthesis before fixation. Data are expressed as fold change in geoMFI over the negative control—mouse IgG1 isotype control for (B), and IgG1 isotype control or normal mouse IgG for (C). Error bars represent SEM of two (C—left) or three (C—right) independent experiments and one-way ANOVA with Tukey's test were used. (D) SDS–PAGE and western blotting were performed on cell and Schistosoma mansoni life stage lysates and using the monoclonal antibodies, with detection by HRP-conjugated secondary antibodies and chemiluminescent substrate on film. Primary antibody concentrations and exposure times are specified for each pane. The six-point star denotes bands that are not antibody-specific, since they appear with secondary antibody and substrate only when SEA and adult worm lysates are stained, likely the result of trace amounts of mouse immunoglobulins in the parasite preparations. SA, streptavidin; ConA, concanavalin A; GSL-II, Griffonia simplicifolia lectin II; WFA, Wisteria floribunda agglutinin; AAL, Aleuria aurantia lectin; Kif, kifunensine; Iso, isotype control antibody or normal mouse IgG; stds, molecular weight standards; Cerc., cercarial lysate; Som., schistosomula lysate; Adult, adult worm lysate; SEA, soluble egg antigen; ns P ≥ 0.05; *P = 0.01–0.05; **P = 0.001–0.01; ***P = 0.0001–0.001; ****P < 0.0001.
To examine the LDN/LDNF content of the cells relative to schistosome life stages and characterize the molecular species carrying these glycans, we prepared lysates of S. mansoni cercariae, 3-day cultured schistosomula, adult worms and soluble egg antigen (SEA) (the latter two prepared from infected mice). We performed SDS–PAGE of protein concentration-standardized cell and parasite life stage lysates and western blotted with anti-LDN and -LDNF (Figure 1D). The anti-LDN and -LDNF were highly specific for L8-GT and L8-GTFT cells, respectively, and stained a range of glycoproteins between 40 and 260 kDa (Figure 1D—panels 1–2). Anti-LDN stained only a few distinct species of SEA and adult worm lysate (Figure 1D—panels 3–4). In contrast, anti-LDNF showed broad-range staining of SEA glycoproteins as well as less-intense reactivity with several species in adult worm lysate, and a few distinct species in schistosomula and cercarial lysates, as demonstrated by using different concentrations of the antibody and exposure times (Figure 1D—panels 5–7). [Note: the non-specific band at 50 kDa marked with a six-point star (Figure 1D—panels 3–7) is likely due to cross-reactivity of the HRP-conjugated anti-mouse-IgG secondary detection reagent with the parasite lysates prepared from mice, and stains when only secondary antibody is used.] Taken together, these results demonstrate that LDN and LDNF antigens are abundantly expressed on a variety of cell-surface L8-GT and L8-GTFT cells glycoproteins, respectively, at levels greater than in the early stages of schistosome infection and comparable with LDNF expression of adult worms and eggs in robustness and molecular complexity. Additionally, the cells present LDN and LDNF on N-glycans in such a way that they can be recognized by monoclonal antibodies originally generated by mice in response to parasite infection.
Recombinantly expressed poly-LDN and -LDNF are cross-reactive with schistosome antigens
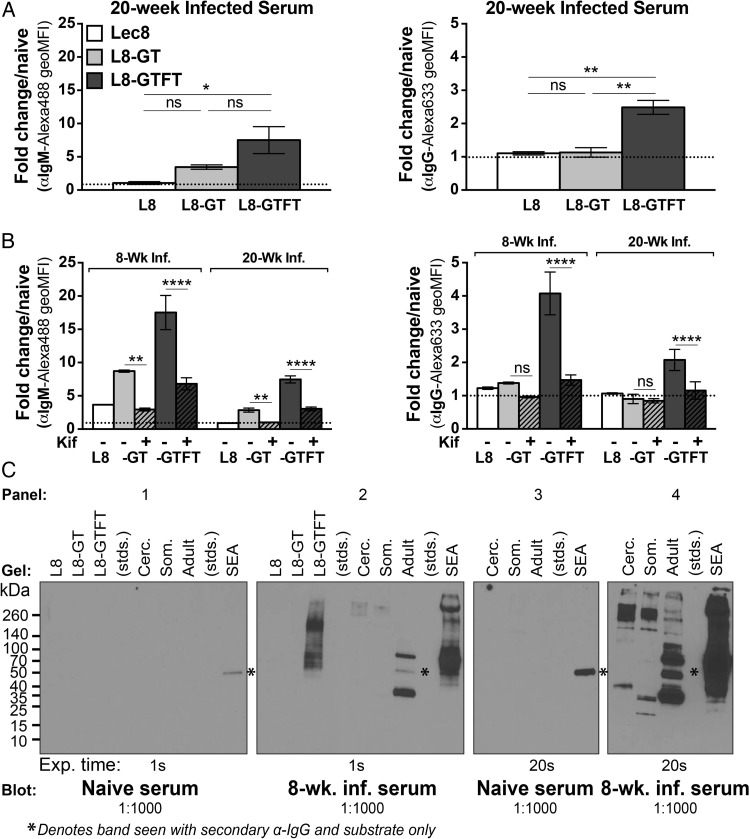
We next asked whether the glycan antigens on the CHO cell derivatives were recognized by antisera from experimental murine S. mansoni infection, a common laboratory model for schistosomiasis. Chronically infected mouse antisera (20-week, low cercarial dose) had significantly more IgM (Figure 2A—left) and IgG (Figure 2A—right) specific for L8-GTFT cells compared with Lec8 and L8-GT cells. Antiserum from both acutely and chronically S. mansoni-infected (8- and 20-week, low cercarial dose) contained IgM specific for N-glycans on L8-GT and L8-GTFT cells (Figure 2B—left), and IgG specific for N-glycans on L8-GTFT cells (Figure 2B—right). Western blotting also showed that, whereas naïve serum had no specific staining of the cell lysates (Figure 2C—panel 1), 8-week infection antisera contained IgG that bound L8-GTFT cell lysate (Figure 2C—panel 2). The infection antisera stained some higher molecular weight species of L8-GTFT cells that were less prominent in the anti-LDNF stain (Figure 1D—panel 2), indicating that the anti-LDNF response during infection may differ in breadth from the monoclonal. Staining of S. mansoni cercariae, schistosomula, worm and egg antigens are shown as a positive control (Figure 2C—panels 2 and 4). These results indicate that by overexpressing parasite glycan epitopes in mammalian cells, we generated an immunogen that mimics the antigenicity presented by S. mansoni during infection.
Fig. 2.
Recombinantly expressed LDN and LDNF are cross-reactive with schistosome antigens. (A and B) Cells were fixed and stained with pooled antisera from naïve, 8- or 20-week low-dose S. mansoni-infected mice on flow cytometry. Bound IgM (left) or IgG (right) was detected with Alexa-488 or Alexa-633-conjugated secondary antibodies. White bars, Lec8 cells; gray bars, L8-GT cells; black bars, L8-GTFT cells. (A) Mean ± SEM of the geoMFI fold change of 20-week infected serum over naïve serum for three experiments is shown and one-way ANOVA with Tukey's multiple comparisons test was used. (B) Mean ± SD of the geoMFI fold change of 8- and 20-week infected serum over naïve serum for two independent experiments with kifunensine- (hashed bars) or mock-treated cells is shown and two-way ANOVA with Tukey's test used to compare the effect of kifunensine treatment independent of the serum used. (C) Cell lysates were western blotted with infected antisera, using parasite life stages as a positive control, and detected with HRP-conjugated anti-mouse IgG at 1:5000. Asterisk denotes non-specific bands that appear with secondary antibody and substrate only when SEA and adult worm lysates are stained. Kif, kifunensine; stds, molecular weight standards; Cerc., S. mansoni cercarial lysate; Som., schistosomula lysate; Adult, adult worm lysate; SEA, soluble egg antigen; Exp., exposure time; ns P ≥ 0.05; *P = 0.01–0.05; **P = 0.001–0.01; ***P = 0.0001–0.001; ****P < 0.0001.
Whole-cell vaccines are immunogenic and induce a sustained anamnestic response
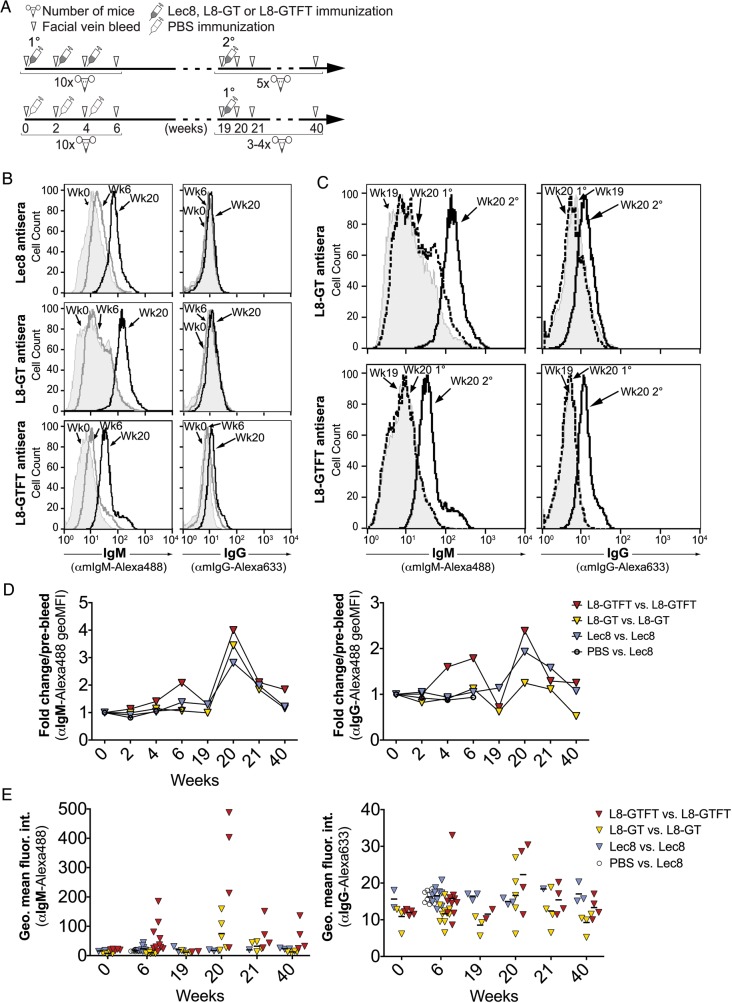
To determine the effectiveness of the engineered cells at inducing an anti-glycan response, we immunized 10 female Swiss-Webster mice with each of the cell lines, or a PBS control. We delivered 106–107 cells (stored frozen and thawed immediately before use) intraperitoneally, without adjuvant, to each mouse (Figure 3A). Three primary immunizations were given at the onset of weeks 0, 2 and 4, followed by a waiting period, and then a secondary immunization at week 19. Facial vein bleeds were taken to obtain serum from each animal at weeks 0 (pre-bleed), 2, 4, 6, 19, 20, 21 and 40, at which point the animals were sacrificed. Bleeds were taken the day before an injection. At 19 weeks, 3–4 PBS-immune mice were given primary immunizations of each cell type to directly compare with the secondary immunizations given at the same time.
Fig. 3.
Whole-cell poly-LDN(F) vaccines are immunogenic and induce a sustained anamnestic response. (A) Freshly-thawed aliquots of 106–107 cells, or PBS, were delivered without adjuvant, intra-peritoneally to female Swiss-Webster mice and facial vein bleeds were collected according to the immunization scheme depicted. Three immunizations were given at 0, 2 and 4 weeks (1°), and one secondary (2°) boost was given at week 19, to the number of mice indicated under each timeline. The mice given PBS immunizations at weeks 0–4 made no detectable response and were therefore used for 1° immunization at 19 weeks. (B–E) To measure immunogenicity of each cell type, antisera from immunized mice was run on flow cytometry against fixed cells of the same type to assess IgM and IgG binding to surface antigens. (B) Histograms representative of two to three experiments are shown for the pooled antisera at week 0 (solid gray), week 6 (thick gray line) and week 20 (thick black line) post-immunization for IgM (left) and IgG (right). (C) The memory response was assessed by comparing pooled week 19 sera (pre-2°; solid gray) with shifts from week 20 sera from 2° immunized mice (thick black line) and 1° immunized mice (which had previously received PBS as mock immunization; dashed black line), representative of 2-3 experiments. (D) The increase in antibody binding to homologous cell types, expressed as fold change in average MFI relative to week 0, was measured for antisera pooled from each group of mice over time, representative of 3 experiments. Yellow triangles, Lec8-immunized vs. Lec8 cells; blue triangles, L8-GT-immunized vs. L8-GT cells; red triangles; L8-GTFT-immunized vs. L8-GTFT cells; open circles, PBS-immunized vs. Lec8 cells (only shown through week 6); black dash, mean for each group. (E) Flow cytometry was also used to assess the range of magnitudes in IgM (left) and IgG (right) response for individual mice, where enough serum was available. Two-way ANOVA with repeated measures was performed on 3-4 mice from each of the L8, L8-GT and L8-GTFT which had complete data at weeks 0, 6, 20 and 40. Within each group, time points were compared with week 0 using Dunnett's multiple comparisons test.
All three of the cell lines were immunogenic based on flow cytometry of pooled immune serum binding to the surface of homologous fixed cells. Representative shifts from weeks 0 (naïve) to weeks 6 (2 weeks after the third immunization) and 20 (1 week after the secondary immunization) are shown (Figure 3B). L8-GT cells induced the greatest IgM shift at week 20 compared with naïve (Figure 3B—left), whereas for IgG, the Lec8 cell immunization induced no perceptible shift and L8-GTFT cells induced the most robust shift at 20 weeks (Figure 3B—right). [Note that for all flow cytometry experiments, the relative levels of IgG and IgM in a sample cannot be quantitatively compared, because different secondary antibodies are used.] To determine whether the cells induced a memory response, we compared pooled immune sera from before (week 19) and 1 week after (week 20) the simultaneous primary and secondary immunization groups (Figure 3C). Both L8-GT and L8-GTFT cells induced an anamnestic response, indicated by comparing the shifts of secondary with primary immunization at 20 weeks. The L8-GT cells appeared to induce more IgM memory than L8-GTFT cells (Figure 3C—left), whereas the reverse was true for IgG memory (Figure 3C—right).
We compared the fold change in mean fluorescence intensity (MFI) relative to week 0 of each of the pooled antisera binding to homologous cells over the course of the experiment (Figure 3D). The Lec8 and L8-GT immunizations induced an increase in IgG and IgM binding that was only detectable by flow cytometry at week 20, whereas L8-GTFT cells induced more robust primary IgG and IgM responses that were detectable at weeks 4 and 6, and peaked at week 20. Additionally, the L8-GTFT IgM and IgG responses were still elevated at week 40 whereas the response to other cell types had declined to levels seen at week 0.
The magnitude of response to cellular immunogens was highly variable among individual mice (Figure 3E). Not all mice were included in the individual analysis because of serum shortage, which accounts for the slight discrepancy between flow cytometry results of pooled and individual sera. However, it was evident that in each group, some mice responded very highly while some hardly responded at all, which could stem from individual differences in ability to respond to the immunogens, or variability in the efficiency of delivery. To assess the relative immunogenicity of the cellular immunogens, we performed two-way ANOVA with repeated measures on the individual mice which had complete longitudinal data at weeks 0, 6, 20 and 40 (n = 3–4 per group). Within each group, time points were compared with week 0 using Dunnett's multiple comparisons test. The IgM and IgG responses to L8-GTFT cells, and the IgG response to L8-GT cells were significantly elevated (P < 0.05) compared with the pre-bleed sera. Mice immunized with PBS did not show any detectable response to Lec8 cells through 6 weeks (Figure 3D and E).
Taken together, flow cytometry data demonstrate that all three cell lines were immunogenic without adjuvant and induced a memory response. L8-GTFT cells, simply by virtue of their engineered glycans, appear to be the most potent immunogen in terms of inducing the highest response to primary immunization, and generating a more sustained IgG memory response than the other cell types.
Lec8-GT and -GTFT cells induce IgM and IgG antibodies specific for LDN and LDNF glycans
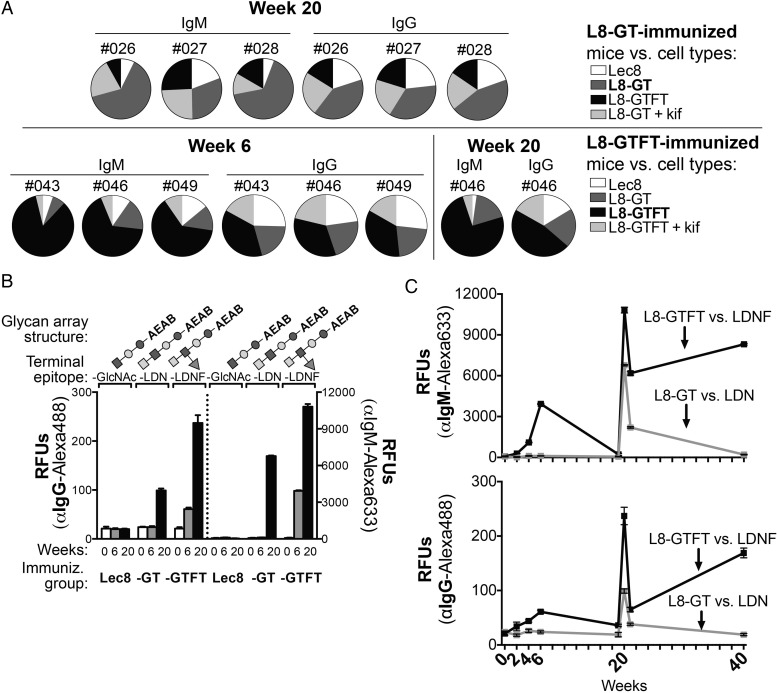
To examine the specificity of the immune response, we first looked at the binding of immune sera from several high-responding individual mice to homologous cell types, heterologous cell types and kifunensine-treated cells on flow cytometry (Figure 4A). Each pie chart represents the sum of raw MFI values for the indicated antisera binding to different cell types. In all L8-GT (top) and—GTFT (bottom)—immunized animals tested, we found the largest portion of binding seen was to the homologous cell type (dark gray for the binding to L8-GT cells, and black for binding to L8-GTFT cells.) In one mouse (#046) that was tested for specificity at weeks 6 and 20, the portion of cell-specific response was amplified at the later time point. This indicates that antibody responses were specific to the cells used for immunization and specific for cell-surface N-glycans.
Fig. 4.
L8-GTFT cells induce long-lasting IgM and IgG antibodies specific for LDNF glycan. (A) Sera from individual L8-GT- (top) and L8-GTFT-immunized (bottom) mice was run on flow cytometry against heterologous cell types (Lec8, white; L8-GT, dark gray; L8-GTFT, black; L8-GT or L8-GTFT treated with kifunensine, light gray) to assess specificity of the response. Individual mouse numbers, isotype and week of the serum sample are indicated above each pie chart, in which the relative binding to each cell type (each slice) is represented as a portion of the sum of geoMFIs of binding for all the cell types (whole pie). Note that naïve serum was also run on each cell type as a negative control, with similar binding to all cell types. (B) The pooled antisera from weeks 0 (white bars), 6 (gray bars) and 20 (black bars) was run on glycan microarrays to assess glycan-specific binding of serum IgG (left y-axis) an IgM (right y-axis). Glycan array structures derived from LNnT, terminating in GlcNAc (glycan ID 13) or with a single unit of LDN (ID 14) or LDNF (ID 16) were printed at 100uM and are shown above each group of bars. Glycan IDs and monosaccharide symbols correspond to Figure 5A, which depicts the entire glycan array with controls—only three selected structures are shown in this figure. Binding is representative of two experiments. (C) Pooled sera from L8-GT (gray) and L8-GTFT-immunized (black) mice at all time points and binding to LDN and LDNF is shown, representative of two experiments. The mean RFUs ± SD are plotted for binding to six replicate spots of each glycan. Kif, kifunensine; RFUs, relative fluorescence units; -GlcNAc; agalacto-Lacto-N-neotetraose (LNnT); -LDN, LacdiNAc made by adding GalNAc to agalacto-LNnT; -LDNF, fucosylated LacdiNAc made by adding fucose to -LDN.
To more directly examine glycan specificity of the immune response to the cells, we probed glycan microarrays with pooled immune sera. Glycan microarrays consist of a collection of glycans that have been functionalized and printed on glass slides (as described in Materials and Methods section and our previous publications) (Heimburg-Molinaro et al. 2011; Song et al. 2012). Slides are then incubated with antibodies or lectins and detected by appropriate fluorescent secondary reagents as relative fluorescent units (RFUs). This yields an extremely sensitive and specific read-out of glycan-binding patterns. [Note that for all glycan array experiments, the relative levels of IgG and IgM in a sample cannot be quantitatively compared, since different secondary antibodies are used.]
We first examined reactivity to three defined glycan structures, chemo-enzymatically synthesized by remodeling lacto-N-neotetraose (LNnT; Galβ1-4GlcNAcβ1-3Galβ1-4Glc) to contain one unit of either LDN or LDNF. Agalacto-LNnT (terminating in GlcNAc) was generated by digestion with β-galactosidase. LDN was generated using recombinant C. elegans β1,4-N-acetylgalactosaminyltransferase (B4GALNT1) to add GalNAc to the non-reducing end, and LDNF was generated using recombinant human α1,3-fucosyltranserase 6 (FUT6) to add fucose to the GlcNAc (Figure 4B; the monosaccharide key can be found at the bottom of Figure 5A).
Fig. 5.
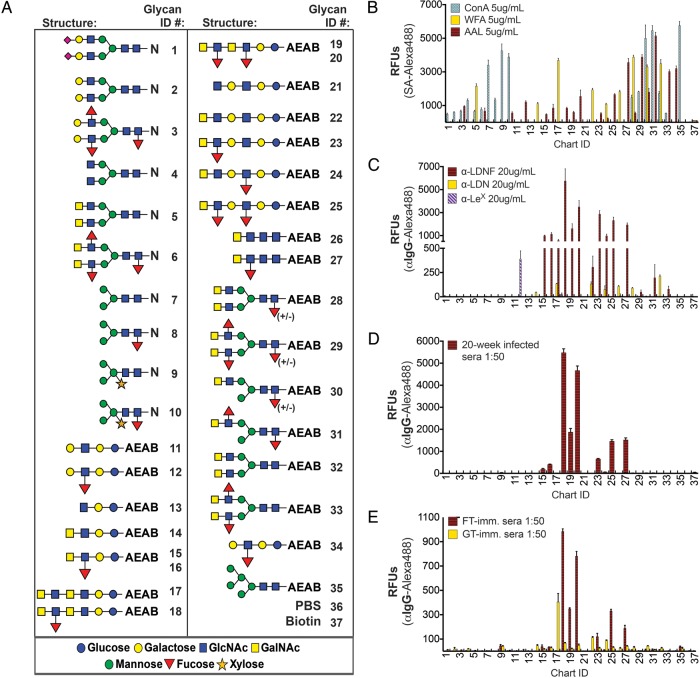
Antibodies to schistosome glycans discriminate among very similar presentations of the epitope. (A) N-linked glycopeptides and AEAB-linked glycans were modified to include several variants of LDN, LDNF and other schistosome antigens and printed on glass slides, called the defined schistosome-type microarray (DSA). LDN-terminating glycans are printed at 50 μM and all other glycans are printed at 100 μM due to variation in reaction yields. Some LDNF-terminating glycans are printed at 50 and 100 μM, in that order, for comparison with LDN. (B) Biotinylated lectins binding tri-mannose (ConA), terminal GalNAc (WFA) and fucose (AAL) were used to quality-control printing of the slides. (C) Anti-schistosomal monoclonal antibodies, (D) antisera from chronically S. mansoni-infected mice and (E) week 20 antisera from L8-GT and L8-GTFT (FT) immunization were tested on the DSA. Streptavidin-Alexa488 or goat anti-mouse-IgG-Alexa488 were used to detect biotinylated lectins and antibodies bound to the slides, respectively. Mean RFUs ± SD of tetra-replicate spots for each glycan ID are shown. N, asparagine; AEAB, 2-amino-N-(2-aminoethyl)-benzamide; RFUs, relative fluorescence units; SA, streptavidin; ConA, concanavalin A; WFA, Wisteria floribunda agglutinin; AAL, Aleuria aurantia lectin.
When we compared pooled serum from each immunization group at weeks 0, 6 and 20, we found that, consistent with flow cytometry data, the L8-GT cells induced detectable LDN-specific antibody only at 20 weeks, whereas the L8-GTFT-immunized mice expressed the highest titer of glycan-specific IgG and IgM to LDNF at both time points (Figure 4B). Lec8-immunized mice made no detectable antibodies to agalacto-LNnT. We examined the IgM (Figure 4C—top) and IgG (Figure 4C—bottom) responses to LDN and LDNF in pooled serum over the course of the whole experiment by plotting the glycan array RFUs at each time point. In the L8-GTFT group, glycan-specific IgG and IgM were detectable beginning at week 2 and steadily increased with repeated immunizations. They peaked at week 20 after the booster immunization, declined at week 21, and were sustained through week 40 when the mice were sacrificed. In contrast, glycan-specific antibodies to LDN were detectable in L8-GT-immunized mice only at weeks 20 and 21, and declined again to undetectable levels by week 40. Based on the glycan array data, we conclude that expression of polymeric LDN and LDNF on the surface of Lec8 cells allowed for production of glycan-specific antibodies in mice, and that L8-GTFT cells induced a robust LDNF-specific primary and secondary response, which was sustained for at least 20 weeks after the last immunization.
Antibodies to parasite glycans discriminate among very similar presentations of the epitopes
Our Defined Schistosome-type Array (DSA) is a collection of chemically defined glycans and glycopeptides containing schistosome-like glycan epitopes, such as LDN, LDNF, Lewis-X (LeX) and core xylose/core α3-fucose (CX/CF), as well as control structures, where each structure is represented by a unique glycan ID on the structure list (Figure 5A) and on the x-axis (Figure 5B–E). A previous version of the DSA consisted of a collection of biantennary N-glycopeptides (glycan ID 1–10) and straight-chain glycans derived from LNnT (ID 11–16). The glycopeptides were isolated from natural glycoproteins and chemoenzymatically modified to contain biantennary LDN, LDNF or LeX using recombinant C. elegans B4GALNT1 and FUT6 (Luyai et al. 2014). LNnT was similarly modified to contain a single unit of LDN, LDNF or LeX. While examining the specificity of the anti-schistosomal immune response on that version of the array, we observed that antibodies to LDNF bound well to LNnT-derived-LDNF (ID 15–16) but not at all to a biantennary N-glycopeptide bearing LDNF (ID 6). This unprecedented finding prompted us to better define the specificity of anti-schistosomal antibodies by expanding the DSA to include multiple variants of LDN and LDNF which represent the variety of “structural contexts” in which LDN(F) might be presented on the parasite (Figure 5A).
To this end, the LNnT-derived-LDN structure (ID 14) was used as starting material for extension catalyzed by a β1,3-N-acetylglucosaminyltransferase activity in normal human serum, and then further modified as above to contain two repeats of LDN (ID 17), with one or two fucose residues added (glycan ID 18–20). LNnT (ID 11) was also extended into LDN and LDNF using a recombinant Helicobacter pylori β1,3-N-acetylglucosaminyltransferase (Peng et al. 2012) (ID 21–25). LDN and LDNF were also synthesized from chitotriose (ID 26–27) and agalacto-NA2 (biantennary N-glycan from chicken glycopeptide) (ID 32–33). To obtain biantennary or mono-antennary LDN and LDNF, human IgG N-glycans were fractionated by charge, and asialo- and mono-sialyl N-glycans were used to either symmetrically or asymmetrically digest their antennae (ID 28–31). All glycans were labeled at the reducing end with a fluorescent tag, purified, and confirmed for correct molecular weight on MALDI-TOF. All structures were printed at 100 µM, except for the new LDN-containing structures, which were printed at 50 µM due to the low efficiency of the serum β1,3-N-acetylglucosaminyltransferase reaction in generating poly-LDN. LNnT-derived-LDNF (ID 15–16) and poly-LDNF (ID 19–20) were therefore printed at both 50 and 100 μM for the purpose of comparing immune response among immunization groups (Figure 5A).
We tested a panel of lectins on the DSA as controls, and their binding patterns verified the successful printing of all desired structures (Figure 5B). For example, ConA bound to all of the N-glycans as expected, since it recognizes a wide variety of biantennary complex-type N-glycans, WFA bound to all of the GalNAc-terminating glycans, and AAL bound to all of the fucose-containing glycans. In contrast to the lectin-binding patterns, monoclonal antibodies generated during parasite infection and polyclonal sera from infected mice can differentiate acutely among very similar structures on the array (Figure 5C and D). The anti-LDNF antibody (Figure 5C) and chronically infected mouse serum (Figure 5D) had highest reactivity with the poly-LDN(F) chains terminating in LDNF (ID 18–20), and also bound to LDNF linked to lactose, N-acetyllactosamine, LeX and chitobiose (ID 15–16, 23, 25, 27). The antibody had little reactivity with the monoantennary or biantennary versions of LDNF (ID 6, 29, 31, 33), and chronically infected mouse serum had none. In contrast, anti-LDN (Figure 5C) reacted more similarly with all of the straight-chain versions of LDN as well as the synthetic biantennary N-glycans (ID 14, 17, 22, 24, 26, 28, 32). Curiously, it did not react with the monoantennary or biantennary glycopeptide versions of LDN (ID 5, 30). We have observed that another anti-schistosomal monoclonal antibody, targeting the LeX glycan, which was recently characterized by Mandalasi et al. (2013) (Figure 5C), is also specific for an LNnT-derived version of LeX (ID 12) over a biantennary glycopeptide (ID 3).
The array profiles of L8-GTFT week 20 secondary immunized antisera (Figure 5E) showed similar specificity to the chronically infected mouse antisera (Figure 5D). For IgG, both immune and infected serum samples showed a clear preference for extended straight-chain vs. biantennary versions of LDNF, with a difference of roughly 100-fold between the highest binders (ID 18, 20) and the N-linked versions (ID 29, 31, 33). The chronically infected serum had about 5-fold higher activity than the L8-GTFT-immune serum, but showed exactly the same pattern of specificity. The IgM response of L8-GTFT-immune serum was identical to IgG in specificity on the DSA, whereas infected mouse IgM bound most highly to extended versions of LDNF but also had high reactivity with LeX, straight-chain and biantennary LDN, and biantennary LDNF (data not shown). In comparison to infection antisera reactivity on the DSA, L8-GTFT immunization appears to have exactly replicated the IgG-binding pattern and biased the IgM response towards extended versions of LDNF vs. biantennary versions.
The L8-GT-immunized group also generated IgG that preferred straight-chain versions of the epitope, and titers of IgG to poly-LDN (ID 17) were similar to the IgG generated to poly-LDNF (ID 19) by L8-GTFT immunization at the week 20 time point (Figure 5E). The anti-LDN IgG and IgM had relatively more reactivity to N-glycan versions of the epitope when compared with the anti-LDNF response, binding about 10-fold less to these (ID 28, 30, 32) than to poly-LDN (ID 17). In comparison to the anti-LDN monoclonal (Figure 5C) and infection antisera IgM (data not shown), the L8-GT cellular immunization seems to have skewed the specificity of the response more towards extended versions of LDN vs. LDN on N-glycans, in a similar fashion to L8-GTFT immunization.
Taken together, the glycan microarray data demonstrate that antibodies generated to parasite glycans, both during infection and immunization, are highly specific for particular presentations of glycan epitopes and that L8-GTFT cells mimicked the pattern of anti-LDNF IgG reactivity displayed by infection antisera.
Lec8-GTFT cell antiserum binds to schistosome glycans
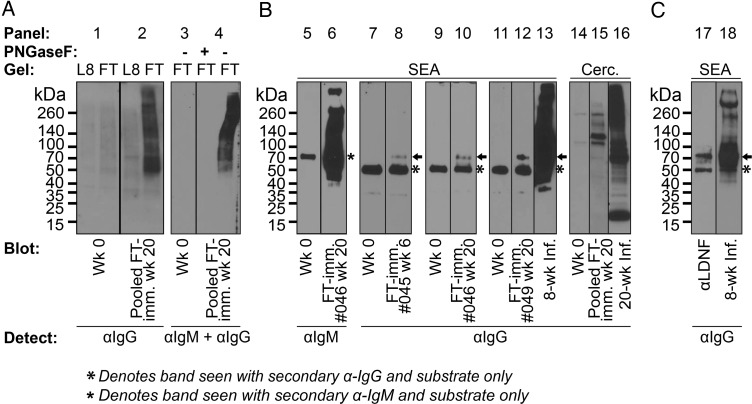
Conceivably, a vaccine to protect against schistosome infection or mitigate its pathology by reducing worm and/or egg burden would need to target the intra-mammalian life stages of the parasite. We used serum from several high-responding individual L8-GTFT-immune mice at the 6- and 20-week time points to explore reactivity with parasite life stages by performing western blots against concentration-standardized cell lysates (Figure 6A) and S. mansoni lysates (Figure 6B). As negative controls, we found that the pre-bleed serum had no specific staining of cells or parasites (Figure 6A and B—panels 1, 3, 5, 7, 9, 11, 14). As a positive control, the L8-GTFT-immune serum reacted with L8-GTFT cell lysate, but not with Lec8 cell lysate or with L8-GTFT cell lysate where N-glycans have been removed with PNGaseF (Figure 6A—panels 2 and 4). In all cases, the immune sera were most highly reactive to SEA when compared with the earlier life stages (Figure 6B—panels 6, 8, 10 and 12), and in at least one mouse there was reactivity to cercarial lysate (Figure 6B—panel 15). This was not surprising given that SEA also demonstrated the most LDNF staining when blotted with anti-LDNF (Figure 1D). In the immune serum samples, IgM binding to SEA was most readily detectable, staining many molecular species from 40 to >260 kDa (Figure 6B—panel 6). A molecular species around 70 kDa in SEA was stained by IgG from at least three different L8-GTFT-immunized mice (Figure 6b—panels 8, 10 and 12). This species may correspond to a ∼70 kDa antigen that was also one of the most highly reactive bands when staining with anti-LDNF and infected mouse serum (Figure 6C—panels 17 and 18). Eight- and 20-week-infected mouse sera are shown staining SEA and cercarial lysate in two of the experiments for comparison (Figure 6B—panels 13 and 16), demonstrating that the anti-parasite titer generated by L8-GTFT immunization was much lower than what is characteristic of infected mice.
Fig. 6.
L8-GTFT cell antisera binds to schistosome glycoproteins. (A) Binding to cell N-glycans was confirmed by western blotting of pre-bleed and week 20 L8-GTFT (FT) antisera against Lec8 and L8-GTFT (FT) cell lysate, mock or PNGaseF-treated to remove N-glycans. (B) Pre-bleed sera and antisera from individual L8-GTFT-immunized mice that responded highly in flow cytometry experiments were used to stain parasite life stage lysates, with infected mouse sera shown as a positive control in some experiments. (C) Low-exposure stains of SEA with anti-LDNF and infected sera are shown for comparison with the molecular weights stained by immune sera in (B). Panes separated by a line were run in the same experiment and membranes were cut to allow for incubation with naïve, immune or infected sera. Membranes separated by space were run on different days under similar conditions. SEA, soluble egg antigen; Cerc., cercarial lysate (B). Blots were detected with HRP-labeled goat anti-mouse IgG or IgM.
These data demonstrate that vaccination of mice with L8-GTFT cells induced a response that is cross-reactive with S. mansoni egg glycoproteins. While the magnitude of the response induced by this vaccination regimen may not be sufficient to protect against schistosomiasis, our studies show that the unadjuvanted cells were immunogenic, successfully mimicked the glycan antigenicity induced by S. mansoni infection and induced antibodies that bound to parasite antigens.
Discussion
Pathogenic helminths possess a wealth of glycans that are antigenic to their mammalian hosts. Translation of these antigenic glycans into vaccine candidates is contingent upon our ability to package them in a way that (i) is immunogenic and (ii) induces an immune response specific for parasite glycans. In this study, we have developed a recombinant cellular engineering platform with which to achieve these ends, and demonstrated their utility in manipulating the anti-glycan immune response and as potential vaccine antigens.
Immunization with cells or cell membrane preparations is an established technique for development of monoclonal antibodies to cell-surface antigens, such as cancer antigens, blood group antigens and glycosphingolipids (Koprowski et al. 1978, 1979; Brockhaus et al. 1981; Hansson et al. 1983; Hirohashi et al. 1985; Iwamori et al. 2009). However, there are few examples in the literature of engineering cell lines to generate immunogens with particular glycosylation patterns (Lee et al. 2002; Ahn et al. 2011), and, to our knowledge, this is the first application of such a strategy for generation of antibodies to the glycan antigens of a eukaryotic pathogen. Glycans are prominent antigens in many pathogenic infections, such as other helminthiases, leishmaniasis and HIV, as well as several autoimmune diseases. Manipulation of mammalian cell lines with well-characterized glycosylation patterns, such as the Lec series mutants, will enable us to better characterize the function of glycosyltransferases from such pathogens, to determine the precise glycoforms targeted by parasite- or virus-neutralizing antibodies and autoantibodies to glycoproteins, and to test the immunogenicity of vaccine candidates in their appropriately glycosylated state. The contributions of this strategy, coupled with glycan microarray technology, to glyco-immunology and vaccinology could be far-reaching.
The cells tested in this study were immunogenic without adjuvant and induced an anamnestic response consisting of IgM and IgG, suggesting that many molecular species in the Lec8 cells were immunogenic. The addition of the polymeric LDNF on cell-surface N-glycans improved the cells' immunogenicity, as evidenced by higher magnitude IgG responses and longer-lasting antibodies than the parent cell lines. This raises interesting questions about the role of LDNF motifs in innate immunity. It is possible that repeating LDNF units, or fucose-containing antigens in general, specifically contribute to a robust, T-cell-dependent anti-glycan response. LDNF is a ligand for DC-SIGN (Van Die et al. 2003; Van Liempt et al. 2006), an internalizing C-type lectin receptor found on DC. LDNF was recently shown to be among the fucosylated glycans which promote DC-SIGN/TLR-4-mediated activation of human DC to a pro-inflammatory phenotype by worm glycolipids (Van Stijn, Meyer et al. 2010). These cells could be used to determine the effect of fucosylated cell-surface motifs on parameters of immunogenicity such as internalization by antigen presenting cells (APC), T-cell proliferation and generation of memory.
In spite of the impressive natural immunogenicity of the cells, western blot and glycan array data showed that both the titer of glycan-specific antibodies and the anti-parasite titer generated by the cells were lower than that of chronically- and acutely-infected mouse serum. The variability of the response among mice was also high. This could be due to the relatively low dosage used, especially since membrane glycoproteins comprise a small percentage of total cellular material. Future studies will adjust the immunization regimen, including increasing the dosage above 107 cells per animal, fixing cells and using adjuvants, with the goal of increasing the magnitude of anti-parasite response.
Immunization with Lec8 cells induced sizable shifts in IgG and IgM on flow cytometry but failed to induce GlcNAc-specific antibodies. The L8-GTFT cells, in contrast, induced glycan-specific antibodies, and when lysates were treated with N-glycanase, the immune serum reactivity was completely lost. This indicates that addition of LDNF created an immunodominant antigen. The immunodominance of LDNF could be due to its carriage on many distinct molecular species and thus linked to many distinct T-cell epitopes, or due to special immunogenic properties of the poly-LDNF itself, as suggested above. Membrane-bound antigen may be a more efficient and more physiological method of stimulating B cell receptors (Carrasco and Batista 2006; Batista and Harwood 2009); however, it is unclear whether this phenomenon is limited to antigens on the surface of APC or whether antigens on other cell membranes have the same effect. The robust IgM anamnestic response we observed for L8-GT and L8-GTFT cells was unexpected. We suspect that LDN(F) glycoconjugates are behaving as T-dependent antigens, but it is possible that poly-LDN(F) could cross-link B-cell receptors, act as a ligand for pattern recognition receptors and/or stimulate T-dependent or T-independent memory in non-classical B cells (reviewed in Defrance et al. 2011; Good-Jacobson and Tarlinton 2012). More work is needed to understand the nature of adaptive immunity to eukaryotic glycan antigens in both vaccination and parasitic infections.
The glycan array studies of L8-GTFT immune serum, infected mouse serum and monoclonal αLDNF antibody presented here demonstrated two extremely interesting findings. First, that antibodies generated to parasite glycans are exquisitely specific for particular forms of their epitope. In the case of LDNF, for example, IgG antibodies generated during chronic infection have a clear preference for LDNF when the trisaccharide is not directly linked to mannose. We observed the same pattern of selectivity with an αLeX antibody, whereas the αLDN antibodies examined in this study had varying levels of binding to mannose-linked LDN, suggesting that such fine specificities are a common feature of anti-glycan antibodies. In agreement with our results, it has previously been noted that most antibodies to N-glycans are IgM, whereas all of the monoclonals used in this study were IgG (Lee et al. 2002). In the case of LDNF, the antibody selectivity could be due to a particular structural presentation of LDNF, such as poly-LDNF, being immunodominant and leading to an abundance of affinity-matured antibodies that are highly specific for that presentation, or because mammals are tolerized to mannose-linked LDNF, which may resemble a select few host glycoproteins (Dell et al. 1995; Van den Eijnden et al. 1995; Van Den Nieuwenhof et al. 2000). The Stanley group found that when immunizing with Lec10 cells producing bisected N-glycans, only mutant mice lacking endogenous bisecting GlcNAc could produce antibodies to the structure (Lee et al. 2002). We hypothesize that the “antigenic” form of LDNF in S. mansoni occurs on the non-reducing termini of glycolipids, O-glycans, and/or of extended (non-mannose-linked) N-glycan chains. Preliminary evidence suggests cercarial LDNF is carried on species other than PNGaseF-sensitive N-glycans, and studies are ongoing to answer this question.
Secondly, the poly-LDNF-expressing cells exactly recapitulated the profile of αLDNF specificities induced during S. mansoni infection of mice. This collection of glycans is by no means exhaustive; nevertheless, among the glycans assayed on our microarray, the immune serum and the infection serum shared the same immunodominant epitope, poly-LDNF. Our group first identified the LDNF on the biantennary N-glycans of S. mansoni, and, at the time, it was noted that glycopeptides with that structure were not reactive with sera from infected hamsters (Srivatsan et al. 1992a). Furthermore, previous experiments in which biantennary LDN and LDNF were synthetically linked to carrier proteins failed to elicit a glycan-specific response, even though the conjugates were immunogenic. Cellular immunogens, in contrast, possess a high density of LDNF carried as polymeric chains. Array results suggest that, unlike synthetic glycoconjugates of biantennary LDNF, this mode of presenting LDNF provides sufficient immunogenicity, and the correct antigenicity, to induce an LDNF-specific antibody response that mimics the murine response to this antigen during parasite infection. Thus, well-defined glycan microarray structures have identified features of anti-glycan antibodies that could be helpful in design of glycan-based vaccines or diagnostics. The addition of more schistosome glycans to the array and screening of stage-specific antisera, which is currently being done by our lab and others (Van Diepen, Smit et al. 2012) will yield additional insights.
We chose to express LDN and LDNF because they are building blocks for several unique schistosome antigens and because antibodies to these epitopes are found in schistosomula-lethal sera of naturally protected hosts (Luyai et al. 2014). Antibodies to LDNF are also implicated as a correlate of protection in vaccination of sheep against Haemonchus contortus, and a potential serodiagnostic for trichinellosis (Vervelde et al. 2003; Aranzamendi et al. 2011). The L8-GTFT immunization group generated both IgM and IgG that bound to S. mansoni life stages, mainly to SEA. This result supports our hypothesis that expression of schistosome glycan epitopes in mammalian cells can recapitulate the immunity generated by the parasite to these epitopes. The finding that immune serum primarily targeted the egg stage was unsurprising, given that LDNF is most abundantly expressed on eggs, which was confirmed in this and previous studies by our lab and others using different clones of αLDNF (Nyame et al. 2002, 2003; Robijn et al. 2005). An ideal schistosomiasis vaccine, however, would likely target the earlier intra-mammalian life stages. There are other antigenic glycans that are expressed more highly on larval stages (Robijn et al. 2005), and the cellular expression system could be adapted to target such antigens. A vaccine targeting one glycan antigen that is highly expressed on the larval or adult worm surface, or a few glycan and protein antigens that are differentially localized and/or expressed on multiple intra-mammalian life stages might be effective against helminths. Many of the glycan epitopes targeted in schistosome infection have been discovered (Srivatsan et al. 1992a, b; Khoo et al. 1995; Van Remoortere et al. 2000; Jang-Lee et al. 2007; Robijn et al. 2007), but few have been characterized in terms of protective ability. The recombinant engineering strategy could be utilized to assess candidate glycoconjugates by co-expressing parasite glycans and proteins, either in a whole-cell platform or as secreted, soluble, parasite-like glycoproteins.
This study serves as a proof-of-concept that glyco-engineered cells, in contrast to synthetic glycoconjugates carrying similar epitopes, induce a long-lived and highly specific antibody response towards an immunodominant surface-expressed glycan. Our work has highlighted the complex epitope specificity of anti-glycan antibodies. This type of information, along with further advances in helminth glycomics and glycan microarray technology, will shape the structural guidelines for design of glycan-based vaccines in the future. In addition to providing new directions for helminth vaccine development, these cells should facilitate the discovery of novel glycosyltransferase activities in a variety of systems, and help us better understand the role of glycan antigens in infectious and autoimmune diseases.
Materials and methods
Materials
General chemicals and glycosyltransferase enzymes were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburg, PA) unless otherwise noted.
Cell culture reagents
DMEM, G418 sulfate and sterile PBS without calcium or magnesium were purchased from Cellgro (Manassas, VA). Fetal bovine serum was purchased from Atlanta Biologicals (Lawrenceville, GA). Glutamine, penicillin/streptomycin and 0.25% trypsin/ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco (Grand Island, NY). Zeocin was purchased from Invivogen (San Diego, CA). Tissue-culture treated flasks were purchased from Corning (Corning, NY). Kifunensine was purchased from Toronto Research Chemicals, Inc. (North York, ON, Canada). Petri dishes for schistosomula culture were obtained from Falcon (Franklin Lakes, NJ).
Animal work
Luer-Lok syringes were obtained from Becton Dickinson & Co. (Franklin Lakes, NJ). Precision Glide needles were obtained from Fisher Scientific (Suwanee, GA). Sterile Goldenrod lancets were obtained from Medipoint (Mineola, NY). Microtainer polymer gel serum collection tubes were obtained from Becton Dickinson & Co. (Franklin Lakes, NJ).
Antibodies and flow cytometry
Peroxidase-conjugated goat anti-mouse IgG (γ) and IgM (µ) were purchased from KPL (Gaithersburg, MD) and Sigma (St. Louis, MO). AlexaFluor-488- and AlexaFluor-633-conjugated secondary goat anti-mouse IgG (H + L) and IgM (µ) were purchased from Invitrogen/MP (Eugene, OR). Mouse IgG1 isotype control was purchased from R&D Systems (Minneapolis, MN). Polystyrene tubes for flow cytometry were purchased from BD Falcon (Franklin Lakes, NJ).
Parasite isolation, lysates and western blots
Complete MINI protease inhibitor tablets (EDTA free) were purchased from Roche Diagnostics (Mannheim, Germany). 70 µm nylon cell strainers were purchased from BD Falcon. Percoll was purchased from GE Healthcare (Piscataway, NJ). Potassium chloride was obtained from J.T. Baker, Inc. (Phillipsburg, NJ). Amicon Ultra-4 centrifugal filter devices were purchased from Millipore (Tullagreen, Carrigtwohill Co., Cork, Ireland). The PNGaseF kit was purchased from New England BioLabs (Ipswitch, MA). Mini-PROTEAN-TGX 4–20% gels for SDS–PAGE, Kaleidoscope Precision-Plus Protein standards, the Trans-Blot Turbo system, and accompanying nitrocellulose membranes, filter paper stacks and transfer buffer were purchased from Bio-Rad (Hercules, CA, USA and membranes, Munich, Germany). Non-fat dried milk was obtained from the grocery store (Publix brand). Pierce BCA protein assay kit, Pierce 660 nm protein assay kit, and SuperSignal West Pico and Femto Chemiluminescent substrates were purchased from Thermo Scientific (Rockford, IL). 4× NuPAGE SDS sample buffer was purchased from Invitrogen (Carlsbad, CA). Metal sieves (425, 180, 106, 45, 20 µm) were obtained from VWR Scientific (West Chester, PA).
Preparation of glycans and microarrays
2-Amino(N-aminoethyl) benzamide (AEAB) was synthesized as previously published (Song et al. 2009). Chicken glycopeptide (NA2) was prepared from eggs as previously published (Seko et al. 1997). Chitin hydrolysate, E-PHA agarose and biotinylated lectins (ConA, AAL, WFA, GSL-II) were purchased from Vector Labs (Burlingame, CA). Chitin oligosaccharides were then fractionated by normal phase HPLC. GDP-Fucose (GDP-Fuc), UDP N-acetylgalactosamine (UDP-GalNAc) and UDP-N-acetylglucosamine (UDP-GlcNAc) were purchased from Kyowa Hakko Kogyo Co. (Tokyo, Japan). Affigel 10 was purchased from Bio-Rad. IgG to the HPC4 epitope tag was purified from supernatant, kind gift from Dr. Chuck Esmon (University of Oklahoma Health Sciences Center). Neuraminidase, Protein A agarose and shrimp alkaline phosphatase (SAP) were purchased from Roche Diagnostics. Recombinant H. pylori β1,3-N-acetylglucosaminyltransferase was a kind gift from Warren Wakarchuk (Peng et al. 2012). Guanidine hydrochloride and iodoacetamide were purchased from Acros Organics (Fair Lawn, NJ). TPCK-treated trypsin was purchased from Worthington Biochemical Corp (Lakewood, NJ). Dialysis tubing was obtained from Spectrum (Rancho Dominguez, CA). Sep-Pak cartridges (reverse phase C18) were purchased from Waters (Milford, MA). Carbograph columns and slide separation chambers were purchased from Grace Davison Discovery Sciences (Deerfield, IL). NHS slides were purchased from Schott (Elmsford, NY). Tris–HCl was obtained from ProMega (Madison, WI).
Methods
Animals and infection with S. mansoni
Female Swiss-Webster mice (6–8 weeks old) were used for immunization. S. mansoni-infected mice were obtained from the Schistosomiasis Resource Center of the Biomedical Research Institute in Rockville, MD. Female Swiss-Webster mice (4–6 weeks old) from Taconic Farms were infected with an average of 30 (low dose) or 200 (high dose) cercaria per mouse, and shipped to our facility. Infected mice were monitored for abdominal distention and piloerection and sacrificed if experiencing excessive stress. High-dose infected mice were sacrificed at 7.5 weeks postinfection for collection of adult worms and eggs, and low-dose infected mice were sacrificed at 20 weeks postinfection, after obtaining acute infection antisera (8 weeks postinfection) and chronic infection antisera (20 weeks postinfection). All sera were obtained by facial vein puncture except the chronic infection sera, which was obtained by cardiac puncture immediately following euthanasia. At the conclusion of our experiments, all immunized mice were euthanized by carbon dioxide inhalation followed by cervical dislocation, and all infected mice were euthanized by intraperitoneal overdose with 300 μL of 65 mg/mL sodium pentobarbital with 200 U/mL heparin sodium salt. All experiments involving mice were approved by the Emory University IACUC.
Isolation of S. mansoni life stages
Schistosoma mansoni-infected snails were provided by the Schistosome Research Reagent Resource Center for distribution by BEI Resources, NIAID, NIH: Schistosoma mansoni, Strain NMRI-exposed Biomphalaria glabrata, Strain NMRI, NR-21962, and maintained under conditions specified in unit 19.1 of Current Protocols in Immunology, “Schistosomiasis” (Lewis 1998). Schistosoma mansoni cercariae and schistosomulae were obtained from infected snails as per the above literature with some modifications. Briefly, snails maintained in a dark room were placed into beakers of conditioned water, under a bright light, for 2 h. The water was filtered through 70 µm nylon cell strainers to collect cercaria. After counting, the cercariae were incubated on ice for 30 min, and centrifuged at 500 × g for 10 min at 4°C. For transformation to schistosomula, the cercarial pellet was resuspended in 5–10 mL of cold DMEM and vortexed on high for 2× 45 s periods, with a 3-min ice incubation in between; or the pellet was resuspended to 1000 cercaria/mL in cold DMEM and transformed by passage 8 times though a syringe and 22-G needle, in 10 mL batches. The suspension of detached schistosomula and tails was allowed to settle and the bottom few milliliters were loaded onto a 40 or 60% percol gradient in DMEM. The gradient was centrifuged for 15 min at 500 × g, 4°C. The supernatant was carefully removed down to the bottom 1–2 mL, and the schistosomula pellet was washed three times by spinning at 300 × g in cold DMEM. The schistosomula were cultured for 3 days in DMEM with 10% FBS, penicillin/streptomycin at a density of 500–1000 organisms/mL in tissue-culture dishes. For preparation of adult worms and eggs, 50 high-dose infected Swiss-Webster mice were sacrificed after 7.5 weeks of infection. The mesenteric veins were perfused with saline (0.85% sodium chloride, 0.75% sodium citrate) as described previously (Lewis 1998). Adult worm pairs were centrifuged twice at 163 × g in ultracentrifuge bottles, and three times in 15 mL conical tubes in a table-top centrifuge at 8 × g, washing with perfusion fluid in between spins, at 4°C, until the worms were well washed. The loosely pelleted worms were then snap-frozen in a minimal amount of perfusion fluid and stored at −80°C. The livers were removed from each mouse immediately following perfusion and stored in cold 1.2% NaCl solution overnight to prevent hatching of eggs. The livers were cut into 1–2 cm sections, processed in a Waring blender, and then loaded onto a series of four sieves, as described in the above literature. Eggs were washed through to the bottom sieve, and then swirled in petri dishes to further purify them from debris. All work with B. glabrata and S. mansoni was approved by the Emory University Office of Occupational Health and Safety, and conducted in BSL-II animal surgery facilities and laboratories.
Cell lines and preparation of immunogens
CHO (Pro-5) cells were obtained from ATCC (CRL-1781). CHO Lec8 cells were obtained from ATCC (CRL-1737). Transfections with glycosyltransferases, selection of stable, clonal cell lines and confirmation of glycosyltransferase activity and N-glycan expression were performed as detailed in Kawar et al. (2005). Cells were thawed from −135°C and grown adherently in tissue culture-treated 75 cm2 flasks with complete DMEM (10% heat-inactivated FBS/2 mM glutamine/100 U/mL penicillin/streptomycin). Lec8-GT cells were additionally cultured with 0.6 mg/mL G418 sulfate, and Lec8-GTFT cells, with G418 and 0.4 mg/mL zeocin. For routine maintenance they were grown to 1–2 × 107 cells/flask and detached for 2 min in 0.25% trypsin/EDTA, which was quenched with complete DMEM and split 1:10–1:20, twice per week. To prepare cells for immunization, they were grown in 225 cm2 flasks to 90% confluence, washed extensively with cold PBS, and then detached by scraping in a minimal volume of cold PBS. The volume was then brought to 50 mL and cells were centrifuged for 5 min at 200 × g. The cell pellet was resuspended in 10 mL cold PBS, counted, and density was adjusted to 2.5 × 106 cells per 200 µL immunization dose. They were frozen at −20°C until use. Kifunensine was dissolved in DMSO to 1 mg/mL and stored at −20°C. Cells were grown for 8 days in complete DMEM with kifunensine at 10 µg/mL, with two splits into fresh kifunensine-containing medium during that period, and then prepared for flow cytometry or lysate as described below. The lack of complex N-glycan expression was confirmed by staining with Concanavalin A (ConA) and anti-glycan monoclonals on flow cytometry, before staining with antisera.
Antibodies
Mouse monoclonal IgGs anti-LDN and anti-LDNF were produced from S. mansoni-infected mice and purified as described (Nyame et al. 2000; Mandalasi 2011; Mandalasi et al. 2013).
Immunization and serum collection
Cellular immunogen preparations in PBS were thawed 30 min before use and gently vortexed to resuspend. 200 µL containing 2.5 × 106 freshly thawed cells in PBS was delivered to each mouse via intraperitoneal injection. Mice were monitored for 30 min after injections and the following day for signs of distress and sacrificed if necessary. Primary cellular immunizations (Figure 3A) were delivered at 0, 2 and 4 weeks. At week 19, secondary (or primary, as a control) immunizations were delivered. 50–100 µL of blood was collected from the facial vein of each mouse using sterile lancets and centrifuged in Microtainer serum collection tubes for 6 min at 3300 × g to isolate serum. For immunized mice, bleeds were taken the day before each primary immunization and 2 weeks after the last, the day before the secondary immunization, and 1 and 2 weeks after, and finally, at 40 weeks, after which all of the mice were euthanized. Serum was stored at −20°C either as individual mouse aliquots or in aliquots pooled from 5 to 10 mice per group. For western blotting, flow cytometry and glycan array experiments, serum was thawed at 4°C and used within 2 weeks of thawing.
Flow cytometry
Batches of 5–10 × 107 cells were detached with trypsin as described above, quenched and then washed three times by centrifugation at 600 × g for 7 min, followed by resuspension in 50 mL of ice-cold, sterile PBS. After each wash, a 25 mL serological pipette was used to bring the cells to single-cell suspension. Cell count and viability were recorded before the last spin and then cells were resuspended in 37.5 mL cold PBS, and 12.5 mL of 8% paraformaldehyde was added, for a final concentration of 2%. They were rotated in the dark, overnight at 4°C. Fixed cells were then washed 3× with 50 mL cold PBS as above. They were stored at 4°C, with 107 cells/mL, in PBS with sodium azide 0.1%, for up to 4 months without any changes noted on flow cytometry. For flow cytometry experiments, 150 μL of cold PBS containing appropriately diluted antibody, lectin or serum sample (1:100) was added to each 5 mL polystyrene tube on ice and then gently mixed with 50 μL containing 5 × 105 fixed cells. Incubations were for 45 min, in the dark, on ice. The samples were brought up with 1 mL cold PBS before centrifuging for 5 min at 200 × g, 4°C, with brakes on 5 out of 10. Supernatants were decanted and the cell pellet resuspended in 200 μL of the appropriate secondary detection reagent diluted at 1:1000—goat α-mouse IgG-488 or 633, gαmIgM-488, or a mixture of the two for serum and monoclonal antibodies, or streptavidin-488 for biotinylated lectins. After the secondary incubation and centrifugation as above, cells were resuspended in 500 μL cold PBS for analysis on the FACSCalibur using CellQuestPro acquisition software. Each stain was performed in duplicate and 10,000 events were collected per sample using FL-1 for -488 and FL-4 for -633 with no compensation. Data was analyzed using FlowJo software by gating on the live cell population in SSC vs. FSC and recording geometric MFI of FL-1 or FL-4 for each live cell population. Statistical analysis was performed using GraphPad Prism using one- or two-way ANOVA, with or without repeated measures, and using Tukey's or Dunnett's post-test multiple comparisons, as indicated in each figure legend. Alpha of 0.05 was used as the cut-off for significance and levels of significance are specified in the figure legends.
Preparation of cell and parasite lysates
Lysates of Lec8, L8-GT and L8-GTFT cells were made by detaching two 75 cm2 flasks of 90% confluent cells as detailed above, quenching, and washing three times with 5 mL room-temperature sterile PBS. After removing supernatant, cells were resuspended in ∼100 μL per 4 × 106 cells of freshly thawed lysis buffer (100 mM sodium cacodylate pH7.0/1.5% triton X-100/1 tablet complete MINI protease inhibitor per 10 mL buffer in ddH20), vortexed vigorously for 30 s, and incubated for 40 min on ice, vortexing once in the middle. The lysate was centrifuged for 10 min at 3220 × g at 4°C to remove cell debris. The supernatant was aliquotted for storage at −20°C, quantified via BCA assay, and used for western blotting within 2 weeks of thawing. N-glycan removal from the cell lysate was performed according to the manufacturer's protocol, using 2.5 μL PNGaseF enzyme for 50 μg of lysate. After inactivating the enzyme, lysates were stored at −20°C until use. For preparation of cercarial lysate, after spinning the chilled cercaria as describe above, the supernatant was removed and the pellet was transferred to 1.5 mL Eppendorf tubes in at most 50 µL of parasite lysis buffer (50 mM Tris buffer, pH 8.0, 2.5% 2-mercaptoethanol, 1% Triton-X-100, 1 mM EDTA and 1 tablet of Complete Mini Protease Inhibitor per 10 mL of lysis buffer) per 10,000 cercaria. This was vortexed, boiled for 15 min (vortexing once during the boiling incubation) and centrifuged at 20,000 × g for 2 min. The supernatant was removed to clean tube, and a small amount of lysis buffer was added to the pellet for another 10 min boiling incubation, after which the spin was repeated and the supernatants were pooled. After culture, schistosomula were washed three times in cold PBS and then lysed as described above for cercaria, using at most 25 µL of parasite lysis buffer per 10,000 schistosomula. Adult worm lysate was made by bringing a freshly-thawed adult worm pellet of ∼0.5 mL up in 5 mL of PBS, spinning at 500 × g for 10 min at 4°C, and adding 3 mL of lysis buffer to the pellet. The worm lysate was made as described above except 1% SDS and 1 mM phenylmethylsulfonylfluoride were used instead of Triton-X-100 and the Complete Mini tablet, respectively, and the SDS was salted out using 100 mM KCl so the lysates could be concentrated in Amicon spin filter tubes (3000 Da MWCO). Triton X-100 was then added back to 1% to the concentrated adult worm lysates. SEA was prepared as per unit 19.1, “Schistosomiasis,” of Current Protocols in Immunology (Lewis 1998). Parasite lysates were quantified by the Pierce 660 nm protein assay and stored in aliquots at −80°C. For experiments where both cell and parasite lysates were compared, their concentrations were standardized by including SEA in both the BCA and Pierce 660 assays.
Western blots
For SDS–PAGE and western blotting, 5–12 µg of cell or parasite lysates (for each experiment the gel was equally loaded) were boiled in 1× NuPAGE SDS sample buffer + 2.5% β-mercaptoethanol for 10 min and then run in 10- or 12-well Mini-PROTEAN-TGX gels at 200 V for 30 min, with 7 µL of protein standards. Protein was transferred to a nitrocellulose membrane using the 10-min High Molecular Weight program in the Trans-Blot Turbo semi-dry transfer system. All subsequent incubations were shaking at ambient temperature. Membranes were stained with 0.1% Ponceau S in 5% acetic acid to check for equal loading and transfer, and destained with TBS wash buffer (20 mM Tris, 300 mM NaCl, 0.1% Tween-20). For staining with serum, membranes were blocked for 2 h or overnight in 2-3% (w/v) milk (de-fatted dried milk in 20 mM Tris, 300 mM NaCl). Incubations with serum (1:500–1:1000 dilutions; same dilution is used where multiple serum samples are compared on the same date) were 1 h in milk diluent (0.5–1% milk in 10 mM Tris, 150 mM NaCl, 0.1% Tween-20). The membranes were then washed three times quickly and three times for 10 min each in TBS wash buffer. Secondary detection antibodies (HRP-conjugated goat anti-mouse-IgG or -IgM) were added for 1 h at 1:3000–5000 in milk diluent. The same wash procedure was repeated, and then SuperSignal West Pico or Femto Chemiluminescent Substrate was added for 30 s. The membranes were dabbed dry and exposed to film for 1 s–3 min (panes shown from the same date used the same substrate and exposure time). When blotting was performed only with monoclonal antibodies and not with serum, a similar protocol was followed except membranes were blocked for 1 h or overnight in 5% bovine serum albumin fraction V (BSA), primary and secondary incubations were in 5% BSA diluents with the secondary antibody at 1:10,000, and three washes were performed for five minutes each after each incubation.
Chemo-enzymatic synthesis of array glycans
Biantennary N-glycopeptides were generated as described in Luyai et al. (2014). All other glycan starting products (LNnT, NA2, chitotriose, released IgG N-glycans) were conjugated to 2-amino(N-aminoethyl) benzamide (AEAB) at the reducing end, as described previously (Heimburg-Molinaro et al. 2011), before further modification with glycosyltransferases. Recombinant HPC4-tagged β1,4-N-acetylgalactosaminyltransferase (B4GALNT) and human α1,3-fucosyltransferase 6 (FUT6) were cloned and expressed in SF9 cells as previously described (De Vries et al. 1997; Kawar et al. 2002). Anti-HPC4-linked Affigel 10 beads were prepared according to the manufacturer's instructions, by incubating for 4 h with 1 mL of a 2 mg/mL αHPC4 IgG solution in 100 mM 3-(N-morpholino)propanesulfonic acid (MOPS) buffer pH7.5, at 4°C, and then washed and stored at 4°C in 50 mM sodium cacodylate, pH7, with 0.2% sodium azide. The beads were washed three times with 100 mM sodium cacodylate, pH7 with 2 mM calcium chloride and 0.1% sodium azide and then rotated for 2 h, slowly, at ambient temperature with freshly-thawed supernatant containing the recombinant B4GALNT or FUT6 from clarified SF9 cell medium, and 2mM calcium chloride. A ratio of ∼60:1 (v/v) supernatant to beads was used. The supernatant was then removed and beads washed three times as above before combining with the glycan acceptor in the reaction mix as specified below.
To synthesize LDN, galactose-terminating AEAB-conjugated glycans were digested with β-galactosidase (β-gal) in 30 mM sodium acetate buffer, pH 5.2 (β-gal was previously dialyzed into 30 mM sodium acetate pH 5.2), with 1 mg glycan per 100 µL reaction, at 37°C. The reaction was boiled and then either ethanol-precipitated or brought up directly in B4GALNT reaction. The volume was increased 3-fold to a final concentration of 50 mM sodium cacodylate, pH 7, 0.1% sodium azide, 25 mM manganese chloride, 1 μL SAP, 2 mM UDP-GalNAc and mixed with a slurry of Affigel beads bound to B4GALNT at a ratio of 5:1 (reaction volume:slurry volume). When GalNAc addition was complete, the supernatant was removed to a clean tube and the reaction volume was increased slightly, maintaining the same buffer conditions, except that no additional manganese chloride was added, and GDP-fucose was added instead of UDP-GalNAc. This reaction mix was added to beads carrying FUT6 enzyme, prepared by the same method described above. All reactions were monitored by mixing 0.5 μL of the reaction with 2,5-dihydroxybenzoic acid matrix on a target plate and analyzing sample on a MALDI-TOF (Bruker Daltonics Ultraflex II TOF/TOF) in reflectron positive (RP) mode. Fresh UDP-GalNAc or GDP-fucose were added as needed and, if reaction was not complete within 36 h, fresh enzyme/beads were prepared for the reaction mix. Poly-LDN and poly-LDNF were prepared using AEAB-LNnT as a starting product and following the LDN chemo-enzymatic synthesis method described above. Fresh human serum was used to catalyze the addition of GlcNAc to LDN (Yates and Watkins 1983; Leppanen et al. 2002). Briefly, 200 nmol of acceptor was added to 5.6 μmol UDP-GlcNAc, 1.6 μmol manganese chloride, ∼7 mg of fresh human serum concentrate (or the 30–65% fraction of ammonium sulfate precipitate from the serum), and this was brought up to 200 μL in 50 mM Tris–HCl, pH 7.4 with 0.04% sodium azide. The reaction was run for 6–9 days at 37°C and then purified using ethanol precipitation, C18 SepPak and Carbograph columns and HPLC as described previously (Heimburg-Molinaro et al. 2011). The LDN/LDNF chemo-enzymatic synthesis was then repeated to obtain two repeats of LDN and addition of 1-2 fucose residues. These were also HPLC purified as described above and the mass of each purified peak as well as position of the fucose was verified by tandem mass spectrometry. Human IgG N-glycans were obtained by purifying IgG from 10 mL of fresh human serum using a protein A agarose column at 4°C, according to the manufacturer's instructions. The serum was centrifuged at 10,000 × g and 2 mL batches of supernatant were adjusted to pH8 using Tris–HCl and loaded onto 2 mL of resin. Tris–HCl was used as wash buffer, and 100 mM glycine for elution into 1 M Tris–HCl, pH 8.0, and the column was regenerated between each batch. The purity was checked on SDS–PAGE and then purified IgG was dialyzed into PBS. The IgG was frozen, lyophilized and then denatured in 8 M guanidine HCl/90 mM dl-dithiothreitol (DTT)/200 mM Tris–HCl, pH 8.2, for 1 h at ambient temperature on an orbital shaker. It was then alkylated using 120 mM iodoacetamide in the same buffer for 30 min. The denatured IgG was dialyzed into water at 4°C overnight in 6–8 kDa MWCO tubing, frozen and lyophilized. Two hundred and twenty-two milligrams of denatured IgG were obtained from the 10 mL of serum. This was dissolved in 50 mM phosphate buffer, pH 8.2, and TPCK-treated trypsin was added to 0.33 mg/mL, and rotated at 37°C for 12 h. The sample was boiled for 5 min to deactivate trypsin and digestion was checked via SDS–PAGE. The pH was adjusted to 7.5 with HCl and sodium azide 0.02% and 50 μL PNGaseF were added. The reaction was incubated at 37°C for 72 h and then boiled for 5 min. The glycans were purified by flowing through a 10 g C18 SepPak column, onto a 1 g Carbograph column, and eluted from the Carbograph with 50% acetonitrile/0.1% trifluoroacetic acid (TFA). The free IgG N-glycans (23 mg) were frozen and lyophilized. Neutral and monosialyl glycan fractions were isolated by purification on DEAE cellulose resin and elution in 2 and 20 mM pyridyl acetate buffer, respectively. The eluted fractions were monitored by phenol sulfuric acid assay for hexose content, and peaks were frozen and lyophilized. A small amount of the neutral and monosialyl fractions was permethylated for MALDI-TOF, and the remainder was conjugated to AEAB. The labeled glycan pools were quantified using a Shimadzu spectrofluorophotometer RF-5301PC against AEAB-lactose standards. 1.6 μmol of monosialyl and 1.1 μmol of neutral glycans were obtained. Both the neutral and monosialyl glycan mixtures were enriched for nonbisected glycans by column purification over 2 mL of E-PHA agarose in PBS. A mixture of non-bisected, +/− core fucosylated glycans were collected between 2 and 6 mL of eluent. These were purified on Carbograph columns. Batches of 200 nmol of the mixture of AEAB-labeled, non-bisected, monosialyl N-glycans were digested with 25 μL β-gal and 25 μL β-N-acetylglucosaminidase from Canavalia ensiformis in 1 mL of 50 mM sodium acetate buffer, pH 5.0, at 37°C for 24 h. Digestion with α-mannosidase was attempted, but we found that mannose could not be removed when the other branch of the glycan was intact. Enzymes were removed by ethanol precipitation and glycans were dried. These were digested with 5 μL neuraminidase and 5 μL β-gal in 300 μL of 50 mM sodium acetate buffer with 4 mM calcium chloride at pH 5.5 for 4 h at 37°C and then ethanol precipitated and Carbograph purified. The nonbisected neutral glycan mixture was simply digested with β-gal as above. The mixture of +/− core fucosylated GlcNAc-terminating biantennary N-glycans (from neutral fraction), and the mixture of +/− core fucosylated asymmetrical Man-/GlcNAc-terminating N-glycans (from monosialyl fraction) were then converted to biantennary and monoantennary, respectively, LDN and LDNF, using the chemo-enzymatic synthesis method described above. The products were HPLC purified using an analytical Porous Graphite Carbon (PGC) column, 25 min gradient of 15–40% acetonitrile in water with 0.1% TFA, and then re-purified using a 15–25% gradient. Purified mono- or biantennary LDN or LDNF of the expected molecular weight were successfully isolated from other contaminating N-glycans via this method, with the +/− core-fucosylated glycans co-eluting in most cases, so they were printed as a mixture. All other AEAB-labeled glycans were HPLC-purified using a PGC column, 15–40% acetonitrile gradient, using fluorescence detection at 330 nm (Shimadzu Scientific Instruments). All molecular weights of purified HPLC fractions were verified by MALDI-TOF in RP mode, and, if necessary, fragmented by MALDI-TOF-TOF. The glycans were quantified by fluorescence as described above and adjusted to 10 µL of 100 µM glycan, or, if not enough was available, 50 µM, before printing.
Preparation and analysis of glycan microarrays
Glycan microarrays were prepared, assayed and analyzed as described in Heimburg-Molinaro et al. (2011) with some modifications. Ten microliters of each glycan at 50 or 100 μM was mixed with 1 μL of 10× phosphate buffer in 384-well plates and printed on NHS-activated glass slides using a Piezo Printer (Piezorray, PerkinElmer). Slides were stored at −20°C until use. Binding assays were performed as per Heimburg-Molinaro et al. for experiments with monoclonal antibodies and biotinylated lectins, at the indicated concentrations. For experiments with serum, serum was diluted 1:50, and wells were washed three times with 200 μL TSM wash buffer, then three times with 200 μL TSM buffer with 5 min of shaking for each wash, after the primary and secondary incubations. The secondary antibodies, goat anti-mouse IgG or IgM, -Alexa488 or -Alexa-633, and streptavidin-Alexa488 were used at 5 μg/mL. Slides were scanned using the ScanArray Express software on a PerkinElmer Proscanner XL4000. ScanArray Express was used to align spots, remove background and quantify fluorescence. An excel macro file was then used to average 4–6 replicate spots for each glycan ID, determine SEM, SD and %CV, and generate bar graphs.
Abbreviations
CeB4GalNAcT, C. elegans β1,4-N-acetylgalactosaminyltransferase; FucT9, human α1,3-fucosyltransferase 9; CHO, Chinese hamster ovary cells; L8-GT, Lec8-GalNAcT cells; L8-GTFT cells; LN, N-acetyl lactosamine, Galβ1-4GlcNAc Gal; LDN, LacdiNAc, GalNAcβ1-4GlcNAc; LDNF, fucosylated LacdiNAc, GalNAcβ1-4(Fucα1-3)GlcNAc; LeX, Lewis X, Galβ1-4(Fucα1-3)GlcNAc; MALDI-TOF, matrix-assisted laser desorption ionization time-of-flight; SA, streptavidin; ConA, concanavalin A; GSL-II, Griffonia simplicifolia lectin II; WFA, Wisteria floribunda agglutinin; AAL, Aleuria aurantia lectin; Kif, kifunensine; Iso, isotype control antibody; stds, molecular weight standards; Cerc., S. mansoni cercarial lysate; Som., schistosomula lysate; Adult, adult worm lysate; SEA, soluble egg antigen; Exp. film, exposure time; DSA, defined schistosome-type microarray; RFUs, relative fluorescence units; GlcNAc, N-acetyl glucosamine; GalNAc, N-acetyl galactosamine; LNnT, agalacto-Lacto-N-neotetraose; AEAB, 2-amino-N-(2-aminoethyl)-benzamide; APC, antigen presenting cell; DC, dendritic cells.
Acknowledgments
The authors would like to gratefully acknowledge NIH grant AI101982 to RDC, and acknowledge Fred Lewis, Matt Tucker and the NIAID Schistosomiasis Resource Center in Rockford, MD for supplying infected snails, infected mice and technical advice. Infected snails and mice were provided by Biomedical Research Institute via the NIAID schistosomiasis resource center under NIH-NIAID Contract No. HHSN272201000005I. These materials can be obtained by contacting BEI Resources. We also thank Yi Lasanajak for printing microarrays. We also thank Chris Cutler, Jean-Philippe Gourdine, Rajindra Aryal, Ziad Kawar, Sean Stowell, Stephen Nnodim Sandra Cummings and Carlos Rivera-Marrero for assistance in performing experiments and helpful discussion.
References
- Ahn HJ, Kim Y-S, Lee C-H, Cho E-W, Yoo H-S, Kim S-H, Ko J-H, Kim SJ. Generation of antibodies recognizing an aberrant glycoform of human tissue inhibitor of metalloproteinase-1 (TIMP-1) using decoy immunization and phage display. J Biotechnol. 2011;151:225–230. doi: 10.1016/j.jbiotec.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Aranzamendi C, Tefsen B, Jansen M, Chiumiento L, Bruschi F, Kortbeek T, Smith DF, Cummings RD, Pinelli E, Van Die I. Glycan microarray profiling of parasite infection sera identifies the LDNF glycan as a potential antigen for serodiagnosis of trichinellosis. Exp Parasitol. 2011;129:221–226. doi: 10.1016/j.exppara.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9:15–27. doi: 10.1038/nri2454. [DOI] [PubMed] [Google Scholar]
- Brockhaus M, Magnani JL, Blaszczyk M, Steplewski Z, Koprowski H, Karlsson K-A, Larson G, Ginsburg V. Monoclonal antibodies directed against the human Leb blood group antigen. J Biol Chem. 1981;256:13223–13225. [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B cell recognition of membrane-bound antigen: An exquisite way of sensing ligands. Curr Opin Immunol. 2006;18:286–291. doi: 10.1016/j.coi.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Caulfield JP, Cianci CML, Mcdiarmid SS, Suyemitsu T, Schmid K. Ultrastructure, carbohydrate, and amino acid analysis of two preparations of the cercarial glycocalyx of schistosoma mansoni. J Parasitol. 1987;73:514–522. [PubMed] [Google Scholar]
- Cummings RD, Etzler ME. Antibodies and lectins in glycan analysis. In: Varki A, Cummings RD, Esko JD, editors. Essentials Glycobiol. 2nd ed. New York: Cold Spring Harbor Laboratory Press; 2009. [PubMed] [Google Scholar]
- Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–336. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- De Gruijl TD, van den Eertwegh AJM, Pinedo HM, Scheper RJ. Whole-cell cancer vaccination: From autologous to allogeneic tumor- and dendritic cell-based vaccines. Cancer Immunol Immunother. 2008;57:1569–1577. doi: 10.1007/s00262-008-0536-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dell A, Morris HR, Easton RL, Panico M, Patankar M, Oehninger S, Koistinen R, Koistinen H, Seppala M, Clark GF. Structural analysis of the oligosaccharides derived from glycodelin, a human glycoprotein with potent immunosuppressive and contraceptive activities. J Biol Chem. 1995;270:24116–24126. doi: 10.1074/jbc.270.41.24116. [DOI] [PubMed] [Google Scholar]
- De Vries T, Palcic MP, Schoenmakers PS, Van Den Eijnden DH, Joziasse DH. Acceptor specificity of GDP-Fuc:GalB1-4GlcNAc-R a3-fucosyltransferase VI (FucTVI) expressed in insect cells as soluble, secreted enzyme. Glycobiolgy. 1997;7:921–927. doi: 10.1093/glycob/7.7.921. [DOI] [PubMed] [Google Scholar]
- Diemert DJ, Pinto AG, Freire J, Jariwala A, Santiago H, Hamilton RG, Periago MV, Loukas A, Tribolet L, Mulvenna J, et al. Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: Implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130:169–76.e6. doi: 10.1016/j.jaci.2012.04.027. [DOI] [PubMed] [Google Scholar]
- Eberl M, Langermans JA, Vervenne RA, Nyame AK, Cummings RD, Thomas AW, Coulson PS, Wilson RA. Antibodies to glycans dominate the host response to schistosome larvae and eggs: Is their role protective or subversive? J Infect Dis. 2001;183:1238–1247. doi: 10.1086/319691. [DOI] [PubMed] [Google Scholar]
- Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 1990;265:15599–15605. [PubMed] [Google Scholar]
- Ellis LA, Reason AJ, Morris HR, Dell A, Iglesias R, Ubeira FM, Appleton JA. Glycans as targets for monoclonal antibodies that protect rats against Trichinella spiralis. Glycobiology. 1994;4:585–592. doi: 10.1093/glycob/4.5.585. [DOI] [PubMed] [Google Scholar]
- Faveeuw C, Mallevaey T, Paschinger K, Wilson IBH, Fontaine J, Mollicone R, Oriol R, Altmann F, Lerouge P, Capron M, et al. Schistosome N-glycans containing core alpha 3-fucose and core beta 2-xylose epitopes are strong inducers of Th2 responses in mice. Eur J Immunol. 2003;33:1271–1281. doi: 10.1002/eji.200323717. [DOI] [PubMed] [Google Scholar]
- Frank S, van Die I, Geyer R. Structural characterization of Schistosoma mansoni adult worm glycosphingolipids reveals pronounced differences with those of cercariae. Glycobiology. 2012;22:676–695. doi: 10.1093/glycob/cws004. [DOI] [PubMed] [Google Scholar]
- Good-Jacobson KL, Tarlinton DM. Multiple routes to B-cell memory. Int Immunol. 2012;24:403–408. doi: 10.1093/intimm/dxs050. [DOI] [PubMed] [Google Scholar]
- Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–1118. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- Grzych J, Capron M, Bazin H, Capron A. In vitro and In vivo effector function of rat IgG2a monoclonal anti-S. mansoni antibodies. J Immunol. 1982;129:2739–2743. [PubMed] [Google Scholar]
- Grzych JM, Capron M, Dissous C, Capron A. Blocking activity of rat monoclonal antibodies in experimental schistosomiasis. J Immunol. 1984;133:998–1004. [PubMed] [Google Scholar]
- Grzych JM, Capron M, Lambert PH, Dissous C, Torres S, Capron A. An anti-idiotype vaccine against experimental schistosomiasis. Nature. 1985;316:74–76. doi: 10.1038/316074a0. [DOI] [PubMed] [Google Scholar]
- Hansson GC, Karlsson K, Larson G, Mckibbinl JM, Herlynll M, Steplewskill Z, Koprowskill H. Mouse monoclonal antibodies against human cancer cell lines with specificities for blood group and related antigens. J Biol Chem. 1983;258:4091–4097. [PubMed] [Google Scholar]
- Harn DA, Mitsuyama M, David JR. Schistosoma manosoni anti-egg monoclonal antibodies protect against cercarial challenge in vivo. J Exp Med. 1984;159:1371–1387. doi: 10.1084/jem.159.5.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimburg-Molinaro J, Song X, Smith DF, Cummings RD. Preparation and analysis of glycan microarrays. Curr. Protoc. Protein Sci. 2011:1–33. doi: 10.1002/0471140864.ps1210s64. Chapter 12, Unit 12.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Clausen H, Yamada T, Shimosato Y, Hakomori S. Blood group A cross-reacting epitope defined by monoclonal antibodies NCC-LU-35 and -81 expressed in cancer of blood group O or B individuals: Its identification as Tn antigen. Proc Natl Acad Sci USA. 1985;82:7039–7043. doi: 10.1073/pnas.82.20.7039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotez PJ, Bethony JM, Diemert DJ, Pearson M, Loukas A. Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8:814–826. doi: 10.1038/nrmicro2438. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Engels D, Fenwick A, Savioli L. Africa is desperate for praziquantel. Lancet. 2010;376:496–498. doi: 10.1016/S0140-6736(10)60879-3. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Fenwick A, Savioli L, Molyneux DH. Rescuing the bottom billion through control of neglected tropical diseases. Lancet. 2009;373:1570–1575. doi: 10.1016/S0140-6736(09)60233-6. [DOI] [PubMed] [Google Scholar]
- Iwamori M, Murata M, Toyoda M, Iwamori Y. Contribution of glycolipids to species-specific antigens on erythrocytes of several animal species as to recognition of antigens with rabbit anti-glycolipids and anti-erythrocyte antisera. Glycoconj J. 2009;26:467–476. doi: 10.1007/s10719-008-9197-6. [DOI] [PubMed] [Google Scholar]
- Jang-Lee J, Curwen RS, Ashton PD, Tissot B, Mathieson W, Panico M, Dell A, Wilson RA, Haslam SM. Glycomics analysis of Schistosoma mansoni egg and cercarial secretions. Mol Cell Proteomics. 2007;6:1485–1499. doi: 10.1074/mcp.M700004-MCP200. [DOI] [PubMed] [Google Scholar]
- Kantelhardt SR, Wuhrer M, Dennis RD, Doenhoff MJ, Bickle Q, Geyer R. Fuc(alpha1-->3)GalNAc-: The major antigenic motif of Schistosoma mansoni glycolipids implicated in infection sera and keyhole-limpet haemocyanin cross-reactivity. Biochem J. 2002;366:217–223. doi: 10.1042/BJ20011678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariuki TM, Farah IO, Wilson Ra, Coulson PS. Antibodies elicited by the secretions from schistosome cercariae and eggs are predominantly against glycan epitopes. Parasite Immunol. 2008;30:554–562. doi: 10.1111/j.1365-3024.2008.01054.x. [DOI] [PubMed] [Google Scholar]
- Kawar ZS, Haslam SM, Morris HR, Dell A, Cummings RD. Novel poly-GalNAcbeta1-4GlcNAc (LacdiNAc) and fucosylated poly-LacdiNAc N-glycans from mammalian cells expressing beta1,4-N-acetylgalactosaminyltransferase and alpha1,3-fucosyltransferase. J Biol Chem. 2005;280:12810–12819. doi: 10.1074/jbc.M414273200. [DOI] [PubMed] [Google Scholar]
- Kawar ZS, Van Die I, Cummings RD. Molecular cloning and enzymatic characterization of a UDP-GalNAc:GlcNAc(beta)-R beta1,4-N-acetylgalactosaminyltransferase from Caenorhabditis elegans. J Biol Chem. 2002;277:34924–34932. doi: 10.1074/jbc.M206112200. [DOI] [PubMed] [Google Scholar]
- Khoo KH, Sarda S, Xu X, Caulfield JP, McNeil MR, Homans SW, Morris HR, Dell A. A unique multifucosylated -3GalNAc beta 1-->4GlcNAc beta 1-->3Gal alpha 1- motif constitutes the repeating unit of the complex O-glycans derived from the cercarial glycocalyx of Schistosoma mansoni. J Biol Chem. 1995;270:17114–17123. doi: 10.1074/jbc.270.29.17114. [DOI] [PubMed] [Google Scholar]
- Ko AI, Dräger UC, Harn DA. A Schistosoma mansoni epitope recognized by a protective monoclonal antibody is identical to the stage-specific embryonic antigen 1. Proc Natl Acad Sci USA. 1990;87:4159–4163. doi: 10.1073/pnas.87.11.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H, Steplewski Z, Herlyn D, Herlyn M. Study of antibodies against human melanoma produced by somatic cell hybrids. Proc Natl Acad Sci USA. 1978;75:3405–3409. doi: 10.1073/pnas.75.7.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprowski H, Steplewski Z, Mitchell K, Herlyn M, Herlyn D, Fuhrer P. Colorectal carcinoma antigens detected by hybridoma antibodies. Somatic Cell Genet. 1979;5:957–971. doi: 10.1007/BF01542654. [DOI] [PubMed] [Google Scholar]
- Lee J, Park S-H, Stanley P. Antibodies that recognize bisected complex N-glycans on cell surface glycoproteins can be made in mice lacking N-acetylglucosaminyltransferase III. Glycoconj J. 2002;19:211–219. doi: 10.1023/A:1024205925263. [DOI] [PubMed] [Google Scholar]
- Leppanen A, Penttila L, Renkonen O, McEver RP, Cummings RD. Glycosulfopeptides with O-glycans containing sialylated and polyfucosylated polylactosamine bind with low affinity to P-selectin. J Biol Chem. 2002;277:39749–39759. doi: 10.1074/jbc.M206281200. [DOI] [PubMed] [Google Scholar]
- Lewis F. Schistosomiasis. Curr. Protoc. Immunol. 1998:19.1.1–19.1.28. doi: 10.1002/0471142735.im1901s28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FYC, Ho VA, Khiem HB, Trach DD, Bay PV, Thang TC, Kossaczka Z, Bryla DA, Shiloach J, Robbins JB, et al. The efficacy of a Salmonella typhi Vi conjugate vaccine in two-to-five-year-old children. N Engl J Med. 2001;344:1263–1269. doi: 10.1056/NEJM200104263441701. [DOI] [PubMed] [Google Scholar]
- Luyai AE, Heimburg-Molinaro J, Prasanphanich NS, Mickum ML, Lasanajak Y, Song X, Nyame AK, Wilkins P, Rivera-Marrero CA, Smith DF, et al. Differential expression of anti-glycan antibodies in schistosome-infected humans, rhesus monkeys and mice. Glycobiology. 2014;24:602–618. doi: 10.1093/glycob/cwu029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandalasi M. Immunity to schistosomiasis: Studies on the correlation between schistosome glycan antigen expression and host humoral responses. Chapter 4: Characterization of the binding specificities of IgG monoclonal antibodies to lacdiNAc (GalNAcβ1-4GlcNAc-R) and fucosylated lacdiNAc (GalNAcβ1-4[Fucα1-3]GlcNAc-R) glycan epitopes derived from splenocytes of Schistosoma mansoni infected mice. Doctoral dissertation. 2011 University of Maryland Eastern Shore, Princess Anne, MD, p. 74. [Google Scholar]
- Mandalasi M, Dorabawila N, Smith DF, Heimburg-molinaro J, Nyame AK, Cummings RD. Development and characterization of a specific IgG monoclonal antibody toward the Lewis x antigen using splenocytes of Schistosoma mansoni infected mice. Glycobiology. 2013;23:877–892. doi: 10.1093/glycob/cwt025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mcmanus DP, Loukas A. Current status of vaccines for schistosomiasis. Clin Microbiol Rev. 2008;21:225–242. doi: 10.1128/CMR.00046-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani a, Sangiolo D, Montemurro F, Aglietta M, Valabrega G. Active immunotherapy in HER2 overexpressing breast cancer: Current status and future perspectives. Ann Oncol. 2013;24:1740–1748. doi: 10.1093/annonc/mdt133. [DOI] [PubMed] [Google Scholar]
- Naus CWA, Booth M, Jones FM, Kemijumbi J, Vennervald BJ, Kariuki CH, Ouma JH, Kabatereine NB, Dunne DW. The relationship between age, sex, egg-count and specific antibody responses against Schistosoma mansoni antigens in a Ugandan fishing community. Trop Med Int Heal. 2003;8:561–568. doi: 10.1046/j.1365-3156.2003.01056.x. [DOI] [PubMed] [Google Scholar]
- Naus CWA, Remoortere A Van, Ouma JH, Kimani G, Dunne DW, Kamerling JP, Deelder M, Hokke CH. Specific antibody responses to three schistosome-related carbohydrate structures in recently exposed immigrants and established residents in an area of Schistosoma mansoni endemicity. Infect Immun. 2003;71:5676–5681. doi: 10.1128/IAI.71.10.5676-5681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyame AK, Kawar ZS, Cummings RD. Antigenic glycans in parasitic infections: Implications for vaccines and diagnostics. Arch Biochem Biophys. 2004;426:182–200. doi: 10.1016/j.abb.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Leppänen AM, Bogitsh BJ, Cummings RD. Antibody responses to the fucosylated LacdiNAc glycan antigen in Schistosoma mansoni-infected mice and expression of the glycan among schistosomes. Exp Parasitol. 2000;96:202–212. doi: 10.1006/expr.2000.4573. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Lewis FA, Doughty BL, Correa-Oliveira R, Cummings RD. Immunity to schistosomiasis: Glycans are potential antigenic targets for immune intervention. Exp Parasitol. 2003;104:1–13. doi: 10.1016/s0014-4894(03)00110-3. [DOI] [PubMed] [Google Scholar]
- Nyame AK, Yoshino TP, Cummings RD. Differential expression of LacdiNAc, Fucosylated LacdiNAc, and Lewis x glycan antigens in intramolluscan stages of Schistosoma manosni. J Parasitol. 2002;88:890–897. doi: 10.1645/0022-3395(2002)088[0890:DEOLFL]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Oelmann S, Stanley P, Gerardy-Schahn R. Point mutations identified in Lec8 Chinese hamster ovary glycosylation mutants that inactivate both the UDP-galactose and CMP-sialic acid transporters. J Biol Chem. 2001;276:26291–26300. doi: 10.1074/jbc.M011124200. [DOI] [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Abe M, Harn DA., Jr Induction of Th2 responses and IgE is largely due to carbohydrates functioning as adjuvants on Schistosoma mansoni egg antigens. J Immunol. 1999;163:6712–6717. [PubMed] [Google Scholar]
- Okano M, Satoskar AR, Nishizaki K, Harn DA., Jr Lacto-N-fucopentaose III found on Schistosoma mansoni egg antigens functions as adjuvant for proteins by inducing Th2-type response. J Immunol. 2001;167:442–450. doi: 10.4049/jimmunol.167.1.442. [DOI] [PubMed] [Google Scholar]
- Omer-Ali P, Magee AI, Kelly C, Simpson AJG. A major role for carbohydrate epitopes preferentially recognized by chronically infected mice in the determination of Schistosoma mansoni schistosomulum surface antigenicity. J Immunol. 1986;137:3601–3607. [PubMed] [Google Scholar]
- Omer Ali P, Mansour M, Woody JN, Smithers SR, Simpson AJ. Antibody to carbohydrate and polypeptide epitopes on the surface of schistosomula of Schistosoma mansoni in Egyptian patients with acute and chronic schistosomiasis. Parasitology. 1989;98(Pt 3):417–424. doi: 10.1017/s0031182000061503. [DOI] [PubMed] [Google Scholar]
- Omer Ali P, Smithers SR, Bickle Q, Phillips SM, Harn D, Simpson AJG. Analysis of the anti-Schistosoma mansoni surface antibody response during murine infection and its potential contribution to protective immunity. J Immunol. 1988;140:3273–3279. [PubMed] [Google Scholar]
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006;416:159–182. doi: 10.1016/S0076-6879(06)16011-5. [DOI] [PubMed] [Google Scholar]
- Peng W, Pranskevich J, Nycholat C, Gilbert M, Wakarchuk W, Paulson JC, Razi N. Helicobacter pylori β1,3-N-acetylglucosaminyltransferase for versatile synthesis of type 1 and type 2 poly-LacNAcs on N-linked, O-linked and I-antigen glycans. Glycobiology. 2012;22:1453–1464. doi: 10.1093/glycob/cws101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanphanich NS, Mickum ML, Heimburg-Molinaro J, Cummings RD. Glycoconjugates in host–Helminth interactions. Front Immunol. 2013;4:240. doi: 10.3389/fimmu.2013.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijn MLM, Koeleman CaM, Wuhrer M, Royle L, Geyer R, Dwek Ra, Rudd PM, Deelder AM, Hokke CH. Targeted identification of a unique glycan epitope of Schistosoma mansoni egg antigens using a diagnostic antibody. Mol Biochem Parasitol. 2007;151:148–161. doi: 10.1016/j.molbiopara.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Robijn MLM, Wuhrer M, Kornelis D, Deelder aM, Geyer R, Hokke CH. Mapping fucosylated epitopes on glycoproteins and glycolipids of Schistosoma mansoni cercariae, adult worms and eggs. Parasitology. 2005;130:67–77. doi: 10.1017/s0031182004006390. [DOI] [PubMed] [Google Scholar]
- Samuelson JC, Caulfield JP. The cercarial glycocalyx of Schistosoma mansoni. J Cell Biol. 1985;100:1423–1434. doi: 10.1083/jcb.100.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seko A, Koketsu M, Nishizono M, Enoki Y, Ibrahim HR, Juneja LR, Kim M, Yamamoto T. Occurrence of a sialylglycopeptide and free sialylglycans in hen's egg yolk. Biochim Biophys Acta. 1997;1335:23–32. doi: 10.1016/s0304-4165(96)00118-3. [DOI] [PubMed] [Google Scholar]
- Song X, Heimburg-Molinaro J, Dahms NM, Smith DF, Cummings RD. Carbohydrate microarrays: Preparation of a mannose-6-phosphate glycan miroarray through fluorescent derivatization, phosphorylation, and immobilization of natural high-mannose N-glycans and application in ligand identification of P-type lectins. Methods Mol Biol. 2012;808:137–148. doi: 10.1007/978-1-61779-373-8_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Xia B, Stowell SR, Lasanajak Y, Smith DF, Cummings RD. Novel fluorescent glycan microarray strategy reveals ligands for galectins. Chem Biol. 2009;16:36–47. doi: 10.1016/j.chembiol.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan S, Patel J, Bozeman E, Imasuen I, He S, Daniels D, Selvaraj P. Allogeneic tumor cell vaccines: The promise and limitations in clinical trials. Hum Vaccin Immunother. 2014;10:52–63. doi: 10.4161/hv.26568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. Schistosoma mansoni synthesizes novel biantennary Asn-linked oligosaccharides containing terminal B-linked N-acetylgalactosamine. Glycobiology. 1992a;2:445–452. doi: 10.1093/glycob/2.5.445. [DOI] [PubMed] [Google Scholar]
- Srivatsan J, Smith DF, Cummings RD. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewis X antigen. J Biol Chem. 1992b;267:20196–20203. [PubMed] [Google Scholar]
- Stanley P. Altered Glycolipids of CHO Cells Resistant to Wheat-Germ Agglutinin. American Chemical Society; 1980. pp. 213–221. Sweeley CC, editor. American Chemical Society Symposium Series No. 128. [Google Scholar]
- The World Health Organization. The Global Burden of Disease: 2004 Update. 2008. http://www.who.int/topics/global_burden_of_disease/en/ [Google Scholar]
- The World Health Organization. Working to Overcome the Global Impact of Neglected Tropical Diseases: First WHO Report on Neglected Tropical Diseases. 2010. http://www.who.int/neglected_diseases/2010report/en/ [Google Scholar]
- Thomas PG, Harn DA., Jr Immune biasing by helminth glycans. Cell Microbiol. 2004;6:13–22. doi: 10.1046/j.1462-5822.2003.00337.x. [DOI] [PubMed] [Google Scholar]
- Van de Vijver KK, Deelder AM, Jacobs W, van Marck EA, Hokke CH. LacdiNAc- and LacNAc-containing glycans induce granulomas in an in vivo model for schistosome egg-induced hepatic granuloma formation. Glycobiology. 2006;16:237–243. doi: 10.1093/glycob/cwj058. [DOI] [PubMed] [Google Scholar]
- Van de Vijver KK, Hokke CH, van Remoortere A, Jacobs W, Deelder AM, Van Marck Ea. Glycans of Schistosoma mansoni and keyhole limpet haemocyanin induce hepatic granulomas in vivo. Int J Parasitol. 2004;34:951–961. doi: 10.1016/j.ijpara.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Van den Eijnden DH, Neeleman AP, Van der Knap WPW, Bakker H, Agterberg M, van Die I. Novel glycosylation routes for glycoproteins: The lacdiNAc pathway. Biochem Soc Trans. 1995;23:175–179. doi: 10.1042/bst0230175. [DOI] [PubMed] [Google Scholar]
- Van Den Nieuwenhof IM, Koistinen H, Easton RL, Koistinen R, Kamarainen M, Morris HR, Die I Van, Seppala M, Dell A, Van Den Eijnden DH. Recombinant glycodelin carrying the same type of glycan structures as contraceptive glycodelin-A can be produced in human kidney 293 cells but not in Chinese hamster ovary cells. Eur J Biochem. 2000;267:4753–4762. doi: 10.1046/j.1432-1327.2000.01528.x. [DOI] [PubMed] [Google Scholar]
- Van Die I, van Vliet SJ, Nyame AK, Cummings RD, Bank CMC, Appelmelk B, Geijtenbeek TBH, van Kooyk Y. The dendritic cell-specific C-type lectin DC-SIGN is a receptor for Schistosoma mansoni egg antigens and recognizes the glycan antigen Lewis x. Glycobiology. 2003;13:471–478. doi: 10.1093/glycob/cwg052. [DOI] [PubMed] [Google Scholar]
- Van Diepen A, Smit CH, van Egmond L, Kabatereine NB, Pinot de Moira A, Dunne DW, Hokke CH. Differential anti-glycan antibody responses in Schistosoma mansoni-infected children and adults studied by shotgun glycan microarray. PLoS Negl Trop Dis. 2012;6:e1922. doi: 10.1371/journal.pntd.0001922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Diepen A, Van der Velden NSJ, Smit CH, Meevissen MHJ, Hokke CH. Parasite glycans and antibody-mediated immune responses in Schistosoma infection. Parasitology. 2012;139:1219–1230. doi: 10.1017/S0031182012000273. [DOI] [PubMed] [Google Scholar]
- Van Liempt E, Bank CMC, Mehta P, Garciá-Vallejo JJ, Kawar ZS, Geyer R, Alvarez Ra, Cummings RD, Kooyk Y Van, van Die I. Specificity of DC-SIGN for mannose- and fucose-containing glycans. FEBS Lett. 2006;580:6123–6131. doi: 10.1016/j.febslet.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Van Remoortere A, van Dam GJ, Hokke CH, can den Eijnden DH, van Die I, Deelder AM. Profiles of immunoglobulin M (IgM) and IgG antibodies against defined carbohydrate epitopes in sera of Schistosoma-infected individuals determined by surface plasmon resonance. Infect Immun. 2001;69:2396–2401. doi: 10.1128/IAI.69.4.2396-2401.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Remoortere A, Hokke CH, van Dam GJ, van Die I, Deelder AM, van den Eijnden DH. Various stages of Schistosoma express Lewis x, LacdiNAc, GalNAcβ1–4 (Fucα1–3)GlcNAc and GalNAcβ1–4(Fucα1–2Fucα1–3)GlcNAc carbohydrate epitopes: Detection with monoclonal antibodies that are characterized by enzymatically synthesized neoglycoproteins. Glycobiology. 2000;10:601–609. doi: 10.1093/glycob/10.6.601. [DOI] [PubMed] [Google Scholar]
- Van Stijn CMW, van den Broek M, Vervelde L, Alvarez Ra, Cummings RD, Tefsen B, van Die I. Vaccination-induced IgG response to Galalpha1-3GalNAc glycan epitopes in lambs protected against Haemonchus contortus challenge infection. Int J Parasitol. 2010;40:215–222. doi: 10.1016/j.ijpara.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Stijn CMW, Meyer S, van den Broek M, Bruijns SCM, van Kooyk Y, Geyer R, van Die I. Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC-SIGN. Mol Immunol. 2010;47:1544–1552. doi: 10.1016/j.molimm.2010.01.014. [DOI] [PubMed] [Google Scholar]
- Vervelde L, Bakker N, Kooyman FNJ, Cornelissen AWCa, Bank CMC, Nyame AK, Cummings RD, van Die I. Vaccination-induced protection of lambs against the parasitic nematode Haemonchus contortus correlates with high IgG antibody responses to the LDNF glycan antigen. Glycobiology. 2003;13:795–804. doi: 10.1093/glycob/cwg107. [DOI] [PubMed] [Google Scholar]
- Weckx LY, Thompson A, Berezin EN, de Faria SM, da Cunha CA, Pride M, Patterson S, Gruber WC, Emini Ea, Scott Da. A phase 3, randomized, double-blind trial comparing the safety and immunogenicity of the 7-valent and 13-valent pneumococcal conjugate vaccines, given with routine pediatric vaccinations, in healthy infants in Brazil. Vaccine. 2012;30:7566–7572. doi: 10.1016/j.vaccine.2012.10.040. [DOI] [PubMed] [Google Scholar]
- Wilson RA, Langermans JAM, Van Dam GJ, Vervenne RA, Hall SL, Borges WC, Dillon GP, Thomas AW, Coulson PS. Elimination of Schistosoma mansoni adult worms by rhesus macaques: Basis for a therapeutic vaccine? PLoS Negl Trop Dis. 2008;2:8. doi: 10.1371/journal.pntd.0000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CAM, Deelder AM, Hokke CH. Repeats of LacdiNAc and fucosylated LacdiNAc on N-glycans of the human parasite Schistosoma mansoni. FEBS J. 2006;273:347–361. doi: 10.1111/j.1742-4658.2005.05068.x. [DOI] [PubMed] [Google Scholar]
- Wuhrer M, Koeleman CAM, Fitzpatrick JM, Hoffmann KF, Deelder AM, Hokke CH. Gender-specific expression of complex-type N-glycans in schistosomes. Glycobioogy. 2006;16:991–1006. doi: 10.1093/glycob/cwl020. [DOI] [PubMed] [Google Scholar]
- Yates AD, Watkins WM. Enzymes involved in the biosynthesis of glycoconjugates: A UDP-2-acetamido-2-deoxy-d-glucose: B-d-galactopyranosyl-(1-4)-saccharide (1-3)-2-acetamido-2-deoxy-B-d-glucopyranosyltransferase in human serum. Carbohydr Res. 1983;120:251–268. doi: 10.1016/0008-6215(83)88020-3. [DOI] [PubMed] [Google Scholar]