Abstract
BACKGROUND
Statin therapy is a proven effective treatment of hyperlipidemia. However, a significant number of patients cannot tolerate statins. This study was conducted to review treatment strategies for patients intolerant to statin therapy with a focus on intermittent statin dosing.
METHODS AND RESULTS
We performed a retrospective analysis of medical records of 1605 patients referred to the Cleveland Clinic Preventive Cardiology section for statin intolerance between January 1995 and March 2010 with at least a six-month follow-up. The changes in lipid profile, achievement of low-density lipoprotein cholesterol (LDL-C) goals and statin tolerance rate were analyzed. 72.5% of patients with prior statin intolerance were able to tolerate a statin for the median follow-up time of 31 months. Patients on intermittent statin dosing (n=149) had significantly lower LDL-C reduction compared to daily dosing group (n=1014) (21.3±4.0% vs 27.7±1.4%, p<0.001). But compared to the statin discontinued group (n=442), they had a significantly higher LDL-C reduction (21.3±4.0% vs 8.3±2.2%, p<0.001), and a significantly higher portion achieved their ATP–III goal of LDL-C (61% vs 44%, p<0.05). There was a trend toward a decrease in all-cause mortality at 8 years for patients on daily and intermittent statin dosing compared with those who discontinued statin (p=0.08).
CONCLUSIONS
The majority of patients with previous statin intolerance can tolerate subsequent trial of statin. A strategy of intermittent statin dosing can be an effective therapeutic option in some patients and may result in reduction in LDL-C and achievement of LDL-C goals.
Keywords: Statin, Lipid, Cholesterol, Intermittent Dosing
INTRODUCTION
Statins are among the most prescribed drugs in the world and are first-line therapy in the management of hyperlipidemia. Their beneficial effects on cardiovascular morbidity and mortality have been demonstrated both in primary and secondary prevention.(1,2) They are generally safe, but in some patients, statin therapy is stopped because of intolerance to the drug that may result in muscle aches and weakness, gastrointestinal symptoms, liver enzyme abnormalities or other non-specific discomforts. The rate of reported statin-related events is about 5% to 10% in randomized, placebo-controlled clinical trials.(3,4) This rate has been reported as high as 20% in observational studies.(5–10) The discrepancy between clinical trials and observational studies can be explained by patient selection in randomized trials which often exclude old patients and those with many comorbidities and enroll few women.
Some adverse effects of statin therapy such as muscle pain and liver enzymes abnormalities are thought to be dose-related.(11)
It is recognized that 20 to 40% LDL-C reduction can be achieved with the lowest daily dose of statins approved. Furthermore, an additional 6 to 7% can be achieved with each doubling of the dose.(12) Nevertheless, in some patients, daily dosing of statin or the lowest approved doses are not tolerated.
The intermittent dosing strategy has been suggested recently and, mostly with rosuvastatin because of its long half-life, high potency and favorable metabolic characteristics.(13–16) However, the previous studies are small. The aim of this study is to review the different treatment strategies used to improve lipid profiles in patients with prior statin intolerance treated at a large tertiary prevention clinic, with a focus on intermittent statin dosing.
METHODS
We reviewed the electronic records of patients referred to the Cleveland Clinic Preventive Cardiology section for statin intolerance between January 1995 and March 2010. We used the Preventive Cardiology Information System (PreCIS) database, which contains information from patients referred to this clinic. At the time of baseline visit, demographic information, medical history, physical exam and laboratory data are obtained and entered into an electronic medical record. Patients are classified as statin intolerant based on their medical history and information provided by themselves or the referring physician. A comprehensive history of statin intolerance including drugs involved, doses and symptoms was taken. We used ACC/AHA definitions for myalgia (muscle ache or weakness without CK elevation), myositis (muscle symptoms with CK elevation) and rhabdomyolysis (muscle symptoms with CK > 10 x ULN and elevation of creatinine).(17)
PRECIS database analyses are approved by the Cleveland Clinic Institutional Review Board. We identified 1605 consecutive patients with documented intolerance to at least 2 statin medications and at least a 6-month follow-up with a stable form of lipid-lowering therapy. Patients were divided into 3 groups based on their statin regimen at the time of their last follow-up visit: no statin, intermittent statin dosing, or daily statin dosing.
Intermittent statin dosing is defined as any statin prescription that is not taken on a daily basis notwithstanding the dose. All subjects received nutritional recommendations and education as a routine part of each patient visit. We evaluated different prescriptive strategies used in those patients to improve their lipid profiles and analyzed the changes in their lipid profile. The primary outcomes of the study were the percent change in fasting lipid profiles from baseline to the last follow-up visit and the percentage of patients achieving the specified NCEP ATP-III LDL-C goals. The secondary outcome was all-cause mortality difference between the different treatment strategies. A direct LDL-C was automatically obtained as a standard practice if triglyceride level was > 250 mg/dL. Otherwise, LDL-C was calculated from the Friedewald equation.(18) No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
STATISTI CAL ANALYSIS
Patient characteristics of the three groups of statin intolerant patients are presented as mean ± standard deviation (SD) for continuous variables with normal distribution (median and 25th and 75th percentiles for non-normally distributed data) and percents for categorical variables. Comparisons of continuous data were made using analysis of variance (ANOVA) for normally distributed data and Kruskal-Wallis for non-normally distributed data. Categorical data were compared using Chi-square or exact tests.
Percent changes in TC, LDL-C, HDL-C and TG from baseline to the last follow-up visit were calculated and were natural logarithmically transformed, if necessary.
Linear regression models were used to calculate least square means, and comparisons of the percent changes in the lipid parameters across statin dosing strategies were performed with adjustment variables including the baseline lipid parameter and other lipid lowering agents (fibrates, ezetimide, niacin, red yeast rice, Metamucil, phytosterols, bile acid resins, flaxseed).
All-cause mortality was assessed, as determined by the Social Security Death Index (SSDI). Kaplan-Meier methods were used to estimate 8-year survival. Time to event for patients who did not have follow-up beyond 8 years or died was censored to the date the SSDI was accessed for this study. Log-rank p values were computed to compare survival rates across statin dosing strategies.
A p value of less than 0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC).
RESULTS
During the median follow-up time of 31 months, 72.5% (n=1163) of the subjects remained on a regular regimen of statin therapy, with 63.2% (n=1014) on a daily regimen, and 9.3% (n=149) on an intermittent statin regimen. Statins were completely discontinued for 27.5% (n=442) of patients. The patients’ demographics are presented in Table 1. The majority of patients were female (n=919, 57%) and Caucasians (n=1347, 84%). There was a higher proportion of men in the daily statin dosing group. Patients in the daily statin group were relatively younger. The study population was relatively obese with a mean body mass index of 30. Based on the Framingham risk score assessment for the determination of the LDL-C goal, 65% of the patients were classified as moderate or high risk for CHD, which corresponds with an LDL goal of less than 130 mg/dL or 100 mg/dL, respectively. Although the proportion of patients with CHD was higher in the daily statin and intermittent groups compared to the statin discontinued group, there was no significant difference in the overall Framingham Risk Score categories. There was a higher proportion of patients with kidney disease (glomerulonephritis, chronic kidney disease or polycystic kidney disease) and liver disease (cirrhosis, hepatitis B or C, fatty liver disease) in the statin discontinued group. Overall, 42% of the patients were treated for secondary prevention, with a higher proportion in the daily statin dosing group. Patients were not at their LDL goal at their first visit to the clinic. The mean LDL was 144±58 mg/dL. Patients in the intermittent statin dosing group had a higher baseline mean LDL-C compared to the other two groups.
Table 1.
Baseline Characteristics of Patients Intolerant to Statin
| Characteristic | Statin Discontinued group N = 442 | Intermittent Statin dosing group N = 149 | Daily Statin dosing group N = 1014 | P value |
|---|---|---|---|---|
| Age, years (mean ± SD) | 61.1 ± 11.9 | 63.5 ± 9.8 | 58.1±11.7 | <0.001 |
|
| ||||
| Male n (%) | 155 (35.1) | 60 (40.3) | 471 (46.4) | <0.001 |
|
| ||||
| Median Follow-up Time ( months) | 26.9 | 25.6 | 36.0 | <0.001 |
|
| ||||
| Caucasians n (%) | 360 (81.4) | 133 (89.3) | 854 (84.2) | 0.10 |
|
| ||||
| BMI, kg/m2 (mean ± SD) | 30.2 ± 6.2 | 29.2 ± 5.6 | 29.8±6.0 | 0.20 |
|
| ||||
| Medical History, n (%) | ||||
| CHD | 81 (18.3) | 36 (24.2) | 254 (25.4) | 0.001 |
| Hypertension | 264 (59.7) | 92 (61.7) | 593 (58.5) | 0.72 |
| Smoking | 46 (10.4) | 14 (9.4) | 101 (10.0) | 0.93 |
| CVD | 45 (10.2) | 10 (6.7) | 73 (7.2) | 0.13 |
| CHF | 30 (6.8) | 8 (5.4) | 66 (6.5) | 0.83 |
| Diabetes mellitus | 99(22.4) | 31 (20.9) | 195 (19.2) | 0.38 |
| PVD | 20 (4.5) | 8 (5.4) | 55 (5.4) | 0.77 |
| Liver disease | 44 (10.0) | 6 (4.0) | 65 (6.4) | 0.02 |
| Kidney disease | 62 (14.0) | 10 (6.7) | 97 (9.6) | 0.01 |
|
| ||||
| Secondary prevention n (%) | 159 (36.0) | 61 (40.9) | 457 (45.1) | 0.005 |
|
| ||||
| Framingham Risk Categories n (%) | ||||
| 0–1 Risk Factor | 88 (19.9) | 24 (16.1) | 214 (21.1) | 0.16 |
| 2+ Risk Factors and ≤ 10% Risk | 171 (38.7) | 48 (32.2) | 383 (37.8) | |
| 2+ Risk Factors and 10–20% Risk | 57 (12.9) | 31 (20.8) | 154 (15.2) | |
| CHD or CHD Risk Equivalents | 126 (28.5) | 46 (30.9) | 263 (25.9) | |
|
| ||||
| Baseline lipid profile mg/dL (mean ± SD) | ||||
| TC | 245 ± 66 | 251 ± 55 | 227 ± 72 | <0.001 |
| LDL | 148 ± 51 | 162 ± 49 | 139 ± 61 | <0.001 |
| HDL | 55 ± 17 | 54 ± 16 | 52 ± 17 | 0.04 |
| TG (median, Q1,Q3) | 151 (106, 232) | 146 (98, 217) | 192 (102, 214) | 0.22 |
|
| ||||
| Type of previous statin intolerance, n (%) | ||||
| Myalgia | 326 (73.8) | 127 (87.2) | 702 (69.2) | <0.001 |
| Myositis | 22 (4.9) | 11 (7.3) | 42 (4.1) | 0.17 |
| Weakness/Fatigue | 67 (15.2) | 25 (16.8) | 113 (11.1) | 0.03 |
| Rhabdomyolysis | 5 (1.1) | 3 (2.0) | 0 (0.0) | 0.18 |
| Elevated hepatic enzymes | 49 (11.1) | 11 (7.4) | 130 (12.8) | 0.14 |
| Gastrointestinal complaints | 50 (11.3) | 19 (12.8) | 84 (8.3) | 0.07 |
| Pancreatitis | 17 (3.8) | 2 (1.3) | 16 (1.6) | 0.02 |
| Joint pain | 38 (8.6) | 20 (13.4) | 97 (9.6) | 0.22 |
| Rash or flushing | 18 (4.1) | 4 (2.7) | 30 (3.0) | 0.50 |
| Neurological symptoms | 13 (2.9) | 12 (8.1) | 39 (3.8) | 0.02 |
|
| ||||
| Previous intolerances to individual statins, n (%) | ||||
| Atorvastatin | 333 (75.3) | 118 (79.2) | 706 (69.6) | 0.01 |
| Cerivastatin | 7 (1.6) | 1 (0.7) | 8 (0.8) | 0.33 |
| Fluvastatin | 28 (6.3) | 6 (4.0) | 13 (1.3) | <0.001 |
| Lovastatin | 75 (17.0) | 24 (16.1) | 93 (9.2) | <0.001 |
| Pravastatin | 123 (27.8) | 49 (32.9) | 148 (14.6) | <0.001 |
| Rosuvastatin | 134 (30.3) | 61 (40.9) | 222 (21.9) | <0.001 |
| Simvastatin | 219 (49.5) | 93 (62.4) | 386 (38.1) | <0.001 |
|
| ||||
| Intolerance to other lipid lowering drugs n (%) | ||||
| Fibrates | 21(4.8) | 16 (10.7) | 32 (3.2) | <0.001 |
| Niacin | 31 (7.0) | 23 (15.4) | 68 (6.7) | 0.001 |
| Ezetimide | 45 (10.2) | 32 (21.5) | 59 (5.8) | <0.001 |
| Bile Acid Sequestrants | 15 (3.4) | 8 (5.4) | 14 (1.4) | 0.002 |
|
| ||||
| Labs | ||||
| Creatinine | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.0) | 0.9 (0.8. 1.0) | 0.77 |
| CK (mg/dL) | 107 (63, 217) | 97 (74, 181) | 114 (70, 181) | 0.81 |
| ALT (IU/L) | 21 (15, 31) | 23 (17, 31) | 22 (17, 32) | 0.20 |
| AST (IU/L) | 24 (19, 31) | 24 (20, 30) | 24 (20, 30) | 0.59 |
| TSH (mIU/L) | 1.9 (1.2, 2.7) | 1.9 (1.4, 2.9) | 1.9 (1.3, 2.8) | 0.56 |
CHD: coronary heart disease; CVD: cerebral vascular disease; PVD: peripheral vascular disease
The profile of statin intolerance is described in table 1. The most common complaint of statin intolerance was myalgia with a higher proportion in the statin intermittent group. There was a higher proportion of patients with pancreatitis (significant elevation of serum amylase or lipase) in the statin discontinued group. The intermittent statin group had more complaints of neurological symptoms. There was no significant difference for all the other symptoms.
Seventy-two percent of patients reported prior intolerance to atorvastatin, 44% to simvastatin, 26% to rosuvastatin and 20% to pravastatin, reflecting the fact that these statins were the most used statins. As would be expected, the proportions of patients intolerant to different statins were higher in the intermittent dosing and the discontinued groups compared to the daily statin dosing group. The intermittent dosing group had higher proportions of patients intolerant to other lipid lowering drugs including fibrates, niacin, ezetimide and bile acid sequestrants.
Of the 1605 patients referred to our clinic for statin intolerance, 72.5% were ultimately able to tolerate some form of long-term statin therapy regimen. Most patients who tolerated long-term statin were on rosuvastatin (43%). Rosuvastatin was the single most used statin in the intermittent dosing group (75.2%). In the daily dosing group, other statins like Atorvastatin (21.5%), pravastatin (18.0%) and simvastatin (20.1%) were also used beside rosuvastsatin (38.3%). Concomitant lipid-lowering drugs were also heavily used in this population, including ezetimide (34%), niacin (21%), and non-prescription drugs such as omega-3 fatty acid (44%) and plant sterols (8%).(Table 2) While there was a heavier use of niacin, bile acid sequestrants, Metamucil, red yeast rice extract and plant sterols in the intermittent dosing and the statin discontinued groups, there was no difference in the use of ezetimide and fibrates between the 3 groups. CoQ10 was used more often in the statin intermittent group. Other medications like ACEI, aspirin and b-blockers were used more often in the statin groups.
Table 2.
Long-term Lipid-lowering and cardiovascular drugs use in the study population
| Medications | Statin Discontinued group N = 442 | Intermittent Statin dosing group N = 149 | Daily Statin dosing group N = 1014 | P |
|---|---|---|---|---|
| Statins (last statin tried) n (%) | <0.001 | |||
| Atorvastatin | 85 (19.1) | 8 (5.4) | 218 (21.5) | |
| Fluvastatin | 12 (2.7) | 3 (2.0) | 4 (0.4) | |
| Lovastatin | 12 (2.7) | 0 (0.0) | 17 (1.7) | |
| Pravastatin | 86 (19.3) | 19 (12.9) | 183 (18.0) | |
| Rosuvastatin | 182 (41.4) | 112 (75.2) | 388 (38.3) | |
| Simvastatin | 65 (14.7) | 7 (4.7) | 204 (20.1) | |
| Cerivastatin | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
|
| ||||
| Concomitant lipid-altering drugs, n (%) | 310 (70.1) | 105 (70.5) | 655 (64.6) | 0.070 |
| Ezetimibe | 140 (31.7) | 46 (30.9) | 359 (35.4) | 0.272 |
| Niacin | 129 (29.2) | 36 (24.2) | 166 (16.4) | <0.001 |
| Bile Acid Sequestrants | 41 (9.3) | 11 (7.4) | 20 (2.0) | <0.001 |
| Colestipol | 1 (0.2) | 2 (1.3) | 1 (0.1) | 0.038 |
| Questran | 5 (1.1) | 4 (2.7) | 2 (0.2) | 0.001 |
| Welchol | 35 (7.9) | 5 (3.4) | 17 (1.7) | <0.001 |
| Fibrate | 39 (8.8) | 11 (7.4) | 88 (8.7) | 0.853 |
| Fenofibrate | 35 (7.9) | 7 (4.7) | 77 (7.6) | 0.404 |
| Gemfibrozil | 4 (0.9) | 5 (3.4) | 15 (1.5) | 0.103 |
| Non-Prescription Drugs | 204 (46.2) | 78 (52.3) | 420 (41.4) | 0.021 |
| Plant Stanol/Sterols | 44 (10.0) | 24 (16.1) | 58 (5.7) | <0.001 |
| Flaxseed oil | 41 (9.3) | 13 (8.7) | 74 (7.3) | 0.413 |
| Omega-3 fatty acids | 151 (34.2) | 58 (38.9) | 335 (33.0) | 0.362 |
| Metamucil | 33 (7.5) | 12 (8.1) | 39 (3.8) | 0.005 |
| Red Yeast Rice extract | 18 (4.1) | 6 (4.0) | 12 (1.2) | 0.001 |
|
| ||||
| Other medications N (%) | ||||
| ACEI / ARB | 133 (30.1) | 54 (36.2) | 390 (38.5) | 0.009 |
| emsp;Aspirin | 175 (39.6) | 75 (50.3) | 470 (46.4) | 0.022 |
| emsp;B-Blocker | 93 (21.0) | 50 (33.6) | 317 (31.3) | <0.001 |
| emsp;Calcium-Blocker | 49 (11.1) | 26 (17.4) | 146 (14.4) | 0.095 |
| emsp;Diuretic | 87 (19.7) | 38 (25.5) | 253 (25.0) | 0.078 |
| emsp;Levothyroxine | 30 (6.8) | 21 (14.1) | 79 (7.8) | 0.015 |
| emsp;CoQ10 | 111 (25.1) | 60 (40.3) | 259 (25.5) | <0.001 |
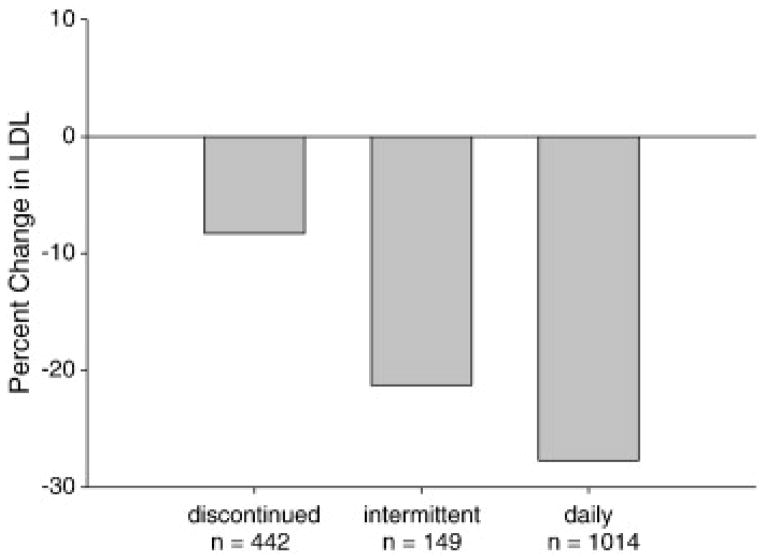
Patients who remained on daily or intermittently statin achieved a higher LDL-C reduction compared to those who discontinued statin (27.7±1.4% vs 21.3±4.0% vs 8.3±2.2%, p<0.001).(Table 3, figure1) Patients on intermittent statin dosing achieved a lower LDL-C reduction compared with patients on daily statin dosing (21.3±4.0% vs 27.7±1.4%, p=0.04). Although the statin discontinued group had a lower reduction in LDL-C than those who stayed on statin, they still achieved significant LDL-C reductions from baseline (8.3±2.2%, p<0.001). Changes in total cholesterol paralleled changes in LDL-C while changes in HDL-C and triglycerides were not statistically significant. (Table 3)
Table 3.
Comparison of Lipoprotein percent Changes between different strategy groups
| Lipoprotein change (%) LS Mean + SE | Statin Discontinued n=442 | Intermittent Statin Dosing n=149 | Daily statin dosing n=1014 |
|---|---|---|---|
| TC | −8.0 ± 1.5*† | −17.2 ± 2.7 | −21.2 ± 0.9 |
| LDL-C | −8.3 ± 2.2*† | −21.3 ± 4.0† | −27.7 ± 1.4 |
| HDL-C | −0.2 ± 1.3† | −0.2 ± 2.3 | 2.9 ± 0.8 |
| TG | −15.1 ± 2.7† | −13.7 ± 5.0 | −21.1 ± 1.7 |
Adjusted for baseline lipoproteins value and other cholesterol lowering agents.
p<0.05 compared with intermittent dosing;
p<0.05 compared with daily dosing
Figure 1.
A greater proportion of patients achieved their ATP-III goals in the daily dosing group compared to the intermittent statin and the statin-discontinued groups (79% vs 61% vs 44%, respectively, p<0.001).(Table 4) More patients achieved their ATP III goals in the intermittent statin dosing group compared with the statin-discontinued group (p=0.002).
Table 4.
Percentage of patients achieving LDL-C Goals by Framingham risk stratification
| Risk Category % (N) | Statin Discontinued Group n= 442 |
Intermittent Statin Dosing Group n= 149 | Standard Statin Dosing Group n= 1014 | Overall n=1605 |
|---|---|---|---|---|
| Low 20 (325) |
69 (88)*† | 94 (24) | 92 (214) | 86 (325) |
| Moderate 38 (608) |
44 (171)* | 44 (48)* | 81 (383) | 68 (608) |
| Moderately high 15 (237) |
45 (57)*† | 82 (31) | 90 (154) | 79 (237) |
| High 27(435) |
28 (126)* | 44 (46) | 57 (263) | 47 (435) |
| Total | 44 (442)*† | 61 (149)* | 79 (1014) | 68 (1605) |
p<0.05 compared with daily dosing;
p<0.05 compared with intermittent dosing
A multivariate analysis was done to determine the factors affecting LDL-C changes. This analysis was adjusted for baseline LDL-C, statin use and other lipid lowering therapies. The analysis shows that the LDL-C change was affected by the patient’s gender (p=0.002), history of CVD (p=0.03) and PVD (p=0.009), use of statin (p=0.004) and niacin (p=0.003) or welchol (p=0.01). All the other parameters were non-significant for the model.
Using All-Cause Mortality as a Dependent Variable, Cox proportional hazard regression analysis was performed to determine whether statin use (both daily and intermittent) was significantly and independently associated with mortality. Significant variables in the final survival model of the multivariable analysis included patient’s age (p<0.001), history of heart failure (p<0.004) and PVD (p<0.04).
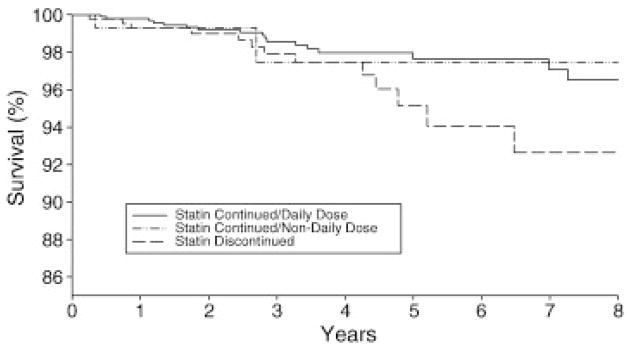
As shown by the Kaplan-Meier Survival Curves, there was a trend toward a decrease in 8-year all-cause mortality in patients on statins compared to those who discontinued statin, which did not attain statistical significance (p=0.08). (Figure 2)
Figure 2.
DISCUSSION
In the present study, we reviewed treatment strategies used in 1605 patients with statin intolerance referred to a single institution and assessed serum lipid levels, tolerance of therapies and all-cause mortality. In particular, we analyzed the tolerance and the efficacy of intermittent statin therapy. The most important finding of this study is that the majority of statin intolerant patients can tolerate some form of statin therapy and that intermittent statin use can be an option for some patients to achieve improvement in serum lipid levels. The other interesting finding was the trend toward a survival benefit in patients who were able to tolerate some form of statin therapy compared to those who discontinued statin.
This study is the largest to date to review different treatment regimens for patients with documented statin intolerance and their impact on LDL-C. It confirms previous reports that the majority of patients with a history of statin intolerance can tolerate subsequent statin trials, achieve significant LDL-C reductions and attain LDL-C goals.
In a recent study Zhang and colleagues explored the frequency of statin discontinuation in one hospital system and analysed data from 107835 patients’ medical records between 1 January 2000 and 31 December 2008.(10) They found that most statin intolerant patients (more than 90%) could ultimately tolerate a statin.
Our study shows that the majority (72.5%) of patients who are “statin intolerant” can tolerate some form of statin therapy with a significant proportion (63.2%) on a daily statin regimen.
Statins have well-documented benefits and their discontinuation has been associated with increased risk for cardiovascular events.(5,19)
The result of this and other studies suggests that for most patients who report statin-related events, especially high-risk patients, statin can safely be represcribed with a high probability of long-term tolerance.
Intermittent statin dosing is regarded as an interesting strategy in patients with statin-related symptoms. Nevertheless, most studies on the efficacy of this strategy are small.(13–16,20–21)
A recent review of several small studies by Marcus et al. suggests that alternate-day dosing of statin may achieve similar levels of LDL-C reduction compared to daily dosing and may improve tolerance.(22) Backes et al showed a mean LDL-C decrease of 34.5% in 51 patients on every-other-day rosuvastatin dosing.(14) Gardala et al also showed a 26 % LDL-C reduction with rosuvastatin 5 to 10 mg twice a week in 40 statin intolerant patients.(15)
Ruisinger et al achieved a 23% LDL-C reduction using rosuvastatin 5 to 20 mg once a week for 4 months in a group of 50 satin intolerant patients.(21)
A randomized, controlled trial of once-weekly rosuvastatin in statin-intolerant patients was published during the writing of this paper. It included 17 patients and showed a 12% LDL reduction and 20% LDL goal achievement over 8 weeks.(23)
Other statins including atorvastatin and simvastatin have also demonstrated effectiveness with intermittent dosing in small studies of limited duration.(24–26)
An important conclusion of this analysis is that patients who could only tolerate intermittent statin regimens (9.3%) could still achieve acceptable LDL-C reduction (21.3±4.0%) and ATP-III LDL-C goals (61%).
Intermittent dosing regimens in this study included different statins with various infrequent dosing strategies (from once weekly to six-days a week). The average LDL-C reduction observed in this study (21.3±4.0%) is consistent with the reductions shown in previous trials. Although some studies with atorvastatin, fluvastatin and rosuvastatin have suggested that every other day dosing needs to be nearly twice the daily dose to yield similar LDL-C lowering (21,27–29), our study shows that similar reductions can be achieved with the same and even low doses, in both daily and intermittent dosing strategies. Intermittent statin dosing can therefore be a useful strategy to improve statin utilization and tolerability. There is presently no data on a large randomized trial that demonstrates equivalent effects on clinical outcomes with intermittent dosing regimens as for the daily dosing. This study does, however, show a trend toward improved survival with any use of statin, either intermittent or daily. We believe that the observed trend, though not statistically significant, reinforces the belief that for patients who need statin therapy, we should strive to find some regimen that can be tolerated even if the dosage is as infrequent as once per week.
Finally, this study also shows that some patients who discontinued statin can still achieve significant LDL-C reduction and reach ATP-III goals with comprehensive lifestyle therapy and non-statin lipid-lowering drugs.
LIMITATIONS
There are several limitations to our study. Firstly, this is an observational study reviewing historical data with no placebo controls in a single centre. Secondly, statin intolerance symptoms were self-reported and not objectively assessed. Thirdly, although a comprehensive medical and lifestyle therapy was provided to this population, non-prescriptive pharmacological interventions such as exercise and diet were not systematically quantified or documented. It’s therefore difficult to determine the contribution of statin alone to the overall LDL-C reduction compared to the cumulative alternative lipid lowering efforts recommended to the patients.
Fourthly, compliance was not objectively assessed because of lack of data. Compliance is an important consideration in assessing the effectiveness of an intervention. Patients who are non-compliant on a daily statin regimen would actually fall into the intermittent dosing group.
Fifthly, the number of patients in the 3 different arms of the study is not balanced and the number of patients with intermittent therapy is particularly small. This makes the comparison among the three groups somewhat difficult.
Lastly, the exact cause of death is not documented because the mortality data was obtained through SSDI. This would have been interesting to know given the differences in liver disease, kidney disease and pancreatitis.
Despite the above-mentioned limitations, this study provides interesting data on the management of this very challenging group of patients. More studies are needed to verify whether intermittent statin regimens reduce cardiac events and prolong survival.
CONCLUSIONS
This study shows that prior statin intolerance does not preclude long-term statin therapy and achievement of ATP-III goals. Most patients with statin intolerance are able to maintain a stable long-term statin treatment regimen. A strategy of intermittent statin dosing can be an effective therapeutic option in some statin intolerant patients and may result in reduction in LDL-C, achievement of LDL-C goals.
Abbreviations List
- TC
Total Cholesterol
- HDL-C
High Density Lipoprotein Cholesterol
- LDL-C
Low Density Lipoprotein Cholesterol
- TG
Triglycerides
- NCEP ATP-III
National Cholesterol Education Program Adult Treatment Panel III
- CVD
Cerebral Vascular Disease
- CHD
Coronary Heart Disease
- PVD
Peripheral Vascular Disease
- ACEI
Angiotensin Converting Enzyme Inhibitor
- ARB
Angiotensin Receptor Blocker
- CoQ10
Coenzyme Q10
References
- 1.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720–732. doi: 10.1016/j.jacc.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 2.Cheng AY, Leiter LA. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Curr Opin Cardiol. 2006;21:400–404. doi: 10.1097/01.hco.0000231412.15049.fb. [DOI] [PubMed] [Google Scholar]
- 3.Davidson MH, Robinson JG. Safety of aggressive lipid management. J Am Coll Cardiol. 2007;49:1753–1762. doi: 10.1016/j.jacc.2007.01.067. [DOI] [PubMed] [Google Scholar]
- 4.Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: a systematic overview of randomized clinical trials. Circulation. 2006;114:2788–97. doi: 10.1161/CIRCULATIONAHA.106.624890. [DOI] [PubMed] [Google Scholar]
- 5.Gomez Sandoval YH, Braganza MV, Daskalopoulou SS. Statin discontinuation in high-risk patients: a systematic review of the evidence. Curr Pharm Des. 2011;17:3669–89. doi: 10.2174/138161211798220891. [DOI] [PubMed] [Google Scholar]
- 6.Fernandez G, Spatz ES, Jablecki C, Phillips PS. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve Clin J Med. 2011;78:393–403. doi: 10.3949/ccjm.78a.10073. [DOI] [PubMed] [Google Scholar]
- 7.Bruckert E, Hayem G, Dejager S, Yau C, Be gaud B. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients— the PRIMO study. Cardiovasc Drugs Ther. 2005;19:403–14. doi: 10.1007/s10557-005-5686-z. [DOI] [PubMed] [Google Scholar]
- 8.Buettner C, Davis RB, Leveille SG, Mittleman MA, Mukamal KJ. Prevalence of musculoskeletal pain and statin use. J Gen Intern Med. 2008;23:1182–6. doi: 10.1007/s11606-008-0636-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maningat P, Breslow JL. Needed: pragmatic clinical trials for statin- intolerant patients. N Engl J Med. 2011;365:2250. doi: 10.1056/NEJMp1112023. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, et al. Discontinuation of statins in routine care settings. A cohort study. Ann Intern Med. 2013;158:526–34. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farmer JA, Torre-Amione G. Comparative tolerability of the HMG-CoA reductase inhibitors. Drug Safety. 2000;23:197–213. doi: 10.2165/00002018-200023030-00003. [DOI] [PubMed] [Google Scholar]
- 12.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 13.Backes JM, Moriarty PM, Ruisinger JF, Gibson CA. Effects of once weekly rosuvastatin among patients with a prior statin intolerance. Am J Cardiol. 2007;100:554–555. doi: 10.1016/j.amjcard.2007.03.059. [DOI] [PubMed] [Google Scholar]
- 14.Backes JM, Venero CV, Gibson CA, Ruisinger JF, Howard PA, Thompson PD, Moriarty PM. Effectiveness and tolerability of every-other-day rosuvastatin dosing in patients with prior statin intolerance. Ann Pharmacother. 2008;42:341–346. doi: 10.1345/aph.1K604. [DOI] [PubMed] [Google Scholar]
- 15.Gadarla M, Kearns AK, Thompson PD. Efficacy of rosuvastatin (5 mg and 10 mg) twice a week in patients intolerant to daily statins. Am J Cardiol. 2008;101:1747–1748. doi: 10.1016/j.amjcard.2008.02.061. [DOI] [PubMed] [Google Scholar]
- 16.Glueck CJ, Aregawi D, Agloria M, Khalil Q, Winiarska M, Munjal J, Gogineni S, Wang P. Rosuvastatin 5 and 10 mg/d: a pilot study of the effects in hypercholesterolemic adults unable to tolerate other statins and reach LDL cholesterol goals with nonstatin lipid-lowering therapies. Clin Ther. 2006;28:933–942. doi: 10.1016/j.clinthera.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 17.ACC/AHA/NHLBI clinical advisory on the use and safety of statins. J Am Coll Cardiol. 2002;40:567–72. doi: 10.1016/s0735-1097(02)02030-2. [DOI] [PubMed] [Google Scholar]
- 18.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 19.Tziomalos K, Athyros VG, Mikhailidis DP. Statin discontinuation: an underestimated risk? [Editorial] Curr Med Res Opin. 2008;24:3059–62. doi: 10.1185/03007990802469102. [DOI] [PubMed] [Google Scholar]
- 20.Mackie BD, Satija S, Nell C, Miller J, 3rd, Sperling LS. Monday, Wednesday, and Friday dosing of rosuvastatin in patients previously intolerant to statin therapy. Am J Cardiol. 2007;99:291. doi: 10.1016/j.amjcard.2006.07.093. [DOI] [PubMed] [Google Scholar]
- 21.Ruisinger JF, Backes JM, Gibson CA, Moriarty PM. Once-a-week rosuvastatin (2. 5 to 20 mg) in patients with a previous statin intolerance. Am J Cardiol. 2009;103:393–394. doi: 10.1016/j.amjcard.2008.09.095. [DOI] [PubMed] [Google Scholar]
- 22.Marcus FI, Baumgarten AJ, Fritz WL, Nolan PE. Alternate-day Dosing with Statins. The American Journal of Medicine. 2013 Feb;126(2):99–104. doi: 10.1016/j.amjmed.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy SP, Barnas GP, Schmidt MJ, Glisczinski MS, Paniagua AC. Efficacy and tolerability of once-weekly rosuvastatin in patients with previous statin intolerance. Journal of Clinical Lipidology. 2011;5:308–315. doi: 10.1016/j.jacl.2011.03.454. [DOI] [PubMed] [Google Scholar]
- 24.Jafari M, Ebrahimi R, Ahmadi-Kashani M, Balian H, Bashir M. Efficacy of alternate-day dosing versus daily dosing of atorvastatin. Journal of Cardiovascular Pharmacology & Therapeutics. 2003;8:123–126. doi: 10.1177/107424840300800205. [DOI] [PubMed] [Google Scholar]
- 25.Kayikcioglu M, Ozerkan F, Soydan I. Effectiveness and safety of alternate-day simvastatin and fenofibrate on mixed hyperlipidemia. Am J Cardiol. 1999;83:1135–1137. doi: 10.1016/s0002-9149(99)00030-2. [DOI] [PubMed] [Google Scholar]
- 26.Athyros VG, Tziomalos K, Kakafika AI, Koumaras H, Karagiannis A, Mikhailidis DP. Effectiveness of ezetimibe alone or in combination with twice a week Atorvastatin (10 mg) for statin intolerant high-risk patients. Am J Cardiol. 2008;101:483–485. doi: 10.1016/j.amjcard.2007.09.096. [DOI] [PubMed] [Google Scholar]
- 27.Matalka MS, Ravnan MC, Deedwania PC. Is alternate daily dose of atorvastatin effective in treating patients with hyperlipidemia? The Alternate Day Versus Daily Dosing of Atorvastatin Study (ADDAS) Am Heart J. 2002;144:674–677. doi: 10.1067/mhj.2002.124399. [DOI] [PubMed] [Google Scholar]
- 28.Rindone JP, Hiller D, Arriola G. A comparison of fluvastatin 40 mg every other day versus 20 mg every day in patients with hypercholesterolemia. Pharmacotherapy. 1998;18:836–839. [PubMed] [Google Scholar]
- 29.Ferrer-Garcia JC, Perez-Silvestre J, Martinez-Mir I, Herrera-Ballester A. Alternate-day dosing of atorvastatin: effects in treating type 2 diabetic patients with dyslipidaemia. Acta Diabetol. 2006;43:75–78. doi: 10.1007/s00592-006-0216-4. [DOI] [PubMed] [Google Scholar]