Summary
Components of neural circuits are often repurposed so that the same biological hardware can be used for distinct computations. This flexibility in circuit operation is required to account for the changes in sensory computations that accompany changes in input signals. Yet we know little about how such changes in circuit operation are implemented. Here we show that a single retinal ganglion cell performs a different computation in dim light – averaging contrast within its receptive field – than in brighter light – when the cell becomes sensitive to fine spatial detail. This computational change depends on interactions between two parallel circuits that control the ganglion cell’s excitatory synaptic inputs. Specifically, steady-state interactions through dendro-axonal gap junctions control rectification of the synapses providing excitatory input to the ganglion cell. These findings provide a clear example of how a simple synaptic mechanism can repurpose a neural circuit to perform diverse computations.
Introduction
The array of neural computations required to explain behavior is far too large to be explained by specialized single-function neural circuits. Instead, the computation performed by a neural circuit often changes as task demands change. Such repurposing has been studied extensively in motor control. Neuromodulators, for example, alter central pattern generator circuits so that common circuit components participate in multiple motor rhythms (Marder and Bucher, 2007). Although similar functional repurposing occurs in circuits throughout the central nervous system, we know much less about the underlying mechanisms.
The optic nerve of the mammalian retina contains the axons of ~20 subtypes of retinal ganglion cells (RGCs; Masland, 2012), through which all visual information is transmitted to the brain. These same RGCs provide the basis for visually-guided behavior under lighting conditions ranging from the darkest night to the brightest day. As the demands of the visual environment change, the computations performed by retinal circuits change correspondingly. Some functional properties of RGCs, like gain (Shapley and Enroth-Cugell, 1984), receptive field size (Barlow et al., 1957), and center/surround ratio (Enroth-Cugell and Lennie, 1975), change with the statistics of the visual environment; other properties have traditionally been considered immutable, and correspondingly are often used to classify RGCs into specific types. On versus Off response polarity and direction selectivity are examples of these more stable functional properties, though recent work has disputed the immutability of even these properties (Geffen et al., 2007; Rivlin-Etzion et al., 2012). Here we show that another property commonly used to classify RGCs – linear vs. nonlinear spatial integration of visual signals contained within their receptive field (Enroth-Cugell and Robson, 1966) – can change with the visual environment.
While functional properties of retinal circuits can change rapidly, the underlying circuit wiring is likely fixed over the course of an ~hour-long physiology experiment. Thus, rapid functional changes arise from light-dependent changes in the operation of common circuit elements. We find here that tonic input via gap junctions controls the rectification of the dominant excitatory synapse onto retinal ganglion cells. This tonic input changes with luminance, and the resulting change in synaptic rectification controls whether ganglion cells integrate inputs across space linearly or nonlinearly. More generally, this work illustrates how fine control of the synaptic operating point, in this case via dendro-axonal gap junctions, can control key computational features of a neural circuit.
Results
Spatial integration depends on mean illumination
We used a flat mount preparation of the mouse retina to characterize how RGCs integrate light inputs across space. By mounting the isolated retina flat in a recording chamber, we could deliver spatially patterned light stimuli to the photoreceptors while measuring the resulting RGC responses. We focused on On alpha RGCs, a physiologically and anatomically well-characterized ganglion cell type (Pang et al., 2003; Murphy and Rieke, 2006; Schwartz et al., 2012).
The spatial dependence of RGC responses was measured using a classic stimulus paradigm designed to characterize cells as linear (‘X’ cells) or nonlinear (‘Y’ cells) integrators over space (Enroth-Cugell and Robson, 1966; Victor and Shapley, 1979). A split-field stimulus with regions of equal positive and negative contrast was modulated sinusoidally in time (at 3.75 Hz) so that the light and dark regions changed sides periodically (Figure 1A). When the light and dark regions of the stimulus each cover exactly half of the receptive field center, linear spatial integration predicts no modulation of the response because responses to the light and dark regions cancel. Nonlinear spatial integration of the same input would result in incomplete cancellation and a response at twice the modulation frequency (a frequency doubled or “F2” response).
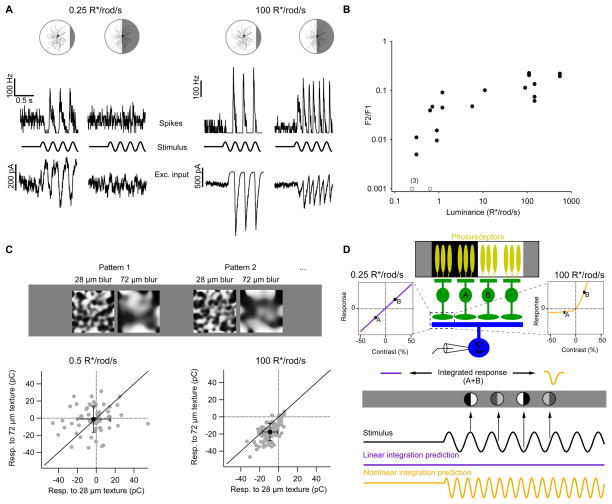
Figure 1. The computation of a retinal ganglion cell changes with luminance.
A, Spike rates (top traces) and excitatory input currents (bottom traces) recorded from On alpha ganglion cells responding to sinusoidally modulated split-field stimuli at 0.5 R*/rod/s and 100 R*/rod/s. Spikes and input currents were recorded from the same cell, and a different cell was recorded at each luminance. B, Power of the frequency doubled (F2) response divided by that of the F1 response as a function of background illumination (see Experimental Procedures). C, Top, examples of textured stimuli from the same random seed with different levels of blurring used in this experimental paradigm (see Experimental Procedures). Bottom, individual responses (gray points) to texture patterns presented at two different levels of blur for the same RGC at two different luminance levels. Solid line is unity. Mean ± standard deviation are shown in black. D, Top, schematic of RGC’s spatial summation of input from multiple presynaptic bipolar cells in response to a sinusoidally-modulated split-field stimulus. Bottom, the predicted response to sinusoidal modulation of a split-field stimulus depends on the contrast response function of the bipolar cell to ganglion cell synapse. All recordings are from whole mount preparations.
RGCs responded at the modulation frequency (an “F1” response) when the border between light and dark regions was far (≥ 50 μm) from the receptive field center (Figure 1B). These responses were robust at both low (0.25–1 photoisomerizations per rod photoreceptor per second or R*/rod/s; Figure 1A, left) and moderate (≥ 100 R*/rod/s; Figure 1A, right) luminance. However, when the border was centered on the receptive field, responses were weak or non-existent at low luminance but strong at moderate luminance (F2 responses; see Experimental Procedures). This change from linear to nonlinear spatial integration was evident in both spike responses and excitatory input currents (Figure 1A). We focused on changes in the spatial integration of excitatory inputs from presynaptic bipolar cells since inhibitory inputs play a minor role in shaping spike responses of these cells under similar stimulus conditions (Murphy and Rieke, 2006; Schwartz et al., 2012). We quantified nonlinearities in spatial integration from the ratio of the frequency-doubled response to the response at the modulation frequency (i.e. the F2/F1 ratio); this ratio increased by more than a factor of 10 across the luminance range tested (Figure 1B; see Linsenmeier and Jakiela, 1979, for a comparison with cat RGCs). The change in F2/F1 ratio with luminance was highly significant (p =1×10−5; Pearson’s correlation test on log-log scale, n = 19 cells).
Nonlinear spatial integration underlies many of the computations of RGCs including sensitivity to second-order motion (Demb et al., 2001), differential motion of object and background (Gollisch and Meister, 2010), and rotations or small translations of texture patterns within the receptive field center (Schwartz et al., 2012). Computations in higher visual areas, like the recognition of form from texture patterns (El-Shamayleh and Movshon, 2011) and the demodulation of spatio-temporal patterns (Rosenberg and Issa, 2011), also depend on nonlinear spatial integration.
We found previously that On alpha RGC responses at high luminance depend on the spatial scale of texture stimuli confined to the receptive field center (Schwartz et al., 2012). The RGC response was modulated maximally by textures containing spatial scales of ~40 μm, much smaller than the ~300 μm diameter of the full receptive field center. Here, we measured the ability of the same On alpha RGCs to encode information about two-dimensional spatial structure within their receptive fields under different luminance conditions. We presented sets of texture patterns with two different levels of spatial blur which we had shown previously to elicit different average responses in the RGC at high luminance (Schwartz et al., 2012) (Figure 1C, see Experimental Procedures). At a luminance of 0.5 R*/rod/s, performance at distinguishing blur levels was not different from chance (55 ± 5% correct; n = 5 cells). The same cells performed substantially better at a mean luminance of 100 R*/rod/s (86 ± 3%; p = 0.003). Thus the change from linear to nonlinear spatial integration confers the On alpha RGCs with sensitivity to fine spatial structure, fundamentally altering the type of information these cells extract from a visual scene. Other computations of these RGCs that depend on nonlinear spatial integration should similarly be impaired or absent at low luminance.
Changes in signal rectification underly computational change
What circuit mechanisms could cause RGC spatial integration to change with luminance? The bipolar cells that provide excitatory synaptic input to RGCs have much smaller receptive fields (~40 μm) than the RGCs themselves (~300 μm) (Schwartz et al., 2012), and the On alpha RGC receives synaptic input from hundreds of bipolar cells within its receptive field center (Schwartz et al., 2012). If the bipolar cells report stimulus contrast without rectification (i.e. with equal and opposite responses to positive and negative contrast), inputs from bipolar cells in the dark and light regions of the split-field stimulus would cancel, resulting in linear spatial integration by the RGC. If instead the bipolar cells provide a rectified signal to the RGC, responses from the dark and light regions of the split-field stimulus would not cancel, resulting in nonlinear spatial integration by the RGC (Figure 1C,D) (Demb et al., 1999; Schwartz et al., 2012). We have shown previously that excitatory synaptic input to the On alpha RGC is rectified in bright conditions (Schwartz et al., 2012), so we hypothesized that a change in the rectification of bipolar cell input across luminance underlies the observed changes in RGC spatial integration.
To test this hypothesis, we measured changes in RGC excitatory synaptic input elicited by increments and decrements of spatially uniform stimuli across a range of luminance (Figure 2). We focused on responses to the step onset since these reflect the adaptational state of the circuit in the prior period of constant light. At low luminance, ±50% contrast steps elicited approximately equal and opposite responses. At higher luminance, the response profile was markedly rectified, with the positive contrast step eliciting a ~4-fold larger response than the negative contrast step (Fig 2A,B). To quantify changes in rectification, we computed a rectification index by dividing the difference between the +50% and −50% contrast responses by their sum (see Experimental Procedures). This index ranges from −1 (negatively rectified) through 0 (non-rectified) to 1 (positively rectified). Rectification increased systematically with increasing luminance (Figure 2C; p = 1×10−10; Pearson’s correlation on log-linear scale, n = 49 cells). Changes in rectification could not be explained as simply a change in dynamic range since a rescaling of the contrast axis did not produce identical contrast-response curves (Supplemental Figure 1). The transition in rectification occurred across the same luminance range as the change in F2/F1 ratio (Figure 1B), consistent with changes in synaptic rectification underlying changes in spatial integration (Figure 1D).
Figure 2. Contrast encoding changes with luminance.
A, Excitatory current responses to positive (black) and negative (gray) 50% contrast steps in On alpha RGCs at different luminance. B, Representative contrast response functions of excitatory input currents to On alpha RGCs each measured at a different luminance. C, Population data for the rectification index of excitatory input (see Experimental Procedures) as a function of background luminance. Open symbols are individual cells (n = 49) and filled symbols are binned means ± s.e.m. All recordings are from whole mount preparations.
Luminance-dependent transitions in relevant circuit components
At low luminance, signals traverse the retina primarily through the rod bipolar circuit, while at higher luminance the cone bipolar circuit also contributes to ganglion cell responses (Figure 3A; Trexler et al., 2005). Signals traversing either circuit must pass through the cone bipolar cell → On alpha RGC synapse, regardless of their origin. The sensitivity of these two circuits in darkness has been explored (Deans et al., 2002; Pang et al., 2007), but their relative contribution at different luminance levels is unclear. Does a transition in signaling between these two circuits contribute to changes in RGC spatial integration? To answer this question we turned to a retinal slice preparation, allowing better access to bipolar and amacrine cells whose somas reside in the inner nuclear layer (see Experimental Procedures). We then measured signal flow through the rod and cone bipolar circuits across a range of luminances.
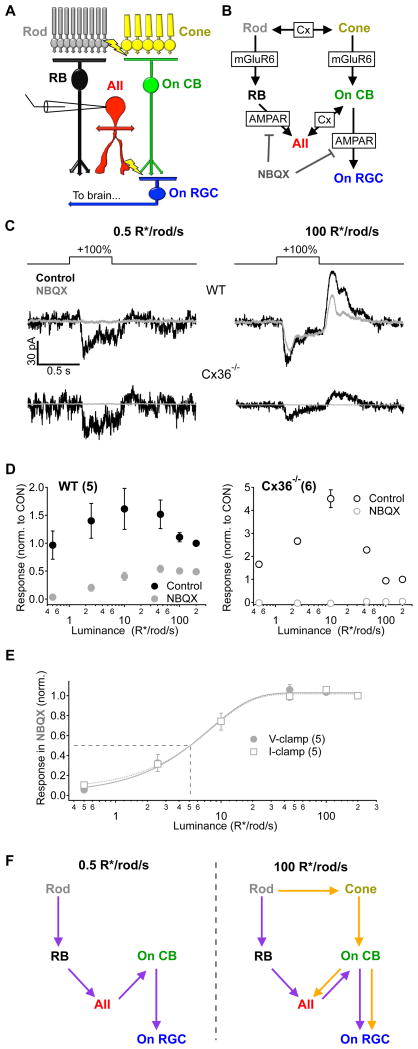
Figure 3. Signal propagation through parallel circuits depends on luminance.
A, Simplified diagram of the parallel circuits that transmit visual information to On alpha RGCs in mouse retina. B, Detailed signaling schematic of the circuit elements and synaptic receptors that mediate transmission within the On alpha RGC circuit. Cx - gap junctions containing connexin proteins, mGluR6 - metabotropic glutamate receptor 6, AMPAR - fast ionotropic glutamate receptors. C, Responses to +100% contrast steps measured in voltage clamp in an AII amacrine cell from both WT (top) and Cx36−/− (bottom) mice before and after bath application of NBQX (10 μM). D, AII amacrine cell responses across luminance (± NBQX) normalized to the control response at 200 R*/rod/s in wild type (WT, left) and Cx36−/−(right) retinas. Responses from AII amacrine cells in Cx36−/− mice were completely eliminated in the presence of NBQX, confirming a decoupling of the two pathways. E, Responses recorded from AII amacrine cells in the presence of NBQX in the voltage clamp configuration (same data is in D; closed circles) or the current clamp configuration (open squares) normalized to the response at 200 R*/rod/s. Fits are sigmoids with the half-max indicated by dotted line. The background eliciting a half-maximal response to 100% contrast steps in the presence of NBQX was 5.1 R*/rod/s, regardless of the recording configuration. F, Schematic illustrating the flow of signals through the rod bipolar (purple) and cone bipolar (orange) circuits at two different luminance levels. Error bars in D and E are s.e.m. across cells. Numbers of cells are indicated in parentheses in panel legends. All recordings are from slice preparations.
The AII amacrine cell receives inputs from both the rod and cone bipolar circuits. Signals from rod bipolar cells are transmitted to the AII amacrine cell via glutamatergic synapses, which contain primarily alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors (AMPARs; Singer and Diamond, 2003; Mørkve et al., 2002). In comparison, signals from the cone bipolar circuit are mediated by connexin36 (Cx36)-containing gap junctions between AII amacrine cell dendrites and On cone bipolar cell axon terminals (Figure 3A,B; Deans et al., 2002; Tsukamoto et al., 2001). On cone bipolar cells themselves receive dendritic input from cones via metabotropic glutamate receptors. Thus input to the AII amacrine cell originating from the rod bipolar circuit can be selectively eliminated by the AMPAR antagonist 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), effectively isolating input from the cone bipolar circuit (i.e. the NBQX-insensitive component; Figure 3B; Cohen, 2000; Murphy and Rieke, 2008; Manookin and Demb, 2006).
We first recorded the combined signals from both circuits in the AII amacrine cell (in response to +100% contrast steps; voltage-clamp) at different luminance levels. We then repeated these measurements in the presence of NBQX to measure signals from the cone bipolar circuit alone (Figure 3C,D). These experiments were conducted using retinas from wild-type (WT) and Cx36−/− mice, in which gap junctions in both the inner and outer retina are disrupted (Deans et al., 2002). At a dim background of 0.5 R*/rod/s, NBQX eliminated the entire light response in both WT and Cx36−/− retinas, indicating that rod signals are transmitted primarily through the rod bipolar circuit at this luminance, with little or no contribution from rod or cone signals traversing the cone bipolar circuit (Figure 3C,D,F). At 100 R*/rod/s, NBQX again eliminated the entire response in AII amacrine cells from Cx36−/− retinas, but much of the signal in WT retinas remained, indicating that both the rod and cone bipolar circuits normally contributed to the response at this luminance (Figure 3C,D,F). The NBQX-insensitive response in WT mice - i.e. the response of the cone bipolar circuit - was half-maximal at a mean luminance of ~5 R*/rod/s (Figure 3E; similar results for current and voltage responses).
The experiments of Figures 2 and 3 show that the balance of signal flow through the rod and cone bipolar circuits and the rectification of the RGC excitatory synaptic input have a similar dependence on luminance. Thus a possible explanation for the change in spatial integration is that the rod and cone bipolar circuits encode contrast differently. We tested this hypothesis by measuring responses to positive and negative contrast steps in AII amacrine cells and type 6 cone bipolar cells (which provide the dominant excitatory input to the On alpha RGC; Schwartz et al., 2012) at low luminance (0.5 R*/rod/s), where input from the rod bipolar circuit dominates, and moderate luminance (100 R*/rod/s), where both rod and cone bipolar circuits provide sizable input (Figure 4).
Figure 4. Luminance alters rectification at the cone bipolar cell to ganglion cell synapse.
A–C, Responses to +50% (gray) and −50% (black) contrast steps in three different cells types at two different luminance levels (solid lines represent the average response, shaded areas represent the s.e.m, number of cells indicated in parentheses in D). D, Rectification index for each cell type at 0.5 R*/rod/s and 100 R*/rod/s (see Experimental Procedures). Error bars represent s.e.m. Paired t-tests were used to compare within-cell data (AII and RGC recordings) and an unpaired t-test was used to compare cone bipolar cells that were recorded at 0.5 R*/rod/s or 100 R*/rod/s and to compare cone bipolar cells and RGCs (*, * and ** represent p-values of < 0.05, 0.01 and 0.001, respectively). AII amacine cell and type 6 cone bipolar cell recordings (A,B,D) are from slice preparations; On alpha RGC recordings (C,D) are from whole mount preparations.
Voltage responses of both AII amacrine cells (Figure 4A,D) and On cone bipolar cells (Figure 4C,D) were negatively rectified (i.e. larger responses to negative contrast steps than positive contrast steps) at both low and moderate luminance levels. In comparison, RGC excitatory inputs exhibited near zero rectification at low light levels, and became positively rectified as luminance was increased to 100 R*/rod/s (Figure 4C,D; see also Figure 2). These data indicate that the change in rectification of the RGC synaptic inputs does not reflect a basic difference in the rectification of the rod bipolar and cone bipolar circuits. Instead, the change in rectification occurs at the synapse between cone bipolar cells and RGCs, a shared element in the two circuits. Such shared elements provide likely sites for interactions of signals from the two circuits.
A steady-state interaction between parallel circuits
Changes in the steady-state signals observed in AIIs, cone bipolar cells and RGCs provided a clue about the interactions responsible for this luminance-dependent change in RGC encoding and synaptic rectification. On alpha RGCs lost excitatory holding current as luminance increased (Figure 4C, 169 ± 57pA, n = 5 cells, p = 0.04), indicating a reduction in maintained glutamate release from presynaptic cone bipolar cells. AII amacrine cells and type 6 cone bipolar cells hyperpolarized across the same luminance range (ΔVAII= −2.94 ± 0.14 mV, n = 9 cells, p = 1×10−4; ΔVCB6= −4.32 ± 0.33 mV, n = 6 cells, p = 0.003; Figure 4A,B and Figure 5G). AII amacrine cells in whole mount and slice preparations exhibited similar behavior, confirming that this aspect of the inner-retinal network had not been compromised by slicing the retina (Supplemental Figure 2G). Taken together, these data indicate that the entire gap-junctionally coupled AII-On cone bipolar cell network hyperpolarizes when luminance increases from 0.5 to 100 R*/rod/s.
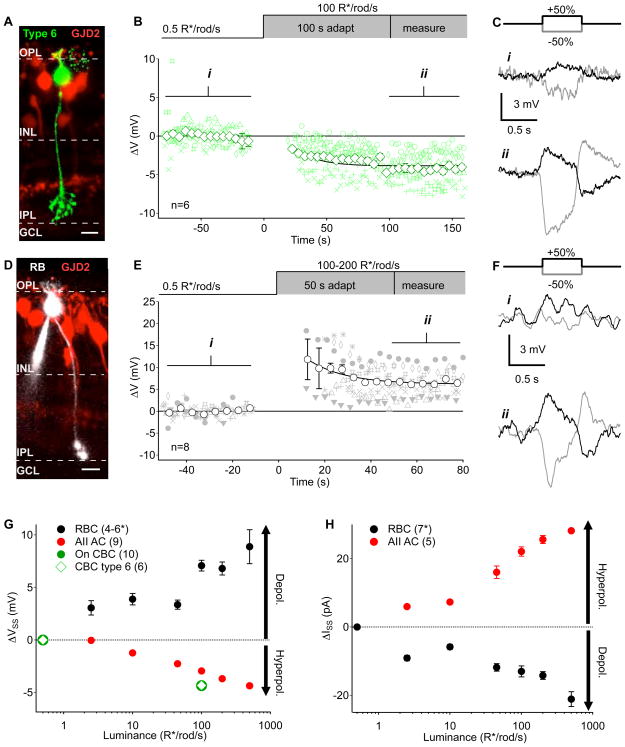
Figure 5. Steady-state voltage changes in the rod and On cone bipolar circuits as a function of luminance.
A, Example of a type 6 On cone bipolar cell reconstructed post-recording in the GJD2-GFP line (Siegert et al., 2009). Scale bar = 10 μm. B, Changes in mean voltage recorded from type 6 On cone bipolar cells before and after a step-wise increase in luminance from 0.5 to 100R*/rod/s. Smaller green symbols are measurements from individual cells (different symbol for each cell) during 1 s preceding a contrast step. Open diamonds are means (error bars are s.e.m.) across cells (n = 6 cells). C, Mean responses to positive (black) and negative (gray) contrast steps from an example cell over the time periods indicated in (i) and (ii). D, Example of a rod bipolar cell reconstructed post-recording in the GJD2-GFP line. Scale bar = 10 μm. E, Changes in mean voltage in rod bipolar cells (n = 8 cells) as in B. F, Example rod bipolar cell contrast responses as in C. G, Current clamp measurements of changes in resting membrane potential (relative to 0.5 R*/rod/s) in rod bipolar cells, AII amacrine cells, and On cone bipolar cells as a function of luminance (as in B,E). Each bipolar cell was only tested at two luminance levels (0.5 R*/rod/s and one other level) to minimize the effects of washout. For rod bipolar cells n is 6, 6, 6, 4, 5 and 4 for the test luminance levels in ascending order. H, Changes in the steady-state holding current (relative to 0.5 R*/rod/s) measured in rod bipolar cells and AII amacrine cells at different luminance levels. Numbers in parentheses indicate number of cells. All error bars represent s.e.m. Recordings are from slice preparations except for the AII amacrine cell data in G which includes data from slice and whole mount (see Supplemental Figure 2G).
Paradoxically, the cells of interest (On RGCs, On cone bipolar cells, and AII amacrine cells) are all classified as On cells – they depolarize in response to positive contrast. Hence, we were surprised that maintained light exposure hyperpolarized the presynaptic AII-On cone bipolar electrical network and reduced tonic excitatory input to postsynaptic RGCs. In the following paragraphs we explore the mechanistic origin of the steady-state hyperpolarization before returning to its impact on synaptic rectification and spatial integration.
Previous work showed that the transition from low to moderate luminance involves a dramatic reduction in gain of the rod bipolar cell → AII synapse (Dunn et al, 2006; Jarsky et al., 2011; Oesch and Diamond, 2011) and recruitment of the cone bipolar circuit (Figure 3). Specifically, tonic depolarization of the rod bipolar cell reduces synaptic gain whereas hyperpolarization increases gain. Close proximity of the rod bipolar cell → AII synapses and AII → cone bipolar cell electrical synapses suggests that a local change in AII voltage produced by a change in gain of the rod bipolar synapse will be efficiently relayed to cone bipolar axon terminals (Figure 3A; Tsukomoto et al., 2001). Is this the case? If so, what synaptic mechanisms are involved?
We first compared changes in rod and cone bipolar cell voltage in response to a step in mean luminance from 0.5 to 100 R*/rod/s. While cone bipolar cells slowly hyperpolarized following the luminance increase (Figure 5B and Supplemental Figure 2A–F), rod bipolar cells depolarized and slowly relaxed to steady-state (Figure 5E; Jarsky et al., 2011). Both cell types maintained their polarity as On cells throughout the change in luminance (Figure 5C,F) despite their opposing changes in resting membrane potential (ΔVCB= −4.34 ± 0.26 mV, n = 10 cells, p = 6×10−4; ΔVRB = +7.26 ± 0.49 mV, n = 9 cells, p = 5×10−4; Figure 5G). Somatic cone bipolar cell recordings reflect a superposition of axonal input from AIIs (via gap junctions) and dendritic input through mGluR6 receptors. If dendritic input to rod and cone bipolar cells exhibit similar kinetics, the slow hyperpolarization of the cone bipolar cell following the increase in luminance could reflect slowly decreasing dendritic input and a more rapid axonal hyperpolarization.
Next, we determined how the excitatory input AII amacrine cells receive from rod bipolar cells depended on mean luminance. Steady-state release at the rod bipolar cell → AII synapse was measured using voltage-clamp recordings from AII amacrine cells (in retinal slices). These recordings revealed a decrease in AII holding current with increasing luminance, consistent with a reduction in glutamate release from rod bipolar cells (Figure 5H). Furthermore, bath application of NBQX reduced the AII holding current more at low luminance levels than at higher levels (Figure 6A,B) - again consistent with a reduction in glutamate release at the higher luminance level. Recordings from AII amacrine cells in Cx36−/− retina also showed a decrease in NBQX-sensitive current as luminance increased (0.5→500R*/rod/s), conditions under which rod bipolar cells provide their only known excitatory input (i.e. dendro-axonal gap junctions are absent; Figure 6B).
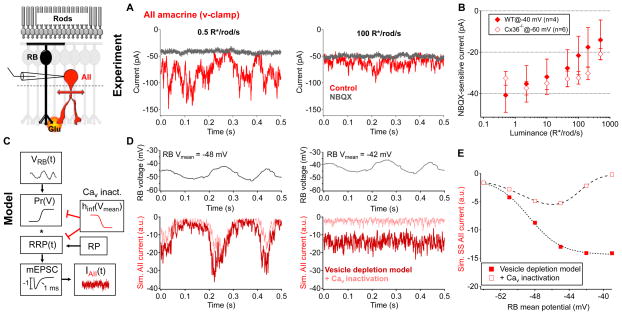
Figure 6. Tonic excitatory transmission at the rod bipolar cell → AII synapse decreases as luminance increases from 0.5 to 500 R*/rod/s.
A, Example current traces from the same AII amacrine cell under steady background illumination of 0.5 R*/rod/s and 100 R*/rod/s in the presence or absence of NBQX (10 μM). B, NBQX(10 μM)-sensitive holding current in AII amacrine cells across luminance in WT (held at −40 mV, n = 4 cells, solid diamonds) and Cx36−/ − (held at −60 mV, n = 6 cells, open diamonds) retinas. Error bars represent s.e.m. across cells. C–E, Stochastic release model of synaptic transmission at the rod bipolar cell → AII synapse (see Experimental Procedures for model details). C–D, Time-varying rod bipolar cell voltage record (model input) were passed through a stochastic synaptic release model; the model included time-dependent vesicle depletion from and replenishment to a readily releasable pool (RRP, red trace) and the addition of presynaptic calcium channel inactivation (pink trace, from Jarsky et al., 2011). The mean voltage of the rod bipolar cell example trace was varied (−54 to −39 mV, 3 mV increments). RP = reserve pool, mEPSC = miniature excitatory postsynaptic current. D, (left)At more hyperpolarized potentials (ex. −48 mV) calcium channel inactivation contributes very little to tonic glutamate releasefrom rod bipolar cells. (r ight) At more depolarized potentials (ex. −42 mV), calcium channel inactivation suppresses tonic glutamate release. E, Steady-state glutamate release/model output as a function of presynaptic resting membrane potential. All data are from slice preparations.
Rod bipolar cells depolarized as luminance increased from 0.5 R*/rod/s (Figure 5E,G), yet the excitatory current in postsynaptic AII amacrine cells was reduced (Figure 6A,B). Presynaptic depression is known to play a prominent role in controlling gain at the rod bipolar cell → AII synapse (Singer and Diamond, 2003; Singer and Diamond, 2006; Dunn and Rieke, 2008) while postsynaptic receptor desensitization contributes minimally (Singer and Diamond, 2003). Extensive studies of the mechanisms underlying presynaptic depression have produced detailed models of glutamate release at this synapse (Jarsky et al., 2011; Oesch and Diamond, 2011) and emphasized prominent roles for vesicle depletion and inactivation of presynaptic Cav channels.
Our experimental conditions were similar to those in Jarsky et al. (2011), allowing us to adopt their physiologically-constrained model (see Experimental Procedures; Figure 6C–E). We used the model to determine how vesicle depletion and Cav channel inactivation influenced the relationship between steady-state synaptic release and mean presynaptic voltage – specifically whether the model could account for the observed reduction in tonic glutamate release with increasing presynaptic depolarization. Presynaptic release rates in this model depend on the probability of release (Pr) and the size and occupancy of the readily-releasable pool (RRP) of neurotransmitter-filled vesicles. Steady-state release specifically reflects an equilibrium between release from the RRP and replenishment of the RRP from the reserve pool (RP). Furthermore, Jarsky and colleagues found that Cav channels expressed at rod bipolar synaptic release sites became inactivated as mean voltage was increased. They concluded that Cav channel inactivation reduced not only the sensitivity of the synapse to presynaptic voltage but also reduced the effective size of the RRP due to tight nano-domain control of release sites by as few as one Cav channel (Jarsky et al., 2010).
We explored how RRP size and CaV channel inactivation influenced steady-state release using either constant voltage steps or actual time-varying voltage traces recorded from rod bipolar cells as input to the model. At each time step, presynaptic voltage was used to calculate the rate of release based on the probability of release curves and vesicle availability (see Experimental Procedures and Supplemental Figure 4); models were run until release rates reached steady-state. In the absence of Cav channel inactivation, increases in presynaptic voltage led to increases in steady-state neurotransmitter release (Figure 6D,E). The maximum steady-state release rate occurring under these conditions is set by the rate at which the RRP is replenished. Incorporation of Cav channel inactivation (as in Jarsky et al., 2011) strongly altered the relationship between mean presynaptic voltage and steady-state release. In this more complete model, tonic glutamate release exhibited a non-monotonic dependence on presynaptic membrane potential (Figure 6D,E) which resembled the u-shaped steady-state signals observed in AIIs when the range of luminance tested includes darkness (Supplemental Figure 4C). Experimentally, tonic release from RBCs was maximal near our dim light level of 0.5 R*/rod/sec. Thus, model and experiment together indicate that the depolarization of RBCs produced by increasing luminance from dim to moderate levels (Figure 5G; see also Jarsky et al., 2011) will reduce tonic glutamate release via vesicle depletion and Cav channel inactivation.
Reduced tonic excitatory input will hyperpolarize the AII amacrine cells, and this hyperpolarization will spread to On cone bipolar cell axon terminals via gap junctions. While a decrease in tonic inhibition onto the cone bipolar cell terminal could also contribute to the luminance-dependent hyperpolarization, we found no evidence for such a mechanism using pharmacological manipulations designed to alter inhibition (Supplemental Figure 3). This is consistent with recent work showing that rectification of the On alpha RGC responses persists in the absence of inhibitory synaptic transmission (Chang and He, 2014). Together these results indicate that adaptation at the rod bipolar → AII synapse controls the voltage, and hence synaptic set point, of the cone bipolar → On RGC synapse though dendro-axonal gap junctions.
Voltage bias alters rectification at the cone bipolar synapse
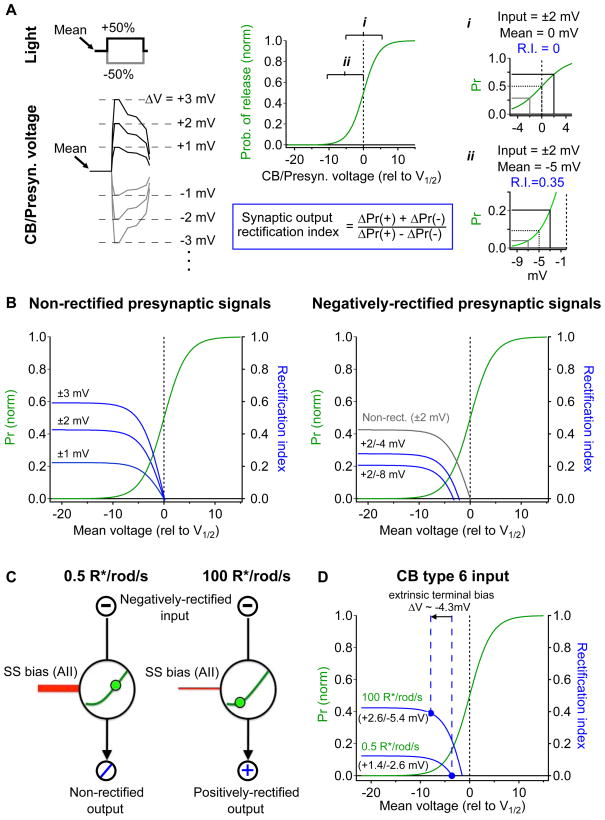
The modeling work described above indicates how mechanisms at the rod bipolar output synapse can cause the On cone bipolar synaptic terminal to hyperpolarize with increasing luminance. How does this hyperpolarization regulate rectification of the On cone bipolar synaptic output and RGC encoding (e.g. see Figure 1D)? To answer this question we consider a simplified model of the On cone bipolar cell synapse that predicts output rectification based on the mean and rectification of the presynaptic signals and rectification of the synapse itself (Figure 7).
Figure 7. Changes in the mean and range of presynaptic cone bipolar cell voltage underlie shifts in synaptic rectification.
A, Simplistic model of synaptic rectification at the On cone bipolar → On alpha RGC synapse. left, Positive and negative steps in luminance drive voltage responses of different sign and sometimes of different amplitude. right, The mean and amplitude of the presynaptic signal drive changes in transmitter release rates, from these changes a synaptic output rectification index was calculated. This instantaneous rectification index depends strongly on mean voltage (compare i and ii). B,D, Instantaneous synaptic rectification index (blue) plotted against the mean presynaptic voltage for a generalized synapse. Green trace represents a ‘probability of release’ curve (taken from rod bipolar cell → AII model in Figure 6). B left, Increasing the amplitude of symmetrical presynaptic voltage signals enhances positive synaptic rectification at negative mean voltages (relative to V1/2). right, Negatively rectified presynaptic voltage signals alter the synaptic rectification range and shift the non-rectified point (i.e. rectification index = 0) to a value negative to V1/2. C, Schematic of the effect of steady-state excitatory input through AII amacrine cells as a bias, shifting the mean voltage and synaptic rectification at the cone bipolar cell output synapse. D, Prediction of the model using our experimentally measured values (from Figure 4).
We modeled synaptic rectification using a probability-of-release curve from the rod bipolar → AII synapse model presented in Figure 6 (see Experimental Procedures). The voltage dependence of the presynaptic Cav channels and the high cooperativity of presynaptic calcium sensors together produce a sigmoidal relationship between presynaptic voltage and the rate of glutamate release (reviewed by Neher and Sakaba, 2008; green curves in Figure 7). As a result, synaptic rectification depends on the mean presynaptic voltage and local curvature of the synaptic input-output function (Figure 7A). For presynaptic voltage signals of equal and opposite sign (non-rectified presynaptic signals), minimal rectification in synaptic output occurs when the mean voltage equals the midpoint (i.e. V1/2) of the synaptic input-output relationship (Figure 7Ai,B left). This holds true across a range of input voltage signals since the input-output relationship is symmetric for changes around its midpoint and hence symmetric inputs lead to symmetric outputs. For negatively-rectified presynaptic signals (i.e. decrement signals larger than increment signals), the mean presynaptic voltage that minimizes output rectification becomes negative to V1/2 (i.e. at a voltage at which synaptic rectification cancels rectification in the presynaptic input signals, Figure 7B right).
Using the measured type 6 cone bipolar cell voltage responses at 0.5 and 100 R*/rod/s (Figure 4C) as inputs, we derived the rectification index of the synaptic output for the measured range of mean presynaptic voltages. We first examined the synaptic rectification index for signals observed at low luminance (0.5 R*/rod/s). Because these presynaptic bipolar cell voltage signals were negatively rectified, the presynaptic resting membrane potential required to reproduce a non-rectified postsynaptic response (i.e. RGC synaptic input; Figure 4C,D) was negative to V1/2 (Figure 7D). Using this mean voltage as a baseline, we then introduced the extrinsic voltage bias we measured in Figure 5 (ΔVCB= −4.3 mV) to estimate the synaptic rectification index in response to presynaptic signals recorded at 100 R*/rod/s. This extrinsic hyperpolarization shifted the synaptic output into a more positively-rectified state and provided an estimated rectification index of 0.39 (Figure 7D), which agreed well with that measured in the RGC synaptic inputs (0.42; Figure 4F). This model demonstrates how hyperpolarization of the cone bipolar cell synaptic terminal, originating in part in the rod bipolar circuit, can contribute to the luminance-dependent change in rectification of the excitatory synaptic currents in On alpha RGCs. Rectification of the excitatory inputs in turn controls how the RGC encodes patterns of light within its receptive field (Fig. 1C,D; Schwartz et al, 2012).
Discussion
Computation in neural circuits often relies on the integration of the outputs of multiple parallel sub-circuits. Here we show that the computation implemented by the circuitry subserving a particular RGC type changes with increasing luminance, and that this change in computation is mediated by interactions between two parallel circuits upstream of the RGC. The result is a fundamental change in the stimulus features encoded in the RGC spike response.
Circuit repurposing alters retinal function
RGCs receive input from hundreds of presynaptic bipolar cells across their dendritic tree, giving rise to the excitatory part of the receptive field center. Integration of the spatial pattern of light within the receptive field center depends on the properties of the cone bipolar cell → RGC synapse. Here we show that changes in the properties of this synapse with background luminance cause a change in how the RGC integrates inputs across space.
At low luminance, mouse On alpha RGCs integrated light near-linearly across their receptive field center (Figure 1); this behavior is similar to linear RGCs in other retinas (Enroth-Cugell and Robson, 1966; Schwartz and Rieke, 2011; Rosenberg and Issa, 2011) and supports a simple encoding framework in which each RGC reports the average signal within its receptive field. At higher luminance, On alpha RGCs exhibited nonlinear spatial integration, and hence became sensitive to fine spatial structure in images presented in their receptive field center (Figure 1). This nonlinear behavior supports the detection of a variety of spatial and spatiotemporal features (Demb et al., 2001; Gollisch and Meister, 2010; Gollisch, 2012; Schwartz and Rieke, 2011; Rosenberg and Issa, 2011); sensitivity to these features is well-predicted from models incorporating rectification at the bipolar output synapses and heterogenous sampling of the bipolar population due to the sparsity of the RGC dendrites (Schwartz et al., 2012).
The change we observe here from linear to nonlinear spatial integration adds to a growing list of stimulus-dependent changes in retinal computations, including changes in the On-Off characteristics of RGCs (Geffen et al., 2007), the direction selectivity of On-Off direction-selective RGCs (Rivlin-Etzion et al., 2012), and the center-surround structure of On alpha RGCs (Farrow et al., 2013). The last of these studies provided a rare link to mechanism, showing that increasing luminance recruited a component of the receptive field surround provided by direct inhibition to the RGC from wide-field amacrine cells. These studies, together with our findings here, emphasize the breadth of functional repurposing of retinal circuits and the diversity of the underlying mechanisms.
What are the ecological implications of the switch from linear to nonlinear spatial integration with increased luminance? While a complete answer to this question will require considering changes in other retinal circuits, we can make a few speculations here. At low light levels, the loss of texture sensitivity and other computations supported by nonlinear spatial integration may be offset by more efficient integration across the entire receptive field center, which helps average out noise associated with quantal fluctuations in photon absorption and hence improves sensitivity to low contrast signals (Hemila et al., 1998). In brighter conditions, when quantal fluctuations are much smaller relative to the mean rate, integration over the full receptive field center may not be required to achieve adequate contrast sensitivity. Additionally, the linearity of the spike responses in dim conditions allows the On alpha RGC to encode information about both positive and negative contrasts, transmitting both kinds of information to downstream cells. In brighter conditions, when rectification truncates the negative contrast responses, the cell signals almost exclusively to positive contrast changes. Negative contrasts are presumably relegated to the Off RGC pathways, so a downstream neuron would have to receive input from both RGC types to have access to information about both positive and negative contrasts.
Synaptic interactions and specializations at bipolar cell synapses
Both rod and cone bipolar cells experienced substantial changes in resting membrane potential over the luminance range we explored (Figure 5). With increasing luminance, rod bipolar cells depolarize, but Cav inactivation accrues and tonic glutamate release declines (Figure 6). This decrease in tonic excitation through the rod bipolar circuit in turn decreases the bias voltage to cone bipolar cells (through dendro-axonal gap junctions with AII dendrites) resulting in increased rectification at the cone bipolar cell output synapse (Figure 7).
This synaptic specialization (i.e. axons electrically coupled to the dendrites of other neurons) is not unique to type 6 cone bipolar cell terminals, but is instead a common feature of most, if not all On cone bipolar cell types (Veruki and Hartveit, 2002; Trexler et al., 2001). Consistent with this observation, we found that other On cone bipolar types hyperpolarized with a step from 0.5 R*/rod/s to 100 R*/rod/s (Supplemental Figure 2 A-F). This suggests that the operating range of many different cone bipolar cell types will be influenced by the rod bipolar circuit. The luminance-dependent hyperpolarization of the AII amacrine cell could also change its tonic glycine release. Consistent with this idea, tonic inhibitory input to Off sustained RGCs, which largely originates from AII amacrine cells (Murphy and Rieke, 2008), decreases with increasing luminance (data not shown). A change in tonic glycine release by the AII could also alter the operating range of Off bipolar cells.
Fine control of presynaptic function alters neural computation
Our work here ties together two broad themes in neural computation. First, modeling work has emphasized the surprisingly complex computational effects that can be achieved by precise tuning of simple mechanisms (Gollisch and Meister, 2010; Priebe and Ferster, 2012). Second, the activity of output neurons in many neural circuits is controlled by convergence of signals from several parallel pathways - a ubiquitous example being converging excitatory and inhibitory inputs. Interactions between signals in such parallel pathways will be a key determinant of circuit computation.
We find that dendro-axonal gap junctions alter the operating point of the primary excitatory input to the RGC, and by doing so control synaptic rectification and integration of light inputs within the RGC receptive field. This adds to a growing appreciation of the diverse roles gap junctions serve in the retina (reviewed by Bloomfield and Volgyi, 2009). Gap junctions between photoreceptors, for example, route light responses generated in a single rod through multiple photoreceptor synapses, and by doing so allow effective transmission of the entire rod voltage response through synapses with limited dynamic range (Attwell et al., 1987; Belgum and Copenhagen, 1988; Hornstein et al., 2005; Li et al., 2012). In this case gap junctions help avoid synaptic rectification, rather than control it as we find here. Recent work has also shown that gap junctions between direction-selective RGCs interact with chemical synapses to shape direction selectivity (Trenholm et al., 2013a; Trenholm et al., 2013b); specifically, electrical synapses help compensate for the delays associated with retinal processing and produce a more veridical representation of motion.
Our expanding appreciation of the diverse roles of gap junctions is not limited to the retina. In addition to their common location between dendrites, electrical synapses are also found near synaptic outputs throughout the brain (Schmitz et al., 2001). Gap junctions have been shown to synchronize or desynchronize networks of neurons (Dugue et al., 2009; Vervake et al., 2010; Traub et al., 2001a), expand and smooth spatial receptive fields (Elyada, 2009), generate persistent firing (Sheffield et al., 2011), and contribute to the initiation of epileptic seizures (Traub et al., 2001b).
Synaptic transmission in other systems, including spiking networks, is similarly highly sensitive to fine changes in presynaptic membrane potential (Awatramani et al., 2005). Parallel pathways could exploit such sensitivity via several mechanisms - including presynaptic voltage bias introduced by gap junctions, as found here, or by tonic presynaptic inhibition. Indeed, a recent study demonstrated that excitability of a population of inhibitory interneurons in the cochlear nucleus is regulated by asymmetrical gap-junctional coupling with excitatory projection neurons (Apostolides and Trussell, 2013). Our work provides a clear link between such changes in synaptic operating point and circuit computation. The components of such circuit interactions - converging parallel circuits, nonlinear synaptic transfer functions, and presynaptic mechanisms that could control synaptic operating point - are common, and hence similar functional changes could be a general feature of neural computation.
Experimental Procedures
Electrophysiology
Experiments were conducted on whole mount and slice (210 μm thick) preparations taken from dark-adapted wild-type or Cx36−/− C57/BL6 mice (Murphy and Rieke, 2006; Sampath et al., 2004). Isolated retina was stored in oxygenated (95% O2/5% CO2) Ames medium (Sigma) at ~32–34° C and, once under the microscope, tissue preparations were perfused by the same Ames solution at a rate of ~8 mL/min. Isolated retinas were either flattened onto polyL-lysine slides (whole mount) or embedded in agarose and sliced as previously described (Dunn et al., 2006; Schwartz et al., 2012; Sampath et al., 2004). Retinal neurons were visualized and targeted for cell-attached and/or whole-cell recordings using infrared light (>950 nm).
Voltage clamp recordings were obtained using pipettes (RGCs: 2–3 MΩ, AII amacrine cells: 5–6 MΩ, bipolar cells: 10–14 MΩ) filled with an intracellular solution containing (in mM): 105 Cs methanesulfonate, 10 TEA-Cl, 20 HEPES, 10 EGTA, 2 QX-314, 5 Mg-ATP, 0.5 Tris-GTP and 0.1 Alexa-750 hydrazide (~280 mOsm; pH ~7.3 with CsOH). Current clamp recordings were conducted using an intracellular solution containing (in mM): 123 K-aspartate, 10 KCl, 10 HEPES, 1 MgCl2, 1 CaCl2, 2 EGTA, 4 Mg-ATP, 0.5 Tris-GTP and 0.1 Alexa-750 hydrazide (~280 mOsm; pH ~7.2 with KOH). NBQX (10 μm; Tocris) or TTX (0.5 μm; Alamone) was added to the perfusion solution as indicated in figures 3, 6, and supplemental figure 3. In voltage clamp recordings, RGCs and bipolar cells were held at the estimated reversal potential for inhibition (~ −60 mV) to isolate excitatory synaptic input. Voltage-clamp recordings from AII amacrine cells were performed near their resting potential (−40 mV) to avoid exposing inputs from rod and cone bipolar circuits to non-physiological driving forces and hence altering the balance of these inputs. Recordings from AII amacrine cells in Cx36−/− mice were held at −60 mV to isolate excitation from rod bipolar cells. On cone bipolar and rod bipolar recordings were kept short (typically <5 min.) to minimize washout effects. Absolute voltage values were not corrected for liquid junction potentials (K+-based = −10.8 mV; Cs+-based = −8.5 mV).
Visual stimuli
Spatial stimulus patterns were displayed on a 800 × 600 pixel OLED array (eMagin) with a pixel size of either 1.2 μm or 1.8 μm and were focused onto the photoreceptors through the microscope condenser. Split-field stimuli (Figure 1A) were modulated sinusoidally at 3.75 Hz from −80% to +80% contrast. Results were similar across a range of temporal frequencies from 0.5 Hz to 8 Hz (data not shown). Texture stimuli (Figure 1C) were created by applying a two-dimensional Gaussian filter to binary random noise patterns and normalizing the resulting pattern to a uniform distribution of contrasts from −100% to +100%. Patterns were 300 μm squares centered on the receptive field of the RGC and were presented for 0.5 s. Uniform stimuli for measuring RGC contrast-response functions (Figure 2) were circles 300 μm in diameter of various contrasts from −100% to +100% centered on the receptive field of the RGC and were presented for 0.5 s. For all experiments using the retinal slice preparation, full field illumination (diameter: 560 μm) was delivered to the tissue from LEDs with peak spectral outputs at 470 or 513 nm.
Analysis
Response amplitudes were quantified by taking the peak current or voltage during the stimulus presentation in figures 2–5 or by integrating the current or voltage throughout the duration of the stimulus presentation in figure 1. F2/F1 ratio (Figure 1B) was computed based on the power of the current traces at the stimulus modulation frequency (F1) and twice the modulation frequency (F2). The noise at each frequency was measured from 2 s of the current trace preceding stimulus onset and subtracted from the power values measured during stimulus presentation. Discriminability of spatial blur patterns (Figure 1C) was determined by a two-alternative forced choice analysis. Each pattern was presented at two different blur values, and discriminability was quantified as the percentage of trials on which the response to the larger blur size exceeded that of the smaller blur size.
Rectification index (RI; Figures 2, 4, 7) was defined as
where r+50 and r−50 were the responses to +50% and −50% contrast. The responses were always of opposite sign, so the index ranged from −1 (complete negative rectification) through 0 (no rectification) to 1 (complete positive rectification).
Two-tailed paired t-tests were used to test significance unless otherwise noted. Pearson’s correlation tests were used to determine if there was significant correlation between the data in Figures 1B and 2C. Because the trends were nonlinear in both cases, we used a logarithmic scaling of data on both axes for Figure 1B and only a logarithmic scaling of luminance data for Figure 2C.
Cell identification
On alpha RGCs, were identified by their large somas (> 15 μm diameter) and their sustained spike responses to a step of light. In some cases, identification was confirmed by dye fills which revealed characteristic alpha cell dendritic morphology with several thick primary dendrites branching several times each and stratifying in sublamina 4 and 5 of the inner plexiform layer. Separate experiments revealed that the cells were labeled by SMI-32, a common feature of alpha cells across mammalian species (Peichl and Boycott, 1987; Bleckert et al., 2014). Rod bipolar cells and AII amacrine cells were identified by soma morphology and electrophysiological characteristics; some were confirmed by dye fills. Cone bipolar cell types were identified by morphology after the recordings, in some cases using the GJD2 transgenic line as a marker of stratification in the inner-plexiform layer (Siegert et al., 2009).
Light adaptation
For experiments at a fixed luminance (Figures 1A,B and 2), data acquisition began after at least 5 minutes of exposure to constant luminance. For experiments in which mean luminance was varied (Fig 1C, Supplemental Figures 1–3. Figures 3–6), data acquisition began 50–100 s (depending on cell type; Figure 5) after a change in mean luminance.
Modeling
A stochastic vesicle release model was created in Igor Pro (WaveMetrics) using parameters described in Jarsky et al. (2011). In short, vesicle release and replenishment rates were calculated in 0.1 ms intervals using the voltage-dependent release curves in Supplemental Figure 4A and a replenishment time constant of 130 ms. For simulations without Cav channel inactivation the probability of release midpoint did not shift with mean voltage, in these simulations we used only the release curve on the far left of Supplemental Figure 4A. The model used 1000 release sites and each event (i.e. miniature excitatory postsynaptic current, mEPSC) had an amplitude of −1, a decay time of 1 ms and a rise time of 0.1 ms. The steady-state Cav channel inactivation (hinf) was modeled as a shift and compression of the release curves and a reduction of available vesicles as described in Jarsky et al. 2011 (Supplemental Figure 4B). Uncorrelated release (B) was also included for mean voltages ≥ −48 mV. For simulating the influence of Cav channel inactivation on synaptic release at a mean voltage of −39 mV (a mean voltage not explicitly reported in Jarsky et al., 2011) we derived values for B and hinf by fitting the explicit data with a sigmoid and taking values from those fits at −39 mV (Supplemental Figure 4B). Additionally, the V1/2 of the release curve used for simulations including Cav channel inactivation at −39 mV was −33.5 mV, a value also extrapolated from their measurements. Differences in experimental conditions - particularly differences in extracellular solutions and uncertainty about the magnitude of RBC voltage changes due to run down during whole cell recordings - precluded a direct comparison of absolute voltages in our RBC recordings and those in the model.
The generalized model of synaptic rectification in Figure 7 uses a probability release curve (when no Cavinactivation is present) from the rod bipolar cell → AII synapse model presented in Figure 6. The synaptic rectification index in Figure 7D was calculated using the average of the peak amplitudes of the presynaptic voltage responses to positive and negative contrast recorded from type 6 cone bipolar cells (Figure 4B).
Cell selection criteria
On alpha RGCs were selected for further analysis based on a sensitivity criterion of 10 spikes in response to a +10% contrast step (0.5 s duration) at 100 R*/rod/s. Recordings from slice preparations were performed within ~3.5 hours of retinal dissection and we specifically targeted neurons that were ≥20 μm below the surface of the slice. These parameters seemed to be particularly important for achieving stable recordings of activity from both the inner and outer retina. Rod bipolar cells were retained for analysis when saturating flashes from darkness produced reliable and robust events both before and after light adaptation. Rod bipolar cell recordings typically lasted 3–4 minutes. On cone bipolar cells were retained when stable baselines could be achieved within <1 minute. Contrast responses in On cone bipolar cells were taken within the first 4 minutes after break-in at 0.5 and/or 100 R*/rod/s. Individual bipolar cells were tested at a maximum of 2 luminance levels (0.5 R*/rod/s and one other value). AII amacrine cell recordings could be targeted particularly deep in the slice (~40–50 μm), and provided stable long lasting recordings (~30 minutes). One AII amacrine cell from WT retina in Figure 3D was excluded from steady-state pharmacological subtraction analysis in Figure 6B due to an obvious jump in holding current just before switching to the solution containing NBQX.
Supplementary Material
Acknowledgments
We thank Jon Cafaro, Jeff Diamond, David Perkel and the anonymous reviewers for helpful comments on earlier versions of the paper, and Mike Ahlquist, Mark Cafaro, Shellee Cunnington and Paul Newman for excellent technical assistance. Support provided by the Helen Hay Whitney Foundation (GWS), HHMI (FR) and NIH (EY11850 to FR).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apostolides PF, Trussell LO. Regulation of interneuron excitability by gap junction coupling with principal cells. Nat Neurosci. 2013;16:1764–1772. doi: 10.1038/nn.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Borges S, Wu SM, Wilson M. Signal clipping by the rod output synapse. Nature. 1987;328:522–524. doi: 10.1038/328522a0. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Barlow HB, Fitzhugh R, Kuffler SW. Change of organization in the receptive fields of the cat’s retina during dark adaptation. J Physiol. 1957;137:338–354. doi: 10.1113/jphysiol.1957.sp005817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgum JH, Copenhagen DR. Synaptic transfer of rod signals to horizontal and bipolar cells in the retina of the toad (Bufo marinus) J Physiol. 1988;396:225–245. doi: 10.1113/jphysiol.1988.sp016960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleckert A, Schwartz GW, Turner MH, Rieke F, Wong RO. Visual space is represented by nonmatching topographies of distinct mouse retinal ganglion cell types. Curr Biol. 2014;24:310–315. doi: 10.1016/j.cub.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, He S. Light adaptation increases response latency of alpha ganglion cells via a threshold-like nonlinearity. Neuroscience. 2013;256C:101–116. doi: 10.1016/j.neuroscience.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Cohen ED. Light-evoked excitatory synaptic currents of X-type retinal ganglion cells. J Neurophysiol. 2000;83:3217–3229. doi: 10.1152/jn.2000.83.6.3217. [DOI] [PubMed] [Google Scholar]
- Deans MR, Volgyi B, Goodenough DA, Bloomfield SA, Paul DL. Connexin36 is essential for transmission of rod-mediated visual signals in the mammalian retina. Neuron. 2002;36:703–712. doi: 10.1016/s0896-6273(02)01046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Haarsma L, Freed MA, Sterling P. Functional circuitry of the retinal ganglion cell’s nonlinear receptive field. J Neurosci. 1999;19:9756–9767. doi: 10.1523/JNEUROSCI.19-22-09756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demb JB, Zaghloul K, Sterling P. Cellular basis for the response to second-order motion cues in Y retinal ganglion cells. Neuron. 2001;32:711–721. doi: 10.1016/s0896-6273(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Dugue GP, et al. Electrical coupling mediates tunable low-frequency oscillations and resonance in the cerebellar Golgi cell network. Neuron. 2009;61:126–139. doi: 10.1016/j.neuron.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Dunn FA, Doan T, Sampath AP, Rieke F. Controlling the gain of rod-mediated signals in the mammalian retina. J Neurosci. 2006;26:3959–3970. doi: 10.1523/JNEUROSCI.5148-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn FA, Rieke F. Single-photon absorptions evoke synaptic depression in the retina to extend the operational range of rod vision. Neuron. 2008;57:894–904. doi: 10.1016/j.neuron.2008.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shamayleh Y, Movshon JA. Neuronal responses to texture-defined form in macaque visual area V2. J Neurosci. 2011;31:8543–8555. doi: 10.1523/JNEUROSCI.5974-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elyada YM, Haag J, Borst A. Different receptive fields in axons and dendrites underlie robust coding in motion-sensitive neurons. Nat Neurosci. 2009;12:327–332. doi: 10.1038/nn.2269. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Lennie P. The control of retinal ganglion cell discharge by receptive field surrounds. J Physiol. 1975;247:551–578. doi: 10.1113/jphysiol.1975.sp010947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. The contrast sensitivity of retinal ganglion cells of the cat. J Physiol. 1966;187:517–552. doi: 10.1113/jphysiol.1966.sp008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow K, et al. Ambient illumination toggles a neuronal circuit switch in the retina and visual perception at cone threshold. Neuron. 2013;78:325–338. doi: 10.1016/j.neuron.2013.02.014. [DOI] [PubMed] [Google Scholar]
- Gollisch T, Meister M. Eye smarter than scientists believed: neural computations in circuits of the retina. Neuron. 2010;65:150–164. doi: 10.1016/j.neuron.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollisch T. Features and functions of nonlinear spatial integration by retinal ganglion cells. J Physiol Paris. 2012 doi: 10.1016/j.jphysparis.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Geffen MN, de Vries SE, Meister M. Retinal ganglion cells can rapidly change polarity from Off to On. PLoS Biol. 2007;5:e65. doi: 10.1371/journal.pbio.0050065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter M, et al. Connectomic reconstruction of the inner plexiform layer in the mouse retina. Nature. 2013;500:168–174. doi: 10.1038/nature12346. [DOI] [PubMed] [Google Scholar]
- Hemila S, Lerber T, Donner K. Noise-equivalent and signal-equivalent visual summation of quantal events in space and time. Vis Neurosci. 1998;15:731–742. doi: 10.1017/s0952523898154123. [DOI] [PubMed] [Google Scholar]
- Hornstein EP, Verweij J, Li PH, Schnapf JL. Gap-junctional coupling and absolute sensitivity of photoreceptors in macaque retina. J Neurosci. 2005;25:11201–11209. doi: 10.1523/JNEUROSCI.3416-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, Tian M, Singer JH. Nanodomain control of exocytosis is responsible for the signaling capability of a retinal ribbon synapse. J Neurosci. 2010;30:11885–11895. doi: 10.1523/JNEUROSCI.1415-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarsky T, et al. A synaptic mechanism for retinal adaptation to luminance and contrast. J Neurosci. 2011;31:11003–11015. doi: 10.1523/JNEUROSCI.2631-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li PH, Verweij J, Long JH, Schnapf JL. Gap-junctional coupling of mammalian rod photoreceptors and its effect on visual detection. J Neurosci. 2012;32:3552–3562. doi: 10.1523/JNEUROSCI.2144-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsenmeier RA, Jakiela HG. Non-linear spatial summation in cat retinal ganglion cells at different background levels. Exp Brain Res. 1979;36:301–309. doi: 10.1007/BF00238913. [DOI] [PubMed] [Google Scholar]
- Manookin MB, Demb JB. Presynaptic mechanism for slow contrast adaptation in mammalian retinal ganglion cells. Neuron. 2006;50:453–464. doi: 10.1016/j.neuron.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Marder E, Bucher D. Understanding circuit dynamics using the stomatogastric nervous system of lobsters and crabs. Annu Rev Physiol. 2007;69:291–316. doi: 10.1146/annurev.physiol.69.031905.161516. [DOI] [PubMed] [Google Scholar]
- Masland RH. The neuronal organization of the retina. Neuron. 2012;76:266–280. doi: 10.1016/j.neuron.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mørkve SH, Veruki ML, Hartveit E. Functional characteristics of non-NMDA-type ionotropic glutamate receptor channels in AII amacrine cells in rat retina. J Physiol. 2002;542:147–165. doi: 10.1113/jphysiol.2002.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Network variability limits stimulus-evoked spike timing precision in retinal ganglion cells. Neuron. 2006;52:511–524. doi: 10.1016/j.neuron.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GJ, Rieke F. Signals and noise in an inhibitory interneuron diverge to control activity in nearby retinal ganglion cells. Nat Neurosci. 2008;11:318–326. doi: 10.1038/nn2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Oesch NW, Diamond JS. Ribbon synapses compute temporal contrast and encode luminance in retinal rod bipolar cells. Nat Neurosci. 2011;14:1555–1561. doi: 10.1038/nn.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, Gao F, Wu SM. Light-evoked excitatory and inhibitory synaptic inputs to ON and OFF alpha ganglion cells in the mouse retina. J Neurosci. 2003;23:6063–6073. doi: 10.1523/JNEUROSCI.23-14-06063.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang JJ, et al. Relative contributions of rod and cone bipolar cell inputs to AII amacrine cell light responses in the mouse retina. J Physiol. 2007;580:397–410. doi: 10.1113/jphysiol.2006.120790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peichl L, Ott H, Boycott BB. Alpha ganglion cells in mammalian retinae. Proc R Soc Lond B Biol Sci. 1987;231:169–197. doi: 10.1098/rspb.1987.0040. [DOI] [PubMed] [Google Scholar]
- Priebe NJ, Ferster D. Mechanisms of neuronal computation in mammalian visual cortex. Neuron. 2012;75(2):194–208. doi: 10.1016/j.neuron.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivlin-Etzion M, Wei W, Feller MB. Visual stimulation reverses the directional preference of direction-selective retinal ganglion cells. Neuron. 2012;76:518–525. doi: 10.1016/j.neuron.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A, Issa NP. The Y cell visual pathway implements a demodulating nonlinearity. Neuron. 2011;71:348–361. doi: 10.1016/j.neuron.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Sampath AP, Rieke F. Selective Transmission of Single Photon Responses by Saturation at the Rod-to-Rod Bipolar Synapse. Neuron. 2004;41:431–443. doi: 10.1016/s0896-6273(04)00005-4. [DOI] [PubMed] [Google Scholar]
- Schmitz D, et al. Axo-axonal coupling. a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- Schwartz GW, Rieke F. Nonlinear spatial encoding by retinal ganglion cells: when 1 + 1 ≠ 2. Journal of General Physiology. 2011;138:283–290. doi: 10.1085/jgp.201110629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GW, et al. The spatial structure of a nonlinear receptive field. Nat Neurosci. 2012;15:1572–1580. doi: 10.1038/nn.3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapley R, Enroth-Cugell C. In: Progress in Retinal Research. Osborne N, Chader G, editors. Vol. 3. Pergamon; London: 1984. pp. 263–346. [Google Scholar]
- Sheffield ME, Best TK, Mensh BD, Kath WL, Spruston N. Slow integration leads to persistent action potential firing in distal axons of coupled interneurons. Nat Neurosci. 2011;14:200–207. doi: 10.1038/nn.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert S, et al. Genetic address book for retinal cell types. Nat Neurosci. 2009;12:1197–1204. doi: 10.1038/nn.2370. [DOI] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Sustained Ca2+ entry elicits transient postsynaptic currents at a retinal ribbon synapse. J Neurosci. 2003;23:10923–10933. doi: 10.1523/JNEUROSCI.23-34-10923.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Diamond JS. Vesicle depletion and synaptic depression at a mammalian ribbon synapse. J Neurophysiol. 2006;95:3191–3198. doi: 10.1152/jn.01309.2005. [DOI] [PubMed] [Google Scholar]
- Traub RD, et al. Gap junctions between interneuron dendrites can enhance synchrony of gamma oscillations in distributed networks. J Neurosci. 2001a;21:9478–9486. doi: 10.1523/JNEUROSCI.21-23-09478.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub RD, et al. A possible role for gap junctions in generation of very fast EEG oscillations preceding the onset of, and perhaps initiating, seizures. Epilepsia. 2001b;42:153–170. doi: 10.1046/j.1528-1157.2001.26900.x. [DOI] [PubMed] [Google Scholar]
- Trenholm S, Schwab DJ, Balasubramanian V, Awatramani GB. Lag normalization in an electrically coupled neural network. Nat Neurosci. 2013a;16:154–156. doi: 10.1038/nn.3308. [DOI] [PubMed] [Google Scholar]
- Trenholm S, McLaughlin AJ, Schwab DJ, Awatramani GB. Dynamic tuning of electrical and chemical synaptic transmission in a network of motion coding retinal neurons. J Neurosci. 2013b;33:14927–14938. doi: 10.1523/JNEUROSCI.0808-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler EB, Li W, Mills SL, Massey SC. Coupling from AII amacrine cells to ON cone bipolar cells is bidirectional. J Comp Neurol. 2001;437:408–422. doi: 10.1002/cne.1292. [DOI] [PubMed] [Google Scholar]
- Trexler EB, Li W, Massey SC. Simultaneous contribution of two rod pathways to AII amacrine and cone bipolar cell light responses. J Neurophysiol. 2005;93:1476–1485. doi: 10.1152/jn.00597.2004. [DOI] [PubMed] [Google Scholar]
- Tsukamoto Y, Morigiwa K, Ueda M, Sterling P. Microcircuits for night vision in mouse retina. J Neurosci. 2001;21:8616–8623. doi: 10.1523/JNEUROSCI.21-21-08616.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veruki ML, Hartveit E. Electrical synapses mediate signal transmission in the rod pathway of the mammalian retina. J Neurosci. 2002;22:10558–10566. doi: 10.1523/JNEUROSCI.22-24-10558.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervaeke K, et al. Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input. Neuron. 2010;67:435–451. doi: 10.1016/j.neuron.2010.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor JD, Shapley RM. The nonlinear pathway of Y ganglion cells in the cat retina. J Gen Physiol. 1979;74:671–689. doi: 10.1085/jgp.74.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.