Abstract
The mammalian lung is a complex organ containing numerous putative stem/progenitor cell populations that contribute to region-specific tissue homeostasis and repair. In this review, we discuss recent advances in identifying and studying these cell populations in the context of lung homeostasis and disease. Genetically engineered mice now allow for lineage tracing of several lung stem and progenitor cell populations in vivo during different types of lung injury repair. Using specific sets of cell surface markers, these cells can also be isolated from murine and human lung and tested in 3D culture systems and in vivo transplant assays. The pathology of devastating lung diseases, including lung cancers, is likely in part due to dysregulation and dysfunction of lung stem cells. More precise characterization of stem cells with identification of new, unique markers; improvement in isolation and transplant techniques; and further development of functional assays will ultimately lead to new therapies for a host of human lung diseases. In particular, lung cancer biology may be greatly informed by findings in normal lung stem cell biology as evidence suggests that lung cancer is a disease that begins in, and may be driven by, neoplastic lung stem cells.
1. INTRODUCTION
The mammalian respiratory system is a highly complex three-dimensional organ historically described as containing over 40 different cell types, each with specialized functions to maintain adequate gas exchange and protect against environmental exposures. During development, the primordial lung undergoes branching morphogenesis to form the proximal conducting airways and distal gas-exchanging alveolar space (Morrisey & Hogan, 2010). The adult murine lung contains several distinct epithelial cell populations with unique anatomical positions and specialized functions (Fig. 8.1). The proximal airway includes the cartilaginous trachea, lined by pseudostratified columnar epithelial cells with submucosal glands interspersed. Noncartilaginous bronchioles, lined with simple columnar epithelium, branch from the trachea in an organized pattern. Secretory Clara cells also line the basement membrane of the airway with ciliated, neuroendocrine, and goblet cell populations (Bertoncello & McQualter, 2013). Lung cell-type terminology is undergoing a transition as the name Clara cell is being replaced by club cell; this review will use the historic term Clara cell. Neuroendocrine cells are present individually as well as in clusters termed neuroendocrine bodies that may play a role in sensing stimuli within the airway lumen (Van Lommel, 2001). Terminal bronchioles lead to the distal alveolar space containing surfactant-producing alveolar type II (AT2) cells and gas-exchanging alveolar type I (AT1) cells (Rock & Hogan, 2011).
Figure 8.1.
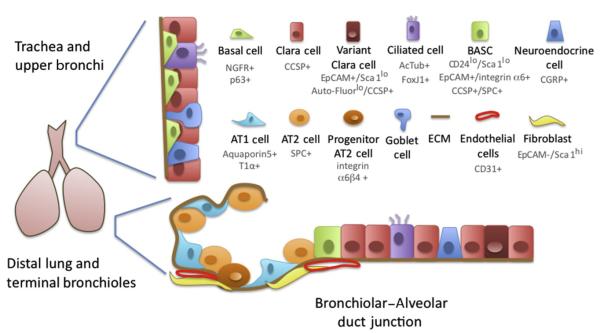
Cell types in the Lung. The proximal region of the murine respiratory system is lined by a pseudostratified epithelium containing secretory CCSP+ Clara cells, mucus-producing goblet cells, and host-defending FoxJ1/Actub+ ciliated cells. Variant Clara cells are thought to give rise to ciliated and Clara cell lineages after injuries such as naphthalene, and are enriched within the EpCAMhi/Sca1lo/Auto-fluorescencelo cells. At the basal edge of the epithelium are the NGFR+/p63+ basal cells, which are thought to be able to give rise to Clara and ciliated cells during repair and in culture. In the more distal bronchioles, ciliated and Clara cells are interspersed with CGRP+ neuroendocrine cells. Alveolar epithelial type 1 cells (AT1 cells), which express T1α and Aquaporin5, and SPC+ alveolar epithelial type 2 cells (AT2 cells) line the alveolar space where gas exchange takes place. An alveolar progenitor cell has been identified that can give rise to AT2 and AT1 cells after injuries such as bleomycin, and is termed integrin α6β4+. At the brochioalveolar duct junction, a rare cell population termed the brochioalveolar stem cells (BASC) coexpresses both CCSP and SPC, and is enriched in the CD24lo/Sca1lo/EpCAM+/integrin α6+ fraction of lung epithelial cells. BASCs are thought to be able to give rise to both Clara and AT2 cell lineages after injury. Alveolar epithelial cells and BASCs are closely associated with mesenchymal cells such as fibroblasts, the extracellular matrix (ECM), and CD31+ endothelial cells. Useful markers for FACS isolation of cell types are indicated.
Diverse experimental approaches have provided evidence that different populations of lung stem/progenitor cells reside in distinct niches and act in region-specific homeostasis and injury repair. Murine mouse models of injury have been utilized to study stem cells because of the low baseline levels of lung cell turnover during homeostasis and the increased rate of proliferation to replace ablated tissue following injury (Rawlins & Hogan, 2006). For example, bleomycin injures the alveolar epithelium, and naphthalene specifically injures the bronchiolar epithelium (Rawlins & Hogan, 2006). For more proximal airway injury, sulfur dioxide inhalation damages the tracheal epithelium (Borthwick, Shahbazian, Krantz, Dorin, & Randell, 2001), while ozone and nitrogen dioxide damage airway epithelial cells (Evans, Johnson, Stephens, & Freeman, 1976; Evans, Shami, Cabral-Anderson, & Dekker, 1986). Using these region-specific epithelial injury mouse models, it is possible to study cellular proliferation and epithelial regeneration. Lineage tracing is another valuable in vivo tool that has been used to study stem cell populations and their role in lung injury and repair without removing them from the lung (Barkauskas et al., 2013; Raiser et al., 2008; Rawlins et al., 2009; Rock et al., 2011; Tropea et al., 2012). Investigators have created mouse models to label stem cell populations of choice, which coupled with the injuries mentioned earlier, allow for detection of the lineage label, which will remain in both the progenitor population and progeny cells after injury repair.
Three-dimensional (3D) culture systems have emerged as an important method of characterizing lung stem cell properties including proliferation, differentiation, and self-renewal (Lee et al., 2012; McQualter, Yuen, Williams, & Bertoncello, 2010; Rock et al., 2009). Fluorescence-activated cell sorting (FACS) can be used to isolate individual stem cell populations (Kim et al., 2005; Lee et al., 2013; McQualter et al., 2009; Rock et al., 2009; Summer, Fitzsimmons, Dwyer, Murphy, & Fine, 2007b; Teisanu, Lagasse, Whitesides, & Stripp, 2009; Zacharek et al., 2011), which can then be grown in clonal 3D assays in the presence of various microenvironmental factors such as Matrigel, nonepithelial cells, and growth factors to assess growth and differentiation properties. An ongoing challenge has been the ability to directly compare the functions of FACS-isolated lung stem cell populations with reparative cells in situ. Limited knowledge of markers distinguishing lung cell types in vivo has prevented precise concordance between the identity of lung cells with stem cell functions in vitro and in vivo. Transplantation assays have also been lacking in lung stem cell biology; currently there is no in vivo transplant assay for freshly sorted stem cells delivered to the lung. Recently, a kidney capsule transplantation model has been utilized as an alternative in vivo method of examining stem cell autonomous properties (Chapman et al., 2011). Subcutaneous injection of multipotent lung cells with Matrigel and the use of ex vivo decellularized lung models have also provided a new means to assess potential progenitor cell function (Longmire et al., 2012; Mou et al., 2012). The development of in vivo or in vitro assays to interrogate the function of stem cells at the single cell level, and the discovery of unique marker sets (rather than single markers) for each lung cell type are critical advances necessary to better identify lung stem cell populations and understand their relative contributions to tissue maintenance and repair.
The pathology of devastating lung diseases including lung cancers, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis (IPF), cystic fibrosis (CF), asthma, bronchopulmonary dysplasia (BPD), and others is likely caused, in part, by dysregulation and dysfunction of lung stem cells. Investigators continue to work to unlock the mystery of the lung stem cell landscape by first identifying the stem cell populations, examining their cell autonomous properties, and trying to better understand their microenvironmental interactions. While much progress has been made over recent years, important questions remain and the complete picture remains unclear. Lung stem cell studies offer a new avenue for developing treatment strategies for lung disease. In this review, we discuss the current knowledge of the lung stem field and the assays and tools used to dissect the complex biology of the lung in homeostasis and disease.
2. ENDOGENOUS LUNG STEM AND PROGENITOR CELLS
Endogenous adult lung stem/progenitor cells are regenerative cell populations important for epithelial cell maintenance and injury repair. In multiple adult organs, tissue-specific stem cells have been identified as multipotent cells with the capacity for long-term self-renewal and the ability to give rise to at least two distinct differentiated lineages. Tissue-specific stem cells are typically quiescent in normal conditions and proliferate during injury repair (Kolios & Moodley, 2013; Potten & Loeffler, 1990), although there are some exceptions such as the proliferative intestinal crypt stem cells (Clevers, 2013). Unipotent stem cells are cells with the ability to self-renew and differentiate into a single restricted lineage (e.g., spermatogonial stem cells). Progenitor cells may be multipotent or unipotent, and they are generally thought to have limited self-renewal capacity. Based on murine studies, it is believed that the lung contains multiple stem cell and progenitor cell populations, some of which reside in distinct regional niches (Bertoncello & McQualter, 2013). Current evidence suggests that different lung stem or progenitor cells may have distinct roles to play during the injury response (e.g., depending on the exact nature or extent of injury) and homeostasis. Microenvironmental signals likely play a key role in triggering specific regenerative functions (Hong, Reynolds, Giangreco, Hurley, & Stripp, 2001), yet they remain to be understood in detail. Perhaps unique from cells of some other organs, lung stem and progenitor cells also tend to have “daily” functional roles in tissue function other than a regenerative role. For example, some lung stem cells secrete proteins critical for gas exchange and become activated to proliferate for a reparative role after injury. The ability to assess self-renewal properties in vivo or in vitro in order to discern lung stem cells from lung progenitor cells is debated and may be influenced by microenvironmental cues. Herein we have attempted to refer to these important cell populations in the lung by the nomenclature used in the primary literature describing them.
In the proximal airway, basal cells have been identified as important stem cells of bronchiolar epithelium. Basal cells can be isolated based on their expression of the markers NGFR (nerve growth factor receptor), p63, cyto-keratin 5 (CK5), cyto-keratin 14 (CK14), and aquaporin 3 (Rock, Randell, & Hogan, 2010). FACS-isolated basal cells (p63+, NGFR+, CK5+) can be cultured in Matrigel and form clonal structures positive for markers of both ciliated and Clara cells. This 3D tracheosphere assay includes the use of growth medium containing EGF (epidermal growth factor), FGF (fibroblast growth factor), and bovine pituitary extract for colony growth. In mouse lineage tracing studies using a CK5–CreER transgenic mouse model, basal cells give rise to ciliated cells in the proximal airways both during homeostasis and after sulfur dioxide inhalation injury (Rock et al., 2009, 2010). Recently, a transient population of p63+/CK5+ cells were reported to migrate from the bronchiolar and peribronchiolar regions and proliferate to repair damaged alveolar lung tissue after H1N1 viral infection in vivo (Kumar et al., 2011). These cells were shown to expand locally into “pods” with an organized spherical shape, and then gain expression of alveolar-specific proteins. It is possible that the p63+/CK5+ migratory cells observed were basal cells, raising the possibility that basal cells can act as progenitors for alveolar epithelium during repair of particular injuries. Another proximal airway stem/progenitor cell population of tracheal submucosal gland duct cells has recently been identified to regenerate submucosal gland tubules, duct cells, and airway surface epithelium after severe hypoxic–ischemic injury (Hegab et al., 2011; Hegab, Nickerson, Ha, Darmawan, & Gomperts, 2012).
In the bronchioles of mouse lungs, two distinct niches have been identified for lung stem cells and progenitor cells. After nitrogen dioxide bronchiolar injury, Clara cells have been shown to give rise to ciliated cells; ciliated cells cannot self-renew (Evans, Cabral-Anderson, & Freeman, 1978; Rawlins & Hogan, 2008). Lineage tracing has shown that Clara cells can self-renew and generate ciliated cells during epithelial homeostasis (Rawlins et al., 2009). Lineage tracing studies labeling calcitonin gene-regulated peptide (CGRP)-expressing neuroendocrine cells showed their ability to self-renew in uninjured adult lung and to differentiate into Clara and ciliated cells after naphthalene injury (Song et al., 2012). The area surrounding the neuroendocrine bodies and the bronchioalveolar duct junction (BADJ) contains a population of Clara cells that are resistant to naphthalene injury. These cells, which have been termed variant Clara cells, can self-renew and differentiate into Clara cells to repair naphthalene bronchiolar epithelial injury (Giangreco, Reynolds, & Stripp, 2002; Reynolds, Giangreco, Power, & Stripp, 2000). The Stripp laboratory identified a population of CD45neg CD31neg CD34neg Epcampos Sca-1low AutoFluorescence (AF)low cells that contained naphthalene-resistant progenitors (Teisanu et al., 2009). In contrast, the AFhigh population contained the naphthalene-sensitive Clara cells (Teisanu et al., 2009). Also located at the BADJ is a population of cells identified as bronchioalveolar stem cells (BASCs) (Kim et al., 2005). BASCs are a rare population of epithelial cells with coexpression of both Clara cell secretory protein (CCSP, also known as Scgb1a1 or CC10) and AT2 cell marker prosurfactant protein C (SPC). BASCs increase in number after naphthalene bronchiolar injury and bleomycin alveolar injury, which suggests their role in repair (Kim et al., 2005). BASCs can be isolated by FACS for cells positive for the stem cell marker Sca-1 and negative for hematopoietic and endothelial cell markers. Recent studies have shown that a robust marker profile for enriching for BASCs utilizes CD45neg CD31neg EpCAMpos Sca-1low CD24low (Zacharek et al., 2011). BASCs self-renew over multiple passages in vitro and differentiate into both bronchiolar and alveolar colonies (Kim et al., 2005; Zacharek et al., 2011). Another similar population was recently isolated with the FACS signature of CD45neg CD31neg EpCAMhigh integrin α6pos CD104pos CD24low, and these cells produce colonies with airway, alveolar, or both airway and alveolar markers in a 3D coculture Matrigel assay with lung mesenchymal cells (McQualter et al., 2010). Work on improving specific cell surface marker expression profiles to better identify and isolate lung stem/progenitor cells is ongoing and necessary to isolate a truly homogenous population. In addition to phenotypic identification, functional assays including lineage tracing, single cell assays, and cell transplantation are necessary to better understand whether the roles of the many putative stem cells in the bronchioles, including Clara cells, variant Clara cells, and BASCs, are distinct during epithelial tissue repair in vivo.
In the distal lung, AT2 cells have long been proposed to be the progenitors of alveolar epithelium (Adamson & Bowden, 1974; Evans, Cabral, Stephens, & Freeman, 1975). Recent work from the Hogan laboratory showed evidence that AT2 cells are stem cells, through genetic lineage tracing experiments and 3D cultures (Barkauskas et al., 2013; Rock et al., 2010). After bleomycin injury, AT2 cells were previously shown to proliferate and were hypothesized to differentiate into AT2 cells and AT1 cells (Aso, Yoneda, & Kikkawa, 1976). Using an SPC-inducible CreER to fate-map AT2 cells, it has been shown that AT2 cells are not the major contributor to alveolar repair after bleomycin injury; instead, a new progenitor cell type has been suggested to be responsible for the majority of the AT2 cell regeneration (Chapman et al., 2011). This integrin α6β4-positive cell population located in the alveolar epithelium has been identified as a progenitor cell population and shown to differentiate into mature SPC positive cells after in vitro colony expansion (Chapman et al., 2011). This cell type is capable of contributing to airway and alveolar structures when transplanted with embryonic lung cells under the kidney capsule in vivo (Chapman et al., 2011). BASCs have also been identified as potential progenitors of alveolar epithelium because of their increased proliferation in vivo after bleomycin injury and their ability to differentiate into SPC-expressing cells in culture (Kim et al., 2005). Lineage tracing studies using a CCSP-CreER mouse model have shown that the percentage of lineage-labeled alveolar cells increased after bleomycin injury (Rock et al., 2011; Tropea et al., 2012). Specifically, after bleomycin lung injury and repair, the percentage of lineage-labeled AT2 and AT1 cells increased, indicating that a CCSP-expressing cell, such as a BASC or Clara cell contributed to alveolar epithelial repair (Rock et al., 2011; Tropea et al., 2012). In homeostasis or following hyperoxia injury, no increase in labeled alveolar cells was seen (Rawlins, Okubo, et al., 2009), suggesting that the CCSP-expressing cell contribution to alveolar repair may be injury-specific and may be dependent on specific microenvironmental factors. Ding et al. (2011) showed that unilateral pneumonectomy stimulates pulmonary capillary endothelial cells to produce angiocrine growth factors VEGFR2 and FGFR1, which are critical to initiate and sustain alveologenesis. This work supports the idea that signals from stromal cells are critical for lung stem cell functions. Together, these studies suggest that bronchiolar cells may be able to contribute to the alveolar lineage(s) under specific conditions and injury repair.
The studies outlined here provide evidence to support region-specific lung stem and progenitor cells in the murine lung; however, much less is known about stem cells in the human lung. Basal cells can be isolated from human lung using the markers NGFR and integrin α6 (Rock et al., 2009) and human AT2 cells have been isolated for many years, most recently using the marker HTII-280 for FACS (Barkauskas et al., 2013). A putative population of c-kit positive human lung stem cells was reported to generate both epithelial and mesodermal lineages in culture (Kajstura et al., 2011). After mouse lung cryoinjury, injected human c-kit positive cells appear capable of contributing to airway, alveoli, and pulmonary vessel repair. These findings present a single multipotent lung stem cell, a concept not widely accepted in the current lung stem cell field. Further characterization and replication using vigorous techniques such as lineage tracing and functional assays are necessary. Recently, a new population of human E-Cad/Lgr6+ putative stem cells has been isolated, expanded, and shown to have self-renewal properties and the ability to differentiate in vitro and in vivo (Oeztuerk-Winder, Guinot, Ochalek, & Ventura, 2012). E-Cad/Lgr6+ single cell transplantations into the kidney capsule produce differentiated bronchioalveolar tissue and retain the ability to self-renew (Oeztuerk-Winder et al., 2012). Human airway epithelial cell cultures have been developed as an important methodology to further study human lung progenitor cells (Fulcher, Gabriel, Burns, Yankaskas, & Randell, 2005). It is critical to continue to work toward linking the exciting mouse lung stem cell discoveries to the human lung and to begin to extrapolate findings to human disease.
3. THE OTHERS: LUNG MESENCHYMAL STROMAL CELLS AND LUNG ENDOTHELIAL PROGENITOR CELLS
Evidence continues to support the idea that adult mesenchymal stromal cells (MSCs) are an important element of epithelial stem/progenitor niches. Critical for regional specification of embryonic lung epithelium, MSCs at the distal tip of the branching epithelium are known to secrete FGF10, a critical component of the signaling network involving Bmp, Wnt, and sonic hedgehog pathways that is necessary for coordinating differentiation in the developing lung (Morrisey & Hogan, 2010). FGF10-positive mesenchymal cells have also been shown to give rise to smooth muscle cells (De Langhe, Carraro, Warburton, Hajihosseini, & Bellusci, 2006; Mailleux et al., 2005; Shan et al., 2008). MSCs have been shown to critically support the proliferation and differentiation of epithelial stem cells in coculture (McQualter et al., 2010). Supporting the importance of this microenvironmental relationship, recent in vivo work used the naphthalene injury model to show that parabronchial mesenchymal cells secrete FGF10 to support epithelial regeneration in the surviving progenitor cells (McQualter et al., 2010).
Several groups have identified resident lung mesenchymal stromal cells (LMSCs) that share some common characteristics with bone marrow-derived MSCs (Bentley et al., 2010; Hennrick et al., 2007; Hoffman et al., 2011; Martin et al., 2008; Summer, Fitzsimmons, Dwyer, Murphy, & Fine, 2007a). LMSCs fulfill the International Society of Cellular Therapy criteria for MSCs, including marker profile and differentiation abilities (Dominici et al., 2006), and are clonogenic when grown in Matrigel with bFGF or HGF (Hegab et al., 2011; Karoubi, Cortes-Dericks, Breyer, Schmid, & Dutly, 2009). Investigators have begun to dissect the role of LMSCs in injury showing that LMSC administration after elastase-induced emphysema resulted in decreased injury and increased survival, likely via a paracrine-mediated anti-inflammatory effect (Hoffman et al., 2011). Ongoing investigation is needed, including in vivo lineage tracing, to better characterize these cells in their in vivo state to test their potential role in homeostasis and injury.
An important cross talk is thought to exist between endothelial cells and epithelial cell progenitors. Angiogenic and angiocrine factor signaling are important in distal lung organogenesis and lung regeneration (Ding et al., 2011; Yamamoto et al., 2007). Coculture of human vascular endothelial cells with a human bronchiolar epithelial cell line in 3D culture has been shown to promote the generation of bronchioalveolar branching structures. This and other ongoing studies suggest a supportive role for endothelium in promoting branching of airway epithelium (Yamamoto et al., 2007). The role of the lung endothelial progenitor cell (EPC) in lung regeneration has begun to be examined, but it has proved difficult because of the inability to discriminate circulating EPCs from bone marrow and tissue resident EPCs. All three types of EPCs may contribute to endothelial cell regeneration and repair of lung injuries such as hyperoxia-induced injury that affect epithelial and endothelial cells (Balasubramaniam et al., 2010; Duong, Erzurum, & Asosingh, 2011). Ongoing work to better classify the in vivo roles of tissue resident EPCs is necessary to better understand their distinct role in development, homeostasis, and injury repair.
4. DIRECTING DIFFERENTIATION: EMBRYONIC STEM CELLS AND INDUCED PLURIPOTENT STEM CELLS
Pluripotent embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells) hold great promise for regeneration of injured tissue and repair of disease states. ES cells are isolated from the inner cell mass of preimplantation blastocysts and under well-defined culture conditions, they can be maintained indefinitely in an undifferentiated state with the ability to give rise to cells of all three embryonic germ layers (Odorico, Kaufman, & Thomson, 2001). Takahashi and Yamanaka discovered that the transcription factors Klf4, Sox2, Oct4, and c-Myc, when introduced into mouse fibroblasts through retroviral transduction, led to clones with pluripotent properties (Takahashi & Yamanaka, 2006) and soon after, reprogrammed human iPS cells were first generated (Takahashi et al., 2007; Yu et al., 2007). The possibility of patient-specific iPS cells offers great hope for generating genetically matched patient-specific lung progenitor cells, with the opportunity for patient-specific drug screening and modeling of human disease.
ES cells resemble the early embryo and investigators have worked toward directed differentiation to lung lineages using a developmental approach. Definitive endoderm progenitor cells of the developing foregut give rise to the differentiated tissue of the thyroid, lung, liver, and pancreas. All lung epithelia must progress through a primordial progenitor stage defined by the onset of expression of homeodomain-containing transcription factor, Nkx2.1 (also known as TTF-1, TITF1), and downregulation of Sox2 along the dorsal–ventral axis of the gut tube (Lazzaro, Price, de Felice, & Di Lauro, 1991; Minoo, Su, Drum, Bringas, & Kimura, 1999; Que, Luo, Schwartz, & Hogan, 2009). Later in development, Sox2 expression increases in the area of the future lung trachea, bronchus, and bronchioles with the Nkx2.1-expressing cells of the embryonic lung giving rise to mature airway epithelium (Que et al., 2009). Sox9, FoxP2, and ID2 are expressed in the distal embryonic lung and mark a multipotent embryonic lung progenitor population (Perl, Kist, Shan, Scherer, & Whitsett, 2005; Rawlins, Clark, Xue, & Hogan, 2009; Shu et al., 2007). Nkx2.1 knock-out mice have lung and thyroid agenesis and malformations of forebrain (Kimura et al., 1996; Minoo et al., 1999). Initial attempts to generate functional lung epithelium from ES cells generated mixed cell populations with the risk of teratoma formation after transplantation because of the remaining undifferentiated pluripotent stem cells (Van Haute et al., 2009). Green et al. (2011) found that dual inhibition of TGF-β and bone morphogenic protein (BMP) signaling after specification of definitive endoderm from pluripotent cells highly enriches anterior foregut endoderm for the next steps in differentiation. Recently, Longmire et al. (2012) and Mou et al. (2012) published important advances and generation of putative lung progenitors from ES cells. Definitive endoderm was derived from mouse ES cells and converted first into foregut endoderm, and then into Nkx2.1+ lung endoderm using precisely-timed BMP, FGF, and WNT signaling. Nkx2.1+ lung endoderm was then converted into multipotent embryonic lung progenitor and airway progenitor cells with the formation of tracheospheres when subcutaneously transplanted into nude mice (Mou et al., 2012). Similarly, Longmire et al. (2012) purified and directed differentiation of ES cells via initial inhibition of TGF-β and BMP signaling, and then via stimulation of BMP and FGF signaling to form definitive endodermal precursors able to recellularize a 3D lung scaffold.
iPS cells can be induced in culture to develop into definitive endoderm, but it has also proved difficult to completely differentiate iPS into lung progenitor cells with phenotypic markers of differentiated epithelium. Several groups have produced patient-specific iPS cells to date, and Mou et al. (2012) have produced human disease-specific immature airway epithelium derived from CF iPS cells. The Rossant laboratory has shown directed differentiation of human pluripotent stem cells into mature airway epithelium that express functional cystic fibrosis transmembrane conductance regulator protein (Wong et al., 2012). Work to better understand the details of ES cell- and iPS cell-directed differentiation in the pulmonary field is ongoing, showing great progress and promise.
5. MSCs: POTENTIAL ROLE OF CELL-BASED THERAPY IN LUNG DISEASE
MSCs have been recently examined as a potential cell-based therapy for lung disease. MSCs secrete growth factors and antimicrobial peptides, have immunomodulatory properties, and exhibit low immunogenicity (Lee, Fang, Krasnodembskaya, Howard, & Matthay, 2011). MSCs can respond, migrate, and facilitate repair of damaged tissue making them an attractive candidate for both prevention and treatment of lung disease. MSCs can be isolated from a variety of human tissues including bone marrow, adipose tissue, and placenta. The International Society of Cellular Therapy defined MSCs in 2006 by three standard criteria: (1) They must adhere to plastic; (2) they must express cell surface markers CD73, CD90, and CD105 and must not express CD45, CD34, CD14, and CD11b; and (3) they must have the capacity to differentiate into osteoblasts, adipocytes, and chondroblasts under in vitro conditions (Dominici et al., 2006). Paracrine-modulating factors secreted by MSCs appear to be responsible for injury repair rather than MSC engraftment (Lee et al., 2011). Recent evidence suggests that in addition to releasing soluble anti-inflammatory factors, the MSCs transfer microvesicles containing mitochondria, protein, and microRNA to other cells (Islam et al., 2012; Lee et al., 2012). MSC-based therapy has been studied in several important and devastating lung diseases, with promising results outlined here.
BPD is a disease with high morbidity and mortality that affects premature infants. BPD leads to distal airway epithelial cell simplification and vascular injury, and most current treatments are palliative (Ghanta, Leeman, & Christou, 2013). Bone marrow-derived MSCs and umbilical cord-derived MSCs have been shown to ameliorate lung injury in hyperoxia mouse models of BPD (Aslam et al., 2009; van Haaften et al., 2009; Vosdoganes, Lim, Moss, & Wallace, 2012). Low levels of MSC cell engraftment and an even greater preventative effect with delivery of cell-free MSC-conditioned media support the importance of a paracrine effect. A possible mechanism of action is stimulation of endogenous lung stem/progenitor cells by MSC-secreted factors, triggering their role in epithelial cell repair (Tropea et al., 2012). The overall mechanisms or specific factors responsible for amelioration of hyperoxia-induced lung injury remain active areas of investigation. No clinical trials have been completed in the United States, but at the time of writing, an open-label, single-center, phase 1 clinical study is underway to evaluate the safety and efficacy of umbilical cord-derived MSC treatment in premature infants with BPD. Ongoing preclinical work is necessary to better understand the mechanisms and to build upon current findings.
The therapeutic potential of MSCs has been tested and holds promise in studies of several other lung diseases such as pulmonary hypertension (PH), acute lung injury (ALI), and pulmonary fibrosis. PH is a progressive disease with ongoing endothelial dysfunction and vascular remodeling. In addition to EPCs as potential therapeutic cells for PH, the efficacy of MSC administration has been demonstrated in murine models of PH (Babar et al., 2007; Lee et al., 2012). MSCs genetically engineered to overexpress endothelial nitric oxide synthase, prostacyclin, or heme oxygenase 1 had even greater reversal effects on PH (Kanki-Horimoto et al., 2006; Liang et al., 2011). Several studies in animal models of ALI and also in a model of ALI in explanted human lungs have shown the beneficial effects of MSC administration, with increased survival, less lung damage, and decreased proinflammatory and increased anti-inflammatory cytokines (Gupta et al., 2007; Lee, Fang, Gupta, Serikov, & Matthay, 2009; Mei et al., 2007; Xu et al., 2007). IPF is a progressive disease currently without any effective therapy. MSC delivery to the bleomycin injury IPF mouse model has shown low levels of MSC engraftment, but significant improvement in lung injury (Aguilar et al., 2009; Kumamoto, Nishiwaki, Matsuo, Kimura, & Matsushima, 2009; Ortiz et al., 2003). Ongoing work continues to explore the possible benefits of treatment with MSCs for these lung diseases with high morbidity and mortality.
MSCs have been utilized in several clinical trials for immune- and inflammatory-related diseases and although efficacy has varied, no safety issues have arisen (Giordano, Galderisi, & Marino, 2007; Uccelli, Moretta, & Pistoia, 2008). Potential concerns of tumor or ectopic tissue formation have not been reported. A recent placebo-controlled, randomized, multicenter trial examined treatment with systemic MSCs for COPD and showed neither any adverse effects nor any differences in pulmonaryfunction tests or quality of life indicators (Weiss, Casaburi, Flannery, Leroux-Williams, & Tashkin, 2013). This clinical trial showed important safety data, and it provides a basis for further clinical trials to test the efficacy of MSC treatment in respiratory diseases. Extensive work continues to examine the role of treatment with MSCs in lung disease in both mouse models of respiratory disease and human clinical trials.
6. STEM CELLS IN LUNG CANCER
Lung cancer is the leading cause of cancer related deaths in the United States and worldwide (Ettinger et al., 2013; Herbst, Heymach, & Lippman, 2008). In the United States alone, nearly 200,000 people will be diagnosed with lung cancer in 2013, and the predicted 5-year survival rate for all patients is a dismal 15% (Ettinger et al., 2013). Evidence suggests that lung cancer may originate from neoplastic lung stem/progenitor cell populations and that certain lung tumors contain cancer stem cells (CSCs). Through mouse models and molecular characterization of lung tumors, research aims to understand the molecular changes that lead to lung cancer development and to find novel ways to target lung cancer and lung CSCs.
Lung cancer is subdivided into two major groups: small cell and nonsmall cell lung cancers (Travis, Brambilla, & Riely, 2013). Small cell lung cancer (SCLC) accounts for ~15% of lung malignancies and is characterized by neuroendocrine cell morphology and gene expression. Nonsmall cell lung cancers (NSCLC) account for the remaining ~85% of lung malignances. Within the NSCLC group, at least three distinct histological subtypes exist: adenocarcinoma, squamous cell carcinoma, and large cell carcinoma. Adenocarcinoma is the predominant subtype, accounting for approximately 50% of NSCLCs. Adenocarcinomas have histology and biomarker expression consistent with distal lung, including expression of CK14, surfactant proteins, and amplification of the transcription factor Nkx2.1 (Herbst et al., 2008; Imielinski et al., 2012; Travis et al., 2013). Squamous cell carcinoma is the next most prevalent subtype, with ~40% of NSCLC patients receiving this diagnosis. Squamous cell carcinomas in the lung are characterized by squamous differentiation, which is most reminiscent of normal basal and secretory cell subtypes found in the more proximal lung and trachea (Herbst et al., 2008; TCGA, 2012; Travis et al., 2013; Wilkerson et al., 2010). Squamous cell carcinomas often express p63, CK5, and the transcription factor Sox2. Lastly, large cell carcinoma accounts for approximately 10% of NSCLCs. Some tumors have areas of histology that represent two or all three subtypes and, often, if the subtypes are commixed, the pathologist will term the lesion “adeno-squamous” (Travis et al., 2013). While smoking is associated with all types of lung tumors, it is most highly correlated with small cell and squamous cell carcinomas (Govindan et al., 2012; Sun, Schiller, & Gazdar, 2007).
Even within clonal tumor populations, it is believed that not all cells are equal in their ability to initiate or propagate disease. Both tumor recurrence following treatment and establishment of tumors at metastatic sites have been attributed to growth and survival of CSCs. Generally stated, the CSC hypothesis predicts that tumors contain a population of cells that are able to self-renew and differentiate, thus giving rise to the phenotypically distinct cells found within the tumor population (Nguyen, Vanner, Dirks, & Eaves, 2012). This hypothesis, coupled with emerging evidence that CSCs are relatively resistant to standard chemotherapies, has led to the theory that identification of CSCs in each type of tumor would enable development of treatments to specifically target the cells responsible for recurrent and metastatic disease (Li, Tiede, Massague, & Kang, 2007). Whether different oncogenic drivers, different cells of origin, or a combination of the two contribute to the various subtypes of lung tumors is unclear. The idea that stem and progenitor cells are tumor “cells of origin” is attractive because stem cells possess inherent self-renewal capacity and do not need to reawaken self-renewal programs during neoplastic transformation (Visvader, 2011). However, an equally plausible explanation for how tumor cells with the capacity to self-renew arise is that a more differentiated cell acquires the capacity for self-renewal through genetic and/or epigenetic mechanisms. Currently in debate is whether lung tumors are more likely to arise from stem or progenitor populations, and if CSCs, when present, maintain phenotypic resemblance to the cell of origin or the normal stem/progenitor cells of the lung.
CSCs are defined functionally by their ability to seed tumors in xenograft or allograft settings. Studies of human lung CSCs are hindered by genetic complexity and limited sample availability of fresh patient tissue. In efforts to expand viable cells for study, adherent lung cancer cell lines have been established from over 200 primary lung cancers (Gazdar, Girard, Lockwood, Lam, & Minna, 2010). The lines appear to recapitulate many features of the primary tumors from which they were derived, including amplification of genes such as Nkx2.1 and Sox2 (Bass et al., 2009; Tanaka et al., 2007; Wistuba et al., 1999). Many studies using human cell lines, or cell line-derived xenografts, have allowed for testing novel therapeutics, and tumor initiation and progression. Drug resistance was modeled efficiently in lung cancer cell lines and found to be mediated through selection of cells within the population that showed altered chromatin state and signaling properties (Sharma et al., 2010). In addition, cell lines have been utilized for several high-throughput synthetic lethal screens to identify novel druggable targets in defined genetic backgrounds (Barbie et al., 2009). However, the utility of cell lines for CSC assays remains in question; therefore, using primary human lung cancer samples remains the goal for many researchers. Patient-derived xenografts (PDXs), where a fresh or cryopreserved piece of resected lung tumor is embedded onto the side of an immunocompromised mouse, help to expand the numbers of cells available from a single tumor for analysis. One PDX study showed that cell surface marker CD166 and the gene Lin28B mark cells enriched for CSC activity in NSCLC; further characterization of this cell population is pending (Zhang et al., 2012). Other studies suggest that CD133 could enrich for human lung CSC activity, though reports on the utility of this marker are conflicting, (Bertolini et al., 2009; Eramo et al., 2008; Meng, Li, Wang, Wang, & Ma, 2009). Aldehyde dehydrogenase activity has also been shown to enrich for lung cancer cells with increased tumorigenic potential (Jiang et al., 2009; Sullivan et al., 2010).
The ability to produce transgenic mice has transformed the cancer research field by allowing for precise control of the genetic drivers of model tumors. The first genetically engineered mouse model (GEMM) of lung cancer, in which oncogenic Kras-G12D can be specifically induced in the murine lung, was described in 2001 (Jackson et al., 2001; Johnson et al., 2001). This model allows for activation of oncogenic Kras by Cre recombinase-mediated excision of the Lox-Stop-Lox cassette preceding the Kras oncogene. Lung specificity is obtained through administration of Cre-expressing viral vector directly to the cells of the airways through inhalation. The Kras-G12D mouse has been bred to many conditional knock-out allele backgrounds, including the tumor suppressor p53, yielding Kras-G12D/p53-null tumors (Jackson et al., 2005). These Kras-G12D/p53-null tumors are more advanced in stage than tumors from the Kras-G12D mice. In similar conditional systems, lung-specific promoters such as CCSP can be used to drive expression of transgenes in a doxycycline-inducible manner. This system has been particularly useful for expression of specific point mutants of epidermal growth factor receptor (EGFR) that were identified in patient samples (Ji et al., 2006; Politi et al., 2006).
GEMMs of lung cancer have allowed for numerous studies that would not have been possible to complete with patient samples. They have been used successfully for preclinical or coclinical trials of targeted therapies for lung cancer such as inhibition of EGFR (Ji et al., 2006; Zhou et al., 2009). GEMMs have also proved useful for studying metastatic spread of the disease (Carretero et al., 2010; Ji et al., 2007; Winslow et al., 2011). Winslow et al. demonstrated in the Kras-G12D/p53-null mice that primary tumors that downregulated Nkx2.1 expression were the tumors that seeded metastases in distant organs. Lastly, GEMMs have allowed for a comprehensive study of drug resistance mechanisms (Oliver et al., 2010). However, to date, GEMMs of lung cancer appear to predominantly represent the adenocarcinoma subtype of the disease. One exception is the Kras-G12D/LKB1-null mouse model, which appears to drive tumors of adenocarcinoma, squamous cell carcinoma, and large cell phenotypes (Ji et al., 2007). Recently, a transgenic mouse that expresses a kinase-dead IKKα transgene in all tissues was shown to develop spontaneous lung squamous cell carcinomas (Xiao et al., 2013). However, no lung-specific tumor suppressor loss or oncogene expression has been able to produce solely squamous cell carcinoma in the mouse.
Experimental data from GEMMs suggest that stem and progenitor cells are primary targets for transformation in the lung and that distinct lung cancer subtypes arise from different lung progenitor populations. The mouse model consisting of lung-specific inactivation of both p53 and Rb (p53-null/Rb-null) leads to highly penetrant SCLC (Park et al., 2011; Sutherland et al., 2011). Because SCLC has neuroendocrine features, it was long proposed that these tumors arise from neuroendocrine bodies within the lung, which are thought to contain their own stem cell population. In support of this cell of origin theory, in the p53-null/Rb-null model infected with Adeno-Cre, the majority of early lesions were composed of proliferating neuroendocrine cells (Park et al., 2011). Likewise, when a variety of cell type-specific Cre viruses were used, it was found that only a virus able to infect neuroendocrine or SPC-expressing cells efficiently drove small cell tumor formation (Sutherland et al., 2011). Furthermore, deletion of Rb and p53 by a Cre driven by the endogenous neuroendocrine gene CGRP was sufficient for SCLC development (Song et al., 2012). For models of adenocarcinoma, the SPC and CCSP-expressing BASCs were shown to be the first cells to proliferate in response to oncogenic Kras activation (Kim et al., 2005). However, lineage tracing studies also suggest that AT2 cells can be the cells of origin for Kras-expressing adenocarcionomas (Xu et al., 2012). For squamous cell carcinoma, It has been proposed that the basal cells of the trachea are the cells of origin (Lu et al., 2010), though the lack of GEMM to recapitulate this disease subtype prevents direct testing of this hypothesis. Expression of Sox2 directed by an inducible CCSP-driven Cre recombinase was able to induce lung tumors that expressed the marker p63, but histologically, these tumors were adenocarcinomas (Lu et al., 2010). This intriguing finding suggests that the CCSP-Cre allele targets a subset of cells, possibly variant Clara cells and BASCs, that are unable to produce a fully squamous phenotype despite expression of a squamous cell carcinoma transcription factor. If a transgenic mouse strain is designed that can specifically target basal cells, it may be possible to drive squamous cell carcinoma formation. Furthermore, derivation of BASC- or variant Clara cell-specific Cre mice will help resolve questions regarding cells of origin for adenocarcinomas.
GEMMs have also allowed the systematic study of CSC phenotypes by allowing for production of virtually unlimited genetically homogenous primary tumor samples. The gold standard for testing CSC potential involves injecting sorted, or otherwise enriched and depleted, tumor cell populations into immunocompromised or syngenic mouse recipients. In order to allow the transplanted tumor cells to engraft into the lung, they are often injected into the tail vein of the mouse, or delivered to the lung by intratracheal (IT) instillation. Using a serial IT transplantation assay, it was demonstrated that the BASC marker Sca-1 enriched for the tumor-propagating potential of the Kras-G12D/p53-null tumors (Fig. 8.2) (Curtis et al., 2010). However, in lung tumor models that rely only upon Kras or EGFR activation, this same sorting strategy did not show consistent CSC enrichment. This research exemplifies the importance of oncogenotype in determining the phenotype of CSC populations. It appears that genotype has a direct influence on whether CSCs retain cell-of-origin marker expression as the cancer evolves. These findings also exemplify the importance of functionally validating the tumor-propagating capacity of sorted populations in each new tumor type under study. Further experiments are needed to understand the importance of Sca-1 or other lung stem cell markers in defining tumor-propagating or metastatic tumor cell populations in each GEMM of lung cancer.
Figure 8.2.
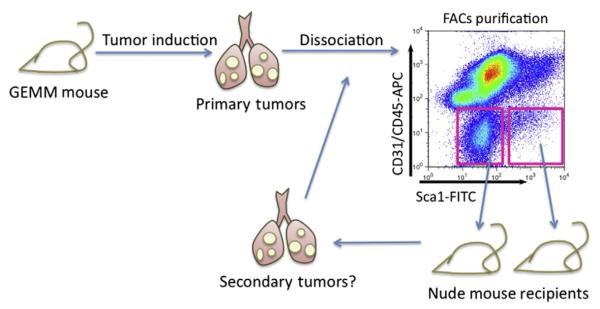
Orthotopic serial transplant assay for lung cancer stem cells. Using genetically engineered mouse models (GEMMs) of lung cancer, tumors with defined genetic characteristics can be induced. These primary tumors can be resected, enzymatically dissociated, and sorted, based on cell surface marker expression. Fluorescence-activated cell sorting (FACS) is typically used to separate tumor cell types. Antibodies against CD31 and CD45 can be used to exclude endothelial cells and hematopoietic cells, respectively, while the remaining tumor cell population can be fractionated using putative cancer stem cell markers such as Sca-1 (x-axis of representative FACS plot shown). FACS-isolated populations can then be introduced into the lungs of immunocompromised mice, such as nude mice, and cancer stem cell activity can be measured by secondary tumor growth. As cancer stem cells are thought to have self-renewal ability, a true cancer stem cell pool should be able to propagate tumors in a serial manner.
7. FUTURE DIRECTIONS
Identification and characterization of the various stem and progenitor cell populations in the lung will allow for the study of the mechanisms through which these cells are maintained and stimulated. Because the lung is an essential organ, understanding these mechanisms and exploiting them therapeutically for lung regenerative medicine holds great promise for improving public health. Research will likely focus now on identification of unique cell surface markers that can be used to enrich for populations by FACS, as well as both in vitro and in vivo assays to fully examine the self-renewal and differentiation potential of each population. Extrapolation of findings in the mouse lung to the human, as well as the design of new mouse strains for lineage-specific gene control will be essential as this field matures. In parallel, the molecular characterization of lung stem cell markers in lung tumors will allow for testing of the CSC hypothesis in the various subtypes and genetic backgrounds of lung tumors. These advances will pave the way toward more personalized lung cancer treatment regimens and a deeper understanding of all lung disease.
ACKNOWLEDGMENTS
We thank members of our laboratory for their discussions and critical comments on the chapter. Work in our laboratory is supported by the Post-Doctoral Fellowship, PF-12-151-01-DMC, from the American Cancer Society (CMF), the Ikaria Advancing Newborn Medicine Grant (KTL), American Medical Association Foundation SEED Grant (KTL), 5 T32HD7466-15 (KTL), RO1 HL090136, U01 HL100402 RFA-HL-09-004, American Cancer Society Research Scholar Grant RSG-08-082-01-MGO, the V Foundation for Cancer Research, a Basil O’Conner March of Dimes Starter Award, and the Harvard Stem Cell Institute (CFK).
REFERENCES
- Adamson I, Bowden D. The type 2 cell as progenitor of alveolar epithelial regeneration. A cytodynamic study in mice after exposure to oxygen. Laboratory Investigation. 1974;30:35–42. [PubMed] [Google Scholar]
- Aguilar S, Scotton C, McNutty K, Nye E, Stamp G, Laurent G, et al. Bone marrow stem cells expressing keratinocyte growth factor via an inducible lentivirus protects against bleomycin-induced pulmonary fibrosis. PLoS One. 2009;4(11):e8013. doi: 10.1371/journal.pone.0008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslam M, Baveja R, Liang O, Fernandez-Gonzalez A, Lee C, Mitsialis S, et al. Bone marrow stromal cells attenuate lung injury in a murine model of neonatal chronic lung disease. American Journal of Respiratory and Critical Care Medicine. 2009;180(11):1122–1130. doi: 10.1164/rccm.200902-0242OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Yoneda K, Kikkawa Y. Morphologic and biochemical study of pulmonary changes induced by bleomycin in mice. Laboratory Investigation. 1976;35:558–568. [PubMed] [Google Scholar]
- Baber S, Deng W, Master R, Bunnell B, Taylor B, Murthy S, et al. Intratracheal mesenchymal stem cell administration attenuates monocrotaline-induced pulmonary hypertension and endothelial dysfunction. American Journal of Physiology. Heart and Circulatory Physiology. 2007;292(2):H1120–H1128. doi: 10.1152/ajpheart.00173.2006. [DOI] [PubMed] [Google Scholar]
- Balasubramaniam V, Ryan S, Seedorf G, Roth E, Heumann T, Yoder M, et al. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2010;298(3):L315–L323. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, et al. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkauskas C, Cronce M, Rackley C, Bowie E, Keene D, Stripp B, et al. Type 2 alveolar cells are stem cells in adult lung. The Journal of Clinical Investigation. 2013;123(7):3025–3036. doi: 10.1172/JCI68782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nature Genetics. 2009;41(11):1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley J, Popova A, Bozyk P, Linn M, Baek A, Lei J, et al. Ovalbumin sensitization and challenge increases the number of lung cells possessing a mesenchymal stromal cell phenotype. Respiratory Research. 2010;11:127. doi: 10.1186/1465-9921-11-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolini G, Roz L, Perego P, Tortoreto M, Fontanella E, Gatti L, et al. Highly tumorigenic lung cancer CD133+ cells display stem-like features and are spared by cisplatin treatment. Proceedings of the National Academy of Sciences. 2009;106(38):16281–16286. doi: 10.1073/pnas.0905653106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertoncello I, McQualter J. Lung stem cells: Do they exist? Respirology. 2013;18(4):587–595. doi: 10.1111/resp.12073. [DOI] [PubMed] [Google Scholar]
- Borthwick D, Shahbazian M, Krantz Q, Dorin J, Randell S. Evidence for stem-cell niches in the tracheal epithelium. American Journal of Respiratory Cell and Molecular Biology. 2001;24:662–670. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- Carretero J, Shimamura T, Rikova K, Jackson AL, Wilkerson MD, Borgman CL, et al. Integrative Genomic and Proteomic Analyses Identify Targets for Lkb1-Deficient Metastatic Lung Tumors. Cancer Cell. 2010;17(6):547–559. doi: 10.1016/j.ccr.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman H, Li X, Alexander J, Brumwell A, Lorizio W, Tan K, et al. Integrin α6β4 identifies an adult distal lung epithelial population with regenerative potential in mice. The Journal of Clinical Investigation. 2011;121(7):2855–2862. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. Stem cells: A unifying theory for the crypt. Nature. 2013;495(7439):53–54. doi: 10.1038/nature11958. [DOI] [PubMed] [Google Scholar]
- Curtis SJ, Sinkevicius KW, Li D, Lau AN, Roach RR, Zamponi R, et al. Primary tumor genotype is an important determinant in identification of lung cancer propagating cells. Cell Stem Cell. 2010;7(1):127–133. doi: 10.1016/j.stem.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Langhe S, Carraro G, Warburton D, Hajihosseini M, Bellusci S. Levels of mesenchymal FGFR2 signaling modulate smooth muscle progenitor cell commitment in the lung. Developmental Biology. 2006;299(1):52–62. doi: 10.1016/j.ydbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Ding B, Nolan D, Guo P, Babazadeh A, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147(3):539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- Duong H, Erzurum S, Asosingh K. Pro-angiogenic hematopoietic progenitor cells and endothelial colony-forming cells in pathological angiogenesis of bronchial and pulmonary circulation. Angiogenesis. 2011;14:411–422. doi: 10.1007/s10456-011-9228-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eramo A, Lotti F, Sette G, Pilozzi E, Biffoni M, Di Virgilio A, et al. Identification and expansion of the tumorigenic lung cancer stem cell population. Cell Death Differ. 2008;15(3):504–514. doi: 10.1038/sj.cdd.4402283. [DOI] [PubMed] [Google Scholar]
- Ettinger DS, Akerley W, Borghaei H, Chang AC, Cheney RT, Chirieac LR, et al. Non-small cell lung cancer, version 2.2013. Journal of the National Comprehensive Cancer Network. 2013;11(6):645–653. doi: 10.6004/jnccn.2013.0084. [DOI] [PubMed] [Google Scholar]
- Evans M, Cabral L, Stephens R, Freeman G. Transformation of alveolar type 2 cells to type 1 cells following exposure to NO2. Experimental and Molecular Pathology. 1975;22(1):142–150. doi: 10.1016/0014-4800(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Evans M, Cabral-Anderson L, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Laboratory Investigation. 1978;38(6):648–653. [PubMed] [Google Scholar]
- Evans M, Johnson L, Stephens R, Freeman G. Renewal of the terminal bronchiolar epithelium in the rat following exposure to NO2 or O3. Laboratory Investigation. 1976;35(3):246–257. [PubMed] [Google Scholar]
- Evans M, Shami S, Cabral-Anderson L, Dekker N. Role of nonciliated cells in renewal of the bronchial epithelium of rats exposed to NO2. The American Journal of Pathology. 1986;123(1):126–133. [PMC free article] [PubMed] [Google Scholar]
- Fulcher M, Gabriel S, Burns K, Yankaskas J, Randell S. Well-differentiated human airway epithelial cell cultures. Methods in Molecular Medicine. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Gazdar AF, Girard L, Lockwood WW, Lam WL, Minna JD. Lung cancer cell lines as tools for biomedical discovery and research. Journal of the National Cancer Institute. 2010;102(17):1310–1321. doi: 10.1093/jnci/djq279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanta S, Leeman K, Christou H. An update on pharmacologic approaches to bronchopulmonary dysplasia. Seminars in Perinatology. 2013;37(2):115–123. doi: 10.1053/j.semperi.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giangreco A, Reynolds S, Stripp B. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchioalveolar duct junction. The American Journal of Pathology. 2002;161(1):173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Galderisi U, Marino I. From the laboratory bench to the patient’s bedside: An update on clinical trials with mesenchymal stem cells. Journal of Cellular Physiology. 2007;211:27–35. doi: 10.1002/jcp.20959. [DOI] [PubMed] [Google Scholar]
- Green M, Chen A, Nostro M, d’Souza S, Lemischka I, Gouon-Evans V, et al. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature Biotechnology. 2011;29(3):267–272. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindan R, Ding L, Griffith M, Subramanian J, Dees ND, Kanchi KL, et al. Genomic Landscape of Non-Small Cell Lung Cancer in Smokers and Never-Smokers. Cell. 2012;150(6):1121–1134. doi: 10.1016/j.cell.2012.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta N, Su X, Popov B, Lee J, Serikov V, Matthay M. Intrapulmonary delivery of bone marrow-derived mesenchymal stem cells improves survival and attenuates endotoxin-induced acute lung injury in mice. The Journal of Immunology. 2007;179:1855–1863. doi: 10.4049/jimmunol.179.3.1855. [DOI] [PubMed] [Google Scholar]
- Hegab A, Ha V, Gilbert J, Zhang K, Malkoski S, Chon A, et al. Novel stem/progenitor cell population from murine tracheal submucosal gland ducts with multipotent regenerative potential. Stem Cells. 2011;29(8):1283–1293. doi: 10.1002/stem.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegab A, Nickerson D, Ha V, Darmawan D, Gomperts B. Repair and regeneration of tracheal surface epithelium and submucosal glands in a mouse model of hypoxic-ischemic injury. Respirology. 2012;17(7):1101–1113. doi: 10.1111/j.1440-1843.2012.02204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennrick K, Keeton A, Nanua S, Kijek T, Goldsmith A, Sajjan U, et al. Lung cells from neonates show a mesenchymal stem cell phenotype. American Journal of Respiratory and Critical Care Medicine. 2007;175(11):1158–1164. doi: 10.1164/rccm.200607-941OC. [DOI] [PubMed] [Google Scholar]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. New England Journal of Medicine. 2008;359(13):1367–1380. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman A, Paxson J, Mazan M, Davis A, Tyagi S, Murthy S, et al. Lung-derived mesenchymal stromal cell post-transplantation survival, persistence, paracrine expression, and repair of elastase-injured lung. Stem Cells and Development. 2011;20(10):1779–1792. doi: 10.1089/scd.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K, Reynolds S, Giangreco A, Hurley C, Stripp B. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. American Journal of Respiratory Cell and Molecular Biology. 2001;24(6):671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the Hallmarks of Lung Adenocarcinoma with Massively Parallel Sequencing. Cell. 2012;150(6):1107–1120. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M, Das S, Emin M, Wei M, Sun L, Westphalen K, et al. Mitochondrial transfer from bone marrow derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes & Development. 2001;15(24):3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EL, Olive KP, Tuveson DA, Bronson R, Crowley D, Brown M, et al. The Differential Effects of Mutant p53 Alleles on Advanced Murine Lung Cancer. Cancer Research. 2005;65(22):10280–10288. doi: 10.1158/0008-5472.CAN-05-2193. [DOI] [PubMed] [Google Scholar]
- Ji H, Li D, Chen L, Shimamura T, Kobayashi S, McNamara K, et al. The impact of human EGFR kinase domain mutations on lung tumorigenesis and in vivo sensitivity to EGFR-targeted therapies. Cancer Cell. 2006;9(6):485–495. doi: 10.1016/j.ccr.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Ji H, Ramsey MR, Hayes DN, Fan C, McNamara K, Kozlowski P, et al. LKB1 modulates lung cancer differentiation and metastasis. Nature. 2007;448(7155):807–810. doi: 10.1038/nature06030. [DOI] [PubMed] [Google Scholar]
- Jiang F, Qiu Q, Khanna A, Todd NW, Deepak J, Xing L, et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Molecular Cancer Research. 2009;7(3):330–338. doi: 10.1158/1541-7786.MCR-08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L, Mercer K, Greenbaum D, Bronson RT, Crowley D, Tuveson DA, et al. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature. 2001;410(6832):1111–1116. doi: 10.1038/35074129. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Rota M, Hall SR, Hosoda T, D’Amario D, Sanada F, et al. Evidence for human lung stem cells. The New England Journal of Medicine. 2011;364(19):1795–1806. doi: 10.1056/NEJMoa1101324. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kanki-Horimoto S, Horimoto H, Mieno S, Kishida K, Watanabe F, Furuya E, et al. Implantation of mesenchymal stem cells overexpressing endothelial nitric oxide synthase improves right ventricular impairments caused by pulmonary hypertension. Circulation. 2006;114:I181–I185. doi: 10.1161/CIRCULATIONAHA.105.001487. [DOI] [PubMed] [Google Scholar]
- Karoubi G, Cortes-Dericks L, Breyer I, Schmid R, Dutly A. Identification of mesenchymal stromal cells in human lung parenchyma capable of differentiating into aquaporin 5-expressing cells. Laboratory Investigation. 2009;89:1100–1114. doi: 10.1038/labinvest.2009.73. [DOI] [PubMed] [Google Scholar]
- Kim C, Jackson E, Woolfenden A, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121(6):823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kimura S, Hara Y, Pineau T, Fernandez-Salguero P, Fox C, Ward J, et al. The T/ebp null mouse: Thyroid-specific enhancer-binding protein is essential for the organogenesis of the thyroid, lung, ventral forebrain, and pituitary. Genes & Development. 1996;10:60–69. doi: 10.1101/gad.10.1.60. [DOI] [PubMed] [Google Scholar]
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85:3–10. doi: 10.1159/000345615. [DOI] [PubMed] [Google Scholar]
- Kumamoto M, Nishiwaki T, Matsuo N, Kimura H, Matsushima K. Minimally cultured bone marrow mesenchymal stem cells ameliorate fibrotic lung injury. The European Respiratory Journal. 2009;34:740–748. doi: 10.1183/09031936.00128508. [DOI] [PubMed] [Google Scholar]
- Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, et al. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147(3):525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzaro D, Price M, de Felice M, Di Lauro R. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development. 1991;113(4):1093–1104. doi: 10.1242/dev.113.4.1093. [DOI] [PubMed] [Google Scholar]
- Lee J, Fang X, Gupta N, Serikov V, Matthay M. Allogenic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(38):16357–16362. doi: 10.1073/pnas.0907996106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fang X, Krasnodembskaya A, Howard J, Matthay M. Concise review: Mesenchymal stem cells for acute lung injury: Role of paracrine soluble factors. Stem Cells. 2011;29(6):913–919. doi: 10.1002/stem.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim J, Gludish D, Roach RR, Saunders AH, Barrios J, et al. Surfactant protein C chromatin-bound green fluorescence protein reporter mice reveal heterogeneity of surfactant protein C expressing lung cells. American Journal of Respiratory Cell and Molecular Biology. 2013;48(3):288–298. doi: 10.1165/rcmb.2011-0403OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Mitsialis S, Aslam M, Vitali S, Vergadi E, Konstantinou G, et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation. 2012;126(22):2601–2611. doi: 10.1161/CIRCULATIONAHA.112.114173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Tiede B, Massague J, Kang Y. Beyond tumorigenesis: Cancer stem cells in metastasis. Cell Research. 2007;17(1):3–14. doi: 10.1038/sj.cr.7310118. [DOI] [PubMed] [Google Scholar]
- Liang O, Mitsialis S, Chang M, Vergadi E, Lee C, Aslam M, et al. Mesenchymal stromal cells expressing heme oxygenase-1 reverse pulmonary hypertension. Stem Cells. 2011;29(1):99–107. doi: 10.1002/stem.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longmire T, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean J, et al. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BLM, et al. Evidence that Sox2 overexpression is oncogenic in the lung. PLoS One. 2010;5(6):e11022. doi: 10.1371/journal.pone.0011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux A, Kelly R, Veltmaat J, De Langhe S, Zaffran S, Thiery J, et al. Fgf10 expression identifies parabronchial smooth muscle cell progenitors and is required for their entry into the smooth muscle cell lineage. Development. 2005;132(9):2157–2166. doi: 10.1242/dev.01795. [DOI] [PubMed] [Google Scholar]
- Martin J, Helm K, Ruegg P, Varella-Garcia M, Burnham E, Majka S. Adult lung side populations have mesenchymal stem cell potential. Cytotherapy. 2008;10:140–151. doi: 10.1080/14653240801895296. [DOI] [PubMed] [Google Scholar]
- McQualter J, Brouard N, Williams B, Baird B, Sims-Lucas S, Yuan K, et al. Endogenous fibroblastic progenitor cells in the adult mouse lung are highly enriched in the sca-1 positive cell fraction. Stem Cells. 2009;27(3):623–633. doi: 10.1634/stemcells.2008-0866. [DOI] [PubMed] [Google Scholar]
- McQualter J, Yuen K, Williams B, Bertoncello I. Evidence of an epithelial stem/progenitor cell hierarchy in the adult mouse lung. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1414–1419. doi: 10.1073/pnas.0909207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei S, McCarter S, Deng Y, Parker C, Liles W, Stewart D. Prevention of LPS-induced acute lung injury in mice by mesenchymal stem cells overexpressing angiopoietin 1. PLoS Medicine. 2007;4:e269. doi: 10.1371/journal.pmed.0040269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Li M, Wang X, Wang Y, Ma D. Both CD133+ and CD133− sub-populations of A549 and H446 cells contain cancer-initiating cells. Cancer Science. 2009;100(6):1040–1046. doi: 10.1111/j.1349-7006.2009.01144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoo P, Su G, Drum H, Bringas P, Kimura S. Defects in tracheoesophageal and lung morphogenesis in Nkx2.1 (−/−) mouse embryos. Developmental Biology. 1999;209(1):60–71. doi: 10.1006/dbio.1999.9234. [DOI] [PubMed] [Google Scholar]
- Morrisey E, Hogan B. Preparing for the first breath: Genetic and cellular mechanisms in lung development. Developmental Cell. 2010;18:8–23. doi: 10.1016/j.devcel.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, et al. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–397. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LV, Vanner R, Dirks P, Eaves CJ. Cancer stem cells: An evolving concept. Nature ReviewsCancer. 2012;12(2):133–143. doi: 10.1038/nrc3184. [DOI] [PubMed] [Google Scholar]
- Odorico J, Kaufman D, Thomson J. Multilineage differentiation from human embryonic stem cell lines. Stem Cells. 2001;19:193–204. doi: 10.1634/stemcells.19-3-193. [DOI] [PubMed] [Google Scholar]
- Oeztuerk-Winder F, Guinot A, Ochalek A, Ventura J. Regulation of human lung alveolar multipotent cells by a novel p38alpha MAPK/mir-17-92 axis. The EMBO Journal. 2012;31(16):3431–3441. doi: 10.1038/emboj.2012.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver TG, Mercer KL, Sayles LC, Burke JR, Mendus D, Lovejoy KS, et al. Chronic cisplatin treatment promotes enhanced damage repair and tumor progression in a mouse model of lung cancer. Genes & Development. 2010;24(8):837–852. doi: 10.1101/gad.1897010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz L, Gambelli F, McBride C, Gaupp D, Baddoo M, Kaminski N, et al. Mesenchymal stem cell engraftment in lung is enhanced in response to bleomycin exposure and ameliorates its fibrotic effects. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:8407–8411. doi: 10.1073/pnas.1432929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-S, Liang M-C, Raiser DM, Zamponi R, Roach RR, Curtis SJ, et al. Characterization of the cell of origin for small cell lung cancer. Cell Cycle. 2011;10(16):2806–2815. doi: 10.4161/cc.10.16.17012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl A, Kist R, Shan Z, Scherer G, Whitsett J. Normal lung development and function after Sox9 inactivation in the respiratory epithelium. Genesis. 2005;41:23–32. doi: 10.1002/gene.20093. [DOI] [PubMed] [Google Scholar]
- Politi K, Zakowski MF, Fan P-D, Schonfeld EA, Pao W, Varmus HE. Lung adenocarcinomas induced in mice by mutant EGF receptors found in human lung cancers respond to a tyrosine kinase inhibitor or to down-regulation of the receptors. Genes & Development. 2006;20(11):1496–1510. doi: 10.1101/gad.1417406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Que J, Luo X, Schwartz R, Hogan B. Multiple roles for Sox2 in the developing and adult mouse trachea. Development. 2009;136(11):1899–1907. doi: 10.1242/dev.034629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiser D, Zacharek S, Roach R, Curtis S, Sinkevicius K, Gludish D, et al. Stem cell biology in the lung and lung cancers: Using pulmonary context and classic approaches. Cold Spring Harbor Symposia on Quantitative Biology. 2008;73:479–490. doi: 10.1101/sqb.2008.73.036. [DOI] [PubMed] [Google Scholar]
- Rawlins E, Clark C, Xue Y, Hogan B. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136(22):3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E, Hogan B. Epithelial stem cells of the lung: Privileged few or opportunities for many? Development. 2006;133(13):2455–2465. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- Rawlins E, Hogan B. Ciliated epithelial cell lifespan in the mouse trachea and lung. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2008;295:L231–L234. doi: 10.1152/ajplung.90209.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawlins E, Okubo T, Xue Y, Brass D, Auten R, Hasegawa H, et al. The role of Scgb1a1+ Clara cells in the long-term maintenance and repair of lung airway, but not alveolar epithelium. Cell Stem Cell. 2009;4(6):525–534. doi: 10.1016/j.stem.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds S, Giangreco A, Power J, Stripp B. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. The American Journal of Pathology. 2000;156(1):269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J, Barkauskas C, Cronce M, Xue Y, Harris J, Liang J, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(52):E1475–E1483. doi: 10.1073/pnas.1117988108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J, Hogan B. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Annual Review of Cell and Developmental Biology. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- Rock J, Onaitis M, Rawlins E, Lu Y, Clark C, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock J, Randell S, Hogan B. Airway basal stem cells: A perspective on their roles in epithelial homeostasis and remodeling. Disease Models & Mechanisms. 2010;3(9–10):545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan L, Subramaniam M, Emanuel R, Degan S, Johnston P, Tefft D, et al. Centrifugal migration of mesenchymal cells in the embryonic lung. Developmental Dynamics. 2008;237(3):750–757. doi: 10.1002/dvdy.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SV, Lee DY, Li B, Quinlan MP, Takahashi F, Maheswaran S, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell. 2010;141(1):69–80. doi: 10.1016/j.cell.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu W, Lu M, Zhang Y, Tucker P, Zhou D, Morrisey E. Foxp2 and Foxp1 cooperatively regulate lung and esophagus development. Development. 2007;134:1991–2000. doi: 10.1242/dev.02846. [DOI] [PubMed] [Google Scholar]
- Song H, Yao E, Lin C, Gacayan R, Chen M-H, Chuang P-T. Functional characterization of pulmonary neuroendocrine cells in lung development, injury, and tumorigenesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):17531–17536. doi: 10.1073/pnas.1207238109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan JP, Spinola M, Dodge M, Raso MG, Behrens C, Gao B, et al. Aldehyde dehydrogenase activity selects for lung adenocarcinoma stem cells dependent on notch signaling. Cancer Research. 2010;70(23):9937–9948. doi: 10.1158/0008-5472.CAN-10-0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summer R, Fitzsimmons K, Dwyer D, Murphy J, Fine A. Isolation of an adult mouse lung mesenchymal progenitor cell population. American Journal of Respiratory Cell and Molecular Biology. 2007;37:152–159. doi: 10.1165/rcmb.2006-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Schiller JH, Gazdar AF. Lung cancer in never smokers - a different disease. Nat Rev Cancer. 2007;7(10):778–790. doi: 10.1038/nrc2190. [DOI] [PubMed] [Google Scholar]
- Sutherland KD, Proost N, Brouns I, Adriaensen D, Song J-Y, Berns A. Cell of origin of small cell lung cancer: Inactivation of Trp53 and Rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19(6):754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Yanagisawa K, Shinjo K, Taguchi A, Maeno K, Tomida S, et al. Lineage-specific dependency of lung adenocarcinomas on the lung development regulator TTF-1. Cancer Research. 2007;67(13):6007–6011. doi: 10.1158/0008-5472.CAN-06-4774. [DOI] [PubMed] [Google Scholar]
- TCGA Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teisanu R, Lagasse E, Whitesides J, Stripp B. Prospective isolation of bronchiolar stem cells based upon immunophenotypic and autofluorescence characteristics. Stem Cells. 2009;27(3):612–622. doi: 10.1634/stemcells.2008-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. Journal of Clinical Oncology. 2013;31(8):992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- Tropea K, Leder E, Aslam M, Lau A, Raiser D, Lee J, et al. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2012;302(9):L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nature Reviews. Immunology. 2008;8:726–736. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- van Haaften T, Byrne R, Bonnet S, Rochefort G, Akabutu J, Bouchentouf M, et al. Airway delivery of mesenchymal stem cells prevents arrested alveolar growth in neonatal lung injury in rats. American Journal of Respiratory and Critical Care Medicine. 2009;180(11):1131–1142. doi: 10.1164/rccm.200902-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haute L, De Block G, Liebaers I, Sermon K, DeRycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Respiratory Research. 2009;5(10):105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Lommel A. Pulmonary neuroendocrine cells (PNEC) and neuroepithelial bodies (NEB): Chemoreceptors and regulators of lung development. Paediatric Respiratory Reviews. 2001;2:171–176. doi: 10.1053/prrv.2000.0126. [DOI] [PubMed] [Google Scholar]
- Visvader JE. Cells of origin in cancer. Nature. 2011;469(7330):314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- Vosdoganes P, Lim R, Moss T, Wallace E. Cell therapy: A novel treatment approach for bronchopulmonary dysplasia. Pediatrics. 2012;130:727–737. doi: 10.1542/peds.2011-2576. [DOI] [PubMed] [Google Scholar]
- Weiss D, Casaburi R, Flannery R, Leroux-Williams M, Tashkin D. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598. doi: 10.1378/chest.12-2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkerson MD, Yin X, Hoadley KA, Liu Y, Hayward MC, Cabanski CR, et al. Lung squamous cell carcinoma mRNA expression subtypes are reproducible, clinically important, and correspond to normal cell types. Clinical Cancer Research. 2010;16(19):4864–4875. doi: 10.1158/1078-0432.CCR-10-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow MM, Dayton TL, Verhaak RGW, Kim-Kiselak C, Snyder EL, Feldser DM, et al. Suppression of lung adenocarcinoma progression by Nkx2–1. Nature. 2011;473(7345):101–104. doi: 10.1038/nature09881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistuba II, Bryant D, Behrens C, Milchgrub S, Virmani AK, Ashfaq R, et al. Comparison of features of human lung cancer cell lines and their corresponding tumors. Clinical Cancer Research. 1999;5(5):991–1000. [PubMed] [Google Scholar]
- Wong A, Bear C, Chin S, Pasceri P, Thompson T, Huan L, et al. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nature Biotechnology. 2012;9:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Jiang Q, Willette-Brown J, Xi S, Zhu F, Burkett S, et al. The pivotal role of IKKα in the development of spontaneous lung squamous cell carcinomas. Cancer Cell. 2013;23(4):527–540. doi: 10.1016/j.ccr.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Rock JR, Lu Y, Futtner C, Schwab B, Guinney J, et al. Evidence for type II cells as cells of origin of K-Ras-induced distal lung adenocarcinoma. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(13):4910–4915. doi: 10.1073/pnas.1112499109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Woods C, Mora A, Joodi R, Brigham K, Iyer S, et al. Prevention of endotoxin-induced systemic response by bone marrow derived mesenchymal stem cells in mice. American Journal of Physiology. Lung Cellular and Molecular Physiology. 2007;293(1):L131–L141. doi: 10.1152/ajplung.00431.2006. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Yun E, Gerber H, Ferrara N, Whitsett J, Vu T. Epithelialvascular cross talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Developmental Biology. 2007;308(1):44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik M, Smuga-Otto K, Antosiewicz-Bourget J, Frane J, Tian S, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zacharek S, Fillmore C, Lau A, Gludish D, Chou A, Ho J, et al. Lung stem cell self-renewal relies on BMI1-dependent control of expression at imprinted loci. Cell Stem Cell. 2011;9(3):272–281. doi: 10.1016/j.stem.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WC, Shyh-Chang N, Yang H, Rai A, Umashankar S, Ma S, et al. Glycine decarboxylase activity drives non-small cell lung cancer tumor-initiating cells and tumorigenesis. Cell. 2012;148(1–2):259–272. doi: 10.1016/j.cell.2011.11.050. [DOI] [PubMed] [Google Scholar]
- Zhou W, Ercan D, Chen L, Yun C-H, Li D, Capelletti M, et al. Novel mutant-selective EGFR kinase inhibitors against EGFR T790M. Nature. 2009;462(7276):1070–1074. doi: 10.1038/nature08622. [DOI] [PMC free article] [PubMed] [Google Scholar]


