Abstract
Objective
The aim of this study was to test the relationships between mood state and rhythm disturbances as measured via actigraphy in bipolar disorder by assessing the correlations between manic and depressive symptoms as measured via Young Mania Rating Scale (YMRS) and 30-item Inventory for Depressive Symptomatology, Clinician-Rated (IDS-C-30) scores and the actigraphic measurements of rhythm, the 24-hour autocorrelation coefficient and circadian quotient
Method
The research was condua ed at the University of Texas Southwestern Medical Center at Dallas from February 2, 2009, to March 30, 2010. 42 patients with a DSM-IV-TR diagnosis of bipolar I disorder were induded in the study. YMRS and the IDS-C-30 were used to determine symptom severity. Subjects wore the actigraph continuously for 7 days. The 24-hour autocorrelation coefficient was used as an indicator of overall rhythmicity. The circadian quotient was used to characterize the strength of a circadian rhythm.
Results
A greater sevefity of manic symptoms correlated with a lower degree of rhythmicity and less robust rhythms of locomotor activity as indicated by lower 24-hour autocorrelation (r = −0.3406, P = .03) and circadian quotient (r = −0.5485, P = .0002) variables, respectively. No relationship was noted between the degree of depression and 24-hour autocorrelation scores (r = −0.1190, P = .45) or circadian quotient (r=0.0083, P= .96). Correlation was noted between the 24-hour autocorrelation and circadian quotient scores (r=0.6347, P<.0001).
Conclusions
These results support the notion that circadian rhythm disturbances are associated with bipolar disorder and that these disturbances may be associated with clinical signatures of the disorder. Further assessment of rhythm disturbances in bipolar disorder is warranted.
Rhythm disruption is a hallmark of bipolar disorder.1 Multiple models have been proposed to explain the disruptions in rhythms associated with bipolar disorder.2-15 While no consensus has been reached on the direction of these disturbances, an integrating concept is that, rather than there being a stable change In the absolute timing of the circadian clock, there is am inherent instability in the circadian timing system and therefore an increased sensitivity toward temporal disorganization of physiologic rhythms In patients with affective dlsorders.16 In support of this concept are several studies that report a wide variability In the phases of circadian rhythms In patients suffering from bipolar disorder.12-15
Actigraphy has previously been used to assess sleep, activity, and circadian variables in bipolar disorder.5,17,18 Findings from these studies support the concept that there is a loss of rhythmicity in those suffering from the Ulness. Disturbances in the levels of locomotor activity are common in both manic and depressive phases of bipolar dlsorder.17 Bipolar subjects show less locomotor activity when depressed and greater locomotor activity when manlc.5,17 Bipolar subjects have also demonstrated less stable circadian activity patterns, a greater fragmentation of activity, and a greater variabiUty In 24-hour rhythm when compared to controls.18
Previous studies were designed to compare the differences in activity patterns and rhythms between bipolar patients and healthy controls or assessed activity levels in specific mood states. The aim of this study was to test the relationships between mood state and rhythm disturbances as measured via actigraphy in bipolar disorder by assessing the correlations between manic and depressive symptoms as measured via Young Mania Rating Scale (YMRS) and 30-item Inventory of Depressive Symptoms, Clinician-Rated scale (IDS-C-30) scores and the actigraphlc measurements of rhythm the 24-hour autocorrelation coefficient and circadian quotient. We hypothesized an association between greater severity of affective symptoms and a decrease in the circadian rhythmicity of activity. We also hypothesized that mania would have a greater Impact on rhythm disturbances than would depression.
METHOD
Subjects
Forty-two subjects diagnosed with bipolar I disorder were included In our evaluation. The research was conducted at the University of Texas Southwestern Medical Center at Dallas (UfSW) from February 2, 2009, to March 30, 2010. Subjects were recruited from various sources throughout Dallas County and represented a broad sampling of subjects diagnosed with the illness. Patients were recruited from county and community hospitals, the university medical center, community mental health clinics, and psychiatric and clinical research groups at UTSW. Subjects with a history of major neurologic Impairment (le, history of cerebrovascular accident), decompensated medical Ulness, mental retardation, traumatic brain injttry, shift work or diurnal changes in work schedule 4 weeks prior to or during the course of the study, travel involving 3 or more time zones occurring 4 weeks prior to or during the course of the study, current use of hypnotic agents for sleep, and a recent history (1 month prior) of substance abuse or dependence were excluded from the study. The study was approved by the Institutional review board of UTSW Medical Center and was consistent with standards for the ethical conduct of human research. All study participants provided written informed consent.
Clinical Assessments
DSM-IV-TR Axis I diagnosis of bipolar I disorder was confirmed by the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I/P).19 All cases were then subject to a best-estimate diagnostic consensus Including a minimum of3 experienced clinicians in order to confrrm the diagnosis of bipolar I disorder. Young Mania Rating Scale (YMRS)20 was used to determine the degree of manic symptoms while the 30-ltem Inventory for Depressive Symptomatology, Clinician-Rated (IDS-C-30)21 was used to determine the degree of depressive symptoms. Demographic and course of illness characteristics were collected on all participants.
Actigraphy
Actigraphy was used to collect data concerning locomotor activlty.22 For this study we utilized the Basic Motionlogger actigraph units (Ambulatory Monitoring, Inc; Ardsley, New York). Data were sampled in 60-second epochs. Subjects wore the apparatus continuously on the nondomlnant wrist for the duration of the study (7 days).
Statistical Analysis
Actigraphlc data were analyzed by using Action 4 circadian rhythm analysis software (Ambulatory Monitoring, Inc; Ardsley, New York). The primary outcome of rhythmicity was the 24-hour autocorrelation coefficient22 that represents the correlation of a time series with its own past and future values and may be talcen as an indicator of the degree of rhythmicity. Higher 24-hour autocorrelation scores indicate a higher degree of rhythmicity. Lower scores Indicate a lower degree of rhythmicity. Actigraph data were also analyzed via cosinor analysls.6,23 The circadian quotient,22 or amplitude-mesor ratio, was then calculated and used as a proxy for the robustness of rhythms. This measure provides an estimation of bow well circumscribed periods of activity and sleep/rest are during the course of the day. Higher circadian quotient scores indicate a more robust rhythm. Lower scores indicate a less robust rhythm. The Pearson test was conducted to test the correlations between the degree of depressive and manic symptomatology, as defined by total scores on the IDS-C-30 and YMRS, respectively, and the actigraphlc variables of Interest (autocorrelation coefficient and circadian quotient). If positive correlations were noted, additional exploratory pairwise analyses via the Pearson test were conducted to test for correlations between autocorrelation coefficient and circadian quotient scores and individual items on clinical rating scales to explore the relationships between circadian rhythm variables and depressive and manic symptomatology. The Pearson test was also used to explore the correlation between the autocorrelation coefficient and circadian quotient scores to explore the relationships between the rhythmicity and rhythm robustness of physical activity. A significance value of .05 was set for all statistical tests.
RESULTS
Subject Demographic and Clinical Characteristics
Table I summarizes the demographic, clinical, and course-of-illness characteristics of the subjects included In the protocol. Twenty-one percent of subjects (n = 9) were medication free, while the remaining 79% of subjects (n = 33) were taking various medication combinations. Twenty-five subjects were talclng mood stabillzers, 18 were taking atypical antipsychotics, and 17 were taking antidepressants. The mean (SD) YMRS score was 13.8 (8.0), while the mean (SD) IDS-C-30 score was 20.7 (12.2). No differences between medicated (21.4 [13.3]) and unmedicated (18.4 [6.9]) groups on IDS-C-30 scores were noted. Differences In YMRS scores (P= .04) were noted, with unmedlcated patients (18.6 [7.2]) having a greater severity of manic symptoms when compared to medicated patients (12.5 [7.8]).
Table 1.
Sample Demographic, Clinical, and Course of Illness Characteristics
| Demographic | Value |
|---|---|
| Bipolar I disorder, N | 42 |
| Age, mean (SD), y | 41.0 (11.2) |
| Gender, n (%) | |
| Male | 15 (36) |
| Female | 27 (64) |
| Ethnicity, n (%) | |
| Caucasian | 25 (60) |
| African American | 14 (33) |
| Hispanic | |
| Clinical characteristics | |
| YMRS score, mean (SD) | 13.8 (8.0) |
| IDS-C-30 score, mean (SD) | 20.7 (122) |
| Medicated, n (%) | 33 (79) |
| Mood stabilizers | 25 (60) |
| Atypical antipsychotics | 18 (43) |
| Antidepressants | 17 (40) |
| Unmedicated, n (%) | 9 (21) |
| Course of illness characteristics | |
| History of hospitalization, n (%) | 29 (69) |
| History of psychosis, n (%) | 28 (67) |
| Age at onset of illness, mean (SD), y | 17.5 (9.6) |
Abbreviations: IDS-C-30 = 30-item Inventory for Depressive Symptomatology, Clinician-Rated; YMRS = Young Mania Rating Scale.
Relationships Between Actigraphic Measurements of Rhythmicity and Mood State Severity
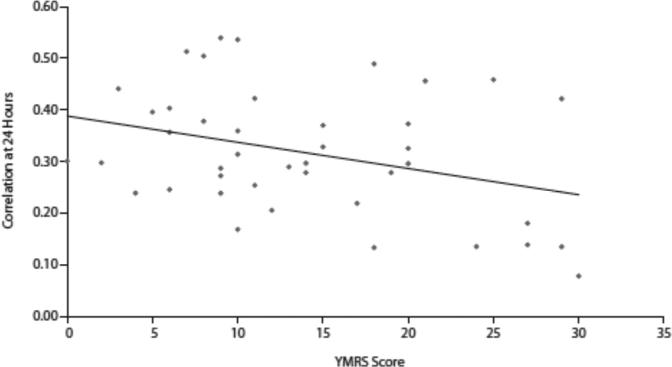
The 24-hour autocorrelation scores were Inversely correlated with YMRS scores (r=−0.3406, P=.03) (Figure 1). Pairwise correlations between 24-hour autocorrelation scores and YMRS items (Table 2) revealed that 24-hour correlation scores were correlated with decreased need for sleep (YMRS item 4) (r= −0.4698, P= .002) and disturbances of thought content (YMRS item 8) (r=−0.3116, P=.04). Trends toward inverse correlations between increased rate and amount of speech (YMRS item 6) and language and thought disorder (YMRS item 7) (both P= .09) and 24-hour autocorrelation scores were noted No correlation between IDS-C-30 scores and 24-hour autocorrelation scores was noted (r=−0.1190, P= .45).
Figure 1.
Correlation Between 24-Hour Autocorrelation Coefficient and YMRS Total Scoresa
Table 2.
Relationship Between YMRS Items, 24-Hour Correlation Coefficient, and Circadian Quotient
| Correlation With 24-Hour Autocorrelation Coefficient |
Correlation With Circadian Quotient |
|||
|---|---|---|---|---|
| YMRS Item | r | P | r | P |
| 1: Elevated mood | –0.1649 | .2968 | –0.1256 | .4279 |
| 2: Increased motor activity and energy | –0.2037 | .1956 | –03737 | .0148 |
| 3: Sexual interest | –0.1452 | .3589 | –02684 | .0857 |
| 4: Sleep | –0.4698 | .0017 | –0.6798 | <.0001 |
| 5: Irritability | –0.0024 | .9882 | –0.2165 | .1686 |
| 6: Speech (rate and amount) | –0.2675 | .0868 | –03957 | .0095 |
| 7: Language and thought disorder | –0.2654 | .0894 | –03047 | .0497 |
| 8: Disturbances of thought content | –0.3116 | .0446 | –03649 | .0175 |
| 9: Disruptive-aggressive behavior | 0.1218 | .4421 | –0.1714 | .2777 |
| 10: Appearance | –0.2311 | .1409 | –0.2125 | .1766 |
| 11: Insight | –0.1273 | .4218 | –0.1251 | .4299 |
Abbreviation: YMRS = Young Mania Rating Scale.
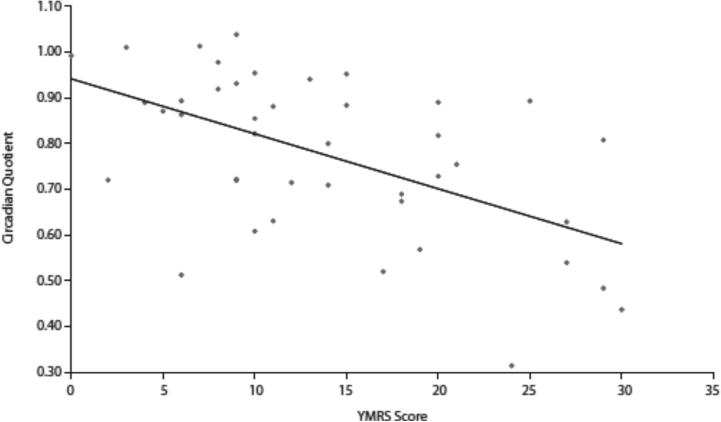
Circadian quotient scores were Inversely correlated with YMRS scores (r=−0.5485, P=.0002) (Figure 2). Pairwise correlations between circadian quotient scores and YMRS items (Table 2) also indicated that circadian quotient scores were Inversely correlated with Increased motor activity and energy(YMRS item 2) (r=−0.3737,P=.Ol),decreased need for sleep (YMRS item 4) (r=−0.6798, P<.OOO1), increased rate and amount of speech (YMRS item 6) (r= −0.3957, P= .O1), language and thought disorder (YMRS item 7) (r= −0.3047, P= .O5), and disturbances of thought content (YMRS item 8) (r= −0.3649, P= .02). No association between IDS-C-30 scores and circadian quotient was noted (r=0.0083, P= .96).
Figure 2.
Correlation Between Circadian Quotient and YMRS Total Scoresa
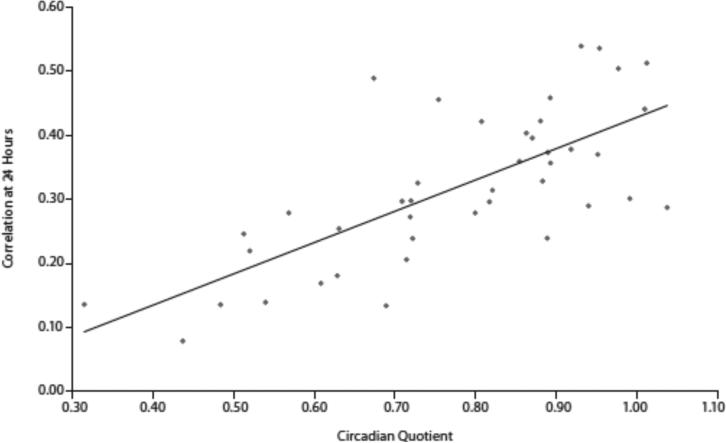
The relationships between severity of mood symptoms and the components used to calculate circadian quotients, amplitude and mesor, were explored. Amplitude did not correlate with either YMRS (r= −0.0879, P= .5799) or IDSC-30 scores (r=0.2274, P=.l475). Mesor did not correlate with either YMRS (r=0.1574, P= .3194) or IDS-C-30 scores (r=0.1674, P=.2893). Post hoc analysis also revealed a positive correlation between 24-hour autocorrelation and circadian quotient (r=0.6347, P< .0001) (Figure 3).
Figure 3.
Correlation Between Circadian Quotient and 24-Hour Autocorrelation Scoresa
In order to test the impact of medication status on actigraphic variables of rhythmicity, we compared the mean values between medicated and unmedicated patients. We found no statistical difference between these groups on either 24-hour autocorrelation (P = .60) or circadian quotient variables (P= .11).
DISCUSSION
The findings of this study suggest a relationship between mood state symptom severity and rhythm disturbances of locomotor activity in subjects suffering from bipolar disorder. The results suggest that a greater severity of manic symptoms is related to a less robust circadian rhythm. Specifically, manic symptoms correlated with a lower degree of rhythmidty and less robust rhythms of locomotor activity, as indicated by lower 24-hour autocorrelation and circadian quotient variables, respectively. While the relationship noted between rhythm disturbances and manic but not depressive symptoms could potentially suggest a mood state phenomenon, these findings could also reflect a decreased acuity of actigraphy to characterize circadian rhythm disturbances.
Several relationships between rhythm disruptions and clinical characteristics of mania were observed. Clinical signatures that correlated with rhythm disturbances included decreased need for sleep, disturbances in content of thought and thought disorder, increase in rate and amount of speech, and increased motor activity and energy were noted. Of particular interest was the significant correlation between rhythm disturbances and a reported decreased need for sleep. These results are suggestive of a possible commonality or shared pathophysiology between sleep disruption and circadian rhythm disturbances and warrant further assessment.
Relationships between various markers of rhythmicity were also noted. A significant correlation between the 24-hour autocorrelation and circadian quotient scores suggests a relationship between the degree of circadian rhythmicity and the robustness of circadian rhythms. Also, while a correlation between circadian quotient and YMRS scores was found, no correlation between the severity of manic symptoms and either amplitude or mesor was noted. This seems to suggest that the relationship between amplitude and mesor variables, rather than the variables individually, is correlated with symptoms of mania.
Limitations with the current analytic methods used to analyze actigrapbic data should be mentioned Many current analytic methods (le, coslnor analysis) require that raw actigrapbic data fit a specific mathematical model or set time frame (ie, 24 hours). Therefore, the use of more refined methods of actlgrapby analysis that do not adhere to predetermined mathematical models or time constraints may better describe rhythm disturbances, especially in a population such as persons with bipolar disorder, in whom there is a great degree of disturbances in diurnal sleep-wake patterns. For example, the use of functional principal components analysis may allow for a more refined quantification of the actigraph data and the flexibility to search for functions representing activity patterns that may distinguish between subgroups and between-subject variability in bipolar patlents.24
We did not find a statistically significant difference between medicated and unmedlcated patients on either 24-bour autocorrelation or circadian quotient scores. lt should also be noted that certain medications used to treat the illness may exert some of their therapeutic action via their influence on endogenous molecular clocks.25-29 For example, both lithium.26,29,30 and valproic acid26 have been shown to influence the rhythmic expression of circadian genes and the rhythmic properties of molecular docks. While unmedicated patients bad higher YMRS scores when compared to medicated patients, no difference in locomotor rhythms were observed between groups. These findings suggest that there may be other factors related to the manic state that more closely correlate with disturbances of rhythm.
The results of this study support the concept of a relationship between biological rhythm disruption and bipolar disorder, but caution should be taken regarding the inference of causality. While locomotor activity is influenced by the central circadian pacemaker, it is affected by many other variables and is not considered a robust marker of the central circadian dock. Future studies would benefit from the use of more refined chronobiologlcal protocols to characterize and quantify rhythm disturbances and functioning of the central circadian dock in bipolar patients. Even with the limitations noted above, actlgrapby does present a unique method to objectively and longitudinally assess cbronobiologlcal variables in bipolar patients.
Overall, our study supports the concept that there is significant mood instability associated with affective states in bipolar disorder. While we did not find a relationship between depression 12-15 and rhythm disturbances, we did fmd relationships between mania and rhythm disturbances.12,13 As has been shown previously,6,18 we have demonstrated the ability to measure rhythm disturbances utilizing actigraphy in patients with bipolar disorder. Ambulatory monitoring of activity patterns in conjunction with other chronobiological biomarkers may be valuable assessments that could potentially serve as physiological biomarkers of the illness and could lead to potential refinement of endophenotypes in bipolar disorder.
■ The findings of this study suggest that disturbances in biological rhythms may be associated with affective state, particularly mania, and may be correlated with other clinical signatures of bipolar disorder.
■ Actigraphy presents a method to objectively and longitudinally assess chronobiology in bipolar patients.
■ Actigraphic assessments may serve as potential objective measures of treatment response and may help to characterize sleep and rhythm disturbances associated with bipolar disorder.
Acknowledgments
Funding/support: NARSAD Young Investigator Award, T32 MH06754305, and NIMH P30 MH089868 grants provided funding support for this research.
Footnotes
Drug names: lithium (Lithobid and others), valproic acid (Depakene and olhers).
Potential conflicts of interest: Dr Tamminga serves as deputy editor for the American Psychiatric Association; is an ad hoc consultant for Astellas. Eli Lilly, Lundbeck, and Pure Tech Ventures; is a council member of the National Institute of Medicine; and is an advisory board member of Intra-cellular Therapies. She is also an unpaid council member of the Brain and Behavior Foundation, the Institute of Medicine, and NAMI; and is an unpaid volunteer and organizer for the International Congress on Schizophrenia Research. Dr Tohen has served as a consultant to Eli Lilly, GlaxoSmithKline, Wyeth, Roche, Merck, Lundbeck, and Otsuka. Dr Suppes has received funding or medications for clinical grants from the National Institute of Mental Health (NIMH), Sunovion, Elan Pharma, and the VA Cooperative Studies Program; has served on consulting/advisory boards of and has received travel expenses from Sunovion; and has received royalties from Jones and Bartlett. Dr Gonzalez reports no conflict of interest.
Previous presentation: Poster presented at the Society of Biological Psychiatry Annual Meeting May 3-5, 2012: Philadelphia, Pennsylvania.
REFERENCES
- 1.American Psychiatric Association . Text Revision. Fourth Edition American Psychiatric Association; Washington, DC: 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 2.Wehr TA, Sack DA, Duncan WC, et al. Sleep and circadian rhythms in affective patients isolated from external time cues. Psychiatry Res. 1985;15(4):327–339. doi: 10.1016/0165-1781(85)90070-8. [DOI] [PubMed] [Google Scholar]
- 3.Kripke DP, Mullaney DJ, Atkinson M, et al. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978;13(3):335–351. [PubMed] [Google Scholar]
- 4.Kripke DF, Judd LL, Hubbard B, et al. The effect of lithium carbonate on the circadian rhythm of sleep in normal human subjects. Biol Psychiatry. 1979;14(3):545–548. [PubMed] [Google Scholar]
- 5.Wehr TA, Muscettola G, Goodwin FK. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm: early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry. 1980;37(3):257–263. doi: 10.1001/archpsyc.1980.01780160027002. [DOI] [PubMed] [Google Scholar]
- 6.Salvatore P, Ghidini S, Zita G, et al. Circadian activity rhythm abnormalities in ill and recovered bipolar I disorder patients. Bipolar Disord. 2008;10(2):256–265. doi: 10.1111/j.1399-5618.2007.00505.x. [DOI] [PubMed] [Google Scholar]
- 7.Linkowski P, Mendlewiz J, Leclercq R, et al. The 24-hour profile of adrenocortkotropin and cortisol in major depressive illness. J Clin Endocrinol Metab. 1985;61(3):429–438. doi: 10.1210/jcem-61-3-429. [DOI] [PubMed] [Google Scholar]
- 8.Linkowski P, Van Cauter E, Leclercq R, et al. ACTH, cortisol and growth hormone 24-hour profiles in major depressive illness. Acta Psychiatr Belg. 1985;85(5):615–623. [PubMed] [Google Scholar]
- 9.Linkowski P, Kerkhofs M, Van Onderbergen A, et al. The 24-hour profiles of cortisol, prolactin, and growth hormone secretion in mania. Arch Gen Psychiatry. 1994;51(8):616–624. doi: 10.1001/archpsyc.1994.03950080028004. [DOI] [PubMed] [Google Scholar]
- 10.Nurnberger JI, Jr, Adkins S, Lahiri DK, et al. Melatonin suppression by light in euthymic bipolar and unipolar patients. Arch Gen Psychiatry. 2000;57(6):572–579. doi: 10.1001/archpsyc.57.6.572. [DOI] [PubMed] [Google Scholar]
- 11.Wood J, Birmaher B, Axelson D, et al. Replicable differences in preferred circadian phase between bipolar disorder patients and control individuals. Psychiatry Res. 2009;166(2-3):201–209. doi: 10.1016/j.psychres.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pflug B, Martin W. Analysis of circadian temperature rhythm in endogenous depressive illness (author's transl) [article in German]. Arch Psychiatr Nevenkr. 1980;229(2):127–143. doi: 10.1007/BF00343078. [DOI] [PubMed] [Google Scholar]
- 13.Tsujimoto T, Yamada N, Shimoda K, et al. Circadian rhythms in depression, part 2: circadian rhythms in inpatients with various mental disorders. J Affect Disord. 1990;18(3):199–210. doi: 10.1016/0165-0327(90)90037-9. [DOI] [PubMed] [Google Scholar]
- 14.Pflug B, Erikson R, Johnsson A. Depression and daily temperature: a long-term study. Ada Psychiatr Scand. 1976;54(4):254–266. doi: 10.1111/j.1600-0447.1976.tb00119.x. [DOI] [PubMed] [Google Scholar]
- 15.Pflug B, Johnsson A, Ekse AT. Manic-depressive stales and daily temperature: some circadian studies. Acta Psychiatr Scand. 1981;63(3):277–289. doi: 10.1111/j.1600-0447.1981.tb00675.x. [DOI] [PubMed] [Google Scholar]
- 16.Souêtre E, Salvati E, Belugou JL, et al. Circadian rhythms in depression and recovery: evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Res. 1989;28(3):263–278. doi: 10.1016/0165-1781(89)90207-2. [DOI] [PubMed] [Google Scholar]
- 17.Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3(1):18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- 18.Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7(2):176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 19.First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version. Patient Edition, (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York, NY: 2002. [Google Scholar]
- 20.Young RC, Biggs JT, Ziegler VE, et al. A rating scale for mania: reliability, validity and sensitivity. Br J Phychiatry. 1978;133(5):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 21.Rush AJ, Giles DE, Schlesser MA, et al. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res. 1986;18(1):65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 22.Ancoli-Israel S, Cole R, Alessi C, et al. The role of actigraphy in the study of sleep and circadian rhythms. Steep. 2003;26(3):342–391. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 23.Erbel-Sieler C, Dudley C, Zhou Y, et al. Behavioral and regulatory abnormalities in mice deficient in the NPAS1 and NPAS3 transcription factors. Proc Natl Acad Sci U S A. 20O4;101(37):13648–13653. doi: 10.1073/pnas.0405310101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang J, Xian H, Licis A, et al. Measuring the impact of apnea and obesity on circadian activity patterns using functional linear modeling of actigraphy data. J Circadian Rhythms. 2011;9(1):11. doi: 10.1186/1740-3391-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gould TD, Manji HK. Glycogen synthase kinase-3: a putative molecular target for lithium mimetic drugs. Neuropsychopharmacology. 2005;30(7):1223–1237. doi: 10.1038/sj.npp.1300731. [DOI] [PubMed] [Google Scholar]
- 26.Johansson AS, Brask I Owe-Larsson B, et al. Valproic acid phase shifts the rhythmic expression of PERIOD2::LUCIFERASE. J Biol Rhythms. 2011;26(6):541–551. doi: 10.1177/0748730411419775. [DOI] [PubMed] [Google Scholar]
- 27.O'Brien WT, Harper AD, Jové F, et al. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. J Neurosci. 2004;24(30):6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenwasser AM, Fecleau ME Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiol Behav. 2005;84(4):537–541. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Osland TM, Fernø J, Håvik B, et al. Lithium differentially affects clock gene expression in serum-shocked NIH-3T3 cells. J Psychophatmacol. 2011;25(7):924–933. doi: 10.1177/0269881110379508. [DOI] [PubMed] [Google Scholar]
- 30.Li J, Lu WQ, Beesley S, et al. Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS ONE. 2012;7(3):e33291. doi: 10.1371/journal.pone.0033292. [DOI] [PMC free article] [PubMed] [Google Scholar]