Abstract
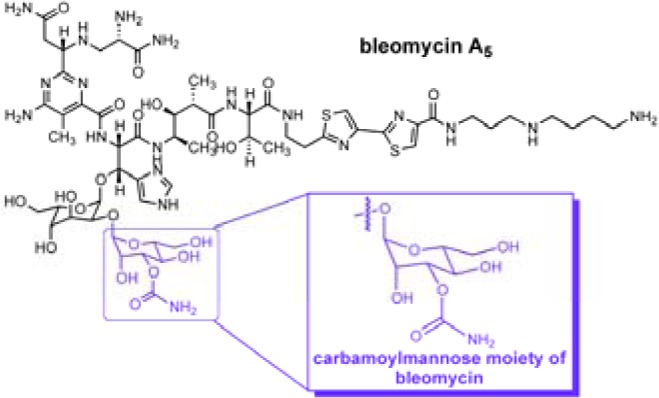
Recently, we reported that both bleomycin (BLM) and its disaccharide, conjugated to the cyanine dye Cy5**, bound selectively to cancer cells. Thus, the disaccharide moiety alone recapitulates the tumor cell targeting properties of BLM. Here, we demonstrate that the conjugate of the BLM carbamoylmannose moiety with Cy5** showed tumor cell selective binding and also enhanced cellular uptake in most cancer cell lines. The carbamoyl functionality was required for tumor cell targeting. A dye conjugate prepared from a trivalent cluster of carbamoylmannose exhibited levels of tumor cell binding and internalization significantly greater than those of the simple carbamoylmannose–dye conjugate, consistent with a possible multivalent receptor.
The bleomycins (BLMs) are glycopeptide-derived antitumor antibiotics consisting of a structurally complex hexapeptide and a disaccharide (Figure 1a).1 Bleomycin was first isolated from a culture broth of Streptomyces verticillus.2 In the United States, bleomycin is used clinically as a mixture of congeners denoted Blenoxane, which consists mainly of bleomycin A2 and B2.3 It is employed for the treatment of several types of cancer, notably squamous cell carcinomas and malignant lymphomas.4 The therapeutically useful effects of bleomycin are believed to be due both to its unique ability to target tumor cells selectively5,6 and its ability to mediate double-strand cleavage of DNA.7
Figure 1.
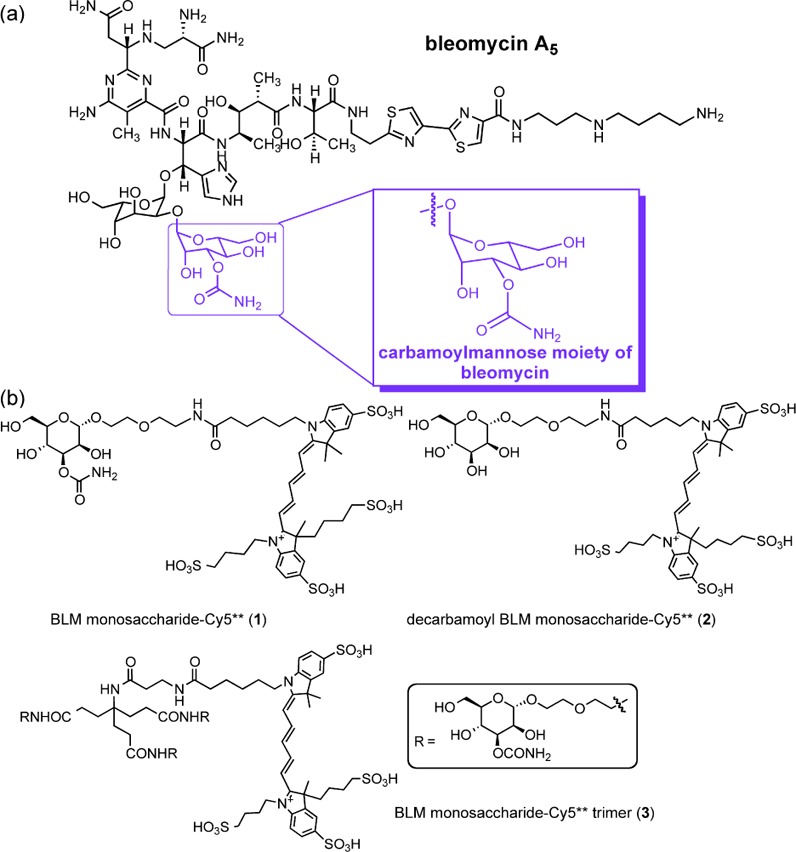
(a) Structure of BLM A5, in which the highlighted domain shows the carbamoylmannose. (b) Structures of BLM monosaccharide bound to Cy5** (1), decarbamoyl BLM monosaccharide bound to Cy5** (2), and the BLM monosaccharide–Cy5** trimer (3).
We have reported previously that the tumor cell targeting ability of BLM requires the presence of the BLM disaccharide moiety, consisting of l-gulose and 3-O-carbamoyl-d-mannose.6 More recently, by the use of the BLM disaccharide conjugated to the cyanine dye Cy5**, we have shown that the disaccharide moiety of BLM is also sufficient to allow tumor targeting and cellular uptake.8 For example, BLM A5 and BLM disaccharide conjugates selectively bound to MCF-7 human breast cancer cells, but not to normal breast cell line MCF-10A. The same selectivity was observed for a variety of cancer and matched normal cell lines.6,8 These findings suggest the possible utility of the BLM disaccharide moiety as part of novel conjugates employed for cancer diagnosis and therapy.
One issue not addressed in any previous study is whether the BLM disaccharide, while small and uncomplicated relative to the natural product itself, actually represents the simplest structural entity capable of selective tumor cell targeting. Here, we demonstrate that 3-O-carbamoyl-d-mannose, one of the two sugars present in the BLM disaccharide, mediates selective targeting of a number of tumor cell lines, and that its efficiency of targeting and/or uptake as a conjugate with Cy5** is generally better than that of the BLM disaccharide. Further, we demonstrate that the carbamoyl moiety is absolutely required for selective tumor cell targeting and describe a trimeric carbohydrate cluster, which displays a still greater efficiency in tumor cell targeting and/or uptake.
Synthesis of BLM Monosaccharide–Dye Conjugates
3-O-Carbamoylmannose, activated as the diphenyl phosphate ester, was prepared by modification of a reported procedure.9 Glycosyl donor 4 was coupled to a CBz-protected commercially available linker (5),8 affording intermediate 6 in 96% yield (Scheme S1 of the Supporting Information). Deacetylation and hydrogenolysis of the primary amine afforded 7, which was treated with the N-hydroxysuccinimide (NHS) ester of Cy5** (8). BLM monosaccharide–Cy5** conjugate 1 was obtained in 36% overall yield (last three steps). The Cy5** conjugate of decarbamoyl BLM monosaccharide (2) was prepared analogously starting from acetylated d-mannose (Scheme S2 of the Supporting Information).
The synthesis of the BLM monosaccharide–Cy5** trimer commenced with hydrogenolysis of the primary amine in 6 and subsequent conjugation to the NHS ester of protected linker 15, which afforded BLM monosaccharide trimer 16 in 40% yield (Scheme S3 of the Supporting Information). Complete deacetylation, followed by hydrogenolysis of the primary amine in 16 and subsequent coupling with the NHS ester of Cy5** (8), provided the BLM monosaccharide–Cy5** trimer conjugate in 33% overall yield for the last three steps.
Cellular Binding/Uptake of the Dye Conjugates
Six cancer cell lines were cultured on 16-well glass chamber slides for 48 h and then treated with 25 μM BLM disaccharide–Cy5** (Figure S1 of the Supporting Information) or BLM monosaccharide–Cy5** (1) conjugate for 1 h. The cells were then washed twice with PBS and fixed with 4% paraformaldehyde. After irradiation for 3 s, fluorescence imaging (Figure 2a) was performed using an inverted microscope. The cells were stained with DAPI to permit evaluation of localization of the conjugates relative to the cell nuclei. It was clear that both BLM disaccharide–Cy5** and BLM monosaccharide–Cy5** conjugates underwent significant binding and uptake in all three cell lines. Analogous results were obtained using DU-145, BT-474, and MCF-7 cell lines (Figure S2 of the Supporting Information). The binding/uptake was found to be specific for the cancer cell lines, as compared with matched normal controls (Figure S3 of the Supporting Information). Quantification of the data (Figure 2b) revealed that the binding/uptake of the BLM monosaccharide was much (∼2-fold) greater in A549 lung cancer cells, A498 kidney cancer cells, and MCF-7 human breast carcinoma cells. The binding/uptake was almost identical for BT-474 human breast ductal carcinoma cells and BxPC-3 human pancreas cells. In comparison, the uptake exhibited by the BLM disaccharide–Cy5** conjugate was much greater in DU-145 human prostate cancer cells.
Figure 2.
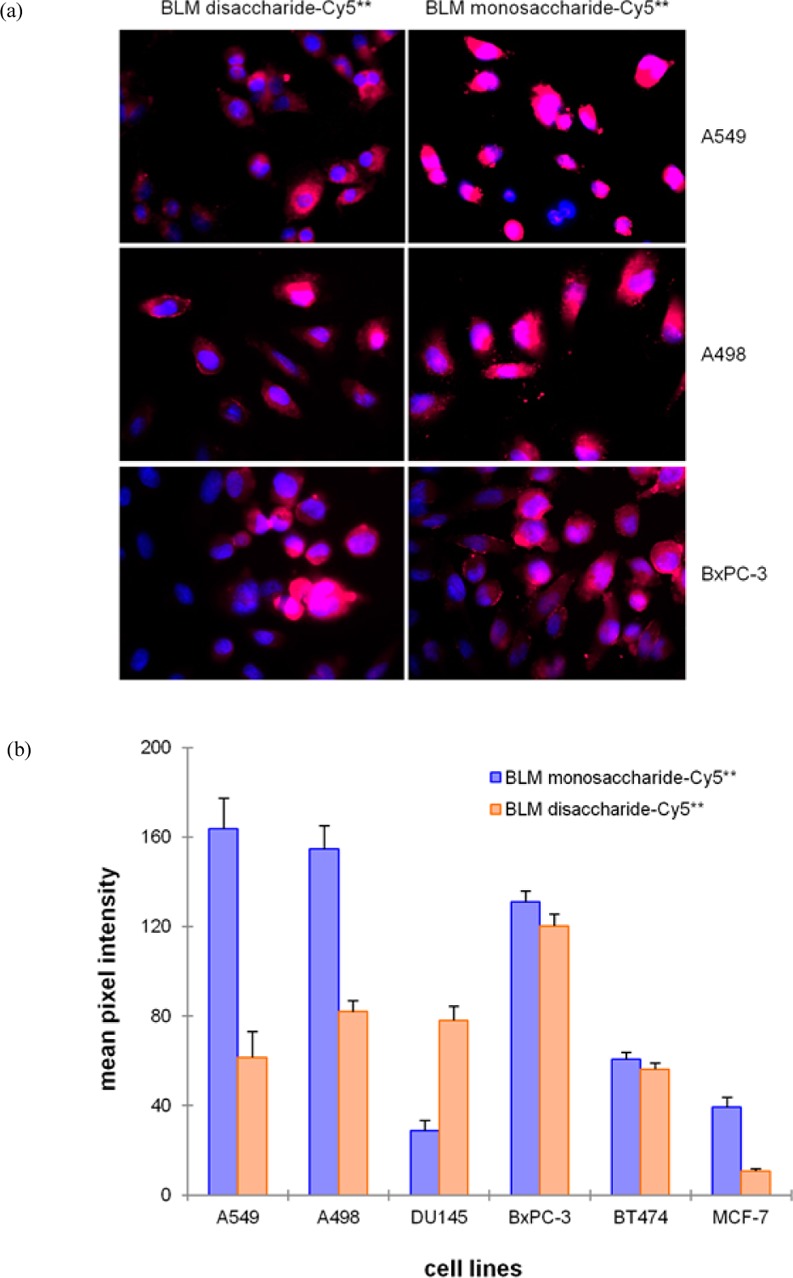
(a) Comparison of binding/uptake of BLM disaccharide–Cy5** and BLM monosaccharide–Cy5** conjugates by A549, A498, and BxPC-3 cell lines. The cells were treated with 25 μM BLM disaccharide–Cy5** (Figure S1 of the Supporting Information) or BLM monosaccharide–Cy5** (1) conjugate at 37 °C for 1 h, washed with PBS, and fixed with 4% paraformaldehyde. The nuclei were stained with 2-(4-amidinophenyl)-6-indolecarbamidine (DAPI). Fluorescence imaging was conducted with a 3 s exposure time. (b) Quantification of the binding/uptake of BLM disaccharide–Cy5** and BLM monosaccharide–Cy5** conjugates in six cancer cells. The cells were treated with 25 μM dye conjugates and irradiated for 3 s prior to being imaged and then analyzed using a Zeiss Axiovert 200M inverted microscope, with a 40× oil objective.
To better define the importance of the carbamoyl group for cellular recognition, binding, and internalization, we conducted an experiment in which four cancer cell lines were treated with 25 μM decarbamoyl BLM monosaccharide–Cy5** (2) or BLM monosaccharide–Cy5** (1) conjugate and then fixed with 4% paraformaldehyde. After the samples had been exposed to a xenon lamp for 3 s, the fluorescent images (Figure 3a and Figure S4 of the Supporting Information) were recorded. None of the four cell lines bound decarbamoyl BLM monosaccharide–Cy5** (2) to a significant extent, indicating that the carbamoyl group is essential for tumor cell binding/uptake. The quantified binding/uptake results are summarized in Figure 3b.
Figure 3.
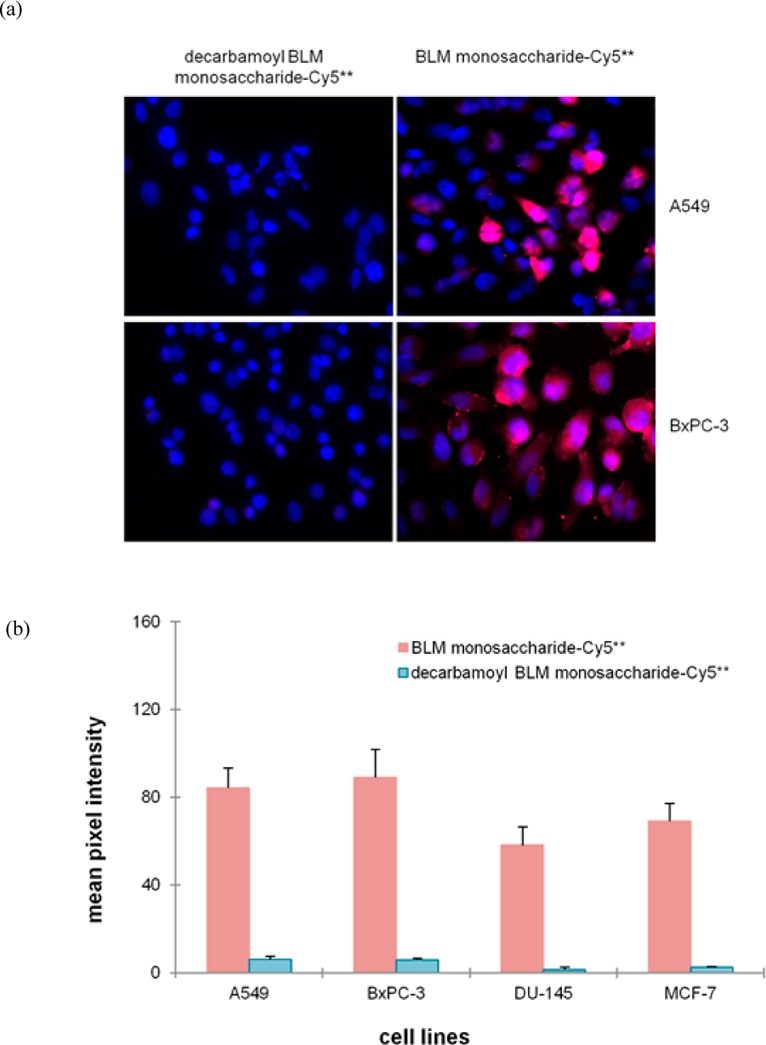
(a) Binding/uptake of decarbamoyl BLM monosaccharide–Cy5** and BLM monosaccharide–Cy5** conjugates in A549 and BxPC-3 cell lines. The cells were treated with 25 μM decarbamoyl BLM monosaccharide–Cy5** or BLM monosaccharide–Cy5** conjugate at 37 °C for 1 h, washed and fixed. The nuclei were stained with DAPI. Fluorescence imaging was conducted with a 3 s exposure time. (b) Quantification of the binding/uptake of decarbamoyl monosaccharide–Cy5** and BLM monosaccharide–Cy5** conjugates in four cancer cell lines. The cells were treated with 25 μM dye conjugates and irradiated for 3 s prior to being imaged.
Cellular receptors for carbohydrates frequently exhibit multivalency. Accordingly, we studied the cellular targeting and uptake of a Cy5** conjugate containing a cluster of three carbamoylmannose molecules (3). The BLM monosaccharide–Cy5** trimer (3) and the BLM monosaccharide–Cy5** conjugate (1) were studied in six cancer cell lines. As shown in Figure 4a for the A498 and A549 cell lines, the binding/uptake of the BLM monosaccharide–Cy5** trimer was greater than that of the BLM monosaccharide–Cy5** conjugate. The same finding was made in four additional cell lines (Figure S5 of the Supporting Information). The quantified data are shown (Figure 4b) and reflect the (1.6–2.3-fold) greater binding/uptake of the BLM monosaccharide–Cy5** trimer in the cell lines, consistent with the targeting of some cell surface carbohydrate receptor.
Figure 4.
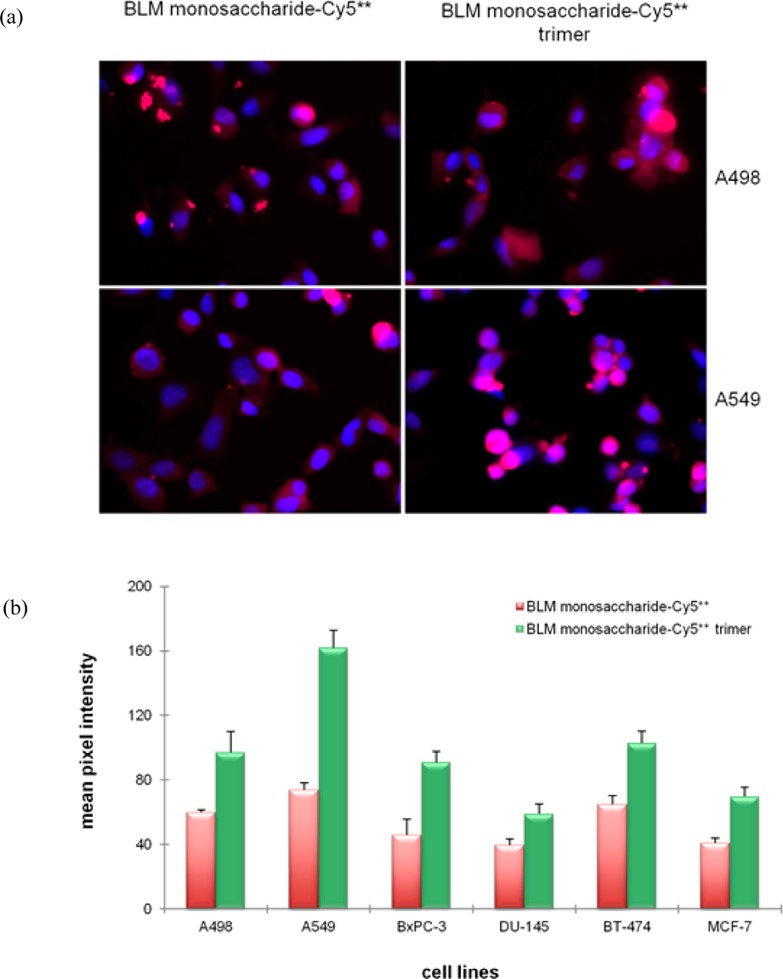
(a) Binding/uptake of BLM monosaccharide–Cy5** and BLM monosaccharide–Cy5** trimer conjugates in A498 and A549 cell lines. Cells were treated with 25 μM BLM monosaccharide–Cy5** conjugate or BLM monosaccharide–Cy5** trimer at 37 °C for 1 h, washed and fixed. The nuclei were stained with DAPI. Fluorescence imaging was conducted with a 3 s exposure time. (b) Quantification of the binding/uptake of the BLM monosaccharide–Cy5** conjugate and the BLM monosaccharide–Cy5** trimer in six cancer cells. The cells were treated with 25 μM dye conjugates and irradiated for 3 s prior to being imaged.
As for the BLM disaccharide–Cy5** conjugate, the BLM monosaccharide–Cy5** conjugate presumably first binds to a cell surface receptor followed by internalization. This model is supported by the results obtained with the BLM monosaccharide–Cy5** trimer, which showed enhanced uptake in each of six cell lines (Figure 4). The lack of cell surface fluorescence presumably indicates that internalization is rapid relative to cell binding. For the BLM disaccharide, evidence in support of this model was obtained by attaching multiple BLM disaccharides to the surface of a microbubble, which was too large to be internalized readily.8 The derivatized microbubbles were shown to be bound specifically to cancer cells. Here, the majority of fluorescence within the cells was found to colocalize with DAPI, suggesting that the conjugates were present within the nucleus. The use of fixed cells for this analysis after a defined period of incubation imposes some limitations on the conclusions that can be drawn from these observations, as does the lack of information regarding the mechanism(s) of cellular uptake and the receptor(s) responsible. While entirely within the realm of speculation at present, the selective recognition of numerous cancer cell lines suggests that the carbamoylmannose moiety may recognize some cell surface structural alteration resulting from a process common to most cancer cells, such as the well-known metabolic shift to energy production from glycolysis as compared with oxidative phosphorylation.
In addition to its enhanced efficiency of uptake, the smaller size and complexity of the BLM monosaccharide relative to the BLM disaccharide should facilitate the preparation of conjugates for potential diagnostic and therapeutic applications. The increase in binding/uptake observed for the BLM monosaccharide–Cy5** trimer provides evidence of the nature of the receptor, and a tool for further studying and exploiting the tumor targeting properties of 3-O-carbamoylmannose.
Supporting Information Available
Procedures for the synthesis and characterization of the BLM monosaccharide–Cy5** conjugates and data pertinent to their interaction with specific cancer cell lines. This material is available free of charge via the Internet at http://pubs.acs.org.
This work was supported by National Institutes of Health Grant CA140471, awarded by the National Cancer Institute.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Hecht S. M. (2012) Bleomycin group antitumor antibiotics. In Anticancer Agents from Natural Products (Cragg G. M., Kingston D. G. I., and Newman D. J., Eds.) 2nd ed., pp 451–478, CRC Press, Boca Raton, FL. [Google Scholar]
- Umezawa H.; Maeda K.; Takeuchi T.; Okami Y. (1966) J. Antibiot. 23, 200–209. [PubMed] [Google Scholar]
- Umezawa H. (1979) Advances in bleomycin studies. In Bleomycin: Chemical, Biochemical and Biological Aspects (Hecht S. M., Ed.) pp 24–36, Springer-Verlag, New York. [Google Scholar]
- Sikic B. I., Rozencweig M., and Carter S. K., Eds. (1985) Bleomycin Chemotherapy, Academic Press, Orlando, FL. [Google Scholar]
- Jones S. E.; Lilien D. L.; O’Mara R. E.; Durie B. G.; Salmon S. E. (1975) Med. Pediatr. Oncol. 1, 11. [DOI] [PubMed] [Google Scholar]
- Chapuis J. C.; Schmaltz R. M.; Tsosie K. S.; Belohlavek M.; Hecht S. M. (2009) J. Am. Chem. Soc. 131, 2438–2439and references therein. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B.; Hecht S. M. (2014) J. Am. Chem. Soc. 136, 4382–4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Schmaltz R. M.; Bozeman T. C.; Paul R.; Rishel M. J.; Tsosie K. S.; Hecht S. M. (2013) J. Am. Chem. Soc. 135, 2883–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boger D. L.; Honda T. (1994) J. Am. Chem. Soc. 116, 5647–5656. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


