Abstract
Renal ischemia-reperfusion leads to acute kidney injury (AKI) that is characterized pathologically by tubular damage and cell death, followed by tubular repair, atrophy and interstitial fibrosis. Recent work suggested the possible presence of DNA damage response (DDR) in AKI. However, the evidence is sketchy and the role and regulation of DDR in ischemic AKI remain elusive. In this study, we demonstrated the induction of phosphorylation of ATM, H2AX, Chk2 and p53 during renal ischemia-reperfusion in mice, suggesting DDR in kidney tissues. DDR was also induced in vitro during the recovery or “reperfusion” of renal proximal tubular cells (RPTCs) after ATP-depletion. DDR in RPTCs was abrogated by supplying glucose to maintain ATP via glycolysis, indicating that the DDR depends on ATP depletion. The DDR was also suppressed by the general caspase inhibitor z-VAD and the overexpression of Bcl-2, supporting a role of apoptosis-associated DNA damage in the DDR. N-acetylcysteine (NAC), an antioxidant, suppressed the phosphorylation of ATM and p53 and, to a less extent, Chk2, but NAC increased the phosphorylation and nuclear foci formation of H2AX. Interestingly, NAC increased apoptosis, which may account for the observed H2AX activation. Ku55933, an ATM inhibitor, blocked ATM phosphorylation and ameliorated the phosphorylation of Chk2 and p53, but it increased H2AX phosphorylation and nuclear foci formation. Ku55933 also increased apoptosis in RPTCs following ATP-depletion. The results suggest that DDR occurs during renal ischemia-reperfusion in vivo and ATP-depletion injury in vitro. The DDR is partially induced by apoptosis and oxidative stress-related DNA damage. ATM, as a sensor in the DDR, may play a cytoprotective role against tubular cell injury and death.
INTRODUCTION
Renal ischemia-reperfusion is a major cause of acute kidney injury (AKI), which leads to high mortality in patients and may progress to chronic kidney disease. Pathologically, ischemic AKI is characterized by sublethal and lethal damages in renal tubules, especially the proximal tubules [1,2]. Cell death in renal tubules in the forms of both apoptosis and necrosis is detected in animal models as well as the kidneys of AKI patients. Interestingly, renal tubules possess the capacity of repair and when the repair is incomplete, fibrosis or “scar” develops, contributing to gradual loss of renal function and chronic deficiency[3,4]. The molecular basis of tubular cell death and repair remains poorly understood.
DNA damage occurs in a variety of conditions such as irradiation, UV exposure, genotoxic chemical challenge, and oxidative stress or free radical insult. In response to DNA damage, cells activate a network of signaling pathways known as DNA damage response (DDR). DDR starts from the activation of the ‘sensor’ protein kinases, including the phosphoinositol 3-kinase-like serine/threonine protein kinases ataxia telangiectasis mutated (ATM), ATM- and Rad3-related (ATR), and DNA-dependent protein kinase (DNA-PK). The ‘sensor’ kinases relay the signal to the ‘executors’ kinases, especially Check-point kinases (Chk1 and Chk2), which then phosphorylate a multitude of proteins to induce cell cycle arrest or, in the presence of severe DNA damage, cell death [5-7].
DNA damage, such as the accumulation of 8-oxo-dG and apoptosis-associated DNA cleavage in renal tubular cells, is known to occur in kidney tissues following ischemiareperfusion [8-10]. In experimental models of renal ischemia-reperfusion, p53 is activated and inhibition of p53 provides renoprotective effects, implying the possibility of DDR [11,12]. However, DDR has not been studied in kidney cells or tissues during renal ischemia-reperfusion. In cisplatin-induced AKI, we have delineated a DDR signaling pathway that involves the activation of ATR followed by Chk2 activation and the phosphorylation of p53, leading to the expression of apoptotic genes, such as PUMA-α to result in tubular cell death [13-15]. Whether DDR occurs in renal ischemia-reperfusion and what role it plays in kidney injury and repair remain largely unknown.
In the present study, we detected DDR in kidney tissues during ischemia-reperfusion in mice and in ATP-depleted renal tubular cells. The DDR was suppressed to various extents by the ATM inhibitor Ku55933, the antioxidant N-acetylcysteine, the general caspase inhibitor z-VAD and overexpression of Bcl-2. It is suggested that DDR occurs in ischemic AKI via multiple mechanisms, including apoptotic DNA fragmentation and oxidative stress.
MATERIALS AND METHODS
Reagents and Antibodies
Antibodies were from the following sources: anti-Chk1 from Epitomics (Burlingame, CA); anti-Chk2, Anti-p53, anti-γH2AX, anti-PARP and anti-phosphorylated Chk1(serine 345) from Cell Signaling Technology (Beverly, MA), anti-phosphrylated Chk2(Thronine-68) from Novus(Littleton, CO), anti-ATM antibody from Abcam(Cambridge, MA), anti-phosphorylated ATM(serine-1981) from Millipore (Billerica, MA); anti-β-actin from Sigma (St. Louis, MO); all secondary antibodies from Jackson ImmunoResearch (West Grove, PA). Ku55933 was purchased from Selleck (Houston, TX). Other reagents and chemicals including azide were purchased from Sigma (St. Louis, MO).
Renal ischemie-reperfusion
Male mice of 8 to 10 weeks old C57BL/6 mice were purchased from Jackson Laboratory (Bar Harbor, MN) and maintained in the animal facility of Charlie Norwood VA Medical Center under a 12-hour light/12-hour dark pattern with free access to food and water. All animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee of Charlie Norwood VA Medical Center. Renal ischemia-reperfusion was induced as detailed recently[16]. Briefly, after anesthetization with pentobarbital (50mg/kg, i.p.), the mice were kept on a Homoeothermic Blanket Control Unit (Harvard Apparatus Ltd, UK) with a rectal probe to monitor and maintain the body temperature at 36.4°C. Flank incisions were made to expose both renal pedicles for bilateral clamping to induce 30 minutes of renal ischemia. The clamps were then released for reperfusion. Kidneys were collected after 48 hours of reperfusion for the following examinations. Color changes of kidneys during the initiation of clamping and after removal of clamps were observed to monitor the renal ischemia and reperfusion. Control animals were subjected to sham operation without renal pedicle clamping. Renal function was monitored by measuring BUN and serum creatinine using analytical kits from Biotron Diagnostics (Hemet,CA) and Stanbio Laboratory (Boerne, TX), respectively.
Immunohistochemistry
Kidneys were collected and fixed in 4% paraformaldehyde, paraffin embedded, and sectioned at 4 μm. The tissue sections were then deparaffinized and rehydrated, Antigen retrieval was conducted by 1 h of incubation at 95°C in 0.01 M sodium citrate, pH 6.0. The tissues were subsequently incubated with 3% H2O2 at 37°C for 30min to block the endogenous peroxidase activity. Then, the tissues were incubated sequentially with a blocking buffer (2% BSA, 0.2% nonfat dry milk, 0.8% Triton X-100, and 2% normal donkey serum) and the anti-γH2AX antibody (1:250 dilution, Cell Signalling, Danvers, USA) in the blocking buffer. After incubation with biotin-labeled secondary antibody, the color was developed with an ABC kit from Vector Laboratories (Burlingame, CA). The staining was evaluated in a blinded manner.
Cell ATP-depletion and recovery
The rat kidney proximal tubular cell line (RPTC) was originally obtained from Dr. U. Hopfer (Case Western Reserve Univ., Cleveland, OH) and cultured in serum-supplemented Ham's F-12/Dulbecco's modified Eagle's medium with 17.5 mM glucose as described previously[17,18]. Stable Bcl-2-overexpressing RPTC were generated as described before [18]. For experiment, the cells were seeded in 35mm collagen-coated dishes. After overnight growth, the cells were washed with phosphate-buffered saline and subjected to ATP depletion by incubation in glucose-free Krebs-Ringer bicarbonate buffer containing 10 mM azide. Following ATP-depletion, the cells were incubated in full culture medium without azide for recovery or ‘reperfusion’.
Apoptosis evaluation
Cells were fixed with 4% paraformaldehyde and the nuclei were stained by Hoechst 33342. The apoptotic cells were identified by the apoptotic morphology under phase contrast microscope and the condensed nuclei under fluorescent microscope.
Immunoblot analysis
A standard protocol of immunoblot analysis was followed. Briefly, the protein concentration of cell lysate was determined by using the BCA reagent (Pierce, Rockford, IL). Equal amounts of proteins (20μg) were loaded for reducing SDS-gel electrophoresis, followed by transferring onto polyvinylidene difluoride membranes. The blots were then incubated sequentially in blocking buffer, primary antibody, and the horseradish peroxidaseconjugated secondary antibody. Finally, the antigens on the blots were revealed using the enhanced chemiluminescence kit from Pierce. All primary antibodies were used at 1:1000 dilutions for immunoblot analysis.
Immunofluorescence
Cells were grown on glass coverslips for indirect immunofluorescence as described previously[13]. Briefly, the cells were fixed with 4% paraformaldehyde and permeabilized with 0.4% Triton X-100 in a blocking buffer. The cells were then exposed to primary antibodies, followed by incubation with Cy3-labeled donkey anti-rabbit secondary antibodies. The nuclei were counter-stained by Hoechst 33342. After washes, the coverslips were mounted on slides with Antifade (Life Technologies, Grand Island, NY) for examination by confocal microscopy using Cy3 and DAPI channels.
MTT assay
After azide treatment, the cells were recovered for 24 h in full culture medium to determine cell viability using MTT assay kit according to the manufacture's instruction (Molecular Probes, Eugene, OR).
Statistics
All data were analyzed by Microsoft Excel 2010 or SPSS. Student t-test or One-way ANOVA analysis was used for statistics and P<0.05 was considered as significantly different.
RESULTS
DDR is induced in kidney tissues during renal ischemia-reperfusion
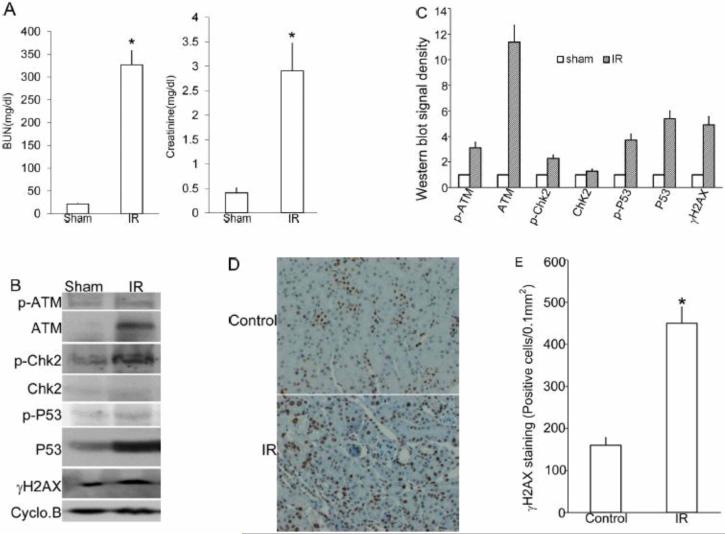
We used a commonly used mouse model of renal ischemia-reperfusion to examine DDR in kidney tissues. As shown in Figure 1A, 30 minutes of bilateral renal ischemia followed by 48 hours of reperfusion induced the loss of renal function as indicated by significant increases in blood urea nitrogen (BUN) and serum creatinine(Figure 1A). To analyze DDR, we first examined the expression of ATM, a DNA damage sensor that is rapidly recruited to the double strand breaks by the Mre11-Rad50-Nbs1 complex to phosphorylate the downstream ‘effector’ protein kinases including Chk2 [19-21]. As shown in Figure 1B and C, ATM expression was barely detectable in control tissues, but it was induced dramatically in mouse kidney tissues after ischemia-reperfusion. ATM phosphorylation at Ser1981 was also moderately induced by renal ischemia-reperfusion. Correlatively, Chk2 phosphorylation was increased, which was accompanied with increases in both total and phosphorylated p53 (ser-15) (Figure 1B). In mammalian cells, histone H2AX phosphorylation at ser139 by ATM is a biochemical hallmark of DDR. Phosphorylated H2AX, also called γH2AX, accumulates at the DNA damage site to recruit DNA repair complex to form nuclei foci [22,23]. γH2AX was low in sham operated kidneys and was induced following ischemia-reperfusion. γH2AX positive tubular epithelial cells were significantly increased in ischemia-reperfusion cortex tissues compared to the sham control (Fig. 1D, E), further implying the induction of DDR following ischemia-reperfusion. Together, these results indicate the induction of DDR during renal ischemia-reperfusion.
Figure 1. DDR is induced in kidney tissues during ischemia-reperfusion in mice.
C57BL/6 male mice were subjected to sham operation or 30 minutes of bilateral renal ischemia, followed by 48 hours of reperfusion. (A) Blood samples were collected for the measurement of blood urea nitrogen and serum creatinine. Data are presented as mean ±SD (n=7); *, p<0.05 vs. sham control. (B) Kidney cortex and outer medulla tissue were collected for immunoblot analysis of phosphorylated (Serine 1981) ATM, total ATM, phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine 15) p53, total p53, phosphorylated H2AX (γH2AX), and cyclophilin B as the protein loading control. (C) Quantitative analysis of the ratio of phosphorylated (Serine 1981) ATM, total ATM, phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine 15) p53, total p53, phosphorylated H2AX (γH2AX) to Cyclophilin B. Data are mean±SD (n=3). (D) Representative images of immunohischemistry staining of γH2AX in kidney cortex, It demonstrates that γH2AX is expressed in the nuclei. (E) Quantitative analysis of γH2AX -positive tubular epithelial cells in Fig.(1D). γH2AX -positive cells were quantified by cell counting in comparable regions of the tissues. Data are shown as means±SD (n=5). *, P<0.05 vs. Sham control.
DDR is induced in RPTCs following azide-induced ATP depletion injury
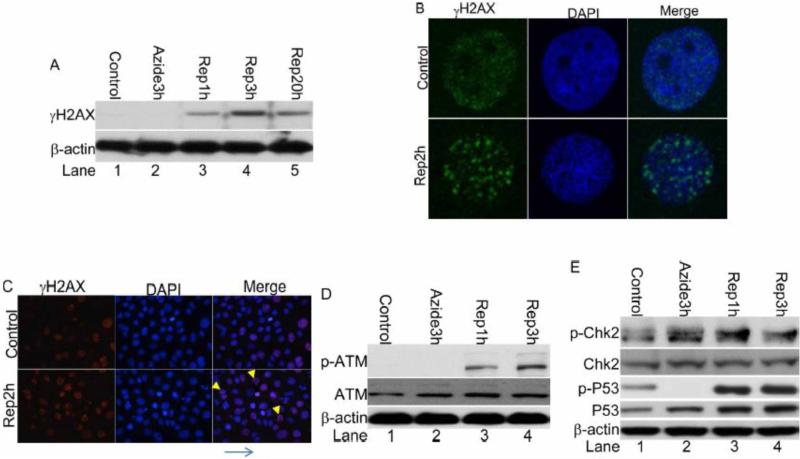
To study the mechanism of DDR in renal ischemia-reperfusion, we used an in vitro model of ‘chemical hypoxia’, in which RPTCs were incubated with azide (an inhibitor of respiratory complex IV) in a glucose-free buffer to induced ATP-depletion. After ATP-depletion, the cells were returned to full culture medium for recovery to mimic reperfusion. This in vitro model was used to investigate cell injury in renal ischemia-reperfusion in our recent studies[17,24,25]. γH2AX was not detected in control cells and was not induced during ATP-depletion (Figure 2A: lane 1, 2). However, after ATP-depleted cells were returned to full culture medium for recovery or ‘reperfusion’, γH2AX was induced at 1 hour, reached a higher level at 3 hours and remained to be elevated after 20 hours (Figure 2A: Lane 3-5). Immunofluorescence showed weak, diffuse signals of γH2AX in the nuclei of most control cells. However, after ATP depletion and recovery, γH2AX accumulated to form nuclear foci (Figure 2B). Interestingly, a significant population of the γH2AX positive nuclei were not apoptotic as indicated by double staining with DAPI (Figure 2C). The phosphorylation of ATM, Chk2 and p53 was increased during recovery of ATP-depleted cells (Figures 2 D, E: Lane 3,4), further confirming the induction of DDR in this in vitro model.
Figure 2. DDR is induced following ATP-depletion injury in RPTC.
RPTC cells were left untreated (control) or treated with 10 mM sodium azide for 3 hours, followed by recovery in full medium for 0, 1, 2, 3 or 20 hours as indicated. (A) Whole cell lysate was collected for immunoblot analysis of phosphorylated H2AX (γH2AX), and β-actin (sample loading control); (B, C) The cells were fixed and subjected to immunofluorescence staining of phosphorylated H2AX (γH2AX). (D) Whole cell lysate was collected for immunoblot analysis of phosphorylated (Serine 1981) ATM, total ATM and β-actin. (E) Whole cell lysate was collected for immunoblot analysis of phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Ser15) p53, total p53, and β-actin.
Glucose added during azide treatment prevents DDR
What triggers DDR during the recovery of ATP-depleted RPTCs? With this question, we first determined if the DDR is related to ATP-depletion. Previous work demonstrated that glucose provided during mitochondrial inhibition enables cellular ATP production via glycolysis [18]. Thus we examined the effect of glucose added during azide treatment. As shown in Figure 3, the addition of 5 and 10 mM glucose during azide treatment attenuated the induction of γH2AX and the phosphorylation of ATM at 1 and 3 hours of recovery(Lane 4,5 vs. 3, Lane 7,8 vs. 6), indicating that DDR in this model is causally related to the initial ATP depletion.
Figure 3. Glucose added during azide treatment prevents DDR.
RPTC cells were left untreated (control) or treated with 10mM sodium azide for 3 hours in the presence or absence of 5 or 10mM D-Glucose, followed by recovery in full medium for 0, 1, or 3 hours. Whole cell lysate was collected for immunoblot analysis of phosphorylated (Serine 1981) ATM and phosphorylated H2AX (γH2AX). β-actin was used as a loading control.
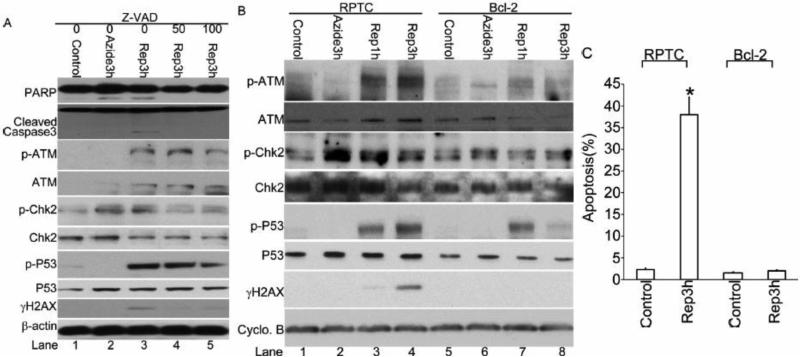
Inhibition of DDR following azide treatment by Z-VAD and Bcl-2
We showed previously that renal ischemia-reperfusion and ATP depletion in RPTCs activate the mitochondrial pathway of apoptosis [17,24,26]. Since apoptosis is associated with extensive internucleosomal DNA cleavage, we reasoned that the DDR observed during the recovery period of ATP-depleted cells may be partly attributable to apoptosis. To test this possibility, the pancaspase inhibitor Z-VAD was added during ATP depletion and the subsequent recovery to suppress apoptosis. As expected, 50 and 100μM Z-VAD blocked the cleavage of caspase 3 and PARP, a biochemical hallmark of apoptosis, during azide treatment and 3 hours of recovery (Figure 4A: lanes 4, 5 vs. 3). Notably, Z-VAD also attenuated γH2AX expression, while the phosphorylation of ATM, Chk2, and p53 was only marginally inhibited by Z-VAD (Figure 4A: lanes 4, 5 vs. 3).
Figure 4. Inhibition of DDR following azide treatment by Z-VAD and Bcl-2.
(A) RPTC cells were left untreated (control) or treated with 10 mM sodium azide for 3 hours followed by recovery of 0, 1, or 3 hours in the presence or absence of 50 or 100μM Z-VAD. Whole cell lysate was collected for immunoblot analysis of phosphorylated (Serine 1981) ATM, phosphorylated H2AX (γH2AX), PARP, cleaved Caspase3, phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine 15) p53, total p53, and β-actin as a loading control. (B) Regular and Bcl-2-overexpressing RPTCs were left untreated (control) or treated with 10 mM sodium azide for 3 hours followed by reperfusion of 0, 1, or 3 hours. Whole cell lysate was collected for immunoblot analysis of phosphorylated (Serine 1981) ATM, phosphorylated H2AX (γH2AX), phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine 15) p53, total p53, and Cyclophilin B as a loading control. (C) The percentage of apoptosis in RPTC and Bcl-2 cells after azide treatment and reperfusion was determined by counting the cells with typical apoptotic morphology.
We further examined the effect of overexpression of Bcl-2, a well-known anti-apoptotic gene. As shown in Figure 4B (Lane 7, 8 vs. 3, 4), Bcl-2 overexpression totally blocked γH2AX induction and significantly blocked ATM, Chk2 and p53 phosphorylation. As shown previously[17,26], azide-induced apoptosis was markedly suppressed in Bcl-2-overexpressing cells (Fig.4C). Together, these results suggest that apoptosis-associated DNA cleavage contributes significantly to the DDR observed in this ATP-depletion/recovery model.
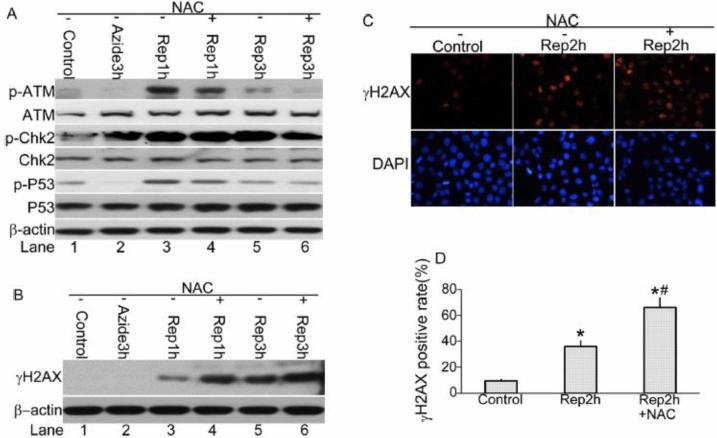
N-acetylcysteine suppresses ATM activation during recovery of ATP-depleted cells
Mitochondria are an important source for reactive oxygen species (ROS) generation inside the cells[27]. The generation of ROS in the form of superoxide anion is only 3 to 5% of total oxygen consumed during normal oxidative phosphorylation[28]. However, under conditions when oxidative phosphorylation is inhibited, this rate of ROS production can be increased greatly[29,30]. Oxidative stress resulting from ROS is a known pathogenic factor in renal ischemia/reperfusion injury [31]. ROS inflict damage on lipids, proteins and DNAs[32-35]. In order to clarify the role of ROS in the DDR observed during recovery of ATP-depleted cells, we examined the effect of N-acetylcysteine (NAC), a general antioxidant. As shown in Figure 5A (Lane 4 vs. 3, Lane 6 vs. 5), NAC suppressed the phosphorylation of ATM (Ser1981), Chk2 (Thr68) and p53 (Ser15) at 1 and 3 hours of recovery following azide-induced ATP depletion. Surprisingly, γH2AX level was not suppressed, but further induced by NAC (Figure 5B). Immunofluorescence staining detected ~35% cells with γH2AX nuclear foci during ATP depletion /recovery, which was increased to over 60% by NAC (Figure 5C, D).
Figure 5. NAC suppresses ATM activation following azide treatment.
RPTC cells were left untreated (control) or treated with 10mM sodium azide for 3hours followed by recovery in the presence or absence of 10mM NAC. (A) Whole cell lysate was collected at the indicated recovery time for immunoblot analysis of phosphorylated (Serine 1981) ATM, phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine 15) p53, total p53, and β-actin as a loading control. (B) Whole cell lysate was collected at the indicated recovery time for immunoblot analysis of phosphorylated H2AX (γH2AX), and β-actin as a loading control. (C) The cells were fixed at 2 hours of recovery and subjected to immunofluorescence staining of γH2AX. (D) The cells with more than 3 γH2AX nuclear foci were counted to determine the percentage of γH2AX foci positive cells. *, P<0.05 vs. Control; #, p<0.05 vs. Rep2h.
NAC increases apoptosis during recovery of ATP-depleted cells
The γH2AX increase by NAC during recovery of ATP-depleted RPTCs in above Figure 5 was intriguing. We noticed that NAC increased apoptosis in this experiment (Figure 6A). For quantification, we counted the cells with typical apoptotic morphology. As shown in Figure 6B, 3 hours of recovery of azide-treated cells induced ~40% apoptosis, which was further increased to ~60% by NAC. With this result, we considered the possibility that the γH2AX induction by NAC is secondary to the higher level of apoptosis and the associated DNA damage. In line with this possibility, the caspase inhibitor Z-VAD diminished γH2AX expression in the presence of NAC (Figure 6C). NAC treatment also decreased cell survival (Figure 6D),
Figure 6. NAC increases apoptosis, induces γH2AX, and decreases cell survival following azide treatment.
RPTCs were left untreated (control) or treated with 10 mM sodium azide for 3 hours and then followed by recovery in the presence or absence of 10 mM NAC. (A, B) At 3 hours of recovery, the cells were stained with Hoechst 33342 to record cellular morphology by phase contrast microscopy and nuclear morphology by fluorescence microscopy (A). (B) The percentage of apoptosis in each condition was determined by counting the cells with typical apoptotic morphology. (C) RTPCs were recovered for 0 or 1hour in the presence or absence of 10 mM NAC, 100 μM Z-VAD, or 10 mM NAC+100 μM Z-VAD. Whole cell lysate was collected for immunoblot analysis of phosphorylated H2AX (γH2AX) and β-actin ( loading control). (D) RPTCs were left untreated (control) or treated with 10 mM sodium azide for 3 hours and then followed by reperfusion of 3 hours in the presence or absence of 10 mM NAC. After the treatment, the cells were recovered for 24 h in full culture medium to determine cell viability by MTT assay. Quantitative data are expressed as mean±SD (n=3). *, P<0.05 vs. Control, #, p<0.05 vs. Azide group.
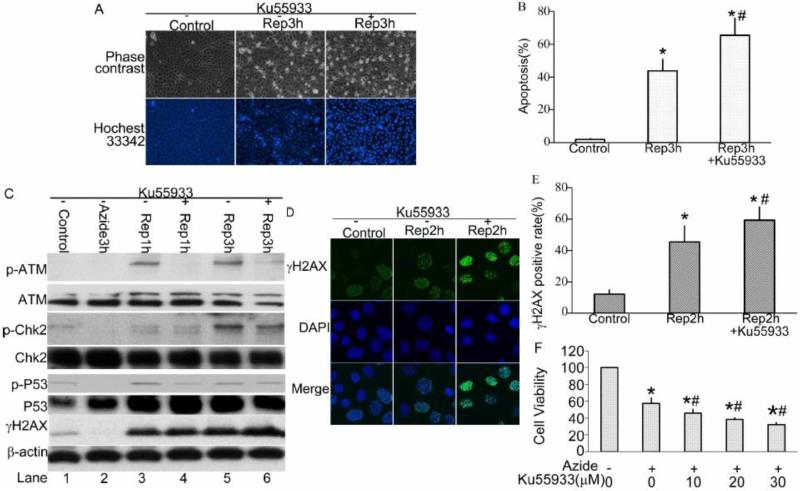
Inhibition of ATM increases apoptosis and γH2AX following ATP-depletion and decreases cell survival
ATM is an early sensor in DDR. Depending on the cellular context, ATM may activate the signaling pathway of cell death or of DNA repair. To determine the role of ATM activation during recovery of ATP-depleted cells, we tested the effect of Ku55933, a specific ATM inhibitor. Ku55933 suppressed ATM phosphorylation during 1 and 3 hours of recovery periods (Figure 7C: Lane 4 vs. 3, Lane 6 vs. 5). To some extent, Ku55933 also suppressed Chk2 and p53 phosphorylation (Figure 7C). Notably, Ku55933 increased apoptosis from ~44% to ~66% (Figure 7A, B). γH2AX expression seemed to be increased by Ku55933 as well, so did γH2AX nuclear foci formation (Figure 7C-E). Finally, we analyzed the effect of Ku55933 on long-term cell survival. To this end, cell viability was analyzed by MTT assay 24 hours after ATP-depletion treatment. As Shown in Figure 7F, Ku55933 decreased cell survival in a dose-dependent manner (Figure 7F). Thus, the inhibition of ATM increased cell death and decreased cell viability in this in vitro model, suggesting that ATM activation plays a cytoprotective role for cell survival.
Figure 7. Inhibition of ATM increases apoptosis and γH2AX and decreases cell survival following azide treatment.
RPTCs were left untreated (control) or treated with 10 mM sodium azide for 3 hours and then followed by recovery of 1, 2 or3 hours in the presence or absence of 10 μM Ku55933. (A, B) The cells were stained with Hoechst 33342 to record cellular morphology by phase contrast microscopy and nuclear morphology by fluorescence microscopy (A). The percentage of apoptosis in each condition was determined by counting the cells with typical apoptotic morphology (B). (C) Whole cell lysate was collected for immunoblot analysis of phosphorylated (Serine 1981) ATM, total ATM, phosphorylated (threonine 68) Chk2, total Chk2, phosphorylated (Serine15) p53, total p53, phosphorylated H2AX(γH2AX), total H2AX and β-actin (loading control). (D, E) RPTCs at 2 hours of reperfusion were fixed for immunofluorescence of γH2AX and nuclear staining with DAPI. The representative images of γH2AX and DAPI staining were recorded (D) and the cells with more than 3 γ-H2AX nuclear foci were counted to determine the percentage of γH2AX positive cells (E). (F) After the treatment, the cells were recovered for 24 h in full culture medium to determine cell viability by MTT assay. Quantitative data are expressed as mean±SD (n=3). *, P<0.05 vs. Control, #, p<0.05 vs. Azide group.
DISCUSSION
Ischemia-reperfusion injury in organs and tissues leads to the development of ischemic diseases, including myocardial infarction, stroke in the brain and acute kidney injury. DNA damage and DDR-related proteins were reported to play important roles during ischemia-reperfusion injury in brain and heart [36-39]. However, the evidence of DDR in renal ischemia-reperfusion injury is sketchy, though the accumulation of 8-oxo-dG and apoptosis-associated DNA cleavage in renal tubular cells is known to occur in kidney tissues following ischemia-reperfusion [8-10]. In the present study, we have demonstrated the evidence of DDR in kidney tissues following ischemicreperfusion injury in mice. Using the in vitro model of ‘chemical hypoxia’, we have further suggested the involvement of ATP-depletion, oxidative stress, and apoptosis in the induction of DDR in renal tubular cells.
In general, the DDR shown in the in vitro model recapitulates the main features of that of in vivo renal ischemia/reperfusion. Especially, the phosphorylation of ATM, H2AX, Chk2 and p53 that are indicative of the activation of these proteins was all increased in both the in vitro and in vivo models. The expression of total proteins showed some differences between these two models. For example, total ATM was markedly induced during renal IRI, but it did not change significantly during recovery or ‘reperfusion’ of ATP-depleted cells (Figures 1, 2). In addition, total p53 accumulation was more evident in reperfused tissues than ATP-depleted cells, whereas p53 phosphorylation at ser-15 was more evident in the cells. These differences may result from the differences between the in vitro and in vivo models. Obviously, kidney tissues are much more complex as there are multiple cell types and during renal IRI, inflammatory cells also infiltrate into renal interstitium[1,2,40].
p53 plays a role in ischemic kindey injury[11,41,42]. P53 and Chk2 regulate the G2/M transition in response to stress and DNA damage as well[43,44]. Enhanced expression of p-ATM and p-Chk2 was reported in the post-ischemic kidneys and G2/M arrest induced by enhanced expression of p-Chk2 is associated with profibrotic cytokines production and kidney fibrosis progression after ischemia reperfusion injury in kidney [45,46]. In this study, up regulated P53 and p-Chk2 both in ischemia-reperfused kidney cortex and ATP-depleted RPTC may participate in cellular repair and recovery by inducing cell cycle arrest, a possibility that needs to be tested in future study.
The signaling pathway activated during DDR is dependent on the type and extent of DNA damage. In our study, ATM showed an early phosphorylation or activation during the recovery of ATP-depleted cells, suggesting that ATM is a DNA damage sensor in this model. However, in the presence of NAC or Ku55933, while ATM activation was inhibited, γH2AX increased. Since both NAC and Ku55933 increased apoptosis during ATP-depletion/recovery, the observed γH2AX induction is likely related to apoptosis-associated DNA damage. Under this condition, other DNA damage sensors such as ATR and DNA-PK may be activated to phosphorylate H2AX, a possibility that needs to be investigated in future studies.
In our study, the addition of glucose during azide treatment suppressed DDR. Glucose is the major substrate of glycolysis that promotes ATP production in RPTCs, especially under conditions of mitochondrial inhibition[47]. The suppressive effect of glucose on DDR in the ‘chemical hypoxia’ model indicates that the DDR is a result of ATP-depletion-triggered cell injury. This is not surprising because ATP-depletion is largely responsible for the initiation of cell injury processes in this model, culminating in mitochondrial damage and ultimate cell death by apoptosis[17,26]. In this regard, our results also support a role of apoptosis in DDR in the ATP-depletion/recovery model. The work from us and others has demonstrated that renal tubular cells undergo apoptosis during ischemia-reperfusion in vivo and ATP-depletion in vitro via the intrinsic pathway mediated by mitochondrial injury [24,47]. In this apoptotic pathway, the activation of Bax and Bak leads to mitochondrial outer membrane permeabilization and the release of apoptotic factors including cytochrome c, which further activates caspases for the development of apoptosis. Bcl-2 antagonizes Bax and Bak to prevent mitochondrial permeabilization and inhibit apoptosis. In the present study, DDR was suppressed by the pancaspase inhibitor Z-VAD and Bcl-2, supporting the involvement of apoptosis in the DDR. Apoptosis is often associated with internucleosomal DNA cleavage, which occurs in kidney tissues during renal ischemia-reperfusion[10]. Thus, the DDR observed in the present study is partly attributable to apoptosis-associated DNA damage.
Although both Z-VAD and Bcl-2 blocked apoptosis in our study, the effect of Z-VAD on DDR was less than that of Bcl-2 (Figure 4). This observation suggests the presence of other mechanisms of DDR induction, in addition to apoptosis. The primary action of Bcl-2 is the protection of mitochondria, resulting in inhibition of caspase activation and apoptosis. Based on this consideration, our results support an apoptosis-independent role of mitochondrial damage in triggering DDR, although the underlying mechanism is currently unclear. It is suggested that there are two phases of DDR activation in the ATP-depletion/recovery model. The initial phase of DDR activation is indicated by the ATM activation, which is mainly due to mitochondrial injury, resulting in a mild γH2AX induction. The inhibitory effect of NAC suggests that oxidative stress may also contribute to the early activation of ATM, probably as a result of mitochondrial injury. The ATM activation in this study leads to the downstream phosphorylation of Chk2 and p53. Some reports show that NAC protects against drug and oxidative stress-induced apoptosis[48-53]. However, NAC has also been shown to induce apoptosis in multiple types of cells by increasing the pro-apoptotic Bax gene expression, or by inhibiting expression of antiapoptotic proteins Bcl-2, or via the ER stress response-signaling pathway[29,54,55]. Our studies show that NAC treatment enhances apoptosis and decreased cell survival following ATP-depletion injury in RPTC (Figure 6). NAC may exert this effect partially by inhibiting ATM, a surviving signaling pathway in this model, but further studies need to delineate the finding. The second phase of DDR is apoptosis or caspase activation related, and is mainly indicated by the massive induction of γH2AX. As discussed above, apoptosis-associated DDR seems less dependent on ATM, because Ku55933 completely blocked ATM activation yet increase γH2AX induction and apoptosis. According to our results, the initial phase of DDR mediated by ATM after mitochondrial injury may be cytoprotective, while the second phase of DDR indicated by massive γH2AX induction is mainly an indication of extensive DNA damage in apoptosis. ATM is known to activate the mechanism of DNA repair in DDR, which may account for the cytoprotective effect of ATM in our study. In addition, ATM may be involved in the regulation of anti-oxidant defense[56]. In support of this possibility, ATM mutation or deficient were reported to affect cellular antioxidant status, including decreased amounts of antioxidant molecules and glutathione biosynthesis in A-T cells and increased concentrations of superoxide anions in the cerebella in ATM–/– mice[57-59].
In conclusion, this study has demonstrated the occurrence of DDR during renal ischemiareperfusion in vivo and ATP-depletion injury in vitro. The DDR may consist of two phases (Figure 8). The first phase of DDR is mediated by ATM and depends on mitochondrial injury, whereas the second phase is indicated by γH2AX induction and is consequent to tubular cell apoptosis. ATM-mediated DDR may have a cytoprotective role. Modulation of the DDR may therefore offer a new strategy for kidney protection during renal ischemia-reperfusion.
Figure 8.
A schematic summary of DDR in cell death and survival following ischemia reperfusion.
Highlights.
DNA damage response (DDR) is induced in renal ischemia-reperfusion.
Apoptosis contributes partially to the observed DDR.
ATM is a sensor in the DDR and plays a cytoprotective role.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bonventre JV, Yang L. Cellular pathophysiology of ischemic acute kidney injury. J Clin Invest. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharfuddin AA, Molitoris BA. Pathophysiology of ischemic acute kidney injury. Nat Rev Nephrol. 2011;7:189–200. doi: 10.1038/nrneph.2011.16. [DOI] [PubMed] [Google Scholar]
- 3.Chawla LS, Kimmel PL. Acute kidney injury and chronic kidney disease: an integrated clinical syndrome. Kidney Int. 2012;82:516–524. doi: 10.1038/ki.2012.208. [DOI] [PubMed] [Google Scholar]
- 4.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, et al. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol. 2010;298:F1078–1094. doi: 10.1152/ajprenal.00017.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McGowan CH, Russell P. The DNA damage response: sensing and signaling. Curr Opin Cell Biol. 2004;16:629–633. doi: 10.1016/j.ceb.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Flynn RL, Zou L. ATR: a master conductor of cellular responses to DNA replication stress. Trends Biochem Sci. 2011;36:133–140. doi: 10.1016/j.tibs.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sancar A, Lindsey-Boltz LA, Unsal-Kacmaz K, Linn S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu Rev Biochem. 2004;73:39–85. doi: 10.1146/annurev.biochem.73.011303.073723. [DOI] [PubMed] [Google Scholar]
- 8.Tsuruya K, Furuichi M, Tominaga Y, Shinozaki M, Tokumoto M, et al. Accumulation of 8-oxoguanine in the cellular DNA and the alteration of the OGG1 expression during ischemiareperfusion injury in the rat kidney. DNA Repair (Amst) 2003;2:211–229. doi: 10.1016/s1568-7864(02)00214-8. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida T, Shimizu A, Masuda Y, Mii A, Fujita E, et al. Caspase-3-independent internucleosomal DNA fragmentation in ischemic acute kidney injury. Nephron Exp Nephrol. 2012;120:e103–113. doi: 10.1159/000337358. [DOI] [PubMed] [Google Scholar]
- 10.Schumer M, Colombel MC, Sawczuk IS, Gobe G, Connor J, et al. Morphologic, biochemical, and molecular evidence of apoptosis during the reperfusion phase after brief periods of renal ischemia. Am J Pathol. 1992;140:831–838. [PMC free article] [PubMed] [Google Scholar]
- 11.Fujino T, Muhib S, Sato N, Hasebe N. Silencing of p53 RNA through transarterial delivery ameliorates renal tubular injury and down-regulates GSK-3beta expression after ischemiareperfusion injury. Am J Physiol Renal Physiol. 2013 doi: 10.1152/ajprenal.00279.2013. [DOI] [PubMed] [Google Scholar]
- 12.Kelly KJ, Plotkin Z, Vulgamott SL, Dagher PC. P53 mediates the apoptotic response to GTP depletion after renal ischemia-reperfusion: protective role of a p53 inhibitor. J Am Soc Nephrol. 2003;14:128–138. doi: 10.1097/01.asn.0000040596.23073.01. [DOI] [PubMed] [Google Scholar]
- 13.Pabla N, Ma Z, McIlhatton MA, Fishel R, Dong Z. hMSH2 recruits ATR to DNA damage sites for activation during DNA damage-induced apoptosis. J Biol Chem. 2011;286:10411–10418. doi: 10.1074/jbc.M110.210989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pabla N, Huang S, Mi QS, Daniel R, Dong Z. ATR-Chk2 signaling in p53 activation and DNA damage response during cisplatin-induced apoptosis. J Biol Chem. 2008;283:6572–6583. doi: 10.1074/jbc.M707568200. [DOI] [PubMed] [Google Scholar]
- 15.Jiang M, Wei Q, Wang J, Du Q, Yu J, et al. Regulation of PUMA-alpha by p53 in cisplatin-induced renal cell apoptosis. Oncogene. 2006;25:4056–4066. doi: 10.1038/sj.onc.1209440. [DOI] [PubMed] [Google Scholar]
- 16.Wei Q, Dong Z. Mouse model of ischemic acute kidney injury: Technical notes and tricks. Am J Physiol. 2012;303:F1487–1494. doi: 10.1152/ajprenal.00352.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saikumar P, Dong Z, Patel Y, Hall K, Hopfer U, et al. Role of hypoxia-induced Bax translocation and cytochrome c release in reoxygenation injury. Oncogene. 1998;17:3401–3415. doi: 10.1038/sj.onc.1202590. [DOI] [PubMed] [Google Scholar]
- 19.Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 2003;22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jazayeri A, Balestrini A, Garner E, Haber JE, Costanzo V. Mre11-Rad50-Nbs1-dependent processing of DNA breaks generates oligonucleotides that stimulate ATM activity. EMBO J. 2008;27:1953–1962. doi: 10.1038/emboj.2008.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiloh Y, Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat Rev Mol Cell Biol. 2013;14:197–210. [PubMed] [Google Scholar]
- 22.Sharma A, Singh K, Almasan A. Histone H2AX phosphorylation: a marker for DNA damage. Methods Mol Biol. 2012;920:613–626. doi: 10.1007/978-1-61779-998-3_40. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka T, Halicka D, Traganos F, Darzynkiewicz Z. Cytometric analysis of DNA damage: phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods Mol Biol. 2009;523:161–168. doi: 10.1007/978-1-59745-190-1_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei Q, Dong G, Chen JK, Ramesh G, Dong Z. Bax and Bak have critical roles in ischemic acute kidney injury in global and proximal tubule-specific knockout mouse models. Kidney Int. 2013;84:138–148. doi: 10.1038/ki.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang M, Wei Q, Dong G, Komatsu M, Su Y, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Z, Wang JZ, Yu F, Venkatachalam MA. Apoptosis-resistance of hypoxic cells: multiple factors involved and a role for IAP-2. Am J Pathol. 2003;163:663–671. doi: 10.1016/S0002-9440(10)63693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halliwell B, Gutteridge JM. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- 28.Boonstra J, Post JA. Molecular events associated with reactive oxygen species and cell cycle progression in mammalian cells. Gene. 2004;337:1–13. doi: 10.1016/j.gene.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 29.Qanungo S, Wang M, Nieminen AL. N-Acetyl-L-cysteine enhances apoptosis through inhibition of nuclear factor-kappaB in hypoxic murine embryonic fibroblasts. J Biol Chem. 2004;279:50455–50464. doi: 10.1074/jbc.M406749200. [DOI] [PubMed] [Google Scholar]
- 30.Roy A, Ganguly A, BoseDasgupta S, Das BB, Pal C, et al. Mitochondria-dependent reactive oxygen species-mediated programmed cell death induced by 3,3′-diindolylmethane through inhibition of F0F1-ATP synthase in unicellular protozoan parasite Leishmania donovani. Mol Pharmacol. 2008;74:1292–1307. doi: 10.1124/mol.108.050161. [DOI] [PubMed] [Google Scholar]
- 31.Basnakian AG, Kaushal GP, Shah SV. Apoptotic pathways of oxidative damage to renal tubular epithelial cells. Antioxid Redox Signal. 2002;4:915–924. doi: 10.1089/152308602762197452. [DOI] [PubMed] [Google Scholar]
- 32.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, et al. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 33.Barzilai A, Yamamoto K. DNA damage responses to oxidative stress. DNA Repair (Amst) 2004;3:1109–1115. doi: 10.1016/j.dnarep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivasan A, Lehmler HJ, Robertson LW, Ludewig G. Production of DNA strand breaks in vitro and reactive oxygen species in vitro and in HL-60 cells by PCB metabolites. Toxicol Sci. 2001;60:92–102. doi: 10.1093/toxsci/60.1.92. [DOI] [PubMed] [Google Scholar]
- 36.Liu PK, Hsu CY, Dizdaroglu M, Floyd RA, Kow YW, et al. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cordis GA, Bagchi D, Riedel W, Stohs SJ, Das DK. Implication of DNA damage during reperfusion of ischemic myocardium. Ann N Y Acad Sci. 1996;793:427–430. doi: 10.1111/j.1749-6632.1996.tb33535.x. [DOI] [PubMed] [Google Scholar]
- 38.Cui J, Holmes EH, Greene TG, Liu PK. Oxidative DNA damage precedes DNA fragmentation after experimental stroke in rat brain. FASEB J. 2000;14:955–967. doi: 10.1096/fasebj.14.7.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shukla PC, Singh KK, Quan A, Al-Omran M, Teoh H, et al. BRCA1 is an essential regulator of heart function and survival following myocardial infarction. Nat Commun. 2011;2:593. doi: 10.1038/ncomms1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinsey GR, Sharma R, Okusa MD. Regulatory T cells in AKI. J Am Soc Nephrol. 2013;24:1720–1726. doi: 10.1681/ASN.2013050502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang D, Liu Y, Wei Q, Huo Y, Liu K, Liu F, Dong Z. Tubular p53 regulates multiple genes to mediate acute kidney injury. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013080902. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, Ashush H, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor WR, Stark GR. Regulation of the G2/M transition by p53. Oncogene. 2001;20:1803–1815. doi: 10.1038/sj.onc.1204252. [DOI] [PubMed] [Google Scholar]
- 44.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 45.Jang HS, Han SJ, Kim JI, Lee S, Lipschutz JH, et al. Activation of ERK accelerates repair of renal tubular epithelial cells, whereas it inhibits progression of fibrosis following ischemia/reperfusion injury. Biochim Biophys Acta. 2013;1832:1998–2008. doi: 10.1016/j.bbadis.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Yang L, Besschetnova TY, Brooks CR, Shah JV, Bonventre JV. Epithelial cell cycle arrest in G2/M mediates kidney fibrosis after injury. Nat Med. 2010;16:535–543. 531p. doi: 10.1038/nm.2144. following 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saikumar P, Dong Z, Weinberg JM, Venkatachalam MA. Mechanisms of cell death in hypoxia/reoxygenation injury. Oncogene. 1998;17:3341–3349. doi: 10.1038/sj.onc.1202579. [DOI] [PubMed] [Google Scholar]
- 48.Gong X, Celsi G, Carlsson K, Norgren S, Chen M. N-acetylcysteine amide protects renal proximal tubular epithelial cells against iohexol-induced apoptosis by blocking p38 MAPK and iNOS signaling. Am J Nephrol. 2010;31:178–188. doi: 10.1159/000268161. [DOI] [PubMed] [Google Scholar]
- 49.Sun L, Gu L, Wang S, Yuan J, Yang H, et al. N-acetylcysteine protects against apoptosis through modulation of group I metabotropic glutamate receptor activity. PLoS One. 2012;7:e32503. doi: 10.1371/journal.pone.0032503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin HM, Zhou DC, Gu HF, Qiao QY, Fu SK, et al. Antioxidant N-acetylcysteine protects pancreatic beta-cells against aldosterone-induced oxidative stress and apoptosis in female db/db mice and insulin-producing MIN6 cells. Endocrinology. 2013;154:4068–4077. doi: 10.1210/en.2013-1115. [DOI] [PubMed] [Google Scholar]
- 51.Kim JH, Lee SS, Jung MH, Yeo HD, Kim HJ, et al. N-acetylcysteine attenuates glycerol-induced acute kidney injury by regulating MAPKs and Bcl-2 family proteins. Nephrol Dial Transplant. 2010;25:1435–1443. doi: 10.1093/ndt/gfp659. [DOI] [PubMed] [Google Scholar]
- 52.Ji YL, Wang H, Zhang C, Zhang Y, Zhao M, et al. N-acetylcysteine protects against cadmium-induced germ cell apoptosis by inhibiting endoplasmic reticulum stress in testes. Asian J Androl. 2013;15:290–296. doi: 10.1038/aja.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Low WK, Sun L, Tan MG, Chua AW, Wang DY. L-N-Acetylcysteine protects against radiation-induced apoptosis in a cochlear cell line. Acta Otolaryngol. 2008;128:440–445. doi: 10.1080/00016480701762490. [DOI] [PubMed] [Google Scholar]
- 54.Guan D, Xu Y, Yang M, Wang H, Wang X, et al. N-acetyl cysteine and penicillamine induce apoptosis via the ER stress response-signaling pathway. Mol Carcinog. 2010;49:68–74. doi: 10.1002/mc.20578. [DOI] [PubMed] [Google Scholar]
- 55.Rieber M, Rieber MS. N-Acetylcysteine enhances UV-mediated caspase-3 activation, fragmentation of E2F-4, and apoptosis in human C8161 melanoma: inhibition by ectopic Bcl-2 expression. Biochem Pharmacol. 2003;65:1593–1601. doi: 10.1016/s0006-2952(03)00147-3. [DOI] [PubMed] [Google Scholar]
- 56.Cosentino C, Grieco D, Costanzo V. ATM activates the pentose phosphate pathway promoting anti-oxidant defence and DNA repair. EMBO J. 2011;30:546–555. doi: 10.1038/emboj.2010.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ditch S, Paull TT. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:15–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuang X, Yan M, Ajmo JM, Scofield VL, Stoica G, et al. Activation of AMP-activated protein kinase in cerebella of Atm−/− mice is attributable to accumulation of reactive oxygen species. Biochem Biophys Res Commun. 2012;418:267–272. doi: 10.1016/j.bbrc.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamsler A, Daily D, Hochman A, Stern N, Shiloh Y, et al. Increased oxidative stress in ataxia telangiectasia evidenced by alterations in redox state of brains from Atm-deficient mice. Cancer Res. 2001;61:1849–1854. [PubMed] [Google Scholar]