Abstract
Purpose of review
The association between obesity and insulin resistance is an area of much interest and enormous public health impact, with hundreds of articles being published in the last year focused on the possible mechanisms that underlie this association. The purpose to this review is to highlight some of the key recent literature with emphasis on emerging concepts.
Recent findings
The specific link between visceral adipose tissue accumulation and insulin resistance continues to be discerned. Visceral adiposity is correlated with accumulation of excess lipid in liver, and results in cell autonomous impairment in insulin signaling. Visceral adipose tissue is also prone to inflammation and inflammatory cytokine production, which also contribute to impairment in insulin signaling. The expansion of visceral adipose tissue and excess lipid accumulation in liver and muscle may result from limited expandability of subcutaneous adipose tissue, due to the properties of its extracellular matrix and capacity for capillary growth.
Summary
Recent studies underscore the need to better understand the mechanisms linking visceral adiposity with liver fat accumulation, the mechanisms by which ectopic fat accumulation cause insulin resistance, and the mechanisms by which the size of adipose tissue depots is determined.
Keywords: adipose tissue expandability, inflammation, insulin resistance, lipotoxicity
INTRODUCTION
Insulin resistance is a requisite precursor for the development of type 2 diabetes mellitus (T2DM), and is associated with hypertension and dyslipidemia [1]. Epidemiological data link T2DM with obesity, and a causal relationship between insulin resistance and weight gain has been gleaned from classical studies in which lean individuals with no previous history of obesity or diabetes became insulin resistant upon experimental overnutrition [2]. These facts reinforce the great importance of understanding the physiological basis for insulin resistance in obesity.
NOT ALL FORMS OF OBESITY RESULT IN INSULIN RESISTANCE
Obesity is the excessive growth of adipose tissue depots arising from the chronic consumption of calories in excess of the energetic needs of the individual. In humans, the expansion of adipose depots results from increased numbers of individual adipocytes (hyperplasia), and from the hypertrophy of adipocytes, in a depot-dependent fashion [3]. Importantly, there is a large individual variation in the size and expandability of different adipose tissue depots in humans. This factor is critically important in understanding the relationship between obesity and insulin resistance, as expansion of some depots is associated with increased risk, whereas expansion of others is associated with decreased risk [4]. Each standard deviation (SD) increase in subcutaneous adipose tissue mass decreases the odds of insulin resistance by 48%, whereas a SD increase in visceral adipose tissue mass increases the odds of insulin resistance by 80% [5▪]. These findings can explain the existence of ‘benign’ and ‘malign’ obesity wherein insulin resistance is not observed in all individuals with high BMIs. They may also explain the very high incidence of insulin resistance and diabetes in ethnic populations that display relatively low BMIs associated with high waist circumferences or waist-to-hip ratios, reflecting elevated visceral obesity [6].
In this context, the mechanisms that control the expandability of subcutaneous adipose tissue, including its high capacity for adipocyte differentiation and lipid storage may be key factors in determining diabetes risk in obesity [7]. The enhanced capacity for formation of adipocytes, inferred by the presence of hyperplasia in subcutaneous adipose tissue [8], correlates with decreased risk of glucose and insulin abnormalities. Furthermore, the gene expression patterns of subcutaneous adipose tissue differ more than the gene expression patterns of skeletal muscle when comparing insulin-sensitive versus insulin-resistant individuals. These results are consistent with variations in subcutaneous adipose tissue being a key factor in determining metabolic disease risk. These differences were found to include genes related to lipid and fatty acid metabolism, inflammation, and cell-cycle regulation [9▪].
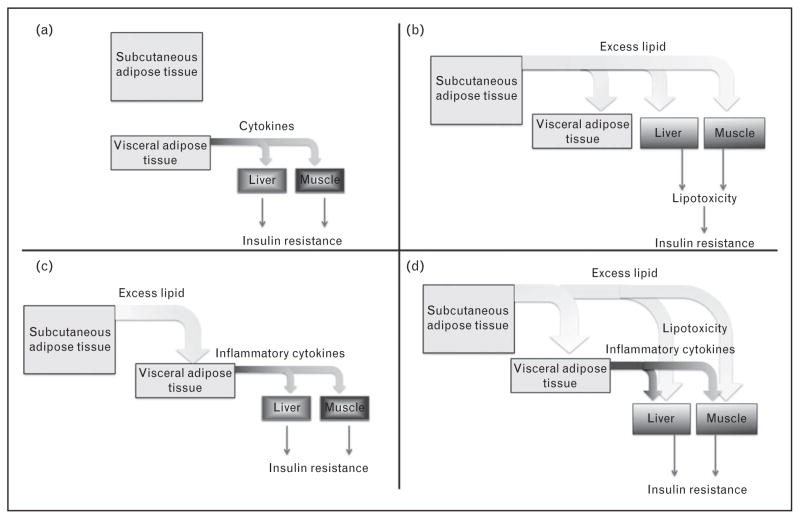
Why is visceral fat accumulation associated with insulin resistance? One possibility is that visceral fat itself is inherently diabetogenic, for example, it secretes adipokines that impair insulin sensitivity in tissues such as liver and muscle, which increase upon expansion of this depot (Fig. 1a). Another possibility is that the accumulation of visceral fat is a surrogate indicator of ectopic lipid accumulation and lipotoxicity, which occur in parallel in liver and muscle, causing insulin resistance in these tissues (Fig. 1b). A third possibility is that excess lipid accumulation in visceral adipose tissue actually causes its acquisition of diabetogenic properties (Fig. 1c); visceral adipose tissue indeed accumulates macrophages that release inflammatory cytokines, which can impair insulin sensitivity. A fourth possibility is one in which lipotoxicity in peripheral tissues and visceral adipose tissue cytokine production, both contribute to systemic insulin resistance (Fig. 1d). Recent data related to these models are reviewed below.
FIGURE 1.
Potential mechanisms by which visceral adiposity might be related to insulin resistance. Description of each model is in the text.
DEFINITION OF INSULIN RESISTANCE
Systemic insulin resistance can be measured as a decreased glucose disposal rate in rodents and humans in response to defined concentrations of insulin [1]. Systemic insulin resistance can result from impaired insulin action in metabolically active organs and tissues, including skeletal muscle, the liver, and adipose tissue. The degree to which systemic insulin resistance is due to impaired insulin action in skeletal muscle, liver, or adipose tissue may vary among individuals.
In skeletal muscle, insulin resistance is manifested as a decrease in glucose transport and a decline in muscle glycogen synthesis in response to circulating insulin. Insulin sensitivity is decreased in myocytes obtained from obese individuals, or cultured myocytes in the presence of adipocyte-derived lipids [10], supporting the concept that accumulation of excess lipids or their metabolic derivatives cause decreased insulin signaling in skeletal muscle [11,12]. Recently, muscle insulin resistance in obese diabetic humans has also been correlated with decreased transcapillary insulin transport [13▪], and found to be present in mice harboring endothelial cell-specific insulin signaling defects [14▪]. It remains to be determined whether obesity causes endothelial cell insulin resistance in muscle. Insulin resistance is also correlated with mitochondrial respiratory chain deficiency in muscle [15], but this may be a consequence, rather than a cause of insulin resistance [16▪].
In the liver, insulin resistance is selective in that insulin fails to suppress gluconeogenesis, but continues to stimulate fatty acid synthesis [17]. Thus, the point at which insulin signaling is disrupted in obesity is downstream of insulin receptor activation. A critical role of the mammalian target of rapamycin complex (mTORC) in hepatic lipogenesis [18], as well as other mechanisms downstream of the serine-threonine protein kinase Akt2 [19▪] may be responsible for this uncoupling of glucose and lipid metabolism in the insulin signaling pathway, which ultimately manifests as hyperglycemia and hyper-triglyceridemia.
In adipose tissue, insulin resistance is manifested as impaired insulin-stimulated glucose transport, as well as impaired inhibition of lipolysis. As in liver, adipocytes exhibit a divergence in insulin signaling whereby the insulin effect on glucose transporter-4 trafficking is blunted, yet its effect on Forkhead box O-1 (FoxO1) nuclear exclusion is preserved [20]. Obesity may produce adipocyte insulin resistance through cell autonomous mechanisms, or as detailed below, through the interactions between the adipocyte and mediators of inflammation.
VISCERAL ADIPOSE TISSUE CYTOKINE PRODUCTION AND INSULIN RESISTANCE
Visceral adipocytes secrete adipose-specific cytokines such as leptin and adiponectin but also inflammatory cytokines such as tumor necrosis factor-α and interleukin (IL)-6. Recent experiments suggest that an increase in the abundance of adipose tissue draining into the portal vein can cause liver and systemic insulin resistance [21▪]. In this study, the capacity of adipose tissue grafted onto the mesentery to induce insulin resistance depended on IL-6 production. The size of the visceral adipose depot and adipocyte size in humans is linked to systemic insulin resistance, as well as increased expression of chemokines and cytokines by immune cells in the tissue [22]. A recent study also revealed a correlation between increased amounts of visceral fat, adipocyte hypertrophy, insulin resistance, and elevated expression of autophagy genes in human omental adipose tissue [23]. These results suggest that the propensity of visceral adipose tissue for increased inflammation, and the subsequent secretion of cytokines that impair insulin signaling may significantly contribute to systemic insulin resistance in central obesity.
Circulating levels of the macrophage-derived apoptosis inhibitor of macrophage protein are also increased with obesity, stimulate lipolysis in adipose tissue, and appear to be necessary for the local recruitment of adipose tissue macrophages [24]. Interleukin-1 receptor 1(IL-1R1) partially mediates the inflammatory signals responsible for adipose inflammation because adipose tissue from IL-1R1(−/−) mice fed a high-fat diet display increased insulin sensitivity, and lower cytokine secretion when compared with wild-type mice [25]. Recent evidence implicates a role for the nucleotide-binding domain, leucine-rich containing family, pyrin domain containing-3 (Nlrp3) inflammasome, an innate immune cell sensor that responds to metabolic danger signals such as lipids and ceramides. A reduction in adipose tissue expression of Nlrp3 is associated with decreased inflammation and an improvement in insulin sensitivity. Mice lacking Nlrp3 display enhanced insulin sensitivity and reduced inflammasome activation, even in the setting of diet-induced obesity [26▪]. Consistent with the concept that mild inflammation is causal in development of insulin resistance, treatment of obese mice with resolvins, endogenous lipid mediators that promote inflammatory resolution, improves glucose tolerance, decreases fasting blood glucose levels, and enhances insulin signaling in adipose tissue [27]. Positive effects of anti-inflammatory agents on controlling glucose levels in human diabetics further reinforces this thinking [28].
EXCESSIVE AND ECTOPIC LIPID DEPOSITION AND INSULIN RESISTANCE
The ability to store calories in excess of immediate energy needs is a biological adaptation with great evolutionary advantage. Many organisms, from worms to mammals store excess calories in the form of triglyceride droplets, which accumulate in diverse cells and tissue types, such as the gut, fat body, and the liver [29,30]. Adipose tissue first appears in evolution surrounding the gut and internal organs, possibly serving to maintain temperature as recent evidence indicates that adipose tissue surrounding the aorta is of brown adipose origin [31]. This suggests that these adipose depots fulfill protective and biomechanical roles. The formation of large subcutaneous adipose depots appears later in evolution, and is critical for storage of large amounts of fat in times of excess calories.
Despite a highly evolved ability to sequester fat, the storage capacity of single adipocytes is finite. Enlarged adipocytes display insulin resistance without much macrophage infiltration into adipose tissue following a short-term high-fat diet [32▪]. Thus, even without inflammatory responses, excess lipid in adipose cells results in insulin resistance. One plausible hypothesis is that excess lipid accumulation in adipocytes, and ectopic lipid accumulation in liver and muscle may lead to insulin resistance through the formation of metabolically toxic products. For example, saturated fatty acids have been shown to increase ceramide production, which appears to contribute to insulin resistance [33]. Lipids such as triacylglycerols are converted to diacylglyerols by adipose triglyceride lipase (ATGL) then hydrolyzed by hormone sensitive lipase (HSL). The expression of ATGL and HSL in skeletal muscle appears to increase the accumulation of intracellular diacylgyercerols that negatively impacts insulin signaling [34]. Hepatic diacylglycerol content shows a strong correlation with systemic insulin resistance especially when present in the setting of nonalcoholic fatty liver disease [35▪]. These lipids may activate signaling pathways, for example, one or more of the protein kinase C proteins that negatively impact upon insulin signal transduction. The products of incomplete fatty acid oxidation may also impair one or more steps in the insulin signaling cascade or in the pathways it regulates.
ADIPOSE TISSUE EXPANDABILITY AND PROTECTION FROM INSULIN RESISTANCE
Impaired storage capability of individual adipose cells leads to ectopic lipid deposition in critical organs including visceral adipose tissue, liver, and muscle [36]. Thus, a critical factor in protecting against insulin resistance is the expandability of adipose tissue, defined as the capacity to form new adipocytes that can accumulate excess energy and protect from adipocyte hypertrophy and ectopic lipid accumulation. The mechanisms that determine adipose tissue expandability are not known, but, like any growing tissue, the capacity to remodel the extracellular matrix, and to adequately increase capillary vascularization to enable oxygen and nutrient supply are necessarily involved.
Several studies have shown the existence of hypoxia in adipose tissue from obese humans [37,38▪], and recent microdialysis of abdominal subcutaneous adipose tissue in humans showed that obesity is associated with lower adipose tissue blood flow [39▪], although evidence of hypoxia was not found in this study. Hypoxic stress in adipose tissue may lead to aberrant remodeling of the extracellular matrix leading to fibrosis and inflammation [40]. Thus, the expansion of capillary networks may be essential to prevent hypoxia, fibrosis, and inflammation in expanding adipose tissue. A recent study in morbidly obese individuals reveals a positive correlation between the angiogenic capacity of subcutaneous tissue and insulin sensitivity, suggesting that insufficient angiogenic growth of subcutaneous adipose tissue may play a role in the pathogenesis of metabolic disease [41]. Elucidating the factors that promote adipose tissue angiogenic expansion is an important area of future research.
On the basis of the above considerations, it is of high importance to elucidate the factors that determine the ability of the individual to expand subcutaneous adipose tissue. One possibility to consider is the natural variation in the number of adipocyte progenitors. These cells have been identified in the mouse adipose tissue stromovascular fraction [42]. In early development, factors such as matrix–cell and cell–cell interactions, as well as angiogenesis are essential for adipocyte differentiation from progenitor cells [43]. More work is required to further characterize the properties of these cells in specific adipose tissue depots in humans.
SUCCESSFUL THERAPEUTIC STRATEGIES HELP ELUCIDATE MECHANISMS OF INSULIN RESISTANCE
Ongoing research into successful therapeutic strategies for improving insulin sensitivity can provide an insight into the mechanisms linking insulin resistance and obesity. Bariatric surgery is a highly effective therapy for obesity and obesity-related comorbidities. The most commonly performed bariatric procedure, Roux-en-Y gastric bypass, results in resolution of T2DM in approximately 80% of patients [44▪]. A recent study using hyperinsulinemic euglycemic clamps in humans demonstrated an improvement in adipose tissue insulin sensitivity with significant suppression of lipolysis after the Roux-en-Y gastric bypass procedure [45]. Although the mechanisms leading to the dramatic improvement in glucose homeostasis are not well understood, it appears that they involve both weight-dependent and independent processes. The procedure results in altered gut anatomy leading to caloric restriction and malabsorption, which both contribute to the sustained weight loss effects. However, this cannot explain the immediate improvement in glycemic control that occurs within a few days after the surgical procedure. Although this is still an active, unresolved area of research, the leading theories involve an incretin effect on insulin action that occurs as a result of bypassing the upper gastrointestinal tract [44▪]. This immediate effect of improvement in glucose homeostasis is not seen in other bariatric procedures that do not include a component of gastrointestinal bypass. Similarly, partial removal of the subcutaneous (liposuction) or visceral (omentectomy) adipose depots do not improve insulin sensitivity [45,46].
Dietary changes are common approaches to weight loss, and although most attempts are unsuccessful due to patient noncompliance, there still remains significant controversy surrounding the best dietary approach. A recent review of several dietary interventions suggests that insulin-resistant individuals derive the most short-term benefit from a low-carbohydrate diet compared with a low-fat diet, likely due to the adverse effect that high levels of carbohydrates have on postprandial insulin and triglyceride levels [47]. Although caloric restriction has been shown to decrease the amounts of adipose cells in skeletal muscle and visceral adipose tissue itself, these effects are almost doubled when weight loss is due to exercise in sedentary overweight patients [48▪]. Rodent studies provide mechanistic insight into the improvements of insulin resistance associated with exercise. Both acute and chronic exercise in a diet-induced obesity rat model lead to suppression of inflammatory signaling in liver, muscle, and adipose tissue that subsequently improved insulin signaling [49].
CONCLUSION
Work to elucidate the mechanisms underlying the relationship between obesity and insulin resistance in humans continues to support the concept that visceral obesity, but not subcutaneous, results in insulin resistance and increased risk of T2DM. The mechanisms by which visceral obesity results in insulin resistance appear to be related to excess lipid accumulation in liver. This may be due to excess fatty acids from visceral adipose tissue draining into the portal vein. Excess lipid accumulation may result in impaired insulin signaling through cell autonomous mechanisms, or through the induction of inflammation and the subsequent production of inflammatory cytokines by macrophages, which impair insulin action. Storage of excess fat in subcutaneous depots mitigates the risk of insulin resistance and T2DM, possibly by preventing accumulation of fat in visceral adipose tissue, liver, and skeletal muscle. Thus, the mechanisms that determine the size and expandability of subcutaneous adipose tissue depots, such as the control of extracellular matrix and capillary expansion, may be important targets for future therapy.
KEY POINTS.
Visceral adipose tissue increases, and subcutaneous adipose tissue decreases the risk of insulin resistance and T2DM in humans.
Visceral adiposity correlates with excess lipid accumulation in liver.
Excess accumulation of lipid may cause insulin resistance through cell autonomous mechanisms, and through the induction of inflammation, and the consequent production of inflammatory cytokines.
Failure to expand subcutaneous adipose tissue in parallel with chronic excess calorie consumption may result from impaired expandability of its extracellular matrix and capillary network, and result in ectopic lipid accumulation.
Acknowledgments
The authors acknowledge valuable discussions with members of our respective laboratories and of the UMASS Diabetes Center of Excellence. We apologize for the inability to cite numerous examples of important work in the field due to space considerations.
Footnotes
There are no conflicts of interest.
Conflicts of interest
Work in the authors’ laboratories has been supported by grants from the National Institutes of Health DK089101 to SC, DK30898 to MPC and UMASS Center for Clinical and Translational Science to OTH.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 142–143).
- 1.Reaven GM. Banting lecture role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 2.Sims EA, Danforth E., Jr Expenditure and storage of energy in man. J Clin Invest. 1987;79:1019–1025. doi: 10.1172/JCI112913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tchoukalova YD, Votruba SB, Tchkonia T, et al. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107:18226–18231. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preis SR, Massaro JM, Robins SJ, et al. Abdominal subcutaneous and visceral adipose tissue and insulin resistance in the Framingham heart study. Obesity (Silver Spring) 2010;18:2191–2198. doi: 10.1038/oby.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5▪.McLaughlin T, Lamendola C, Liu A, Abbasi F. Preferential fat deposition in subcutaneous versus visceral depots is associated with insulin sensitivity. J Clin Endocrinol Metab. 2011;96:E1756–E1760. doi: 10.1210/jc.2011-0615. Using computerized tomography to quantify adipose depot size, and normalizing for BMI, the data in this study clarify that regional distribution of fat-favoring subcutaneous depots is associated with lower risk for insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagami T, Qiao Q, Carstensen B, et al. Age, body mass index and type 2 diabetes-associations modified by ethnicity. Diabetologia. 2003;46:1063–1070. doi: 10.1007/s00125-003-1158-9. [DOI] [PubMed] [Google Scholar]
- 7.Kursawe R, Eszlinger M, Narayan D, et al. Cellularity and adipogenic profile of the abdominal subcutaneous adipose tissue from obese adolescents: association with insulin resistance and hepatic steatosis. Diabetes. 2010;59:2288–2296. doi: 10.2337/db10-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffstedt J, Arner E, Wahrenberg H, et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia. 2010;53:2496–2503. doi: 10.1007/s00125-010-1889-3. [DOI] [PubMed] [Google Scholar]
- 9▪.Elbein SC, Kern PA, Rasouli N, et al. Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes. 2011;60:1019–1029. doi: 10.2337/db10-1270. This study demonstrates that in patients matched for BMI, differences in systemic insulin sensitivity are accompanied by more pronounced changes in gene expression in adipose tissue that in skeletal muscle, pointing to the possible pathogenic role of adipose tissue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovalik JP, Slentz D, Stevens RD, et al. Metabolic remodeling of human skeletal myocytes by cocultured adipocytes depends on the lipolytic state of the system. Diabetes. 2011;60:1882–1893. doi: 10.2337/db10-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koves TR, Ussher JR, Noland RC, et al. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell metab. 2008;7:45–56. doi: 10.1016/j.cmet.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 12.Petersen KF, Dufour S, Savage DB, et al. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc Natl Acad Sci U S A. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13▪.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. American journal of physiology. Endocrinol metab. 2011;301:E252–E263. doi: 10.1152/ajpendo.00186.2011. Review of data related to insulin action on the arterial vasculature as well as recent data on the regulation of endothelial cell vesicular transport by insulin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14▪.Kubota T, Kubota N, Kumagai H, et al. Impaired insulin signaling in endothelial cells reduces insulin-induced glucose uptake by skeletal muscle. Cell metab. 2011;13:294–307. doi: 10.1016/j.cmet.2011.01.018. Using cell-specific knockout models demonstrates that insulin signaling in endothelial cells is involved in the regulation of glucose uptake by skeletal muscle. [DOI] [PubMed] [Google Scholar]
- 15.Patti ME, Corvera S. The role of mitochondria in the pathogenesis of type 2 diabetes. Endocr Rev. 2010;31:364–395. doi: 10.1210/er.2009-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16▪.Sleigh A, Raymond-Barker P, Thackray K, et al. Mitochondrial dysfunction in patients with primary congenital insulin resistance. J clin invest. 2011;121:2457–2461. doi: 10.1172/JCI46405. Demonstrate that mitochondrial disfunction can occur in humans as a consequence of disrupted insulin signaling; does not rule out the possibility that impaired mitochondrial function may also cause alterations in insulin signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19▪.Wan M, Leavens KF, Saleh D, et al. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. Finds a previously unrecognized insulin-Akt2 signaling pathway that stimulates anabolic lipid metabolism independent of Foxa2, FoxO1 and mTORC1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez E, Flier E, Molle D, et al. Hyperinsulinemia leads to uncoupled insulin regulation of the GLUT4 glucose transporter and the FoxO1 transcription factor. Proc Natl Acad Sci U S A. 2011;108:10162–10167. doi: 10.1073/pnas.1019268108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21▪.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60:56–63. doi: 10.2337/db10-0697. Using mice with fat transplanted into either to the parietal peritoneum or to the mesenterium, find that only mice receiving the portal drained fat transplant develop impaired glucose tolerance and hepatic insulin resistance. This supports the concept that excess visceral adipose tissue drainage into liver causes insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hardy OT, Perugini RA, Nicoloro SM, et al. Body mass index-independent inflammation in omental adipose tissue associated with insulin resistance in morbid obesity. Surg Obes Relat Dis. 2011;7:60–67. doi: 10.1016/j.soard.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovsan J, Bluher M, Tarnovscki T, et al. Altered autophagy in human adipose tissues in obesity. J Clin Endocrinol Metab. 2011;96:E268–E277. doi: 10.1210/jc.2010-1681. [DOI] [PubMed] [Google Scholar]
- 24.Kurokawa J, Nagano H, Ohara O, et al. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011;108:12072–12077. doi: 10.1073/pnas.1101841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGillicuddy FC, Harford KA, Reynolds CM, et al. Lack of interleukin-1 receptor I (IL-1RI) protects mice from high-fat diet-induced adipose tissue inflammation coincident with improved glucose homeostasis. Diabetes. 2011;60:1688–1698. doi: 10.2337/db10-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. Show that weight loss in obese individuals with T2DM is associated with a reduction in adipose tissue expression of Nlrp3. Find that ablation of Nlrp3 in mice enhances insulin signaling in obese mice. These results suggest that Nlrp3 inflammasome contributes to obesity-induced inflammation and insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hellmann J, Tang Y, Kosuri M, et al. Resolvin D1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goldfine AB, Fonseca V, Jablonski KA, et al. The effects of salsalate on glycemic control in patients with type 2 diabetes: a randomized trial. Ann Intern Med. 2010;152:346–357. doi: 10.1059/0003-4819-152-6-201003160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottaviani E, Malagoli D, Franceschi C. The evolution of the adipose tissue: a neglected enigma. Gen Comp Endocrinol. 2011;174:1–4. doi: 10.1016/j.ygcen.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 30.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 31.Fitzgibbons TP, Kogan S, Aouadi M, et al. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–H1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32▪.Lee YS, Li P, Huh JY, et al. Inflammation is necessary for long-term but not short-term high-fat diet-induced insulin resistance. Diabetes. 2011;60:2474–2483. doi: 10.2337/db11-0194. Find that insulin resistance occurs after only 3 days of high-fat diet, in wild-type as well as inflammation-resistant mice, suggesting that the initial stage of obesity-induced insulin resistance is independent of inflammation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holland WL, Bikman BT, Wang LP, et al. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121:1858–1870. doi: 10.1172/JCI43378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badin PM, Louche K, Mairal A, et al. Altered skeletal muscle lipase expression and activity contribute to insulin resistance in humans. Diabetes. 2011;60:1734–1742. doi: 10.2337/db10-1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35▪.Kumashiro N, Erion DM, Zhang D, et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc Natl Acad Sci U S A. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. Examine liver from obese, non diabetic individuals and find hepatic diacylglycerol content to be the best predictor of insulin resistance, and to be also strongly correlated with activation of hepatic PKC epsilon. Support the hypothesis that insulin resistance is caused by increased hepatic diacylglycerol content and cell autonomous impairment of insulin signaling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Le KA, Mahurkar S, Alderete TL, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60:2802–2809. doi: 10.2337/db10-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabon B, Nagele A, Reddy D, et al. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪.Pasarica M, Rood J, Ravussin E, et al. Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J Clin Endocrinol Metab. 2010;95:4052–4055. doi: 10.1210/jc.2009-2377. In-situ adipose tissue oxygenation was measured with a Clark electrode in lean and obese individuals. Insulin sensitivity was positively correlated with tissue oxygen partial pressure and capillary density. This study suggests that low capillary density may contribute to adipose tissue dysfunction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39▪.Goossens GH, Bizzarri A, Venteclef N, et al. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. Developed a system for continuous monitoring of oxygen partial pressure and adipose tissue blood flow using microdialysis. Find that increases in adipose tissue blood flow induced by glucose ingestion were blunted in obese, insulin resistant individuals. Point to adipose tissue blood flow as a potentially important variable associated with in insulin resistance. [DOI] [PubMed] [Google Scholar]
- 40.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gealekman O, Guseva N, Hartigan C, et al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011;123:186–194. doi: 10.1161/CIRCULATIONAHA.110.970145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 43.Han J, Lee JE, Jin J, et al. The spatiotemporal development of adipose tissue. Development. 2011;138:5027–5037. doi: 10.1242/dev.067686. [DOI] [PubMed] [Google Scholar]
- 44▪.Varela JE. Bariatric surgery: a cure for diabetes? Curr Opin Clin Nutr Metab Care. 2011;14:396–401. doi: 10.1097/MCO.0b013e3283468e50. Reviews most recent findings on the relationship between different modes of surgically induced weight loss and T2DM. [DOI] [PubMed] [Google Scholar]
- 45.Curry TB, Roberts SK, Basu R, et al. Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab. 2011;300:E746–E751. doi: 10.1152/ajpendo.00596.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fabbrini E, Tamboli RA, Magkos F, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139:448–455. doi: 10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee MW, Fujioka K. Dietary prescriptions for the overweight patient: the potential benefits of low-carbohydrate diets in insulin resistance. Diabetes Obes Metab. 2011;13:204–206. doi: 10.1111/j.1463-1326.2010.01328.x. [DOI] [PubMed] [Google Scholar]
- 48▪.Murphy JC, McDaniel JL, Mora K, et al. Preferential reductions in intermuscular and visceral adipose tissue with exercise-induced weight loss as compared to calorie restriction. J Appl Physiol. 2012;112:79–85. doi: 10.1152/japplphysiol.00355.2011. Analyzed intermuscular adipose tissue (IMAT) and visceral adipose tissue (VAT) by MRI in sedentary men and women subjected to caloric restriction or exercise-induced weight loss. With comparable degrees of total fat mass loss, exercise induced greater loss in IMAT and VAT, and loss of fat in these depots correlated with weigh loss induced enhancement of insulin sensitivity. Thus, exercise-induced is preferable to calorie restriction-induced weight loss in that it preferentially affects depots associated with insulin resistance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliveira AG, Carvalho BM, Tobar N, et al. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60:784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]