Abstract
Nearly two-thirds of the population in the United States is overweight or obese, and this unprecedented level of obesity will undoubtedly have a profound impact on overall health, although little is currently known about the effects of obesity on the brain. The objective of the current study was to investigate cerebral oxidative stress and cognitive decline in the context of diet-induced obesity (DIO). We demonstrate for the first time that DIO induces higher levels of reactive oxygen species (ROS) in the brain, and promotes cognitive impairment. Importantly, we also demonstrate for the first time in these studies that both body weight and adiposity are tightly correlated with the level of ROS. Interestingly, ROS were not correlated with cognitive decline in this model. Alterations in the antioxidant/detoxification Nrf2 pathway, superoxide dismutase, and catalase were not significantly altered in response to DIO. A significant impairment in glutathione peroxidase was observed in response to DIO. Taken together, these data demonstrate for the first time that DIO increases the level of total and individual ROS in the brain, and highlight a direct relationship between the amount of adiposity and the level of oxidative stress within the brain. These data have important implications for understanding the negative effects of obesity on the brain, and are vital to understanding the role of oxidative stress in mediating the effects of obesity on the brain.
Keywords: adipose, brain, cognitive impairment, free radicals, neurodegeneration, oxidative stress
Introduction
In the United States (and projected to be worldwide), over one-third of the population is obese and another third of the population is overweight (1). This level of adiposity is unprecedented in human history, and is certain to have a dramatic, and as yet, undefined impact on human health. Obesity has already been shown to increase risk for many diseases including type II diabetes, metabolic syndrome, cardiovascular disease, and some forms of cancer (3–9). The objective of this study was to investigate the impact of diet induced obesity (DIO) on the levels of oxidative stress in the brain, as well as overall brain function.
It has previously been established that oxidative stress promotes neurodegeneration through a combination of increased reactive oxygen species (ROS) formation and decreased antioxidant capacity (10–13). The role of oxidative stress in a variety of experimental models, and epidemiological studies of age-related neurodegenerative diseases is well documented (14–18). In particular, studies link increased oxidative stress to declines in cognitive function (14–18). Interestingly, a number of epidemiological studies have revealed cognitive impairment is correlated with saturated fat intake (19–22). Rodent studies by our laboratory and others have shown consumption of a high fat diet results in increased protein oxidation and a decrease in cognitive performance (23, 24), suggesting increased levels of oxidative stress in the context of DIO.
In the current study we examined for the first time, the links between DIO with the levels of total ROS and specific ROS such as superoxide and peroxynitrite. Our study demonstrates for the first time that DIO exacerbates the levels of ROS in the brain and the increased levels of ROS correlates with adiposity. Increased ROS did not appear to be due to changes in Nrf2 signaling, but likely in part due in part to decreased glutathione peroxidase levels. The implications of DIO and increases in cerebral oxidative stress, and associated cognitive decline, are discussed.
Materials and Methods
Animals and Dietary Treatments
All animal experiments were approved by the Institutional Animal Care and Use Committee of Pennington Biomedical Research Center. Male C57Bl/6 mice (Charles River Laboratories) were used at 2–4 months of age. Diets were purchased from Research Diets (New Brunswick, NJ). At the start of the experiment, mice were randomly assigned to either the high fat diet (HF; D12451) or control diet (CD; D12450B). There were no significant differences in body weight between groups before the experimental diets were administered as all mice had been maintained on a regular chow diet. The HF diet provided 45% kcals from fat while the CD provided 10% kcals from fat (Table 1). The CD was developed as an appropriate, balanced control to the HF diet: both contained the necessary mineral and vitamin mixes, 20% kcals from protein and the fat source was soybean oil and lard (Table 1). Mice were housed in standard caging with a 12:12 light/dark cycle and ad libitum access to water and their respective diet unless otherwise noted. Mice were maintained on the HF diet or CD for 7–8 months. Total body adiposity was measured via nuclear magnetic resonance (NMR) spectroscopy (Minispec, Brucker Optics, Billerica MA) before conclusion of the experiment.
Table 1.
Diets used for the current study
| Component (kcal%) | CD | HF |
|---|---|---|
| Fat | 10% | 60% |
| Protein | 20% | 20% |
| Carbohydrate | 70% | 20% |
| Ingredient (kcal) | ||
| Lard | 180 | 2205 |
| Soybean Oil | 225 | 225 |
| Corn Starch | 1260 | 0 |
| Maltodextrin 10 | 140 | 500 |
| Sucrose | 1400 | 275 |
| Casein | 800 | 800 |
| L-Cysteine | 12 | 12 |
| Cellulose, BW200 | 0 | 0 |
| Mineral Mix | 0 | 0 |
| Vitamin Mix | 40 | 40 |
| Total (kcal) | 4057 | 4057 |
Behavioral Battery
Three weeks before the termination of the experiment, mice (n=9–12/group) were subjected to a behavioral battery including: rotarod, fear conditioning, and a stress learning swim (SLS) task. On Day 1 of the behavioral battery, motor abilities were analyzed using a Five Station Rota-Rod Treadmill for Mouse (Med-Associates, St. Albans, VT). Each mouse underwent three trials with a 30 minute inter-trial interval (ITI) and were evaluated for the amount of time they were able to remain on the rotarod as it accelerated from 4 rpm to 40 rpm during a 5 minute session. Following the rotarod, mice were given one day of rest and then a fear conditioning paradigm was administered. The first day included a 12.5 minute session: the first 5 minutes allowed the mouse to acclimate to the box which included a drop of anise extract below the grid floor, adding an olfactory cue. The next 7.5 minutes included 5 stimuli sessions (delay conditioning): a 30 second tone (85 dB, 4 kHz) co-terminating with a shock (0.5 mA × 1 s), and then a 60 second ITI, free of the tone and shock. On the second day of fear conditioning (contextual fear test), mice were returned to the boxes under the same conditions for a ten-minute trial, but they did not receive a shock or tone. On the last day of fear conditioning (tone fear test), mice were brought to a different room containing testing boxes with new contextual cues compared to the previous days; the first 5 minutes allowed the animal to acclimate to the new environment without a cue but the last 5 minutes of the trial included a steady tone (85 dB, 4 kHz). On each day, freezing time in 30 second intervals was measured using Video Freeze software (Med Associates, St. Albans, VT). Next, mice were given four days of rest before the stress learning swim (SLS) test. On both days of the SLS test, a large cylinder (approximately 18 cm × 13 cm) was filled with room temperature water, a few centimeters below the top of the cylinder so that the mouse was unable to grab the sides and high enough so the mouse was unable to reach the bottom with its tail. During the five minute trial, behavior was characterized every 15 seconds to be: active swimming (movement of all four legs), passive swimming (only one or two legs active), or essentially immobile (floating/very slight movements to keep head above water).
Serum and Tissue Collection
After 7–8 months of dietary treatment, mice were fasted overnight, euthanized by isoflurane anesthesia, exsanguinated via cardiac puncture, perfused with phosphate buffered saline (PBS, pH 8.0) and decapitated. Brains were rapidly removed following decapitation; the right hemisphere was removed and post-fixed in formalin. The frontal cortex and hippocampus from the left hemisphere were manually dissected and frozen on dry ice for biochemical measures (see below).
Oxidative Stress Measurements
Electron paramagnetic resonance (EPR) spectroscopy
The frontal cortex was used for EPR measurements in order to calculate total reactive oxygen species (ROS), superoxide, and peroxynitrite levels in the brain, as done previously on other tissues (38–41). Briefly, the first spin probe used in order to measure total ROS was 1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine (CMH). The tissue was incubated at 37°C with 6.6 μl of CMH (200 μM) for 30 minutes; measurements from the probe media were performed using a BenchTop EPR spectrophotometer e-scan R (Noxygen Science Transfer and Diagnostics, Elzach, Germany). Next, the tissue was incubated with 1.5 μl of polyethylene glycol covalently linked to superoxide dismutase (PEG-SOD; 50 U/μl) for 30 minutes, then CMH for an additional 30 minutes for superoxide measurement. Lastly, the tissue was incubated with 30 μl of 1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine (CPH; 500 μM) for 30 minutes for peroxynitrite measurement. Each time, probe media was collected and analyzed as done previously (41).
Analysis of the Cellular Detoxification Systems
Frontal cortex and hippocampus were homogenized and analyzed for superoxide dismutase, catalase, and glutathione peroxidase activity levels using assay kits from Cayman Chemical. All assays were performed according to the manufacturer’s instructions. As stated by Cayman Chemical, the assay kits were based on the following reactions described below. The Cayman Chemical Superoxide Dismutase Assay Kit measures the dismutation of superoxide radicals generated by xanthine oxidase and hypoxanthine. The Cayman Chemical Catalase Assay Kit is based on the reaction of catalase with methanol in the presence of hydrogen peroxide. This produces formaldehyde which is measured spectrophotometrically with 4-amino-3-hydrazino-5-mercapto-1,2,4-triazole (Purpald) as the chromogen. The Cayman Chemical Glutathione Peroxidase Assay Kit was used in order to measure all of the glutathione-dependent peroxidases in the brain homogenates. Measured indirectly, oxidized glutathione (GSSG) is produced from the reduction of hydroperoxide by GPx which is then recycled to its reduced state by GR and NADPH. The oxidation of NADPH to NADP+ is accompanied by a decrease in absorbance at 340 nm. The rate of decrease in the A340 is directly proportional to the GPx activity in the sample (X).
Nrf2 and Keap1 Western Blotting
A subgroup of frontal cortex homogenates and hippocampal homogenates were used for Western blotting in order to measure Nrf2 and Keap1 protein expression following administration of the HF diet or CD. As done previously in our laboratory (42), samples were homogenized in a Tris-buffered saline (pH 7.4) lysis buffer containing 0.1% Triton X-100, 5 mM EDTA, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). The following primary antibodies were used for this study: anti-Nrf2 (1:200, Abcam, Cambridge, MA), anti-Keap1 (1:200, Cell Signaling Technology, Danvers, MA), and anti-beta actin (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA). Band density was measured and normalized for each blot then data were calculated and presented as percent expression in young CD mice.
Analysis of Serum 8-isoprostanes
Serum collected at euthanization was analyzed using a Cayman Chemical 8-Isoprostane Express EIA Kit according to the manufacturer’s instructions. Briefly, this is a competitive enzyme immunoassay based on the competition between 8-isoprostane and 8-isoprostane acetylcholinesterase (AChE). The 8-isoprostane-AChE (tracer) is kept constant in the experiment and therefore, the amount of tracer that binds the 8-isoprostane-specific rabbit antiserum binding sites is inversely proportional to the concentration of 8-isoprostane added to the plate (serum). A substrate to AChE is then added to the plate producing a color change enzymatic reaction, detected by reading the absorbance at 412 nm. The concentration of serum 8-isoprostane was calculated according to the manufacturer’s instructions.
Statistical Analysis
All data are shown as mean ± standard error of measurement. All measures were analyzed by two-way ANOVA and Fisher’s Least Significant Difference (LSD) post hoc analysis using SPSS © statistical software. As performed previously by our laboratory (10), protein expression values generated by Western blot (ratios of expression over β-actin) were normalized to percent young control to reconcile data from multiple blots. Statistical significance for all analyses was accepted at p < 0.05, and labels including “a, b, c, d” refer to statistical significance between the respective groups labeled. Statistical analysis of correlations between EPR results, body weight, adiposity, and cognitive test results were performed using SPSS © statistical software. The Pearson correlation coefficient squared (r2) and statistical significance (p) are reported.
Results
Body Composition
In our first set of analyses, we analyzed the impact of control diet (CD) and high fat (HF) diet towards body weight and adiposity (Figure 1). Before diets were administered, there were no significant differences in total body weight between groups (data not shown). Total body weights and body composition (as measured by NMR) were determined one week before euthanization. HFD significantly increased body weight, adiposity, and to lesser extent muscle mass (Figure 1) as expected. This increase in muscle for Young HF mice is typically found and likely necessary to support the significant increase in adiposity.
Figure 1. Total Body Weights and Adiposity.

Young HF animals had significantly greater body weight, fat and muscle compared to Young CD mice. *p < 0.05 compared to young CD mice.
Cellular Detoxification System
In our next set of experiments, we analyzed the impact of CD and HF diet consumption towards the cellular detoxification system by measuring catalase (Figure 2), glutathione peroxidase (Figure 3), and superoxide dismutase (Figure 4) activity in the cortex and hippocampus. Interestingly, the only antioxidant enzyme activity which was altered was glutathione peroxidase (Figure 3), which was selectively decreased in the cerebral cortex. No declines in any antioxidant enzymes were observed in the hippocampus.
Figure 2. Catalase activity is not altered in response to HFD.
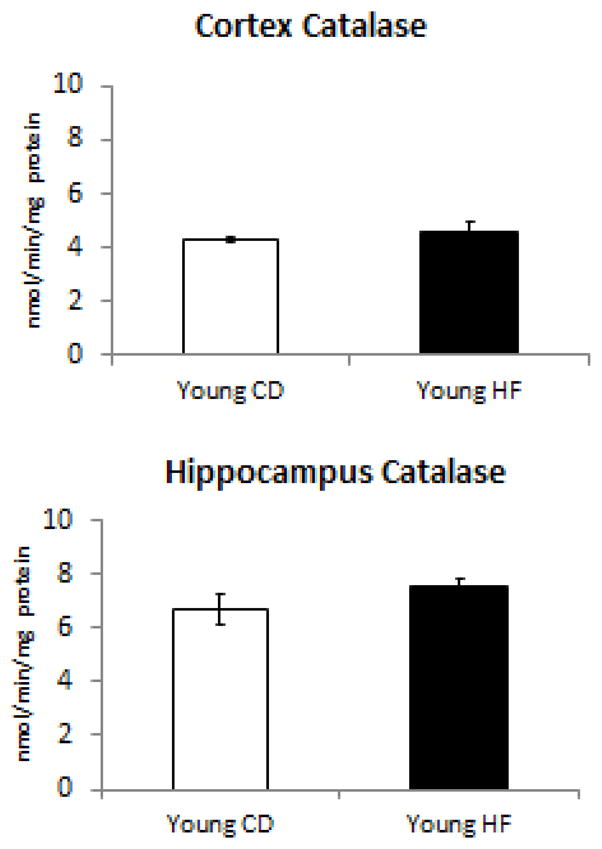
Young HFD mice did not have any change in the amount of catalase activity within either the cerbrocortex or hippocampus.
Figure 3. Glutathione peroxidase activity is altered in response to HFD.

Glutathione peroxidase activity is significantly decreased within the cerebrocortex but not the hippocampus of young mice fed a HFD. *p < 0.05 compared to young CD mice.
Figure 4. Superoxide dismutase activity is not altered in response to HFD.
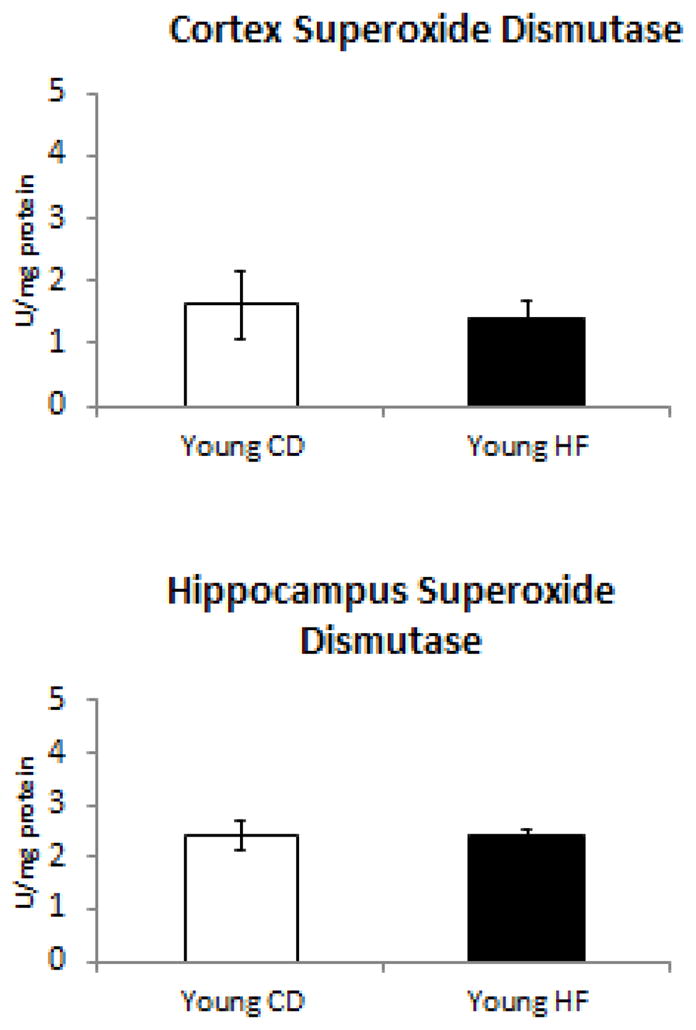
Young HFD mice did not have any change in the amount of superoxide dismutase activity within either the cerbrocortex or hippocampus.
Nrf2 Signaling
Because the Nrf2 signaling pathway has been tightly linked to the regulation of oxidative stress (23, 25, 26), we analyzed Nrf2 signaling in each of our experimental groups. Cortical and hippocampal protein levels of Nrf2, Keap 1, and HO-1 were evaluated using Western blotting for a subgroup of mice within each of the four groups (n=5–6 for each group). A great variability in cortical Nrf2 protein levels within each group was observed, particularly for the young CD mice (Figure 5). No significant differences in Nrf2 or Keap1 (Figure 5) were found in either the hippocampus or cortex of animals fed a HFD.
Figure 5. Nrf2 and Keap1 Protein Levels.
Nrf2 and Keap1 levels were not significantly changed in response to HFD in either the cerebrocortex or hippocampus.
Oxidative Stress Measurements
In the next set of analyses we measured, or the first time, cerebral ROS levels in young mice fed a control or high fat diet (Figure 5). Oxidative stress measurements as determined by EPR revealed a significant increase in all ROS parameters examined (total ROS, superoxide, and peroxynitrite) in response to DIO mice (Figure 6) within the cerebrocortex. The increase in superoxide was the most dramatic effect of DIO, although each EPR measurement revealed a greater than 2.5 fold elevation in DIO as compared to animals fed a control diet.
Figure 6. Cortical Oxidative Stress Levels.

A significant upregulation of all species (total ROS, superoxide, and peroxynitrite) was observed for HF diet-treated mice compared to control diet-treated mice. *p < 0.05 compared to young CD mice.
Serum Oxidative Stress Evaluation
8-isoprostane has been proposed as a reliable biomarker of oxidative stress. We evaluated levels of 8-isoprostane in serum in order to determine the potential contribution of peripheral oxidative stress to our observed CNS oxidative stress. No significant elevation in 8-isoprostane was observed in the serum of DIO mice in the current study (Figure 7).
Figure 7. Serum 8-Isoprostanes.

The serum level of 8-isoprostanes were not significantly changed in response to HFD.
Behavioral Battery
We sought to identify the impact of HF diet consumption towards cognitive function (Figure 8). No significant differences were observed on the rotarod test between groups (Figure 8). On average over the three trials, mice spent 65–80 seconds on the rotarod suggesting no major differences in motor ability due to dietary treatment. No significant differences were observed on Day 1 or Day 2 of the Fear Conditioning Test (Figure 8). However, significant differences in freezing behavior were observed on Day 3 of Fear Conditioning in animals fed HFD (Figure 8). The third day of the Fear Conditioning Test is the “Tone Test” which provided a measure of associative learning. Overall, animals froze less on Day 3 compared to the previous two days since they were placed in a novel environment (Figure 8C). However, when the tone was presented after five minutes, the Young CD mice froze significantly longer than Young HF animals, revealing a diet-dependent effect for this task. The significant decrease in freezing time suggests an even greater impairment in associating the tone and shock from the first day due to DIO.
Figure 8. Behavioral Battery Performance.
No significant differences were observed on the rotarod test between groups. Significant differences in freezing behavior were observed on Day 3 of the Fear Conditioning Test with animals fed HFD performing worse than animals on a CD. *p < 0.05 compared to young CD mice.
Correlation Analysis
Lastly, we evaluated potential correlations between oxidative stress measures, adiposity, and cognitive testing results. A subgroup of animals within each group that received behavioral testing as well as EPR were included in these analyses. As shown in Table 2, cortical levels of total ROS, superoxide, and peroxynitrite were significantly correlated with each other (p<0.01) consistent with all ROS being increased comparably. Total ROS and superoxide levels also revealed a significant correlation (p<0.05 and p<0.01, respectively) to adiposity, but not body weight suggesting the fat itself was more predictive than body weight to increase oxidative stress. While oxidative stress levels did not correlate with performance on the behavioral tasks, it is evident that DIO resulted in increased cerebral oxidative stress levels.
Table 2.
Correlations between adiposity, ROS, and brain function
| Total ROS | Superoxide | Peroxynitrite | Body Weight | Adiposity | Fear Cond. Day 3 | SLST Day 1 | SLST Day 2 | |
|---|---|---|---|---|---|---|---|---|
| Total ROS | r2 = 0.947 p < 0.0001 |
r2 = 0.951 p < 0.0001 |
r2 = 0.806 p =0.006 |
r2 = 0.903 p < 0.0001 |
r2 = 0.277 p = 0.224 |
r2 = 0.121 p = 0.444 |
r2 = 0.001 p = 0.953 |
|
| Superoxide | r2 = 0.947 p < 0.0001 |
r2 = 0.884 p = 0.002 |
r2 = 0.841 p = 0.004 |
r2 = 0.780 p = 0.009 |
r2 = 0.120 p = 0.447 |
r2 = 0.104 p = 0.479 |
r2 = 0.002 p = 0.928 |
|
| Peroxynitrite | r2 = 0.951 p < 0.0001 |
r2 = 0.884 p = 0.002 |
r2 = 0.695 p = 0.020 |
r2 = 0.920 p = 0.001 |
r2 = 0.312 p = 0.192 |
r2 = 0.039 p = 0.671 |
r2 = 0.005 p = 0.883 |
|
| Body Weight | r2 = 0.806 p = 0.006 |
r2 = 0.841 p = 0.004 |
r2 = 0.695 p = 0.020 |
r2 = 0.556 p = 0.054 |
r2 = 0.110 p = 0.469 |
r2 = 0.084 p = 0.528 |
r2 = 0.002 p = 0.924 |
|
| Adiposity | r2 = 0.903 p < 0.0001 |
r2 = 0.780 p = 0.009 |
r2 = 0.920 p = 0.001 |
r2 = 0.556 p = 0.054 |
r2 = 0.317 p = 0.188 |
r2 = 0.052 p = 0.623 |
r2 = 0.031 p = 0.704 |
|
| Fear Cond. Day 3 | r2 = 0.277 p = 0.224 |
r2 = 0.120 p = 0.447 |
r2 = 0.312 p = 0.192 |
r2 = 0.110 p = 0.469 |
r2 = 0.317 p = 0.188 |
r2 = 0.166 p = 0.365 |
r2 = 0.145 p = 0.399 |
|
| SLST Day 1 | r2 = 0.121 p = 0.444 |
r2 = 0.104 p = 0.479 |
r2 = 0.039 p = 0.671 |
r2 = 0.084 p = 0.528 |
r2 = 0.052 p = 0.623 |
r2 = 0.166 p = 0.365 |
r2 = 0.226 p = 0.281 |
|
| SLST Day 2 | r2 = 0.001 p = 0.953 |
r2 = 0.002 p = 0.928 |
r2 = 0.005 p = 0.883 |
r2 = 0.002 p = 0.924 |
r2 = 0.031 p = 0.704 |
r2 = 0.145 p = 0.399 |
r2 = 0.226 p = 0.281 |
Discussion
Our results demonstrate for the first time evidence for DIO elevating the levels of total ROS in the brain. Animals fed the HF diet had significantly higher levels of total ROS, superoxide, and peroxynitrite compared to CD mice. Prominent cellular antioxidant defense systems were evaluated in the hippocampus and cortex of mice following control or high fat diet administration. Most notable was the significant decrease in cortical glutathione peroxidase activity in mice fed the high fat diet. This reduced activity supports the observed increase in total ROS and superoxide levels measured by EPR in the cerebrocortex of Young HF mice. However, no significant differences were found for superoxide dismutase in the cortex or hippocampus. Based on the EPR data which revealed increased oxidative stress levels for animals fed the high fat diet and not the control diet, it appears the reduced glutathione peroxidase activity levels may be more important in promoting oxidative stress than other antioxidant pathways. However, future studies are necessary to determine which other factors in are contributing to the significant increase in cortical total ROS, superoxide, and peroxynitrite following DIO.
One of the molecules known to play a key role in oxidative stress is the transcription factor, Nrf2. The Nrf2 signaling pathway is a master regulator of multiple antioxidant and detoxification pathways (26), and is known to be involved in regulating oxidative stress in the brain in a variety of settings (27–29), as well as modulating the complexities of obesity in tissues other than the brain (30–32). In a previous study by our laboratory, the Nrf2 system was evaluated in 24-month old male C57Bl/6 mice following consumption of a high fat lard-based diet (HFL) for 16 weeks. Consumption of the HFL diet resulted in significantly decreased Nrf2 DNA binding activity, a decrease in the Nrf2 responsive pathway proteins, HO-1 and NQO-1, and decreased Nrf2 protein expression (but not significant)(23). In the current study we also observed a trend, but not significant level, of reduced cortical Nrf2 protein levels for animals fed the HF diet. We did not observe a significant difference in cortical or hippocampal Keap1 or HO-1 (data not shown). The lack of a statistically significant change in Nrf2 and its related proteins suggests other pathways must be involved in the observed increase in DIO-induced cerebral oxidative stress.
In addition to exacerbating cerebral oxidative stress, DIO promoted a decline in cognitive ability. Significant results for the Fear Conditioning task were selective to Day 3 revealing differences in associative learning during the tone test. The young mice exhibited an increased freezing response following presentation of the tone, but a significant decrease in freezing response was determined for the Young HF mice compared to the Young CD mice. These data indicate the existence of a DIO effect on impairing cognitive function. No significant differences in freezing response between groups were found on Day 2 suggesting a lack of contextual memory deficits. This is also supported by the lack of significant differences in hippocampal superoxide dismutase, catalase, and glutathione peroxidase activity. However, additional tests for hippocampal function and oxidative stress analyses must be done in the future to better understand the effects of the CD and HF diets for this brain region. Upon further analysis over the three days of testing, the possibility of impaired hearing and/or sensory perception was ruled out. This is demonstrated by the results of training on Day 1, shown in Figure 8A, which reveals similar increases in freezing due to the presentation of the tone and then shock.
In summary, the current data demonstrated DIO increased cerebrocortical oxidative stress, and that this level of oxidative stress was highly related to the level of adiposity. Understanding the downstream effects of increased ROS, and identifying the basis for obesity induced elevations in ROS, are the next essential steps to advancing our understanding of how obesity modulates brain function. The impairments in cognitive function following obesity may not only be important to understanding how obesity alters cognitive function in adults, but may also be important to understanding how obesity may increase the risk for age-related neurodegenerative conditions such as Alzheimer’s disease. The current study suggest that any contribution of obesity as a mediator of neurodegeneration may preferentially originate within the cerebral cortex. Studies are currently underway to understand the influence of vascular and neurotransmitter disturbances as the triggers by which elevations in oxidative stress, and the development of cognitive decline, occur in response to DIO.
Acknowledgments
This work utilized the facilities of the Cell Biology and Bioimaging Core that are supported in part by COBRE (NIH 2P20-RR021945) and NORC (NIH 2P30-DK072476) center grants from the National Institutes of Health, the Pennington Animal Metabolism and Behavior Core, and funds from the Hibernia National Bank/Edward G Schleider Chair (JNK).
List of Abbreviations
- AD
Alzheimer’s Disease
- CD
Control Diet
- CMH
1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethyl-pyrrolidine
- CPH
1-hydroxy-3-carboxy-2,2,5,5-tetramethylpyrrolidine
- DHE
Dihydroethidium
- DIO
Diet-induced obesity
- DNA
Deoxyribonucleic acid
- EDTA
Ethylenediaminetetraacetic acid
- EPR
Electron Paramagnetic Resonance
- HF
High fat
- HFL
High fat lard
- HO-1
Heme oxygenase-1
- Keap1
Kelch-like ECH-associated protein 1
- NMR
Nuclear Magnetic Resonance
- NQO-1
NAD(P)H dehydrogenase, quinone 1
- Nrf2
Nuclear factor (erythroid-derived 2)-like 2
- PBS
Phosphate buffered saline
- PEG-SOD
Polyethylene glycol, covalently linked to superoxide dismutase
- ROS
Reactive oxygen species
- SLS
Stress learning swim
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, et al. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Census Bureau. SAotUSsEW, DC. 2011 < http://www.census.gov/compendia/statab/>.
- 3.Flegal KM, et al. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303(3):235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 4.Malnick SD, Knobler H. The medical complications of obesity. QJM. 2006;99(9):565–79. doi: 10.1093/qjmed/hcl085. [DOI] [PubMed] [Google Scholar]
- 5.Cordain L, et al. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81(2):341–54. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, Shigenaga MK, Hagen TM. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993;90(17):7915–22. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L, et al. Aging, cancer and nutrition: the DNA methylation connection. Mech Ageing Dev. 2003;124(10–12):989–98. doi: 10.1016/j.mad.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Keller JN. Age-related neuropathology, cognitive decline, and Alzheimer’s disease. Ageing Res Rev. 2006;5(1):1–13. doi: 10.1016/j.arr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Lakatta EG. Age-associated cardiovascular changes in health: impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7(1):29–49. doi: 10.1023/a:1013797722156. [DOI] [PubMed] [Google Scholar]
- 10.Bruce-Keller AJ, et al. NOX activity in brain aging: exacerbation by high fat diet. Free Radic Biol Med. 2010;49(1):22–30. doi: 10.1016/j.freeradbiomed.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemma C, et al. Oxidative Stress and the Aging Brain: From Theory to Prevention. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Boca Raton (FL): 2007. [PubMed] [Google Scholar]
- 12.Head E, Rofina J, Zicker S. Oxidative stress, aging, and central nervous system disease in the canine model of human brain aging. Vet Clin North Am Small Anim Pract. 2008;38(1):167–78. vi. doi: 10.1016/j.cvsm.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariani E, et al. Oxidative stress in brain aging, neurodegenerative and vascular diseases: an overview. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;827(1):65–75. doi: 10.1016/j.jchromb.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 14.Bueler H. Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson’s disease. Exp Neurol. 2009;218(2):235–46. doi: 10.1016/j.expneurol.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer’s disease. Biochim Biophys Acta. 2010;1801(8):924–9. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cenini G, et al. Association between frontal cortex oxidative damage and beta-amyloid as a function of age in Down syndrome. Biochim Biophys Acta. 2012;1822(2):130–8. doi: 10.1016/j.bbadis.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Floyd RA, Hensley K. Oxidative stress in brain aging. Implications for therapeutics of neurodegenerative diseases. Neurobiol Aging. 2002;23(5):795–807. doi: 10.1016/s0197-4580(02)00019-2. [DOI] [PubMed] [Google Scholar]
- 18.Umur EE, et al. Increased iron and oxidative stress are separately related to cognitive decline in elderly. Geriatr Gerontol Int. 2011;11(4):504–9. doi: 10.1111/j.1447-0594.2011.00694.x. [DOI] [PubMed] [Google Scholar]
- 19.Kalmijn S, et al. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42(5):776–82. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- 20.Morris MC, et al. Dietary fat intake and 6-year cognitive change in an older biracial community population. Neurology. 2004;62(9):1573–9. doi: 10.1212/01.wnl.0000123250.82849.b6. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J, et al. Cognitive performance is associated with macronutrient intake in healthy young and middle-aged adults. Nutr Neurosci. 2006;9(3–4):179–87. doi: 10.1080/10284150600955172. [DOI] [PubMed] [Google Scholar]
- 22.Parrott MD, Greenwood CE. Dietary influences on cognitive function with aging: from high-fat diets to healthful eating. Ann N Y Acad Sci. 2007;1114:389–97. doi: 10.1196/annals.1396.028. [DOI] [PubMed] [Google Scholar]
- 23.Morrison CD, et al. High fat diet increases hippocampal oxidative stress and cognitive impairment in aged mice: implications for decreased Nrf2 signaling. J Neurochem. 2010;114(6):1581–9. doi: 10.1111/j.1471-4159.2010.06865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White CL, et al. Effects of high fat diet on Morris maze performance, oxidative stress, and inflammation in rats: contributions of maternal diet. Neurobiol Dis. 2009;35(1):3–13. doi: 10.1016/j.nbd.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baird L, Dinkova-Kostova AT. The cytoprotective role of the Keap1-Nrf2 pathway. Arch Toxicol. 2011;85(4):241–72. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 26.Lee JM, Johnson JA. An important role of Nrf2-ARE pathway in the cellular defense mechanism. J Biochem Mol Biol. 2004;37(2):139–43. doi: 10.5483/bmbrep.2004.37.2.139. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, et al. Melatonin activates the Nrf2-ARE pathway when it protects against early brain injury in a subarachnoid hemorrhage model. J Pineal Res. 2012 doi: 10.1111/j.1600-079X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, et al. Histone deacetylase inhibition activates transcription factor Nrf2 and protects against cerebral ischemic damage. Free Radic Biol Med. 2012;52(5):928–36. doi: 10.1016/j.freeradbiomed.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salminen A, et al. Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer’s disease. Prog Neurobiol. 2012;96(1):87–95. doi: 10.1016/j.pneurobio.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 30.Sykiotis GP, et al. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr Opin Clin Nutr Metab Care. 2011;14(1):41–8. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chartoumpekis DV, et al. Nrf2 represses FGF21 during long-term high-fat diet-induced obesity in mice. Diabetes. 2011;60(10):2465–73. doi: 10.2337/db11-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meher AK, et al. Nrf2 deficiency in myeloid cells is not sufficient to protect mice from high-fat diet-induced adipose tissue inflammation and insulin resistance. Free Radic Biol Med. 2012;52(9):1708–1715. doi: 10.1016/j.freeradbiomed.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao H, et al. Superoxide reacts with hydroethidine but forms a fluorescent product that is distinctly different from ethidium: potential implications in intracellular fluorescence detection of superoxide. Free Radic Biol Med. 2003;34(11):1359–68. doi: 10.1016/s0891-5849(03)00142-4. [DOI] [PubMed] [Google Scholar]
- 34.Pestana RR, et al. Reactive oxygen species generated by NADPH oxidase are involved in neurodegeneration in the pilocarpine model of temporal lobe epilepsy. Neurosci Lett. 2010;484(3):187–91. doi: 10.1016/j.neulet.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 35.Douglas RM, et al. Neuronal death during combined intermittent hypoxia/hypercapnia is due to mitochondrial dysfunction. Am J Physiol Cell Physiol. 2010;298(6):C1594–602. doi: 10.1152/ajpcell.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Javeshghani D, et al. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. J Am Soc Hypertens. 2009;3(5):295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Csont T, et al. Hypercholesterolemia increases myocardial oxidative and nitrosative stress thereby leading to cardiac dysfunction in apoB-100 transgenic mice. Cardiovasc Res. 2007;76(1):100–9. doi: 10.1016/j.cardiores.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal D, et al. Role of proinflammatory cytokines and redox homeostasis in exercise-induced delayed progression of hypertension in spontaneously hypertensive rats. Hypertension. 2009;54(6):1393–400. doi: 10.1161/HYPERTENSIONAHA.109.135459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elks CM, et al. Chronic NF-{kappa}B blockade reduces cytosolic and mitochondrial oxidative stress and attenuates renal injury and hypertension in SHR. Am J Physiol Renal Physiol. 2009;296(2):F298–305. doi: 10.1152/ajprenal.90628.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ebenezer PJ, et al. Diet-induced renal changes in Zucker rats are ameliorated by the superoxide dismutase mimetic TEMPOL. Obesity (Silver Spring) 2009;17(11):1994–2002. doi: 10.1038/oby.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elks CM, et al. A blueberry-enriched diet attenuates nephropathy in a rat model of hypertension via reduction in oxidative stress. PLoS One. 2011;6(9):e24028. doi: 10.1371/journal.pone.0024028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pistell PJ, et al. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219(1–2):25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]




