Abstract
Inducing significant axon growth or regeneration after spinal cord injury has been difficult, primarily due to the poor growth supportive environment and low intrinsic growth ability of neurons within the CNS. Neurotrophins alone have been shown to readily induce regeneration of sensory axons after dorsal root lesions, however if neurotrophin gradients are expressed within the spinal cord these axons fail to terminate within appropriate target regions. Under such conditions, addition of a “stop” signal reduces growth into deeper dorsal laminae to support more specific targeting. Such neurotrophin gradients alone lose their effectiveness when lesions are within the spinal cord, requiring a combined treatment regime. Construction of pathways using combined treatments support good regeneration when they increase the intrinsic growth properties of neurons, provide a bridge across the lesion site, and supply a growth supportive substrate to induce axon growth out of the bridge and back into the host. Neurotrophin gradients distal to the bridge greatly enhance axon outgrowth. In disorders where neuronal circuits are lost, construction of preformed growth supportive pathways sustain long distance axon growth from a neuronal transplant to distal target locations.
Keywords: Axon regeneration, Spinal cord injury, Neurotrophin, Gradient, Guidance molecules
1. Introduction
Neurotrauma and disease in the adult mammalian central nervous system (CNS) cause axon disruption, neuronal death, target denervation, and functional deficits. To recover the normal circuitry, injured axons and those of transplanted neurons need to grow through a lesion, with its physical barriers of cysts and a dense scar, along a pathway with insufficient growth supporting molecules and the presence of inhibitory molecules to reinnervate their target neurons. Little marked spontaneous axonal regeneration or neuronal replacement occurs in the damaged CNS. The experimental evidence to date indicates that injured axons regenerate amongst cells transplanted to bridge a CNS lesion but rarely beyond [82]. Those of transplanted neurons elongate short distances and terminate their growth when they encounter the lesioned environment [18]. To entice axons to regenerate across a lesion and towards distal targets a number of growth and guidance molecules have been examined, particularly neurotrophins. In this review we will examine the use of growth and guidance molecules to direct the growth of regenerating or sprouting axons within the adult CNS or out of bridging transplants.
The possibility that axons could be directed to grow towards a specific source was first described early in the last century by Ramon y Cajal [56], who observed that peripheral nerve axons would extend within a fibrin clot from the proximal tip of a lesioned nerve to the distal cut end. Even if the distal stump was placed in a disadvantageous location for axonal growth, many axons would often follow a circuitous path to find the distal stump. They also observed that CNS axons grew into peripheral nerve grafts. Based on these findings, they concluded that neurons have a regenerative capacity as long as appropriate “neutritive” and “orienting” substances were provided to them. Similar observations led Sperry [71] to establish the chemoaffinity hypothesis for axonal guidance.
Over the years, many axonal growth and guidance molecules have been identified. They display a myriad of functions, ranging from those that support axonal growth and fasciculation to those that have both chemoaffinitive and chemorepulsive properties, depending on the neuronal subpopulation [16,69,76]. During development two primary schemes are used to deliver guidance information to growing axons. They consist of either long-range (diffusible) factors secreted at intermediate locations or short-range (contact) factors expressed on cells along the pathway. At long distances, the diffusible factors establish gradients emanating from the point of secretion [16,76]. These gradients provide directional information to the growth cone by inducing asymmetry within the cytoskeletal network to drive growth either up (chemoattractive) or down (chemorepulsive) the gradient. Several of these axonal growth and guidance molecules have successfully been used in the adult lesioned spinal cord to entice axons growth past the lesion site or to induce sprouting of axons.
2. Guidance factor gradients direct axon regeneration
Numerous studies have shown that strategic application of neurotrophins provides directional cues to entice axons to regenerate past the lesion site towards distal targets. To maximize directional growth of these axons, neurotrophins are often applied in a manner to generate a gradient with the highest expression level near the presumptive target location [8,64,75,87]. Such gradients can easily be generated by expression of neurotrophins using viral vectors. A single injection can induce a fairly extensive gradient extending several millimeters from the injection site, due most likely to the diffusion of both the injected virus and the neurotrophin produced by the transduced cells [8,30,75]. Others and we have used such a gradient to induce regeneration of sensory axons into the spinal cord after dorsal root lesions [65,73,86]. In this model, lesioned dorsal root axons regenerate within the peripheral part of the pathway leading to the spinal cord, but terminate their growth upon contact with the spinal cord at the dorsal root entry zone (DREZ). Over expression of nerve growth factor (NGF) within the dorsal horn leads to robust regeneration of nociceptive axons that extend through the DREZ and throughout the entire dorsal horn [65,73,74]. These axons terminated their growth within the area of neurotrophin expression, not abiding endogenous targeting signals, thus terminating within both appropriate lamina (I & II) and ectopic locations (deeper dorsal lamina, see Fig. 1C and E). Likewise, sprouting of non-lesioned endogenous spinal cord pathways towards the NGF source was also observed, which can lead to unwanted plasticity and detrimental function should synapse formation occur [10,64,72]. Neurotrophin-induced sprouting of non-lesioned endogenous pathways is potentially an unavoidable response which could be difficult to define anatomically or behaviorally.
Fig. 1.
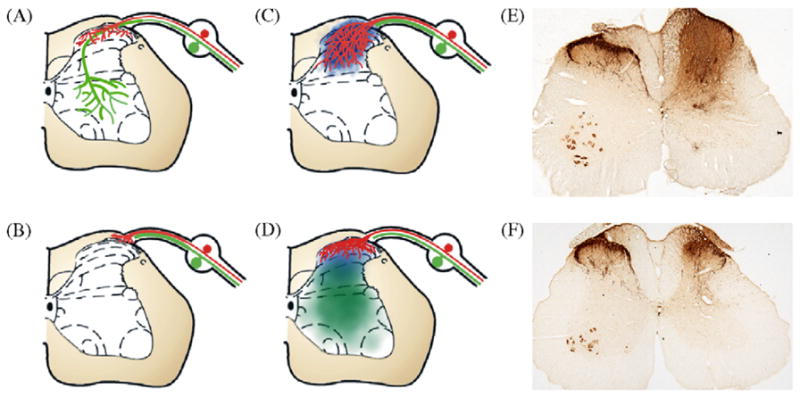
Recapitulation of developmental-like guidance cues can target regenerating sensory axons. Schematic illustration of the DREZ model used to examine regeneration of sensory afferents into the spinal cord. (A) Peptidergic nociceptive axons (red) in the normal spinal cord terminate in laminae I and II of the dorsal horn; whereas, large myelinated axons (green) extend to more ventral regions. (B) Dorsal root rhizotomy results in severing of both nociceptive and proprioceptive axons. These axons are capable of regenerating in the peripheral nerve up to, but not into, the spinal cord. (C) Injecting NGF adenovirus into the dorsal horn of the spinal cord (blue) supports the regeneration of sensory afferents through the DREZ and throughout the entire dorsal horn of the spinal cord, a pattern unlike normal. (D) To target regenerating axons to their normal laminae, NGF adenovirus was injected dorsally, while semaphorin 3A-encoding adenovirus was injected ventrally. This produced slightly overlapping gradients that supported regeneration of peptidergic nociceptive axons into the upper dorsal horn exclusively, generating a reinnervation pattern very similar to normal. (E) Photo of spinal cord treated with NGF dorsally and GFP ventrally (right side, as in C) showing extensive regeneration of sensory nociceptive axons throughout the entire dorsal horn. (F) Photo of spinal cord treated with NGF dorsally and Semaphorin 3A ventrally (right side, as in E) axon regeneration is restricted to the upper dorsal horn similar to the sham lesion side (left side of cord in E and F; Tang et al. [74]).
To limit the regeneration or sprouting of axons into ectopic regions, a stop signal can be induced within these regions. During development such stop signals are under tight genetic control that regulates their expression within precise spatial and temporal domains [16,76]. This regulation supports growth along appropriate pathways, while limiting or preventing axon growth into ectopic regions. One such stop signal for nociceptive axons is semaphorin 3A. During development it is expressed within regions of the spinal cord or periphery where NGF-responsive nociceptive axons fail to grow and potentially limits nociceptive axon growth to lamina I and II of the dorsal horn [48,81]. To determine if semaphorin 3A would restrict NGF-induced regeneration of nociceptive axons to the appropriate targets in the upper dorsal horn (lamina I and II), slightly overlapping gradients of NGF and semaphorin 3A were established with the semaphorin gradient slightly ventral to the NGF gradient (Fig. 1D). This configuration provides a growth supportive NGF rich environment near the DREZ which becomes more inhibitory as axons regenerate into the deeper dorsal lamina. Since high concentrations of NGF can antagonize the chemorepulsive effect of semaphorin [20,81], we carefully balanced our titers to ensure good functional expression of both transgenes [72]. We also observed the best results when the injection of semaphorin 3A was delayed 3 days after the NGF viral injections. This most likely allows NGF to prime regenerating axons to help support their growth into the spinal cord. Co-expression of both NGF with semaphorin, but not with green fluorescent protein (GFP), limited regeneration of nociceptive axons to the more appropriate target regions of the upper dorsal horn, while preventing growth into deeper dorsal laminae (Fig. 1F) [74]. This anatomical result was associated with restoration of a more normal-like synaptic patterning and almost complete restoration of thermal nociceptive latencies. Likewise, co-expression of NGF with semaphorin, but not GFP, reduced sprouting of non-lesioned nociceptive axons and reduced its detrimental behavioral effects observed by either injury, expression of NGF alone or with GFP [10,72].
High levels of neurotrophin expressed over multiple target locations could mask endogenous guidance properties within the adult nervous system and prevent specific targeting of regenerating axons. Systemic application of artemin, a member of the GDNF family of neurotrophin, not only induced robust regeneration of sensory axons across the DREZ, but also supported growth of multiple sensory populations to appropriate dorsal laminae [79]. Since this neurotrophin was administered systemically, it could not attract axons to regenerate to the targets using a chemotropic mechanism, but most likely increased the intrinsic growth capability of these axons allowing them to overcome the inhibitory environment at the DREZ. Interestingly, it did not disrupt their ability to cue onto endogenous guidance mechanism within the dorsal horn. Artemin not only supported targeting to appropriate lamina, but also supported targeting of somatosensory axons to appropriate dermatomal targets within the dorsal horn (Eric Frank, personal communication). Artemin has also not been observed to enhance sprouting from non-injured sensory axons within the spinal cord, suggesting it could be clinically useful in treatment of dorsal root injuries.
3. Bridging the lesion to enhance regeneration
Unlike peripheral or dorsal root injuries, lesions to the CNS result in extensive inhibitory scar formation at the lesion site [68] and the axon target site [46]. Inducing regeneration across such a lesion has proven extremely difficult. In the 1980s, Aguayo and co-workers observed, with neuroanatomical tracing, that injured adult rat spinal cord axons and primary afferents regenerated into a peripheral nerve graft to bridge a complete transection gap in the thoracic spinal cord [60,61]. Subsequent experiments using animal models of spinal cord, dorsal and ventral root, optic nerve, and brain injury have been performed. In addition to peripheral nerves, bridging materials of rat, mouse, dog, monkey, and human Schwann cells alone [2] and when inserted into semipermeable polyacrylonitrile/polyvinylchoride polymer channels [32,83] or resorbable tubular scaffolds made of high molecular weight poly(L-lactic acid) and 10% poly(L-lactic acid oligomers) [51], olfactory ensheathing glia alone [33,36,37,40,55,57,62,67] and when in endogenous matrix [41], porous polymer capsules [14], and biodegradable dextran sulphate–gelatin co-precipitate tubular scaffolds [63], fetal spinal cord alone [5,9,59] and in guidance channels [3], and bone marrow stromal cells [28] have shown some of the best results in providing a supportive substrate for axon regeneration in experimental spinal cord injury.
4. Combining treatments further enhances axon regeneration
Although axons are often observed growing into these bridges, a consistent finding was limited axon growth out of the bridges back into the host tissue. This has led to various combinatorial approaches being evaluated to entice axon growth out of bridges and back into the host tissue. Neurotrophin or growth factor treatment within the host tissue just distal to the bridge has proven quite effective for enticing axon regeneration through/from a peripheral nerve [52,53] and multiple peripheral nerves routed from white to gray matter [12,23], Schwann cell [4,7,26], or fetal spinal cord [15,44] bridges and back into the host cord. Removal of the inhibitory extracellular matrix chondroitin sulfate proteoglycan with the bacterial enzyme chondroitinase ABC has also been observed to aid regenerating axon outgrowth from peripheral nerve [29], Schwann cell [11], or fetal spinal cord [38] bridges. Combining chondroitinase ABC [22,78] with intraspinal transplants of olfactory ensheathing cells [58] also promoted regenerating axon outgrowth from Schwann cell bridges. This also occurred through Schwann cell [54] or fetal spinal cord [50] bridges when spinal cord cAMP levels were altered by intraspinal dibutyryl cAMP delivery and systemic administration of rolipram, the cAMP-specific phosphodiesterase 4 inhibitor.
Many of the cell types that provide bridges for lesioned axons endogenously express high levels of neurotrophins. As has been seen with Schwann cells, exogenous administration of [32,83,85] and engineering to express [25,47] neurotrophins further enhance the regenerative potential of these cell types. Fibroblasts or bone marrow stromal cells typically express low levels of neurotrophin and transplantation of non-modified cells induce little to no axon regeneration. However, when any of these cells are engineered to express high levels of neurotrophin using viral vectors, the numbers of axons regenerating into the grafts significantly increase [6,43]. As observed with other bridging cells very few axons grow back into the host cord. To induce regeneration of sensory axons through a NT-3 expressing bone marrow stromal cell graft within a dorsal column lesion to the cervical cord, Taylor et al. [75] injected lentivirus encoding NT-3 rostral to the graft border. When the rostral NT-3 gradient came in contact with the graft significant numbers of axons were observed extending out of the graft. If the rostral NT-3 gradient failed to contact the graft border no axons were observed growing out of the graft. This indicated that even a small gap between the NT-3 expressing graft and the rostral NT-3 gradient fails to induce sensory axon growth out of the graft.
Axon regeneration is also highly dependent on the intrinsic growth state of neurons; in which adult neurons have a relatively poor intrinsic growth state when compared to embryonic neurons. This intrinsic growth state can be increased by application of dibutyryl cAMP and rolipram or by performing a conditioning lesion to the peripheral part of the sensory neuron [49,70]. Enhanced outgrowth from a neurotrophin expressing bridge has been observed with either treatment [43,75]. A triple combined treatment of NT-3 expressing bone marrow stromal cells, sciatic nerve conditioning lesion, and the rostral injection of NT-3 into either the nucleus gracilis or the reticular formation was used to assess the targeting of regenerating dorsal column sensory axons [1]. In either group, axons grew into the source of NT-3, whether an appropriate target (nucleus gracilis) or an inappropriate target (reticular formation). Furthermore, regenerating axons within the nucleus gracilis reached a level about 27% that of the original axon density; however, even though synapses were identified, no post-synaptic recording was evoked by sciatic nerve stimulation [1]. Interestingly, this triple combination of treatments induced sensory axon regeneration when administration started either 6 weeks or 15 months after midcervical lesions [35]. These results show that increasing the intrinsic growth potential of neurons combined with construction of a supportive bridge and neurotrophin gradient can effectively induce regeneration of either acutely or chronically injured adult sensory axons.
Unlike what was seen in the adult rat [12], combining multiple peripheral nerves grafts from injured white matter to gray matter with fibrin glue containing fibroblast growth factor 1 was not found to be anatomically or functionally therapeutic when used in non-human primates with lesioned spinal cords [39]. However, this strategy was reported to markedly improve function 2 years post-treatment for a person with chronic SCI [13]. Examination 1 year after autologous Schwann cells were transplanted into an individual with chronic SCI revealed that the treatment was safe and feasible [66]. Reports that olfactory ensheathing glia transplants were safe, feasible, and led to improved function have been made [27,31,42,45]. However, these results need to be carefully considered in light of the number of issues that have been raised about them. These include the SCI recipient selection, preclinical results, cell types transplanted and where, use of methylprednisolone, medical complications, and post-surgery assessments [19]. Lastly, cysts were filled and relatively modest changes in sensation and pain occurred when pieces of human fetal spinal cord were grafted into the spinal cords of a small number of patients with syringomyelia in combination with detethering, draining the cysts, and immunosuppression [21,80]. However, spasticity as well as gait and proprioceptive disturbances were observed. A Phase I/II open-label and non-randomized study of autologous bone marrow stromal cells transplanted acutely and subchronically into patients with complete spinal cord injuries when combined with granulocyte macrophage-colony stimulating delivery also was seen to be safe [84].
5. Directing long distance growth of axons from transplanted neurons
Relatively long distance gradient pathways of neurotrophins or guidance molecules have been shown to promote long distance directed axon growth from neuronal transplants to a particular target location. These experiments primarily focused on early postnatal or embryonic neuronal transplants [8,34,87]. It is known that microtransplantation of neurons into the intact brain or spinal cord, in such a way that no glial scar forms, will promote long distance axon growth within white matter tracts [17,18,24,77]. Interestingly, if these neurons are transplanted into the correct region they can re-establish appropriate targets. However, if the transplants are relatively large, near a lesion site, or induce glial scar formation, axons fail to grow a significant distance. To get axons from relatively large neuronal transplants to grow long distances (>5 mm) or across a lesion, gradient pathways were established within the corpus callosum, so that the concentration increased with distance from the transplant site (Fig. 2) [34,87]. When DRG neuronal transplants were placed at the low concentration end of a NGF and fibroblast growth factor-2 gradient robust axon growth up the gradient, across the corpus callosum and into the contralateral hemisphere was observed. In these experiments the pathway within the contralateral hemisphere turned 90° leading to targets in either the cortex or the striatum. Approximately 50% of the axons following the pathway likewise turned and grew towards either the cortex or the striatal targets [87]. This turning could be enhanced by co-expression of semaphorin 3A slightly lateral to the turning point (Fig. 2C). Recently we have been investigating reconstruction of the nigrostriatal pathway in a hemi-Parkinson’s rat model and have observed robust growth of E14 dopaminergic axons along gradient pathways of GDNF and GFR-α1, as well as, netrin-1. Likewise with partial lesions of the substantia nigra in mice, a gradient of GDNF placed into the striatum, is sufficient to induce robust growth of dopaminergic axons into the striatum, and reduced amphetamine-induced rotational behavior [77].
Fig. 2.
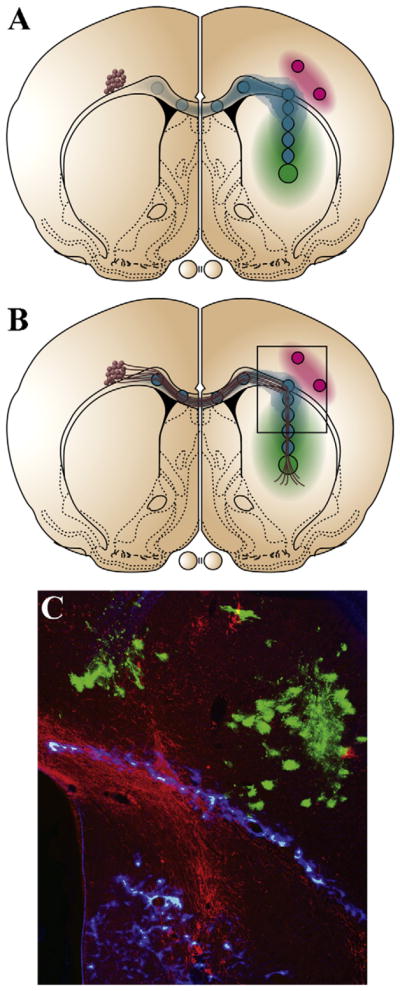
Targeting the growth of transplanted neurons. Schematic diagram of coronal rat brain cross sections. (A) Adenovirus encoding a target neurotrophic source (green) was injected into the striatum on the right side. Nine smaller injections of a second adenovirus were used to create a pathway to enhance growth (blue) from the striatum through the corpus callosum to the contralateral hemisphere. In some experiments semaphorin 3A-encoding adenovirus was injected above the corpus callosum just lateral to the turn. One week after viral injections, DRG neurons (brown) were transplanted above the corpus callosum in the hemisphere contralateral to the target. (B) Pathway supported axons grew along the corpus callosum into the contralateral hemisphere and turned toward the target neurotrophic source. Without semaphorin, only ~50% of the axons turned; and with semaphorin ~80% turned into the striatum. (C) Representative photo of DRG axons (red) turning into the striatum towards an NGF target (blue overlay from adjacent section). Semaphorin 3A expressing cells (green) increase the number of axons turning into the striatum by reducing their growth past the turning site (Ziemba et al. [87]).
In summary, the construction of neurotrophin gradients distal to the lesion supports regeneration of sensory axons into the spinal cord after dorsal root rhizotomies and out of cellular bridges after CNS lesions. Such cellular bridges can be composed of tissues or cells that support spontaneous regeneration, or cells that typically do not support regeneration if engineered to express growth factors. Although neurotrophins can be used to direct axon growth to a target, overexpression across multiple target locations or within the wrong target area can lead to inappropriate targeting of axons. Finally the construction of preformed guidance pathways can be used to direct axons out of neuronal transplants towards a specific target location.
Footnotes
Conflict of interest statement
The authors are not aware of any memberships, affiliations, funding, or financial holdings that could be perceived as affecting the objectivity of this review.
References
- 1.Alto LT, Havton LA, Conner JM, Hollis ER, II, Blesch A, Tuszynski MH. Hemotropic guidance facilitates axonal regeneration and synapse formation after spinal cord injury. Nat Neurosci. 2009;12:1106–1113. doi: 10.1038/nn.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews MR, Stelzner DJ. Evaluation of olfactory ensheathing and Schwann cells after implantation into a dorsal injury of adult rat spinal cord. J Neurotrauma. 2007;24:1773–1792. doi: 10.1089/neu.2007.0353. [DOI] [PubMed] [Google Scholar]
- 3.Bamber NI, Li HY, Aebischer P, Xu XM. Fetal spinal cord tissue in guidance channels promotes longitudinal axonal growth in the partially lesioned adult rat spinal cord. Neural Plast. 1999;6:103–121. doi: 10.1155/NP.1999.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bamber NI, Li H, Lu X, Oudega M, Aebischer P, Xu XM. Neurotrophins BDNF and NT-3 promote axonal re-entry into the distal host spinal cord through Schwann cell-seeded mini-channels. Eur J Neurosci. 2001;13:257–268. [PubMed] [Google Scholar]
- 5.Bernstein JJ, Goldberg WJ. Fetal spinal cord homografts ameliorate the severity of lesion-induced hind limb behavioral deficits. Exp Neurol. 1987;98:633–644. doi: 10.1016/0014-4886(87)90271-8. [DOI] [PubMed] [Google Scholar]
- 6.Blesch A, Tuszynski MH. Transient growth factor delivery sustains regenerated axons after spinal cord injury. J Neurosci. 2007;27:10535–10545. doi: 10.1523/JNEUROSCI.1903-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blits B, Oudega M, Boer GJ, Bartlett Bunge M, Verhaagen J. Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience. 2003;118:271–281. doi: 10.1016/s0306-4522(02)00970-3. [DOI] [PubMed] [Google Scholar]
- 8.Bonner JF, Blesch A, Neuhuber B, Fischer I. Promoting directional axon growth from neural progenitors grafted into the injured spinal cord. J Neurosci Res. 2009 doi: 10.1002/jnr.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bregman BS, Kunkel-Bagden E, Reier PJ, Dai HN, McAtee M, Gao D. Recovery of function after spinal cord injury: mechanisms underlying transplant-mediated recovery of function differ after spinal cord injury in newborn and adult rats. Exp Neurol. 1993;123:3–16. doi: 10.1006/exnr.1993.1136. [DOI] [PubMed] [Google Scholar]
- 10.Cameron AA, Smith GM, Randall DC, Brown DR, Rabchevsky AG. Genetic manipulation of intraspinal plasticity after spinal cord injury alters the severity of autonomic dysreflexia. J Neurosci. 2006;26:2923–2932. doi: 10.1523/JNEUROSCI.4390-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chau CH, Shum DK, Li H, Pei J, Lui YY, Wirthlin L, Chan YS, Xu XM. Chondroitinase ABC enhances axonal regrowth through Schwann cell-seeded guidance channels after spinal cord injury. Faseb J. 2004;18:194–196. doi: 10.1096/fj.03-0196fje. [DOI] [PubMed] [Google Scholar]
- 12.Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- 13.Cheng H, Liao KK, Liao SF, Chuang TY, Shih YH. Spinal cord repair with acidic fibroblast growth factor as a treatment for a patient with chronic paraplegia. Spine. 2004;29:E284–288. doi: 10.1097/01.brs.0000131217.61390.2c. [DOI] [PubMed] [Google Scholar]
- 14.Chuah MI, Choi-Lundberg D, Weston S, Vincent AJ, Chung RS, Vickers JC, West AK. Olfactory ensheathing cells promote collateral axonal branching in the injured adult rat spinal cord. Exp Neurol. 2004;185:15–25. doi: 10.1016/j.expneurol.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 15.Coumans JV, Lin TT, Dai HN, MacArthur L, McAtee M, Nash C, Bregman BS. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curinga G, Smith GM. Molecular/genetic manipulation of extrinsic axon guidance factors for CNS repair and regeneration. Exp Neurol. 2008;209:333–342. doi: 10.1016/j.expneurol.2007.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–683. doi: 10.1038/37776. [DOI] [PubMed] [Google Scholar]
- 18.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19:5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobkin BH, Curt A, Guest J. Cellular transplants in China: observational study from the largest human experiment in chronic spinal cord injury. Neurorehabil Neural Repair. 2006;20:5–13. doi: 10.1177/1545968305284675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dontchev VD, Letourneau PC. Nerve growth factor and semaphorin 3A signaling pathways interact in regulating sensory neuronal growth cone motility. J Neurosci. 2002;22:6659–6669. doi: 10.1523/JNEUROSCI.22-15-06659.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Falci S, Holtz A, Akesson E, Azizi M, Ertzgaard P, Hultling C, Kjaeldgaard A, Levi R, Ringden O, Westgren M, Lammertse D, Seiger A. Obliteration of a posttraumatic spinal cord cyst with solid human embryonic spinal cord grafts: first clinical attempt. J Neurotrauma. 1997;14:875–884. doi: 10.1089/neu.1997.14.875. [DOI] [PubMed] [Google Scholar]
- 22.Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fraidakis MJ, Spenger C, Olson L. Partial recovery after treatment of chronic paraplegia in rat. Exp Neurol. 2004;188:33–42. doi: 10.1016/j.expneurol.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 24.Gaillard A, Prestoz L, Dumartin B, Cantereau A, Morel F, Roger M, Jaber M. Reestablishment of damaged adult motor pathways by grafted embryonic cortical neurons. Nat Neurosci. 2007;10:1294–1299. doi: 10.1038/nn1970. [DOI] [PubMed] [Google Scholar]
- 25.Golden KL, Pearse DD, Blits B, Garg MS, Oudega M, Wood PM, Bunge MB. Transduced Schwann cells promote axon growth and myelination after spinal cord injury. Exp Neurol. 2007;207:203–217. doi: 10.1016/j.expneurol.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guest JD, Hesse D, Schnell L, Schwab ME, Bunge MB, Bunge RP. Influence of IN-1 antibody and acidic FGF-fibrin glue on the response of injured corticospinal tract axons to human Schwann cell grafts. J Neurosci Res. 1997;50:888–905. doi: 10.1002/(SICI)1097-4547(19971201)50:5<888::AID-JNR24>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 27.Guest JD, Herrera LP, Qian T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord. 2006;44:135–142. doi: 10.1038/sj.sc.3101820. [DOI] [PubMed] [Google Scholar]
- 28.Hofstetter CP, Schwarz EJ, Hess D, Widenfalk J, El Manira A, Prockop DJ, Olson L. Marrow stromal cells form guiding strands in the injured spinal cord and promote recovery. Proc Natl Acad Sci USA. 2002;99:2199–2204. doi: 10.1073/pnas.042678299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Houle JD, Tom VJ, Mayes D, Wagoner G, Phillips N, Silver J. Combining an autologous peripheral nervous system “bridge” and matrix modification by chondroitinase allows robust, functional regeneration beyond a hemisection lesion of the adult rat spinal cord. J Neurosci. 2006;26:7405–7415. doi: 10.1523/JNEUROSCI.1166-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu X, Cai J, Yang J, Smith GM. Sensory axon targeting is increased by NGF gene therapy within the lesioned adult femoral nerve. Exp Neurol. 2009 doi: 10.1016/j.expneurol.2009.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang H, Chen L, Xi H, Wang Q, Zhang J, Liu Y, Zhang F. Olfactory ensheathing cells transplantation for central nervous system diseases in 1,255 patients. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2009;23:14–20. [PubMed] [Google Scholar]
- 32.Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379–393. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 33.Jeffery ND, Lakatos A, Franklin RJ. Autologous olfactory glial cell transplantation is reliable and safe in naturally occurring canine spinal cord injury. J Neurotrauma. 2005;22:1282–1293. doi: 10.1089/neu.2005.22.1282. [DOI] [PubMed] [Google Scholar]
- 34.Jin Y, Ziemba KS, Smith GM. Axon growth across a lesion site along a preformed guidance pathway in the brain. Exp Neurol. 2008;210:521–530. doi: 10.1016/j.expneurol.2007.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kadoya K, Tsukada S, Lu P, Coppola G, Geschwind D, Filbin MT, Blesch A, Tuszynski MH. Combined intrinsic and extrinsic neuronal mechanisms facilitate bridging axonal regeneration one year after spinal cord injury. Neuron. 2009;64:165–172. doi: 10.1016/j.neuron.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kalincík T, Choi EA, Féron F, Bianco J, Sutharsan R, Hayward I, Mackay-Sim A, Carrive P, Waite PM. Olfactory ensheathing cells reduce duration of autonomic dysreflexia in rats with high spinal cord injury. Auton Neurosci. 2009 doi: 10.1016/j.autneu.2009.10.001. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 37.Keyvan-Fouladi N, Raisman G, Li Y. Functional repair of the corticospinal tract by delayed transplantation of olfactory ensheathing cells in adult rats. J Neurosci. 2003;23:9428–9434. doi: 10.1523/JNEUROSCI.23-28-09428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim BG, Dai HN, Lynskey JV, McAtee M, Bregman BS. Degradation of chondroitin sulfate proteoglycans potentiates transplant-mediated axonal remodeling and functional recovery after spinal cord injury in adult rats. J Comp Neurol. 2006;497:182–198. doi: 10.1002/cne.20980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Levi AD, Dancausse H, Li X, Duncan S, Horkey L, Oliviera M. Peripheral nerve grafts promoting central nervous system regeneration after spinal cord injury in the primate. J Neurosurg. 2002;96:197–205. doi: 10.3171/spi.2002.96.2.0197. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Decherchi P, Raisman G. Transplantation of olfactory ensheathing cells into spinal cord lesions restores breathing and climbing. J Neurosci. 2003;23:727–731. doi: 10.1523/JNEUROSCI.23-03-00727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med. 2006;29:191–203. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu P, Yang H, Jones LL, Filbin MT, Tuszynski MH. Combinatorial therapy with neurotrophins and cAMP promotes axonal regeneration beyond sites of spinal cord injury. J Neurosci. 2004;24:6402–6409. doi: 10.1523/JNEUROSCI.1492-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lynskey JV, Sandhu FA, Dai HN, McAtee M, Slotkin JR, MacArthur L, Bregman BS. Delayed intervention with transplants and neurotrophic factors supports recovery of forelimb function after cervical spinal cord injury in adult rats. J Neurotrauma. 2006;23:617–634. doi: 10.1089/neu.2006.23.617. [DOI] [PubMed] [Google Scholar]
- 45.Mackay-Sim A, Feron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, Nowitzke A, Perry C, Silburn PA, Urquhart S, Geraghty T. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Massey JM, Amps J, Viapiano MS, Matthews RT, Wagoner MR, Whitaker CM, Alilain W, Yonkof AL, Khalyfa A, Cooper NG, Silver J, Onifer SM. Increased chondroitin sulfate proteoglycan expression in denervated brainstem targets following spinal cord injury creates a barrier to axonal regeneration overcome by chondroitinase ABC and neurotrophin-3. Exp Neurol. 2008;209:426–445. doi: 10.1016/j.expneurol.2007.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menei P, Montero-Menei C, Whittemore SR, Bunge RP, Bunge MB. Schwann cells genetically modified to secrete human BDNF promote enhanced axonal regrowth across transected adult rat spinal cord. Eur J Neurosci. 1998;10:607–621. doi: 10.1046/j.1460-9568.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- 48.Messersmith EK, Leonardo ED, Shatz CJ, Tessier-Lavigne M, Goodman CS, Kolodkin AL. Semaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron. 1995;14:949–959. doi: 10.1016/0896-6273(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 49.Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- 50.Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oudega M, Gautier SE, Chapon P, Fragoso M, Bates ML, Parel JM, Bunge MB. Axonal regeneration into Schwann cell grafts within resorbable poly(alpha-hydroxyacid) guidance channels in the adult rat spinal cord. Biomaterials. 2001;22:1125–1136. doi: 10.1016/s0142-9612(00)00346-x. [DOI] [PubMed] [Google Scholar]
- 52.Oudega M, Hagg T. Nerve growth factor promotes regeneration of sensory axons into adult rat spinal cord. Exp Neurol. 1996;140:218–229. doi: 10.1006/exnr.1996.0131. [DOI] [PubMed] [Google Scholar]
- 53.Oudega M, Hagg T. Neurotrophins promote regeneration of sensory axons in the adult rat spinal cord. Brain Res. 1999;818:431–438. doi: 10.1016/s0006-8993(98)01314-6. [DOI] [PubMed] [Google Scholar]
- 54.Pearse DD, Pereira FC, Marcillo AE, Bates ML, Berrocal YA, Filbin MT, Bunge MB. cAMP and Schwann cells promote axonal growth and functional recovery after spinal cord injury. Nat Med. 2004;10:610–616. doi: 10.1038/nm1056. [DOI] [PubMed] [Google Scholar]
- 55.Ramer LM, Au E, Richter MW, Liu J, Tetzlaff W, Roskams AJ. Peripheral olfactory ensheathing cells reduce scar and cavity formation and promote regeneration after spinal cord injury. J Comp Neurol. 2004;473:1–15. doi: 10.1002/cne.20049. [DOI] [PubMed] [Google Scholar]
- 56.Ramon y Cajal S. Degeneration and Regeneration of the Nervous System. Hafner; New York: 1928. [Google Scholar]
- 57.Ramón-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- 58.Ramón-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reier PJ, Stokes BT, Thompson FJ, Anderson DK. Fetal cell grafts into resection and contusion/compression injuries of the rat and cat spinal cord. Exp Neurol. 1992;115:177–188. doi: 10.1016/0014-4886(92)90245-l. [DOI] [PubMed] [Google Scholar]
- 60.Richardson PM, McGuinness UM, Aguayo AJ. Axons from CNS neurones regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- 61.Richardson PM, McGuinness UM, Aguayo AJ. Peripheral nerve autografts to the rat spinal cord: studies with axonal tracing methods. Brain Res. 1982;237:147–162. doi: 10.1016/0006-8993(82)90563-7. [DOI] [PubMed] [Google Scholar]
- 62.Richter MW, Fletcher PA, Liu J, Tetzlaff W, Roskams AJ. Lamina propria and olfactory bulb ensheathing cells exhibit differential integration and migration and promote differential axon sprouting in the lesioned spinal cord. J Neurosci. 2005;25:10700–10711. doi: 10.1523/JNEUROSCI.3632-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rochkind S, Shahar A, Fliss D, El-Ani D, Astachov L, Hayon T, Alon M, Zamostiano R, Ayalon O, Biton IE, Cohen Y, Halperin R, Schneider D, Oron A, Nevo Z. Development of a tissue-engineered composite implant for treating traumatic paraplegia in rats. Eur Spine J. 2006;15:234–245. doi: 10.1007/s00586-005-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romero MI, Rangappa N, Li L, Lightfoot E, Garry MG, Smith GM. Extensive sprouting of sensory afferents and hyperalgesia induced by conditional expression of nerve growth factor in the adult spinal cord. J Neurosci. 2000;20:4435–4445. doi: 10.1523/JNEUROSCI.20-12-04435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Romero MI, Rangappa N, Garry MG, Smith GM. Functional regeneration of chronically injured sensory afferents into adult spinal cord after neurotrophin gene therapy. J Neurosci. 2001;21:8408–8416. doi: 10.1523/JNEUROSCI.21-21-08408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saberi H, Moshayedi P, Aghayan HR, Arjmand B, Hosseini SK, Emami-Razavi SH, Rahimi-Movaghar V, Raza M, Firouzi M. Treatment of chronic thoracic spinal cord injury patients with autologous Schwann cell transplantation: an interim report on safety considerations and possible outcomes. Neurosci Lett. 2008;443:46–50. doi: 10.1016/j.neulet.2008.07.041. [DOI] [PubMed] [Google Scholar]
- 67.Sasaki M, Lankford KL, Zemedkun M, Kocsis JD. Identified olfactory ensheathing cells transplanted into the transected dorsal funiculus bridge the lesion and form myelin. J Neurosci. 2004;24:8485–8493. doi: 10.1523/JNEUROSCI.1998-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 69.Song HJ, Poo MM. Signal transduction underlying growth cone guidance by diffusible factors. Curr Opin Neurobiol. 1999;9:355–363. doi: 10.1016/s0959-4388(99)80052-x. [DOI] [PubMed] [Google Scholar]
- 70.Spencer T, Filbin MT. A role for cAMP in regeneration of the adult mammalian CNS. J Anat. 2004;204:49–55. doi: 10.1111/j.1469-7580.2004.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tang XQ, Tanelian DL, Smith GM. Semaphorin3A inhibits nerve growth factor-induced sprouting of nociceptive afferents in adult rat spinal cord. J Neurosci. 2004;24:819–827. doi: 10.1523/JNEUROSCI.1263-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tang XQ, Cai J, Nelson KD, Peng XJ, Smith GM. Functional repair after dorsal root rhizotomy using nerve conduits and neurotrophic molecules. Eur J Neurosci. 2004;20:1211–1218. doi: 10.1111/j.1460-9568.2004.03595.x. [DOI] [PubMed] [Google Scholar]
- 74.Tang XQ, Heron P, Mashburn C, Smith GM. Targeting sensory axon regeneration in adult spinal cord. J Neurosci. 2007;27:6068–6078. doi: 10.1523/JNEUROSCI.1442-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Taylor L, Jones L, Tuszynski MH, Blesch A. Neurotrophin-3 gradients established by lentiviral gene delivery promote short-distance axonal bridging beyond cellular grafts in the injured spinal cord. J Neurosci. 2006;26:9713–9721. doi: 10.1523/JNEUROSCI.0734-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 77.Thompson LH, Grealish S, Kirik D, Björklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- 78.Vavrek R, Pearse DD, Fouad K. Neuronal populations capable of regeneration following a combined treatment in rats with spinal cord transection. J Neurotrauma. 2007;24:1667–1673. doi: 10.1089/neu.2007.0290. [DOI] [PubMed] [Google Scholar]
- 79.Wang R, King T, Ossipov MH, Rossomando AJ, Vanderah TW, Harvey P, Cariani P, Frank E, Sah DW, Porreca F. Persistent restoration of sensory function by immediate or delayed systemic artemin after dorsal root injury. Nat Neurosci. 2008;11:488–496. doi: 10.1038/nn2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wirth ED, 3rd, Reier PJ, Fessler RG, Thompson FJ, Uthman B, Behrman A, Beard J, Vierck CJ, Anderson DK. Feasibility and safety of neural tissue transplantation in patients with syringomyelia. J Neurotrauma. 2001;18:911–929. doi: 10.1089/089771501750451839. [DOI] [PubMed] [Google Scholar]
- 81.Wright DE, White FA, Gerfen RW, Silos-Santiago I, Snider WD. The guidance molecule semaphorin III is expressed in regions of spinal cord and periphery avoided by growing sensory axons. J Comp Neurol. 1995 Oct 2;361:321–333. doi: 10.1002/cne.903610209. [DOI] [PubMed] [Google Scholar]
- 82.Xu XM, Onifer SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol. 2009;169:171–182. doi: 10.1016/j.resp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- 84.Yoon SH, Shim YS, Park YH, Chung JK, Nam JH, Kim MO, Park HC, Park SR, Min BH, Kim EY, Choi BH, Park H, Ha Y. Complete spinal cord injury treatment using autologous bone marrow cell transplantation and bone marrow stimulation with granulocyte macrophage-colony stimulating factor: phase I/II clinical trial. Stem Cells. 2007;25:2066–2073. doi: 10.1634/stemcells.2006-0807. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L, Ma Z, Smith GM, Wen X, Pressman Y, Wood PM, Xu XM. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57:1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang Y, Dijkhuizen PA, Anderson PN, Lieberman AR, Verhaagen J. NT-3 delivered by an adenoviral vector induces injured dorsal root axons to regenerate into the spinal cord of adult rats. J Neurosci Res. 1998;54:554–562. doi: 10.1002/(SICI)1097-4547(19981115)54:4<554::AID-JNR12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 87.Ziemba K, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult central nervous system. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


