Abstract
The construction of genetically encoded cellular mimics in compartments containing organized synthetic cytosols is desirable for the development of artificial cells. Phase separated aqueous domains were placed within water-in-oil emulsion droplets in a manner compatible with transcription and translation machinery. Aqueous two-phase and three-phase systems (ATPS and A3PS) were assembled with dextran, poly(ethylene glycol), and Ficoll. Aqueous two-phase systems were capable of supporting the cell-free expression of protein within water droplets, whereas the aqueous three-phase-based system did not give rise to detectable protein synthesis. The expressed protein preferentially partitioned to the dextran-enriched phase. The system could serve as a foundation for building cellular mimics with liquid organelles.
Introduction
Cellular life is organized on many levels. Several metabolic processes follow a cyclic, circadian rhythm, and the behavior of cellular populations emerges from the coordinated activity of individual cells. Within the cell, various forms of spatial organization help drive cellular chemistry. Membrane bound organelles, protein microcompartments, and transiently forming metabolons all facilitate metabolic flux down desired paths.1 Protein lipidation increases the likelihood that cognate proteins interact by restricting diffusion to the two-dimensional space of the membrane. Similarly, preferential partitioning to specific aqueous phases can lead to significant enhancements in enzymatic activity.2 Although the latter example of aqueous phase separation has not been extensively investigated in biological systems, mixtures of aqueous polymer solutions do phase separate in vitro,3 inside of living cells,4 and were likely present from the earliest stages of evolution.5 Therefore, it seems probable that contemporary cells exploit these “liquid organelles” to facilitate metabolic chemistry.6
One approach toward gaining insight into the organizational features of cellular life is to construct mimetic systems in the laboratory that display similar organization and behavior.7,8 Several recent studies have defined a set of chemical conditions that give rise to two coexisting aqueous phases in lipid vesicles.9,10 Others demonstrated that transcription–translation (T/T) can be carried out in vesicles11 and in bulk aqueous two-phase systems (ATPS).12,13 By combining these approaches, it should be possible to produce genetically encoded proteins that distribute between coexisting phases as a model for cellular microcompartments.
In most traditional giant vesicle preparation strategies (i.e., those that do not involve a water-in-oil (w/o) emulsion step), macromolecule encapsulation efficiency is low and varies considerably from vesicle-to-vesicle within a batch.14−17 This can be understood in terms of the low fraction of total volume that is encapsulated within the vesicle population; generally the vast majority of the aqueous solution volume is outside of the vesicles, with ≪1% inside the vesicles.11 Additionally, macromolecules are generally encapsulated at concentrations less than would be anticipated based on their external concentrations during vesicle formation.18 These problems are compounded when encapsulating the over 100 components needed for T/T. Although recent studies have shown that a few individual vesicles within a population can encapsulate all of the necessary components for protein expression, most vesicles in the population are not competent for transcription–translation.14,15 Polymer condensation due to macromolecular crowding does facilitate encapsulation, but the majority of total solute molecules still remain outside the vesicles.18,19 For vesicles formed by gentle hydration or electroformation, encapsulation of ATPS requires making vesicles under conditions where the system exists as a single phase by, for example, heating or diluting the solution. After vesicle formation, the sample is cooled or concentrated, respectively.5 Alternatively, microfluidic-based protocols were developed to permit control over the volume and contents of droplets in oil.20 Recently, osmotically driven phase separation in cell lysate droplets produced coacervates capable of accommodating T/T.21 Despite these advances, direct encapsulation of more than two phases enriched in different polymers—which poses additional challenges for both phase-transition and microfluidic approaches—has not been demonstrated.
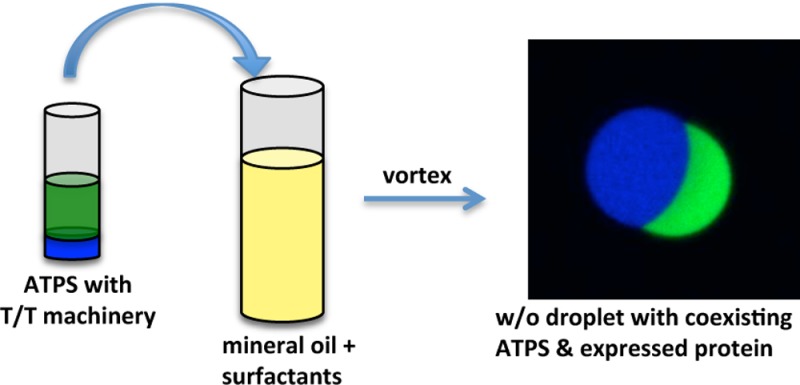
To construct cellular mimics containing organized artificial cytosols and functional T/T machinery, the use of w/o emulsions was explored. Unlike the inefficiency of encapsulation in vesicles, w/o emulsions give nearly complete encapsulation, meaning that the internal aqueous conditions faithfully represent the starting aqueous phase. Therefore, the position on the phase diagram is known, and the properties of the phases, such as solute partitioning, interfacial tensions, and viscosity, can be acquired from bulk measurements. Nevertheless, it was unclear whether simply replacing the water in a w/o emulsion recipe with a preformed ATPS or aqueous three-phase system (A3PS) would result in the encapsulation of multiple phases within each droplet of the emulsion or a mixture of single-phase droplets that contain the different phases.
Herein we describe a simple method to generate aqueous multiphase systems within w/o droplets that is compatible with T/T and does not require the use of a microfluidic device. A phase-separated polymer solution was used in place of the aqueous portion of a traditional w/o droplet-generating protocol based on mechanical mixing of aqueous solution and mineral oil in the presence of a standard surfactant mixture.22,23 Surfactant-stabilized droplets containing ATPS and A3PS with dextran, poly(ethylene glycol) (PEG), and Ficoll were produced in this manner. T/T was performed and efficiently produced fluorescent protein in the ATPS droplets. This platform could serve as a foundation for genetically encoded targeting to liquid organelles within the cytosolic space of artificial cells.
Results and Discussion
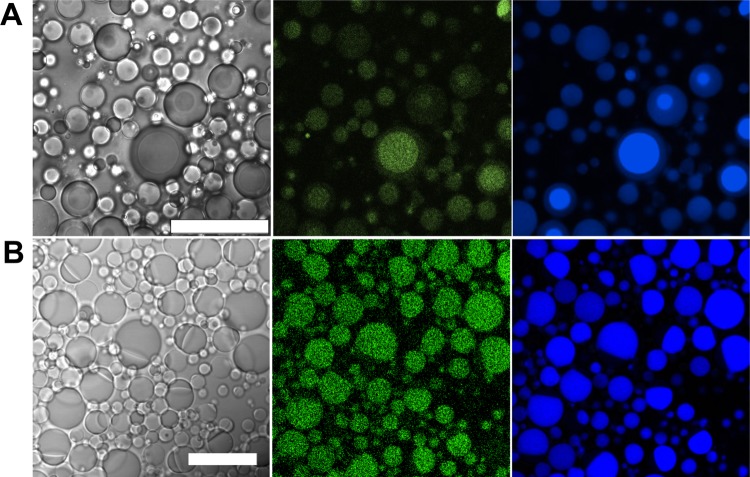
A standard w/o emulsion composition of 4.5% (v/v) Span 80, 0.5% (v/v) Tween 80 in 0.95 mL of mineral oil23, and 50 μL of an ATPS consisting of 10% (w/w) dextran 10 kDa and 7% (w/w) PEG 8 kDa was chosen to evaluate the potential of w/o droplets in encapsulating multiple aqueous phases.9 [Note: surfactant and polymer percent concentrations are given in (v/v) and (w/w), respectively.] In bulk samples of this ATPS, the PEG-rich phase volume is approximately four times larger than that of the dextran-rich phase; mechanical mixing generates a cloudy dispersion of one phase in the other. A room-temperature aliquot of a well-mixed ATPS in which both phases were present as a dispersion was added to mineral oil that contained the surfactant mixture and vortexed to generate an emulsion. Optical microscopy revealed that 91 ± 2% of 233 droplets contained both PEG-rich and dextran-rich aqueous phases, surrounded by mineral oil (Figure 1, Figure S1A). Droplets were not identical in size nor composition, reflecting a distribution of encapsulated ATPS phase volumes. In contrast, hydrating lipids in an ATPS with vortexing produced only vesicles with a single aqueous phase in the internal volume (Figure S2).
Figure 1.
Optical microscope images of w/o emulsion droplets containing a PEG/dextran ATPS. A single droplet is shown in A, and a wider view with several droplets is shown in B. Images on the left and right are transmitted (DIC) and confocal fluorescence channels, respectively. ATPS: 10% dextran 10 kDa, 7% PEG 8 kDa. Labeled polymers were added to aid visualization of the phases: Alexa 488 labeled PEG 5 kDa (false-colored green) and Alexa 647 labeled dextran 10 kDa (false-colored blue). Scale bar = 10 μm.
Next, two additional polymer compositions chosen to fall on or near the same tie-line were encapsulated in w/o droplets so that the phase compositions would be similar to the ATPS described above but differ in relative volume.6 Relative phase volume in the bulk ATPS may influence the ability to encapsulate both phases within an emulsion droplet and/or the relative sizes of the resulting encapsulated phase microcompartments. The two new compositions were 19.8% dextran, 2.8% PEG and 18.5% dextran, 5.5% PEG, resulting in larger volume dextran phases (ca. 30 and 40% of the total bulk aqueous volume, respectively). Increasing the dextran phase only slightly to ∼30% of the total aqueous volume had no observable effect on encapsulation efficiency, with 88% of 201 droplets containing both aqueous phases. However, increasing the volume of the dextran-enriched phase further to 40% resulted in a drop in ATPS encapsulation efficiency to 58% (177 droplets evaluated). The three different ATPS in w/o droplets were similar morphologically, with variability between individual droplets within a batch (Supporting Information, Figure S1A,B,C). Within droplets, the ATPS phases were either side-to-side so that each aqueous phase interfaced with both the oil-surfactant phase and the other aqueous polymer phase, or concentric with one phase surrounding the other and in contact with the oil–surfactant phases. Some of the droplets assumed a non spherical, budding morphology similar to that previously observed with ATPS-containing vesicles.5,10 The relative volumes of each phase were heterogeneous but generally consistent with the starting aqueous compositions in the sense that ATPS compositions with a greater dextran volume, for example, typically showed droplets with a larger dextran enriched aqueous phase than ATPS compositions with a smaller dextran volume.
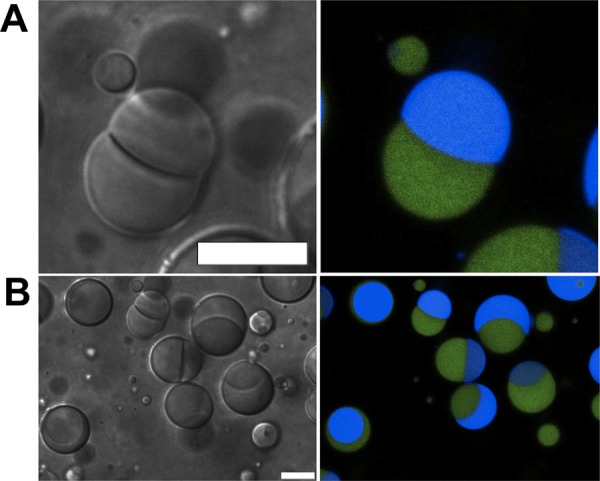
We next sought to determine whether the same method could be exploited to produce an A3PS inside of w/o droplets. A3PS consisting of 19.8% dextran 10 kDa, 2.8% PEG 8 kDa, 5% Ficoll 400 kDa was vortexed with mineral oil containing 4.5% Span 80, 0.5% Tween 80. Three aqueous phases were clearly visible in the droplets (Figure 2, Figure S3). The Ficoll-rich phase was found between the PEG-rich and dextran-rich phases, consistent with its position in bulk A3PS. The efficiency of the process was similar to that of ATPS with 86% of 227 water droplets containing all three aqueous phases. The assembly of analogous A3PS vesicles, e.g., by gentle hydration of lipids, would be significantly more difficult and to our knowledge has never been demonstrated. A3PS droplets in Figure 2 show an interfacial accumulation of the labeled dextran used for visualization. This may be due to interactions between the polymer(s) and surfactants.24 Span 80 and Tween 80 are appreciably soluble in oil and water,25 respectively, and likely were not fully localized to the interface. The fluorescent dextran used in these experiments was larger (40 kDa) than the majority of the dextran that makes up this phase (10 kDa), which may contribute to its different interfacial partitioning.
Figure 2.
A3PS in w/o emulsion droplets. A3PS consisted of 19.8% dextran, 2.8% PEG, and 5% Ficoll. Transmitted light (left) and fluorescence (right) microscopy images are shown. Aqueous phases were visualized with Alexa 488 labeled PEG (green) and Alexa 647 labeled dextran 40 kDa (blue). Scale bar = 10 μm.
To gain insight into whether aqueous multiphase encapsulation efficiency was related to the properties of the oil–water interface, w/o emulsions were prepared using a different surfactant composition. Decreasing Span 80 from 4.5% to 2% and increasing Tween 80 from 0.5 to 3% resulted in stable emulsions over the time course of the experiments; however, the droplets no longer efficiently entrapped the two aqueous phases. Droplets only possessed dextran or PEG enriched phases but not both (Figure S4). Although the oil–water interface likely contributes to the behavior of the system as a whole, other factors may be of importance; the surfactants may influence the phase behavior and/or solubility of the polymers in the aqueous and oil phases.18
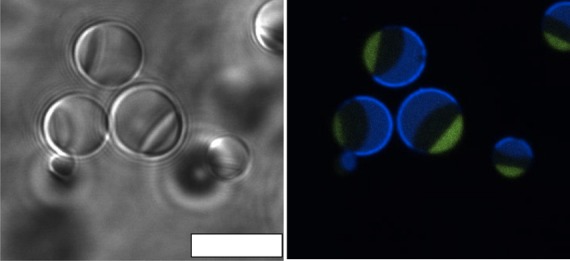
We next attempted to construct cellular mimetic droplets with an artificial, phase separated cytoplasm. ATPS and A3PS were assembled on ice and diluted 2-fold with DNA and the PURE system,26 consisting of T7 RNA polymerase and Escherichia coli translation machinery. The DNA coded for monomeric yellow fluorescent protein (mYPet).27 An aliquot of the aqueous solution was then used to generate the w/o emulsion. In this setup, ATPS encapsulation efficiency was lower (23% of 276 droplets) because of dilution with T/T machinery. Therefore, the starting polymer solutions were doubled in concentration so that the 2-fold dilution with the T/T components would bring the polymer concentrations back to the levels that gave high efficiency encapsulation. This method significantly improved the efficiency of two-phase encapsulation and consequently increased our ability to see expression in two-phase droplets. After incubation at 37 °C for 2 h, fluorescence microscopy showed protein expression in two of the three ATPS emulsion systems (Figure 3, Figure S5–S9). ATPS-containing droplets with mYPet fluorescence made up 49% and 66.5%, respectively, of all droplets in 10% dextran 10 kDa/7% PEG 8 kDa (216 droplets counted) and 19.8% dextran 10 kDa/2.8% PEG 8 kDa (230 droplets). The ATPS that failed to support protein expression was also the least efficient in coentrapment within w/o droplets, i.e., 18.5% dextran, 5.5% PEG. Protein expression was not observed in A3PS. This may be due to the greater similarity between the two polysaccharide-enriched phases, i.e., Ficoll and dextran. It is possible that some T/T components that were colocalized in the dextran-rich phase of the PEG/dextran ATPS were localized to different phases in the A3PS. In other words, in the exploited ATPS compositions, partitioning was between a hydrophilic polysaccharide (dextran) and a more hydrophobic poly(ethylene oxide) (PEG). Under such conditions, T/T machinery would be expected to preferentially partition to the less hydrophobic dextran phase. In the A3PS there was more than one such phase. We anticipate that further optimization, e.g., reducing the collective volumes of the dextran-rich and Ficoll-rich phases, or replacing the Ficoll-rich phase with a different third phase more chemically distinct from the dextran-rich phase, could ultimately enable expression in an A3PS.
Figure 3.
Cell-free protein expression inside of w/o droplets containing an ATPS. The ATPS and w/o emulsions were prepared at room temperature and contained T/T machinery and DNA encoding the fluorescent protein mYPet. Reactions were initiated by incubation at 37 °C. The ATPS was (A) 10% dextran, 7% PEG and (B) 19.8% dextran, 2.8% PEG. From left to right, the images are transmitted light (DIC), the fluorescence of expressed mYPet, and the fluorescence of Alexa 647-labeled dextran 10 kDa. Expression was either for 4 h (A) or 6 h (B). The expressed protein preferentially partitioned to the dextran-enriched phase. Scale bars are 50 μm (A) and 25 μm (B).
Conclusions
Cellular life is organized to an extent that has yet to be well mimicked by artificial, bottom-up constructions. Part of the problem thus far has been an incompatibility between some biological molecules and aqueous organization methods that rely on lengthy protocols or heating steps.9 W/o emulsions overcome many of these problems by not requiring a heating step, by being extremely efficient in encapsulating molecules, and by necessitating little time to implement. This is important because of the fragility of the T/T machinery of the PURE system, which loses activity at 37 °C within 3 h.28,29 Here we have shown that ATPS and A3PS can be encapsulated with high efficiency within w/o droplets stabilized with commonly exploited surfactants. Therefore, routinely used w/o emulsion generation protocols are compatible with the encapsulation of multiple aqueous phases. Further, specific ATPS compositions are capable of converting the information encoded in DNA to functional protein, and the expressed protein preferentially partitioned to a specific aqueous phase. Consequently, it should be possible to build artificial cells with genetically encoded targeting to liquid organelles in the future. Also, since w/o emulsions can be stabilized with natural lipids in place of synthetic surfactants and that such emulsion droplets can be converted to vesicles,30 the methodology described herein should be modifiable for the generation of vesicles containing aqueous multiphase systems compatible with T/T machinery.
Acknowledgments
This work was supported by the Armenise-Harvard foundation, CIBIO, the NSF (MCB-1244180), and the NIH (R01 GM078352).
Supporting Information Available
Supporting Information containing microscopy images with wider fields of view, direct encapsulation of ATPS in POPC lipid vesicles, modified emulsion compositions, time courses of expression, and control reactions is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Agapakis C. M.; Boyle P. M.; Silver P. A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 2012, 8, 527–535. [DOI] [PubMed] [Google Scholar]
- Strulson C. A.; Molden R. C.; Keating C. D.; Bevilacqua P. C. RNA catalysis through compartmentalization. Nat. Chem. 2012, 4, 941–6. [DOI] [PubMed] [Google Scholar]
- Vekilov P. G. Phase diagrams and kinetics of phase transitions in protein solutions. J. Phys. Condens. Mater. 2012, 24, 193101. [DOI] [PubMed] [Google Scholar]
- Brangwynne C. P. Phase transitions and size scaling of membrane-less organelles. J. Cell Biol. 2013, 203, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating C. D. Aqueous phase separation as a possible route to compartmentalization of biological molecules. Acc. Chem. Res. 2012, 45, 2114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby-Phelps K. The physical chemistry of cytoplasm and its influence on cell function-an update. Mol. Biol. Cell 2013, 24, 2593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzieciol A. J.; Mann S. Designs for life: Protocell models in the laboratory. Chem. Soc. Rev. 2012, 41, 79–85. [DOI] [PubMed] [Google Scholar]
- Ichihashi N.; Matsuura T.; Kita H.; Sunami T.; Suzuki H.; Yomo T. Constructing partial models of cells. Cold Spring Harb. Perspect. Biol. 2010, 2, a004945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long M. S.; Jones C.; Helfrich M. R.; Mangeney-Slavin L. K.; Keating C. D. Dynamic microcompartmentation within synthetic cells. Proc. Natl. Acad. Sci. U. S. A. 2005, 102, 2262–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimova R.; Lipowsky R. Lipid membranes in contact with aqueous phases of polymer. Soft Matter 2012, 8, 6409–6415. [Google Scholar]
- Sunami T.; Matsuura T.; Suzuki H.; Yomo T. Synthesis of functional proteins within liposomes. Methods Mol. Biol. 2010, 607, 243–256. [DOI] [PubMed] [Google Scholar]
- Albertsson P. Å.Partition of Cell Particles and Macromolecules, 3rd ed.; Wiley Interscience: New York, 1986. [Google Scholar]
- Marszal E.; Suchova M.; Konecnyt P.; Scouten W. H. Study of cell-free protein synthesis in aqueous two-phase systems. J. Mol. Recognit. 1995, 8, 151–156. [DOI] [PubMed] [Google Scholar]
- Stano P.; D’Aguanno E.; Bolz J.; Fahr A.; Luisi P. L. A remarkable self-organization process as the origin of primitive functional cells. Angew. Chem., Int. Ed. Engl. 2013, 52, 13397–400. [DOI] [PubMed] [Google Scholar]
- Stano P.; Carrara P.; Kuruma Y.; Pereira de Souza T.; Luisi P. L. Compartmentalized reactions as a case of soft-matter biotechnology: Synthesis of proteins and nucleic acids inside lipid vesicles. J. Mater. Chem. 2011, 21, 18887–18902. [Google Scholar]
- Luisi P. L.; Allegretti M.; Pereira de Souza T.; Steiniger F.; Fahr A.; Stano P. Spontaneous protein crowding in liposomes: A new vista for the origin of cellular metabolism. ChemBioChem 2010, 11, 1989–92. [DOI] [PubMed] [Google Scholar]
- Sun B.; Chiu D. T. Determination of the encapsulation efficiency of individual vesicles using single-vesicle photolysis and confocal single-molecule detection. Anal. Chem. 2005, 77, 2770–2776. [DOI] [PubMed] [Google Scholar]
- Dominak L. M.; Keating C. D. Polymer encapsulation within giant lipid vesicles. Langmuir 2007, 23, 7148–54. [DOI] [PubMed] [Google Scholar]
- Dominak L. M.; Keating C. D. Macromolecular crowding improves polymer encapsulation within giant lipid vesicles. Langmuir 2008, 24, 13565–13571. [DOI] [PubMed] [Google Scholar]
- Boreyko J. B.; Mruetusatorn P.; Rettererac S. T.; Collier C. P. Aqueous two-phase microdroplets with reversible phase transitions. Lab Chip 2013, 13, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Sokolova E.; Spruijt E.; Hansen M. M. K.; Dubuc E.; Groen J.; Chokkalingam V.; Piruska A.; Heus H. A.; Huck W. T. S. Enhanced transcription rates in membrane-free protocells formed by coacervation of cell lysate. Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 11692–11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tawfik D. S.; Griffiths A. D. Man-made cell-like compartments for molecular evolution. Nat. Biotechnol. 1998, 16, 652–657. [DOI] [PubMed] [Google Scholar]
- Hall B.; Micheletti J. M.; Pooja S.; Ogle K.; Pollard J.; Ellington A. D. Design, Synthesis, and Amplification of DNA Pools for In Vitro Selection. Curr. Protoc. Mol. Biol. 2009, 24.2.1–24.2.27. [DOI] [PubMed] [Google Scholar]
- Tajwani R. W.; Joshi H. N.; Varia S. A.; Serafuddin A. T. M. Study of phase behavior of poly(ethylene glycol)–polysorbate 80 and poly(ethylene glycol)–polysorbate 80–water mixtures. J. Pharm. Sci. 2000, 89, 946–50. [DOI] [PubMed] [Google Scholar]
- The Merck Index: An Encyclopedia of Chemicals, Drugs and Biologicals, 11th ed.; Merck & Co., Inc.: Rahway, NJ, 1989; p 8691. [Google Scholar]
- Shimizu Y.; Inoue A.; Tomari Y.; Suzuki T.; Yokogawa K.; Nishikawa K.; Ueda T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001, 19, 751–5. [DOI] [PubMed] [Google Scholar]
- Lentini R.; Forlin M.; Martini L.; Del Bianco C.; Spencer A. C.; Torino D.; Mansy S. S. Fluorescent proteins and in vitro genetic organization for cell-free synthetic biology. ACS Synth. Biol. 2013, 2, 428–489. [DOI] [PubMed] [Google Scholar]
- Stogbauer T.; Windhager L.; Zimmer R.; Radler J. O. Experiment and mathematical modeling of gene expression dynamics in a cell free system. Integr. Biol. 2012, 4, 494–501. [DOI] [PubMed] [Google Scholar]
- Chizzolini F.; Forlin M.; Cecchi D.; Mansy S. S. Gene position more strongly influences cell free protein expression from operons than T7 transcriptional promoter strength. ACS Synth. Biol. 2013, 10.1021/sb4000977. [DOI] [PubMed] [Google Scholar]
- Pautot S.; Frisken B. J.; D. Weitz A. Engineering Asymmetric Vesicles. Proc. Natl. Acad. Sci. U. S. A. 2003, 19, 10718–10721. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.