Abstract
To identify guidance molecules to promote long-distance growth of dopaminergic axons from transplanted embryonic ventral mesencephalon (VM) tissue, three pathways were created by expressing green fluorescent protein (GFP), glial cell line-derived neurotrophic factor (GDNF), or a combination of GDNF/GDNF receptor α1 (GFRα1) along the corpus callosum. To generate the guidance pathway, adenovirus encoding these transcripts was injected at four positions along the corpus callosum. In all groups, GDNF adenovirus was also injected on the right side 2.5 mm from the midline at the desired transplant site. Four days later, a piece of VM tissue from embryonic day 14 rats was injected at the transplant site. All rats also received daily subcutaneous injections of N-acetyl-L-cysteinamide (NACA; 100 μg per rat) as well as chondroitinase ABC at transplant site (10 U/ml, 2 μl). Two weeks after transplantation, the rats were perfused and the brains dissected out. Coronal sections were cut and immunostained with antibody to tyrosine hydroxylase (TH) to identify and count dopaminergic fibers in the corpus callosum. In GFP-expressing pathways, TH+ fibers grew out of the transplants for a short distance in the corpus callosum. Very few TH+ fibers grew across the midline. However, pathways expressing GDNF supported more TH+ fiber growth across the midline into the contralateral hemisphere. Significantly greater numbers of TH+ fibers grew across the midline in animals expressing a combination of GDNF and GFRα1 in the corpus callosum. These data suggest that expression of GDNF or a combination of GDNF and GFRα1 can support the long-distance dopaminergic fiber growth from a VM transplant, with the combination having a superior effect.
Keywords: axon growth, ventral mesencephalon (VM), transplantation, neurotrophic factors, gene therapy
Transplantation is a promising strategy for treating central nervous system disorders, such as neurodegenerative diseases like Parkinson's and Huntington's diseases, as well as traumatic injuries of the brain and spinal cord. The purpose of transplantation is to replace the cells lost in trauma or degenerative diseases and to bridge the lesion cavity in spinal cord injury. Various transplantation techniques have been investigated using bio-materials, fetal tissue, peripheral nerve grafts, and different type of cells including Schwann cells, olfactory ensheathing cells, stem cells, and cells modified to secrete neurotrophic factors (David and Aguayo, 1981; Tuszynski et al., 1994; Xu et al., 1995; Diener and Bregman, 1998; Ramon-Cueto et al., 1998; McDonald et al., 1999; Jin et al., 2002; Cai et al., 2007). Transplants of fetal tissue for Parkinson's and Huntington's diseases as well as spinal cord injury have been examined in both basic and clinic studies (Bjorklund et al., 1980; Theele et al., 1996; Rabinovich et al., 2003; Gaura et al., 2004; Oertel et al., 2004; Mendez et al., 2008). For Parkinson's disease, which results from degeneration of dopamine neurons projecting from the substantia nigra (SN) to the striatum, dopaminergic neurons from fetal ventral mesencephalon (VM) have been transplanted directly into their target, the striatum, rather than into their true origin, the SN, in most cases. This leads to an incomplete recovery of function both in animal models and in human PD patients, because the transplanted neurons provide a dopamine source to the striatum but do not reestablish the degenerated neural circuit (Winkler et al., 2000).
To restore the circuit that is lost in PD truly, dopaminergic neurons that are transplanted into the SN should extend axons to the striatum. However, the distance between the SN and the striatum, coupled with the inhibitory environment of adult CNS, makes it difficult to effect such axon growth (Schwab et al., 1993). In theory, expression of appropriate molecules could support and direct long-distance growth of dopaminergic fibers from VM transplants in the SN to their striatal target. Our previous study showed that preformed guidance pathways created by injection of viral vectors can direct axon growth from transplanted DRG neurons to desired target locations (Ziemba et al., 2008). In that study, we demonstrated that axons from dorsal root ganglion neurons transplanted into the corpus callosum could be directed to grow a long distance toward a target with a combination of fibroblast growth factor (FGF) and nerve growth factor (NGF). In the present study, we used the same technique to examine which molecules might support long-distance dopaminergic axon growth along a preformed pathway. One candidate molecule for such growth enhancement is glial cell line-derived neurotrophic factor (GDNF), which is known to increase the survival, differentiation, fiber outgrowth, and dopamine release from fetal midbrain dopaminergic neurons both in vitro and in vivo (Lin et al., 1993; Johansson et al., 1995). PI-linked GDNF receptor α1 (GFRα1) has been shown to direct neurite outgrowth of sensory and sympathetic neurons in the presence of GDNF and may have a similar effect on dopaminergic neurons (Ledda et al., 2002).
In this study, molecular pathways were created along the corpus callosum by multiple injections of adenovirus encoding GDNF, GDNF plus GFRα1, or green fluorescent protein (GFP) before fetal VM tissue was transplanted at one end of the corpus callosum. Because injection of adenoviral vectors in vivo may have some toxic effects, N-acetyl-L-cysteinamide (NACA), a cell-permeable antioxidant and glutathione precursor, was injected into some study animals to reduce viral toxic effects and oxidative stress. Another procedural concern is the trauma induced by the large (22-guage) spinal needle for transplantation of VM tissue. This likely induces expression of growth-inhibitory chondroitin sulfate proteoglycans (CSPGs), so the enzyme chondroitinase ABC (ChABC) was injected at the transplant site right before VM transplantation in some animals to decrease the subsequent expression of CSPGs. We found that the combination of GDNF and GFRα1 along the desired pathway and NACA and ChABC treatments was best able to promote robust, long-distance growth of dopaminergic fibers from VM transplants.
MATERIALS AND METHODS
Female adult Sprague-Dawley rats (225–250 g; Harlan)were used for all experiments. All procedures were carried out under the supervision of the Institutional Animal Care and Use Committee and according to the NIH Guide for the care and use of laboratory animals. Animals were maintained under conditions of controlled light and temperature, with food and water available ad libitum.
Pathway Setup
Rats were anesthetized with a mixture of ketamine (80 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) and placed into a stereotactic frame after shaving their heads, and the skin was cleaned with betadine, followed by 70% ethanol. Animals were randomly divided into the following groups: GFP adenovirus (Ad-GFP; n = 3), Ad-GDNF (n = 4), and Ad-GDNF/GFRα1 (n = 4). The guidance pathways were created by injecting each adenovirus along the corpus callosum with a transplantation site in the right corpus callosum (Fig. 1). Using Bregma as a landmark, holes were drilled into the skull along the coronal plane of Bregma to allow injections at the following coordinates, depths relative to dura: transplant site, +2.5 mm lateral (right side), −2.8 mm deep; pathway within the corpus callosum, ± 0.5 mm lateral, 3.2 mm deep, ± 1.5 mm lateral, 2.9 mm deep. Adenovirus concentrations were 2 × 106 pfu/μl, except for combination Ad-GDNF/GFR α1 injections, for which the concentration of each virus was 1 × 106 pfu/μl. Injection volumes ranged from 0.4 μl at transplantation site (+ 2.5 mm), to 0.6 μl at +1.5 mm, to 0.8 μl at +0.5 mm, to 1.0 μl at −0.5 mm to 1.2 μl at −1.5 mm and increased along the pathway to create a gradient effect. Volumes were injected at a rate of 0.4 μl/min using a 10-μl Hamiltom syringe with a 30-gauge beveled needle, and the needle remained in place for 2 min at the end of each injection.
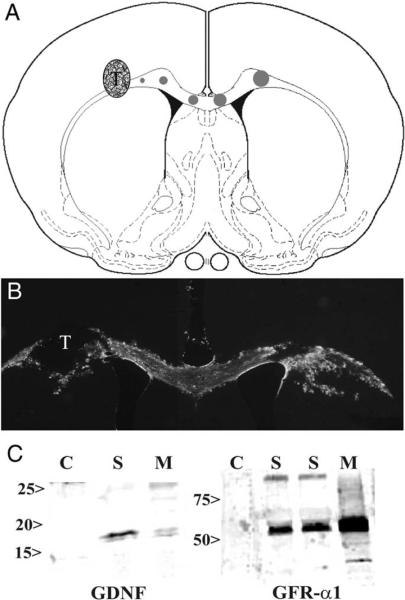
Fig. 1.
A: Diagram showing locations for adenovirus injections and VM transplant. Circles along the corpus callosum show different pathways of Ad-GFP, Ad-GDNF, or Ad-GDNF/GFRα1 from left to right. The circle size represents the volume injected, ranging from 0.4 μl (smallest circle) increasing by 0.2 μl for consecutively larger circles to the final volume of 1.2 μl (largest circle). VM transplant (T) is the stippled circle within the corpus callosum just left of the smallest circle. B: GFP expression along the corpus callosum of the GFRα1 adenovirus coexpressing a GFP reporter. The VM transplant (T) is shown within the non-GFP region on the left side of the corpus callosum. C: Western blot analysis of U373 astrocytoma cell line transfected with adenovirus encoding GDNF or GFRα1. Blots show no expression in GFP transfected controls (C). Protein was identified in tissue culture supernatants (S) and cell monolayers (M).
VM Dissection and Transplantation
Three to four days after adenovirus injection, animals received transplants of embryonic day 14 (E14) ventral mesencephalon (VM) tissue. Immediately prior to transplantation, E14 embryos were removed from a pregnant Sprague-Dawley rat and the ventral mesencephalic brain region (approximately 1 mm × 1 mm) was dissected out of each fetus and kept in ice-cold dissection buffer (calcium- and magnesium-free HBSS, HEPES, pH 7.2–7.4) until transplant. Once each rat was anesthetized, VM was implanted as a whole tissue chunk using a modified 22-guage spinal needle lowered to −3.0 mm from skull level, then pulled up to −2.8 mm before ejecting the tissue. Each tissue chunk was ejected slowly by depressing the needle's plunger ~1 mm every 20 sec for a total distance of ~10 mm. The needle was kept in place for 5 min after ejection, raised 0.4 mm and kept there for another 5 min, before slowly raising it all the way out of the brain to be sure the transplant remained in place.
NACA and ChABC Treatment
Animals in all three experimental groups were treated with subcutaneous N-acetyl-L-cysteinamide and chondroitinase ABC at the transplant site. In another set of experimentsw, to compare both NACA and ChABC treatments, animals with Ad-GDNF/GFRα1 pathways were randomly divided into two subgroups receiving either ChABC treatment or NACA treatment only (n = 4/group). For NACA treatment, rats received daily subcutaneous injections of NACA (100 μg/rat) beginning immediately after adenovirus injection and ending 1 day after VM transplantation. For ChABC treatment, 2 μl of chondroitinase ABC (Sigma, St. sLouis, MO; 10 U/ml) was injected at the transplant site right before VM transplantation.
Immunocytochemistry
At 2 weeks post-transplantation, rats were perfused transcardially with saline followed by 4% paraformaldehyde. Brains were removed and postfixed in 4% paraformaldehyde overnight, then transferred to a 30% sucrose solution for 2 days before cryosectioning. Coronal sections (30 μm) were cut with a cryostat and divided into five serial sets. Floating sections were washed with phosphate-buffered saline (PBS), incubated with 3% H2O2 for 10 min, blocked with 5% normal goat serum for 1 hr, then transferred into the primary antibody and incubated overnight at room temperature on a shaker. Antibody to tyrosine hydroxylase (TH; 1:4,000; Chemicon, Temecula, CA) was used for staining to identify transplanted VM and dopaminergic fibers along the corpus callosum. On the following day, sections were rinsed and incubated in biotin-SP-conjugated affinipure goat anti-mouse IgG (1:1,200; Jackson Immunoresearch, West Grove, PA) for 1 hr, then incubated in Vectastain Elite ABC reagents (1:100; Vector, Burlingame, CA) for 1 hr and developed for 5–10 min in a 0.05% solution of 3,3,8-diaminobenzidine (DAB) and 0.01% H2O2. Sections were mounted on the glass slides, air dried, dehydrated and coverslipped with Permount (Fisher, Fair Lawn, NJ). Some sections were used for immunofluorescent labeling to assess CSPG digestion in the brain. Anti-proteoglycan Di-4S (2B6; 1:200; Seikagaku Biobusiness Corporation, Tokyo, Japan) was used for identifying 4-sulfated chondroitin following ChABC digestion. Primary antibody was applied overnight at room temperature. Secondary antibody was goat anti-mouse Texas red (1:1,000; Jackson Immunoresearch) and was applied for 1 hr. Sections were mounted on slides and coverslipped with antifade permount for fluorescence microscopy.
GDNF ELISA From Lentivirus Injection
Animals were injected with GDNF/Ad (2.5 × 105 pfu/μl, 1 μl) into the striatum. Seven days later, the striatum was isolated and processed according to the manufacturer's instructions (Promega, Madison, WI). Protein assays were performed, and 10 μg/ml (100 μl/well) of each sample was used for the assay. Each assay was done in triplicate. After development, 96-well plates were read at 450 nm using a BioTech E12a microplate reader.
Western Blots of Tissue Culture Supernatants and Cell Monolayers
The in vitro expression of GDNF and GFRα1 was evaluated 72 hr after virus infection of U373 cells. One milliliter of culture supernatant was precipitated by 0.1 volume of 0.5% sodium deoxycholate and 0.1 volume of TCA. Proteins were pelleted by centrifugation at 14,000 rpm, washed in 80% acetone, dried, and resuspended in 100 μl Laemmli's buffer. Twenty microliters of each sample was loaded for the Western blot. Cell monolayers were washed twice in PBS and cells scraped off plate and homogenized manually with a dounce in 200 μl of 1% SDS in Tris-EDTA buffer with proteinase inhibitors (10 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 mM PMSF) and sonicated using a Branson sonifier 450 (VWR Scientific, West Chester, PA). After centrifuging at 14,000 rpm, supernatant was assayed for protein concentration using a BCA kit (Pierce, Rockford, IL), diluted with 3× Laemmli's buffer, and 50 μg of protein was loaded for each sample. After running the sample in either 14% GDNF or 10% GFRα1 SDS-polyacrylamide gel, proteins were transferred to polyvinylidene difluoride membranes. Membranes were blocked using 5% nonfat dry milk in TBS with 0.05% Tween 20 (TBST). GDNF was identified by M2 anti-Flag antibody (1:500; Sigma), and adenovirus-mediated GFR-α1 expression was detected by goat anti-GFRα1 (1:500; R&D Systems, Minneapolis, MN) antibody. After a 3-hr incubation in primary antibody, the membranes were washed five times for 10 min each in TBST and incubated in goat anti-mouse (GDNF) or donkey anti-goat (GFRα1) IgG (1:7,500; Promega) conjugated with alkaline phosphatase for 1 hr. Membranes were washed as described above and developed using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium solutions (Boehringer Mannheim, Indianapolis, IN).
Quantification of Axonal Growth
To quantify axon growth, TH+ fibers were counted manually at ×200 total magnification at the following points along the pathway: in the corpus callosum 0.5 and 1.5 mm from midline ipsilateral (+) and contralateral (−) to transplant. For each animal, these counts were conducted and averaged over three sections. All counts were carried out by observers blinded to treatment.
Statistical Analysis
One-way ANOVA was used to test for differences in the number of TH+ axons at the different points, followed by Tukey post hoc analysis. Differences were considered statistically significant at P ≤ 0.05.
RESULTS
VM Transplant Procedure
Our previous studies examining growth of DRG axons across a preformed pathway along the corpus callosum used a cell suspension for the transplant; however, to preserve the cellular integrity of the SN better, many laboratories use ventral mesenecephalic tissue chunks (Olanow et al., 1996; Kordower et al., 1997), which show very good survival. In addition, previous studies indicated that the pressure of injecting cells can increase single-cell dispersion within the host tissue. To examine cell dispersion of VM transplants in the corpus callosum in normal rats, two types of VM transplants, cell suspensions and whole tissue chunks, were injected into the right corpus callosum. Two weeks after transplantation, both cell suspensions and chunks survived very well without the addition of any supportive growth factors; however, neurons were observed to diffuse within the corpus callosum when injected in suspension (Fig. 2A). This pattern was in stark contrast to tissue chunks, which remained within a tight bundle (Fig. 2C). After either transplant procedure, TH+ axon were not observed growing from the transplant along the untreated corpus callosum (Fig. 2B,D).
Fig. 2.
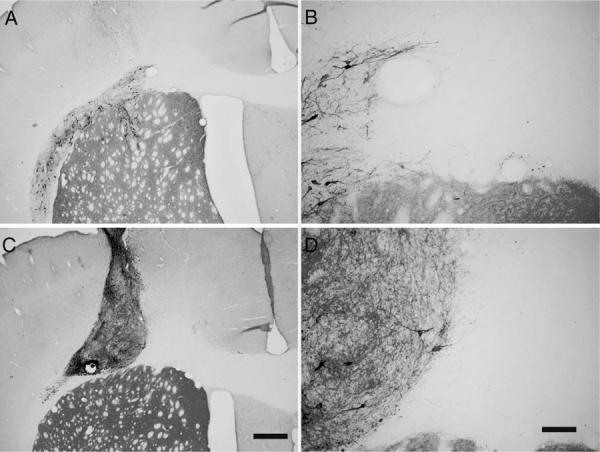
Transplants of VM within the corpus callosum. Transplants of both VM suspension and VM chunks at one side of the corpus callosum survive very well 2 weeks after transplantation. A represents VM cell suspension; B is a higher magnification of A. C represents a VM chunk. D is a higher magnification of C. There were no TH+ axons found along the corpus callosum. Scale bars = 500 μm in C; 100 μm in D.
Comparison of Axon Growth Along Various Pathways
For the first study, two experimental and control groups were established to examine directed growth of dopaminergic axons across the corpus calosum. Pathways were created along the corpus callosum with multiple injections of adenovirus encoding GFP, GDNF, or GDNF/GFRα1. These pathways extended in from the transplant along the corpus callosum and into the contralateral hemisphere (Fig. 1B). Expression of adenoviral transgenes was also verified both in vitro (Fig. 1C) and in vivo by ELISA analysis. Transduction of an astrocyte cell line (U373 cells) showed very nice expression of either GDNF or GFR-α1, within the supernatant or monolayers, respectively. The expression of GFR-α1, but not GDNF, on the cell monolayer shows that most of the GFR-α1 remains attached to the cells themselves and was not released into the tissue culture supernatant as seen with GDNF. In addition, ELISA analysis of GDNF expression 7 days after GDNF/Ad injections showed 925.79 pg GDNF/mg tissue, whereas controls showed about a 30-fold lower expression of GDNF (32.11 pg/mg).
To induce axons growth out of the transplant and across the corpus callosum, an expression gradient was created by increasing the amount of virus injected with distance from the transplant (Ziemba et al 2008). All rats were injected with ChABC at the transplant sites and NACA subcutaneously. In the GFP pathway group, few (7 ± 3) TH+ fibers grew out of the transplants for short distances along the pathway in the corpus callosum. Among these, very few TH+ fibers (2 ± 0) reached the midline (Fig. 3A). With GDNF pathways, many TH+ fibers (16 ± 3) grew out of the transplants. Some grew along the corpus callosum and reached the midline (7 ± 2), but few grew across the midline into the contralateral side (Fig. 3B). In the pathway expressing both GDNF and GFRα1, many TH+ fibers (26 ± 2) grew out of the transplant, with about half crossing the midline into the contralateral side (Figs. 3C, 4A–C). Some TH+ fibers also extended into the contralateral striatum (Fig. 4D). The numbers of TH+ axons at all points along the pathway were significantly higher for GDNF/GFRα1 compared with either GDNF or GFP pathways (Fig. 5A).
Fig. 3.
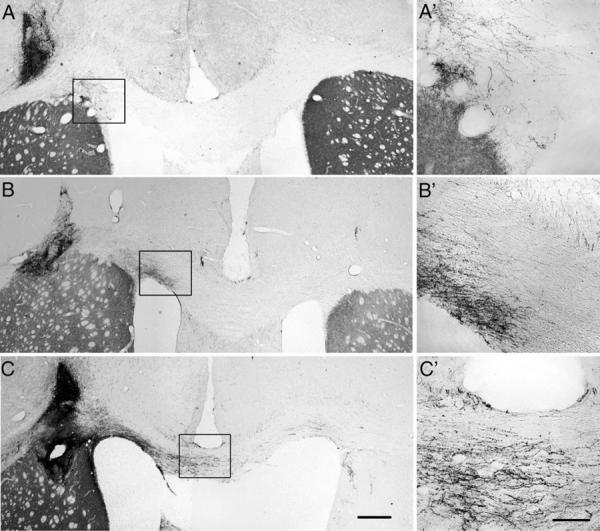
Growth of TH+ axons along the different corpus callosal pathways. Three pathways were created along the corpus callosum with multiple injections of adenovirus encoding GFP, GDNF, or GDNF/GFRα1 with the volume increasing from left to right. All animals received chondroitinase ABC (10 U/ml, 2 μl) injections at the transplantation site just prior to VM transplantation as well as daily subcutaneous injections of NACA (100 μg/rat) beginning immediately after virus injections and ending 1 day after transplantation. A: With GFP pathways, TH+ fibers were found near the VM transplants, but very few TH+ fibers grew along the corpus callosum to reach to the midline. B: With GDNF pathways, some TH+ fibers grew along the corpus callosum toward the midline, but few of them grew across the midline to the contralateral side. C: With GDNF/GFRα1 pathways, many TH+ axons grew from the transplant along the corpus callosum across the midline into the contralateral side of the corpus callosum. A′–C′: Higher magnification of boxed areas showing growing axons. Scale bars = 500 μm in C (applies to A–C); 150 μm in C′ (applies to A′–C′.
Fig. 4.
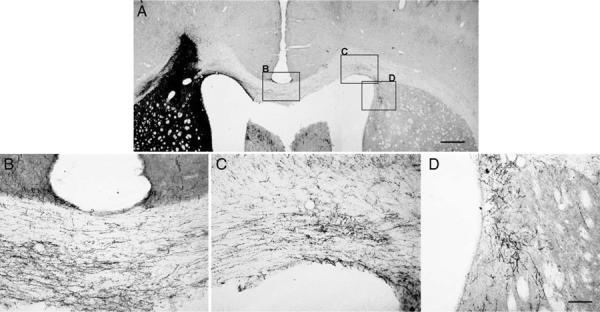
TH+ axons along the GDNF/GFRα1 pathway. Combined with ChABC and NACA treatments, many TH+ axons grew along the GDNF/GFRα1 pathway, across the midline to the contralateral side, and some also grew into the striatum of the contralateral side (A). B shows TH+ axons at the midline of the corpus callosum. C shows some TH+ axons that grew across the midline to the contralateral side of the corpus callosum. D shows some TH+ axons that grew into the contralateral striatum. Scale bars = 500 μm in A; 100 μm in D (applies to B,D).
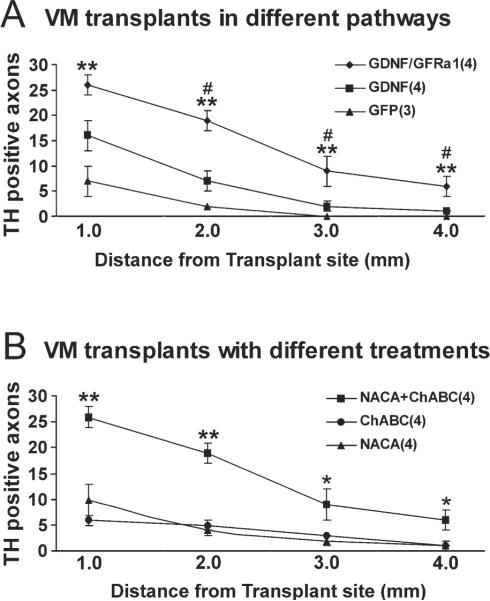
Fig. 5.
A: More axons grew along pathways of GDNF/GFRα1 than GDNF or GFP. With ChABC and NACA treatment, the GDNF/GFRα1 pathway supported the growth of more axons from the transplant along the pathway, across the midline, and toward the contralateral side. Significantly higher numbers of TH+ axons were found in the GDNF/GFRα1 treatment group at almost all pathway points compared with GDNF and GFP pathways, with GDNF at 1.0 mm from the transplantation site being the only nonsignificant point P = 0.065. There was also no significant difference between GDNF and GFP pathways at any points (P > 0.05). # P < 0.05 for GDNF/GFRα1 vs. GDNF; ★ P < 0.01 for GDNF/GFRα1 vs. GFP. B: Combination of GDNF/GFRα1, ChABC, and NACA is required to promote robust growth. Along the GDNF/GFRα1 pathway, a single treatment with ChABC or NACA did not support robust TH+ axons outgrowth along the pathway. However, combining ChABC with NACA significantly increased the number of TH+ axons at all points on both transplantation side and contralateral side. All error bars are standard error of the mean. ★P < 0.01, ★★P < 0.01 for GDNF/GFRα1 vs. ChABC or NACA.
At the closest point measured near the transplant, GDNF/GFR α1 was significantly different from GFP [F(2,8), 11.245, P = 0.004], but not GDNF, which showed good initial growth from the transplant. At every other point, GDNF/GFR α1 was significantly different from pathways for either GDNF or GFP (Fig. 5A). This difference indicates that, although GDNF supports growth out of the transplant, it was not capable of supporting long-distance growth across the corpus callosum, which required the addition of GFRα1. Surprisingly, there was no difference in TH+ axon outgrowth between GDNF and GFP groups (P > 0.05).
Comparison of Axon Growth With NACA, ChABC, or Both Treatments
All experiments described above, including controls, were done with NACA and ChABC treatments. With this combined treatment, the greatest TH+ axons growth occurred along a GDNF/GFRα1 pathway compared with GFP or GDNF alone. The addition of ChABC at the implantation site is thought to reduce the inhibitory environment surrounding the transplant, whereas NACA is thought to reduce the toxic effect of adenovirus. Because the two treatments involve different mechanisms, we examined whether treatment with either ChABC or NACA alone could promote TH+ fiber growth along a growth-supportive pathway of GDNF/GFRα1. Comparing the number of TH+ fibers at all points along the corpus callosum showed that the combined treatment of ChABC and NACA with GDNF/GFRα1 pathway significantly increased axon outgrowth compared with the GDNF/GFRa1 pathway with individual treatments of either ChABC or NACA (Fig. 5B).
Morphology of Effect of ChABC Treatments
Transplants of VM tissue chunks may damage brain tissue around the transplant site and increase expression of inhibitory CSPGs. The enzyme ChABC digests CSPGs, forming degradation products that can be detected by the antibody 2B6. Staining the sections from ChABC or non-ChABC treatment groups with 2B6 antibody showed positive staining along the corpus callosum on the transplantation side in ChABC-treated animals (Fig. 6A). No 2B6 staining was found in non-ChABC-treated animals (Fig. 6B).
Fig. 6.
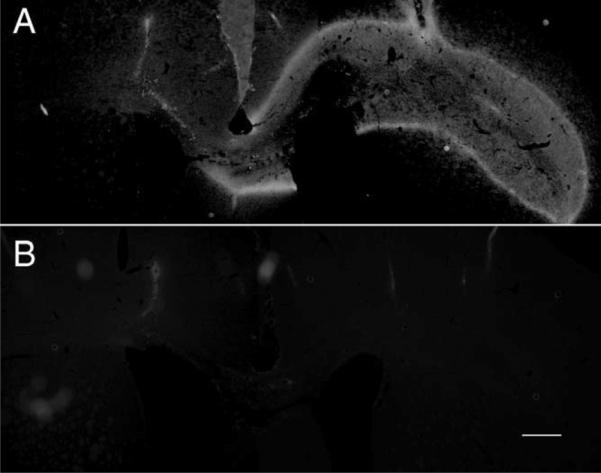
Immunochemical staining for CSPG. 2B6 antibody can specifically recognize four sulfated chondroitin and dermatan sulfate following chondroitinase ABC digestion of various proteoglycans. Injected ChABC at the transplantation site shows 2B6+ staining along the corpus callosum on the transplantation side (A). Without treatment, no 2B6+ staining was found (B). Scale bar = 500 μm.
DISCUSSION
For any transplantation therapy to work, the importance of cell survival is paramount. In this study, we found that both dissociated cells and whole VM tissue chunks survived very well for up to 2 weeks when transplanted into the corpus callosum of normal animals. Single-cell injections showed more dispersion along the corpus callosum, most likely as a result of injection pressure, which could force the cells to create fissure within the white matter tract. No dopa-minergic fibers grew along the corpus callousm from either tissue chunks or dissociated cells without the creation of a growth-supportive pathway prior to transplant. For this study, we choose tissue chunks because the density of TH-positive neurons was higher, and neurons appeared less dispersed within the transplant area.
To reconstruct pathways in the CNS, axons have to be guided to distal targets. We have previously examined this response for DRG neurons (Ziemba et al., 2008), and in this study we have begun examining potential factors to promote dopaminergic axon growth within the adult brain. Similarly to what we observed previously with DRG neurons, few TH-positive fibers grew out of the transplants and only for short distances in untreated or GFP pathway groups. In the GDNF group, many TH-positive fibers initially grew along the corpus callosum and reached the midline, but very few were able to cross the midline into the contralateral side of the corpus callosum. In the GDNF/GFRα1 group, however, many TH-positive axons grew across the midline into the contralateral side, and some of them extended into the striatum. Even though the GDNF/GFRα1 pathway showed very nice growth into the contralateral hemisphere, there was a gradual decrease in the numbers of axons with distance, in which only about 20% of the axons exiting the transplant grew to the end of the pathway. This decrease was dramatically different from what we observed with axon growth from DRG neurons along a fibroblast-growth factor (FGF2) and NGF pathway, in which the vast majority of axons grew to the end of the pathway (Ziemba et al., 2008). The growth of TH-positive axons also appeared to be much more sensitive to endogenous CSPGs and inflammation induced by adenovirus compared with growth of DRG axon.
Since administration of adenovirus in vivo induces a strong immune response, and transplant techniques may cause some damage at the transplantation site, two treatments were used to reduce these side effects. The compound NACA was chosen to reduce viral toxic effects and oxidative stress. NACA, a cell-permeable antioxidant and glutathione precursor, is the amine form of the well-characterized N-acetylcysteine (NAC) and is metabolized via the same pathways as NAC (Wu et al., 2008). The CNS is particularly vulnerable to oxidative stress. Oxidative stress increases in both brain and spinal cord after trauma (Juurlink and Paterson, 1998). Treatment with NAC following spinal cord ischemia-reperfusion injury in rabbits significantly decreases both tissue damage and motor dysfunction (Cakir et al., 2003). NAC showed neuroprotective effects on brain injury by preventing trauma-induced oxidative brain tissue damage (Hicdonmez et al., 2006). NAC administration also has been shown to attenuate the inflammatory response after brain injury (Chen et al., 2008). However, NAC has limited bioavailability because, under physiological conditions, it is charged and therefore does not cross the blood–brain barrier or cellular membranes or enter the mitochondrial matrix, where it would be of most use. NACA overcomes this disadvantage of NAC. In the current study, treatment with NACA alone did not show any effect on dopaminergic axon growth. However, when combined with reduction of CSPGs and expression of GDNF and GFRα1 along the pathway, NACA treatment improved overall dopaminergic growth.
Because whole VM tissue chunks (1 mm × 1 mm) were used for transplantation, a 22-gauge spinal needle was necessary for the transplantation procedure. Such a large needle causes damage around the transplantation site and likely increases expression of CSPGs. CSPGs are a major component of glial scars in the CNS following injury in mature animals (McKeon et al., 1991; Jones et al., 2003). Studies have demonstrated that CSPGs are extremely inhibitory to axonal outgrowth (Snow et al., 1990; Dou and Levine, 1994). The enzyme ChABC removes many of the sugar chains from CSPGs and effectively blocks their inhibitory properties. Application of ChABC to brain or spinal cord injury sites increases both axon regeneration and functional recovery (Yick et al., 2000; Bradbury et al., 2002; Fouad et al., 2005). In this study, ChABC was injected at transplant sites before VM transplantation to reduce the concentration of growth-inhibitory CSPGs. Immunostaining with the antibody 2B6, which specifically recognizes sulfated chondroitin produced by digestion of CSPG by ChABC, showed positive staining around the transplant site in treated animals. Treatment with ChABC alone did not enhance axon growth from VM transplants, but the combination of ChABC and NACA treatments improved long-distance axon growth along pathways expressing GDNF and GFRα1.
Our previous studies showed that transplanted DRG neurons can be directed to grow a long distance in the brain with neurotrophic factor support (Ziemba et al., 2008). Axons from DRG neurons can grow out of the transplants for only a very short distance without such support (Jin et al., 2008). In the present study, although dopaminergic neurons survived well within transplants, no dopaminergic axons extended out of the transplants in the absence of a preformed growth-supportive pathway. GDNF was first shown to promote survival and growth of developing dopaminergic neurons in vitro (Lin et al., 1993) and has also been demonstrated to increase survival and fiber outgrowth from fetal VM transplantation in rodent PD models (Sinclair et al., 1996; Apostolides et al., 1998; Mehta et al., 1998; Yurek, 1998; Hebb et al., 2003; Mendez et al., 2005). In this study, we showed that expression of GDNF alone was not enough to result in robust axon growth along a desired pathway. GDNF signaling is mediated through a multicomponent receptor complex including the tyrosine kinase receptor RET and one of the glycosylphosphatidylinositol (GPI)-linked GDNF family receptors (GFRα1–α4). In vitro studies show that there is preferential binding between GDNF and GFRα1 (Enomot, 2005). GFRα1 is expressed as both membrane-bound and secreted forms by accessory nerve cells and peripheral targets of developing sensory and sympathetic neurons during the period of target innervation (Ledda et al., 2002). Directional growth of sensory and sympathetic axons can be promoted by a local source of GFRα1. Ledda and colleagues demonstrated that an attractive effect occurred when soluble GFRα1 was combined with GDNF. Furthermore, in the presence of GDNF, a spatially confined source of GFRα1 acted as a local directional cue for growing sensory axons, orienting and stabilizing them along sites of GFRα1 expression; however, GFRα1 itself had no effect on the direction of axon growth in the absence GDNF (Ledda et al., 2002). For our studies, we hypothesized that GPI-linked GFRα1 expressed by astrocytes along the corpus callosum would act as a GDNF sink to concentrate GDNF along the surface of transduced astrocytes, thus generating a bound pathway of GDNF. In this study, we have shown for the first time that a combination of GDNF and soluble GFRα1 can support robust dopaminergic axon growth along a preformed pathway from fetal VM transplants.
In summary, this study has demonstrated that adenovirus-mediated expression of GDNF or a combination of GDNF and GFRα1 can support long-distance growth of dopaminergic axons from VM transplants in the corpus callosum. The combination pathway has a superior effect, and the addition of NACA and ChABC treatments further enhances the extent of fiber outgrowth. Future studies will examine whether creating a path expressing GDNF and GFRα1 between a VM transplant in the SN and the denervated striatum can lead to reconstruction of the nigrostriatal pathway and functional recovery in models of Parkinson's disease.
REFERENCES
- Apostolides C, Sanford E, Hong M, Mendez I. Glial cell line-derived neurotrophic factor improves intrastriatal graft survival of stored dopaminergic cells. Neuroscience. 1998;83:363–372. doi: 10.1016/s0306-4522(97)00369-2. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Schmidt RH, Stenevi U. Functional reinnervation of the neostriatum in the adult rat by use of intraparenchymal grafting of dissociated cell suspensions from the substantia nigra. Cell Tissue Res. 1980;212:39–45. doi: 10.1007/BF00234031. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Cai J, Ziemba KS, Smith GM, Jin Y. Evaluation of cellular organization and axonal regeneration through linear PLA foam implants in acute and chronic spinal cord injury. J Biomed Mater Res A. 2007;83:512–520. doi: 10.1002/jbm.a.31296. [DOI] [PubMed] [Google Scholar]
- Cakir O, Erdem K, Oruc A, Kilinc N, Eren N. Neuroprotective effect of N-acetylcysteine and hypothermia on the spinal cord ischemia-reperfusion injury. Cardiovasc Surg. 2003;11:375–379. doi: 10.1016/S0967-2109(03)00077-2. [DOI] [PubMed] [Google Scholar]
- Chen G, Shi J, Hu Z, Hang C. Inhibitory effect on cerebral inflammatory response following traumatic brain injury in rats: a potential neuroprotective mechanism of N-acetylcysteine. Mediators Inflamm. 2008;2008:716458. doi: 10.1155/2008/716458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Diener PS, Bregman BS. Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat. J Neurosci. 1998;18:779–793. doi: 10.1523/JNEUROSCI.18-02-00779.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou CL, Levine JM. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J Neurosci. 1994;14:7616–7628. doi: 10.1523/JNEUROSCI.14-12-07616.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto H. Regulation of neural development by glial cell line-derived neurotrophic factor family ligands. Anat Sci Int. 2005;80:42–52. doi: 10.1111/j.1447-073x.2005.00099.x. [DOI] [PubMed] [Google Scholar]
- Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T, Pearse DD. Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci. 2005;25:1169–1178. doi: 10.1523/JNEUROSCI.3562-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaura V, Bachoud-Levi AC, Ribeiro MJ, Nguyen JP, Frouin V, Baudic S, Brugieres P, Mangin JF, Boisse MF, Palfi S, Cesaro P, Samson Y, Hantraye P, Peschanski M, Remy P. Striatal neural grafting improves cortical metabolism in Huntington's disease patients. Brain. 2004;127:65–72. doi: 10.1093/brain/awh003. [DOI] [PubMed] [Google Scholar]
- Hebb AO, Hebb K, Ramachandran AC, Mendez I. Glial cell line-derived neurotrophic factor-supplemented hibernation of fetal ventral mesencephalic neurons for transplantation in Parkinson disease: long-term storage. J Neurosurg. 2003;98:1078–1083. doi: 10.3171/jns.2003.98.5.1078. [DOI] [PubMed] [Google Scholar]
- Hicdonmez T, Kanter M, Tiryaki M, Parsak T, Cobanoglu S. Neuroprotective effects of N-acetylcysteine on experimental closed head trauma in rats. Neurochem Res. 2006;31:473–481. doi: 10.1007/s11064-006-9040-z. [DOI] [PubMed] [Google Scholar]
- Jin Y, Fischer I, Tessler A, Houle JD. Transplants of fibroblasts genetically modified to express BDNF promote axonal regeneration from supraspinal neurons following chronic spinal cord injury. Exp Neurol. 2002;177:265–275. doi: 10.1006/exnr.2002.7980. [DOI] [PubMed] [Google Scholar]
- Johansson M, Friedemann M, Hoffer B, Stromberg I. Effects of glial cell line-derived neurotrophic factor on developing and mature ventral mesencephalic grafts in oculo. Exp Neurol. 1995;134:25–34. doi: 10.1006/exnr.1995.1033. [DOI] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003;182:399–411. doi: 10.1016/s0014-4886(03)00087-6. [DOI] [PubMed] [Google Scholar]
- Kordower JH, Goetz CG, Freeman TB, Olanow CW. Dopaminergic transplants in patients with Parkinson's disease: neuroanatomical correlates of clinical recovery. Exp Neurol. 1997;144:41–46. doi: 10.1006/exnr.1996.6386. [DOI] [PubMed] [Google Scholar]
- Ledda F, Paratcha G, Ibanez CF. Target-derived GFRalpha1 as an attractive guidance signal for developing sensory and sympathetic axons via activation of Cdk5. Neuron. 2002;36:387–401. doi: 10.1016/s0896-6273(02)01002-4. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V, Hong M, Spears J, Mendez I. Enhancement of graft survival and sensorimotor behavioral recovery in rats undergoing transplantation with dopaminergic cells exposed to glial cell line-derived neurotrophic factor. J Neurosurg. 1998;88:1088–1095. doi: 10.3171/jns.1998.88.6.1088. [DOI] [PubMed] [Google Scholar]
- Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, Tierney T, Holness R, Dagher A, Trojanowski JQ, Isacson O. Dopamine neurons implanted into people with Parkinson's disease survive without pathology for 14 years. Nat Med. 2008;14:507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel J, Samii M, Walter GF. Fetal allogeneic dopaminergic cell suspension grafts in the ventricular system of the rat: characterization of transplant morphology and graft–host interactions. Acta Neuropathol. 2004;107:421–427. doi: 10.1007/s00401-004-0823-5. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Kordower JH, Freeman TB. Fetal nigal transplantation as a therapy for Parkinson's disease. Trends Neurosci. 1996;19:102–109. doi: 10.1016/s0166-2236(96)80038-5. [DOI] [PubMed] [Google Scholar]
- Rabinovich SS, Seledtsov VI, Poveschenko OV, Senuykov VV, Taraban VY, Yarochno VI, Kolosov NG, Savchenko SA, Kozlov VA. Transplantation treatment of spinal cord injury patients. Biomed Pharmacother. 2003;57:428–433. doi: 10.1016/j.biopha.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Ramon-Cueto A, Plant GW, Avila J, Bunge MB. Long-distance axonal regeneration in the transected adult rat spinal cord is promoted by olfactory ensheathing glia transplants. J Neurosci. 1998;18:3803–3815. doi: 10.1523/JNEUROSCI.18-10-03803.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab ME, Kapfhammer JP, Bandtlow CE. Inhibitors of neurite growth. Annu Rev Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- Sinclair SR, Svendsen CN, Torres EM, Martin D, Fawcett JW, Dunnett SB. GDNF enhances dopaminergic cell survival and fibre outgrowth in embryonic nigral grafts. Neuroreport. 1996;7:2547–2552. doi: 10.1097/00001756-199611040-00029. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol. 1990;109:111–130. doi: 10.1016/s0014-4886(05)80013-5. [DOI] [PubMed] [Google Scholar]
- Theele DP, Schrimsher GW, Reier PJ. Comparison of the growth and fate of fetal spinal iso- and allografts in the adult rat injured spinal cord. Exp Neurol. 1996;142:128–143. doi: 10.1006/exnr.1996.0184. [DOI] [PubMed] [Google Scholar]
- Tuszynski MH, Peterson DA, Ray J, Baird A, Nakahara Y, Gage FH. Fibroblasts genetically modified to produce nerve growth factor induce robust neuritic ingrowth after grafting to the spinal cord. Exp Neurol. 1994;126:1–14. doi: 10.1006/exnr.1994.1037. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A, Dunnett SB. Transplantation in the rat model of Parkinson's disease: ectopic vs. homotopic graft placement. Prog Brain Res. 2000;127:233–265. doi: 10.1016/s0079-6123(00)27012-x. [DOI] [PubMed] [Google Scholar]
- Wu W, Abraham L, Ogony J, Matthews R, Goldstein G, Ercal N. Effects of N-acetylcysteine amide (NACA), a thiol antioxidant on radiation-induced cytotoxicity in Chinese hamster ovary cells. Life Sci. 2008;82:1122–1130. doi: 10.1016/j.lfs.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Yick LW, Wu W, So KF, Yip HK, Shum DK. Chondroitinase ABC promotes axonal regeneration of Clarke's neurons after spinal cord injury. Neuroreport. 2000;11:1063–1067. doi: 10.1097/00001756-200004070-00032. [DOI] [PubMed] [Google Scholar]
- Yurek DM. Glial cell line-derived neurotrophic factor improves survival of dopaminergic neurons in transplants of fetal ventral mesencephalic tissue. Exp Neurol. 1998;153:195–202. doi: 10.1006/exnr.1998.6884. [DOI] [PubMed] [Google Scholar]
- Ziemba KS, Chaudhry N, Rabchevsky AG, Jin Y, Smith GM. Targeting axon growth from neuronal transplants along preformed guidance pathways in the adult CNS. J Neurosci. 2008;28:340–348. doi: 10.1523/JNEUROSCI.3819-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]