Abstract
Genetic and epigenetic (DNA methylation, histone modifications, microRNA expression) crosstalk promotes inactivation of tumor suppressor genes or activation of oncogenes by gene loss/hypermethylation or duplications/hypomethylation, respectively. The 8p11-p12 chromosomal region is a hotspot for genomic aberrations (chromosomal rearrangements, amplifications and deletions) in several cancer forms, including breast carcinoma where amplification has been associated with increased proliferation rates and reduced patient survival. Here, an integrative genomics screen (DNA copy number, transcriptional and DNA methylation profiling) performed in 229 primary invasive breast carcinomas identified substantial coamplification of the 8p11-p12 genomic region and the MYC oncogene (8q24.21), as well as aberrant methylation and transcriptional patterns for several genes spanning the 8q12.1-q24.22 genomic region (ENPP2, FABP5, IMPAD1, NDRG1, PLEKHF2, RRM2B, SQLE, TAF2, TATDN1, TRPS1, VPS13B). Taken together, our findings suggest that MYC activity and aberrant DNA methylation may also have a pivotal role in the aggressive tumor phenotype frequently observed in breast carcinomas harboring 8p11-p12 regional amplification.
Keywords: breast cancer, DNA amplification, DNA methylation, 8p11-p12, MYC
Introduction
Genomic instability and epigenetic modulations, that is, DNA methylation, histone modifications, microRNA expression, contribute to the neoplastic phenotype by deregulating key gene functions that permit cells to bypass regulatory mechanisms controlling and maintaining normal cellular physiology.1 Recently, genetic and epigenetic crosstalk has shown to be one of several major driving forces behind tumor initiation and progression.2, 3, 4, 5, 6 However, DNA methylation is considered by some to be a secondary event which locks genes in their inactive/active states only after gene silencing/activation has been achieved by other means.7, 8, 9, 10
Several well-characterized DNA regions have been investigated extensively in breast cancer for their role in genetic modulations, interactions in molecular pathways and association with unfavorable clinical outcome. These include the 8p11-p12, 8q24 (MYC), 11q13 (CCND1), 17q12 (ERBB2, GRB7, STARD3) and 20q13 (ZNF217, MYBL2, STK6) amplicons, some of which have become major molecular targets for breast cancer treatment. Regional amplification of the 8p11-p12 genomic region is a common genetic event in solid tumors, for example, breast carcinoma,11, 12, 13 pleuropulmonary blastoma,14 lung cancer and esophageal squamous cell carcinomas,15, 16, 17, 18 urinary bladder cancer,19, 20 osteosarcoma21 and pancreatic adenocarcinoma.17 In breast cancer cell lines, the initiation site and structure of the 8p11-p12 DNA rearrangement involved different mechanisms of gene activation, thereby resulting in the activation of different combinations of candidate genes.22
To further define the role, 8p11-p12 regional amplification may have on breast cancer pathophysiology, we examined genome-wide copy number alterations, DNA methylation patterns and transcriptional changes in 229 primary invasive breast tumors. Here, we demonstrate that ∼50% of 8p11-p12-amplified tumors also harbor MYC amplification, as well as, hypomethylation of genes located in close proximity to the MYC gene.
Results and discussion
Amplification of the 8p11-p12 genomic region is a common genetic event in breast carcinoma with clinical implications. To assess aberrant transcriptional and DNA methylation patterns in invasive breast carcinomas harboring the 8p11-p12 amplicon, an integrative analysis was performed using DNA copy number, DNA methylation and transcriptome data from 229 primary invasive breast cancer samples previously presented in our work,23, 24 including our own unpublished data. The DNA copy number analysis using array-comparative genomic hybridization data showed recurrent copy number alterations on chromosome bands 8p11-p12 in 83 tumors (36%), including 47/83 high-level gains/amplifications, 20/83 low-level gains and 16/83 heterozygous losses. Copy number alterations were confirmed using a set of overlapping BAC clones building a contig over the 8p11-p12 genomic region. On average, there was a five-fold increase in the number of amplifications observed in lesions containing the 8p11-p12 amplicon compared with those lacking the amplicon (P=1.8E−13). In general, amplification of the 8p11-p12 genomic region was predominantly coamplified with 1q, 8q, 11q, 12p, 16p, 17q or 20q, but also occurred as the sole region of amplification in two cases. Notably, 53% (n=24) of 8p11-p12-amplified tumors were coamplified with the MYC gene, whereas only 20% (n=9) and 18% (n=8) were coamplified with the CCND1 and ERBB2 genes, respectively (Figure 1). Extensive research has been carried out on the coamplification of 8p11-p12 and CCND1, but few studies have investigated 8p11-p12 and MYC interactions.22, 25
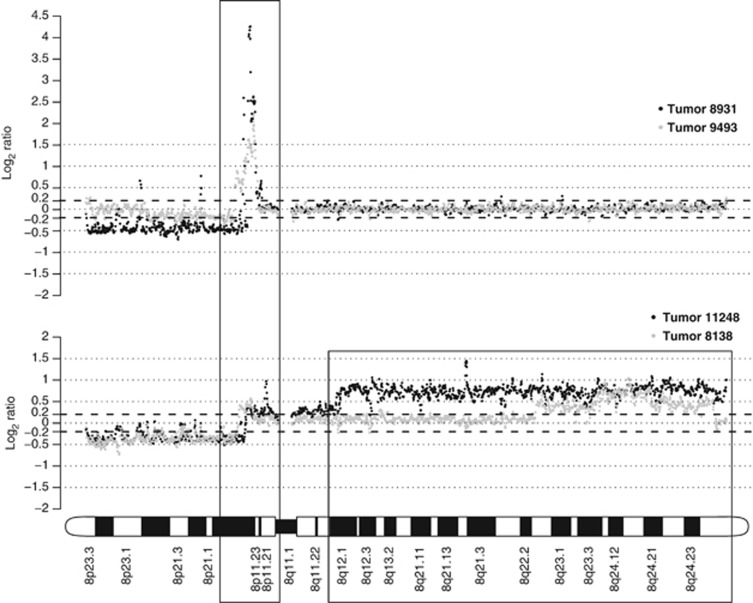
Figure 1.
Array-CGH genomic profiles showing recurrent DNA amplification of the 8p11-p12 genomic region in breast carcinoma. The top panel shows focal amplification (log2 ratio>0.5) of the 8p11-p12 region in two breast tumors. Black dots depict BAC clones spanning chromosome 8 for tumor 8931 and gray dots for tumor 9493. The bottom panel shows amplification of the 8p11-p12 and 8q regions. Black dots depict BAC clones spanning chromosome 8 for tumor 11248 and gray dots for tumor 8138. The x-axis shows chromosome 8 from the 8p telomere to the 8q telomere. The y-axis shows the log2 ratio value for each BAC clone (tumor gDNA versus normal control gDNA).
In accordance with published studies, genetic aberrations of the 8p11-p12 region (gain, loss and amplification; P=5.0E−6), including amplification (P=4.0E−5) or loss (P=0.005), were associated with reduced overall survival rates.26 Conversely, genomic gain was not indicative of unfavorable patient outcome (P=0.08). The amplicon was most prevalent in tumors of large pathologic size (P=0.0002), high genomic grade index status (P=0.0004) and high S-phase fraction (P=0.002; Table 1). There was no significant difference in histologic type, axillary lymph node status, estrogen/progesterone receptor status, human epidermal growth factor receptor 2 (HER2)/neu receptor status, triple negative status or molecular breast cancer subtype. These findings are consistent with previous reports showing high cell proliferation (high Ki-67) and high tumor grade in breast carcinomas harboring the 8p11-p12 amplicon. However, Gelsi–Boyer et al. 26 did not find a connection with amplification and tumor size. Recently, several studies have found an association between the luminal B molecular subtype and DNA amplification of two genes (ZNF703 and FGFR1) within the 8p11-p12 amplicon. Interestingly, tumors harboring these genetic alterations were also resistant to endocrine therapy.27, 28, 29, 30 However, we show that ∼80% of the breast tumors analyzed here were luminal B subtype/estrogen receptor-positive regardless of 8p11-p12 amplicon status. Furthermore, ZNF703 was generally upregulated in breast carcinomas, particularly in estrogen receptor-positive tumors, compared with normal breast tissue.24 Functional studies have provided additional evidence for biological effects in vitro and in vivo using small-interfering RNA-mediated knockdown of candidate genes within the 8p11-p12 genomic region.27, 28, 29, 30, 31, 32, 33 Eight genes (BAG4, C8orf4, DDHD2, ERLIN2, LSM1, PPAPDC1B, WHSC1L1 and ZNF703) have thereby emerged as targets with oncogenic potential.
Table 1. Correlation between 8p11-p12 DNA amplification and clinicopathological features in breast carcinoma.
| Characteristics |
Number of tumors (%) |
|||
|---|---|---|---|---|
| Total tumors (n=229) | Neutral DNA dosagea (n=71) | DNA amplificationa (n=45) | P-value | |
| Age | ||||
| Mean | 59 | 60 | 60 | |
| Range | 30–88 | 37–79 | 30–88 | |
| Histologic type | 0.7 | |||
| Ductal | 136 (59) | 52 (73) | 21 (47) | |
| Lobular | 22 (10) | 7 (10) | 4 (9) | |
| Other | 26 (11) | 12 (17) | 3 (7) | |
| Not available | 45 (20) | 0 (0) | 17 (38) | |
| Axillary lymph node status | 0.2 | |||
| pN0 | 82 (36) | 38 (54) | 12 (27) | |
| pN1 | 84 (37) | 33 (46) | 19 (42) | |
| Not available | 63 (28) | 0 (0) | 14 (31) | |
| Pathologic tumor size | 0.0002 | |||
| pT1 | 51 (22) | 22 (31) | 4 (9) | |
| pT2 | 89 (39) | 35 (49) | 12 (27) | |
| pT3 | 51 (22) | 11 (15) | 20 (44) | |
| pT4 | 6 (3) | 3 (4) | 0 (0) | |
| Not available | 32 (14) | 0 (0) | 9 (20) | |
| S-phase fraction | 0.002 | |||
| ⩽6.1 | 112 (49) | 59 (83) | 18 (40) | |
| >6.1 | 69 (30) | 12 (17) | 16 (36) | |
| Not available | 48 (21) | 0 (0) | 11 (24) | |
| GGI status | 0.0004 | |||
| Low | 45 (20) | 31 (44) | 6 (13) | |
| High | 73 (32) | 26 (37) | 29 (64) | |
| Not available | 111 (48) | 14 (20) | 10 (22) | |
| Estrogen receptor | 0.8 | |||
| Negative | 60 (26) | 14 (20) | 10 (22) | |
| Positive | 166 (72) | 57 (80) | 34 (76) | |
| Not available | 3 (1) | 0 (0) | 1 (2) | |
| Progesterone receptor | 0.7 | |||
| Negative | 108 (47) | 31 (44) | 21 (47) | |
| Positive | 118 (52) | 40 (56) | 23 (51) | |
| Not available | 3 (1) | 0 (0) | 1 (2) | |
| HER2/neu status | 0.6 | |||
| Negative | 199 (87) | 61 (86) | 37 (82) | |
| Positive | 30 (13) | 10 (14) | 8 (18) | |
| Not available | 0 (0) | 0 (0) | 0 (0) | |
| Triple negative status | 1.0 | |||
| Yes | 41 (18) | 9 (13) | 5 (11) | |
| No | 186 (81) | 62 (87) | 39 (87) | |
| Not available | 2 (1) | 0 (0) | 1 (2) | |
| Subtype | 0.9 | |||
| Luminal subtype A | 2 (1) | 1 (1) | 0 (0) | |
| Luminal subtype B/HER2- | 101 (44) | 47 (66) | 31 (69) | |
| Luminal subtype B/HER2+ | 13 (6) | 8 (11) | 4 (9) | |
| HER2/ER- | 18 (8) | 10 (14) | 5 (11) | |
| Basal-like | 16 (7) | 5 (7) | 5 (11) | |
| Normal-like | 0 (0) | 0 (0) | 0 (0) | |
| Not available | 79 (34) | 0 (0) | 0 (0) | |
Abbreviations: GGI status, genomic grade index; HER2, human epidermal growth factor receptor 2.
P-values were calculated using the Fisher's exact test (neutral DNA dosage versus DNA amplification).
Tumor specimens included in the analysis with both array-CGH and gene expression microarray data are available.
To delineate whether aberrant methylation patterns may also has a role in the evolution of breast tumors harboring the 8p11-p12 amplicon, we performed genome-wide DNA methylation analysis on 22/229 tumors (11 tumors harboring the amplicon and 11 tumors lacking the amplicon) using the 450k Infinium Methylation Beadchip (Illumina Inc., San Diego, CA, USA). Of the 382 815 cytosine sites remaining after filtering, ⩽1% (n=2066) were differentially methylated in tumors harboring the 8p11-p12 amplicon compared with samples lacking the amplicon. Eighty-nine percent of aberrantly-methylated cytosine sites were hypermethylated (n=1847) and 11% (n=219) of sites were hypomethylated. The promoter regions (200 and 1500 bp upstream transcriptional start sites, 5′ untranslated region and the first exon) were tightly linked with hypermethylation (n=352 sites, 92%), whereas fewer methylation events occurred further downstream in the body of genes and at the 3′ untranslated region region. The highest number of aberrantly-methylated cytosine sites surrounded CpG islands (n=408) with fewer sites found in the CpG shores (up to 2 kb from CpG islands, n=172) and shelves (2–4 kb from CpG islands, n= 48). The majority of aberrant methylation patterns occurred within genes and intergenic regions, whereas few microRNA transcripts were found (Figure 2). We found that differential methylation occurred on all chromosomes including the X chromosome in 8p11-p12-amplified tumors, where hypermethylation ranged from 72–98% and the highest hypomethylation rates were found on chromosomes 8 and 9 with 28% and 24%, respectively.
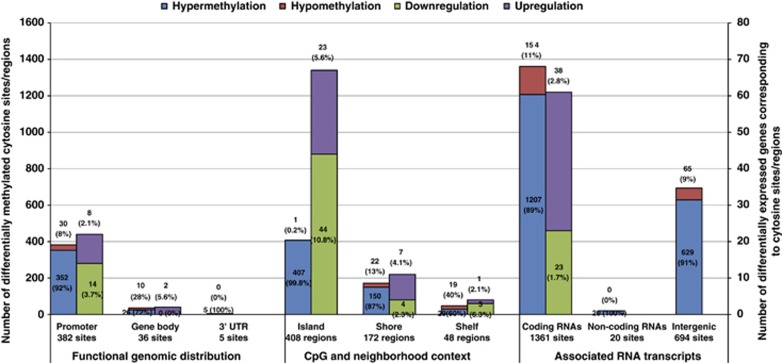
Figure 2.
DNA methylation patterns in 8p11-p12-amplified tumors. The distribution of aberrant methylation (hyper- and hypomethylation, Q<0.05) and gene expression patterns (downregulation and upregulation, Q<0.01) among the 2066 differentially-methylated cytosine sites in 8p11-p12-amplified tumors. Transcripts were categorized into functional genomic regions (promoter region (between 200 and 1500 bp upstream of transcriptional start sites, 5′ untranslated region, first exon), gene body and 3′ untranslated region region) and regions surrounding CpG islands (CpG islands, 2 kb from CpG islands (CpG shores) and 2–4 kb from CpG islands (CpG shelves)).
Few of the methylation events resulted in aberrant gene expression patterns in 8p11-p12-amplified tumors (n=61, 4.5% of aberrantly-methylated coding RNAs), although disparate methylation-transcriptional patterns were observed for 23/61 genes (38%); 20/23 genes were hypermethylated and overexpressed and 3/23 genes were hypomethylated and underexpressed (Table 2). Univariate Cox regression analysis showed that aberrant transcriptional patterns for 47/61 genes influenced overall survival rates. In addition, only one gene located at 8p11-p12 showed differential methylation and gene expression patterns, that is, BRF2 was hypermethylated but overexpressed owing to BRF2 gene amplification in 7/11 cases. Gene Ontology enrichment analysis of the genes with aberrant DNA methylation and gene expression patterns revealed several cancer-related processes, for example, cell differentiation, DNA replication, cell migration and cell adhesion (Table 3).
Table 2. Differentially-methylated genes in 8p11-p12-amplified breast tumors.
| Gene symbol | Chromosome | Delta beta valuea | Gene expression (n=22)b | Gene expression (n=150)c | Cox coefficient (n=150)d | Cox P-value (n=150)d | DNA copy number 8p11-p12-amplified tumors (n=11)e amplification/loss/normal | DNA copy number 8p11-p12 nonamplified tumors (n=11)f amplification/loss/normal |
|---|---|---|---|---|---|---|---|---|
| BAMBI | 10p12.1 | Hypomethylated | Overexpressed | NS | 0/0/11 | 0/0/11 | ||
| CXCL12 | 10q11.21 | Hypermethylated | Underexpressed | −0.498 | 3.76E−05 | 0/0/11 | 0/0/11 | |
| HTRA1 | 10q26.13 | Hypermethylated | Underexpressed | −0.400 | 1.57E−04 | |||
| CRYAB;HSPB2 | 11q23.1 | Hypermethylated | Underexpressed | Underexpressed | NS | 0/4/7 | 0/0/11 | |
| PTHLH | 12p11.22 | Hypermethylated | Underexpressed | Underexpressed | NS | 1/0/10 | 0/0/11 | |
| HOXC13 | 12q13.13 | Hypermethylated | Overexpressed | 0.397 | 3.96E−04 | 0/1/10 | 0/0/11 | |
| PABPC3 | 13q12.13 | Hypermethylated | Overexpressed | 0.643 | 1.12E−06 | 0/4/7 | 0/0/11 | |
| TRAPPC6B | 14q21.1 | Hypermethylated | Overexpressed | 0.866 | 1.80E−05 | 0/2/9 | 0/0/11 | |
| BMP4 | 14q22.2 | Hypermethylated | Underexpressed | −0.379 | 0.001 | 0/3/8 | 0/0/11 | |
| BATF | 14q24.3 | Hypomethylated | Underexpressed | −0.502 | 0.002 | 0/4/7 | 0/0/11 | |
| ELL3 | 15q15.3 | Hypomethylated | Overexpressed | 0.432 | 0.009 | 0/0/11 | 0/0/11 | |
| SEPHS2 | 16p11.2 | Hypomethylated | Overexpressed | 0.495 | 0.003 | 0/0/11 | 0/0/11 | |
| SPN | 16p11.2 | Hypermethylated | Overexpressed | 0.254 | 0.064 | 0/0/11 | 0/0/11 | |
| SPAG9 | 17q21.33 | Hypermethylated | Overexpressed | Overexpressed | 0.628 | 0.000 | 1/0/10 | 0/0/11 |
| DHX40 | 17q23.1 | Hypermethylated | Overexpressed | 0.599 | 0.004 | 2/0/9 | 0/0/11 | |
| CCDC47 | 17q23.3 | Hypomethylated | Overexpressed | 0.671 | 0.001 | |||
| ICAM2 | 17q23.3 | Hypermethylated | Underexpressed | Underexpressed | −0.441 | 0.012 | 1/0/10 | 0/0/11 |
| CYGB | 17q25.1 | Hypermethylated | Underexpressed | −0.433 | 0.013 | 0/0/11 | 0/0/11 | |
| NFIX | 19p13.2 | Hypermethylated | Underexpressed | −0.289 | 0.023 | 0/0/11 | 0/0/11 | |
| NFIC | 19p13.3 | Hypermethylated | Underexpressed | NS | 0/1/10 | 0/0/11 | ||
| CACNG6 | 19q13.42 | Hypermethylated | Overexpressed | NS | 0/0/11 | 0/0/11 | ||
| PHGDH | 1p12 | Hypermethylated | Underexpressed | 0.313 | 0.002 | 0/0/11 | 0/0/11 | |
| CHI3L2 | 1p13.3 | Hypermethylated | Underexpressed | NS | 0/0/11 | 0/0/11 | ||
| COL11A1 | 1p21.1 | Hypermethylated | Overexpressed | NS | 0/1/10 | 0/0/11 | ||
| AGL | 1p21.2 | Hypomethylated | Overexpressed | Overexpressed | 0.337 | 0.013 | 0/1/10 | 0/0/11 |
| PODN | 1p32.3 | Hypermethylated | Underexpressed | −0.366 | 0.001 | 0/2/9 | 0/0/11 | |
| MMP23A;MMP23B | 1p36.33 | Hypermethylated | Underexpressed | −0.415 | 0.003 | 0/1/10 | 0/0/11 | |
| MMP23B | 1p36.33 | Hypermethylated | Underexpressed | −0.415 | 0.003 | 0/1/10 | 0/0/11 | |
| EXOC8 | 1q42.2 | Hypomethylated | Overexpressed | Overexpressed | 0.734 | 2.58E−05 | 1/1/9 | 0/0/11 |
| SYCP2 | 20q13.33 | Hypomethylated | Overexpressed | Overexpressed | 0.447 | 3.15E−05 | 2/0/9 | 0/0/11 |
| GREB1 | 2p25.1 | Hypomethylated | Overexpressed | −0.268 | 0.050 | 0/2/9 | 0/1/10 | |
| C2orf40 | 2q12.2 | Hypermethylated | Underexpressed | Underexpressed | −0.268 | 0.005 | ||
| SATB2 | 2q33.1 | Hypermethylated | Overexpressed | NS | 0/0/11 | 0/0/11 | ||
| KIF1A | 2q37.3 | Hypermethylated | Overexpressed | NS | 0/0/11 | 0/0/11 | ||
| TF | 3q22.1 | Hypermethylated | Underexpressed | NS | 0/1/10 | 0/0/11 | ||
| TAPT1 | 4p15.32 | Hypomethylated | Overexpressed | NS | ||||
| SORBS2 | 4q35.1 | Hypomethylated | Underexpressed | Underexpressed | −0.326 | 0.004 | ||
| PIK3R1 | 5q13.1 | Hypermethylated | Overexpressed | 0.536 | 3.96E−04 | 0/0/11 | 0/0/11 | |
| CARTPT | 5q13.2 | Hypermethylated | Underexpressed | NS | ||||
| PCSK1 | 5q15 | Hypermethylated | Underexpressed | −0.326 | 0.002 | 0/1/10 | 0/0/11 | |
| PAM | 5q21.1 | Hypermethylated | Underexpressed | Underexpressed | −0.340 | 0.024 | 0/0/11 | 0/0/11 |
| DMXL1 | 5q23.1 | Hypermethylated | Overexpressed | Overexpressed | 0.620 | 9.99E−06 | 0/0/11 | 0/0/11 |
| H2AFY | 5q31.1 | Hypermethylated | Overexpressed | Overexpressed | 0.831 | 3.15E−07 | 0/0/11 | 0/0/11 |
| DOCK2 | 5q35.1 | Hypomethylated | Underexpressed | NS | 0/0/11 | 0/0/11 | ||
| SCGB3A1 | 5q35.3 | Hypermethylated | Underexpressed | −0.240 | 0.001 | 0/0/11 | 0/0/11 | |
| USP49 | 6p21.1 | Hypermethylated | Overexpressed | 0.310 | 0.031 | 1/0/10 | 0/0/11 | |
| SCAND3 | 6p22.1 | Hypermethylated | Overexpressed | 0.492 | 0.015 | |||
| ID4 | 6p22.3 | Hypermethylated | Overexpressed | 0.492 | 1.95E−05 | 0/0/11 | 0/0/11 | |
| RARS2;ORC3L | 6q15 | Hypomethylated | Overexpressed | 0.822 | 1.85E−05 | 2/1/8 | 0/0/11 | |
| LRP11 | 6q25.1 | Hypomethylated | Overexpressed | 0.638 | 3.05E−04 | 0/1/10 | 0/0/11 | |
| C7orf28A | 7p22.1 | Hypomethylated | Overexpressed | 0.775 | 1.85E−05 | 0/0/11 | 0/0/11 | |
| LFNG | 7p22.3 | Hypermethylated | Underexpressed | −0.668 | 4.55E−06 | 0/0/11 | 0/0/11 | |
| PON3 | 7q21.3 | Hypermethylated | Underexpressed | Underexpressed | −0.305 | 3.19E−05 | 1/0/10 | 0/0/11 |
| NRCAM | 7q31.1 | Hypomethylated | Overexpressed | 0.336 | 0.024 | 0/0/11 | 0/0/11 | |
| NDUFA5 | 7q31.32 | Hypermethylated | Overexpressed | 0.771 | 1.48E−04 | 0/1/10 | 0/0/11 | |
| LEP | 7q32.1 | Hypermethylated | Overexpressed | 0.357 | 0.003 | 0/0/11 | 0/0/11 | |
| RARRES2 | 7q36.1 | Hypermethylated | Underexpressed | −0.272 | 0.020 | 0/0/11 | 0/0/11 | |
| BRF2 | 8p11.23 | Hypermethylated | Overexpressed | Overexpressed | NS | 7/2/2 | 0/0/11 | |
| IMPAD1 | 8q12.1 | Hypomethylated | Overexpressed | Overexpressed | 1.067 | 2.70E−07 | ||
| FABP5 | 8q21.13 | Hypermethylated | Overexpressed | NS | 1/0/10 | 0/0/11 | ||
| PLEKHF2 | 8q22.1 | Hypomethylated | Overexpressed | Overexpressed | 0.393 | 4.47E−04 | 3/0/8 | 0/0/11 |
| VPS13B | 8q22.2 | Hypomethylated | Overexpressed | 0.878 | 7.06E−06 | |||
| RRM2B | 8q22.3 | Hypomethylated | Overexpressed | Overexpressed | 0.452 | 0.001 | 3/0/8 | 0/0/11 |
| TRPS1 | 8q23.3 | Hypermethylated | Overexpressed | Overexpressed | 0.320 | 0.008 | 6/0/5 | 0/0/11 |
| TRPS1 | 8q23.3 | Hypomethylated | Overexpressed | Overexpressed | 0.320 | 0.008 | 6/0/5 | 0/0/11 |
| ENPP2 | 8q24.12 | Hypermethylated | Underexpressed | Underexpressed | −0.258 | 0.017 | 2/0/9 | 0/0/11 |
| TAF2 | 8q24.12 | Hypomethylated | Overexpressed | 1.164 | 1.34E−06 | 2/0/9 | 0/0/11 | |
| SQLE | 8q24.13 | Hypomethylated | Overexpressed | Overexpressed | 0.566 | 2.95E−07 | 2/0/9 | 0/0/11 |
| TATDN1 | 8q24.13 | Hypomethylated | Overexpressed | Overexpressed | 0.783 | 2.89E−06 | 5/0/6 | 0/0/11 |
| NDRG1 | 8q24.22 | Hypomethylated | Overexpressed | 0.843 | 5.03E−11 | 2/1/8 | 0/0/11 | |
| WDR44 | Xq24 | Hypomethylated | Overexpressed | 0.937 | 4.75E−06 | 3/0/8 | 0/0/11 |
Abbreviation: NS, not statistically significant.
Genes not correlating between DNA methylation and transcriptional patterns are shown in bold text.
Delta beta value (8p11-p12-amplified tumors versus nonamplified tumors) >0.14 are indicated by hypermethylation and <−0.14 are indicated by hypomethylation; Bonferroni adjusted P-value P<0.05.
Gene expression microarray log2 ratio for the 22 tumors (8p11–p12-amplified tumors versus nonamplified tumors) >0.58 are indicated by overexpression and <−0.58 are indicated by underexpression.
Gene expression microarray log2 ratio for the 150 tumors (8p11-p12-amplified tumors versus nonamplified tumors) > 0.58 are indicated by overexpression and < −0.58 are indicated by underexpression.
Univariate Cox proportional hazard regression models using the gene expression data for the 150 tumors and overall survival rates.
Array-CGH log2 ratio thresholds set at ⩾+0.5, −0.2 and between +0.5 and −0.2 for amplification, loss and normal copy number, respectively.
Array-CGH log2 ratio thresholds set at ⩾+0.5, –0.2 and between +0.5 and −0.2 for amplification, loss and normal copy number, respectively.
Table 3. Significantly enriched Gene Ontology (GO) terms identified by integrated DNA methylation and expression profiling in 8p11-p12-amplified breast tumors.
| Category | GO term | P-value | Gene count |
|---|---|---|---|
| Biological process | |||
| GO:0032099 | Negative regulation of appetite | 8.32E−05 | 2 |
| GO:0045671 | Negative regulation of osteoclast differentiation | 2.48E−04 | 2 |
| GO:0045060 | Negative thymic T-cell selection | 2.48E−04 | 2 |
| GO:0008343 | Adult feeding behavior | 4.93E−04 | 2 |
| GO:0006935 | Chemotaxis | 0.00203 | 4 |
| GO:0007281 | Germ cell development | 0.002871 | 2 |
| GO:0006260 | DNA replication | 0.003236 | 4 |
| GO:0007420 | Brain development | 0.003323 | 3 |
| GO:0030335 | Positive regulation of cell migration | 0.007046 | 2 |
| GO:0006366 | Transcription from RNA polymerase II promoter | 0.014531 | 3 |
| GO:0016337 | Cell-cell adhesion | 0.030651 | 2 |
| GO:0008544 | Epidermis development | 0.042861 | 2 |
| Molecular function | |||
| GO:0043169 | Cation binding | 0.011569 | 2 |
| GO:0008083 | Growth factor activity | 0.013173 | 3 |
| GO:0001104 | RNA polymerase II transcription factor activity | 0.013842 | 3 |
| GO:0005179 | Hormone activity | 0.021768 | 2 |
| GO:0004252 | Serine-type endopeptidase activity | 0.030247 | 3 |
| GO:0006351 | Transcription regulator activity | 0.038615 | 2 |
| Cellular component | |||
| GO:0005615 | Extracellular space | 3.46E−05 | 10 |
| GO:0005576 | Extracellular region | 2.89E−04 | 17 |
| GO:0043005 | Neuron projection | 0.002871 | 2 |
| GO:0009897 | External side of plasma membrane | 0.079513 | 2 |
| GO:0005634 | Nucleus | 0.081344 | 22 |
| GO:0005794 | Golgi apparatus | 0.08346 | 6 |
| GO:0005768 | Endosome | 0.096152 | 2 |
| GO:0005578 | Proteinaceous extracellular matrix | 0.107112 | 3 |
| GO:0005737 | Cytoplasm | 0.33593 | 19 |
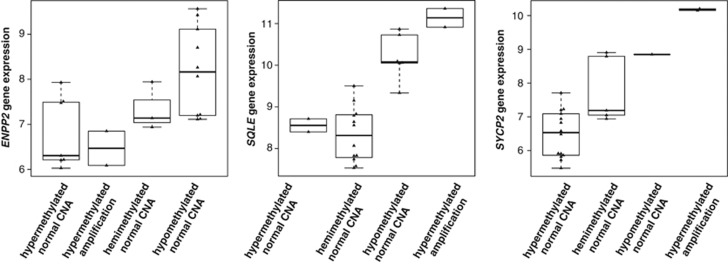
Interestingly, 11 genes spanning the 8q12.1-q24.22 genomic region were differentially methylated and expressed, of which nine genes (IMPAD1, NDRG1, PLEKHF2, RRM2B, SQLE, TAF2, TATDN1, TRPS1, VPS13B) were hypomethylated and overexpressed, the ENPP2 gene was hypermethylated and underexpressed and the FABP5 gene was hypermethylated but overexpressed. As the 11 genes were also coamplified with the 8p11-p12 region in at least one tumor specimen, we examined whether DNA copy number, DNA methylation or both had an impact on gene expression (Figure 3). We found that hypomethylation alone frequently enhanced gene expression patterns. However, hypomethylation and DNA amplification of the same transcript further enhanced expression levels. These findings suggest that genes in the 8q region are frequently targeted by more than one mechanism for activation in breast tumors harboring 8p11-p12 amplification. Consequently, ENPP2 was the only example showing lower expression levels when hypermethylated (at four different cytosine sites in the promoter region) despite amplification of the gene in 2/11 samples harboring the 8p11-p12 amplicon. These results indicate that aberrant methylation patterns may be a secondary event to further lock genes in their inactive or active states only after they have already been silenced or activated by other means.7, 8, 9, 10 The ENPP2 gene was an exception to this phenomenon because hypermethylation occurred at four different cytosine sites in the promoter region of the gene, resulting in lower transcriptional levels despite amplification of the gene in 2/11 samples harboring the 8p11-p12 amplicon. However, 8/11 genes (FABP5, NDRG1, PLEKHF2, RRM2B, SQLE, TAF2, TATDN1, TRPS1) may not be distinctive of 8p11-p12 amplification as they were also differentially regulated in MYC coamplified tumors.
Figure 3.
The effect of aberrant DNA copy number and DNA methylation on gene expression. Box plots showing the relationship between DNA copy number (CNA), methylation status and gene expression for three candidate genes (ENPP2, SQLE and SYCP2) in 22 tumor samples. X-axis, methylation and CNA status; Y-axis, gene expression signal intensity.
Several of the aberrantly-methylated genes spanning the 8q arm have been previously associated with cancer-related processes. In addition to gene amplification shown in the present study, VPS13B frameshift mutations have also been identified in gastric and colorectal cancers, as well as TRPS1-LASP1, PLEC1-ENPP2 and TATDN1-GSDMB fusion genes in breast carcinoma.34, 35, 36 Recently, gain of TRPS1, TATDN1 and SQLE DNA copy numbers in estrogen receptor-positive, ERBB2-amplified breast tumors have been reported, and elevated SQLE levels were associated with distant metastasis-free survival.37, 38, 39 TRPS1, a transcription factor that belongs to the GATA gene family, in which, protein expression is inhibited by androgens via the androgen receptor in prostate cancer and demonstrates high expression levels in both normal breast and tumor tissue. In breast tumors, TRPS1 expression is associated with ER, PgR, GATA3, HER2/neu expression and favorable clinical outcome.40, 41 Elevated NDRG1 protein levels have been associated with shorter disease-free and overall survival, cell differentiation and breast cancer progression.42, 43, 44 In contrast, Han et al.45 demonstrated that NDRG1 methylation in breast cancer is associated with a more aggressive phenotype. Interestingly, substantial NDRG1 phosphorylation is found in Akt inhibitor-resistant breast cancer cell lines, which can be reversed by the mTORC1/2 inhibitor, MLN0128, in breast cancer xenograft models.46, 47 p53-mediated induction of DNA damage-associated genes, such as RRM2B, can promote resistance of cancer cells to genotoxic therapy, which can be prevented by inhibiting histone deacetylases that can in turn inhibit ataxia telangiectasia-mutated kinase and p53 activation and their downstream targets.48, 49, 50 The TAF2 gene is involved in general transcription processes and is the DNA binding component of the transcription factor II D transcription factor complex.51
In summary, we have identified the enrichment of MYC amplification and hypomethylation of genes on cytoband 8q in 8p11-p12-amplified tumors. These findings indicate that the aggressive phenotype observed in invasive breast tumors harboring the 8p11-p12 amplicon may not only be a consequence of altered activity of amplified genes in the genomic region, but also a result of MYC coamplification and aberrant DNA methylation patterns on chromosome 8q.
Materials and methods
Tumor specimens
Primary invasive breast carcinoma specimens (n=229) corresponding to 185 patients diagnosed from 1988–1999 were obtained from the fresh-frozen tumor bank at the Sahlgrenska University Hospital Oncology Lab in accordance with the Declaration of Helsinki and approved by the Medical Faculty Research Ethics Committee (Gothenburg, Sweden). The 229 cases were compiled from three independent array-comparative genomic hybridization microarray datasets, including two published (138/229 tumors) and one unpublished (91/229 tumors) studies.23, 24 The clinicopathological features of the 229 cases are shown in Table 1. Each tumor specimen was assessed for the presence of malignant cells using May–Grünwald Giemsa staining (Chemicon International, Temecula, CA, USA) on touch preparations. Highly representative specimens containing >70% neoplastic cell content were included in the microarray and fluorescence in situ hybridization analyses.
Genomic and transcriptome profiling
Genomic profiling of the tumor specimens was performed using whole-genome tiling 38K array-comparative genomic hybridization microarrays, as previously described.23, 24 Data preprocessing, normalization and data analysis were performed as previously described using log2 ratio thresholds set at +0.2, ⩾+0.5, −0.2 and ⩽−1.0 for low-level gain, high-level gain/amplification, heterozygous loss and homozygous deletion (henceforth referred to as gain, amplification, loss and deletion), respectively.24 Total RNA samples from 150/229 tumor specimens were isolated and profiled using Illumina HumanHT-12 Beadchips (Illumina Inc.) as previously described.24 Enriched gene ontology terms associated with differentially regulated genes were set to P<0.05, analyzed further using the gene ontology database (http://www.geneontology.org). The dataset was stratified into the molecular breast cancer subtypes using the five centroids (normal-like, basal-like, luminal subtype A, luminal subtype B and human epidermal growth factor receptor 2/estrogen receptor-negative (HER2/ER−)) and genomic grade index (high, low), as previously described.52, 53, 54 Luminal subtype B was further stratified according HER2 status as determined by array-comparative genomic hybridization; HER2+ was set to log2 ratio ⩾+0.5 and HER2− was set to log2 ratio <+0.5.55 Univariate Cox proportional hazard models were calculated for statistically significant genes using overall survival.
Fluorescence in situ hybridization
Probe labeling and hybridization were performed as described elsewhere56 using locus-specific bacterial artificial chromosome (BAC; BACPAC Resources, Oakland, CA, USA) probes to verify gene amplification. Dual-color fluorescence in situ hybridization was performed on touchprint and metaphase preparations using cohybridized biotin-16-deoxyuridine triphosphate (dUTP) and dioxigenin-11-dUTP-labeled probes. Analysis was performed using a Leica DMRA2 fluorescent microscope (Leica, Wetzler, Germany) equipped with an ORCA Hamamatsu CCD (charged-couple devices) camera (Hamamatsu City, Japan) and filter cubes specific for fluorescein isothiocyanate, Rhodamine and ultraviolet for DAPI visualization. Digitalized black and white images were acquired using the Leica CW4000 software package (Leica).
DNA methylation profiling
In total, 22/229 tumor samples harboring (n=11) or lacking the 8p11-p12 amplicon (n=11) were profiled using Illumina Infinium Human Methylation 450 Beadchips (Illumina Inc) according to the manufacturer's instructions. The estimated methylation level for specific cytosine sites (average beta) was calculated as a ratio between the intensities of methylated and unmethylated alleles and ranged from 0 (null methylated) to 1 (completely methylated). Delta beta values were calculated using (average beta values8p11–p12-amplified tumors–average beta values8p11-p12 nonamplified tumors). Cytosine sites located on the Y chromosome or containing single-nucleotide polymorphisms were removed. Differential DNA methylation was determined using the IMA package in R/Bioconductor (Bioconductor, FHCRC, Seattle, WA, USA) with thresholds set at: ⩾±0.14 delta beta value and Bonferroni adjusted at P<0.05.57
Acknowledgments
This work was supported by grants from the Swedish Cancer Society (KH), King Gustav V Jubilee Clinic Cancer Research Foundation (KH), the Wilhelm and Martina Lundgren Research Foundation (TZP), Serena Ehrenström Foundation for Cancer Research/Torsten and Sara Jansson Research Foundation (TZP), Assar Gabrielsson Research Foundation for Clinical Cancer Research (TZP) and Lars Hierta's Memorial Research Foundation (TZP).
The authors declare no conflict of interest.
References
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Baylin SB. The cancer epigenome: its origins, contributions to tumorigenesis, and translational implications. Proc Am Thorac Soc. 2012;9:64–65. doi: 10.1513/pats.201201-001MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteller M. Aberrant DNA methylation as a cancer-inducing mechanism. Annu Rev Pharmacol Toxicol. 2005;45:629–656. doi: 10.1146/annurev.pharmtox.45.120403.095832. [DOI] [PubMed] [Google Scholar]
- Gal-Yam EN, Saito Y, Egger G, Jones PA. Cancer epigenetics: modifications, screening, and therapy. Annu Rev Med. 2008;59:267–280. doi: 10.1146/annurev.med.59.061606.095816. [DOI] [PubMed] [Google Scholar]
- Huang Y, Nayak S, Jankowitz R, Davidson NE, Oesterreich S. Epigenetics in breast cancer: what's new. Breast Cancer Res. 2011;13:225. doi: 10.1186/bcr2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund AH, van Lohuizen M. Epigenetics and cancer. Genes Dev. 2004;18:2315–2335. doi: 10.1101/gad.1232504. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Costello JF, Plass C. Methylation matters. J Med Genet. 2001;38:285–303. doi: 10.1136/jmg.38.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuks F. DNA methylation and histone modifications: teaming up to silence genes. Curr Opin Genet Dev. 2005;15:490–495. doi: 10.1016/j.gde.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Heard E, Disteche CM. Dosage compensation in mammals: fine-tuning the expression of the X chromosome. Genes Dev. 2006;20:1848–1867. doi: 10.1101/gad.1422906. [DOI] [PubMed] [Google Scholar]
- Adelaide J, Chaffanet M, Imbert A, Allione F, Geneix J, Popovici C, et al. Chromosome region 8p11-p21: refined mapping and molecular alterations in breast cancer. Genes Chromosomes Cancer. 1998;22:186–199. doi: 10.1002/(sici)1098-2264(199807)22:3<186::aid-gcc4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Theillet C, Adelaide J, Louason G, Bonnet-Dorion F, Jacquemier J, Adnane J, et al. FGFRI and PLAT genes and DNA amplification at 8p12 in breast and ovarian cancers. Genes Chromosomes Cancer. 1993;7:219–226. doi: 10.1002/gcc.2870070407. [DOI] [PubMed] [Google Scholar]
- Thor AD, Eng C, Devries S, Paterakos M, Watkin WG, Edgerton S, et al. Invasive micropapillary carcinoma of the breast is associated with chromosome 8 abnormalities detected by comparative genomic hybridization. Hum Pathol. 2002;33:628–631. doi: 10.1053/hupa.2002.124034. [DOI] [PubMed] [Google Scholar]
- Roque L, Rodrigues R, Martins C, Ribeiro C, Ribeiro MJ, Martins AG, et al. Comparative genomic hybridization analysis of a pleuropulmonary blastoma. Cancer Genet Cytogenet. 2004;149:58–62. doi: 10.1016/S0165-4608(03)00284-X. [DOI] [PubMed] [Google Scholar]
- Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG, et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet. 2009;41:1238–1242. doi: 10.1038/ng.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelens MC, Kok K, van der Vlies P, van der Vries G, Sietsma H, Timens W, et al. Genomic aberrations in squamous cell lung carcinoma related to lymph node or distant metastasis. Lung cancer. 2009;66:372–378. doi: 10.1016/j.lungcan.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Mahmood SF, Gruel N, Nicolle R, Chapeaublanc E, Delattre O, Radvanyi F, et al. PPAPDC1B and WHSC1L1 are common drivers of the 8p11-12 amplicon, not only in breast tumors but also in pancreatic adenocarcinomas and lung tumors. Am J Pathol. 2013;183:1634–1644. doi: 10.1016/j.ajpath.2013.07.028. [DOI] [PubMed] [Google Scholar]
- Tonon G, Wong KK, Maulik G, Brennan C, Feng B, Zhang Y, et al. High-resolution genomic profiles of human lung cancer. Proc Natl Acad Sci USA. 2005;102:9625–9630. doi: 10.1073/pnas.0504126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon R, Richter J, Wagner U, Fijan A, Bruderer J, Schmid U, et al. High-throughput tissue microarray analysis of 3p25 (RAF1) and 8p12 (FGFR1) copy number alterations in urinary bladder cancer. Cancer Res. 2001;61:4514–4519. [PubMed] [Google Scholar]
- Williams SV, Platt FM, Hurst CD, Aveyard JS, Taylor CF, Pole JC, et al. High-resolution analysis of genomic alteration on chromosome arm 8p in urothelial carcinoma. Genes Chromosomes Cancer. 2010;49:642–659. doi: 10.1002/gcc.20775. [DOI] [PubMed] [Google Scholar]
- Knuutila S, Bjorkqvist AM, Autio K, Tarkkanen M, Wolf M, Monni O, et al. DNA copy number amplifications in human neoplasms: review of comparative genomic hybridization studies. Am J Pathol. 1998;152:1107–1123. [PMC free article] [PubMed] [Google Scholar]
- Paterson AL, Pole JC, Blood KA, Garcia MJ, Cooke SL, Teschendorff AE, et al. Co-amplification of 8p12 and 11q13 in breast cancers is not the result of a single genomic event. Genes Chromosomes Cancer. 2007;46:427–439. doi: 10.1002/gcc.20424. [DOI] [PubMed] [Google Scholar]
- Möllerstrom E, Delle U, Danielsson A, Parris T, Olsson B, Karlsson P, et al. High-resolution genomic profiling to predict 10-year overall survival in node-negative breast cancer. Cancer Genet Cytogenet. 2010;198:79–89. doi: 10.1016/j.cancergencyto.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Parris TZ, Danielsson A, Nemes S, Kovacs A, Delle U, Fallenius G, et al. Clinical implications of gene dosage and gene expression patterns in diploid breast carcinoma. Clin Cancer Res. 2010;16:3860–3874. doi: 10.1158/1078-0432.CCR-10-0889. [DOI] [PubMed] [Google Scholar]
- Kwek SS, Roy R, Zhou H, Climent J, Martinez-Climent JA, Fridlyand J, et al. Co-amplified genes at 8p12 and 11q13 in breast tumors cooperate with two major pathways in oncogenesis. Oncogene. 2009;28:1892–1903. doi: 10.1038/onc.2009.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelsi-Boyer V, Orsetti B, Cervera N, Finetti P, Sircoulomb F, Rouge C, et al. Comprehensive profiling of 8p11-12 amplification in breast cancer. Mol Cancer Res. 2005;3:655–667. doi: 10.1158/1541-7786.MCR-05-0128. [DOI] [PubMed] [Google Scholar]
- Holland DG, Burleigh A, Git A, Goldgraben MA, Perez-Mancera PA, Chin SF, et al. ZNF703 is a common Luminal B breast cancer oncogene that differentially regulates luminal and basal progenitors in human mammary epithelium. EMBO Mol Med. 2011;3:167–180. doi: 10.1002/emmm.201100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircoulomb F, Nicolas N, Ferrari A, Finetti P, Bekhouche I, Rousselet E, et al. ZNF703 gene amplification at 8p12 specifies luminal B breast cancer. EMBO Mol Med. 2011;3:153–166. doi: 10.1002/emmm.201100121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA, et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res. 2010;70:2085–2094. doi: 10.1158/0008-5472.CAN-09-3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Mu X, Huang O, Xie Z, Jiang M, Geng M, et al. Luminal breast cancer cell lines overexpressing ZNF703 are resistant to tamoxifen through activation of Akt/mTOR signaling. PLoS One. 2013;8:e72053. doi: 10.1371/journal.pone.0072053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard-Pierrot I, Gruel N, Stransky N, Vincent-Salomon A, Reyal F, Raynal V, et al. Characterization of the recurrent 8p11-12 amplicon identifies PPAPDC1B, a phosphatase protein, as a new therapeutic target in breast cancer. Cancer Res. 2008;68:7165–7175. doi: 10.1158/0008-5472.CAN-08-1360. [DOI] [PubMed] [Google Scholar]
- Yang ZQ, Liu G, Bollig-Fischer A, Giroux CN, Ethier SP. Transforming properties of 8p11-12 amplified genes in human breast cancer. Cancer Res. 2010;70:8487–8497. doi: 10.1158/0008-5472.CAN-10-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZQ, Streicher KL, Ray ME, Abrams J, Ethier SP. Multiple interacting oncogenes on the 8p11-p12 amplicon in human breast cancer. Cancer Res. 2006;66:11632–11643. doi: 10.1158/0008-5472.CAN-06-2946. [DOI] [PubMed] [Google Scholar]
- An CH, Kim YR, Kim HS, Kim SS, Yoo NJ, Lee SH. Frameshift mutations of vacuolar protein sorting genes in gastric and colorectal cancers with microsatellite instability. Hum Pathol. 2012;43:40–47. doi: 10.1016/j.humpath.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Edgren H, Murumagi A, Kangaspeska S, Nicorici D, Hongisto V, Kleivi K, et al. Identification of fusion genes in breast cancer by paired-end RNA-sequencing. Genome Biol. 2011;12:R6. doi: 10.1186/gb-2011-12-1-r6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte I, Batty EM, Pole JC, Blood KA, Mo S, Cooke SL, et al. Structural analysis of the genome of breast cancer cell line ZR-75-30 identifies twelve expressed fusion genes. BMC Genomics. 2012;13:719. doi: 10.1186/1471-2164-13-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin SF, Teschendorff AE, Marioni JC, Wang Y, Barbosa-Morais NL, Thorne NP, et al. High-resolution aCGH and expression profiling identifies a novel genomic subtype of ER negative breast cancer. Genome Biol. 2007;8:R215. doi: 10.1186/gb-2007-8-10-r215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helms MW, Kemming D, Pospisil H, Vogt U, Buerger H, Korsching E, et al. Squalene epoxidase, located on chromosome 8q24.1, is upregulated in 8q+ breast cancer and indicates poor clinical outcome in stage I and II disease. Br J Cancer. 2008;99:774–780. doi: 10.1038/sj.bjc.6604556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircoulomb F, Bekhouche I, Finetti P, Adelaide J, Ben Hamida A, Bonansea J, et al. Genome profiling of ERBB2-amplified breast cancers. BMC Cancer. 2010;10:539. doi: 10.1186/1471-2407-10-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GT, Jhamai M, van Weerden WM, Jenster G, Brinkmann AO. The TRPS1 transcription factor: androgenic regulation in prostate cancer and high expression in breast cancer. Endocr Relat Cancer. 2004;11:815–822. doi: 10.1677/erc.1.00853. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Bao Y, Litton J, Xiao L, Zhang HZ, Warneke CL, et al. Expression and relevance of TRPS-1: a new GATA transcription factor in breast cancer. Horm Cancer. 2011;2:132–143. doi: 10.1007/s12672-011-0067-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotovati A, Abu-Ali S, Kage M, Shirouzu K, Yamana H, Kuwano M. N-myc downstream-regulated gene 1 (NDRG1) a differentiation marker of human breast cancer. Pathol Oncol Res. 2011;17:525–533. doi: 10.1007/s12253-010-9342-y. [DOI] [PubMed] [Google Scholar]
- Mao XY, Fan CF, Wei J, Liu C, Zheng HC, Yao F, et al. Increased N-myc downstream-regulated gene 1 expression is associated with breast atypia-to-carcinoma progression. Tumour Biol. 2011;32:1271–1276. doi: 10.1007/s13277-011-0232-z. [DOI] [PubMed] [Google Scholar]
- Nagai MA, Gerhard R, Fregnani JH, Nonogaki S, Rierger RB, Netto MM, et al. Prognostic value of NDRG1 and SPARC protein expression in breast cancer patients. Breast Cancer Res Treat. 2011;126:1–14. doi: 10.1007/s10549-010-0867-2. [DOI] [PubMed] [Google Scholar]
- Han LL, Hou L, Zhou MJ, Ma ZL, Lin DL, Wu L, et al. Aberrant NDRG1 methylation associated with its decreased expression and clinicopathological significance in breast cancer. J Biomed Sci. 2013;20:52. doi: 10.1186/1423-0127-20-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokmen-Polar Y, Liu Y, Toroni RA, Sanders KL, Mehta R, Badve S, et al. Investigational drug MLN0128, a novel TORC1/2 inhibitor, demonstrates potent oral antitumor activity in human breast cancer xenograft models. Breast Cancer Res Treat. 2012;136:673–682. doi: 10.1007/s10549-012-2298-8. [DOI] [PubMed] [Google Scholar]
- Sommer EM, Dry H, Cross D, Guichard S, Davies BR, Alessi DR. Elevated SGK1 predicts resistance of breast cancer cells to Akt inhibitors. Biochem J. 2013;452:499–508. doi: 10.1042/BJ20130342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson C, Omolo B, Chu H, Zhou Y, Sambade MJ, Peters EC, et al. A prognostic signature of defective p53-dependent G1 checkpoint function in melanoma cell lines. Pigment Cell Melanoma Res. 2012;25:514–526. doi: 10.1111/j.1755-148X.2012.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo ML, Sy AJ, Xue L, Chi M, Lee MT, Yen T, et al. RRM2B suppresses activation of the oxidative stress pathway and is up-regulated by p53 during senescence. Sci Rep. 2012;2:822. doi: 10.1038/srep00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurn KT, Thomas S, Raha P, Qureshi I, Munster PN. Histone deacetylase regulation of ATM-mediated DNA damage signaling. Mol Cancer Ther. 2013;12:2078–2087. doi: 10.1158/1535-7163.MCT-12-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkowska M, Kokoszynska K, Rychlewski L, Wyrwicz L. Structural bioinformatics of the general transcription factor TFIID. Biochimie. 2013;95:680–691. doi: 10.1016/j.biochi.2012.10.024. [DOI] [PubMed] [Google Scholar]
- Hu Z, Fan C, Oh DS, Marron JS, He X, Qaqish BF, et al. The molecular portraits of breast tumors are conserved across microarray platforms. BMC Genomics. 2006;7:96. doi: 10.1186/1471-2164-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi S, Haibe-Kains B, Desmedt C, Lallemand F, Tutt AM, Gillet C, et al. Definition of clinically distinct molecular subtypes in estrogen receptor-positive breast carcinomas through genomic grade. J Clin Oncol. 2007;25:1239–1246. doi: 10.1200/JCO.2006.07.1522. [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Wirapati P, Loi S, Harris A, Fox S, Smeds J, et al. Gene expression profiling in breast cancer: understanding the molecular basis of histologic grade to improve prognosis. J Natl Cancer Inst. 2006;98:262–272. doi: 10.1093/jnci/djj052. [DOI] [PubMed] [Google Scholar]
- Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thurlimann B, Senn HJ. Strategies for subtypes—dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helou K, Wallenius V, Qiu Y, Ohman F, Stahl F, Klinga-Levan K, et al. Amplification and overexpression of the hepatocyte growth factor receptor (HGFR/MET) in rat DMBA sarcomas. Oncogene. 1999;18:3226–3234. doi: 10.1038/sj.onc.1202658. [DOI] [PubMed] [Google Scholar]
- Wang D, Yan L, Hu Q, Sucheston LE, Higgins MJ, Ambrosone CB, et al. IMA: an R package for high-throughput analysis of Illumina's 450K Infinium methylation data. Bioinformatics. 2012;28:729–730. doi: 10.1093/bioinformatics/bts013. [DOI] [PMC free article] [PubMed] [Google Scholar]