Abstract
Despite progresses in diagnosis and treatment, pancreatic cancer continues to have the worst prognosis of all solid malignant tumors. Recent evidences suggest that the metastasis-promoting protein S100P stimulates pancreatic tumor proliferation, survival, invasion and metastasis progression through extracellular functions. Moreover, its expression is strongly correlated with poor prognosis in patients with several types of cancer although the entire molecular mechanism responsible for the diverse biological functions is not fully understood. We showed that extracellular S100P stimulates pancreatic carcinoma BxPC3 cell line by promoting cell proliferation. We also demonstrated that S100P induces, in this cell line, the phosphorylation of IκBα and the secretion of matrix metalloproteinase 9 (MMP-9). In addition, treatment with S100P protected cells from injuries induced by the cytotoxic agent Gemcitabine. On the basis of these results, we developed function-blocking anti-S100P monoclonal antibodies (mAbs) that abolished all of its in vitro activities. Furthermore, in vivo treatment with the candidate 2H8 antibody decreased tumor growth and liver metastasis formation in a subcutaneous and orthotopic BxPC3 tumor model. We conclude here that a therapeutic strategy blocking the extracellular activity of S100P by means of specific mAbs could be an attractive therapeutic approach as a single agent or in combination with target-directed or chemotherapeutic drugs to treat pancreatic cancer.
Keywords: S100P, monoclonal antibody, therapy, metastasis, pancreatic cancer, diagnosis
Introduction
Pancreatic adenocarcinoma is one of the most lethal human cancers with a 5-year survival of less than 5%.1 Because of the difficulty in the detection at an early stage, the majority of tumors are either locally advanced or metastatic upon diagnosis. Pancreatic tumors show a high aggressive nature invading mainly regional lymph nodes and liver, and less often the lungs and visceral organs.2 This high metastatic potential associated with a strong chemoresistance, turns pancreatic carcinoma into one of the most deadly cancer types. In the last two decades, strategies including surgery, radiation and chemotherapy, have failed to improve long-term survival.3 The current standard of treatment, the nucleoside analog Gemcitabine, prolongs survival by only several months. Thus, recent efforts have focused on the application of novel targeted agents based on a better understanding of the mechanisms involved in tumor progression.
Recent studies shown that S100P, a 95-aminoacid protein member of the S100 family of EF-hand calcium-binding proteins, promoted cancer progression via specific roles in survival, cell proliferation, angiogenesis and metastasis,4 thus demonstrating that this protein could be a promising therapeutic candidate in pancreatic adenocarcinoma. In addition, overexpression of S100P in different types of cancer, including breast,5 colon,6 prostate,7 lung,8 gastric9 and cholangiocarcinoma,10 when compared with their matched normal tissues, correlates with poor prognosis in cancer patients.
Other studies also suggested an important role of S100P in the acquisition of chemoresistance. In vitro data shown that S100P is functionally important for pancreatic cancer cell resistance to 5-fluoracil treatment.11 In vivo, S100P has been shown to have a specific role in resistance to bifunctional alkylating agents, doxorubicin,6 5-fluoracil12 and cisplatin,13 analyzing data from microarray studies.
Some S100 proteins can be released by different cellular tumor components and exert regulatory effects on several cell types acting through the Receptor for Advanced Glycation End products (RAGE) via MAPkinase and NFκB pathways.14, 15 Arumugam et al.16 demonstrated that the small molecule Cromolyn can bind to S100P and block the interaction with the receptor. Treatments with Cromolyn in combination with Gemcitabine inhibited tumor growth and metastasis in mouse models of human pancreatic cancer. Even though, many of the biological effects have been described, the mechanisms by which extracellular S100P exerts these effects are not completely understood.
In the current study, we focused on the development of monoclonal antibodies (mAbs) against S100P, directed to block both in vitro and in vivo activities. Here, we demonstrated that S100P has an important role in tumor growth and liver metastases formation by using xenograft tumor models derived from BxPC3 cell line and we were able to abrogate these effects treating animals with specific anti-S100P mAbs. Furthermore, antibodies increased the sensitivity of BxPC3 when the cells were exposed to Gemcitabine and S100P combination. Finally, we have generated tools for detecting S100P protein as a biomarker in plasma and in tumor samples.
In conclusion, therapeutic combinations between S100P mAbs and target-directed or chemotherapeutics drugs could be good approaches for pancreatic cancer treatment.
Results
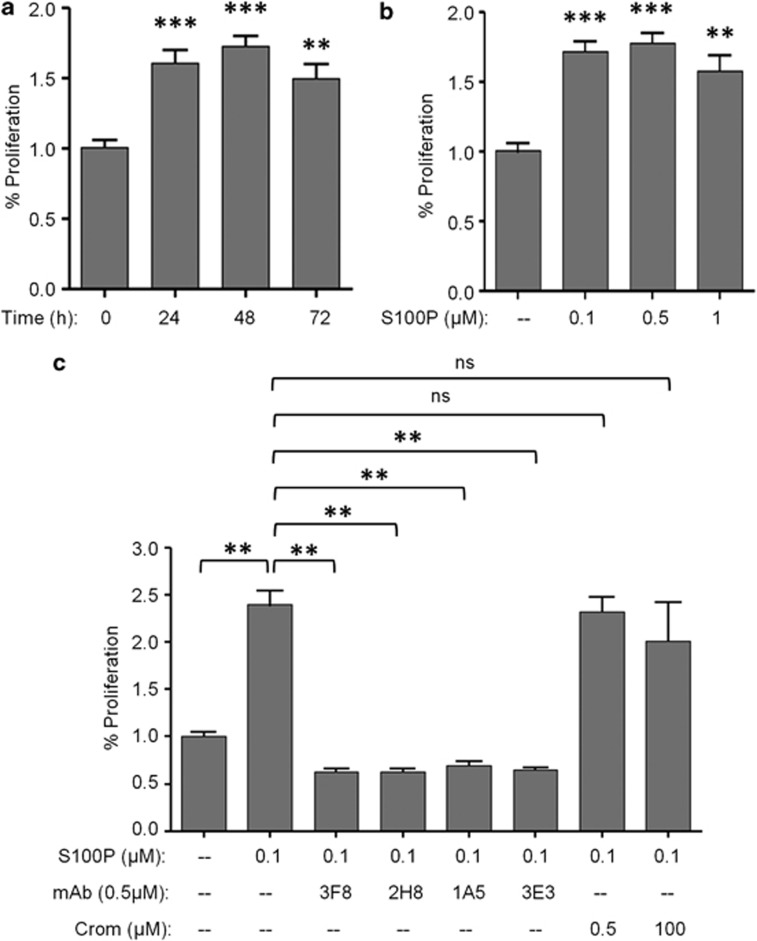
mAbs against S100P block the induction of cell proliferation mediated by extracellular S100P
Previous studies have shown that S100P stimulates pancreatic cancer cell growth in vitro.11 To further extend these studies and to find more effective and specific inhibitors, we focused our work on obtaining neutralizing mAbs against S100P. To determine whether the inhibition of the extracellular function of S100P affected the proliferation of tumor cells, we tested the effect of several mAbs on BrdU incorporation by using the BxPC3 cell line. S100P was tested in a time and in a dose-dependent range, exhibiting a significant increased proliferation activity compared with non-treated cells. In particular, when 100 nM of recombinant S100P was added to the cells, proliferation was increased 1.7-fold compared with those observed in unstimulated cells (Figures 1a and b). We next investigated the in vitro neutralizing activity of mAbs, compared with the effect of Cromolyn. We used Cromolyn at the same concentration than the antibodies or at the most referenced concentration (100 μM) in each in vitro assay. To evaluate this approach, 100 nM of S100P was incubated with 500 nM of each mAb or Cromolyn for 2 h prior to the addition to the cells. After 48 h of incubation, treatments with the antibodies abolished the increase in cell proliferation induced by S100P (Figure 1c). This neutralizing activity was statistically significant, whereas Cromolyn did not show any neutralizing effect.
Figure 1.
Neutralizing effect of anti-S100P mAbs in S100P-induced proliferation. (a) Increased time-dependent proliferation induced by S100P protein (100 nM) in BxPC3 cell line. (b) Dose-dependent response of S100P in BxPC3 proliferation. Cells were treated with S100P (0.1–0.5–1 μM) for 48 h. (c) Neutralizing activity of mAbs (3F8, 2H8, 1A5 ad 3E3) at 500 nM on BxPC3 proliferation assay. Cromolyn (0.5 and 100 μM) was used as a referenced blocking product of the extracellular activity of S100P. Before stimulation, antibodies and Cromolyn were incubated with S100P (100 nM) for 2 h at 37 °C. Each data point was normalized to the basal proliferation of the cells without S100P, which represents 100% proliferation. Bars show the mean±s.e.m. of at least three independent experiments. ns P>0.05, **P<0.01, ***P<0.001.
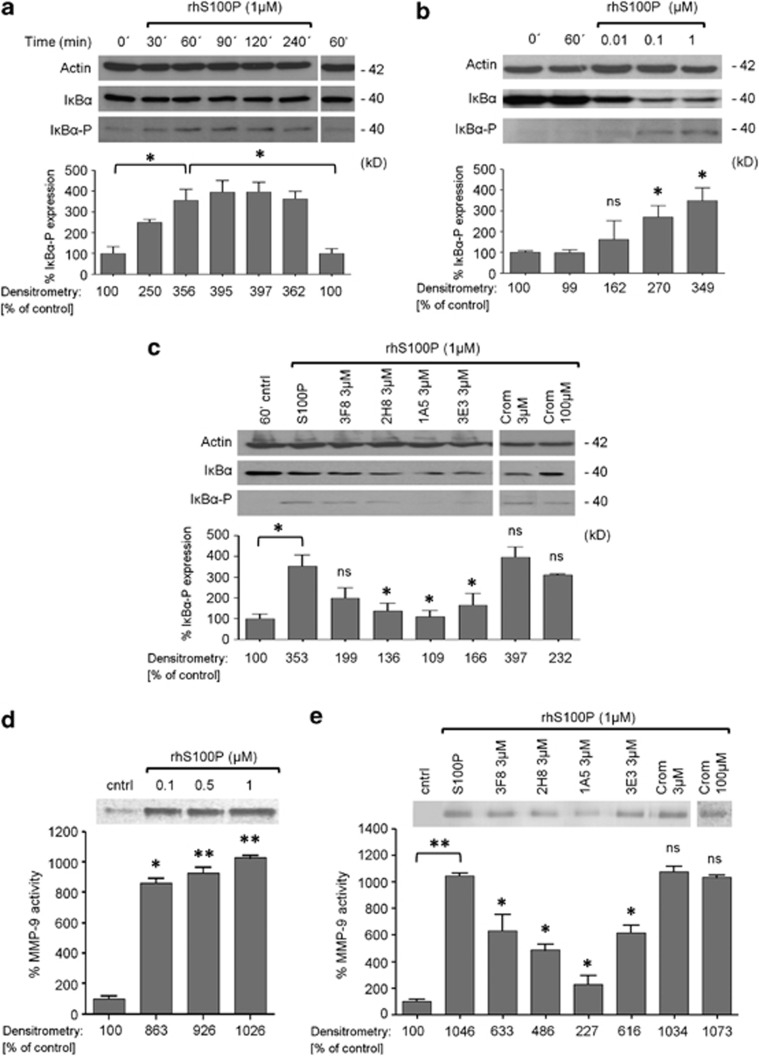
Extracellular S100P stimulates IκB signaling pathway and MMP-9 secretion in BxPC3 cells and mAbs against S100P block these activities
Previous studies revealed that several S100 family members, exert their biologically induced effects (cell proliferation, migration and survival) in tumor cells, leading to the activation of extracellular-regulated kinases, transcription factors as NFκB and the production and secretion of several Matrix Metalloproteinase 9 (MMP-9), but the entire mechanism of action is not fully understood.12, 17 Accordingly, we examined the effects of extracellular S100P on signal mechanisms. Treatment of BxPC3 cells with S100P induced IκBα phosphorylation in a time- and concentration-dependent manner with a noticeable effect after 60 min (Figures 2a and b). To evaluate the specificity and the efficacy of our mAbs by blocking this observed effect, S100P (1 μM) was incubated with 3 μM of each antibody for 2 h prior to the addition to the cells. As shown in Figure 2c, all anti-S100P mAbs were more effective that Cromolyn in blocking the IκBα phosphorylation induced by S100P.
Figure 2.
Extracellular S100P stimulates IκBα signaling pathway and MMP-9 secretion in BxPC3 cells. Cells were treated with the recombinant protein in culture medium without supplements. All western-blot signal intensities were normalized to those of β-actin to account for differences in lane loading. (a) Levels of IkB-alpha phosphorylation in BxPC3 treated with S100P (1 μM) for the indicated periods of time. Sixty minutes of stimulation without S100P was used as a basal control. (b) Dose-dependent IkB-alpha phosphorylation induced by S100P (indicated concentrations) in BxPC3-treated cells for 60 min. (c) Neutralizing effect of mAbs 3F8, 2H8, 1A5 and 3E3 (3 μM) on the IkB-alpha phosphorylation induced by the S100P protein. Cromolyn (3 μM and 100 μM) was used as a control. Before stimulation, antibodies and Cromolyn were incubated with S100P (1 μM) for 2 h at 37 °C. (d) Proteolytic activity of MMP-9 in BxPC3 conditioned media. BxPC3 cells were treated with S100P (0.1–0.5–1 μM) in culture medium without supplements for 72 h and supernatants were analyzed by gelatin zymography. Cleared bands represent MMP-9 secretion. Each data point was normalized to the basal secretion level of the cells (without S100P stimulation) that represents 100% MMP-9 secretion. (e) mAbs 3F8, 2H8, 1A5 and 3E3 (3 μM) neutralize the secretion of active forms of MMP-9 induced by the S100P protein. Cromolyn (3 and 100 μM) was used as a control. Before stimulation, antibodies and Cromolyn were incubated with S100P (1 μM) for 2 h at 37 °C. Bars show the mean±s.e.m. of at least three independent experiments. ns P>0.05, *P<0.05, **P<0.01.
According to the literature, extracellular S100 proteins induce the secretion to the microenvironment of MMPs by several cells as macrophages,18 tumor cells19 and endothelial cells20 to provide the perfect environment for tumor progression and subsequent metastasis formation. On the basis of these results, we demonstrated the ability of the extracellular S100P to increase the production of MMPs on BxPC3 cell line. As shown in Figure 2d, after 72 h of treatment, S100P induced a marked concentration-dependent increase of MMP-9 secretion in this pancreatic cell line. In addition, this effect was blocked when S100P (1 μM) was incubated with 3 μM of each mAb for 2 h prior to the addition to the cells (Figure 2e).
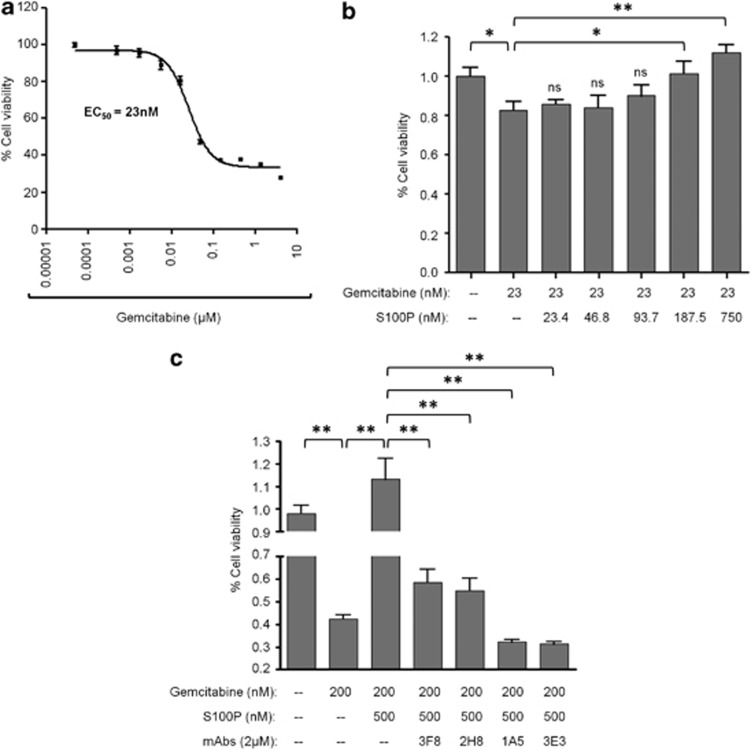
Extracellular S100P increases survival of BxPC3 exposed to Gemcitabine and anti-S100P mAbs block this activity
S100P expression was correlated with chemoresistance by some studies6, 12 being proposed as a marker to predict the effectiveness of some chemotherapeutics drugs.21 Other authors demonstrated that extracellular S100A14 increased the survival of the KYSE180 esophageal squamous carcinoma cell line exposed to the cytotoxic agent Doxorubicin.22 To gain insights into a possible similar effect of S100P in pancreatic carcinoma cells, we exposed BxPC3 cells to Gemcitabine alone and in combination with increased concentrations of S100P. First, we determined the EC50 of Gemcitabine (Figure 3a). Next, we combined this concentration (23 nM) with different doses of recombinant human S100P (0–750 nM). The addition of S100P protected cells from damage induced by Gemcitabine in a concentration-dependent manner with a statistically significant effect for S100P concentrations from 180 nM onwards (Figure 3b). To further confirm the protective effect of S100P and the efficacy of our neutralizing mAbs, a 10-fold increase of Gemcitabine (200 nM) was combined with 500 nM of S100P. As shown, antibodies significantly abrogated the effect of S100P and sensitized the cells to the cytotoxic effect of Gemcitabine (Figure 3c).
Figure 3.
Extracellular S100P protects BxPC3 cells against cell death induced by Gemcitabine. (a) Effect on BxPC3 cell viability induced by Gemcitabine. Cells were incubated with the chemotherapeutic drug at different doses (from 4 μM to 5 nM, dil 1:3) for 72 h. Cell viability was analyzed by MTT assay. The error bars represent mean±s.e.m. (n=6). (b) Dose-dependent increase of cell survival induced by human recombinant S100P (doses from 750 nM to 23 nM, dil 1:4) in BxPC3 cells exposed to Gemcitabine (constant dose of 23 nM) for 72 h. (c) Blocking effect of mAbs 3F8, 2H8, 1A5 and 3E3 (2 μM) of the cell survival induced by S100P (500 nM) on cells exposed to a high dose of Gemcitabine (200 nM). Before stimulation, antibodies were incubated with S100P for 2 h at 37 °C. Percentage of viability was determined in comparison to the positive control (cells without compounds) that represents the 100% of viability. Bars show the mean±s.e.m. *P<0.05, **P<0.01.
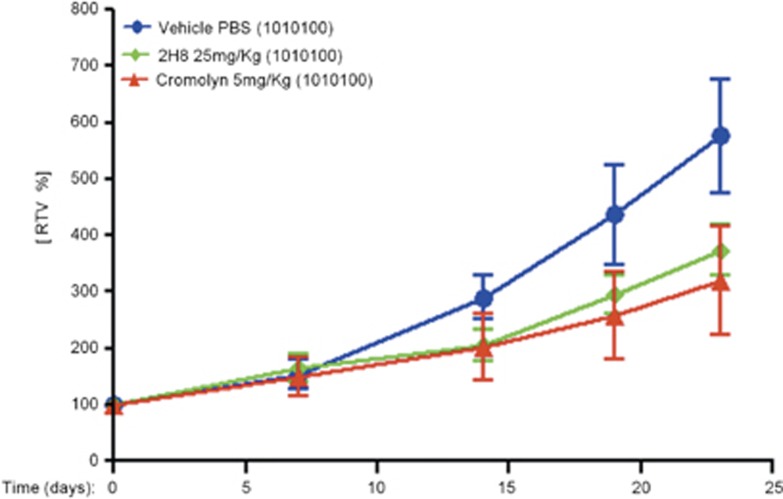
Decreased tumor growth in response to extracellular S100P blockade with 2H8 mAb in subcutaneous BxPC3 tumor model
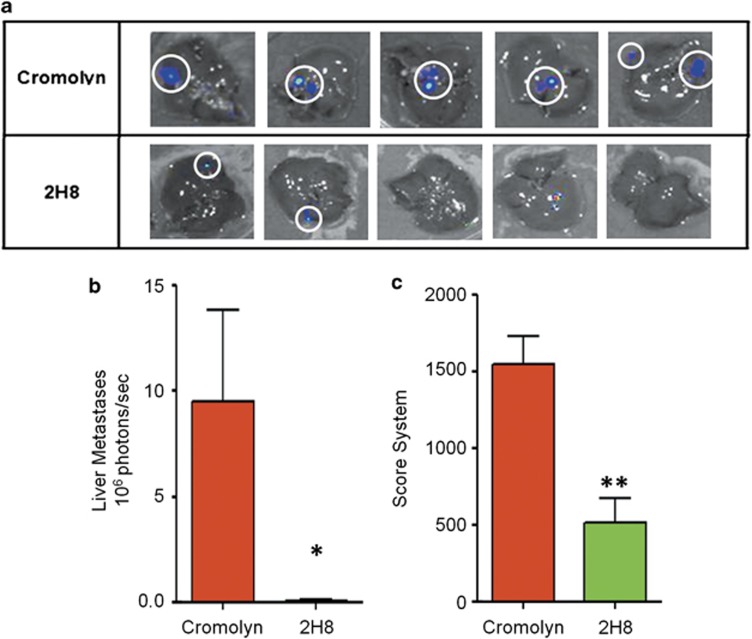
To address the question of whether a mAb could serve as a therapeutic agent in vivo to inhibit tumor growth, we treated athymic mice bearing subcutaneous BxPC3 tumors. The efficacy of the selected mAb for in vivo assays (called 2H8) was compared with control group (vehicle, PBS) and with animals treated with Cromolyn. At the end of the experiment, the control group exhibited a maximum tumor growth with mean relative tumor volume of 576% respect to the initial volume (before treatment). Tumor volume changes in Cromolyn or 2H8 injected mice showed a maximum mean RTV of 320% and 374%, respectively, respect to the initial volume. We observed that treatment either with 2H8 or Cromolyn induced a notable decrease in tumor development compared with the control group (Figure 4). In addition, these differences were reflected on the calculated T/C ratios of tumor volume, 55% for Cromolyn and 65% for 2H8 mAb, respectively.
Figure 4.
Effect of 2H8 mAb in tumor growth using a subcutaneous BxPC3 xenograft model. Female, athymic, nude mice were inoculated subcutaneously with 4 × 106 cells in 0.1 ml culture medium without supplements, into the right upper flank of mice. When tumors reached 65–160 mm3, treatment was initiated. Treatment groups had five animals. PBS (vehicle), 2H8 (25 mg/kg) and Cromolyn (5 mg/kg) was given three times a week (1010100) by intratumoral route (20 μl). Final formulation buffer was given as vehicle control. Graph of relative tumor volume shows the activity of the 2H8 and Cromolyn compared with the control group.
2H8 mAb has neutralizing activity on liver metastasis formation in BxPC3-luciferase orthotopic tumor model
Besides its role in tumor growth, S100P protein has been correlated with a higher metastatic potential. Previous studies showed that a shRNA against S100P inhibited angiogenesis, reduced tumor growth and inhibited metastasis formation by >50% in a mouse model of NSCLC cells.23 Furthermore, treatments with Cromolyn alone and in combination with Gemcitabine inhibited pancreatic tumor growth and metastasis.16 On the basis of those observations, we wanted to investigate the anti-metastatic activity of the 2H8 mAb by blocking the extracellular function of S100P in comparison with Cromolyn.
For this purpose, tumor pieces of 3–5 mm3 from previous orthotopic BxPC3-luciferase tumors were implanted into the pancreas of athymic mice. Treatments with Cromolyn or with 2H8 mAb started 2 days after implantation and continued for 4 weeks. Tumor growth and metastasis were analyzed weekly by bioluminescence imaging. At the end of the experiment, animals were killed and an exhaustive analysis of the disease staging was done based on a TMPN classification and scoring system (Table 1) modified from Hennig et al.24 Scores from each category were multiplied with each other because patterns in medicine follow multiplicative, rather than additive, rules.
Table 1. Staging and Scoring System.
| Stage description | Description | Score |
|---|---|---|
| Primary tumor | ||
| T0 | No tumor | 1 |
| T1 | Small tumor (tumor d<7 mm) | 2 |
| T2 | Large tumor without infiltration (tumor d>7 mm) | 3 |
| T3 | Large tumor with infiltration but still visible margins | 4 |
| T4 | Diffuse and infiltrating tumor | 5 |
| Organ metastases | ||
| M0 | No liver or lung metastases | 1 |
| M1Li | Liver metastases | 5 |
| M1Lu | Lung metastases | 5 |
| M1 | Liver and lung metastases | 10 |
| Peritoneal metastases | ||
| P0 | No peritoneal metastases | 1 |
| P1 | Less than five peritoneal metastases or one with d<7 mm | 3 |
| P2 | More than five peritoneal metastases or one with d>7 mm | 4 |
| P3 | Malignant ascites | 5 |
| P4 | Diaphragm/kidney/intestine/adrenal metastases | 3+3+3+3+P0/1/2/3 |
| Lymph node metastases | ||
| N0 | No lymph node metastases | 1 |
| N1 | Peripancreatic lymph node metastases | 3 |
| N2 | Regional lymph node metastases (for example, mesenteric, mediastinal) | 5 |
Scores for the primary tumor (T), organ metastases (M) peritoneal metastases (P) and lymph node metastases (N) were multiplied to calculate the total tumor scores for each animal. Score P4 value is the sum of the corresponding P0, P1, P2, P3 (P0/1/2/3) plus an additional value of three for metastasis presence in the diaphragm, three for kidney, three for intestine and three for adrenal glands.
Measurements of liver metastases and calculations of photons/second emitted by the isolated organs revealed a more efficient therapeutic effectiveness (statistically significant) in animals treated with the antibody than animals treated with Cromolyn at the best dosing schedule referenced in the bibliography16 for this model (Figures 5a and b).
Figure 5.
Effect of 2H8 mAb in disease staging and liver metastasis incidence using an orthotopic BxPC3-luciferase tumor model. (a) In vivo imaging of liver metastasis from animals treated with Cromolyn (reference compound) and 2H8 mAb. (b) Photon emission quantification of liver metastases. (c) Score system of the disease staging at the end of the experiment, according to a primary tumor (T), organ metastases (M), peritoneal metastases (P) and lymph node metastases (N) classification (see Table 1). Graphs show mean±s.e.m. *P<0.05, **P<0.01.
As shown in Figure 5c, the total score corresponding to the disease staging (TMPN classification) was lower in animals treated with 2H8 compared with Cromolyn group. In addition, these differences are reflected on the T/C ratios of total score calculated; 32% for 2H8 mAb compared with Cromolyn, suggesting a significant clinical benefit of the use of mAbs for anti-S100P cancer therapies.
Determination of S100P protein as a plasmatic biomarker
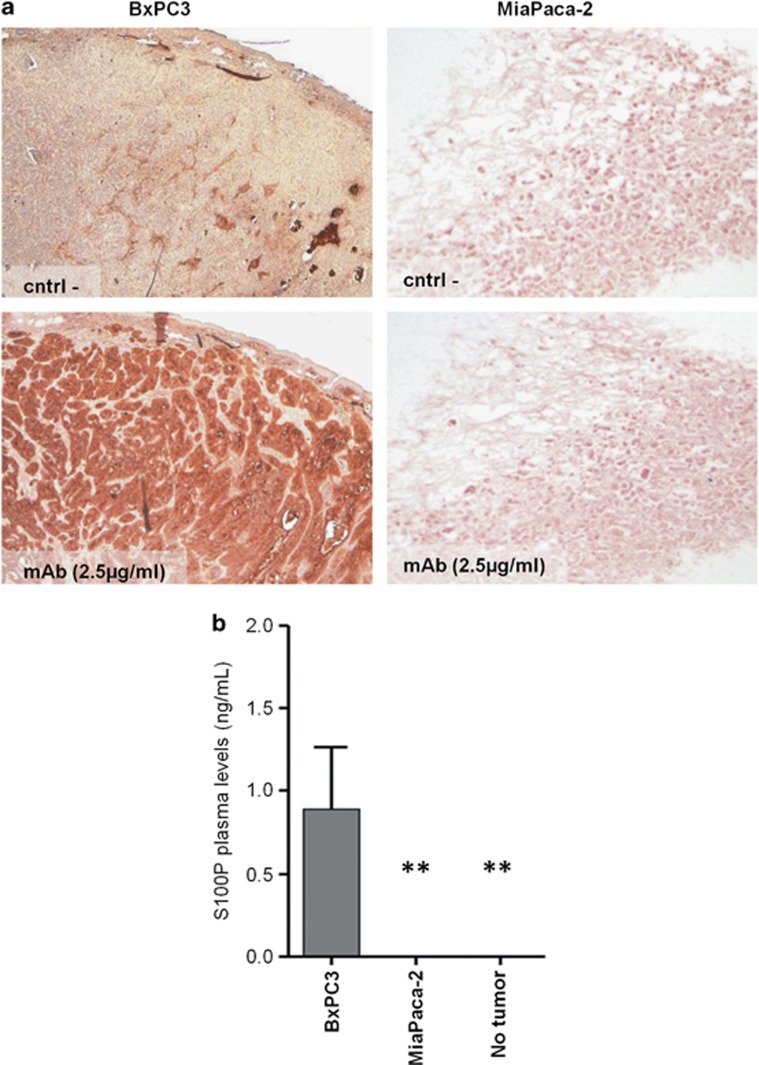
Previous studies have pointed that S100P could be a diagnosis and a poor prognosis marker when found expressed in tissue samples.25 More recently, a study that links elevated serum levels of S100P with an unfavorable prognosis in patients with colorectal cancer were published.26 Taken together, we quantified the levels of S100P in plasma samples obtained from different xenograft tumor models of pancreatic adenocarcinoma cell lines, BxPC3 (S100P positive expression) and MiaPACA-2 (no S100P expression) (Figure 6a). Previously, we quantified the secretion of S100P protein to the culture medium from either BxPC3 or MiaPACA-2 cells showing levels of 10 ng/ml and null detection of the protein, respectively.
Figure 6.
Determination of S100P as a plasmatic biomarker. (a) Immunohistochemical analysis of S100P expression and distribution, in subcutaneous tumors derived from pancreatic adenocarcinoma BxPC3 and MiaPACA-2 cell lines. Sections without anti-S100P antibody were used as a negative control. (b) Plasma levels of S100P protein on two xenograft tumor models, BxPC3 (positive expression of S100P) and MiaPACA-2 (negative expression of S100P) in athymic mice compared with S100P levels in animals without tumor were determined by a sandwich ELISA method. Graph of plasma levels shows the mean±s.e.m. **P<0.01.
As shown in Figure 6b, the quantification of S100P protein in plasma samples from animals bearing MiaPACA-2 and BxPC3 subcutaneous tumors and animals without tumor demonstrated the consistent statistically significant differences between the presence and non-presence of S100P-expressing tumors.
Discussion
Pancreatic cancer is one of the most common causes of adult cancer death. The high incidence of metastasis at initial diagnosis and the lack of adequate therapies make it one of the types of cancer with worst prognostic.27, 28 Current strategies combining surgery with cytotoxic agents as Gemcitabine have proven modest efficacy in patients without prolonging the survival rate remarkably.
In this regard, and taking into consideration the limitations of these therapies, new targeting and therapeutic approaches are essential to improve pancreatic cancer survival.
It has been reported that S100P is highly expressed in >90% of pancreatic tumors regulating a variety of intracellular and extracellular processes, including cell proliferation, invasion, survival, drug resistance and differentiation.14 In addition to its effect on cancer cell growth, S100P has an important role in cancer metastasis. It has also been reported that silencing S100P by siRNA resulted in a reduction in invasiveness in vitro and metastasis in vivo.11 These data demonstrate that S100P could be a promising therapeutic target although its mechanism of action is not fully understood.
Cromolyn is the only described inhibitor of the extracellular function of S100P. Originally, Cromolyn was developed as an anti-inflammatory drug used for prophylactic treatment of bronchial asthma and allergic rhinitis.29 In addition, it has been reported that Cromolyn binds specifically to other members of the S100 protein family (S100A1, S100A12 and S100A13).30, 31 Recently, Cromolyn has been shown to block the interaction with RAGE, and to inhibit tumor growth. Also Cromolyn, increased the effectiveness of Gemcitabine to reduce the volume of metastases in BxPC3 orthotopic tumor model.16 However, these authors observed potential limitations. They saw that Cromolyn reduced the total volume of distant metastases without affecting the number of metastases, indicating that the influence of this drug appears to be mainly on cancer cell growth rather than directly on metastasis formation. Another limitation of Cromolyn would be its specificity, because it may interact with other molecules (other members of the S100 family or other undisclosed targets) that can have equal or more importance than the S100P in pancreatic cancer development.
Here, we extend previous studies about the role played by S100P in tumor growth and metastasis, demonstrating that extracellular S100P blockade with a specific mAb: (i) inhibits tumor proliferation induced by S100P, (ii) blocks the increased survival induced by S100P to the cytotoxic effect of Gemcitabine, (iii) blocks the extracellular mechanism of action of S100P on tumor cells and (iv) reduces tumor growth and liver metastasis formation in a pancreatic tumor model, giving insights into a new strategy to treat pancreatic tumors.
An important finding of this study involves the role played by S100P on tumor cell proliferation using the pancreatic adenocarcinoma BxPC3 cell line as a model. According to the bibliography16 our data showed that addition of recombinant human S100P to the cells increased cell proliferation, and that neutralizing S100P with some specific mAbs, obtained in our lab, leads to a blockade of this process.
The molecular mechanism by which extracellular S100P promotes its activity have not been extensively delineated, although an association between S100P, activation of NFκB and tumor cell proliferation and invasion has been demonstrated.32 In this context and taking as starting point the fact that other S100 proteins (S100A4, S100A14, S100A8/A9) stimulate cellular activities via the secretion of several target proteins as for example, MMPs,33 TNF-α34 or ILs,35 we investigated the presence of any of these regulators, specifically MMPs, in pancreatic tumor cells.
We showed that addition of S100P protein to the cell culture activates the IκBα signaling pathway in a time and dose-dependent manner. Specifically, the NFκB pathway has been associated with the regulation of the expression of MMPs in several cell types36, 37, 38 thus facilitating the degradation of the extracellular matrix and the in vivo tumor invasion and metastasis. At this point, we demonstrated that effectively S100P induced the secretion of MMP-9 in a dose-dependent manner.
To extend the knowledge regarding the inhibitory capacity of anti-S100P mAbs on the in vitro activity of the extracellular S100P protein, we tested the blockade of IκBα activation and MMP-9 secretion, two points in its downstream signaling pathway, showing in both cases a potent decrease by our antibodies.
RAGE is the only described receptor for S100P.39 S100P is secreted by pancreatic cancer cells and interacts with RAGE leading to the activation of extracellular-regulated kinases and NFκB signaling, consistently with increased cell proliferation, migration, survival and tumor growth.12 In this context, we demonstrated the inhibition of the S100P-induced proliferation on BxPC3 cells using a function-blocking anti-RAGE antibody (data not shown) suggesting the hypothesis that extracellular S100P could lead its mechanism of action via the receptor RAGE.
S100P overexpression has recently been shown to correlate with chemoresistance.6, 12 In addition, S100A14 acting extracellularly, increased the survival of an esophageal squamous carcinoma cell line exposed to Doxorubicin.22 Accordingly, we observed that S100P also protected BxPC3 cells from injuries induced by Gemcitabine. Furthermore, our antibodies neutralized this protective effect of S100P and sensitized the cells to the presence of the chemotherapeutic agent, opening new approaches for combinational treatments in pancreatic cancer.
We further sought to determine whether specific mAbs against S100P have a critical role in neutralizing tumor growth and metastasis in both a subcutaneous and orthotopic mouse tumor model of BxPC3 cell line. For this, we selected one of our antibodies, 2H8 and compared its activity with the Cromolyn at the best referenced dosing schedule.16
First, we investigated the effect of the two drugs on the subcutaneous development of tumors in athymic nude mice obtaining a remarkable reduction in tumor growth with similar effect between Cromolyn and 2H8 mAb compared with the control group.
Next we wanted to test the effectiveness in blocking the metastasis formation on an orthotopic tumor model. Our results indicated that 2H8 mAb inhibited almost completely the presence of hepatic metastases in size and number. In addition, we observed, analyzing a TMPN classification a statistically significant reduction on the disease staging of 2H8-treated animals compared with mice treated with Cromolyn, confirming that anti-S100P antibodies act as potent inhibitors of both tumor growth and tumor metastasis, whereas Cromolyn acts only as an inhibitor of tumor growth and not as an antimetastatic agent.
Our observed results about the influence of Cromolyn on cancer cell growth rather than directly on metastasis (number of metastases) are in agreement with previous results published by Arumugam et al.16 using the same dose of the drug (5 mg/kg). Taking into account the implication of S100P both in tumor growth and metastasis formation, its specific neutralization with antibodies will be more effective than Cromolyn due to its unspecificity. Probably, on subcutaneous model Cromolyn affect tumor development blocking S100P and other proteins and cells implicated directly in tumor cell growth (its effectiveness could be due to the sum of the effects). On the other hand, on orthotopic model these target proteins could not have an essential function on metastasis formation and therefore Cromolyn does not have the same activity.
We can affirm that Cromolyn is more effective in vivo than in vitro. It has been reported that Cromolyn interacts with S100P and many other proteins and affect other tumor components, as immune mast cells, and therefore this could be the reason of its high activity in tumor models compared with cell models.16
Therefore, we demonstrate for the first time that treatments with mAbs against S100P induced a marked delay in tumor growth and liver metastasis formation on BxPC3 pancreatic tumors and therefore therapies using antibodies against S100P could be considered as promising strategies to treat pancreatic cancer.
Finally, our results further indicate that S100P could be also considered as a good plasmatic biomarker and by extension for any other biofluid because it allowed us to discriminate between animals without or with S100P-expressing tumors.
Taken together, we have elucidated a therapeutic strategy to combat pancreatic cancers by blocking extracellular S100P with a first in class mAb. A more extensive knowledge of the role played by S100P in the interaction between cancer and stromal cells in promoting tumor development and metastasis will be essential to improve the efficacy of the treatment.
Materials and methods
Ethical animal procedures
All procedures involving experimental animals were approved by the ‘Ethical Committee of Animal Experimentation' of the animal facility place at Science Park of Barcelona (Platform of Applied Research in Animal Laboratory) and by the Catalonian and Spanish regulatory laws and guidelines governing experimental animal care.
Production of human recombinant S100P and mAb generation
To generate the S100P recombinant protein, a cDNA encoding the full-length sequence of human S100P was obtained by RT–PCR from mRNA of the HeLa cell line as we described previously.20 The primers used in the PCR reaction were 5′-actcacatatgacggaactagagagccatgggcatgatc-3′ and 5′-actcatgagctcatcatttgagtcctgccttctcaaagtactt-3′. mAb fusion, ELISA screening and subcloning were performed using standard technologies.20
Cell culture
Myeloma P3 × 63Ag8.653 (ECACC, Salisbury, UK) cells were cultured in RPMI 1640 (PAA, Pasching, Austria) supplemented with 10% FCS (PAA; Australian origin) plus 2 mM GlutaMAX-I (Invitrogen, Scotland, UK). Human pancreatic adenocarcinoma cell line BxPC3 (ECACC) was cultured at 37 °C in a humidified 5% CO2-atmosphere in DMEM High-Glucose (Sigma, St Louis, MO, USA), supplemented with 10% FCS (Sigma). BxPC3 cells stably transfected with luciferase were kindly provided by Dr Adela Mazo (Universitat de Barcelona) and were cultured in the presence of 100 μg/ml of hygromicyn B (Invitrogen) as a selective agent.
Western blot analysis
BxPC3 cells were stimulated with different concentrations of S100P (0.03–3 μM) for 60 min To test the inhibitory effect of the anti-S100P mAbs, 2 μM of each was incubated for 2 h with S100P protein (1 μM) at 37 °C, prior to the addition to the cells. SDS–PAGE and western blot analyses were performed as described previously.20
The following antibodies were used: 4B12 mouse mAb anti-human S100P (Leitat Technological Center, Barcelona, Spain) at 1 μg/ml; peroxidase-conjugated mAb anti-human β-Actin (Sigma) at a 1:25 000 dilution; mouse mAb anti-human phospho-IκB-α (Ser32/36) (Cell Signaling Technology, Danvers, MA, USA), at a 1:1000 dilution; rabbit polyclonal antibody anti-human IκB-α (Cell Signaling Technology), at a 1:1000 dilution. Goat anti-mouse (Jackson ImmunoResearch, Baltimore, PA, USA) at 0.04 μg/ml and goat anti-rabbit (Sigma) at a 1:25 000 dilution, were used as secondary antibodies.
Bands were quantified using the NIH ImageJ imaging software. Quantification of the protein expression was performed by densitometric analysis referring the results to the control cells in the non-stimulated condition (that represents 100% of expression). All signals intensities were normalized to β-Actin.
Proliferation assay
Proliferation studies on BxPC3 cell line were analyzed using the Cell Proliferation ELISA Biotrak System kit (GE Healthcare, Buckinghamshire, UK) according to the manufacturer's instructions. Three thousand cells per well were seeded in culture medium supplemented with 3% FCS. S100P protein (100 nM) alone or in combination with the mAbs (500 nM) was incubated for 2 h before adding to the culture. After 48 h at 37 °C, the proliferation was determined by measuring the absorbance at 450 nm using a Multiskan Ascent spectrophotometer (Thermo Corporation, Roskilde, Denmark). Data analysis was performed by normalizing the results with the negative control (untreated cells) that was considered as 100% of proliferation.
MMP secretion assay
Gelatin zymography analysis was performed as described previously33 with some modifications: 24-well culture plates were seeded with BxPC3 cells at a density of 250 000 cells/well in complete medium. After 24 h, cells were stimulated with S100P (0.5, 1, 3 μM) in serum-free medium. For blocking experiments, mAbs at 2 μM were incubated with S100P (1 μM) for 2 h at 37 °C prior to the addition to the cells. After 72 h at 37 °C, supernatants were resolved in a non-reducing 8% SDS–PAGE gel copolymerized with Type A gelatin from porcine skin (Sigma) at a final concentration of 1 mg/ml. After running, MMPs present in the gel were activated for 24 h, gels were stained and bands were quantified using the NIH ImageJ imaging software. Quantification of the protein expression was performed by densitometric analysis referring the results to the control cells in the non-stimulated condition (that represents 100% of expression).
Cytotoxic effect of Gemcitabine and mAbs
The cytotoxic effect of Gemcitabine alone or in combination with S100P was determined by MTT assay as instructed by the manufacturer (Calbiochem, Darmstadt, Germany). BxPC3 cells were seeded at a density of 12 000 cells/well and 24 h later, Gemcitabine alone (from 4 μM to 5 nM, dil 1:3) or in combination with S100P at different doses (from 3 μM–46 nM, dil 1:2) were added and incubated for 72 h. For blocking experiments, S100P was incubated with mAbs at the indicated doses for 2 h prior to the addition to the cells. Data analysis was performed by normalizing the results with the negative control (untreated cells) that was considered as 100% of viability. Curves were adjusted using a sigmoid dose-response (variable slope) equation, and EC50 values were obtained from the equation: Y=Bottom+(Top−Bottom)/(1+10^(LogEC50−X*HillSlope)), where X is the logarithm of concentration and Y is the response. Y starts at Bottom and goes to Top with a sigmoid shape.
Tumor growth studies in nude mice
Mice for tumor models (athymic (Hsd:Athymic Nude-Foxn1nu; 6–7 weeks old)) were from Harlan Laboratories Models, S.L. (Barcelona, Spain).
Subcutaneous tumor model
Exponentially growing BxPC3 cell line (4 × 106 cells) was subcutaneously injected into the right flank of nude animals. Tumor growth was monitored by measuring the diameter of the tumors with a caliper, and the tumor volumes were calculated according to the formula: 0.5 × length × width2. When tumor volume reached about 120 mm3, mice were randomly divided into three groups (n=5). Mice were treated three times per week by intratumoral route with PBS (control group), 2H8 mAb at 25 mg/Kg and Cromolyn at 5 mg/Kg (130 μM). We calculated the treatment-to-control ratio (of sample means) at the end of experiment and it corresponds to the observed relative tumor volume at a given time for the treatment and control groups, respectively.
Orthotopic tumor model
Pieces of 3–5 mm3 from BxPC3-luciferase tumors previously grown orthotopically were implanted into the pancreas of athymic mice, which were randomly divided into three groups. Treatment by intraperitoneal injection with Cromolyn (5 mg/kg body weight) or 2H8 mAb (25 mg/kg) started 2 days after tumor implantation and continued for 4 weeks. Cromolyn was given daily and anti-S100P mAb was given three times per week. The effect on tumor growth and metastasis were analyzed by weekly bioluminescence imaging (Xenogen IVIS-200 Optical In Vivo Imaging System). At the end of the assay, animals were killed and the disease staging was analyzed based on Hennig method24 with some modifications (Table 1).
Immunohistochemical analysis of S100P protein expression
At the end of the in vivo experiments, BxPC3 tumors were surgically removed, formalin fixed and paraffin embedded. S100P protein was detected in 5 μm thickness sections using 4B12 mAb and a labeled streptavidin-biotin method after antigen retrieval, as previously described.40, 41 Sections were deparaffinized and boiled for 15 min in 0.01 M citrate buffer (pH 6.0) for antigen retrieval. Unspecific tissue peroxidases were blocked by 3% (v/v) H2O2, followed by incubation in blocking solution PBS plus 5% goat serum (Vector, Burlingame, CA, USA). Then, sections were incubated overnight at 4 °C with the 4B12 mAb at a concentration of 5 μg/ml. Biotinylated secondary antibody was added, followed by an avidin–horseradish peroxidase reagent. Finally, sections were incubated with NovaRed (Vector) for 10 min at 4 °C, used as chromogen and mounted using DPX non-aqueous mounting medium (Sigma). As a negative control, we replaced the primary antibody for PBS with 5% goat serum.
Determination of secreted S100P protein by sandwich ELISA assay
To measure the presence of S100P in plasma samples from animals with or without tumor, a sandwich ELISA assay was performed. Ninety-six microtiter dishes (Maxisorb, NUNC, Roskilde, Denmark) were coated with 10 μg/ml of 3E3 mAb, overnight at 4 °C. After removing the coating, dishes were washed twice with washing buffer (PBS-0.1% Tween-20) and were incubated for 2 h at 37 °C in blocking buffer (PBS containing 1% of skimmed milk). Plasma samples diluted 1:2 in blocking buffer were added to the wells and were incubated for 2 h at 37 °C. Biotinylated mAb anti-S100P (4B12) at 10 μg/ml was added to the wells and was incubated for 2 h at 37 °C. Then, streptavidin-HRP (DAKO, Glostrup, Denmark) at a dilution of 1:1000 was added to each well and was incubated for 1 h at 37 °C. The ELISA was developed by adding Tetramethylbenzidine substrate (Sigma) before stopping the reaction with 1 M of HCl. Absorbance was measured at 450 nm using a Multiskan Ascent spectrophotometer (Thermo Corporation). A standard curve was constructed by plotting absorbance values versus human S100P concentrations of recombinant protein (serial 1:3 dilutions in blocking buffer starting at 50 ng/ml).
Statistical analysis
In all studies, values are expressed as mean± s.e.m. as indicated. Statistical analyses were performed by the two-tailed nonparametric Mann–Whitney U-test, using the GraphPad Prism software, version 5.04 for Windows. Differences were considered statistically significant at P<0.05.
Acknowledgments
The work was supported by Grants from ACC1Ó (Project number: TECRD12–1–0010). Our research group hold the Quality Mention SGR2009–261-GRE from the ‘Generalitat de Catalunya'.
The authors declare no conflict of interest.
References
- Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- Yachida S, Jones S, Bozic I, Antal T, Leary R, Fu B, et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature. 2010;467:1114–1117. doi: 10.1038/nature09515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino KM, Hall MD, Goldsborough AS, Callaghan R, Gottesman MM. Collateral sensitivity as a strategy against cancer multidrug resistance. Drug Resist Updat. 2012;15:98–105. doi: 10.1016/j.drup.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Hu H, Tong X, Jiang Q, Zhu H, Zhang S. Calcium-binding protein S100P and cancer: mechanisms and clinical relevance. J Cancer Res Clin Oncol. 2012;138:1–9. doi: 10.1007/s00432-011-1062-5. [DOI] [PubMed] [Google Scholar]
- Guerreiro Da Silva ID, Hu YF, Russo IH, Ao X, Salicioni AM, Yang X, et al. S100P calcium-binding protein overexpression is associated with immortalization of human breast epithelial cells in vitro and early stages of breast cancer development in vivo. Int J Oncol. 2000;16:231–240. [PubMed] [Google Scholar]
- Bertram J, Palfner K, Hiddemann W, Kneba M. Elevated expression of S100P, CAPL and MAGE 3 in doxorubicin-resistant cell lines: comparison of mRNA differential display reverse transcription-polymerase chain reaction and subtractive suppressive hybridization for the analysis of differential gene expression. Anticancer Drugs. 1998;9:311–317. doi: 10.1097/00001813-199804000-00004. [DOI] [PubMed] [Google Scholar]
- Averboukh L, Liang P, Kantoff PW, Pardee AB. Regulation of S100P expression by androgen. Prostate. 1996;29:350–355. doi: 10.1002/(SICI)1097-0045(199612)29:6<350::AID-PROS2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Beer DG, Kardia SL, Huang CC, Giordano TJ, Levin AM, Misek DE, et al. Gene-expression profiles predict survival of patients with lung adenocarcinoma. Nat Med. 2002;8:816–824. doi: 10.1038/nm733. [DOI] [PubMed] [Google Scholar]
- Shyu RY, Huang SL, Jiang SY. Retinoic acid increases expression of the calcium-binding protein S100P in human gastric cancer cells. J Biomed Sci. 2003;10:313–319. doi: 10.1007/BF02256450. [DOI] [PubMed] [Google Scholar]
- Hamada S, Satoh K, Hirota M, Kanno A, Ishida K, Umino J, et al. Calcium-binding protein S100P is a novel diagnostic marker of cholangiocarcinoma. Cancer Sci. 2011;102:150–156. doi: 10.1111/j.1349-7006.2010.01757.x. [DOI] [PubMed] [Google Scholar]
- Arumugam T, Simeone DM, Van Golen K, Logsdon CD. S100P promotes pancreatic cancer growth, survival, and invasion. Clin Cancer Res. 2005;11:5356–5364. doi: 10.1158/1078-0432.CCR-05-0092. [DOI] [PubMed] [Google Scholar]
- Arumugam T, Simeone DM, Schmidt AM, Logsdon CD. S100P stimulates cell proliferation and survival via receptor for activated glycation end products (RAGE) J Biol Chem. 2004;279:5059–5065. doi: 10.1074/jbc.M310124200. [DOI] [PubMed] [Google Scholar]
- Shiota M, Tsunoda T, Song Y, Yokomizo A, Tada Y, Oda Y, et al. Enhanced S100 calcium-binding protein P expression sensitizes human bladder cancer cells to cisplatin. BJU Int. 2011;107:1148–1153. doi: 10.1111/j.1464-410X.2010.09535.x. [DOI] [PubMed] [Google Scholar]
- Tothova V, S100P GibadulinovaA. a peculiar member of S100 family of calcium-binding proteins implicated in cancer. Acta Virol. 2013;57:238–246. doi: 10.4149/av_2013_02_238. [DOI] [PubMed] [Google Scholar]
- Pan X, Arumugam T, Yamamoto T, Levin PA, Ramachandran V, Ji B, et al. Nuclear factor-kappaB p65/relA silencing induces apoptosis and increases gemcitabine effectiveness in a subset of pancreatic cancer cells. Clin Cancer Res. 2008;14:8143–8151. doi: 10.1158/1078-0432.CCR-08-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Logsdon CD. Effect of cromolyn on S100P interactions with RAGE and pancreatic cancer growth and invasion in mouse models. J Natl Cancer Inst. 2006;98:1806–1818. doi: 10.1093/jnci/djj498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li M, Jin J, Bai Y, Yang C. Knockdown of S100A4 decreases tumorigenesis and metastasis in osteosarcoma cells by repression of matrix metalloproteinase-9. Asian Pac J Cancer Prev. 2011;12:2075–2080. [PubMed] [Google Scholar]
- Zhang F, Banker G, Liu X, Suwanabol PA, Lengfeld J, Yamanouchi D, et al. The novel function of advanced glycation end products in regulation of MMP-9 production. J Surg Res. 2011;171:871–876. doi: 10.1016/j.jss.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namba T, Homan T, Nishimura T, Mima S, Hoshino T, Mizushima T. Up-regulation of S100P expression by non-steroidal anti-inflammatory drugs and its role in anti-tumorigenic effects. J Biol Chem. 2009;284:4158–4167. doi: 10.1074/jbc.M806051200. [DOI] [PubMed] [Google Scholar]
- Hernandez JL, Padilla L, Dakhel S, Coll T, Hervas R, Adan J, et al. Therapeutic targeting of tumor growth and angiogenesis with a novel anti-S100A4 monoclonal antibody. PLoS One. 2013;8:e72480. doi: 10.1371/journal.pone.0072480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu GD, Azorsa DO, Kiefer JA, Rojas AM, Tuzmen S, Barrett MT, et al. Functional evidence implicating S100P in prostate cancer progression. Int J Cancer. 2008;123:330–339. doi: 10.1002/ijc.23447. [DOI] [PubMed] [Google Scholar]
- Jin Q, Chen H, Luo A, Ding F, Liu Z. S100A14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE) PLoS One. 2011;6:e19375. doi: 10.1371/journal.pone.0019375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulk E, Hascher A, Liersch R, Mesters RM, Diederichs S, Sargin B, et al. Adjuvant therapy with small hairpin RNA interference prevents non-small cell lung cancer metastasis development in mice. Cancer Res. 2008;68:1896–1904. doi: 10.1158/0008-5472.CAN-07-2390. [DOI] [PubMed] [Google Scholar]
- Hennig R, Ventura J, Segersvard R, Ward E, Ding XZ, Rao SM, et al. LY293111 improves efficacy of gemcitabine therapy on pancreatic cancer in a fluorescent orthotopic model in athymic mice. Neoplasia. 2005;7:417–425. doi: 10.1593/neo.04559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez M, Gil-Longo J, Campos-Toimil M. Calcium binding proteins. Adv Exp Med Biol. 2012;740:461–482. doi: 10.1007/978-94-007-2888-2_19. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang YN, Lin GL, Qiu HZ, Wu B, Wu HY, et al. S100P, a potential novel prognostic marker in colorectal cancer. Oncol Rep. 2012;28:303–310. doi: 10.3892/or.2012.1794. [DOI] [PubMed] [Google Scholar]
- Fang Y, Yao Q, Chen Z, Xiang J, William FE, Gibbs RA, et al. Genetic and molecular alterations in pancreatic cancer: Implications for personalized medicine. Med Sci Monit. 2013;19:916–926. doi: 10.12659/MSM.889636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arumugam T, Ramachandran V, Sun D, Peng Z, Pal A, Maxwell DS, et al. Designing and developing S100P inhibitor 5-methyl cromolyn for pancreatic cancer therapy. Mol Cancer Ther. 2013;12:654–662. doi: 10.1158/1535-7163.MCT-12-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storms W, Kaliner MA. Cromolyn sodium: fitting an old friend into current asthma treatment. J Asthma. 2005;42:79–89. [PubMed] [Google Scholar]
- Okada M, Tokumitsu H, Kubota Y, Kobayashi R. Interaction of S100 proteins with the antiallergic drugs, olopatadine, amlexanox, and cromolyn: identification of putative drug binding sites on S100A1 protein. Biochem Biophys Res Commun. 2002;292:1023–1030. doi: 10.1006/bbrc.2002.6761. [DOI] [PubMed] [Google Scholar]
- Shishibori T, Oyama Y, Matsushita O, Yamashita K, Furuichi H, Okabe A, et al. Three distinct anti-allergic drugs, amlexanox, cromolyn and tranilast, bind to S100A12 and S100A13 of the S100 protein family. Biochem J. 1999;338 (Pt 3:583–589. [PMC free article] [PubMed] [Google Scholar]
- Fuentes MK, Nigavekar SS, Arumugam T, Logsdon CD, Schmidt AM, Park JC, et al. RAGE activation by S100P in colon cancer stimulates growth, migration, and cell signaling pathways. Dis Colon Rectum. 2007;50:1230–1240. doi: 10.1007/s10350-006-0850-5. [DOI] [PubMed] [Google Scholar]
- Pazzaglia L, Ponticelli F, Magagnoli G, Gamberi G, Ragazzini P, Balladelli A, et al. Activation of metalloproteinases-2 and -9 by interleukin-1alpha in S100A4-positive liposarcoma cell line: correlation with cell invasiveness. Anticancer Res. 2004;24:967–972. [PubMed] [Google Scholar]
- Fujiya A, Nagasaki H, Seino Y, Okawa T, Kato J, Fukami A, et al. The role of S100B in the interaction between adipocytes and macrophages. Obesity (Silver Spring) 2013;22:371–379. doi: 10.1002/oby.20532. [DOI] [PubMed] [Google Scholar]
- Koy M, Hambruch N, Hussen J, Pfarrer C, Seyfert HM, Schuberth HJ. Recombinant bovine S100A8 and A9 enhance IL-1beta secretion of interferon-gamma primed monocytes. Vet Immunol Immunopathol. 2013;155:162–170. doi: 10.1016/j.vetimm.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Bond M, Chase AJ, Baker AH, Newby AC. Inhibition of transcription factor NF-kappaB reduces matrix metalloproteinase-1, -3 and -9 production by vascular smooth muscle cells. Cardiovasc Res. 2001;50:556–565. doi: 10.1016/s0008-6363(01)00220-6. [DOI] [PubMed] [Google Scholar]
- Kim H, Koh G. Lipopolysaccharide activates matrix metalloproteinase-2 in endothelial cells through an NF-kappaB-dependent pathway. Biochem Biophys Res Commun. 2000;269:401–405. doi: 10.1006/bbrc.2000.2308. [DOI] [PubMed] [Google Scholar]
- Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20:4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- Arumugam T, Logsdon CD. S100P: a novel therapeutic target for cancer. Amino Acids. 2011;41:893–899. doi: 10.1007/s00726-010-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlen P, Puisieux A. Metastasis: a question of life or death. Nat Rev Cancer. 2006;6:449–458. doi: 10.1038/nrc1886. [DOI] [PubMed] [Google Scholar]
- Donato R. RAGE: a single receptor for several ligands and different cellular responses: the case of certain S100 proteins. Curr Mol Med. 2007;7:711–724. doi: 10.2174/156652407783220688. [DOI] [PubMed] [Google Scholar]