Abstract
With nearly 1,500,000 new patients diagnosed every year in the USA, cancer poses a considerable challenge to healthcare today. Oral cancer is responsible for a sizeable portion of deaths due to cancer, primarily because it is diagnosed at a late stage when the prognosis is poor. Current methods for diagnosing oral cancer need to be augmented by better early detection, monitoring and screening modalities. A new approach is needed that provides real-time, accurate, noninvasive diagnosis. The results of early clinical trials using in vivo optical coherence tomography for the diagnosis of oral dysplasia and malignancy are encouraging.
Keywords: early detection, gold nanoparticles, noninvasive diagnostics, optical coherence tomography, oral cancer
Clinical need
According to the American Cancer Society, 1,437,180 patients were diagnosed with cancer in the year 2008, with 35,310 being oral cancers [1]. In the same year, 552,200 people were expected to succumb to cancer in the USA, with 7590 of those from oral cancer alone [1]. Oral cancer is the 8th most common cancer among white males and the 6th most common cancer among black men living in the USA [1]. Approximately 9000 deaths occur as a result of these malignancies [1]. More deadly than breast cancer, cervical cancer and prostate cancer, it has been estimated that oral cancer kills one person, every hour of every day [2,3].
Accounting for 96% of all oral cancers, squamous cell carcinoma (SCC) is usually preceded by dysplasia presenting as white epithelial lesions on the oral mucosa (leukoplakia). Leukoplakias develop in 1–4% of the population [4]. Malignant transformation, which is quite unpredictable, develops in 1–40% of leukoplakias over 5 years [4]. Dysplastic lesions in the form of erythroplakias (red lesions) carry a 90% risk for malignant conversion [4]. Tumor detection is further complicated by a tendency towards field cancerization, leading to multicentric lesions, which may not all be clinically visible [5]. Current diagnostic techniques require a surgical biopsy of lesions. Benign lesions are often biopsied, reducing patient motivation to agree to further diagnostic biopsies in the future. Conversely, many lesions are only detected by biopsy at an advanced stage, when treatment options and outcome are far from optimal. Of all oral cancer cases documented by the National Cancer Institute (NCI) Surveillance, Epidemiology, and End Results Program, advanced lesions outnumbered localized lesions by more than 2:1. The 5-year survival rate is 75% for those with localized disease at diagnosis, but only 16% for those with metastasis [4,6]. Despite significant advances in cancer treatment, early detection of cancer and its curable precursors remains the best way to ensure patient survival and quality of life.
In anatomical sites, such as the oral cavity, early recognition of malignancy is problematic owing to the frequent lack of gross signs or obvious symptoms. In many cases, detection is further hampered by poor visual access, difficulty in determining which of the encountered dysplastic regions will transform into malignancy and the inability to perform adequate or regularly repeated screening in high-risk patients. A modality for the direct, noninvasive early detection, diagnosis and monitoring of oral dysplasia and malignancy as well as the screening of high-risk populations is urgently required to identify treatment needs at early, more treatable stages of pathological development.
Importance of early detection
The prevention of oral cancer and its associated morbidity and mortality hinges upon the early detection of neoplastic lesions, allowing for histologic evaluation and treatment as necessary. Although basic oral cancer examination to achieve early detection requires only a 90-s visual and tactile examination, too few practitioners, and dentists in particular, are conducting these exams [7]. Moreover, the identification of high-risk individuals would permit the development and implementation of efficient chemoprevention and molecular targeting strategies.
There is a general consensus that the clinical stage at the time of diagnosis is the most important predictor of recurrence and death in head and neck cancer patients. The time of diagnosis is influenced by multiple clinical and sociodemographic variables, including patient reluctance to consult a healthcare professional owing to lack of access that is all too common, especially in patients with low socioeconomic status, as well as professional delay in diagnosing and treating the disease. Studies have demonstrated that dentists and other healthcare providers are in desperate need of systemic educational updates in oral cancer prevention and early detection, as they are remiss in the provision of oral examinations and in the detection of early oral cancers [8]. Clinicians can increase survival rates if a cancerous lesion is detected at an early stage or if a precursor lesion (dysplasia) is discovered and treated prior to malignant progression [9]. Recent models determining the value of a population-based oral cancer screening program show it to be a promising health promotion strategy (especially in highrisk individuals) with significant increases in quality-adjusted life years saved, which await further economic appraisal [10].
Existing diagnostic tools for oral cancer
Existing clinical diagnostic tools developed for the early detection of oral cancer include tolonium chloride or toluidine blue (TB) dye, Oral CDx® brush biopsy kits, ViziLite®, salivary diagnostics and several imaging devices, such as Velscope® and multispectral optical imaging systems. To date, none have shown equivalency or been confirmed to be superior to clinical examination [11,12].
Visual examination & biopsy
Since 11% of dentists and 45% of physicians do not feel adequately trained to complete an effective oral cancer examination, this results in a failure to examine for oral cancer [13]. The current approach to detecting the transformation of leukoplakia/erythroplakia to SCC is regular surveillance combined with biopsy or surgical excision. However, visual examination provides very poor diagnostic accuracy and biopsy techniques – the current gold standard – are invasive and unsuitable for regular screening of high-risk sectors of the population [1,2,14-17]. Adequate visual identification and biopsy of all such lesions to ensure that they are all recognized and diagnosed is difficult, given the often multifocal nature of oral malignancy [15].
Vital staining
Several studies have investigated the use of vital staining with agents such as Lugol iodine, TB and tolonium chloride for detection of oral malignancy [18-23]. Although the sensitivity of these agents in the hands of experts generally approximates 90%, specificity of these agents is poor; sensitivity rapidly decreases when this modality is used by nonexperts, such as screeners in field units [18-23]. From recent studies a relationship between TB staining and genetic changes associated with the progression of potentially malignant lesions to oral cancer – such as allelic loss or loss of heterozygosity (LOH) – was demonstrated [24]. Furthermore, the authors demonstrated in a longitudinal study that TB identified LOH-positive lesions that subsequently progressed to oral cancer [24].
Chemiluminescence: ViziLite
This noninvasive screening tool consists of an acetic acid wash and a single use ‘chemi-light stick’ that generates a moderately short wavelength light with peak outputs near 430, 540 and 580 nm for illumination of the oral cavity (ViziLite). It is based on the rationale that the typically greater nuclear content density and mitochondrial matrix of abnormal squamous epithelial cells will reflect light and appear white when viewed under a diffuse low-energy wavelength light. Normal epithelium will absorb the light and appear dark [25]. The majority of studies investigating chemiluminescence evaluate subjective perceptions of characteristics of intraoral lesions, including brightness, sharpness and texture versus routine clinical examination. Results have been contradictory [11,26]. Recently a combination of both TB and ViziLite systems (ViziLite Plus with TB system) has been introduced. A new chemiluminescence device (MicroLux DL) has also recently been introduced on the market [27].
Oral brush cytology
The brush biopsy (CDx) was designed for use on clinical lesions that would otherwise not be subjected to biopsy because the level of suspicion for carcinoma, based upon clinical features, was low. Using cytological examination of ‘brush biopsy’ samples as a noninvasive method of oral diagnosis has been shown to provide moderate sensitivity levels of detection of oral epithelial dysplasia or SCC (70–90%) but poor specificity (3–44%). Thus, this approach is of limited diagnostic value without augmentation by traditional biopsy [28-44].
Spectroscopy
Spectroscopy provides information regarding the biochemical composition and/or the structure of the tissue that conveys diagnostic information. Malignancy-related biochemical and morphologic changes perturb tissue absorption, fluorescence and scattering properties [45,46]. Thus, biochemical information can be obtained by measuring absorption/reflectance, fluorescence or Raman scattering signals [45-50]. Structural and morphological information may be obtained by techniques that assess the elastic scattering properties of tissue [49,51-53].
For decades the use of tissue autofluorescence has been described to screen and diagnose precancers and early cancer lesions in organs such as the lung, uterine cervix, skin and, more recently, the oral cavity [49,54-57]. The concept behind tissue autofluorescence is that changes in the structure (e.g., hyperkeratosis, hyperchromatin and increased cellular/nuclear pleomorphism) and metabolism (e.g., concentration of flavin adenine dinucleotide [FAD] and nicotinamide adenine dinucleotide) of the epithelium, as well as changes of the subepithelial stroma (e.g., composition of collagen matrix and elastin), alter their interaction with light. Specifically, these epithelial and stromal changes can alter the distribution of tissue fluorophores and as a consequence the way the tissues fluoresce after stimulation with intense light (typically, blue light excitation at 400–460 nm). The autoflorescence signal can be directly visualized by the clinician [54-58].
One of the tissue fluorescence imaging systems that has been marketed to dental offices is the Velscope system. In the oral cavity, normal oral mucosa emits a pale green autofluorescence when viewed through the instrument handpiece, while abnormal tissue displays a decreased autofluorescence and appears darker with respect to the surrounding healthy tissue [55-57]. Studies have shown that Velscope can improve lesions’ contrast and, therefore, improve the clincian’s ability to distinguish between mucosal lesions and healthy mucosa [55-57]. In a recent study using 56 patients with oral lesions and 11 normal volunteers, normal tissue could be discriminated from dysplasia and invasive cancer with a 95.9% sensitivity and 96.2% specificity in the training set, and with a 100% sensitivity and 91.4% specificity in the validation set. Disease probability maps qualitatively agreed with both clinical impression and histology [57]. Further clinical studies are needed in diverse populations to fully evaluate the clinical usefulness of this promising approach.
Structural and morphological information may be obtained by spectroscopic techniques that assess the elastic-scattering properties of tissue [45]. Pursuant to encouraging preliminary data, clinical trials of elastic scattering spectroscopy sometimes in combination with fluorescence spectroscopy or imaging, are underway [53]. Other studies combine spectroscopy with polarized light and/or fluorescence imaging, and/or in vivo microscopy. Devices under development and testing include the FastEEM4® System, the Identafi™ and the PS2-oral®. These clinical studies are still at a relatively early stage and preliminary results are encouraging [46-50,54,59-64]. Remicalm’s Identafi technology combines anatomical imaging with fluorescence, fiber optics and confocal microscopy with the goal of precisely mapping the location and determining the extent of the disease in the area being screened. A study in 124 subjects determined a sensitivity of 82% and a specificity of 87% for differentiating between neoplastic and non-neoplastic sites in the oral cavity. Results differed between different sampling depths and keratinized versus non-keratinized tissues [63].
Significant challenges to the use of diagnostic spectroscopy include the often low signal-to-noise ratio, difficulty in identifying the precise source of signals, data quantification issues, and establishing definitive diagnostic milestones and end points, especially given the wide range of tissue types contained within the oral cavity. Limited tissue penetration and concerns regarding mutagenicity when using UV light present further clinical challenges. The abundance of data/information generated in association with our incomplete understanding of the carcinogenesis process tend to render data analysis and interpretation very complex, however, the development of diagnostic algorithms may be able to mitigate this challenge [46,63].
In vivo confocal imaging
In vivo confocal imaging resembles histological tissue evaluation, except that 3D subcellular resolution is achieved noninvasively and without stains. In epithelial structures, resolutions of 1 μm have been achieved with a 200–400-μm field of view [65-69]. While this technology can provide detailed images of tissue architecture and cellular morphology, a very small field of view and limited penetration depth of 250–500 μm considerably reduce the clinical usefulness of this approach.
Photosensitizers
Topical or systemic application of photosensitizers can selectively render pathologic tissues fluorescent when exposed to specific wavelengths of light, this technique has extensively been used for skin and esophageal cancer [70,71]. This induced fluorescence can be used to identify and delineate areas of pathology. Although the fluorescence may be strong enough to be detected with the naked eye [72,73], usually some sort of fluorescence detection device is used to enhance fluorescence visualization and assist with accurate lesion mapping. While many agents are under investigation, or in clinical use outside of the USA, the US FDA approval for photo-sensitizing drugs remains limited. Some promising agents for pho-todetection include aminolevulinic acid (ALA; Levulan®), hexyl aminolevulinate (Hexvix®), methyl aminolevulinate (Metvix®), tetra(meta-hydroxyphenyl)chlorin (mTHPC®), as well as porfimer sodium (Photofrin®) [72-76]. In a blinded clinical study of 20 patients with oral neoplasms, the diagnostic sensitivity using unaided visual fluorescence diagnosis or fluorescence microscopy approximated 93%. Diagnostic specificity was 95% for visual diagnosis, improving to 97% using fluorescence microscopy. The differences between healthy tissue versus dysplasia versus malignancy were all significant (p < 0.05) [73].
Advantages of a photosensitizer-based diagnostics approach include the capability for 3D surface and subsurface mapping of lesion margins using available imaging technologies, the ability to inspect large surface areas, noninvasiveness and the capability for subsequent photodestruction of the photosensitized lesion. Depending on the photosensitizer used and its mode of application (systemic vs topical), limitations include systemic photo-sensitization over prolonged periods of time, limited penetration depth, the need for specialized fluorescence detection and mapping equipment, and lack of specificity when inflammation or scar tissues are present.
Optical coherence tomography
Optical coherence tomography (OCT) is an imaging modality capable of providing noninvasive cross-sectional imaging of biological tissue [77-79]. Frequently, OCT is compared with ultrasound imaging because both technologies employ backscattered signals reflected from different layers within the tissue to reconstruct structural images. In contrast to conventional medical imaging modalities, OCT provides images with high resolution (micrometer scale) in real time. These images can be obtained noninvasively in vivo by the use of flexible fiberoptic OCT probes. OCT has a wide range of potential applications in diagnosing diseases in various structures, such as the eye, skin, gastrointestinal, respiratory and genitourinary tracts, cardiology and the oral cavity [80-100]. OCT penetration depths in soft tissue are approximately 2–3 mm, enabling high-resolution imaging of the surface and subsurface tissues as well as any structures accessible by endoscopic probes with near histopathological level resolution [101]. This permits in vivo noninvasive imaging of the macroscopic characteristics of epithelial and subepithelial structures, including depth and thickness, histopathological appearance and peripheral margins.
Optical coherence tomography was first used to visualize human tissue in 1991. It has been since refined and accepted as a necessary imaging modality in ophthalmology and non-ophthalmologic OCT systems are increasingly becoming available for medical use. Conventional time domain OCT is based on a scanning optical delay line, which limits its imaging speed [102]. A relatively new technology of OCT, Fourier domain OCT, which measures the magnitude and delay of backscattered light by spectral analysis of the interference pattern, can achieve a 50–500-fold increase in imaging speed and a much higher sensitivity compared with the time domain OCT technique [103,104]. Two methods have been developed for the Fourier domain techniques: a spectrometer-based system that uses a high-speed line-scan camera [105,106] and a swept-laser source-based system that uses a fast wavelength scanning laser [107-110]. At the working wavelength of 800 nm for ophthalmology imaging, the spectrometer-based system is dominant since it can use the economically priced high-speed line-scan cameras and superluminescent diode (SLD) light sources with broad bandwidths for high axial resolutions [105,106]. At the working wavelength of 1310 nm, a customized large-array line-scan camera has to be fabricated for this system, making the swept-source-based approach a better choice for this wavelength region. Swept-source OCT (SSOCT) systems have reduced sensitivity roll-off with imaging depth compared with spectrometer-based systems [104]. In addition, SSOCT has the advantages of a simple system setup and the capability for balanced detection. A narrower spectral line width can also be achieved without crosstalk, resulting in a larger imaging range.
Clinical studies using OCT for oral premalignancy & malignancy
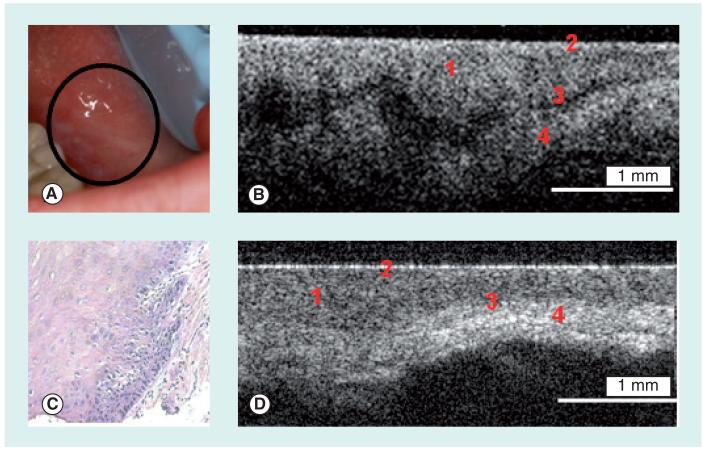
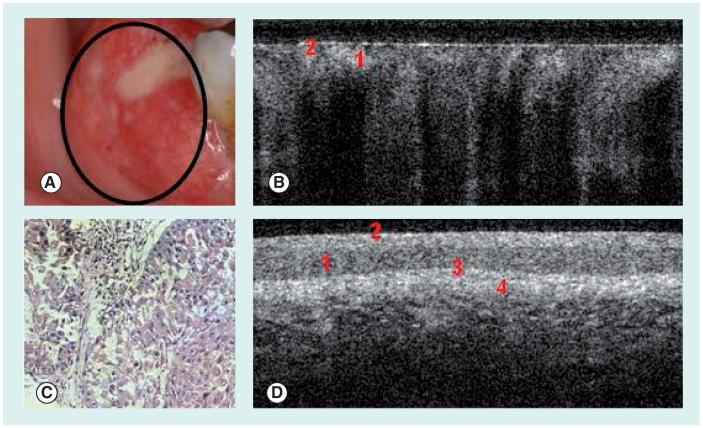
In a recent independent, blinded study, the clinical diagnostic capability of in vivo OCT for oral dysplasia and malignancy was investigated in 50 patients with oral leukoplakia or erythroplakia lesions [111]. The OCT image of a dysplastic lesion (Figure 1B) parallels histopathological status (Figure 1C), showing epithelial thickening, loss of stratification in lower epithelial strata, epithelial downgrowth and loss of epithelial stratification as compared with healthy oral mucosa (Figure 1D). Figures 2A & C show clinical appearance and histopathology, respectively, of an area of SCC on the buccal mucosa. In the OCT image (Figure 2B), the epithelium is highly variable in thickness, with areas of erosion and extensive downgrowth and invasion into the subepithelial layers. The basement membrane is not visible as a coherent landmark. Statistical analysis of the data confirmed the capability of in vivo OCT for detecting and diagnosing oral premalignancy and malignancy in human subjects, with excellent intraobserver agreement between OCT scores (Cohen’s kappa of 0.872), interobserver agreement for OCT (Cohen’s kappas of 0.870), agreement between OCT and histopathology (Cohen’s kappa of 0.896). For detecting carcinoma in situ or SCC versus non-cancer, sensitivity was 93.1% and specificity was 93.1%; for detecting SCC versus all other pathologies, sensitivity was 93.1% and specificity was 97.3%. Anticipated difficulties with movement artifacts were successfully avoided by seating patients in a reclining dental chair with headrest, neck and arm support. OCT imaging was rapid, unproblematic and well-received by all patients, with the imaging protocol adding only a few minutes to visit duration. An added benefit of the imaging procedure was improved patient compliance for necessary biopsy procedures after viewing OCT images. Thus, the introduction of OCT imaging techniques to routine patient visits should be well-received by patients and clinicians alike.
Figure 1. Dysplastic and normal buccal mucosa.
(A) Photograph, (B) in vivo optical coherence tomography image and (C) hematoxylin and eosin (10×) of dysplastic buccal mucosa. (D) In vivo optical coherence tomography image of normal buccal mucosa. 1: Stratified squamous epithelium; 2: Keratinized epithelial surface layer; 3: Basement membrane; 4: Submucosa.
Reprinted with permission from Wiley-Liss, Inc., a subsidiary of John Wiley & Sons, Inc. [8].
Figure 2. Squamous cell carcinoma of the buccal mucosa.
(A) Photograph, (B) in vivo optical coherence tomography image and (C) hematoxylin and eosin (10×) of buccal mucosa with squamous cell carcinoma. (D) In vivo optical coherence tomography image of normal buccal mucosa.
1: Stratified squamous epithelium; 2: Keratinized epithelial surface layer; 3: Basement membrane; 4: Submucosa.
Reprinted with permission of Wiley-Liss, Inc. a subsidiary of John Wiley & Sons, Inc. [8].
Other studies utilized direct analysis of OCT scan profiles rather than image-based criteria as a means of delineating oral cancer lesions [100,101]. Specifically, the lateral variation of A-scan profiles to demonstrate two parameters of the OCT signal was used. One of the parameters is the decay constant in the exponential fitting of the OCT signal intensity along depth. This decay constant decreases as the A-scan point moves laterally across the margin of a lesion. The other parameter is the standard deviation of the SSOCT signal intensity fluctuation in an A-scan. This parameter increased significantly when the A-scan point was moved across the transition region between the normal and abnormal portions. Such parameters may well be useful for establishing an algorithm for detecting and mapping the margins of oral cancer lesions. This capability has a huge clinical significance because of the field cancerization effect in the oral cavity and because of the need to better define excisional margins during surgical removal of oral premalignancy and malignancy.
Optical coherence tomography is also under investigation for a host of innovative applications in related anatomical sites, such as the upper GI tract – especially Barrett’s esophagus – and in otolaryngology. These topics fall outside the scope of this article, but we encourage readers to peruse some of these outstanding and informative papers.
Expert commentary
The need for improved early detection and diagnosis of oral lesions, and the importance of adequate noninvasive monitoring and screening tools is obvious. Early clinical trials using in vivo OCT imaging have demonstrated the potential for effective OCT-based oral diagnosis and for developing a strong diagnostic algorithm for mapping oral lesions based on data from OCT scans. Thus, these studies support the concept that OCT will be a very useful tool for the early detection and diagnosis of oral lesions, as well as regular monitoring of suspect lesions in the oral cavity and rapid, low-cost screening of high-risk populations. Particularly attractive to clinicians are the ease and speed of imaging, the ability to view immediately and at high-resolution the surface and subsurface microanatomy of the tissues, and ease of image interpretation [110]. In its early stages, as OCT is introduced into a wider clinical setting and undergoes further testing and optimization, its initial primary use may be as a screening and diagnostic tool in a single episode of care ‘see and treat’ protocol for preinvasive oral cancer. As the technology and techniques evolve, this modality should progressively reduce the need for biopsy, define surgical margins and provide a direct evaluation of the effectiveness of therapy.
Five-year view
The potential for OCT-based diagnostics in the oral cavity is excellent. The penetration depth of this modality in oral hard and soft tissues is adequate for most dental applications. Ongoing innovations include 3D imaging, Fourier-domain OCT, permitting up to 100-times faster acquisition of 3D OCT images and spectral OCT, providing enhanced image contrast and permitting spatially-resolved detection and quantification of changes within the tissues. Polarization-sensitive OCT can be used to enhance collagen imaging, and Doppler OCT for adding a vascular component to the imaging data. Improvements in OCT probe technologies, including emerging microelectromechanical system-based techniques, which permit the construction of very small probes with high-resolution suitable for endoscopic use, are significant factors permitting better and faster access to imaging sites. Several manufacturers are working on the development of OCT systems specifically for use in the oral cavity, and over the next 5 years we expect to see several oral OCT devices become available to clinicians.
As OCT resolution capabilities improve, a need has arisen for better contrast levels in the images. Several different approaches to this challenge are currently under investigation. Gold nano-particles are particularly promising in vivo OCT contrast agents because they are biocompatible, easy to synthesize and functional with additional modalities. Doubtless, OCT contrast-enhancing agents that can be used clinically will be a topic of great interest over the next 5 years.
Key issues.
Oral cancer contributes to a large portion of deaths occurring due to cancer each year. This is primarily due to the fact that most oral cancers are detected late, when treatment options are poor.
Current methods rely primarily on visual diagnosis and conformational biopsies.
A new clinical modality that is direct, noninvasive, quick, and cost effective to screen at-risk populations is needed.
Optical coherence tomography (OCT) has shown promise in early clinical trials to effectively diagnose oral dysplasia and malignancies.
OCT is easy to use and relatively inexpensive.
OCT has the potential to be easily integrated into a primary use for oral cancer screenings.
Means of combining OCT with other diagnostic entities, such as acoustics, nanoparticles or molecular markers, are important concepts for the future.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Michael DeCoro, Beckman Laser Institute, 1002 Health Sciences Rd East, University of California, Irvine, CA 92612, USA mdecoro@uci.edu.
Petra Wilder-Smith, Beckman Laser Institute, 1002 Health Sciences Rd East, University of California, Irvine, CA 92612, USA, Tel.: +1 949 824 4713, Fax: +1 949 824 8413..
References
Papers of special note have been highlighted as:
•• of considerable interest
- 1.American Cancer Society . Cancer Facts and Figures. American Cancer Society Report; 2008. pp. 1–4. [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun J. CA Cancer J. Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J. Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 4.Regezi JA, Sciubba J. Oral Pathology. WB Saunders Co; NY, USA: 1993. pp. 77–90. [Google Scholar]
- 5.Acha A, Ruesga MT, Rodriguez MJ, Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. [PubMed] [Google Scholar]
- 6.Poate T, Buchanan J, Hodgson T, et al. An audit of the efficacy of the oral brush biopsy technique in a specialist oral medicine unit. Oral Oncol. 2004;40:829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Horowitz AM. Perform a death-defying act: the 90-second oral cancer examination. J. Am. Dent. Assoc. 2001;132(Suppl.):36S–40S. [PubMed] [Google Scholar]
- 8.Horowitz AM, Drury TF, Goodman HS, Yellowitz JA. Oral pharyngeal cancer prevention and early detection. Dentists’ opinions and practices. J. Am. Dent. Assoc. 2000;131(4):453–462. doi: 10.14219/jada.archive.2000.0201. [DOI] [PubMed] [Google Scholar]
- 9••.Alfano MC, Horowitz AM. Professional and community efforts to prevent morbidity and mortality from oral cancer. J. Am. Dent. Assoc. 2001;132(Suppl.):24S–29S. doi: 10.14219/jada.archive.2001.0385. Provides an excellent evaluation of approaches to educating and mobilizing the dental profession and the public regarding oral cancer.
- 10.Downer MC, Jullien JA, Speight PM. An interim determination of health gain from oral cancer and precancer screening: preselecting high risk individuals. Community Dent. Health. 1998;15(2):72–76. [PubMed] [Google Scholar]
- 11••.Lingen MW, Kalmar JR, Karrison T, Speight PM. Critical evaluation of diagnostic aids for the detection of oral cancer. Oral Oncol. 2008;44:10–22. doi: 10.1016/j.oraloncology.2007.06.011. Examines the literature associated with current oral cancer screening and case-finding aids. The characteristics of an ideal screening test are outlined and the authors pose several questions for clinicians and scientists to consider in the evaluation of current and future studies of oral cancer detection and diagnosis.
- 12.Trullenque-Eriksson A, Muñoz-Corcuera M, Campo-Trapero J, et al. Analysis of new diagnostic methods in suspicious lesions of the oral mucosa. Med. Oral Pathol. Oral Cir. Bucal. 2009;14(5):E210–E216. [PubMed] [Google Scholar]
- 13.Petersen PE, Yamamoto T. Community improving the oral health of older people: the approach of the WHO global oral health programme. Dent. Oral Epidemiol. 2005;33(2):81–92. doi: 10.1111/j.1600-0528.2004.00219.x. [DOI] [PubMed] [Google Scholar]
- 14.California Department of Health Services . Cancer Surveillance Section Annual Report. Mar, 1999. [Google Scholar]
- 15.Slaughter DP, Southwick HW, Smejkal W. Field cancerization in oral stratified squamous epithelium. Cancer. 1953;6:963–968. doi: 10.1002/1097-0142(195309)6:5<963::aid-cncr2820060515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 16.Hunter KD, Parkinson EK, Harrison PR. Profiling early head and neck cancer. Nat. Rev. Cancer. 2005;5(2):127–135. doi: 10.1038/nrc1549. [DOI] [PubMed] [Google Scholar]
- 17.Epstein JB, Feldman R, Dolor RJ, Porter SR. The utility of tolonium chloride rinse in the diagnosis of recurrent or second primary cancers in patients with prior upper aerodigestive tract cancer. Head Neck. 2003;25(11):911–921. doi: 10.1002/hed.10309. [DOI] [PubMed] [Google Scholar]
- 18.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2002;68(10):617–621. [PubMed] [Google Scholar]
- 19.Silverman S, Migliorati C, Barbosa J. Toluidine blue staining in the detection of oral precancerous and malignant lesions. Oral Surg. Oral Med. Oral Pathol. 1984;57:379–382. doi: 10.1016/0030-4220(84)90154-3. [DOI] [PubMed] [Google Scholar]
- 20.Epstein J, Scully C, Spinelli U. Toluidine blue and Lugol’s iodine solution for the assessment of oral malignant disease and lesions at risk of malignancy. J. Oral Pathol. Med. 1992;21:160–163. doi: 10.1111/j.1600-0714.1992.tb00094.x. [DOI] [PubMed] [Google Scholar]
- 21.Patton LL. The effectiveness of community-based visual screening and utility of adjunctive diagnostic aids in the early detection of oral cancer. Oral Oncol. 2003;39(7):708–723. doi: 10.1016/s1368-8375(03)00083-6. [DOI] [PubMed] [Google Scholar]
- 22.Onofre MA, Sposto MR, Navarro CM. Reliability of toluidine blue application in the detection of oral epithelial dysplasia and in situ and invasive squamous cell carcinomas. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2001;91(5):535–540. doi: 10.1067/moe.2001.112949. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Williams M, Poh CF, et al. Toluidine blue staining identifies high-risk primary oral premalignant lesions with poor outcome. Cancer Res. 2005;65:8017–8021. doi: 10.1158/0008-5472.CAN-04-3153. [DOI] [PubMed] [Google Scholar]
- 24.Farah CS, McCullough MJ. A pilot case control study on the efficacy of acetic acid wash and chemiluminescent illumination (ViziLite) in the visualisation of oral mucosal white lesions. Oral Oncol. 2007;43(8):820–824. doi: 10.1016/j.oraloncology.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Patton LP, Epstein JB, Kerr RA. Adjunctive techniques for oral cancer examination and lesion diagnosis: a systematic review of the literature. J. Am. Dent. Assoc. 2008;139:896–905. doi: 10.14219/jada.archive.2008.0276. [DOI] [PubMed] [Google Scholar]
- 26.Epstein JB, Silverman S, Jr, Epstein JD, Lonky SA, Bride MA. Analysis of oral lesion biopsies identified and evaluated by visual examination, chemiluminescence and toluidine blue. Oral Oncol. 2008;44:538–544. doi: 10.1016/j.oraloncology.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Poate TW, Buchanan JA, Hodgson TA, et al. An audit of the efficacy of the oral brush biopsy technique in a specialist oral medicine unit. Oral Oncol. 2004;40(8):829–834. doi: 10.1016/j.oraloncology.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Acha A, Ruesga MT, Rodriguez MJ, Martinez de Pancorbo MA, Aguirre JM. Applications of the oral scraped (exfoliative) cytology in oral cancer and precancer. Oral Med. Oral Pathol. Oral Cir. Bucal. 2005;10(2):95–102. [PubMed] [Google Scholar]
- 29.Ogden GR, Cowpe JG, Green MW. Detection of field change in oral cancer using oral exfoliative cytologic study. Cancer. 1991;68:1611–1615. doi: 10.1002/1097-0142(19911001)68:7<1611::aid-cncr2820680724>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 30.El-Naggar AK, Mao L, Staerkel G, et al. Genetic heterogeneity in saliva from patients with oral squamous carcinomas: implications in molecular diagnosis and screening. J. Mol. Diagn. 2001;3:164–170. doi: 10.1016/S1525-1578(10)60668-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Epstein JB, Zhang L, Rosin M. Advances in the diagnosis of oral premalignant and malignant lesions. J. Can. Dent. Assoc. 2002;68:617–621. [PubMed] [Google Scholar]
- 32.Izarzugaza MI, Esparza H, Aguirre JM. Epidemiological aspects of oral and pharyngeal cancers in the Basque Country. J. Oral Pathol. Med. 2001;30:521–526. doi: 10.1034/j.1600-0714.2001.300902.x. [DOI] [PubMed] [Google Scholar]
- 33.Sciubba JJ. Improving detection of precancerous and cancerous oral lesions: computer-assisted analysis of the oral brush biopsy. J. Am. Dent. Assoc. 1999;130:1445–1457. doi: 10.14219/jada.archive.1999.0055. [DOI] [PubMed] [Google Scholar]
- 34.Ogden GR, Cowpe JG, Green M. Cytobrush and wooden spatula for oral exfoliative cytology. A comparison. Acta Cytol. 1992;36:706–710. [PubMed] [Google Scholar]
- 35.Jones AC, Pink FE, Sandow PL, Stewart CM, Migliorati CA, Baughman RA. The cytobrush plus cell collector in oral cytology. Oral Surg. Oral Med. Oral Pathol. 1994;77:95–99. [PubMed] [Google Scholar]
- 36.Nichols ML, Quinn FB, Jr, Schnadig VJ, et al. Interobserver variability in the interpretation of brush cytologic studies from head and neck lesions. Arch. Otolaryngol. Head Neck Surg. 1991;117:1350–1355. doi: 10.1001/archotol.1991.01870240042006. [DOI] [PubMed] [Google Scholar]
- 37.Rick GM, Slater L. Oral brush biopsy: the problem of false positives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003;96:252. doi: 10.1016/s1079-2104(03)00362-7. [DOI] [PubMed] [Google Scholar]
- 38.Remmerbach TW, Weidenbach H, Muller C, et al. Diagnostic value of nucleolar organizer regions (AgNORs) in brush biopsies of suspicious lesions of the oral cavity. Anal. Cell. Pathol. 2003;25:139–146. doi: 10.1155/2003/647685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boyle JO, Mao L, Brennan JA, et al. Gene mutations in saliva as molecular markers for head and neck squamous cell carcinomas. Am. J. Surg. 1994;168:429–432. doi: 10.1016/s0002-9610(05)80092-3. [DOI] [PubMed] [Google Scholar]
- 40.Rosas SL, Koch W, da Costa Carvalho MG, et al. Promoter hypermethylation patterns of p16, O6-methylguanine-DNAmethyltransferase, and death-associated protein kinase in tumors and saliva of head and neck cancer patients. Cancer Res. 2001;61:939–942. [PubMed] [Google Scholar]
- 41.Rosin MP, Epstein JB, Berean K, et al. The use of exfoliative cell samples to map clonal genetic alterations in the oral epithelium of high-risk patients. Cancer Res. 1997;57:5258–5260. [PubMed] [Google Scholar]
- 42.Okami K, Imate Y, Hashimoto Y, Kamada T, Takahashi M. Molecular detection of cancer cells in saliva from oral and pharyngeal cancer patients. Tokai J. Exp. Clin. Med. 2002;27:85–89. [PubMed] [Google Scholar]
- 43.Huang MF, Chang YC, Liao PS, Huang TH, Tsay CH, Chou MY. Loss of heterozygosity of p53 gene of oral cancer detected by exfoliative cytology. Oral Oncol. 1999;35:296–301. doi: 10.1016/s1368-8375(98)00119-5. [DOI] [PubMed] [Google Scholar]
- 44••.Bigio IJ, Bown SG. Spectroscopic sensing of cancer and cancer therapy: current status of translational research. Cancer Biol. Ther. 2004;3(3):259–267. doi: 10.4161/cbt.3.3.694. Reviews briefly the most common methods of diagnostic optical spectroscopy, and reviews recent clinical translational research invoking scattering spectroscopy as the enabling technology.
- 45.McGee SA, Mirkovic J, Mardirossian V, et al. Model-based spectroscopic analysis of the oral cavity: impact of anatomy. J. Biomed. Opt. 2008;13(6):064034. doi: 10.1117/1.2992139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Culha M, Stokes D, Vo-Dinh T. Surface-enhanced Raman scattering for cancer diagnostics: detection of the BCL2 gene. Expert Rev. Mol. Diagn. 2003;3(5):669–675. doi: 10.1586/14737159.3.5.669. [DOI] [PubMed] [Google Scholar]
- 47.Choo-Smith LP, Edwards HG, Endtz HP, et al. Medical applications of Raman spectroscopy: from proof of principle to clinical implementation. Biopolymers. 2002;67(1):1–9. doi: 10.1002/bip.10064. [DOI] [PubMed] [Google Scholar]
- 48.Bigio IJ, Mourant JR. Ultraviolet and visible spectroscopies for tissue diagnostics: fluorescence spectroscopy and elastic-scattering spectroscopy. Phys. Med. Biol. 1997;42(5):803–814. doi: 10.1088/0031-9155/42/5/005. [DOI] [PubMed] [Google Scholar]
- 49.Farrell TJ, Patterson MS, Wilson B. A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med. Phys. 1992;19(4):879–888. doi: 10.1118/1.596777. [DOI] [PubMed] [Google Scholar]
- 50.Sokolov K, Follen M, Richards-Kortum R. Optical spectroscopy for detection of neoplasia. Curr. Opin. Chem. Biol. 2002;6:651–658. doi: 10.1016/s1367-5931(02)00381-2. [DOI] [PubMed] [Google Scholar]
- 51.Sharwani A, Jerjes W, Salih V, et al. Assessment of oral premalignancy using elastic scattering spectroscopy. Oral Oncol. 2006;42(4):343–349. doi: 10.1016/j.oraloncology.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 52••.Upile T, Jerjes W, Betz CS, El Maaytah M, Wright A, Hopper C. Optical diagnostic techniques in the head and neck. Dent. Update. 2007;34(7):410–412. 415–416, 419–420. doi: 10.12968/denu.2007.34.7.410. Compares findings from recent prospective studies on microendoscopy, fluorescence spectroscopy and elastic scattering spectroscopy with histopathology to assess if those techniques can be used as an adjunct or alternative to histopathology in defining tissue involvement.
- 53.Lane PM, Gilhuly T, Whitehead P, et al. Simple device for the direct visualization of oral-cavity tissue fluorescence. J. Biomed. Opt. 2006;11(2):024006. doi: 10.1117/1.2193157. [DOI] [PubMed] [Google Scholar]
- 54••.Poh CF, Ng SP, Williams PM, et al. Direct fluorescence visualization of clinically occult high-risk oral premalignant disease using a simple hand-held device. Head Neck. 2007;29:71–76. doi: 10.1002/hed.20468. Three representative cases in which occult lesions were identified with fluorescence visualization underwent longitudinal follow-up, resulting in the diagnosis of a primary dysplasia in case 1, a second primary cancer in case 2 and cancer recurrence in case 3.
- 55.Poh CF, Zhang L, Anderson DW, et al. Fluorescence visualization detection of field alterations in tumor margins of oral cancer patients. Clin. Cancer Res. 2006;12(22):6716–6722. doi: 10.1158/1078-0432.CCR-06-1317. [DOI] [PubMed] [Google Scholar]
- 56••.Roblyer D, Kurachi C, Stepanek V, et al. Objective detection and delineation of oral neoplasia using autofluorescence imaging. Cancer Prev. Res. 2009;2(5):423–431. doi: 10.1158/1940-6207.CAPR-08-0229. Current approaches to autofluorescence imaging rely on subjective interpretation. This report validates an algorithm to objectively delineate neoplastic oral mucosa using autofluorescence imaging in 56 patients with oral lesions and 11 normal volunteers.
- 57.Rosin MP, Poh CF, Guillard M, Williams PM, Zhang L, MacaUlay C. Visualization and other emerging technologies as change makers for oral cancer prevention. Ann. NY Acad. Sci. 2007;1098:167–183. doi: 10.1196/annals.1384.039. [DOI] [PubMed] [Google Scholar]
- 58.Schwarz RA, Gao W, Redden Webber C, et al. Noninvasive evaluation of oral lesions using depth-sensitive optical spectroscopy simple device for the direct visualization of oral-cavity tissue fluorescence. Cancer. 2009;115(8):1669–1679. doi: 10.1002/cncr.24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Veld DC, Witjes MJ, Sterenborg HJ, Roodenburg JL. The status of in vivo autofluorescence spectroscopy and imaging for oral oncology. Oral Oncol. 2005;41:117–131. doi: 10.1016/j.oraloncology.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 60.Wagnieres GA, Star WM, Wilson BC. In vivo fluorescence spectroscopy and imaging for oncological applications. Photochem. Photobiol. 1998;68(5):603–632. [PubMed] [Google Scholar]
- 61.Ramanujam N. Fluorescence spectroscopy of neoplastic and non-neoplastic tissues. Neoplasia. 2000;2(1-2):89–117. doi: 10.1038/sj.neo.7900077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwarz RA, Gao W, Redden Weber C, et al. Noninvasive evaluation of oral lesions using depth-sensitive optical spectroscopy. Cancer. 2009;115(8):1669–1679. doi: 10.1002/cncr.24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rahman M, Chaturvedi P, Gillenwater AM, Richards-Kortum R. Low-cost, multimodal, portable screening system for early detection of oral cancer. J. Biomed. Opt. 2008;13(3):0305020. doi: 10.1117/1.2907455. [DOI] [PubMed] [Google Scholar]
- 64.Inoue H, Igari T, Nishikage T, Ami K, Yoshida T, Iwai T. A novel method of virtual histopathology using laser-scanning confocal microscopy in-vitro with untreated fresh specimens from the gastrointestinal mucosa. Endoscopy. 2000;32:439–443. doi: 10.1055/s-2000-654. [DOI] [PubMed] [Google Scholar]
- 65.White WM, Rajadhyaksha M, Gonzalez S, Fabian RL, Anderson RR. Noninvasive imaging of human oral mucosa in vivo by confocal reflectance microscopy. Laryngoscope. 1999;109:1709–1717. doi: 10.1097/00005537-199910000-00029. [DOI] [PubMed] [Google Scholar]
- 66.Clark AM, Gillenwater AM, Collier TG, et al. Confocal microscopy for real-time detection of oral cavity neoplasia. Clin. Cancer Res. 2003;9:4714–4721. [PubMed] [Google Scholar]
- 67.Thong PS, Olivo M, Kho KW, et al. Laser confocal endomicroscopy as a novel technique for fluorescence diagnostic imaging of the oral cavity. J. Biomed. Opt. 2007;12(1):014007. doi: 10.1117/1.2710193. [DOI] [PubMed] [Google Scholar]
- 68.Maitland KC, Gillenwater AM, Williams MD, El-Naggar AK, Descour MR, Richards-Kortum RR. In vivo imaging of oral neoplasia using a miniaturized fiber optic confocal reflectance microscope. Oral Oncol. 2008;44(11):1059–1066. doi: 10.1016/j.oraloncology.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kennedy JC, Pottier RH. Endogenous protoporphyrin IX, a clinically useful photosensitizer for photodynamic therapy. J. Photochem. Photobiol. B. 1992;14(4):275–292. doi: 10.1016/1011-1344(92)85108-7. [DOI] [PubMed] [Google Scholar]
- 70.Cassas A, Fukuda H, Battle A. Dougherty TJ, editor. Hexyl ALA ALA-based photodynamic therapy in epithelial tumors: in vivo and in vitro models. Proc. SPIE. 2002;3909:114–123. Optical methods for tumor treatment and detection: mechanisms and techniques in photodynamic therapy IX. [Google Scholar]
- 71.Ebihara A, Liaw L-H, Krasieva TB, et al. Detection and diagnosis of oral cancer by light-induced fluorescence. Lasers Surg. Med. 2003;32(1):17–24. doi: 10.1002/lsm.10137. [DOI] [PubMed] [Google Scholar]
- 72.Chang CJ, Wilder-Smith P. Topical application of photofrin for photodynamic diagnosis of oral neoplasms. Plast. Reconstr. Surg. 2005;115(7):1877–1886. doi: 10.1097/01.prs.0000164684.69899.7b. [DOI] [PubMed] [Google Scholar]
- 73.Leunig A, Rick K, Stepp H, et al. Fluorescence imaging and spectroscopy of 5-aminolevulinic acid induced protoporphyrin IX for the detection of neoplastic lesions in the oral cavity. Am. J. Surg. 1996;172(6):674–677. doi: 10.1016/s0002-9610(96)00312-1. [DOI] [PubMed] [Google Scholar]
- 74.Leunig A, Mehlmann M, Betz C, et al. Detection of squamous cell carcinoma of the oral cavity by imaging 5-aminolevulinic acid-induced protoporphyrin IX fluorescence. Laryngoscope. 2000;110(1):78–83. doi: 10.1097/00005537-200001000-00015. [DOI] [PubMed] [Google Scholar]
- 75.Leunig A, Mehlmann M, Betz C, et al. Fluorescence staining of oral cancer using a topical application of 5-aminolevulinic acid: fluorescence microscopic studies. J. Photochem. Photobiol. B. 2001;60(1):44–49. doi: 10.1016/s1011-1344(01)00117-8. [DOI] [PubMed] [Google Scholar]
- 76.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fercher AF. Optical coherence tomography. J. Biomed. Opt. 1996;1:157–173. doi: 10.1117/12.231361. [DOI] [PubMed] [Google Scholar]
- 78.Schmitt JM. Optical coherence tomography (OCT): a review. IEEE J. Sel. Topics Quantum Electron. 2001;7(2):931–935. [Google Scholar]
- 79.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye with optical coherence tomography. Arch. Ophthamol. 1994;112:1584–1589. doi: 10.1001/archopht.1994.01090240090031. [DOI] [PubMed] [Google Scholar]
- 80.Schmitt JM, Yadlowsky M, Bonner RF. Subsurface imaging if living skin with optical coherence tomography. Dermatology. 1995;191:93–98. doi: 10.1159/000246523. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi K, Izatt JA, Kulkarni MD, Willis J, Sivak MV. High-resolution cross-sectional imaging of the gastrointestinal tract using optical coherence tomography: preliminary results. Gastrointest. Endosc. 1998;47:515–523. doi: 10.1016/s0016-5107(98)70254-8. [DOI] [PubMed] [Google Scholar]
- 82.Yelbuz TM, Choma MA, Thrane L, Kirby ML, Izatt JA. Optical coherence tomography: a new high-resolution imaging technology to study cardiac development in chick embryos. Circulation. 2002;106:2771–2774. doi: 10.1161/01.cir.0000042672.51054.7b. [DOI] [PubMed] [Google Scholar]
- 83.Tearney GJ, Brezinski ME, Southern JF, et al. Optical biopsy in human urologic tissue using optical coherence tomography. J. Urol. 1997;157:1915–1919. [PubMed] [Google Scholar]
- 84.Colston BW, Everett MJ, Silva LB, et al. Imaging of hard and soft tissue structure in oral cavity by optical coherence tomography. Appl. Opt. 1998;37:3582–3585. doi: 10.1364/ao.37.003582. [DOI] [PubMed] [Google Scholar]
- 85.Zagaynova EV, Streltsova OS, Gladkova ND, et al. In vivo optical coherence tomography feasibility for bladder disease. J. Urol. 2002;167:1492–1496. [PubMed] [Google Scholar]
- 86.Pitris C, Jesser C, Boppart SA, et al. Feasibility of optical coherence tomography for high resolution imaging of human gastrointestinal tract malignancies. J. Gastroenterol. 2000;35:87–92. doi: 10.1007/s005350050019. [DOI] [PubMed] [Google Scholar]
- 87.Gonzalo N, Serruys PW, Okamura T, et al. Optical coherence tomography patterns of stent restenosis. Am. Heart J. 2009;158(2):284–293. doi: 10.1016/j.ahj.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 88.López-Guajardo L, Benitez-Herreros J, Teus-Guezala M. Optical coherence tomography as a method for studying sutureless microincisional vitrectomy sclerotomies. Am. J. Ophthalmol. 2009;148(2):321–322. doi: 10.1016/j.ajo.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 89.Arvanitakis M, Hookey L, Tessier G, et al. Intraductal optical coherence tomography during endoscopic retrograde cholangiopancreatography for investigation of biliary strictures. Endoscopy. 2009;41(8):696–701. doi: 10.1055/s-0029-1214950. [DOI] [PubMed] [Google Scholar]
- 90.Coxson HO, Mayo J, Lam S, Santyr G, Parraga G, Sin DD. New and current clinical imaging techniques to study chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2009 doi: 10.1164/rccm.200901-0159PP. [DOI] [PubMed] [Google Scholar]
- 91••.Ridgway JM, Armstrong WB, Guo S, et al. In vivo optical coherence tomography of the human oral cavity and oropharynx. Arch. Otolaryngol. Head Neck Surg. 2006;132(10):1074–1081. doi: 10.1001/archotol.132.10.1074. Optical coherence tomographic (OCT) imaging was combined with endoscopic photography of the oral cavity and oropharynx in 41 patients during operative endoscopy. This report demonstrates a composite series of in vivo OCT images of the oral cavity and oropharynx in a variety of normal regions and pathologic states.
- 92.Wang KK, Zhu TC. Reconstruction of in-vivo optical properties for human prostate using interstitial diffuse optical tomography. Opt. Express. 2009;17(14):11665–11672. doi: 10.1364/oe.17.011665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cucchiara S, Di Nardo G. Optical coherence tomography in children with coeliac disease. Dig. Liver Dis. 2009;41(9):630–631. doi: 10.1016/j.dld.2009.05.017. [DOI] [PubMed] [Google Scholar]
- 94.Williamson JP, James AL, Phillips MJ, Sampson DD, Hillman DR, Eastwood PR. Quantifying tracheobronchial tree dimensions: methods, limitations and emerging techniques. Eur. Respir. J. 2009;34(1):42–55. doi: 10.1183/09031936.00020408. [DOI] [PubMed] [Google Scholar]
- 95.Mogensen M, Thrane L, Jørgensen TM, Andersen PE, Jemec GB. OCT imaging of skin cancer and other dermatological diseases. J. Biophotonics. 2009;2(6-7):442–451. doi: 10.1002/jbio.200910020. [DOI] [PubMed] [Google Scholar]
- 96.Ozawa N, Sumi Y, Chong C, Kurabayashi T. Evaluation of oral vascular anomalies using optical coherence tomography. Br. J. Oral Maxillofac. Surg. 2009;47(8):622–626. doi: 10.1016/j.bjoms.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 97.Strebel J, Ender A, Paqué F, henmann M, Attin T, Schmidlin PR. In vivo validation of a three-dimensional optical method to document volumetric soft tissue changes of the interdental papilla. J. Periodontol. 2009;80(1):56–61. doi: 10.1902/jop.2009.080288. [DOI] [PubMed] [Google Scholar]
- 98.Baek JH, Na J, Lee BH, Choi E, Son WS. Optical approach to the periodontal ligament under orthodontic tooth movement: a preliminary study with optical coherence tomography. Am. J. Orthod. Dentofacial. Orthop. 2009;135(2):252–259. doi: 10.1016/j.ajodo.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 99.Tsai MT, Lee HC, Lu CW, et al. Delineation of an oral cancer lesion with swept-source optical coherence tomography. J. Biomed. Opt. 2008;13(4):044012. doi: 10.1117/1.2960632. [DOI] [PubMed] [Google Scholar]
- 100.Tsai MT, Lee HC, Lee CK, et al. Effective indicators for diagnosis of oral cancer using optical coherence tomography. Opt Express. 2008;16(20):15847–15862. doi: 10.1364/oe.16.015847. [DOI] [PubMed] [Google Scholar]
- 101.Bouma BE, Tearney GJ. Handbook of Optical Coherence Tomography. Marcel Dekker; NY, USA: 2002. [Google Scholar]
- 102.Drexler WM, Fujimoto JG. Optical Coherence Tomography Technology and Applications. Springer; NY, USA: 2008. [Google Scholar]
- 103.Leitgeb RA, Hitzenberger CK, Fercher AF. Performance of fourier domain vs. time domain optical coherence tomography. Opt. Express. 2003;11:889–894. doi: 10.1364/oe.11.000889. [DOI] [PubMed] [Google Scholar]
- 104.Wojtkowski M, Srinivasan V, Ko T, et al. Ultrahigh-resolution, high-speed, Fourier domain optical coherence tomography and methods for dispersion compensation. Opt. Express. 2004;12:2404–2422. doi: 10.1364/opex.12.002404. [DOI] [PubMed] [Google Scholar]
- 105.Cense B, Nassif N, Chen T, et al. Ultrahigh-resolution high-speed retinal imaging using spectral-domain optical coherence tomography. Opt. Express. 2004;12:2435–2447. doi: 10.1364/opex.12.002435. [DOI] [PubMed] [Google Scholar]
- 106.Yun S, Tearney G, de Boer J, Iftimia N, Bouma B. High-speed optical frequency-domain imaging. Opt. Express. 2003;11:2953–2963. doi: 10.1364/oe.11.002953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang J, Nelson JS, Chen Z. Removal of a mirror image and enhancement of the signal-to-noise ratio in Fourier-domain optical coherence tomography using an electro-optic phase modulator. Opt. Lett. 2005;30:147–149. doi: 10.1364/ol.30.000147. [DOI] [PubMed] [Google Scholar]
- 108.Huber R, Wojtkowski M, Taira K, Fujimoto J, Hsu K. Amplified, frequency swept lasers for frequency domain reflectometry and OCT imaging: design and scaling principles. Opt. Express. 2005;13:3513–3528. doi: 10.1364/opex.13.003513. [DOI] [PubMed] [Google Scholar]
- 109.Yasuno Y, Madjarova VD, Makita S, et al. Three-dimensional and high-speed swept-source optical coherence tomography for in vivo investigation of human anterior eye segments. Opt. Express. 2005;13:10652–10664. doi: 10.1364/opex.13.010652. [DOI] [PubMed] [Google Scholar]
- 110••.Wilder-Smith P, Lee K, Guo S, et al. In vivo diagnosis of oral dysplasia and malignancy using optical coherence tomography: preliminary studies in 50 patients. Lasers Surg. Med. 2009;41:353–357. doi: 10.1002/lsm.20773. Diagnostic ability of OCT imaging versus histopathology was investigated in 50 patients with oral lesions. Two blinded, prestandardized investigators separately diagnosed each lesion based on OCT and histopathology. This study demonstrated the excellent capability of in vivo OCT for detecting and diagnosing oral premalignancy and malignancy in human subjects.
Website
- 201.US Department of Health and Human Services A national call to action to promote oral health. www.nidr.nih.gov/sgr/nationalcalltoaction.htm