Abstract
The RecQ helicases are conserved from bacteria to humans and play a critical role in genome stability. In humans, loss of RecQ gene function is associated with cancer predisposition and/or premature aging. Recent data have shown that the RecQ helicases function during two distinct steps during DNA repair; DNA end resection and resolution of double Holliday junctions (dHJs). RecQ functions in these different processing steps has important implications for its role in repair of double-strand breaks (DSBs) that occur during DNA replication, meiosis and at specific genomic loci such as telomeres.
Keywords: RecQ, Werner Syndrome, Bloom Syndrome, Rothmund-Thomson Syndrome, Homologous Recombination, DNA repair
Introduction
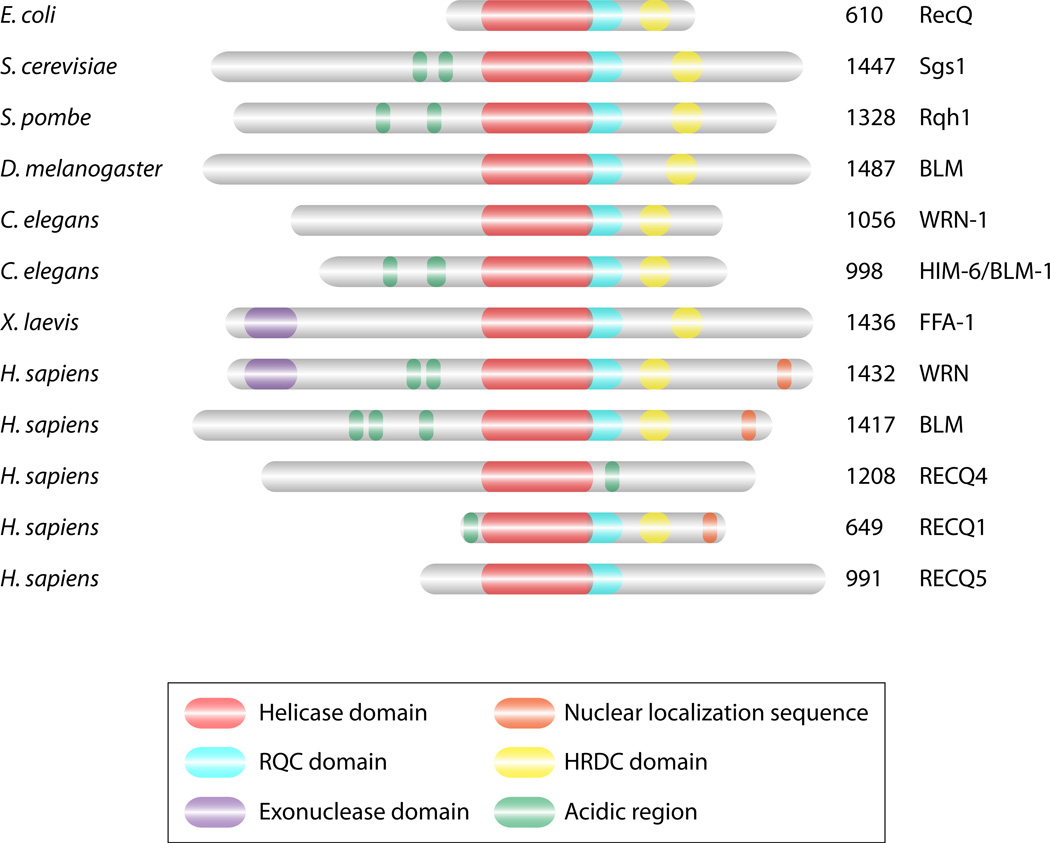
Unrepaired DNA damage can lead to genomic instability. When a DSB occurs, the DNA ends need to be processed for efficient repair from a homologous chromosome. Repair of DSBs requires many proteins with enzymatic activities such as nucleases, helicases, ligases, topoisomerases, etc. One important family of enzymes is DNA helicases and these enzymes function by unwinding complementary strands of DNA. The RecQ DNA helicases, conserved from bacteria to humans (Figure 1), are critical to ensure proper repair of DNA damage. Bacteria and budding yeast have one RecQ homologue, RecQ and Sgs1 respectively. In humans, there are five RecQ homologues and mutations in three of these genes (BLM, WRN, RTS/RECQ4) are associated with cancer predisposition and/or premature aging, Bloom, Werner, and Rothmund-Thomson syndromes, respectively (39, 74, 111, 121, 159).
Figure 1.
Structural features of RecQ helicases. The RecQ proteins have many structural domains that are conserved from bacteria through humans. The RecQ proteins all have a core helicase domain (red). Most RecQ proteins also contain conserved HRDC (Helicase and RNAse D C-terminal; yellow) and RQC (RecQ C-terminal; turquoise) domains that are thought to mediate interactions with nucleic acid or other proteins respectively. Many RecQ proteins have acidic regions (green) that enable protein-protein interactions while some of the RecQ proteins have nuclear localization sequences (NLS; orange). WRN and FFA-1 protein are unique in that they also contain an exonuclease domain (EXO; purple). The number of amino acids for each protein are indicated on the right.
Unwinding of double-stranded DNA (dsDNA) is necessary in many different processing steps. One of the challenges in studying the function of the RecQ helicases is that mutation of the RECQ genes leads to a pleiotropic phenotype, exhibiting traits that include increased chromosomal rearrangements, increased sister chromatid exchanges, premature aging, etc. The RecQ helicases have been pinpointed to function in both early and late recombination steps where they promote homologous recombination (HR) and prevent crossover events. This review will focus on the recent findings that the RecQ proteins function at multiple DNA processing steps and how these functions enable these proteins to act on many different biological substrates.
RecQ and human disease
In humans, mutations in three of the five members of the RecQ family lead to the separate genetic diseases, Bloom (BLM), Werner (WRN), and Rothmund-Thomson syndromes (RTS/RECQ4) (120) (Table 1). The other two RecQ helicases, RECQ1/RECQL1 and RECQL5 have not been associated with heritable diseases. However, a single nucleotide polymorphism in RECQ1 correlates with decreased survival of pancreatic cancer patients (84) and Recql5 knockout mice also display increased cancer rates (60).
Table 1.
Clinical features of RecQ disorders
| Syndrome (gene) | Main Clinical Features | Cancer Predisposition |
|---|---|---|
| Blooms syndrome (BLM) | Dwarfism, beaked nose, narrow face, pigmentation, redness, and dilated blood vessels in skin, mental retardation, type-II diabetes, immunodeficiency, lung problems, low or no fertility | Early onset with normal distribution of tissue and type |
| Werner syndrome (WRN) | Bilateral cataracts, hoarseness, skin alterations, thin limbs, premature gray/loss of hair, pinched facial features, short stature, osteoporosis, hypogonadism, diabetes, soft tissue calcification | Early onset of primary sarcomas and mesenchymal tumors |
| Rothmund-Thomson syndrome (RECQ4) | Poikiloderma, juvenile cataracts, growth retardation, skeletal dysplasia, sparse scalp hair, hypogonadism | Early onset of osteosarcomas |
| RAPADILINO syndrome (RECQ4) | Growth retardation, bone malformation such as limbs, radial defects such as hypoplasia and aplasia of thumbs and radius, cleft or highly arched palate | Lymphoma and osteosarcoma |
| Baller-Gerold syndrome (RECQ4) | Craniosynostosis, radial aplasia and hypoplasia, poikiloderma, growth retardation | N.A. |
Bloom syndrome
Bloom syndrome is a rare autosomal recessive genetic disorder characterized by growth retardation, light sensitivity, immunodeficiency, male infertility, and increased cancer (14, 120) (Table 1). However, it is the predisposition of Bloom patients to develop cancer, frequently occurring by the forth decade of life, which is the primary cause of death. This disease is due to mutations in the BLM gene and there are approximately 230 documented cases of Bloom syndrome patients (39, 48). From the extensive mutational analysis of the BLM gene in these patients, it was concluded that this disease likely originated with a small number of founder mutations (48). The increased cancer incidence correlates with chromosomal breaks and sister chromatid exchanges, both of which are increased in Bloom patient cells (47).
Mouse models of Bloom syndrome have been created to mimic the phenotype observed in humans with this disease. Blm is expressed during embryogenesis in mice in most tissue types with highest expression in spleen, thymus, testis, and ovaries (29). Disruption of the Blm gene, by insertion of a neomycin cassette in the region upstream to the helicase domain, leads to embryonic lethality at 13.5 days gestation in homozygous mutant mice, suggesting that in mice Blm expression is essential during embryogenesis (29). When embryos are analyzed at 9.5 days post conception (dpc), Blm−/− mice are 50% smaller than WT or heterozygous Blm+/− mice and this size discrepancy continues until death at 13.5 dpc, indicating that, like humans with Bloom disease, Blm−/− mice also display growth retardation (29). Also consistent with the human disease, embryonic fibroblasts derived from Blm−/− mice display increased sister chromatid exchanges, which can be seen in metaphase spreads of differentially stained chromosomes (29).
To mimic more closely the mutations found in humans, another Blm mutant mouse was generated. This mutant mouse mimics the predominant mutation found in Ashkenazi Jews, a truncation in the helicase domain of the BLM gene (39, 52). In this mouse model, exons 10, 11, and 12 were replaced (39, 52). Complete disruption of exons 10, 11, and 12 leads to embryonic lethality (52). However, mice heterozygous for this truncation are more susceptible to cancer when combined with other heterozygous mutations such as those that inactivate tumor-suppressor genes like Apc (52). This mouse model demonstrates that haplo-insufficiency is adequate to promote tumor formation.
Additional mouse models, created using ES cells with Cre-loxP excision, were also generated that have altered Blm transcripts. Using this technology, a few different Blm mutant alleles were made including one that truncated the protein similar to an allele observed in several Bloom patients (92). Blm mice with this truncation are viable and do not display growth retardation; however, they do show increased sister chromatid exchange (92). Unlike WT mice, by 20 months these Blm mice exhibit about a 30% rate of cancer development. Furthermore, similar to Bloom patients, the cancers observed in these mice represent a broad range (i.e. sarcomas, lymphomas, and carcinomas) (92). Consistent with increased sister chromatid exchange, ES cells derived from these mice have a 18-fold increase in loss-of-heterozygosity, which is one of the mechanisms that can lead to complete loss-of-function of tumor-suppressor genes in cancer (92).
Together, these mouse models demonstrate that the Blm protein normally functions by repressing sister chromatid exchange, which when unregulated, leads to loss-of-heterozygosity, providing a mechanism to explain why Bloom patients are particularly susceptible to a wide range of cancers.
Werner syndrome
Werner syndrome leads to premature aging, such as an early onset of diseases like cataracts and osteoporosis, as well as genomic instability causing predisposition to tumor formation (120) (Table 1). Interestingly, Werner patients typically exhibit normal development until adolescence and subsequently symptoms emerge in their early 20s that result in death around 46–54 years of age (53). Cells derived from Werner patients show an increased frequency of chromosomal rearrangements such as translocations, inversions, and deletions (42, 43, 119). However, Werner patient tumors have mesenchymal origin, such as sarcomas, which are distinct from those of Bloom patients (61). The vast majority of patients with Werner syndrome have been linked to a founder mutation in Japan (53).
Several mouse models have been created to mimic the phenotype observed in Werner patients. In one mouse model, the helicase domain was disrupted (specifically helicase domains III and IV) resulting in a truncated but stable protein (81). ES cells derived from these mice are not sensitive to many DNA damaging agents (i.e. UV, gamma irradiation, mitomycin C) but are sensitive to camptothecin, which specifically inhibits topoisomerase I (81). Disappointingly, this mutant mouse does not display the premature aging phenotype observed in Werner syndrome.
A breakthrough in recapitulating Werner syndrome in mice occurred when the effect of the Wrn mutation was examined in animals altered for telomere function (27). In a study by Chang et al, Wrn−/− mice were combined with null alleles of Terc, which encodes the telomerase RNA needed for telomere lengthening (27). After homozygous null mice were interbred for 4–6 generations, approximately 60% of the 14–16 week old Terc−/− Wrn−/− mice displayed features of premature aging (i.e. hair loss, cataract formation, hypogonadism) after a normal early adulthood (27). Many of these mice also showed early-onset osteoporosis, type II diabetes, and decreased wound healing. These results demonstrate that many of the key features observed in Werner patients could be explained, at least in part, by the role of WRN in maintenance of telomeres. By fluorescence in situ hybridization (FISH) studies, it was shown that cells derived from these mice had fused chromosome arms and shortened telomeres. Furthermore, mouse embryonic fibroblasts (MEFs) derived from these mice exhibit increased 53BP1 foci, a marker for DSB repair, and increased gamma-H2AX foci, an indicator of DSBs. Interestingly, the Terc−/− Wrn−/− mice are not cancer prone. However, Terc−/− Wrn−/− mice that were crossed to each other for 1–3 generations do develop many osteosarcomas and soft tissue sarcomas (27). Therefore, creation of the Werner mouse model demonstrated that, unlike the other RecQ homologues, WRN’s distinct role in telomere maintenance is likely inter-related to the clinical features observed in Werner patients.
RECQ4-associated diseases
Mutations in RECQ4 are associated with three unrelated disorders; Rothmund-Thomson syndrome (RTS), RAPADILINO syndrome, and Baller-Gerold syndrome (BGS) (75, 126, 127, 136) (Table 1). All of these disorders are characterized by growth retardation and radial defects. However, RAPADILINO syndrome patients do not exhibit poikiloderma, which is characteristic of both RTS and BGS. RAPADILINO is most prevalent in Finland, unlike the other two RECQ4 disorders (126). RTS is the best characterized of the RECQ4 diseases and these patients also have skeletal abnormalities, skin disorders, light sensitivity, and age prematurely (75, 120, 127). Rothmund-Thomson patients are especially susceptible to forming bone and skin cancer and their cells display increased chromosomal rearrangements like translocations and deletions (95).
Several mouse models have been created to mimic RTS. Since most Rothmund-Thomson patients have mutations in RECQ4 that predominately map to the helicase region, the mouse models have focused on this region. In one model, RECQ4 exon 13, which encodes motif III of the helicase domain, was deleted (59). Although the majority of the mice die within two weeks after birth, those that survived are smaller than wild-type mice (59). These mice also display skin abnormalities such as hair loss, graying hair, and thin, dry skin. However, unlike Rothmund-Thomson patients, these mice do not exhibit poikiloderma, osteosarcomas, and cataracts (59).
Another viable mouse model was created that more closely mimics RTS by disrupting the helicase domain encoded by exons 9 through 13 of Recq4 (93). This disruption leads to a truncated protein due to a premature stop codon and 16% of these mice die within 24 hours of birth. The surviving mice develop hypo-/hyper-pigmentation on their tails and show an increased incidence of skeletal defects in their limbs (93). Unlike RTS patients, all of these mice have palatal patterning defects and a wild-type life span. Like the Blm mutant mouse model, when the Recq4 mutant is combined with a mutant Apc tumor suppressor gene, the double mutant mice are more likely to develop cancer (93). MEFs derived from the Recq4 mutant mice exhibit chromosome instability resulting in aneuploidy. These mouse models for RTS demonstrate that many aspects of the phenotype observed in humans are also seen in mice with mutations in Recq4.
Structure of RecQ helicases, complex formation and binding partners
RecQ helicases have been found in bacteria, fungi, animals and plants, and their copy number ranges from one in E. coli and S. cerevisiae to up to seven in Arabidopsis (reviewed in (57)). They all share a similar overall structural organization where the helicase domain, containing a DEAH box, functions to unwind DNA in an ATP and Mg++ dependent manner (Figure 1). RecQ helicases belong to the SF2 superfamily (51), are involved in the resolution of DNA structures and travel on single-stranded DNA (ssDNA) in a 3'→5' direction (reviewed in (4)). The RQC domain (RecQ C-terminal) is characteristic of most RecQ helicases and is believed to mediate protein-protein interactions; however, it is absent in RECQ4 and RECQ5. The HRDC (Helicase and RNase D C-terminal) domain (80 amino acids) found at the C-terminus of many RecQ helicases, allows interactions with nucleic acid (88), but is absent from RECQ1, RECQ4 and RECQ5. The HRDC domain forms a structural scaffold; however, models of the HRDC domain have indicated that the surface properties are not equivalent in different RecQ helicases suggesting that they could be involved in the positioning of a specific substrate through steric interactions or secondary contacts with the DNA (88). In addition to the helicase, RQC, and HRDC domains, some members possess a 3'→5' exonuclease domain in their N-terminus (WRN and Xenopus FFA-1), a nuclear localization signal in their C-terminus (BLM and WRN) or acidic patches (BLM, WRN, RECQ1, RECQ4, Rqh1 or Sgs1) (Figure 1) (4, 73, 139, 152).
The quaternary structure of several RecQ helicases has been investigated. BLM forms principally hexameric ring structures, but a four-fold symmetric square form is also detected perhaps representing a distinct oligomeric species or a side view of the hexameric form (71). Unlike BLM, E. coli active RecQ exists as a monomeric protein (154, 160), while RECQ1 can form monomers or dimers (101, 107). Therefore, RecQ enzymes may adopt different structures depending on the cofactor bound to them (reviewed in (139)).
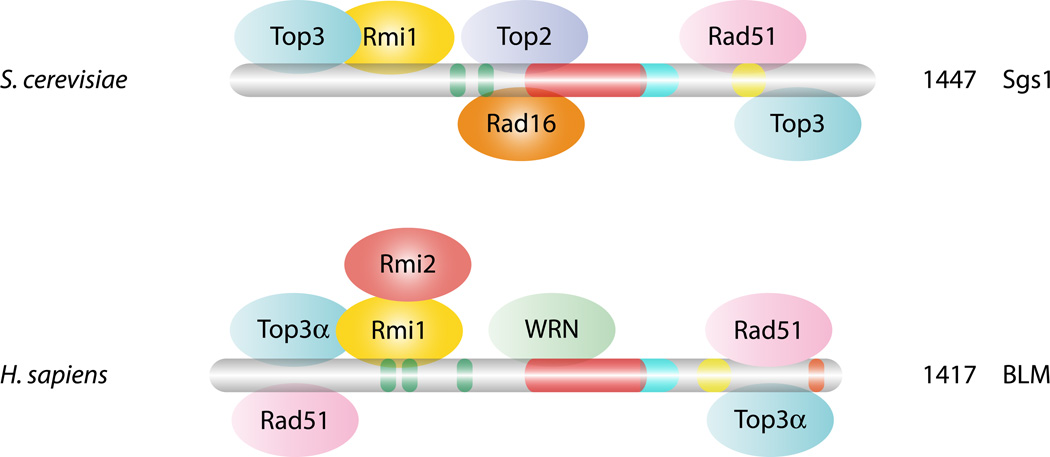
An evolutionarily conserved feature of RecQ helicases is their physical association with the Top3 type IA topoisomerase (Figure 2). In budding and fission yeast, Sgs1 and Rqh1, respectively, interact physically with Top3 (1, 8, 41, 45). Of the two isoforms of TOP3 in humans, BLM interacts with TOP3α (68, 151) while RECQ5 interacts with either TOP3α or TOP3β (125). Similar interactions for the other human RecQ homologues have either not been tested or not been found. Recently, a second conserved partner (Rmi1/Nce4-BLAP75) was identified in yeast (26, 100) and human cells (158) (Figure 2). RMI1 proteins contain an OB-fold found in many DNA binding proteins like RPA or Cdc13 (132), and biochemical studies have shown that Rmi1 forms a stable heteromeric complex with Sgs1-Top3 (BLM-TOP3α). Genetically, rmi1 mutants behave like top3 mutants (45, 141) and exhibit premature DNA damage checkpoint activation (158). Rmi1 is a structure-specific DNA binding protein with preferences for cruciforms that may help target Sgs1-Top3 to appropriate substrates (100). Rmi1 stimulates Top3 by promoting its interaction with ssDNA and its superhelical relaxation activity (28). Recently, in mammalian cells, an additional partner, RMI2 (BLAP18), was found to interact with RMI1 through two OB-fold domains. RMI2, an integral component of the BLM complex, is important for the stability, localization and function of the BLM complex in vivo (128, 153). It has been hypothesized that RMI multi-OB-folds mediate two modes of BLM action: via RPA-mediated protein-DNA interaction, where it promotes unwinding and via RMI-mediated protein-protein interactions, which promotes dissolution.
Figure 2.
Conserved interaction between DNA repair and recombination proteins with Sgs1 and BLM. In yeast, Sgs1 physically interacts with many different repair and recombination proteins as indicated such as Top3 and Rmi1. Many of these interactions are evolutionarily conserved and are shown with the BLM protein. BLM also interacts with one of the other RecQ helicases, WRN. Mlh1 has an interaction site in the C-terminal region of both proteins (not shown). The colors for Sgs1 and BLM are the same as in Figure 1.
In addition to Top3 and the Rmi subunits, RecQ helicases interact with many proteins involved in DNA replication and repair, although the exact natures of these interactions are poorly understood. As we are limiting this review to Sgs1, BLM and WRN, only those proteins participating in DNA repair/replication are mentioned. Sgsl interacts with the type II topoisomerase Top2 (146), with Rad16/Pso5, a protein responsible for repair of silent DNA (118), with the Rad51 recombinase (150), with the mismatch proteins Mlh3 (142) and Mlh1 (2, 36), as well as with Srs2 helicase and Mre11 nuclease, both of which can form various subcomplexes with Sgs1 (30)(Figure 2). On the mammalian side, BLM and WRN interact with each other (140), with RPA (18, 19, 124) and p53 (13, 129, 143). In addition, BLM interacts with the largest subunit of chromatin assembly factor 1 (CAF-1) (67) and, like Sgs1, also interacts with RAD51 (150) and MLH1 (79) (Figure 2).
Recently, another intriguing conserved interaction was discovered between Sgs1 and WRN and jumonji domain proteins, where in yeast, Gis1 is a DNA damage-responsive effector capable of activating genes under conditions of stress (106, 133). Since the jumonji domain contains histone demethylase activity (134, 156), it raises the question of a connection between DNA repair and histone demethylation. In yeast, Gis1 interacts with many proteins including the Nfi1/Siz2 SUMO ligase to form a complex. In mammals, several related jumonji interacting proteins are found in promyelocytic leukaemia nuclear bodies (PML NBs), which also contain transcription factors, BLM, WRN and a SUMO ligase (13, 143, 161). Perhaps the jumonji interacting proteins in yeast are acting in a fashion similar to that of PML NBs, where the recruitment of transcription factors modulates their activity in response to specific signals. However, it is unknown whether the activities of the RecQ helicases themselves are modulated in these bodies by post-translational modification.
Double-strand break repair by homologous recombination
Many types of DNA damage can lead directly or indirectly to the formation of DSBs. When DNA repair is mis-regulated, mutations and genomic rearrangements can occur which are precursors to tumorigenesis and even cellular death. One mechanism to repair DSBs is homologous recombination (HR), which uses a homologous template for repair and is generally considered an error-free mechanism. In the absence of RecQ helicase function, genome instability is increased, consistent with an important function for these enzymes in HR. The RecQ proteins function at multiple repair steps in HR and therefore, this repair pathway will be the focus of this review.
When a DSB occurs, the DNA ends are first recognized by the Mre11-Rad50-Xrs2 complex in yeast or the MRE11-RAD50-NBS1 complex in multi-cellular eukaryotes (DSB repair is reviewed in (87)). The DNA ends are then resected in a 5’ to 3’ direction by the exonuclease activity of Mre11/MRE11 in conjunction with the endonuclease Sae2/CtIP, revealing 3’ ssDNA overhangs. These initial processing steps are critical since they target the DNA to be repaired using a homologous template and inhibit other repair mechanisms such as non-homologous end joining. The DNA ends are then further resected by one of two pathways, one that utilizes Exo1/EXO1 and the other that uses Sgs1 and Dna2. The surprising involvement of the RecQ proteins in this processing step will be discussed in depth below. The ssDNA is subsequently coated by RPA, the ssDNA binding protein. Rad52 then recruits Rad51 to the RPA-coated ssDNA, displacing RPA and leading to the formation of a Rad51 nucleoprotein filament. This Rad51-ssDNA filament performs a homology search followed by strand invasion leading to D-loop formation. Several RecQ helicases have also been shown to function in disruption of Rad51 nucleoprotein filaments or by preventing D-loop formation. After formation of the D-loop, branch migration occurs. This structure can be resolved by second end capture of the homologous chromosome by two different mechanisms: 1) a process called synthesis dependent strand annealing (SDSA) where the DNA strand re-anneals to the original template or 2) by formation of double-Holliday junctions (dHJs) that can be resolved by the Sgs1-Top3-Rmi1/BLM-TOP3-RMI1 (BLAP75)-RMI2 (BLAP18) complex. Therefore, the RecQ proteins perform distinct functions during HR: aiding in the resection of the DSB, inhibiting Rad51 filaments and D-loop formation, and resolving dHJs. Some of these steps are also conserved in meiosis (104).
Role of yeast and human helicases in DNA end resection
After a DSB forms, DNA end resection leads to the formation of 3’ ssDNA tails (147). Although many of the key players involved in DNA end resection were known, it was hypothesized that there may be additional proteins involved. For example, in E. coli the RecBCD, a complex of helicases and a nuclease, is sufficient for generating 3’ ssDNA tails. However, in eukaryotes there was no corresponding helicase known to function in resection. Further analysis in yeast revealed that the Sgs1 complex and one of its human homologues, BLM, are also involved in the extensive resection that occurs after MRX and Sae2 protein function (55, 94, 103, 162). The involvement of Sgs1 and BLM in this process was unexpected since BLM has a well-established later role in resolution of dHJs (152).
One challenge in identifying additional proteins involved in DNA end resection is the visualization of ssDNA. To circumvent this problem, gene conversion can be blocked at an inducible DSB site by either disrupting RAD51 or by eliminating donor sequences needed for strand invasion to occur. Using these approaches, two independent groups found that Sgs1 functions in DNA resection since sgs1Δ cells have a significantly slower rate of ssDNA formation (94, 162). In addition to the helicase activity of Sgs1, the Sgs1-interacting partners, Rmi1 and Top3 are also necessary for end resection suggesting that the entire Sgs1 complex is involved in this step (94, 162).
A breakthrough in our understanding of the resection pathway occurred when it was discovered that sgs1Δ exo1Δ double mutants showed substantially decreased DNA resection activity compared to the single sgs1Δ or exo1Δ strains (94, 162). These results suggest that Sgs1 and Exo1 function in different pathways that each contributes to end resection. Since RecBCD is the main pathway for end resection in bacteria, it was hypothesized that the Sgs1 helicase may also function with a corresponding nuclease during resection. Further analysis revealed that this nuclease is Dna2 (162). This result was very surprising since Dna2 functions in Okazaki fragment processing (5).
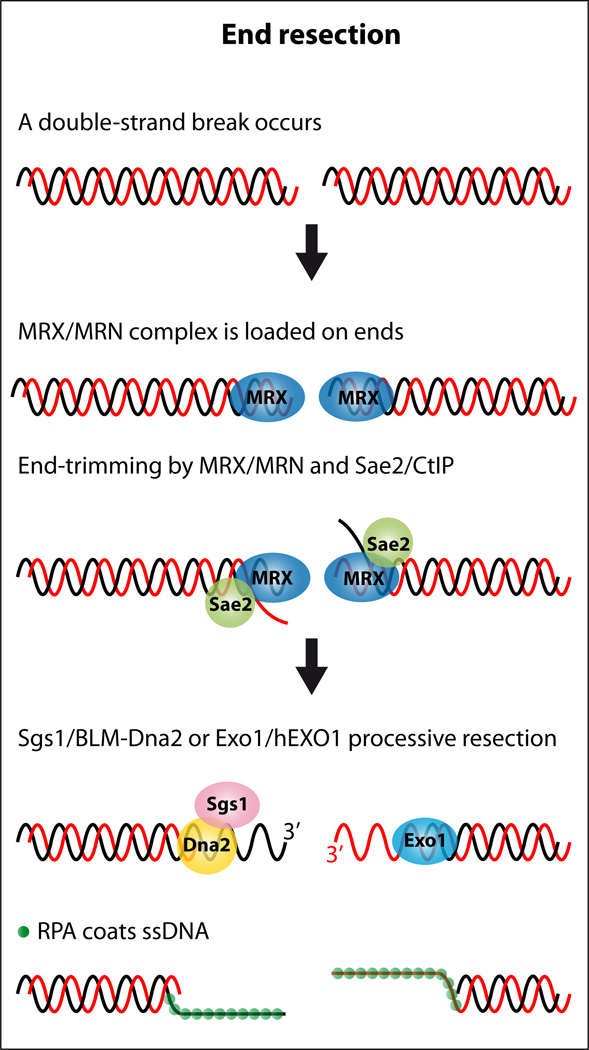
Interestingly, when a DSB is induced in cells where both Sgs1 and Exo1 resection pathways are blocked, DNA fragments accumulate that are 50–100 bp shorter than the initial cut fragment (94, 162). Subsequent analysis of these ssDNA intermediates revealed that their formation is dependent upon the MRX complex and Sae2 (94, 162). These observations lead to a two-step model for DNA end resection where the ends are first resected by the MRX complex and Sae2 and subsequently resected by either the Sgs1-Dna2 or Exo1 pathway (Figure 3). The combined resection activities of these proteins ensure that enough ssDNA is generated which the ssDNA binding protein RPA then coats.
Figure 3.
Model for RecQ function during end resection. After a DSB, the DNA ends are recognized by Mre11-Rad50-Xrs2 (MRX) in yeast or MRE11-RAD50-NBS1 (MRN) in mammals. The DNA ends are partially resected by Mre11/MRE11 nucleases, in collaboration with Sae2/CtIP endonucleases, leaving short 3’ single-stranded DNA tails. These DNA ends can be further resected by utilizing Sgs1/BLM helicases and Dna2/DNA2 nucleases or via a parallel pathway that uses Exo1/EXO1 nucleases. The ssDNA created is coated by RPA.
Inhibition of DNA end resection has profound consequences on the integrity of the DNA damage response. For example, blocking both end-resection pathways leads to increased susceptibility to DNA damaging agents, increased gross chromosomal rearrangements, and the inability to activate the DNA damage checkpoint under DNA damaging conditions (55). Importantly, these defects are not specific to yeast but are also observed in human cells. For example, co-depleted BLM/EXO1 cells exposed to the DNA damaging agent camptothecin have less activated Chk1, a DNA damage checkpoint protein, decreased RPA2 phosphorylation, and decreased survival rates (55). BLM/EXO1 co-depleted cells also have fewer co-localizing H2AX foci, a marker of DSBs, and the ssDNA binding protein RPA suggesting that less ssDNA is created when these two pathways are blocked in mammalian cells, which is similar to that observed in yeast (55).
In vitro analysis of purified BLM and human Exo1 nuclease (hExo1B) have also been examined for their resection phenotype on synthetic DNA substrates (103). Unlike the in vivo observations for yeast and human cells, purified BLM and hExo1B collaborate in vitro to resect 5’ to 3’ dsDNA templates (103). The resection activity of BLM and hExo1B promotes hRad51 joint molecule formation. Importantly, the resection activity is specific to BLM since no other RecQ homologue stimulates resection (103). It remains an open question as to why the in vivo analysis suggesting that Sgs1/BLM function independently of Exo1/EXO1 and the in vitro analysis suggesting that BLM stimulates hExo1B activity do not correspond.
Role of RecQ homologues in the recognition and resolution of secondary DNA structures
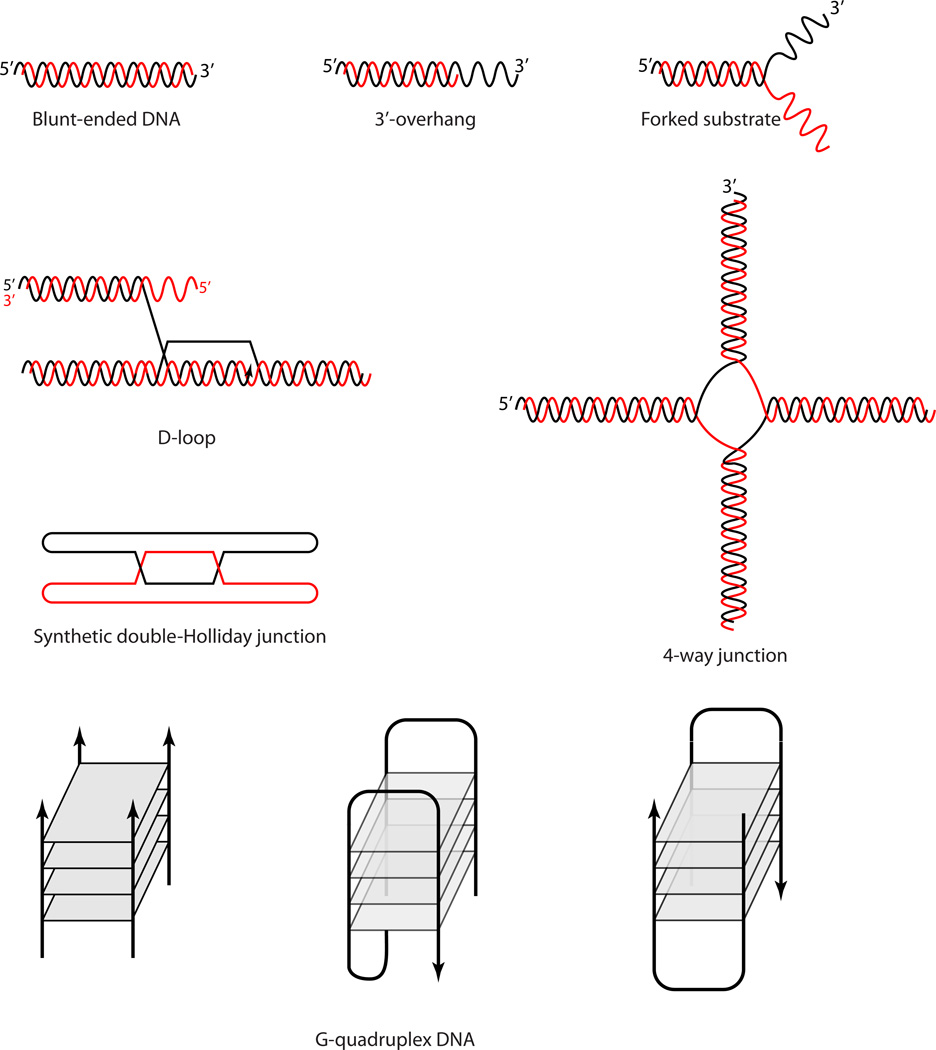
Purification and characterization of RecQ proteins from bacteria, yeast, plants and mammals has revealed that RecQ helicases unwind a wide variety of DNA substrates, with a marked preference for Holliday junctions (HJs), G-quadruplexes and D-loops (7, 23, 56, 98, 130, 135) (Figure 5). The helicase not only binds ssDNA, it can also bind blunt four-stranded junction structures indicating that it does not always require a free 3' DNA tail (7, 23). Interestingly, although early work on a truncated purified version of Sgs1 indicated poor processivity of the helicase (9), recently an unprecedented strong helicase activity for the full length Sgs1 protein (23), far beyond that of the BLM and WRN homologues, has been shown (19, 124). In addition, the full-length Sgs1 unwinds a wider variety of substrates with even greater efficiency than the truncated form, although it still has a preference for unwinding HJs (23).
Figure 5.
DNA structures unwound by one or multiple RecQ helicases. The RecQ proteins unwind a diverse set of DNA structures in vitro such as blunt ended DNA (however, not the preferred substrate), 3’ overhangs, forked substrates (such as those that arise during DNA replication), displacement-loops (D-loops, that arise during strand invasion), 4-way junctions (similar to a Holliday junction, HJ), synthetic dHJs (mimicking a recombination intermediate), or structures capable of forming G-quadruplex DNA (for example, predicted in rDNA and telomere sequences).
An early task of recombinational repair is homology search and strand invasion, which can be divided into a few key steps (Figure 4). In one of the first steps, Rad51 coats the ssDNA replacing RPA. Subsequently, Rad51 nucleo-protein filaments search for complementary sequences in dsDNA. During strand invasion and pairing, the non-complementary strand of the duplex is displaced creating a displacement loop (D-loop), a process that can occur at collapsed replication forks (Figure 4). In vitroRad51 efficiently forms D-loops with either a 5’ or a 3’ invaded end. However, only the 3’ invaded end is proficient for priming new DNA synthesis to allow extension of the D-loop by DNA polymerase. The cross-strand structures formed during this process can also branch migrate. In vitro studies indicate that the RecQ helicases may regulate many steps during strand invasion and exchange. For example, RECQ5 can disrupt the initial step of RAD51 filament formation (60), similar to the Srs2 protein in yeast (77, 137). It has also been shown that several RecQ helicases can disrupt D-loops. For example, C. elegans WRN-1 and A. thaliana RECQ2 unwind D-loops (63, 76). In vitromammalian BLM and RECQ1 preferentially melt naked D-loops and show an affinity for those with a 5’ invaded end. Therefore, these helicases selectively dissociate recombination intermediates whose polarity is unfavorable for polymerase extension (3, 135) (21). Interestingly, BLM disruption of D-loops is restricted to filaments containing an inactive ADP-bound form of RAD51, suggesting that the filament remains inactive and susceptible to BLM dissociation until the cell is fully prepared for HR. During the latter steps of the strand exchange reaction, human RECQ1 efficiently promotes 3' → 5' three-stranded branch migration of the D-loop (20).
Figure 4.
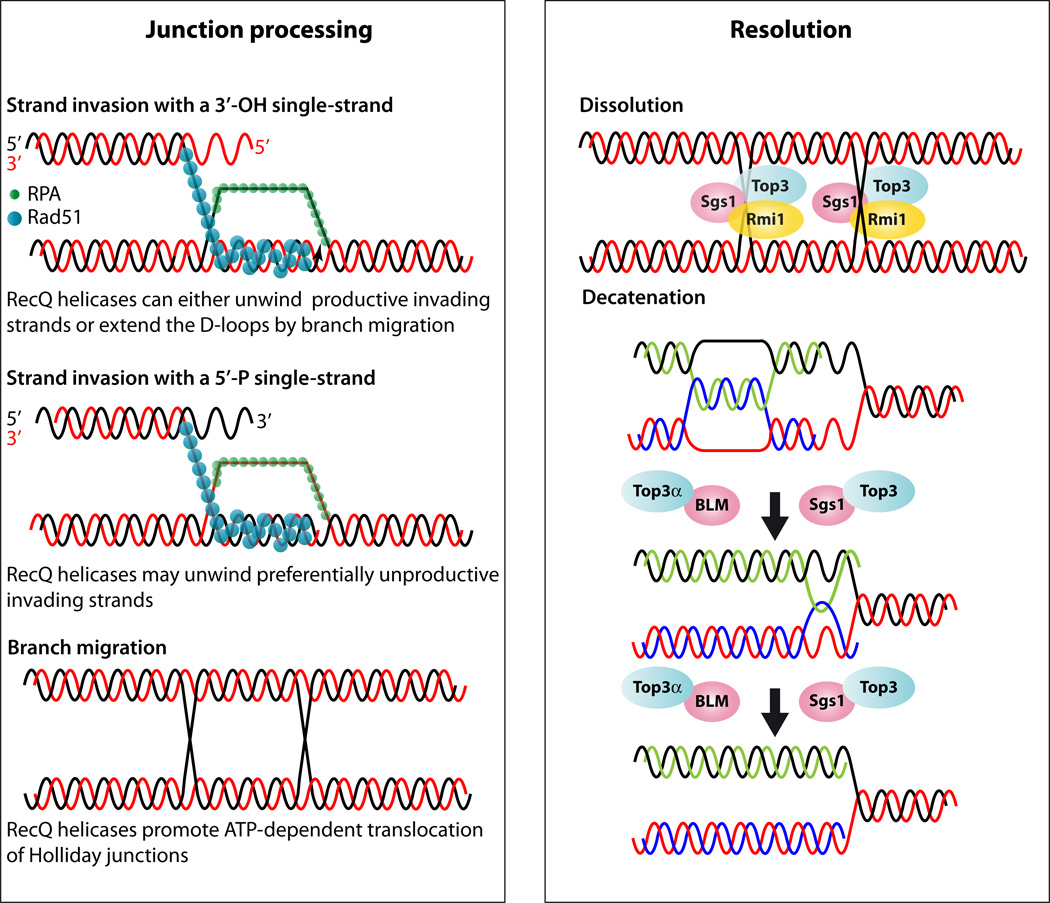
Model for RecQ function during junction processing and resolution. Junction processing: The Rad52 group of proteins recruits Rad51 and displaces RPA leading to Rad51 filament formation. Rad51 filaments perform homology search and strand invasion leading to D-loop formation followed by branch migration. Rad51 coated DNA can invade using 5’ or 3’ DNA ends; however, only the 3’ invading end is proficient for homologous recombination. The RecQ proteins can unwind the 5’ end and thus abort the unproductive reaction but also the 3’ end after it is extended to promote synthesis dependent strand annealing (not shown). Alternatively, second end capture of the homologous chromosome can lead to double Holliday junction formation (dHJ). The RecQ proteins function by promoting translocation and branch migration of dHJs. Resolution: Dissolution of dHJs utilize the Sgs1-Top3-Rmi1/BLM-TOP3α-RMI1(BLAP75) complex. In the figure the yeast proteins are shown bound to each HJ and if they move toward each other dissolution can occur. However, the precise biochemical reaction is not known. During DNA replication, hemicatenenes are thought to form behind the replication fork (17). In vitromaximal decatenation of this structure is achieved when Rmi1 and Rmi2 are added to the reaction.
For recombination to proceed efficiently, branch migration of the D-loop junction is necessary and the subsequent processing of the HJ determines the outcome of the recombination reaction. In prokaryotes, RecQ or RuvA/RuvB promote ATP-dependent branch migration of HJs. In yeast, replication fork arrest results in accumulation of HJs in the absence of Rqh1, which can either impede sister chromatid segregation or lead to the formation of recombinants through HJ resolution (37). In vitroBLM and WRN promote the ATP-dependent translocation of HJs and binding of recombinant p53 attenuates their ability to unwind synthetic HJs (157). RECQ1 predominantly branch migrates HJs in an ATP-dependent fashion in human nuclear extracts (83).
It has long been suspected that a double Holliday junction (dHJ) could be resolved by a topoisomerase partnered with a helicase by convergent branch migration of the HJs (50, 102). Genetic analysis of yeast Top3 and Sgs1 supports this hypothesis. Deletion of Sgs1 increases both spontaneous and DSB-induced crossovers, suggesting that Sgs1 with Top3 removes dHJ intermediates from a crossover-producing repair pathway (64, 114). In an elegant in vitro system, it was shown that BLM and TOP3α resolve recombination intermediates containing a dHJ through a mechanism called double-junction dissolution, a process that prevents exchange of flanking sequences (152). This mechanism is conserved in flies (108), but it is important to note that the in vitro systems only allow the readout of the dissolution reaction, while a dHJ could equally be branch migrated in either direction or apart from one another leading to an increase in the distance between the two junctions. However, the dissolution reaction is highly specific for BLM and depends upon a functional HRDC domain (149). The discovery of BLAP75/RMI1 as a third member of the Sgs1-Top3 (BLM-TOP3α) complex led to the notion of a dissolvasome. Under physiological conditions, dHJ dissolution depends completely on RMI1 to limit DNA crossover formation (112). The conserved N-terminal third of RMI1 mediates complex formation with TOP3α and BLM and acts by recruiting TOP3α to dHJs. Hence, the activity of RMI1 is specific for dissolution catalyzed by TOP3α (22, 113, 148). Recently, the newest member of this complex, RMI2, was shown to stimulate the dHJ resolution capability (128).
G-quadruplex DNAs, which can form into non-Watson-Crick structures within guanine-rich DNA sequences, are another DNA structure that can be preferentially unwound by RecQ helicases (Figure 5). These sequences are often found in telomeric or rDNA repeats. With the exception of RECQ1 (110), the RecQ family of DNA helicases are able to unwind G-quadruplex DNA. The efficient unwinding of G-G paired DNA by Sgs1 is ATP and Mg2+ -dependent and requires a short 3' single-stranded tail. Diminished ability to unwind G-G paired regions may explain the deleterious effect of mutations in Sgs1 on rDNA stability, and the accelerated aging of yeast lacking Sgs1 as well as humans deficient in the WRN helicase (130). In mammals, there is a direct relationship between the helicase activities of BLM and WRN and their G-quadruplex binding capabilities (85). BLM and Sgs1 preferentially unwind G4 DNA relative to HJs. This substrate preference reflects binding affinity and maps to the helicase domain. These observations suggest that, in addition to their roles in promoting recombination to restart a stalled fork, BLM and Sgs1 also function in DNA replication to remove G4 DNA structures, which could hinder fork progression (62).
Meiotic functions
There are several observations that point to a role for the RecQ family in meiosis. For example, in budding yeast, sgs1Δ mutants exhibit both reduced tetrad formation and spore viability (44, 146). Human females homozygous for BLM mutations display reduced fertility and males are infertile (reviewed in (46)). In addition, BLM protein detected by immunofluorescence, frequently colocalizes as discrete foci on synapsed cores with the recombinases RAD51 and DMC1, providing cytological evidence that BLM functions in meiotic recombination as well (97). Furthermore, evidence from other eukaryotes also supports the notion that RecQ family members are involved in meiosis. For example, mutations in the Bloom’s homologue in flies (mus309) and worms (him-6) are infertile (54, 58, 78).
In budding yeast, the decrease in spore viability in sgs1 mutants was attributed to meiosis I missegregation (146) and precocious separation of sister chromatids (116), while heteroallelic recombination and crossovers were not strongly affected (145). However, further studies indicate that, in the absence of Sgs1, there is a long delay in the appearance of four-spored asci dependent on the initiation of recombination (44). Indeed, disruption of RED1 or RAD17two checkpoint genes, partially alleviates the poor sporulation of sgs1 mutants. Interestingly, only the region of Sgs1 spanning amino acids 126 to 596 is required for the meiotic function--the helicase function per se is dispensable (96). However, another study suggests that helicase activity is required in meiosis perhaps reflecting complex interactions between the domains of Sgs1 (90). An important breakthrough in understanding Sgs1 meiotic function came from the discovery that the absence of Sgs1 leads to an increase in both the number of interhomologue connections and the number of meiotic crossovers without affecting the frequency of noncrossover events (115). These observations assign a new role for Sgs1 in the negative regulation of meiotic crossovers at an early stage of meiotic prophase. A similar function was later found for both spontaneous and DSB-induced mitotic events (64, 114). In subsequent meiotic studies, the sgs1-C795 allele, which truncates the protein after amino acid 795, has been used instead of the complete deletion. Although the sgs1-C795 mutants are indistinguishable from the null mutant with respect to the synapsis and crossover phenotypes, they sporulate like wild-type cells, exhibit significantly improved spore viability compared to sgs1Δ (115) and they grow as well as wild-type cells (99). It is therefore possible that some meiotic functions of Sgs1 are mediated via interactions with other partners like Top2 or Top3, whose interacting sites are still present in the sgs1-C795 mutant.
The molecular mechanisms by which Sgs1 regulates meiotic crossovers was further revealed when it was found that the SIC/ZMM proteins, responsible for homologous chromosomes synapsis and crossovers, antagonize the anti-crossover activity of Sgs1 (66). Elegant genetic and molecular work reached the conclusion that Sgs1 and Mus81/Mms4 can disrupt the formation of aberrant joint molecules resulting from secondary strand-invasion events, therefore enhancing the repair efficiency of breaks (65, 104). These results suggest that, in meiosis, Sgs1 does not act primarily as a dissolvase (108, 152), but preferentially disrupts D-loop structures (3, 7, 105). Interestingly, the role of Sgs1 in meiosis is not conserved. Compelling data from fission yeast indicate that, unlike Sgs1, Rqh1 promotes meiotic recombination (34). It is tempting to speculate that this difference between RecQ meiotic function in S. cerevisiae and S. pombe is related to the absence of the synaptonemal complex and the lack of crossover interference in fission yeast.
Repair of replicative damage
Many of the activities performed by the RecQ helicases are also important at multiple steps during DNA replication. The RecQ helicases help enable the association of the polymerases with the replication fork, unwind DNA structures that potentially leads to replication fork stalling (i.e. G-quadruplexes and hairpin structures), and resolve hemicatenane-like structures that can form during repair of replicative damage. In the absence of RecQ activity, errors lead to stalled or collapsed replication forks, which need to be repaired to maintain genomic integrity and to prevent DSBs. RecQ proteins may help unwind inappropriately paired nucleic acids during replication to prevent inappropriate HR events and therefore, maintain genomic stability.
One of the first lines of evidence suggesting that the RecQ proteins can function in the repair of replicative damage comes from the observation in yeast that expression of the SGS1 gene is cell cycle regulated and peaks in S phase (40). Similarly, BLM is cell cycle regulated and its concentration peaks during S phase (38, 49), while the other mammalian RecQ homologues show no such regulation. RecQ proteins also interact with many DNA replication proteins. For example, in yeast, Sgs1 physically interacts with topoisomerases I and II and RPA (31, 45, 146). Sgs1 is also found in chromatin immunoprecipitation (ChIP) experiments at unperturbed replication forks (31). Similarly, the human homologue WRN interacts with hRPA, PCNA, POLδ, and hTOPO1 and copurifies with the DNA replication complex in ES cells (18, 69, 81, 82). Indeed, upon hydroxyurea treatment, WRN localizes to discrete nuclear foci that coincide with those formed by RPA, suggesting that WRN prevents aberrant recombination events at sites of stalled replication forks by dissociating recombination intermediates (32, 70). The BLM proteins interact with p53 and RAD51 at stalled replication forks (122). BLM also forms foci that co-localize with PCNA at stalled replication forks and with the BASC complex (BRCA1-associated genome surveillance complex)(144). Finally, RECQ4 is an integral component of the MCM complex, where MCM10 is important for the RECQ4 interaction with the other replisome components, MCM2–7 (155).
What role(s) do the RecQ helicases play at the replication fork? Perhaps the topological constraints of DNA replication generate catenane-like structures that require DNA helicases and topoisomerases for resolution. In this scenario, the RecQ proteins act on DNA structures enabling replication to continue efficiently. Without the concerted action of the RecQ helicases with DNA topoisomerases, aberrant replication structures arise. In fact, disruption of RecQ helicases leads to the accumulation of abnormal replication intermediates in both BLM and WRN deficient cells (89, 109). Surprisingly, in budding yeast, SGS1 disrupted cells progress through S phase faster than wild-type (138). However, not all genomic loci replicate faster since rDNA actually replicates more slowly in sgs1Δ (138). Furthermore, disruption of SGS1 causes contractions at tri-nucleotide repeats (72). Both rDNA and tri-nucleotide repeat sequences are capable of forming G-quadruplexes, suggesting that such sequences in these repetitive units require RecQ helicase activity to replicate efficiently. In addition, the reliance on other repair mechanisms, such as sister-chromatid recombination or single-strand annealing in the absence of RecQ helicases may lead to slower processivity during the replication of highly repetitive elements.
The RecQ helicases also function in the repair of replication-induced damage (Figure 4). Our understanding of the RecQ proteins in DNA replication comes mostly from experiments that utilize drugs that stall or collapse replication forks. For example, hydroxyurea (HU) is frequently used to deplete dNTP pools and therefore, initially stall and then subsequently collapse forks. Methyl methanesulfonate (MMS) alkylates DNA and also leads to replication fork stalling. Using these drugs to perturb replication, Sgs1 was shown to contribute to the polymerases ability to efficiently immuno-precipitate with the replication fork (31). When replication fork damage is induced with MMS, X-shaped molecules form at replication origins, which can be visualized by 2D gel electrophoresis. These X-structures likely contain ssDNA since their formation is sensitive to Mung Bean nuclease (86). Complete disruption of SGS1 or its helicase domain, leads to the accumulation of these X-structures at damaged forks (10, 86). Furthermore, X-structure accumulation in sgs1Δ cells is dependent upon the recombination protein Rad51 and a post-replication repair protein Rad18 suggesting that a template-switching mechanism is important for their formation (17, 86). The Rad18-mediated post-replicative repair pathway requires the SUMO-conjugating enzyme Ubc9 and sumoylated PCNA, suggesting that assembly/disassembly of proteins at the site of replicative damage is an important contributor to X-structure formation (17). Sgs1 itself is also an Ubc9-dependent SUMO target (16). Interestingly, it is possible to isolate mutants of SGS1 that encode proteins defective in repair of replicative damage but do not influence recombination at other loci, suggesting that the function of Sgs1 in repair of replicative damage is distinct from its function in resolution of HR intermediates (10).
When recombination or replication intermediates are not properly resolved during S phase, cells are unable to undergo sister-chromatid disjunction. In this scenario, anaphase bridges (or ultra-fine bridges), representing incomplete chromosome segregation, connect the two nuclei. Interestingly, BLM, along with TOP3α and hRMI1, localize to these bridges and is needed for their resolution (24). The anaphase bridges observed also contain the PICH helicase (6), where PICH is first recruited to the bridges followed by BLM, which then resolves them (24). Intriguingly, FANCD2/I, proteins that localize to DNA damage site and are ubiquitinated, are located at the termini of a subset of BLM-dependent ultra-fine bridges (25).
Disruption of RecQ function during replicative repair also activates the DNA damage checkpoint. The intra-S phase checkpoint is largely mediated by Rad53 in yeast or CHK2 in humans. Rad53 is activated by DNA damage and becomes phosphorylated leading to a signaling cascade enabling the damage signal to be transduced. Sgs1 is important in one pathway used for Rad53 activation (Rad24 being the other in a parallel pathway) (12). Sgs1 directly binds Rad53, suggesting that Sgs1 and Rad53 interact at damaged DNA structures for checkpoint activation (12, 40). Sgs1 and Rad53 also form foci that co-localize with the replication factor Orc2 (40). In mammalian cells, ATR, an upstream kinase to CHK2, binds to and phosphorylates BLM (35). Furthermore, when replication forks are stalled with HU, BLM foci co-localize with another kinase, CHK1, and 53BP1, a p53 interacting protein in the DNA damage response (123). When CHK1 mRNA is depleted, the formation of BLM and 53BP1 foci at replication forks is disrupted, suggesting that CHK1 recruits BLM to sites of replicative damage (123). In summary, both yeast and mammalian RecQ proteins activate the DNA damage checkpoint in response to replication fork stalling and collapse.
Telomeres
Maintenance of telomere sequences at chromosome ends is critical for genomic stability. Telomeres consist of G-rich repetitive DNA sequences. In the absence of telomerase, telomeres shorten after each cell division until the cell senesces. To bypass senescence, telomeres can be elongated by different mechanisms, for example, one that utilizes telomerase, an RNA-protein complex, or secondly by alternative lengthening of telomeres (ALT), which is predominately DNA recombination mediated. In budding yeast, lengthening of telomeres by ALT requires the recombination protein Rad52. In human tumors, cells often evade senescence by activating either pathway to extend telomere length. The RecQ proteins have been implicated to function in telomere maintenance (11). The WRN RecQ helicase has a well-established role at telomeres in humans. WRN is important for lagging strand synthesis and for efficient replication of G-rich telomeric DNA (33). As mentioned previously, the WRN mutant mouse only recapitulates Werner syndrome when combined with telomerase mutant alleles, suggesting that loss of WRN’s function at telomeres is critical in the development of this syndrome.
Similar to processed DSBs, telomere ends have 3’ overhangs due to the protrusion of the G strand over the complementary C-strand. In yeast, the length of the single-stranded G-tails is approximately 12–14 nucleotides and these G tails form in mice and humans as well and are considerably longer 75–300 nucleotides (80, 131). Studies in yeast have shown that formation of the G-tails is dependent on the MRX complex (80). Interestingly, disruption of the MRX complex does not completely abolish G-tail formation suggesting that other proteins are also involved in the 5’ to 3’ processing of telomere ends (80).
One of the defining features of telomeres is their G-rich nature, which potentially enables the formation of G-quadruplexes, which are guanine-guanine interactions stabilized by inter-strand pairings between four DNA strands. As discussed above, several RecQ proteins unwind G-quadruplex DNA in vitro including BLM, WRN, and Sgs1 (85, 130). In addition, like its role at DSBs (55, 94, 103, 162), Sgs1 is important in resection at telomeres. In budding yeast, an inducible cut site was used to generate a DSB adjacent to the left telomere of chromosome VII to monitor a single telomere for formation of ssDNA and lengthening (15). After induction of the DSB, the fragment distal to the break is lost and the short telomeric “seed” sequence is elongated. Wild type cells can elongate this telomere seed sequence, but cells that are unable to form 3’ tails do not. Interestingly, using this assay, all the proteins required for resection of DSBs are also necessary for telomeric C-strand degradation and telomere elongation (i.e. Sae2, MRX, Sgs1, Exo1, and Dna2) (15). Similar to what occurs at DSBs, Sgs1 and Dna2 function together in a pathway that is parallel to Exo1 in the resection of telomeric DNA (15). Surprisingly, the wild type chromosomes observed in multiple mutants blocking both pathways (i.e. exo1Δ sgs1Δ, sae2Δ exo1Δ), did not exhibit any changes in their telomere lengths even though the cells were largely defective in extending the DSB-induced short telomere (15). Together, these results indicate that, similar to DSBs at other sites, resection of telomere ends is also partially mediated by Sgs1. Interestingly, it was recently shown that sumoylation of Srs2 (91) in budding yeast or Rqh1 in fission yeast (117) is important for telomere-telomere recombination. Importantly, Sgs1 sumoylation did not influence recombination at other genomic loci suggesting that the role of Sgs1 at telomeres may be distinct from its role at other loci (91).
Concluding remarks
The RecQ helicases have a diverse role in genome maintenance that, when unregulated in humans, can lead to premature aging and tumorigenesis. Although the diseases associated with mutations in the RecQ helicases are distinct, they are fundamentally characterized by increased cancer incidence, which is likely attributable to RecQ function in DNA repair. Recently, RecQ proteins were shown to function in resection of DSBs. This resection activity is also important for the ALT pathway, which is used during telomere extension in the absence of telomerase. However, it is unknown whether this function is important during the repair of replicative damage or during meiosis. In meiosis, it is likely that the RecQ role in dissolution is the predominate function. The RecQ proteins, in vitrounwind a diverse set of DNA substrates suggesting that it will be a continuing challenge to dissect out its specific functions at the DNA structures that it encounters. Furthermore, the role of post-translational modification of RecQ, such as sumoylation, may be critical in the regulation of this important helicase family. Finally, insight into precise biochemical function of this helicase family will be revealed by analyzing the specific structures that form from RecQ dysfunction, such as X-structures that arise during DNA replication or the anaphase-bridges found during mitosis.
Future Issues (up to 8).
Is there a role for the RecQ proteins in end resection during meiosis or during DNA replication?
Are the phenotypes observed in human patients with RecQ disorders attributed to the different functions of the RecQ protein at different processing steps?
What is the DNA-protein structure of the anaphase bridges and do they occur in other eukaryotes?
Is the role of sumoylation of RecQ proteins only important at telomeres or is it also important for its regulation in general such as during DNA replication or recombination?
How are RecQ proteins recruited during repair of different DNA structures? How do they function in the processing of DNA lesions?
What is the specificity of the RecQ helicases in organisms with multiple RecQ proteins?
Acknowledgments
We thank Peter Thorpe and Michael Chang for helpful comments and careful reading of the manuscript. This study was supported by ANR-07-BLAN-0350-01 (SG) and by National Institute Health grants GM50237 (RR), GM078840 (KAB), GM088413 (KAB).
Mini glossary of Keywords
- Genome instability
the aberrant rearrangement of chromosomal material that is often a prerequisite to cancer and aging.
- DNA helicase
an enzyme that unwinds complementary duplex DNA
- DNA topoisomerase
enzymes that alter DNA topology by catalyzing strand passage
- Homologous recombination (HR)
a DNA repair mechanism that utilizes a homologous sequence as the template for repair
- Loss-of-heterozygosity
deletion, or mutation or recombination events that result in loss of the wild-type allele in a heterozygote.
- Single-stranded DNA (ssDNA)
stretches of single-stranded DNA that can be coated by Replication Protein A (RPA) complex or the Rad51 recombinase
- Double-stranded DNA (dsDNA)
two base-paired complementary strands of DNA
- Double-strand break (DSB)
DNA damage that results in a break of both strands of DNA
- Holliday junctions (HJ)
cross-strand exchange between two DNA molecules that results in a four-way junction
- Double Holliday junction (dHJ)
two adjacent Holliday junctions formed between four strands of DNA
- Displacement loop (D-loop)
the single stranded DNA formed when two strands of dsDNA are separated by the invasion of a third strand that anneals by base-pair complementation
- MRX/MRN
Mre11-Rad50-Xrs2 in yeast or Mre11-Rad50-Nbs1 in humans, a complex responsible for recognizing and processing DNA ends
- Synthesis dependent strand annealing
a recombination process that occurs when an extended strand is displaced and base paired with a complementary single strand to create a duplex without a crossover
- G-quadruplex
four-stranded structures formed by Hoogsteen basepairing of G-rich sequences
- Sister-chromatid exchange
reciprocal recombination between two sister chromatids
- Resection
degradation of one of the complementary strands of DNA
- Meiosis
specialized division that creates haploid products from a diploid cell
- X-structures
replication intermediates that form in the presence of DNA damaging agents that contain stretches of ssDNA
- Telomeres
sequences that protect the ends of chromosomes from degradation and prevent the recognition of chromosome ends as DNA damage
- Sumoylation
post-translational covalent attachment of a small ubiquitin-like molecule (SUMO)
Footnotes
Disclosure Statement
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
References
- 1.Ahmad F, Stewart E. The N-terminal region of the Schizosaccharomyces pombe RecQ helicase, Rqh1p, physically interacts with Topoisomerase III and is required for Rqh1p function. Mol. Genet. Genomics. 2005;273:102–114. doi: 10.1007/s00438-005-1111-3. [DOI] [PubMed] [Google Scholar]
- 2.Argueso J, Kijas A, Sarin S, Heck J, Waase M, Alani E. Systematic mutagenesis of the Saccharomyces cerevisiae MLH1 gene reveals distinct roles for Mlh1p in meiotic crossing over and in vegetative and meiotic mismatch repair. Mol. Cell. Biol. 2003;23:873–886. doi: 10.1128/MCB.23.3.873-886.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachrati C, Borts R, Hickson I. Mobile D-loops are a preferred substrate for the Bloom's syndrome helicase. Nucleic Acids Res. 2006;34:2269–2279. doi: 10.1093/nar/gkl258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachrati C, Hickson I. RecQ helicases: suppressors of tumorigenesis and premature aging. Biochem. J. 2003;374:577–606. doi: 10.1042/BJ20030491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae SH, Bae KH, Kim JA, Seo YS. RPA governs endonuclease switching during processing of Okazaki fragments in eukaryotes. Nature. 2001;412:456–461. doi: 10.1038/35086609. [DOI] [PubMed] [Google Scholar]
- 6.Baumann C, Korner R, Hofmann K, Nigg EA. PICH, a centromere-associated SNF2 family ATPase, is regulated by Plk1 and required for the spindle checkpoint. Cell. 2007;128:101–114. doi: 10.1016/j.cell.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 7.Bennett R, Keck J, Wang J. Binding specificity determines polarity of DNA unwinding by the Sgs1 protein of S. cerevisiae. J. Mol. Biol. 1999;289:235–248. doi: 10.1006/jmbi.1999.2739. [DOI] [PubMed] [Google Scholar]
- 8.Bennett R, Noirot-Gros M, Wang J. Interaction between yeast sgs1 helicase and DNA topoisomerase III. J. Biol. Chem. 2000;275:26898–26905. doi: 10.1074/jbc.M003137200. [DOI] [PubMed] [Google Scholar]
- 9.Bennett RJ, Sharp JA, Wang JC. Purification and characterization of the Sgs1 DNA helicase activity of Saccharomyces cerevisiae. J. Biol. Chem. 1998;273:9644–9650. doi: 10.1074/jbc.273.16.9644. [DOI] [PubMed] [Google Scholar]
- 10.Bernstein KA, Shor E, Sunjevaric I, Fumasoni M, Burgess RC, et al. Sgs1 function in the repair of DNA replication intermediates is separable from its role in homologous recombinational repair. EMBO J. 2009;28:915–925. doi: 10.1038/emboj.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhattacharyya S, Sandy A, Groden J. Unwinding protein complexes in ALTernative telomere maintenance. J. Cell Biochem. 2010;109:7–15. doi: 10.1002/jcb.22388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bjergbaek L, Cobb JA, Tsai-Pflugfelder M, Gasser SM. Mechanistically distinct roles for Sgs1p in checkpoint activation and replication fork maintenance. EMBO J. 2005;24:405–417. doi: 10.1038/sj.emboj.7600511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blander G, Kipnis J, Leal JFM, Yu CE, Schellenberg GD, Oren M. Physical and functional interaction between p53 and the Werner's syndrome protein. J. Biol. Chem. 1999;274:29463–29469. doi: 10.1074/jbc.274.41.29463. [DOI] [PubMed] [Google Scholar]
- 14.Bloom D. Congenital telangiectatic erythema resembling lupus erythematosus in dwarfs; probably a syndrome entity. AMA Am. J. Dis. Child. 1954;88:754–758. [PubMed] [Google Scholar]
- 15.Bonetti D, Martina M, Clerici M, Lucchini G, Longhese MP. Multiple pathways regulate 3' overhang generation at S. cerevisiae telomeres. Mol Cell. 2009;35:70–81. doi: 10.1016/j.molcel.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Branzei D, Sollier J, Liberi G, Zhao X, Maeda D, et al. Ubc9- and mms21-mediated sumoylation counteracts recombinogenic events at damaged replication forks. Cell. 2006;127:509–522. doi: 10.1016/j.cell.2006.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Branzei D, Vanoli F, Foiani M. SUMOylation regulates Rad18-mediated template switch. Nature. 2008;456:915–920. doi: 10.1038/nature07587. [DOI] [PubMed] [Google Scholar]
- 18.Brosh RM, Jr, Orren DK, Nehlin JO, Ravn PH, Kenny MK, et al. Functional and physical interaction between WRN helicase and human replication protein A. J. Biol. Chem. 1999;274:18341–18350. doi: 10.1074/jbc.274.26.18341. [DOI] [PubMed] [Google Scholar]
- 19.Brosh RM, Li JL, Kenny MK, Karow JK, Cooper MP, et al. Replication protein A physically interacts with the Bloom's syndrome protein and stimulates its helicase activity. J. Biol. Chem. 2000;275:23500–23508. doi: 10.1074/jbc.M001557200. [DOI] [PubMed] [Google Scholar]
- 20.Bugreev D, Brosh RJ, Mazin A. RECQ1 possesses DNA branch migration activity. J. Biol. Chem. 2008;283:20231–20242. doi: 10.1074/jbc.M801582200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bugreev D, Yu X, Egelman E, Mazin A. Novel pro- and anti-recombination activities of the Bloom's syndrome helicase. Genes Dev. 2007;21:3085–3094. doi: 10.1101/gad.1609007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bussen W, Raynard S, Busygina V, Singh A, Sung P. Holliday junction processing activity of the BLM-Topo IIIalpha-BLAP75 complex. J Biol Chem. 2007;282:31484–31492. doi: 10.1074/jbc.M706116200. [DOI] [PubMed] [Google Scholar]
- 23.Cejka P, Kowalczykowski SC. The full-length Saccharomyces cerevisiae Sgs1 protein is a vigorous DNA helicase that preferentially unwinds holliday junctions. J. Biol. Chem. 2010;285:8290–8301. doi: 10.1074/jbc.M109.083196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan KL, North PS, Hickson ID. BLM is required for faithful chromosome segregation and its localization defines a class of ultrafine anaphase bridges. EMBO J. 2007;26:3397–3409. doi: 10.1038/sj.emboj.7601777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan KL, Palmai-Pallag T, Ying S, Hickson ID. Replication stress induces sister-chromatid bridging at fragile site loci in mitosis. Nat. Cell Biol. 2009;11:753–760. doi: 10.1038/ncb1882. [DOI] [PubMed] [Google Scholar]
- 26.Chang M, Bellaoui M, Zhang C, Desai R, Morozov P, et al. RMI1/NCE4, a suppressor of genome instability, encodes a member of the RecQ helicase/Topo III complex. EMBO J. 2005;24:2024–2033. doi: 10.1038/sj.emboj.7600684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang S, Multani AS, Cabrera NG, Naylor ML, Laud P, et al. Essential role of limiting telomeres in the pathogenesis of Werner syndrome. Nat. Genet. 2004;36:877–882. doi: 10.1038/ng1389. [DOI] [PubMed] [Google Scholar]
- 28.Chen C, Brill S. Binding and Activation of DNA Topoisomerase III by the Rmi1 Subunit. J. Biol. Chem. 2007;282:28971–28979. doi: 10.1074/jbc.M705427200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chester N, Kuo F, Kozak C, O'Hara CD, Leder P. Stage-specific apoptosis, developmental delay, and embryonic lethality in mice homozygous for a targeted disruption in the murine Bloom's syndrome gene. Genes Dev. 1998;12:3382–3393. doi: 10.1101/gad.12.21.3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chiolo I, Carotenuto W, Maffioletti G, Petrini J, Foiani M, Liberi G. Srs2 and Sgs1 DNA helicases associate with Mre11 in different subcomplexes following checkpoint activation and CDK1-mediated Srs2 phosphorylation. Mol. Cell. Biol. 2005;25:5738–5751. doi: 10.1128/MCB.25.13.5738-5751.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobb JA, Bjergbaek L, Shimada K, Frei C, Gasser SM. DNA polymerase stabilization at stalled replication forks requires Mec1 and the RecQ helicase Sgs1. EMBO J. 2003;22:4325–4336. doi: 10.1093/emboj/cdg391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Constantinou A, Tarsounas M, Karow JK, Brosh RM, Bohr VA, et al. Werner's syndrome protein (WRN) migrates Holliday junctions and co-localizes with RPA upon replication arrest. EMBO Rep. 2000;1:80–84. doi: 10.1093/embo-reports/kvd004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crabbe L, Verdun RE, Haggblom CI, Karlseder J. Defective telomere lagging strand synthesis in cells lacking WRN helicase activity. Science. 2004;306:1951–1953. doi: 10.1126/science.1103619. [DOI] [PubMed] [Google Scholar]
- 34.Cromie G, Hyppa R, Smith G. The fission yeast BLM homolog Rqh1 promotes meiotic recombination. Genetics. 2008;179:1157–1167. doi: 10.1534/genetics.108.088955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davies SL, North PS, Dart A, Lakin ND, Hickson ID. Phosphorylation of the Bloom's syndrome helicase and its role in recovery from S-phase arrest. Mol. Cell. Biol. 2004;24:1279–1291. doi: 10.1128/MCB.24.3.1279-1291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dherin C, Gueneau E, Francin M, Nunez M, Miron S, et al. Characterization of A Highly Conserved Binding Site of Mlh1 Required for Exonuclease I-Dependent Mismatch Repair. Mol. Cell. Biol. 2008;29:907–918. doi: 10.1128/MCB.00945-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Doe C, Dixon J, Osman F, Whitby M. Partial suppression of the fission yeast rqh1(−) phenotype by expression of a bacterial Holliday junction resolvase. EMBO J. 2000;19:2751–2762. doi: 10.1093/emboj/19.11.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dutertre S, Ababou M, Onclercq R, Delic J, Chatton B, et al. Cell cycle regulation of the endogenous wild type Bloom's syndrome DNA helicase. Oncogene. 2000;19:2731–2738. doi: 10.1038/sj.onc.1203595. [DOI] [PubMed] [Google Scholar]
- 39.Ellis NA, Groden J, Ye TZ, Straughen J, Lennon DJ, et al. The Bloom's syndrome gene product is homologous to RecQ helicases. Cell. 1995;83:655–666. doi: 10.1016/0092-8674(95)90105-1. [DOI] [PubMed] [Google Scholar]
- 40.Frei C, Gasser SM. The yeast Sgs1p helicase acts upstream of Rad53p in the DNA replication checkpoint and colocalizes with Rad53p in S-phase-specific foci. Genes Dev. 2000;14:81–96. [PMC free article] [PubMed] [Google Scholar]
- 41.Fricke W, Kaliraman V, Brill S. Mapping the DNA topoisomerase III binding domain of the Sgs1 DNA helicase. J. Biol. Chem. 2001;276:8848–8855. doi: 10.1074/jbc.M009719200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fukuchi K, Martin GM, Monnat RJ., Jr Mutator phenotype of Werner syndrome is characterized by extensive deletions. Proc. Natl. Acad. Sci. U S A. 1989;86:5893–5897. doi: 10.1073/pnas.86.15.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fukuchi K, Tanaka K, Nakura J, Kumahara Y, Uchida T, Okada Y. Elevated spontaneous mutation rate in SV40-transformed Werner syndrome fibroblast cell lines. Somat. Cell Mol. Genet. 1985;11:303–308. doi: 10.1007/BF01534688. [DOI] [PubMed] [Google Scholar]
- 44.Gangloff S, de Massy B, Arthur L, Rothstein R, Fabre F. The essential role of yeast topoisomerase III in meiosis depends on recombination. EMBO J. 1999;18:1701–1711. doi: 10.1093/emboj/18.6.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gangloff S, McDonald J, Bendixen C, Arthur L, Rothstein R. The yeast type I topoisomerase Top3 interacts with Sgs1, a DNA helicase homolog: a potential eukaryotic reverse gyrase. Mol. Cell. Biol. 1994;14:8391–8398. doi: 10.1128/mcb.14.12.8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.German J. Bloom syndrome: a mendelian prototype of somatic mutational disease. Medicine. 1993;72:393–406. [PubMed] [Google Scholar]
- 47.German J, Archibald R, Bloom D. Chromosomal Breakage in a Rare and Probably Genetically Determined Syndrome of Man. Science. 1965;148:506–507. doi: 10.1126/science.148.3669.506. [DOI] [PubMed] [Google Scholar]
- 48.German J, Sanz MM, Ciocci S, Ye TZ, Ellis NA. Syndrome-causing mutations of the BLM gene in persons in the Bloom's Syndrome Registry. Hum. Mutat. 2007;28:743–753. doi: 10.1002/humu.20501. [DOI] [PubMed] [Google Scholar]
- 49.Gharibyan V, Youssoufian H. Localization of the Bloom syndrome helicase to punctate nuclear structures and the nuclear matrix and regulation during the cell cycle: comparison with the Werner's syndrome helicase. Mol. Carcinog. 1999;26:261–273. doi: 10.1002/(sici)1098-2744(199912)26:4<261::aid-mc5>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 50.Gilbertson L, Stahl F. A test of the double-strand break repair model for meiotic recombination in Saccharomyces cerevisiae. Genetics. 1996;144:27–41. doi: 10.1093/genetics/144.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 1993;3:419–429. [Google Scholar]
- 52.Goss KH, Risinger MA, Kordich JJ, Sanz MM, Straughen JE, et al. Enhanced tumor formation in mice heterozygous for Blm mutation. Science. 2002;297:2051–2053. doi: 10.1126/science.1074340. [DOI] [PubMed] [Google Scholar]
- 53.Goto M. Hierarchical deterioration of body systems in Werner's syndrome: implications for normal ageing. Mech. Ageing Dev. 1997;98:239–254. doi: 10.1016/s0047-6374(97)00111-5. [DOI] [PubMed] [Google Scholar]
- 54.Grabowski MM, Svrzikapa N, Tissenbaum HA. Bloom syndrome ortholog HIM-6 maintains genomic stability in C. elegans. Mech. Ageing Dev. 2005;126:1314–1321. doi: 10.1016/j.mad.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 55.Gravel S, Chapman JR, Magill C, Jackson SP. DNA helicases Sgs1 and BLM promote DNA double-strand break resection. Genes Dev. 2009;22:2767–2772. doi: 10.1101/gad.503108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harmon F, Kowalczykowski S. RecQ helicase, in concert with RecA and SSB proteins, initiates and disrupts DNA recombination. Genes Dev. 1998;12:1134–1144. doi: 10.1101/gad.12.8.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hartung F, Puchta H. The RecQ gene family in plants. J. Plant Physiol. 2006;163:287–296. doi: 10.1016/j.jplph.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Hodgkin J, Horvitz H, Brenner S. Nondisjunction Mutants of the Nematode Caenorhabditis elegans. Genetics. 1979;91:67–94. doi: 10.1093/genetics/91.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hoki Y, Araki R, Fujimori A, Ohhata T, Koseki H, et al. Growth retardation and skin abnormalities of the Recql4-deficient mouse. Hum. Mol. Genet. 2003;12:2293–2299. doi: 10.1093/hmg/ddg254. [DOI] [PubMed] [Google Scholar]
- 60.Hu Y, Raynard S, Sehorn MG, Lu X, Bussen W, et al. RECQL5/Recql5 helicase regulates homologous recombination and suppresses tumor formation via disruption of Rad51 presynaptic filaments. Genes Dev. 2007;21:3073–3084. doi: 10.1101/gad.1609107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang S, Lee L, Hanson NB, Lenaerts C, Hoehn H, et al. The spectrum of WRN mutations in Werner syndrome patients. Hum. Mutat. 2006;27:558–567. doi: 10.1002/humu.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huber MD, Lee DC, Maizels N. G4 DNA unwinding by BLM and Sgs1p: substrate specificity and substrate-specific inhibition. Nucleic Acids Res. 2002;30:3954–3961. doi: 10.1093/nar/gkf530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hyun M, Bohr V, Ahn B. Biochemical characterization of the WRN-1 RecQ helicase of Caenorhabditis elegans. Biochemistry. 2008;47:7583–7593. doi: 10.1021/bi800197m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ira G, Malkova A, Liberi G, Foiani M, Haber J. Srs2 and Sgs1-Top3 suppress crossovers during double-strand break repair in yeast. Cell. 2003;115:401–411. doi: 10.1016/s0092-8674(03)00886-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jessop L, Lichten M. Mus81/Mms4 endonuclease and Sgs1 helicase collaborate to ensure proper recombination intermediate metabolism during meiosis. Mol. Cell. 2008;31:313–323. doi: 10.1016/j.molcel.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jessop L, Rockmill B, Roeder G, Lichten M. Meiotic Chromosome Synapsis-Promoting Proteins Antagonize the Anti-Crossover Activity of Sgs1. PLoS Genet. 2006;2 doi: 10.1371/journal.pgen.0020155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiao R, Bachrati C, Pedrazzi G, Kuster P, Petkovic M, et al. Physical and functional interaction between the Bloom's syndrome gene product and the largest subunit of chromatin assembly factor 1. Mol. Cell. Biol. 2004;24:4710–4719. doi: 10.1128/MCB.24.11.4710-4719.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson F, Lombard D, Neff N, Mastrangelo M, Dewolf W, et al. Association of the Bloom syndrome protein with topoisomerase IIIalpha in somatic and meiotic cells. Cancer Res. 2000;60:1162–1167. [PubMed] [Google Scholar]
- 69.Kamath-Loeb A, Johansson E, Burgers P, Loeb L. Functional interaction between the Werner Syndrome protein and DNA polymerase delta. Proc. Natl. Acad. Sci. U S A. 2000;97:4603–4608. doi: 10.1073/pnas.97.9.4603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Karow J, Constantinou A, Li J, West S, Hickson I. The Bloom's syndrome gene product promotes branch migration of holliday junctions. Proc. Natl. Acad. Sci. U S A. 2000;97:6504–6508. doi: 10.1073/pnas.100448097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karow JK, Newman RH, Freemont PS, Hickson ID. Oligomeric ring structure of the Bloom's syndrome helicase. Curr. Biol. 1999;9:597–600. doi: 10.1016/s0960-9822(99)80264-4. [DOI] [PubMed] [Google Scholar]
- 72.Kerrest A, Anand RP, Sundararajan R, Bermejo R, Liberi G, et al. SRS2 and SGS1 prevent chromosomal breaks and stabilize triplet repeats by restraining recombination. Nat. Struct. Mol. Biol. 2009;16:159–167. doi: 10.1038/nsmb.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khakhar RR, Cobb JA, Bjergbaek L, Hickson ID, Gasser SM. RecQ helicases: multiple roles in genome maintenance. Trends Cell Biol. 2003;13:493–501. doi: 10.1016/s0962-8924(03)00171-5. [DOI] [PubMed] [Google Scholar]
- 74.Kitao S, Ohsugi I, Ichikawa K, Goto M, Furuichi Y, Shimamoto A. Cloning of two new human helicase genes of the RecQ family: biological significance of multiple species in higher eukaryotes. Genomics. 1998;54:443–452. doi: 10.1006/geno.1998.5595. [DOI] [PubMed] [Google Scholar]
- 75.Kitao S, Shimamoto A, Goto M, Miller RW, Smithson WA, et al. Mutations in RECQL4 cause a subset of cases of Rothmund-Thomson syndrome. Nat. Genet. 1999;22:82–88. doi: 10.1038/8788. [DOI] [PubMed] [Google Scholar]
- 76.Kobbe D, Blanck S, Demand K, Focke M, Puchta H. AtRECQ2, a RecQ helicase homologue from Arabidopsis thaliana, is able to disrupt various recombinogenic DNA structures in vitro. Plant J. 2008;55:397–405. doi: 10.1111/j.0960-7412.2008.03511.x. [DOI] [PubMed] [Google Scholar]
- 77.Krejci L, Van Komen S, Li Y, Villemain J, Reddy MS, et al. DNA helicase Srs2 disrupts the Rad51 presynaptic filament. Nature. 2003;423:305–309. doi: 10.1038/nature01577. [DOI] [PubMed] [Google Scholar]
- 78.Kusano K, Johnson-Schlitz DM, Engels WR. Sterility of Drosophila with mutations in the Bloom syndrome gene--complementation by Ku70. Science. 2001;291:2600–2602. doi: 10.1126/science.291.5513.2600. [DOI] [PubMed] [Google Scholar]
- 79.Langland G, Kordich J, Creaney J, Goss K, Lillard-Wetherell K, et al. The Bloom's syndrome protein (BLM) interacts with MLH1 but is not required for DNA mismatch repair. J. Biol. Chem. 2001;276:30031–30035. doi: 10.1074/jbc.M009664200. [DOI] [PubMed] [Google Scholar]
- 80.Larrivee M, LeBel C, Wellinger RJ. The generation of proper constitutive G-tails on yeast telomeres is dependent on the MRX complex. Genes Dev. 2004;18:1391–1396. doi: 10.1101/gad.1199404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lebel M, Leder P. A deletion within the murine Werner syndrome helicase induces sensitivity to inhibitors of topoisomerase and loss of cellular proliferative capacity. Proc. Natl. Acad. Sci. U S A. 1998;95:13097–13102. doi: 10.1073/pnas.95.22.13097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lebel M, Spillare EA, Harris CC, Leder P. The Werner syndrome gene product co-purifies with the DNA replication complex and interacts with PCNA and topoisomerase I. J. Biol. Chem. 1999;274:37795–37799. doi: 10.1074/jbc.274.53.37795. [DOI] [PubMed] [Google Scholar]
- 83.LeRoy G, Carroll R, Kyin S, Seki M, Cole M. Identification of RecQL1 as a Holliday junction processing enzyme in human cell lines. Nucleic Acids Res. 2005;33:6251–6257. doi: 10.1093/nar/gki929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li D, Frazier M, Evans DB, Hess KR, Crane CH, et al. Single nucleotide polymorphisms of RecQ1, RAD54L, and ATM genes are associated with reduced survival of pancreatic cancer. J. Clin. Oncol. 2006;24:1720–1728. doi: 10.1200/JCO.2005.04.4206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li JL, Harrison RJ, Reszka AP, Brosh RM, Jr, Bohr VA, et al. Inhibition of the Bloom's and Werner's syndrome helicases by G-quadruplex interacting ligands. Biochemistry. 2001;40:15194–15202. doi: 10.1021/bi011067h. [DOI] [PubMed] [Google Scholar]
- 86.Liberi G, Maffioletti G, Lucca C, Chiolo I, Baryshnikova A, et al. Rad51-dependent DNA structures accumulate at damaged replication forks in sgs1 mutants defective in the yeast ortholog of BLM RecQ helicase. Genes Dev. 2005;19:339–350. doi: 10.1101/gad.322605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lisby M, Rothstein R. Choreography of recombination proteins during the DNA damage response. DNA Repair (Amst) 2009;8:1068–1076. doi: 10.1016/j.dnarep.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu Z, Macias M, Bottomley M, Stier G, Linge J, et al. The three-dimensional structure of the HRDC domain and implications for the Werner and Bloom syndrome proteins. Structure. 1999;7:1557–1566. doi: 10.1016/s0969-2126(00)88346-x. [DOI] [PubMed] [Google Scholar]
- 89.Lonn U, Lonn S, Nylen U, Winblad G, German J. An abnormal profile of DNA replication intermediates in Bloom's syndrome. Cancer Res. 1990;50:3141–3145. [PubMed] [Google Scholar]
- 90.Louis E, Borts R. Meiotic recombination: too much of a good thing? Curr. Biol. 2003;13:R953–R955. doi: 10.1016/j.cub.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 91.Lu CY, Tsai CH, Brill SJ, Teng SC. Sumoylation of the BLM ortholog, Sgs1, promotes telomere-telomere recombination in budding yeast. Nucleic Acids Res. 2009 doi: 10.1093/nar/gkp1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo G, Santoro IM, McDaniel LD, Nishijima I, Mills M, et al. Cancer predisposition caused by elevated mitotic recombination in Bloom mice. Nat. Genet. 2000;26:424–429. doi: 10.1038/82548. [DOI] [PubMed] [Google Scholar]
- 93.Mann MB, Hodges CA, Barnes E, Vogel H, Hassold TJ, Luo G. Defective sister-chromatid cohesion, aneuploidy and cancer predisposition in a mouse model of type II Rothmund-Thomson syndrome. Hum. Mol. Genet. 2005;14:813–825. doi: 10.1093/hmg/ddi075. [DOI] [PubMed] [Google Scholar]
- 94.Mimitou EP, Symington LS. Sae2, Exo1 and Sgs1 collaborate in DNA double-strand break processing. Nature. 2008:770–775. doi: 10.1038/nature07312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miozzo M, Castorina P, Riva P, Dalpra L, Fuhrman Conti AM, et al. Chromosomal instability in fibroblasts and mesenchymal tumors from 2 sibs with Rothmund-Thomson syndrome. Int. J. Cancer. 1998;77:504–510. doi: 10.1002/(sici)1097-0215(19980812)77:4<504::aid-ijc5>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 96.Miyajima A, Seki M, Onoda F, Shiratori M, Odagiri N, et al. Sgs1 helicase activity is required for mitotic but apparently not for meiotic functions. Mol. Cell. Biol. 2000;20:6399–6409. doi: 10.1128/mcb.20.17.6399-6409.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moens PB, Freire R, Tarsounas M, Spyropoulos B, Jackson SP. Expression and nuclear localization of BLM, a chromosome stability protein mutated in Bloom's syndrome, suggest a role in recombination during meiotic prophase. J. Cell Sci. 2000;113(Pt 4):663–672. doi: 10.1242/jcs.113.4.663. [DOI] [PubMed] [Google Scholar]
- 98.Mohaghegh P, Karow JK, Brosh Jr RM, Jr, Bohr VA, Hickson ID. The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases. Nucleic Acids Res. 2001;29:2843–2849. doi: 10.1093/nar/29.13.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mullen J, Kaliraman V, Brill S. Bipartite structure of the SGS1 DNA helicase in Saccharomyces cerevisiae. Genetics. 2000;154:1101–1114. doi: 10.1093/genetics/154.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mullen J, Nallaseth F, Lan Y, Slagle C, Brill S. Yeast Rmi1/Nce4 controls genome stability as a subunit of the Sgs1-Top3 complex. Mol. Cell. Biol. 2005;25:4476–4487. doi: 10.1128/MCB.25.11.4476-4487.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muzzolini L, Beuron F, Patwardhan A, Popuri V, Cui S, et al. Different quaternary structures of human RECQ1 are associated with its dual enzymatic activity. PLoS Biol. 2007;5:e20. doi: 10.1371/journal.pbio.0050020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nasmyth KA. Molecular genetics of yeast mating type. Annu. Rev. Genet. 1982;16:439–500. doi: 10.1146/annurev.ge.16.120182.002255. [DOI] [PubMed] [Google Scholar]
- 103.Nimonkar AV, Ozsoy AZ, Genschel J, Modrich P, Kowalczykowski SC. Human exonuclease 1 and BLM helicase interact to resect DNA and initiate DNA repair. Proc. Natl. Acad. Sci. U S A. 2008;105:16906–16911. doi: 10.1073/pnas.0809380105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oh S, Lao J, Taylor A, Smith G, Hunter N. RecQ helicase, Sgs1, and XPF family endonuclease, Mus81-Mms4, resolve aberrant joint molecules during meiotic recombination. Mol. Cell. 2008;31:324–336. doi: 10.1016/j.molcel.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Opresko P, Sowd G, Wang H. The Werner syndrome helicase/exonuclease processes mobile D-loops through branch migration and degradation. PLoS ONE. 2009;4:e4825. doi: 10.1371/journal.pone.0004825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pedruzzi I, Burckert N, Egger P, De Virgilio C. Saccharomyces cerevisiae Ras/cAMP pathway controls post-diauxic shift element-dependent transcription through the zinc finger protein Gis1. EMBO J. 2000;19:2569–2579. doi: 10.1093/emboj/19.11.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Pike A, Shrestha B, Popuri V, Burgess-Brown N, Muzzolini L, et al. Structure of the human RECQ1 helicase reveals a putative strand-separation pin. Proc. Natl. Acad. Sci. U S A. 2009;106:1039–1044. doi: 10.1073/pnas.0806908106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Plank J, Wu J, Hsieh T. Topoisomerase IIIalpha and Bloom's helicase can resolve a mobile double Holliday junction substrate through convergent branch migration. Proc. Natl. Acad. Sci. U S A. 2006;103:11118–11123. doi: 10.1073/pnas.0604873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Poot M, Hoehn H, Runger TM, Martin GM. Impaired S-phase transit of Werner syndrome cells expressed in lymphoblastoid cell lines. Exp. Cell Res. 1992;202:267–273. doi: 10.1016/0014-4827(92)90074-i. [DOI] [PubMed] [Google Scholar]
- 110.Popuri V, Bachrati C, Muzzolini L, Mosedale G, Costantini S, et al. The human recq helicases, blm and recq1, display distinct DNA substrate specificities. J. Biol. Chem. 2008 doi: 10.1074/jbc.M709749200. [DOI] [PubMed] [Google Scholar]
- 111.Puranam KL, Blackshear PJ. Cloning and characterization of RECQL, a potential human homologue of the Escherichia coli DNA helicase RecQ. J. Biol. Chem. 1994;269:29838–29845. [PubMed] [Google Scholar]
- 112.Raynard S, Bussen W, Sung P. A double Holliday junction dissolvasome comprising BLM, topoisomerase IIIalpha, and BLAP75. J. Biol. Chem. 2006;281:13861–13864. doi: 10.1074/jbc.C600051200. [DOI] [PubMed] [Google Scholar]
- 113.Raynard S, Zhao W, Bussen W, Lu L, Ding Y, et al. Functional Role of BLAP75 in BLM-Topoisomerase III{alpha}-dependent Holliday Junction Processing. J. Biol. Chem. 2008;283:15701–15708. doi: 10.1074/jbc.M802127200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Robert T, Dervins D, Fabre F, Gangloff S. Mrc1 and Srs2 are major actors in the regulation of spontaneous crossover. EMBO J. 2006;25:2837–2846. doi: 10.1038/sj.emboj.7601158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Rockmill B, Fung JC, Branda SS, Roeder GS. The Sgs1 helicase regulates chromosome synapsis and meiotic crossing over. Curr. Biol. 2003;13:1954–1962. doi: 10.1016/j.cub.2003.10.059. [DOI] [PubMed] [Google Scholar]
- 116.Rockmill B, Voelkel-Meiman K, Roeder GS. Centromere-proximal crossovers are associated with precocious separation of sister chromatids during meiosis in Saccharomyces cerevisiae. Genetics. 2006;174:1745–1754. doi: 10.1534/genetics.106.058933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rog O, Miller KM, Ferreira MG, Cooper JP. Sumoylation of RecQ helicase controls the fate of dysfunctional telomeres. Mol. Cell. 2009;33:559–569. doi: 10.1016/j.molcel.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 118.Saffi J, Feldmann H, Winnacker E, Henriques J. Interaction of the yeast Pso5/Rad16 and Sgs1 proteins: influences on DNA repair and aging. Mutat. Res. 2001;486:195–206. doi: 10.1016/s0921-8777(01)00093-3. [DOI] [PubMed] [Google Scholar]
- 119.Salk D, Bryant E, Au K, Hoehn H, Martin GM. Systematic growth studies, cocultivation, and cell hybridization studies of Werner syndrome cultured skin fibroblasts. Hum. Genet. 1981;58:310–316. doi: 10.1007/BF00294930. [DOI] [PubMed] [Google Scholar]
- 120.Seki M, Otsuki M, Ishii Y, Tada S, Enomoto T. RecQ family helicases in genome stability. Cell Cycle. 2008;7:2472–2478. doi: 10.4161/cc.7.16.6462. [DOI] [PubMed] [Google Scholar]
- 121.Seki M, Yanagisawa J, Kohda T, Sonoyama T, Ui M, Enomoto T. Purification of two DNA-dependent adenosinetriphosphatases having DNA helicase activity from HeLa cells and comparison of the properties of the two enzymes. J. Biochem. 1994;115:523–531. doi: 10.1093/oxfordjournals.jbchem.a124369. [DOI] [PubMed] [Google Scholar]
- 122.Sengupta S, Linke SP, Pedeux R, Yang Q, Farnsworth J, et al. BLM helicase-dependent transport of p53 to sites of stalled DNA replication forks modulates homologous recombination. EMBO J. 2003;22:1210–1222. doi: 10.1093/emboj/cdg114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sengupta S, Robles AI, Linke SP, Sinogeeva NI, Zhang R, et al. Functional interaction between BLM helicase and 53BP1 in a Chk1-mediated pathway during S-phase arrest. J. Cell Biol. 2004;166:801–813. doi: 10.1083/jcb.200405128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shen JC, Gray MD, Oshima J, Loeb LA. Characterization of Werner syndrome protein DNA helicase activity: directionality, substrate dependence and stimulation by replication protein A. Nucleic Acids Res. 1998;26:2879–2885. doi: 10.1093/nar/26.12.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shimamoto A, Nishikawa K, Kitao S, Furuichi Y. Human RecQ5beta, a large isomer of RecQ5 DNA helicase, localizes in the nucleoplasm and interacts with topoisomerases 3alpha and 3beta. Nucleic Acids Res. 2000;28:1647–1655. doi: 10.1093/nar/28.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Siitonen HA, Kopra O, Kaariainen H, Haravuori H, Winter RM, et al. Molecular defect of RAPADILINO syndrome expands the phenotype spectrum of RECQL diseases. Hum. Mol. Genet. 2003;12:2837–2844. doi: 10.1093/hmg/ddg306. [DOI] [PubMed] [Google Scholar]
- 127.Siitonen HA, Sotkasiira J, Biervliet M, Benmansour A, Capri Y, et al. The mutation spectrum in RECQL4 diseases. Eur. J. Hum. Genet. 2009;17:151–158. doi: 10.1038/ejhg.2008.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Singh T, Ali A, Busygina V, Raynard S, Fan Q, et al. BLAP18/RMI2, a novel OB-fold-containing protein, is an essential component of the Bloom helicase-double Holliday junction dissolvasome. Genes Dev. 2008;22:2856–2868. doi: 10.1101/gad.1725108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Spillare EA, Robles AI, Wang XW, Shen JC, Yu CE, et al. p53-mediated apoptosis is attenuated in Werner syndrome cells. Genes Dev. 1999;13:1355–1360. doi: 10.1101/gad.13.11.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sun H, Bennett RJ, Maizels N. The Saccharomyces cerevisiae Sgs1 helicase efficiently unwinds G-G paired DNAs. Nucleic Acids Res. 1999;27:1978–1984. doi: 10.1093/nar/27.9.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tahara H, Kusunoki M, Yamanaka Y, Matsumura S, Ide T. G-tail telomere HPA: simple measurement of human single-stranded telomeric overhangs. Nat. Methods. 2005;2:829–831. doi: 10.1038/nmeth797. [DOI] [PubMed] [Google Scholar]
- 132.Theobald D, Mitton-Fry R, Wuttke D. Nucleic acid recognition by OB-fold proteins. Annu. Rev. Biophys. Biomol. Struct. 2003;32:115–133. doi: 10.1146/annurev.biophys.32.110601.142506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tronnersjo S, Hanefalk C, Balciunas D, Hu G, Nordberg N, et al. The jmjN and jmjC domains of the yeast zinc finger protein Gis1 interact with 19 proteins involved in transcription, sumoylation and DNA repair. Mol. Genet. Genomics. 2007;277:57–70. doi: 10.1007/s00438-006-0171-3. [DOI] [PubMed] [Google Scholar]
- 134.Tsukada Y, Fang J, Erdjument-Bromage H, Warren M, Borchers C, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 135.Van Brabant AJ, Ye T, Sanz M, German IJ, Ellis NA, Holloman WK. Binding and melting of D-loops by the bloom syndrome helicase. Biochemistry. 2000;39:14617–14625. doi: 10.1021/bi0018640. [DOI] [PubMed] [Google Scholar]
- 136.Van Maldergem L, Siitonen HA, Jalkh N, Chouery E, De Roy M, et al. Revisiting the craniosynostosis-radial ray hypoplasia association: Baller-Gerold syndrome caused by mutations in the RECQL4 gene. J. Med. Genet. 2006;43:148–152. doi: 10.1136/jmg.2005.031781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Veaute X, Jeusset J, Soustelle C, Kowalczykowski SC, Le Cam E, Fabre F. The Srs2 helicase prevents recombination by disrupting Rad51 nucleoprotein filaments. Nature. 2003;423:309–312. doi: 10.1038/nature01585. [DOI] [PubMed] [Google Scholar]
- 138.Versini G, Comet I, Wu M, Hoopes L, Schwob E, Pasero P. The yeast Sgs1 helicase is differentially required for genomic and ribosomal DNA replication. EMBO J. 2003;22:1939–1949. doi: 10.1093/emboj/cdg180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Vindigni A, Hickson I. RecQ helicases: multiple structures for multiple functions? HFSP J. 2009;3:153–164. doi: 10.2976/1.3079540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.von Kobbe C, Karmakar P, Dawut L, Opresko P, Zeng X, et al. Colocalization, physical, and functional interaction between Werner and Bloom syndrome proteins. J Biol Chem. 2002;277:22035–22044. doi: 10.1074/jbc.M200914200. [DOI] [PubMed] [Google Scholar]
- 141.Wallis JW, Chrebet G, Brodsky G, Rolfe M, Rothstein R. A hyper-recombination mutation in S. cerevisiae identifies a novel eukaryotic topoisomerase. Cell. 1989;58:409–419. doi: 10.1016/0092-8674(89)90855-6. [DOI] [PubMed] [Google Scholar]
- 142.Wang T, Kung W. Supercomplex formation between Mlh1-Mlh3 and Sgs1-Top3 heterocomplexes in meiotic yeast cells. Biochem. Biophys. Res. Commun. 2002;296:949–953. doi: 10.1016/s0006-291x(02)02034-x. [DOI] [PubMed] [Google Scholar]
- 143.Wang XW, Tseng A, Ellis NA, Spillare EA, Linke SP, et al. Functional interaction of p53 and BLM DNA helicase in apoptosis. J. Biol. Chem. 2001;276:32948–32955. doi: 10.1074/jbc.M103298200. [DOI] [PubMed] [Google Scholar]
- 144.Wang Y, Cortez D, Yazdi P, Neff N, Elledge SJ, Qin J. BASC, a super complex of BRCA1-associated proteins involved in the recognition and repair of aberrant DNA structures. Genes Dev. 2000;14:927–939. [PMC free article] [PubMed] [Google Scholar]
- 145.Watt P, Hickson I, Borts R, Louis E. SGS1, a homologue of the Bloom's and Werner's syndrome genes, is required for maintenance of genome stability in Saccharomyces cerevisiae. Genetics. 1996;144:935–945. doi: 10.1093/genetics/144.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Watt P, Louis E, Borts R, Hickson I. Sgs1: a eukaryotic homolog of E. coli RecQ that interacts with topoisomerase II in vivo and is required for faithful chromosome segregation. Cell. 1995;81:253–260. doi: 10.1016/0092-8674(95)90335-6. [DOI] [PubMed] [Google Scholar]
- 147.White CI, Haber JE. Intermediates of recombination during mating type switching in Saccharomyces cerevisiae. EMBO J. 1990;9:663–673. doi: 10.1002/j.1460-2075.1990.tb08158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wu L, Bachrati CZ, Ou J, Xu C, Yin J, et al. BLAP75/RMI1 promotes the BLM-dependent dissolution of homologous recombination intermediates. Proc. Natl. Acad. Sci. U S A. 2006;103:4068–4073. doi: 10.1073/pnas.0508295103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wu L, Chan K, Ralf C, Bernstein D, Garcia P, et al. The HRDC domain of BLM is required for the dissolution of double Holliday junctions. EMBO J. 2005;24:2679–2687. doi: 10.1038/sj.emboj.7600740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu L, Davies S, Levitt N, Hickson I. Potential role for the BLM helicase in recombinational repair via a conserved interaction with RAD51. J. Biol. Chem. 2001;276:19375–19381. doi: 10.1074/jbc.M009471200. [DOI] [PubMed] [Google Scholar]
- 151.Wu L, Davies SL, North PS, Goulaouic H, Riou JF, et al. The Bloom's syndrome gene product interacts with topoisomerase III. J. Biol. Chem. 2000;275:9636–9644. doi: 10.1074/jbc.275.13.9636. [DOI] [PubMed] [Google Scholar]
- 152.Wu L, Hickson I. The Bloom's syndrome helicase suppresses crossing over during homologous recombination. Nature. 2003;426:870–874. doi: 10.1038/nature02253. [DOI] [PubMed] [Google Scholar]
- 153.Xu D, Guo R, Sobeck A, Bachrati C, Yang J, et al. RMI, a new OB-fold complex essential for Bloom syndrome protein to maintain genome stability. Genes Dev. 2008;22:2843–2855. doi: 10.1101/gad.1708608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu H, Deprez E, Zhang A, Tauc P, Ladjimi M, et al. The Escherichia coli RecQ helicase functions as a monomer. J. Biol. Chem. 2003;278:34925–34933. doi: 10.1074/jbc.M303581200. [DOI] [PubMed] [Google Scholar]
- 155.Xu X, Rochette PJ, Feyissa EA, Su TV, Liu Y. MCM10 mediates RECQ4 association with MCM2-7 helicase complex during DNA replication. EMBO J. 2009;28:3005–3014. doi: 10.1038/emboj.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yamane K, Toumazou C, Tsukada Y, Erdjument-Bromage H, Tempst P, et al. JHDM2A, a JmjC-containing H3K9 demethylase, facilitates transcription activation by androgen receptor. Cell. 2006;125:483–495. doi: 10.1016/j.cell.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 157.Yang Q, Zhang R, Wang X, Spillare E, Linke S, et al. The processing of Holliday junctions by BLM and WRN helicases is regulated by p53. J. Biol. Chem. 2002;277:31980–31987. doi: 10.1074/jbc.M204111200. [DOI] [PubMed] [Google Scholar]
- 158.Yin J, Sobeck A, Xu C, Meetei AR, Hoatlin M, et al. BLAP75, an essential component of Bloom's syndrome protein complexes that maintain genome integrity. EMBO J. 2005;24:1465–1476. doi: 10.1038/sj.emboj.7600622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yu CE, Oshima J, Fu YH, Wijsman EM, Hisama F, et al. Positional cloning of the Werner's syndrome gene. Science. 1996;272:258–262. doi: 10.1126/science.272.5259.258. [DOI] [PubMed] [Google Scholar]
- 160.Zhang X, Dou S, Xie P, Hu J, Wang P, Xi X. E. coli RecQ is a rapid, efficient and monomeric helicase. J. Biol. Chem. 2006 doi: 10.1074/jbc.M513089200. [DOI] [PubMed] [Google Scholar]
- 161.Zhong S, Hu P, Ye T, Stan R, Ellis N, Pandolfi P. A role for PML and the nuclear body in genomic stability. Oncogene. 1999;18:7941–7947. doi: 10.1038/sj.onc.1203367. [DOI] [PubMed] [Google Scholar]
- 162.Zhu Z, Chung WH, Shim EY, Lee SE, Ira G. Sgs1 helicase and two nucleases Dna2 and Exo1 resect DNA double-strand break ends. Cell. 2008;134:981–994. doi: 10.1016/j.cell.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]