Abstract
Objectives
Suppressive anti-retroviral therapy (ART) substantially decreases HIV transmission in clinical research settings. We sought to measure the frequency and correlates of periods of transmission risk among individuals taking ART during multiple years of observation in rural, southwestern Uganda.
Design
Observational cohort study
Methods
We collected sexual behavior and viral load data in a Ugandan cohort of people living with HIV/AIDS from the time of ART initiation. We defined each 90-day visit as a potential transmission period if HIV-1 RNA was > 400 copies/mL and the participant reported sexual transmission risk behavior, defined as unprotected sexual contact with ≥1 HIV-uninfected partners or partners of unknown serostatus in the prior 90 days.
Results
We evaluated data from 463 individuals on ART over a median 3.5 years of observation and 5,293 total study visits. During that time, over half (259, 56%) had detectable viremia or reported sexual transmission risk behavior at least once. However only 23 (5%) had both simultaneously, at 28 (<1%) of all visits. Transmission sexual behavior was reported at 6% of visits with detectable viremia. In multivariable regression modeling, correlates of transmission risk periods included younger age, lower CD4 count, low household asset ownership and increased internalized stigma.
Conclusions
Although detectable viremia and/or sexual transmission risk behavior occurred in over half of individuals, ART reduced periods of HIV transmission risk by over 90% during up to six years of observation time. These findings provide further support for provision of ART, along with interventions to promote long-term adherence, to reduce HIV transmission in HIV-endemic settings.
Keywords: HIV/AIDS, Anti-retroviral therapy, sub-Saharan Africa, treatment as prevention, Uganda
BACKGROUND
Observational studies [1, 2], clinical trials [3], mathematical models [4], and population studies [5] have demonstrated decreased transmission risk among people living with HIV/AIDS (PLWHA) taking suppressive anti-retroviral therapy (ART). Because transmission risk approaches zero when viral detection is below 400 copies/mL [1, 3], sexual transmission is theoretically limited to sexual exposures between sero-discordant partners when the HIV-positive partner is actively viremic. Yet, the frequency and correlates of these events at the individual-level during long-term ART are poorly characterized. To address this question, we analyzed data from a cohort of PLWHA initiating ART in rural Uganda to estimate the frequency and correlates of simultaneous viremia and sexual transmission risk behavior. Our goal was to measure the potential impact and durability of ART to mitigate transmission risk for PLWHA on long-term treatment in an HIV-endemic, resource-limited setting.
METHODS
Study participants
PLWHA initiating ART were enrolled from the public HIV clinic in Mbarara, Uganda into the Uganda AIDS Rural Treatment Outcomes (UARTO) study, an observational, prospective cohort study beginning in 2005 (NCT01596322). The clinic is a PEPFAR-supported, government-run center with an active census over 10,000 patients, serving the largely rural, southwestern Ugandan region. Patients are seen at clinical visits every 1–3 months and are offered free ART, opportunistic infection medications, basic laboratory testing and counseling services. Data for the study were collected on separate days from clinical visits by trained research staff in private rooms apart from the HIV clinic. At baseline and every three months, we collected blood for CD4+ T-lymphocyte count and HIV-1 RNA viral load determinations, and we conducted structured interviews to collect data on sexual activity, partner serostatus, and condom use during the past 90 days. We defined each 90-day visit as a potential transmission period if HIV-1 RNA was > 400 copies/mL and the participant reported sexual transmission risk behavior, defined as unprotected sexual contact with ≥1 HIV-uninfected partners or partners of unknown serostatus in the prior 90 days. We also collected data on demographic and economic characteristics (household size, marital status, educational attainment, household asset ownership [6], food insecurity [7], and travel time to clinic), clinical characteristics (self-reported opportunistic infections, depression symptom severity using a modified version of the Hopkins Symptom Checklist depression subscale [8], and self-reported hospitalization within the past 90 days), and socio-behavioral characteristics (hazardous drinking as defined by a positive screen on the 3-item consumption subset of the Alcohol Use Disorders Identification Test [AUDIT-C] [9], and internalized stigma using the Internalized AIDS-Related Stigma Scale [10]).
Ethics Statement
All participants gave written informed consent. Study procedures were reviewed and approved by institutional review committees at Partners Healthcare, the University of California at San Francisco, and the Mbarara University of Science and Technology, and the Ugandan National Council of Science and Technology.
Statistical analyses
We measured the frequency of sexual transmission risk behavior, HIV-1 RNA viremia, and the simultaneous occurrence of both (defined as a transmission risk period) at each quarterly observation visit. We fit multivariable logistic regression models using generalized estimating equations to estimate correlates of transmission risk periods. We tabulated the number of persons lost-to-follow-up at each six-month period after enrollment, and assessed for attrition bias by comparing demographic characteristics of those lost to follow up or disenrolled by two years of enrollment to those with two or more years of observation time, using Fisher exact tests for categorical variables and Mann-Whitney tests for non-normally distributed continuous variables. All statistical analyses were performed with Stata 11.2 (Statacorp, College Station, TX, USA).
RESULTS
Five-hundred eight participants were enrolled in the UARTO study between June 2005 and June 2011, out of which 463 participants had at least one follow-up visit and were included in these analyses. Included participants were 70% women, with a median age of 34 years (IQR 29 – 40), a median enrollment CD4 count of 136 cells/mm^3, and a median enrollment viral load of log10 5.1 copies/mL (Supplementary Table 1). Regimens at the time of initiation were: zidovudine/lamivudine/nevirapine (64%), nevirapine/stavudine/lamivudine (25%), zidovudine/lamivudine/efavirenz (9%), and other (2%).
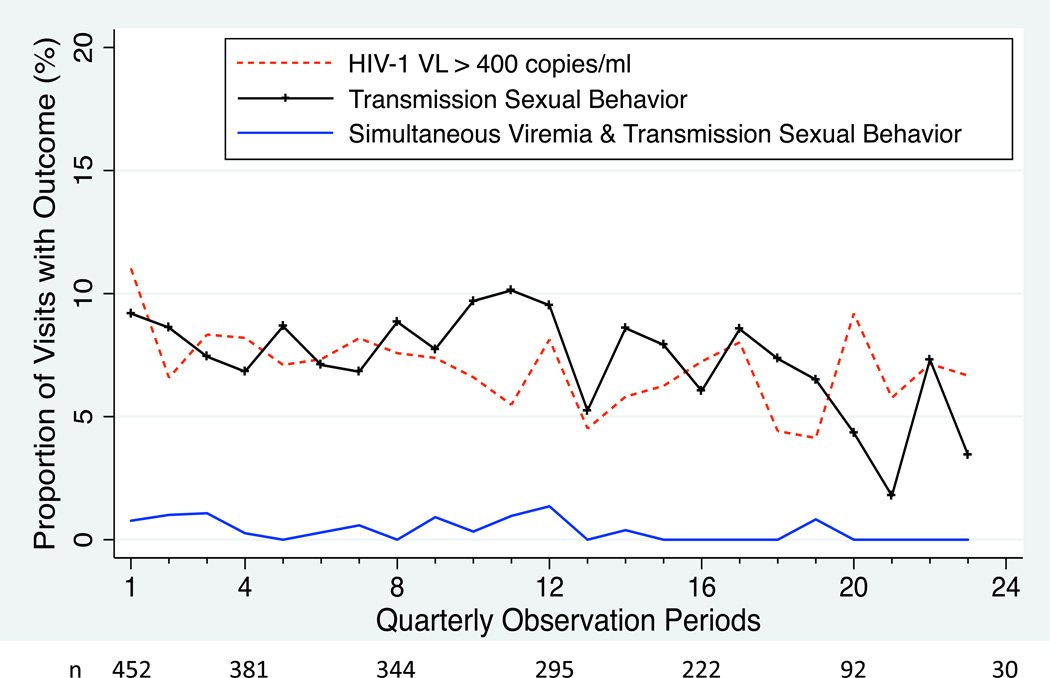
Out of 508 enrolled participants, 39 (8%) were deceased during follow-up, 69 (14%) were lost-to-follow-up or disenrolled, and 401 (79%) were active in the study at time of censoring. We censored 251 of 6,174 (1.0%) visits because data on either viral load or sexual behavior was missing, for a total of 5,923 observations in 463 participants contributing at least one study visit (median of 3.5 years of observation time, IQR 2.2 – 4.2). During follow-up, 259 participants (59%) had either detectable viremia or self-reported sexual transmission risk behavior at least once (136 participants [36%] with detectable viremia and 143 [31%] reported sexual transmission risk behavior). However, only 23 participants (5% of total cohort, 20 women and 3 men) had ≥1 potential transmission period. During a total of 5,923 three-month observation periods, we found detectable viremia during 426 periods (7%) and self-reported sexual transmission risk behavior at 454 periods (8%). However, simultaneous viremia and sexual transmission risk behavior were identified at only 28 periods (< 0.5%, Figure 1). Detectable viremia was found at 28 (6%) of the 445 visits where risky sexual behavior was reported and viral load data was available.
Figure 1.
Prevalence of HIV RNA-1 viremia > 400 copies/mL (dotted line), reported sexual transmission risk behavior (unprotected sex with a sero-discordant partner or partner of unknown serostatus [hatched line]), and simultaneous occurrence of both (solid line) in a cohort of PLWHA on ART in southwestern Uganda during up to six years of observation time.
In multivariable logistic regression models, age (adjusted odds ratio [AOR] 0.9 per each increasing year of age, 95%CI 0.8–0.9), CD4 count (AOR 0.6 per each increase in 100 cells/mm^3, 95%CI 0.4–0.9), relative household asset wealth (AOR 0.3, 95%CI 0.1–0.8, for the 2nd through 4th quartiles versus lowest quartile of asset ownership), and internalized stigma (AOR 2.6, 95%CI 1.1–6.3 for each increasing point on the internalized stigma scale) had statistically significant associations with simultaneous viremia and sexual transmission risk behavior (Table 1).
Table 1.
Correlates of the simultaneous occurrence of HIV-1 RNA viremia > 400 copies/mL and sexual transmission risk behavior, defined as unprotected sex with an HIV-negative partner or partner of unknown serostatus, among PLWHA on ART in southwestern Uganda. Results for both univariable and multivariable models are shown.
| Univariable Models | Multivariable Model | |||||
|---|---|---|---|---|---|---|
| Characteristic | Odds Ratio | 95%CI | p-value | AOR | 95%CI | p-value |
| Age (per year) | 0.86 | 0.80–0.93 | <0.01 | 0.87 | 0.80–0.94 | 0.01 |
| Household size | 0.91 | 0.78–1.06 | 0.24 | 1.07 | 0.92–1.26 | 0.38 |
| Female gender | 2.58 | 0.79–8.43 | 0.12 | 2.20 | 0.48–10.03 | 0.31 |
| Completed primary school or greater | 1.11 | 0.36–3.46 | 0.85 | 1.50 | 0.40–5.48 | 0.55 |
| Married | 0.73 | 0.33–1.63 | 0.44 | 0.98 | 0.39–2.46 | 0.97 |
| Household asset ownership (2–4th quartile vs. 1st quartile as reference) | 0.33 | 0.15–0.70 | 0.004 | 0.33 | 0.13–0.82 | 0.02 |
| Travel time to clinic less than 1 hour | 2.10 | 0.94–4.70 | 0.07 | 1.76 | 0.71–4.32 | 0.22 |
| Internalized stigma (score greater than 0 on the Internalized AIDS Stigma Score) | 2.70 | 1.20–6.08 | 0.02 | 2.53 | 1.04–6.13 | 0.04 |
| Any food insecurity | 2.10 | 0.78–5.59 | 0.14 | 1.46 | 0.52–4.11 | 0.27 |
| Probable depression | 2.51 | 0.87–7.26 | 0.09 | 1.57 | 0.40–6.19 | 0.52 |
| Hospitalized in the past three months | 2.43 | 0.60–9.90 | 0.22 | 2.00 | 0.41–9.67 | 0.39 |
| Any history of an opportunistic infection | 1.07 | 0.46–2.47 | 0.88 | 1.62 | 0.65–4.04 | 0.31 |
| Dry season | 1.13 | 0.56–2.27 | 0.74 | 1.27 | 0.57–2.83 | 0.56 |
| CD4 count (each increase of 100 cells/mm^3) | 0.75 | 0.56–1.00 | 0.05 | 0.62 | 0.43–0.89 | 0.01 |
| Positive screen for hazardous alcohol use | 1.10 | 0.80–1.53 | 0.56 | 1.31 | 0.91–1.88 | 0.15 |
| Time on art (years) | 0.77 | 0.57–1.05 | 0.10 | 0.88 | 0.57–1.37 | 0.60 |
The number of participants lost-to-follow-up or disenrolled was 37 by 6 months, 44 by 1 year, 58 by 2 years, and 67 by 3 years. Compared to participants in the study with more than two years of observation time, participants lost-to-follow-up or disenrolled before two years were older (median age 35 vs. 30 years, p<0.001), but there were no other statistically significant differences in baseline characteristics, including proportion who reported sexual transmission risk behavior at the baseline visit (13% vs. 21%, p=0.10).
DISCUSSION
In this long-term cohort study, we found that provision of ART to PLWHA accessing care at a public HIV clinic in rural, southwestern Uganda durably reduced the risk of HIV transmission over a median of 3.5 years of observation time. Though over half of participants had episodic detectable viremia or reported sexual transmission risk behavior, less than 5% ever experienced them simultaneously. Moreover, during over 5,000 quarters of observation time, participants reported sexual transmission risk behavior at approximately 8% of periods but were found with concurrent viremia at only 0.5% of periods. In other words, ART provided a sustained, > 90% reduction in periods of HIV transmission risk by imparting undetectable viral loads to individuals when risky sexual behavior was reported. Our findings corroborate prior work describing the enormous potential of ART to prevent transmission in HIV-endemic, resource-limited settings.
Sexual transmission risk behavior in resource limited settings has been generally observed to decline after initiation of ART [11], and studies of HIV sexual transmission risk between discordant couples in Uganda have shown impressive declines in transmission [2, 12]. However, sexual transmission risk behavior has remained elevated in some populations during treatment, including those aware of its efficacy to prevent transmission [13–15]. Indeed, we found that approximately one in three participants reported at least one episode of sexual transmission risk behavior during a median of 3.5 years of observation time. Further, approximately one in three participants experienced virologic rebound during follow-up. However, unlike other studies in which sexual transmission risk behavior has reportedly occurred at high rates among those who are viremic [16], we found the co-occurrence of sexual transmission risk behavior and viremia to be exceptionally rare. Moreover, the rate of simultaneous viremia and sexual transmission risk behavior (~0.5% of periods of observation) occurred at nearly the rate that would be expected if the two individual events were independent. In sum, although 5–10% of participants had either sexual transmission risk behavior or detectable viremia at each quarterly visit, their co-occurrence was rare (Figure 1). These findings suggest that achieving continued reductions in secondary HIV transmission will be feasible, but might be strengthened by interventions to maintain long-term ART adherence.
We identified four factors independently associated with periods of HIV transmission risk: younger age, lower CD4 count, low household asset ownership and increased internalized stigma. This confluence of factors contributes to a large body of evidence describing the challenges of care delivery and the poor outcomes associated with lower socioeconomic status and stigmatization of PLWHA in resource limited settings. It also reinforces that younger patients, those with advanced disease stage, and those of lower socioeconomic status might be particularly high-yield targets for interventions to improve adherence and foster safer sexual behavior. The association between internalized HIV stigma and sexual transmission risk behavior was consistent with previously reported associations between stigma, serostatus disclosure [17], and poor ART adherence [18], and corroborates the notion that interventions to reduce stigma might also have an appreciable impact on HIV transmission.
We recruited patients from a prototypical, PEPFAR-supported, publicly operated clinic in rural Uganda, and the findings should be generalized to similar populations. Five important considerations relevant to our study were: (a) participants in the study undergo real-time adherence monitoring with electronic adherence monitors, and thus might be subject to a Hawthorne effect, which would increase adherence levels and decreases rates of viremia; (b) we lack confirmatory data about HIV transmission to sexual partners which would corroborate our assumptions; (c) we assumed that quarterly viral load measurements reflected persistent virologic control in the preceding three-month period, which may not always be the case; (d) we used self-reported sexual behavior as a primary outcome of interest, which would likely under-estimate sexual transmission risk behavior due to social desirability bias [19]. However, given the independent association between viremia and sexual transmission risk behavior, if the rate of reported sexual transmission risk behavior were to double, this would only increase our estimate of potential transmission risk from < 0.5% of all periods to approximately 1% (and leave our primary conclusions unchanged); and (e) we had relatively rare missing data in that only (251/6174, 1%) of visits were missing viral load or sexual behavior data when the other was present, and relatively few participants (67/508, 13%) were lost to follow up or disenrolled by three years of observation time. Community-based tracking of missing patients at this clinic has demonstrated that approximately one in three transfer care to another HIV facility, while another one in three are found to have died [20]. In sum, few patients who default from care in this setting remain alive and off treatment.
In sum, we found that, although both detectable viremia and sexual transmission risk behavior independently occurred in over half of PWLHA people on treatment, ART reduced periods of potential HIV transmission risk by over 90% in a population of PLWHA in Uganda during up to six years of observation time. These findings provide further support for the provision of ART to all PLWHA meeting guidelines to reduce HIV transmission in HIV-endemic settings.
Supplementary Material
Acknowledgements
We thank the UARTO participants and staff who made this study possible.
sources of funding
This work was supported by U.S. National Institutes of Health (NIH) R01MH054907 and P30AI27763. The authors also acknowledge the following additional sources of support: K23MH087228 (Haberer); K23MH096620 (Tsai); K23MH079713 (Weiser); K24MH087227 (Bangsberg); and K23MH099916 (Siedner). Additional study funding was provided by the Mark and Lisa Schwartz Family Foundation, the Sullivan Family Foundation, and the Bacca Foundation.
Footnotes
Conflict of interest
The authors have no conflicts of interest to report.
Author contributions:
Mark Siedner took the lead on conception and design, performed data analysis, and wrote the first draft of the manuscript.
Nicholas Musinguzi took the lead on data analysis and interpretation and performed critical revision of the manuscript.
Alexander Tsai assisted with concept design, data analysis and interpretation, and performed critical revision of the manuscript.
Conrad Muzoora assisted with study design, performed data collection, and performed critical revision of the manuscript.
Annet Kembabazi assisted in study concept design and performed critical revision of the manuscript.
Sheri Weiser participated in study conception and designed, preformed data acquisition, and performed manuscript editing and revision.
John Bennett assisted with data collection and interpretation and performed critical revision of the manuscript.
Peter Hunt participated in study conception and design, performed data acquisition and performed manuscript editing and revision.
Jeffrey Martin participated in study conception and design, performed data acquisition, assisted in data analysis and interpretation and performed manuscript editing and revision.
Jessica Haberer participated in study conception and design, performed data acquisition and performed manuscript editing and revision.
David Bangsberg was the principal investigator and developer of the UARTO study. He participated in study conception, design and manuscript editing and revision.
REFERENCES
- 1.Attia S, Egger M, Muller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23:1397–1404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds SJ, Makumbi F, Nakigozi G, Kagaayi J, Gray RH, Wawer M, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25:473–477. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eaton JW, Johnson LF, Salomon JA, Barnighausen T, Bendavid E, Bershteyn A, et al. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS medicine. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanser F, Barnighausen T, Grapsa E, Zaidi J, Newell ML. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339:966–971. doi: 10.1126/science.1228160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data--or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 7.Swindale A, Bilinsky P. Development of a universally applicable household food insecurity measurement tool: process, current status, and outstanding issues. The Journal of nutrition. 2006;136:1449S–1452S. doi: 10.1093/jn/136.5.1449S. [DOI] [PubMed] [Google Scholar]
- 8.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL): a self-report symptom inventory. Behavioral science. 1974;19:1–15. doi: 10.1002/bs.3830190102. [DOI] [PubMed] [Google Scholar]
- 9.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Archives of internal medicine. 1998;158:1789–1795. doi: 10.1001/archinte.158.16.1789. [DOI] [PubMed] [Google Scholar]
- 10.Tsai AC, Weiser SD, Steward WT, Mukiibi NF, Kawuma A, Kembabazi A, et al. Evidence for the reliability and validity of the internalized AIDS-related stigma scale in rural Uganda. AIDS Behav. 2013;17:427–433. doi: 10.1007/s10461-012-0281-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Venkatesh KK, Flanigan TP, Mayer KH. Is expanded HIV treatment preventing new infections? Impact of antiretroviral therapy on sexual risk behaviors in the developing world. AIDS. 2011;25:1939–1949. doi: 10.1097/QAD.0b013e32834b4ced. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bunnell R, Ekwaru JP, Solberg P, Wamai N, Bikaako-Kajura W, Were W, et al. Changes in sexual behavior and risk of HIV transmission after antiretroviral therapy and prevention interventions in rural Uganda. AIDS. 2006;20:85–92. doi: 10.1097/01.aids.0000196566.40702.28. [DOI] [PubMed] [Google Scholar]
- 13.Crepaz N, Hart TA, Marks G. Highly active antiretroviral therapy and sexual risk behavior: a meta-analytic review. JAMA. 2004;292:224–236. doi: 10.1001/jama.292.2.224. [DOI] [PubMed] [Google Scholar]
- 14.de Walque D, Kazianga H, Mead O. Antiretroviral therapy perceived efficacy and risky sexual behavior: Evidence from Mozambique. Economic Development and Cultural Change. 2012;61:97–126. [Google Scholar]
- 15.Kembabazi A, Bajunirwe F, Hunt PW, Martin JN, Muzoora C, Haberer JE, et al. Disinhibition in Risky Sexual Behavior in Men, but Not Women, during Four Years of Antiretroviral Therapy in Rural, Southwestern Uganda. PLoS ONE. 2013;8:e69634. doi: 10.1371/journal.pone.0069634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Durham MD, Buchacz K, Richardson J, Yang D, Wood K, Yangco B, et al. Sexual risk behavior and viremia among men who have sex with men in the HIV Outpatient Study (HOPS), USA, 2007 – 2010. Journal of acquired immune deficiency syndromes. 2013 doi: 10.1097/QAI.0b013e31828c20d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsai AC, Bangsberg DR, Kegeles SM, Katz IT, Haberer JE, Muzoora C, et al. Internalized Stigma, Social Distance, and Disclosure of HIV Seropositivity in Rural Uganda. Ann Behav Med. 2013 doi: 10.1007/s12160-013-9514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rintamaki LS, Davis TC, Skripkauskas S, Bennett CL, Wolf MS. Social stigma concerns and HIV medication adherence. AIDS Patient Care STDS. 2006;20:359–368. doi: 10.1089/apc.2006.20.359. [DOI] [PubMed] [Google Scholar]
- 19.Schroder KE, Carey MP, Vanable PA. Methodological challenges in research on sexual risk behavior: I. Item content, scaling, and data analytical options. Annals of behavioral medicine : a publication of the Society of Behavioral Medicine. 2003;26:76–103. doi: 10.1207/s15324796abm2602_02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng EH, Bangsberg DR, Musinguzi N, Emenyonu N, Bwana MB, Yiannoutsos CT, et al. Understanding reasons for and outcomes of patients lost to follow-up in antiretroviral therapy programs in Africa through a sampling-based approach. Journal of acquired immune deficiency syndromes. 2010;53:405–411. doi: 10.1097/QAI.0b013e3181b843f0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.