The effect of elevated CO2 on the growth of tobacco under high light (16 h), continuous water and nutrient supply was investigated. Biomass production depended strongly on the size of the root bed. Inhibition by a small root bed was higher at 700 than at 360 ppm CO2. Relative growth rates showed a head-start of the high-CO2 plants which gave rise to a persistently higher biomass production. Root-bed size and CO2 concentration were mirrored by the quantitative cytokinin patterns of the various plant parts. Amounts of the cytokinins moving from the root to the shoot were higher in high-CO2 plants.
Keywords: Biomass portioning, C/N ratio, cytokinins, elevated CO2, growth, root-bed volume, tobacco.
Abstract
The extent of growth stimulation of C3 plants by elevated CO2 is modulated by environmental factors. Under optimized environmental conditions (high light, continuous water and nutrient supply, and others), we analysed the effect of an elevated CO2 atmosphere (700 ppm, EC) and the importance of root-bed size on the growth of tobacco. Biomass production was consistently higher under EC. However, the stimulation was overridden by root-bed volumes that restricted root growth. Maximum growth and biomass production were obtained at a root bed of 15 L at ambient and elevated CO2 concentrations. Starting with seed germination, the plants were strictly maintained under ambient or elevated CO2 until flowering. Thus, the well-known acclimation effect of growth to enhanced CO2 did not occur. The relative growth rates of EC plants exceeded those of ambient-CO2 plants only during the initial phases of germination and seedling establishment. This was sufficient for a persistently higher absolute biomass production by EC plants in non-limiting root-bed volumes. Both the size of the root bed and the CO2 concentration influenced the quantitative cytokinin patterns, particularly in the meristematic tissues of shoots, but to a smaller extent in stems, leaves and roots. In spite of the generally low cytokinin concentrations in roots, the amounts of cytokinins moving from the root to the shoot were substantially higher in high-CO2 plants. Because the cytokinin patterns of the (xylem) fluid in the stems did not match those of the shoot meristems, it is assumed that cytokinins as long-distance signals from the roots stimulate meristematic activity in the shoot apex and the sink leaves. Subsequently, the meristems are able to synthesize those phytohormones that are required for the cell cycle. Root-borne cytokinins entering the shoot appear to be one of the major control points for the integration of various environmental cues into one signal for optimized growth.
Introduction
Current CO2 scenarios (IPCC 2013) predict CO2 concentrations of 700 ppm at the end of the 21st century (Prentice et al. 2001). Such an increase will affect natural and agricultural ecosystems as ‘green CO2 sinks’ (Drake et al. 1997; Krupa 2003; Lindroth 2010; Hikosaka et al. 2011) and thus may have far-reaching economic and political consequences. Knowledge of the reaction of plants to elevated CO2 concentrations is essential for assessing the potential capacity of green CO2 sinks.
In numerous laboratory and field studies, different responses of plants to elevated CO2 concentrations have been observed (Makino and Mae 1999; Poorter and Navas 2003; Long et al. 2006; for a review see Körner 2006). Apart from the CO2 concentration itself, these effects were modulated by a variety of factors, e.g. light intensity, temperature, and water and nutrient (especially nitrogen) supply (McConnaughay et al. 1993; Stitt and Krapp 1999; Ainsworth and Long 2005; Johnsen 2006; Reich et al. 2006; for a review see Kant et al. 2012). Differences in the reactions of plants to elevated CO2 depend also on the experimental approach: chamber studies with plants grown in pots versus field studies using free-air concentration enrichment (Long et al. 2006; Ainsworth et al. 2008).
For C3 plants, an initial and transitory increase of the rates of photosynthesis and plant growth was generally observed upon exposure to elevated CO2 concentrations, a phenomenon that has been termed ‘acclimation’ (Long et al. 2004). Acclimation to elevated CO2 results from a decrease of Rubisco activity, for which several explanations have been presented, in particular an internal feedback mechanism termed ‘sink limitation’. If production of photosynthates in the leaves exceeds utilization by the various sink activities of a plant, e.g. root and shoot growth, export via the phloem decreases and carbohydrates accumulate as starch in the chloroplasts (Thomas and Strain 1991). This in turn inhibits chloroplastic metabolism, in particular photosynthesis (Stitt 1991; Paul and Foyer 2001). Thus, the response of a plant to elevated CO2 should be governed by the activities of its sinks (Reekie et al. 1998). High sink activities can only be expected under optimal growth conditions, which hardly occur in nature. Under optimal light, high CO2 concentration and sufficient water supply, macronutrients may readily become limiting. Deterioration of the plant's nutrient status (in particular nitrogen) due to an attenuation of nutrient uptake might affect growth and thus sink activities, and in turn cause ‘acclimation’ to elevated CO2 (Makino et al. 1997; Nakano et al. 1997; Sicher and Bunce 1997; Curtis et al. 2000). Decreasing nutrient uptake could result from a declining nutrient concentration in the root bed (Geiger et al. 1999; Stitt and Krapp 1999) or by a dwindling uptake capacity of the roots (Thomas and Strain 1991). Both reasons often coincide in pot experiments with too small root-bed volumes that restrict root growth (Arp 1991; Berntson et al. 1993; McConnaughay et al. 1993; Rabha and Uprety 1998; Zhu et al. 2006; Yang et al. 2007, 2010). This is important as elevated CO2 concentrations stimulate root growth, in particular fine root production (Norby et al. 2004; Stiling et al. 2013), and thus a small root-bed size should restrict root growth much more at elevated than at ambient CO2 concentrations.
Both increased root growth in response to elevated CO2 on the one hand and limited root growth by root-bed size on the other hand must be balanced by the plant. It must be able to recognize the ‘nutrient status’ of the root and of the shoot and ‘translate’ this information into a growth response (Berntson et al. 1993; McConnaughay et al. 1993). To adapt growth to the resource status, plants rely on specific long-distance growth signals that mediate the communication between the root and the shoot. At least with respect to nitrogen as the most important macronutrient, translation of the ‘resource status’ of the root into a long-distance signal—cytokinins—has been shown (Beck and Wagner 1994; Beck 1999; Yong et al. 2000; Miyawaki et al. 2004).
Cytokinins are mitogenic signals that control the cell cycle (Francis and Sorrell 2001; Hartig and Beck 2005; Harashima and Schnittger 2010; Dudits et al. 2011) and thus activity of meristems. Reduced cytokinin contents in transgenic tobacco and Arabidopsis plants resulted in slow-growing, stunted shoots with small leaves but an enhanced root system (Werner et al. 2001, 2003, 2008; Yang et al. 2003).
Of course, cytokinins are not the only long-distance and cellular signals that adapt plant growth to its resource status, but they may be the most prominent ones. Evidence is increasing that it is not the cytokinin load exported from the root to the shoot which directly controls the activity of the shoot meristems, but that the meristematic cells convert such exogenous into intracellular cytokinin signals, which however are also under the control of other signals (Bürkle et al. 2003; Motyka et al. 2003). Bishopp et al. (2011) showed that cytokinins moving from the shoot to the root via the phloem play a role in vascular patterning of the root apex.
In the present study, the growth response of tobacco plants to an elevated CO2 concentration was analysed under controlled conditions (optimal water and nutrient supply as well as light conditions). In contrast to similar studies reported so far, an optimized nutrient solution was continuously flushed, day and night, through the root beds of pure quartz sand, keeping the nutrient concentration around the roots constant and thus avoiding effects of changing nutrient concentrations.
With that approach, especially the improved nutrient supply, the following hypotheses were examined: (i) under our conditions, the size of the root bed is the crucial factor that controls growth of the plant under ambient as well as elevated CO2 concentrations, and therefore (ii) acclimation of plants to elevated CO2 can be prevented by a sufficiently large root bed. (iii) We further hypothesize that cytokinins are involved in growth stimulation under conditions where acclimation is avoided. To this end, cytokinins in different plant parts and in the xylem sap were quantified comparing tobacco plants grown under ambient and elevated CO2 and cultivated in growth limiting and non-limiting root beds.
Methods
Plant growth and experimental setup
Plant growth
Nicotiana tabacum cv. Samsun was grown in two accurately controlled walk-in climate chambers (York International, volume 11.5 m3). The light period of 14 h at 26 °C and 70 % relative humidity was followed by a 10-h dark period at 19 °C and 60 % relative humidity. The light intensity at the top of the pots was 700 µmol photons m−2 s−1 (MT 400 DL/BH E-40 lamps; Iwasaki Electric Co., Tokyo, Japan). The dark phase included 30 min ‘dawn’ and ‘dusk’ with increasing or decreasing light intensities. The CO2 concentration in the control chamber was 360 ppm (atmospheric ambient concentration, ‘AC’) whereas in the other chamber a permanent CO2 concentration of 700 ppm CO2 (elevated, ‘EC’) was provided (the CO2 concentration was automatically controlled with a gas exchange analyser, BINOS; Leybold-Heraeus).
Tobacco seeds were germinated in transparent boxes (Phytotray II; Sigma) on agar in the respective climate chambers at 360 or 700 ppm CO2. The medium for germination contained 0.5 % (w/v) inorganic Murashige–Skoog medium, pH 5.7 (Murashige and Skoog 1962), and 0.5 % (w/v) agar to which a small quantity of active carbon powder was added to avoid infestation by fungi. After sowing, the boxes were closed and covered with a net (mesh size 8 mm) to mitigate illumination strength. After 1 week, the boxes were opened once per day to allow exchange with the atmosphere of the climate chamber.
Twelve to 15 days after sowing, when the cotyledons had completely unfolded, the seedlings were transferred to sand culture. The root bed consisted of pure quartz sand (grain size 0.7–1.2 mm) that—after washing with one pot volume of deionized water—was rinsed with half of the pot volume of diluted nutrient solution (nutrient solution : water = 1 : 3). The nutrient solution contained 3 mM K2HPO4, 4 mM KNO3, 4 mM Mg(NO3)2, 2 mM MgSO4, 4 mM CaSO4, 0.02 mM Fe-EDTA, 50 µM KCl, 20 µM H3BO4, 2 µM MnSO4, 2 µM ZnSO4, 0.5 µM CuSO4 and 0.5 µM MoO3 (pH 6.0, adjusted with H2SO4; Dertinger et al. 2003). After transfer to the sand culture, the pots were initially covered with cellophane for maintaining high air humidity.
Two days later, the cellophane was replaced by a shading net, under which the plantlets were kept for 12 days. The root bed was continuously percolated with nutrient solution with a pump (IPC-N; Ismatec Laboratoriumstechnik GmbH, Wertheim, Germany). To maintain the nutrient concentration around the roots constant, the root bed was percolated with 300 mL of nutrient solution per 1 L of root-bed volume and day. During the first 2 weeks, the plantlets were supplied with a half concentrated nutrient solution. Thereafter, undiluted nutrient solution was supplied. To provide equal growth conditions for all plants, the pots were turned around and their places in the climate chambers were exchanged twice a week.
Experimental setup
For close comparison, tobacco plants were cultivated at the same time under the two CO2 concentrations in pots of 1, 5, 10, 15 and 20 L volume until an age of 61 days. Five replicates were grown per pot volume (triplicates in the 20-L pot experiment). A more detailed time kinetics of growth and biomass partitioning under AC and EC was made with the plants in the 15-L pots.
Biomass (dry weights) and relative growth rates (RGRs) of the plantlets were investigated at the ages of 1, 2, 3, 4, 5, 6, 7, 8, 9, 12 and 15 days after sowing in 5-fold replicates samples of 10 plants each. The older plants were examined in triplicate at 21, 28, 35, 42, 48 and 61 days after sowing. The leaves were numbered from the bottom to the top, beginning with the first leaf following the cotyledons. For comparing parameters of older plants, the parameter ‘physiological age’ was used, which was defined by the number of leaves and the area of the biggest leaf (see Table 1).
Table 1.
Number of leaves, leaf length, leaf width and plant age of N. tabacum cv. Samsun grown at 360 and 700 ppm CO2, respectively, in 15-L sand culture. The leaves were counted from bottom to top. Leaf length and leaf width of the biggest leaf per plant were determined.
| Number of leaves |
Biggest leaf (leaf number) | Leaf length × leaf width (mm × mm) |
Plant age (days) | ||
|---|---|---|---|---|---|
| 360 ppm CO2 | 700 ppm CO2 | 360 ppm CO2 | 700 ppm CO2 | ||
| 11 | 12 | 7 | 105 × 80 | 130 × 100 | 28 |
| 15 | 16 | 8 | 170 × 125 | 195 × 145 | 35 |
| 22 | 23 | 9 | 210 × 180 | 250 × 200 | 42 |
| 25 | 25 | 10 | 240 × 200 | 280 × 220 | 49 |
Xylem sap was collected at two sites of 35-day-old plants: at the base of the stem just above leaf 4 and at the petiole of a source leaf (leaf 8). The effect of transpiration (measured with a porometer) was compensated by pressurizing the root with nitrogen in a root pressure chamber (Passioura 1980). Before cutting, the pressure in the root chamber was slightly higher than necessary for compensating the transpiration to avoid embolism. Immediately after cutting, the flow rate was adjusted by reducing the pressure on the root. The first 100 µL of the sap samples were discarded. Xylem sap was subsequently collected for 1 h and stored at −20 °C until cytokinin analysis.
Measurements
Leaf area was calculated by the following equation:
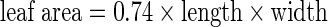 |
[see Supporting Information].
Plants were consistently harvested in the middle of the light period. Dry weights were determined after drying the material at 80 °C to constant weight. Relative growth rate was calculated by the equation
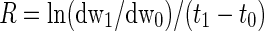 |
where dw1 is the dry weight of the plant at the time of measurement t1 and dw0 the dry weight at the starting time t0.
Carbon and nitrogen contents were determined with an element analyser (CHN-O-Rapid; Elementar Analysesysteme GmbH, Hanau, Germany). The dried material was homogenized in a ball mill (Schwingmühle MM2000; Retsch GmbH & Co. KG, Haan, Germany) and aliquots of the powder were analysed. Acetanilide (71.09 % carbon and 10.36 % nitrogen) was used as the standard.
For cytokinin analysis, the procedure of Wagner and Beck (1993) modified by Hartig and Beck (2005) was followed. This method is more complex than the currently used detection by liquid chromatography-mass spectrometry. It has carefully been elaborated for several plant tissues—among others for tobacco—and for analysis of xylem sap using cross-reactivity of the diverse antibodies as internal control [seeSupporting Information]. It separates the cytokinins (also the aromatic ones), its nucleotides and glucosides and thus provides a comprehensive view of the entire cytokinin pattern. Plant material, as shown in Supporting Information, was extracted with 80 % aqueous methanol in the cold and the extracts were purified by reverse-phase (RP) column chromatography (Bakerbond spe™ octadecyl (C18) disposable extraction column; J. T. Baker, Deventer, Holland) with 80 % methanol. The cytokinins were fractionated by anion exchange chromatography on a DEAE Sephadex™ A-25 column (Amersham Pharmacia Biotech AB, Uppsala, Sweden) into two fractions, using 40 mM NH4OAc buffer (pH 6.5) as the eluent: fraction (i) containing the bases (t-Z, DHZ, IP) + ribosides (t-ZR, DHZR, IPA) + glucosides (Z9G, ZOG, ZROG, DHZ9G, DHZOG, DHZROG) and fraction (ii) with the nucleotides (ZN, DHZN, IPN). To collect fraction (i), a 1.5-mL RP cartridge (Sep-Pak®; Waters Corporation, Milford, MA, USA) was coupled to the anion exchange column. The nucleotides were subsequently eluted from the anion exchanger with 6 % formic acid (v/v) into another RP cartridge from which they were collected with 80 % aqueous methanol. The individual cytokinins were separated by preparative RP-HPLC (column: 250 mm long, 4.6 mm i.d., 5 µm Hypersil® ODS; Muderer & Wochele, Berlin, Germany) and collected in 36 fractions. Elution was carried out with a gradient of acetonitrile as the non-polar phase and 0.1 % (v/v) aqueous triethylammonium acetate in bi-distilled water, pH 6, as the polar phase (for the protocol, see Wagner and Beck 1993). The different cytokinins were quantified by competitive enzyme-linked immunosorbent assay (ELISA) using three phosphatase-coupled antibodies (anti-t-ZR, anti-DHZR and anti-IPA; Wagner and Beck 1993). The bases and 9-glucosides could be determined due to their cross-reactivity with the three antibodies (Weiler and Zenk 1976; Wagner and Beck 1993). The O-glucosides, which did not show cross-reactivity, were quantified as free bases or ribosides after removal of the O-glucosyl residues with β-glucosidase (Wang et al. 1977). The nucleotides were analysed as ribosides after dephosphorylation with alkaline phosphatase followed by ELISA.
The yield of the whole procedure determined with internal standards was more than 90 % and the reliability of the ELISA was examined according to Pengelly (1986) and Crozier et al. (1986). The sources of the chemicals and the standard substances as well as the preparation of the standard solutions have been described in detail by Wagner and Beck (1993).
Statistics
The approach encompassed two variables: two CO2 concentrations and five different volumes of the root beds. Assessment of the effects of root-bed size on plant growth required comparison under identical environmental conditions, i.e. five root-bed variants (with replications) each under ambient and elevated CO2, respectively. Owing to spatial limitations, the entire setup encompassing the subprojects ‘Age-dependence of the effect of the CO2 concentration on the growth of tobacco plants’ and ‘Cytokinin patterns in tobacco plants grown under limiting/non-limiting root-bed conditions and at ambient or elevated CO2’ could be performed only once. Therefore, the question of pseudoreplication (Hurlbert 1984; Waller et al. 2013) arises. Since we had only two walk-in climate chambers with sophisticated control of environmental parameters, we cannot principally rule out pseudoreplication of the CO2 effect with our sample and replication sets. However, due to the fact that for a given root-bed volume all environmental factors including the composition of the root bed were carefully controlled and maintained while only the CO2 concentration was different, we think that the presented results are trustworthy. Furthermore, the CO2 concentrations were addressed as ‘ambient’ and ‘elevated’ and correlations with the absolute values of these concentrations were not established, and thus the danger of erroneous results due to pseudoreplication should be low.
Statistical analyses were carried out with SigmaStat (Systat Software GmbH, Germany). The number of replicates varied between 3 and 30 (and between 2 and 5 for cytokinin determination), as shown in the figures. As the data were not Gaussian distributed and the variances were not homogeneous (as tested by Kolmogorov–Smirnov's test), statistical differences between data from AC and EC plants (weights, shoot–root and shoot–leaf ratios, the leaves' carbon and nitrogen contents and their ratios, and the concentration of cytokinins) were examined using the Wilcoxon–Mann–Whitney test. Statistically significant results were indicated in the figures by asterisks: 0 < P ≤ 0.05*, 0.05 < P ≤ 0.01** and 0.01 < P ≤ 0.001***.
Results
Influence of root-bed volume and CO2 concentration on plant growth rates, morphology, biomass production and carbon/nitrogen status
Tobacco plants were grown under ambient or elevated CO2 concentration in root-bed volumes ranging from 1 to 20 L. Figure 1A illustrates that the size of the plants and the leaf areas of the 61-day-old plants increased with the pot size of up to 15 L. The dry weights of plants increased linearly with the pot size at both CO2 concentrations (Fig. 1D). At the same pot size, plants grown at elevated CO2 (Fig. 1C) were larger and had higher dry weights, but had only one leaf more than plants grown at ambient CO2 (Fig. 1B). The pot size of 15 L was optimal for growth at both CO2 concentrations and a further increase to 20 L had no further effect on the size and biomass production.
Figure 1.
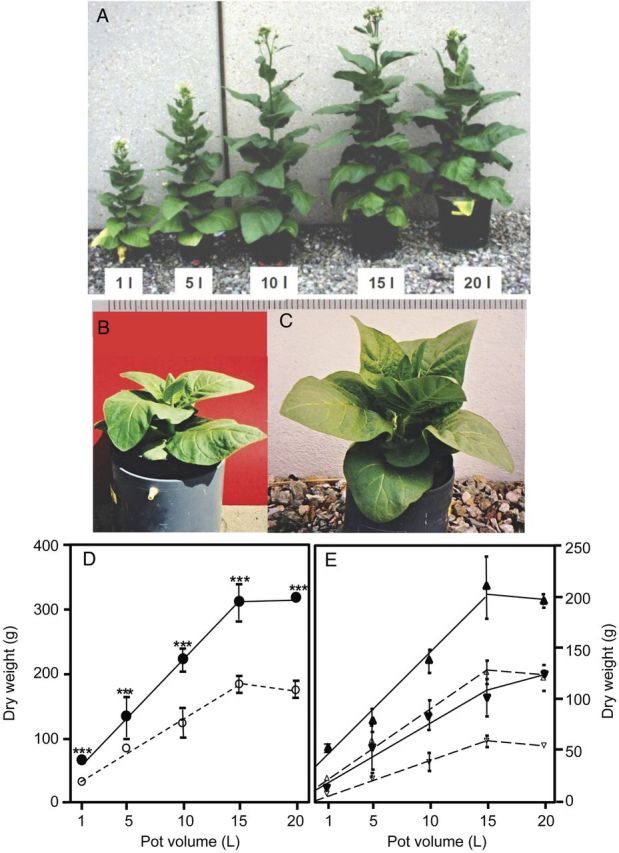
(A) Sixty-one-day-old tobacco plants grown in different pot volumes at 700 ppm CO2, (B) 35-day-old tobacco plants grown at 350 ppm or (C) at 700 ppm CO2. (D) Dry weights of entire 61-day-old tobacco plants grown at 360 ppm (open circles) or 700 ppm CO2 (closed circles), respectively (means of n = 5 and n = 3 for the 20-L pot and standard deviation), and (E) shoot (open upright triangle, black upright triangle) and root (open inverted triangle, black inverted triangle) dry weights of the 61-day-old tobacco plants grown in the indicated pot volumes under ambient (360 ppm; open upright triangle, open inverted triangle) and elevated (700 ppm; black upright triangle, black inverted triangle) CO2 concentrations. Mean values ± standard deviation are presented (n = 5 for 1-, 5-, 10- and 15-L pots, n = 3 for 20-L pots). Asterisks show statistical significances between plants grown under the two CO2 concentrations.
Depending on their position on the stem and their physiological role as source, expanding or sink leaves, the net CO2 uptake rates of the individual leaves differed. While the CO2 concentration had no influence on net CO2 uptake by older, fully expanded leaves, younger, still growing leaves showed higher assimilation rates under elevated CO2 [see Supporting Information].
In order to investigate the influence of root-bed size at ambient and elevated CO2 on biomass partitioning, the shoot and root dry weights were determined (Fig. 1E). All plants allocated more biomass to the shoot than to the root.
Alleviating root growth limitation by increasing the volume of the root bed and promoting root growth by elevated CO2 should result in enhanced nutrient uptake from the continuously supplied nutrients. This was examined by the carbon/nitrogen (C/N) ratio of the plants, of which three leaves (leaves 6, mature; 10, still expanding; and 20, young) were selected for determination of their carbon and nitrogen contents (Fig. 2A–C). Mainly due to the enhanced nitrogen content, the C/N ratios declined with increasing root-bed volumes, demonstrating the expected effect (Fig. 2C). Leaves of high-CO2 plants had consistently higher C/N ratios than those of ambient-CO2 plants. At both CO2 conditions, the C/N ratios increased with leaf age.
Figure 2.
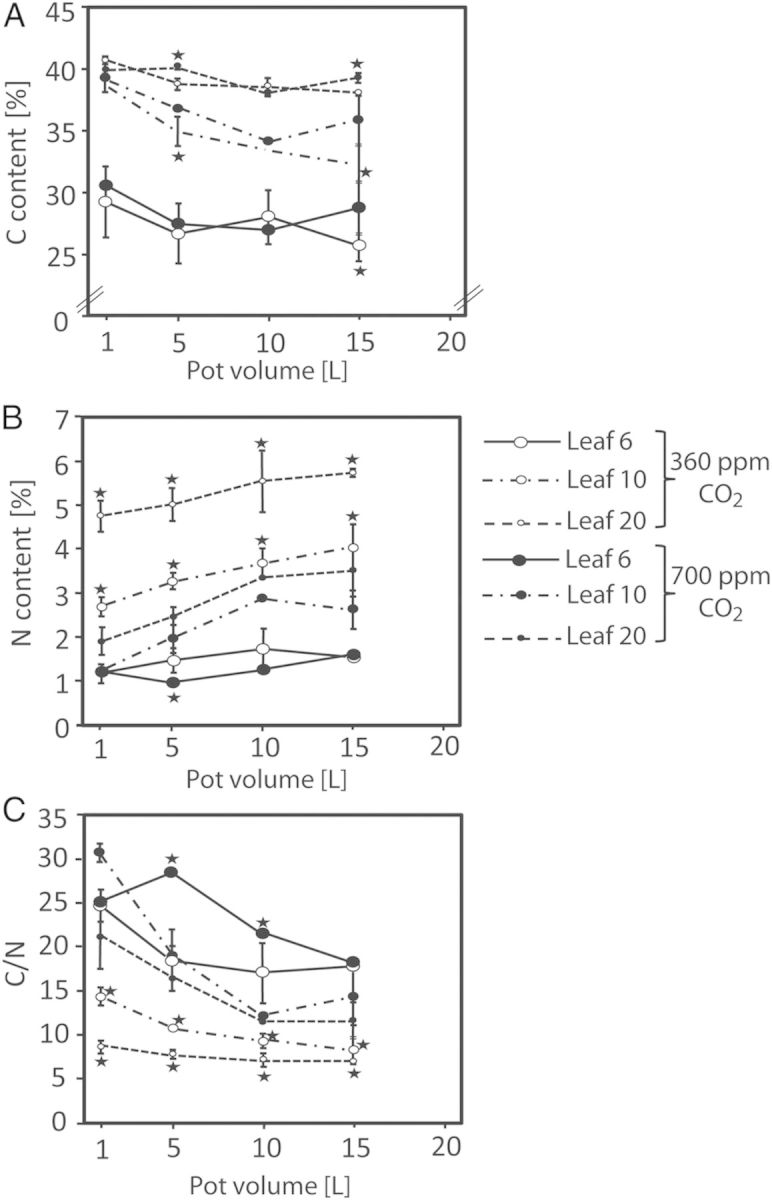
(A and B) Carbon and nitrogen content (%), and (C) C/N ratio of leaves 6, 10 and 20 (numbered from the bottom) of 61-day-old tobacco plants grown in different pot volumes under ambient (360 ppm) and elevated (700 ppm) CO2 concentration (means of n = 5 and standard deviation). Asterisks as in Fig. 1.
Cytokinin patterns in tobacco plants grown under limiting/non-limiting root-bed conditions and at ambient or elevated CO2
Cytokinins are known as signals from the root to the shoot, which respond to the supply of nutrients to and their concentrations in the root. However, also the meristems of the shoot are capable of cytokinin production and the fully expanded leaves, which upon transpiration receive cytokinins via the xylem sap, must be able to metabolize these phytohormones or reload it to the phloem (Beck 1999; Yong et al. 2000). For a better understanding of the effect of root growth restriction and the response to the CO2 concentration on the cytokinin signal, we investigated (i) the cytokinin patterns of tobacco plants grown in a restricted or optimal root-bed volume under ambient or elevated CO2 and (ii) examined the effect of the CO2 concentration separately in an experiment designed for an assessment of the significance of cytokinins as a root signal.
Cytokinin patterns in organs of tobacco plants
Cytokinins of plants from 1- and 15-L pots that had been grown in the described climate chambers under 360 and 700 ppm CO2, respectively, were analysed (Fig. 3).
Figure 3.
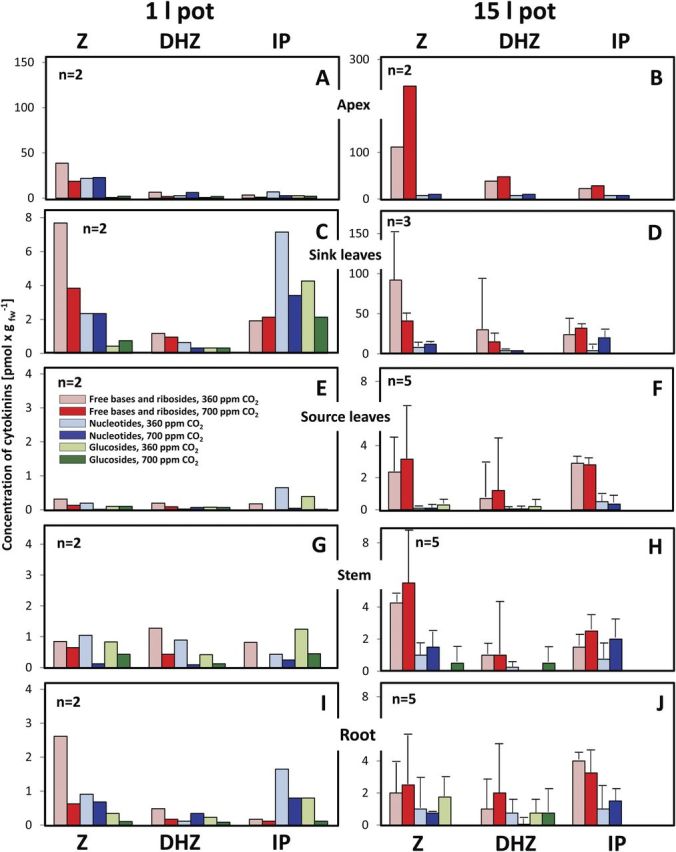
Concentrations of cytokinins in the apices (A, B), sink leaves (C, D), source leaves (E, F), stems (G, H) and roots (I, J) of 42-day-old tobacco plants grown in 1-L sand (A, C, E, G, I) or 15-L sand culture (B, D, F, H, J) under 360 and 700 ppm CO2, respectively (means of at least two independent experiments ± standard deviation). The concentrations of the free bases and ribosides as well as of the glucosides of each of the measured families were combined. Z, members of the t-zeatin family; DHZ, members of the dihydrozeatin family; IP, members of the isopentenyladenine family.
The age of 42 days was chosen, as it still represents the late phase of vegetative growth. For the sake of clarity, the concentrations of the so-called active cytokinins, namely the free bases and the ribosides of each of the three examined cytokinin families, were combined (Mok and Mok 2001). Nucleotides which by dephosphorylation can readily give rise to active cytokinin species as well as the inactive glucosylated species are shown separately.
A clear positive effect of root-bed size was observed in the concentrations of members of all three cytokinin families in the meristematic tissues of the shoot, namely the apices and the unfolding small leaves around the shoot apex (‘sink leaves’). On average, concentrations were up to 6-fold higher in meristems of plants from the 15-L pots, with extremes of 28- and 35-fold higher concentrations for individual cytokinin species (compare Fig. 3A and B and Fig. 3C and D). trans-Z and its derivatives were the most prominent cytokinin species in apices and sink leaves with concentrations up to 37 pmol g−1 fresh weight (t-zeatinriboside) in plants from 1-L pots (Fig. 3A) and up to 140 pmol g−1 fresh weight (t-zeatin) in those from 15-L pots (Fig. 3B). Concentrations of dihydrozeatin and isopentenyladenine and their derivatives were on average 4- to 5-fold lower. Cytokinin concentrations were much lower in mature leaves, stems and roots, and with one exception (t-zeatinriboside in the stems of plants from 15-L pots) out of more than 100 values lower than 2.5 pmol g−1 fresh weight. Differences between the cytokinin concentrations in mature leaves, stems and roots of plants from 1- and 15-L pots were small with a slightly higher concentration in plants from the larger pots. The concentrations in roots as the major cytokinin-producing organs were surprisingly low, whereby representatives of the isopentenyladenine group equalled those of the t-Z family. It should be mentioned, however, that the concentrations do not reflect the total amounts, which—due to the different biomasses—are less conclusive. Substantial concentrations of the inactive O- and N-glycosides of the t-zeatin and dihydrozeatin families were not found in any of the organs, irrespective of pot size or CO2 concentration. Also, a consistent effect of CO2 concentration on the cytokinin patterns could not be detected in spite of several significant differences in the concentrations of individual cytokinin species.
Effect of CO2 concentration on cytokinin signal
While an increase of root-bed size had only a small positive effect on cytokinin concentrations in the roots, a strong effect was observed in the shoot apex and the sink leaves. Also, the difference between the cytokinin concentrations in ambient- and high-CO2 plants from the same root-bed volume was negligible in the roots but high in the meristematic tissues of the shoot. Thus, the question arises as to how cytokinin translocation from the roots by the xylem stream can control growth and development of the shoot. Using the root pressure chamber (Passioura 1980), we collected xylem fluid from 35-day-old tobacco plants grown in root beds of 10 L in the described climate chambers. The technical limitation in this experiment was the size and number of root pressure chambers. Xylem fluid from the entire stem was collected for 1 h at the natural flow rates above the fourth node and from comparable plants from the petiole of a mature leaf (leaf 8). The cytokinin patterns of these xylem fluids [Supporting Information] were compared with those of the roots and the corresponding mature leaves, respectively (Table 2). Multiplication of the cytokinin concentrations with the fresh weight of the investigated plant organs yielded the total amounts. Likewise, the flow rates of cytokinins were calculated from the concentrations in the xylem fluid and the respective measured transpiration rates. The cytokinin concentrations in the transpiration stream and the total amounts of transported cytokinins were higher in the high-CO2 plants than in the ambient-CO2 plants. The cytokinin patterns in the fluid from the base of the stem did not exactly match the patterns in the root where the concentrations and amounts of the t-zeatin group were significantly higher. Comparison of the cytokinin patterns of the xylem fluids from the petioles of mature leaf no. 8 with those of the fluids collected at the bottom of the stems showed two major differences: the concentrations of the cytokinins were considerably lower and the xylem fluid in the petiole appeared to be substantially depleted of the dihydrozeatin and the isopentenyladenine cytokinins [Supporting Information]. But the quantitative cytokinin patterns in the xylem fluid from the petioles from ambient- and high-CO2 plants were almost identical.
Table 2.
Effect of CO2 concentration on the concentration of cytokinins (Cks; Z, members of the t-zeatin family; DHZ, members of the dihydrozeatin family; IP, members of the isopentenyladenine family) in mature leaves and roots as well as the xylem sap of petioles from mature leaves and stems (see explanations in the text for further details on the collection of xylem sap) from 35-day-old tobacco plants grown in root beds of 10 L. Units corresponding to italic values are indicated in italics.
| Cks in mature leaf (pmol g FW−1) (pmol leaf−1) |
Transpiration (nL cm−2 s−1) (mL h−1 leaf−1) | Cks in xylem sap (nM) (pmol leaf−1 h−1) |
|||||
|---|---|---|---|---|---|---|---|
| Z | DHZ | IP | Z | DHZ | IP | ||
| 360 | 1.11 | 1.76 | 2.31 | 10.6 ± 1.6 | 0.89 | 0.44 | 0.42 |
| 4.91 | 7.80 | 10.23 | 5.51 ± 0.92 | 4.73 | 2.42 | 2.31 | |
| 700 | 0.78 | 1.17 | 1.51 | 8.04 ± 1.61 | 0.74 | 0.44 | 0.28 |
| 7.58 | 11.37 | 14.68 | 5.84 ± 1.11 | 4.32 | 2.57 | 1.63 | |
|
Cks in the roots (pmol g FW−1) (pmol per root system) |
Transpiration (mL h−1 shoot−1) |
Cks in xylem sap (nM) (pmol shoot−1 h−1) |
|||||
| Z | DHZ | IP | Z | DHZ | IP | ||
| 360 | 11.1 | 4.3 | 4.3 | 15.2 ± 0.68 | 4.5 | 3.3 | 4.3 |
| 160 | 62.1 | 62.5 | 68.2 | 49.9 | 64.6 | ||
| 700 | 7.7 | 1.9 | 2.1 | 14.5 ± 2.37 | 6.2 | 3.8 | 4.7 |
| 193 | 47.3 | 53.3 | 90.0 | 55.2 | 67.4 | ||
Age dependence of the effect of CO2 concentration on the growth of tobacco plants
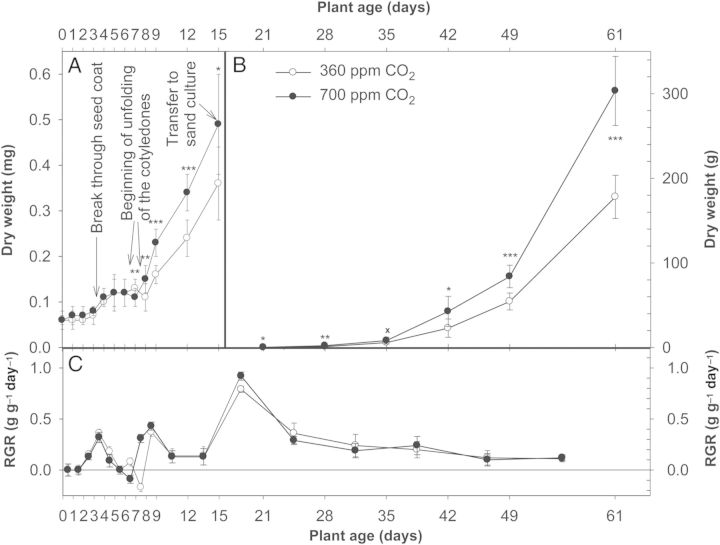
The results described so far revealed a growth-stimulating effect of elevated CO2 on the growth of tobacco plants. However, the question remained about the onset and age dependence of that effect. Germination, seedling development and growth of tobacco plants were therefore followed under ambient or elevated CO2 concentrations at a sufficiently large root bed (Fig. 4). Germination (on agar) started 3 days after sowing irrespective of the CO2 concentration (Fig. 4A). Under both CO2 concentrations, apparent RGRs increased from day 1 to day 4 mainly due to water uptake. Thereafter, RGR decreased to zero prior to unfolding of the cotyledons (Fig. 4C). After development of the cotyledons (days 6 and 7 under elevated CO2 and days 7 and 8 under ambient CO2), the seed coat was shed decreasing RGR to values below zero, which also indicated biomass loss by respiration. After the start of photosynthesis, dry weights and RGRs increased substantially, whereby the increase at elevated CO2 showed a head start of 1 day over that at ambient CO2. Already 8 days after sowing, seedlings grown under 700 ppm CO2 were significantly heavier than those grown under 360 ppm CO2. The higher initial RGR of high-CO2 seedlings, however, was caught up within 24 h by the ambient-CO2 plantlets, leading to equal RGRs from day 9 to day 13 after sowing. Nevertheless, the initially higher RGR of the high-CO2 seedlings was sufficient to produce higher biomasses than the ambient-CO2 plants during the entire pre-flowering development.
Figure 4.
Increase of dry weights and the RGRs of tobacco plants after germination and growth under ambient (360 ppm) and elevated (700 ppm) CO2 concentration. (A) Dry weights (mg) of the seedlings after sowing (mean of n = 30 and standard deviation), (B) dry weights (g) of the tobacco plants after transfer to 15-L sand culture (mean of n = 5 and standard deviation) and (C) RGR (g g−1 day−1) calculated from the dry weights. Asterisks as in Fig. 2.
After transfer of the seedlings from agar to the 15-L sand culture, a third wave of increasing and decreasing RGRs was observed for both sets of plants between day 15 and day 21 after sowing (Fig. 4C). The maximum RGR of the high-CO2 plantlets exceeded that of the plantlets grown under ambient CO2 until day 22 while it was slightly lower during the following 2 weeks. From day 35 to day 61, the RGRs of plants of both sets were identical but decreased slightly.
Taken together, absolute biomass production was consistently higher under elevated than under ambient CO2. However, the major effects of elevated CO2 on RGR were observed during unfolding of the cotyledons and after transfer of the seedlings from the agar to the sand culture.
Biomass allocation within the shoot and morphological characterization of tobacco plants grown at ambient or elevated CO2 concentrations
As shown before, the effect of elevated CO2 on growth and biomass production differed with the developmental stages of the plants. In order to examine the morphological differences of plants grown at ambient or elevated CO2 in more detail, the leaf number and leaf size of the plants (grown in 15-L pots) were examined at the ages of 28, 35, 42 and 49 days (Table 1). Plants grown under elevated CO2 concentration developed faster until the age of 42 days since they had one leaf more than those under ambient CO2. Elevated CO2 also stimulated the expansion of individual leaves (Table 1). However, the final leaf number at the emergence of flower buds was not increased by elevated CO2: at the age of 49 days, plants under both growth conditions had an equal leaf number.
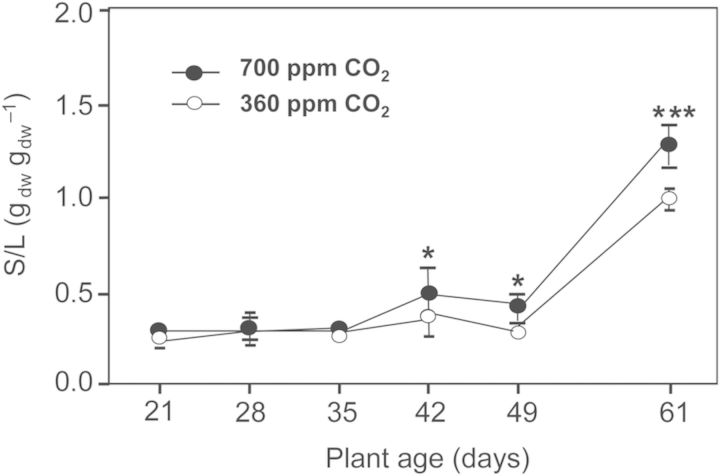
Biomass allocation to the stem and leaves, expressed as the stem/:leaves ratio (St/L in Fig. 5), was initially identical in high-CO2 and ambient-CO2 plants (Fig. 5). From 42 days on, the high-CO2 plants allocated more biomass to the stem than the ambient-CO2 plants. These differences were significant and increased slightly with plant age.
Figure 5.
Stem:leaves ratios (St/L) of tobacco plants grown in 15-L sand culture under ambient (360 ppm) and elevated (700 ppm) CO2 concentration at different plant ages (means of n = 5 and standard deviation). Asterisks as in Fig. 2.
Influence of CO2 concentration on the nutrient status of tobacco leaves
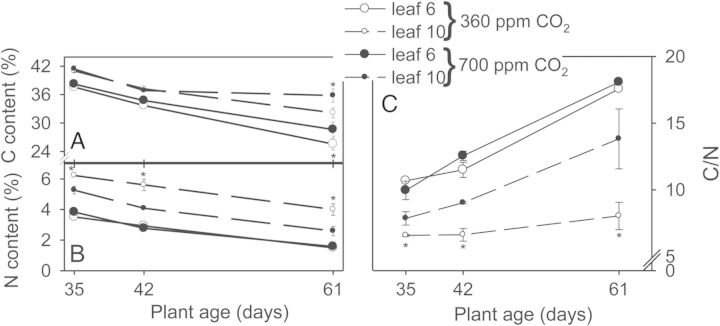
The C/N ratios of two leaves each (leaves 6 and 10) were determined at the ages of 35, 42 and 61 days (Fig. 6). The contents of both carbon and nitrogen of all leaves decreased with age (Fig. 6A and B), but the carbon content of the EC leaves decreased less than that of the AC plants. An effect of the CO2 concentration on the nitrogen content was only observed in young leaves, the nitrogen content of which was lower in the high-CO2 plants. Since the age-dependent decrease of the portion of carbon was considerably larger than that of nitrogen in high-CO2 plants, the C/N ratio decreased substantially with increasing plant age. This decrease was less pronounced in ambient-CO2 plants (Fig. 6C).
Figure 6.
Age-dependent change of (A) carbon and (B) nitrogen content (%), and (C) C/N ratio of leaf 6 and leaf 10 of tobacco plants grown in 15-L sand culture under ambient (360 ppm) and elevated (700 ppm) CO2 concentrations (means of n = 3 and standard deviation). Asterisks as in Fig. 1.
Discussion
In the present comprehensive study, the question of pseudoreplication must be addressed (see also the subsection Statistics): rather than duplicating the entire experimental setup, replicates were confined to the (great number of) samples. This appears permissible, as the results are interpreted to reflect the plants' response to an elevated but not to a particular CO2 concentration. In addition, growth conditions were carefully controlled during the entire experiment to minimize errors resulting from an unexpected environmental factor. Although the problem of pseudoreplication (sensu Hurlbert 1984) thus cannot be principally ruled out, the results are not at odds with the general view of the effects of an increasing CO2 atmosphere on the growth of plants. They provide, however, further insight into the role of nutrient acquisition in the response of the plant to an improved carbon source and in the signals involved in the adaptation of plants to the expected future environment.
Elevated CO2 concentration enhanced growth at all root-bed sizes by a factor of between 2.1 and 1.6, with a mean of 1.8. Root-bed volumes smaller than 15 L greatly inhibited biomass production irrespective of the CO2 concentration. When reviewing the effects of elevated CO2 on photosynthesis and biomass production of a variety of plants that were cultivated under controlled conditions in different root-bed volumes or in the field, Arp (1991) found a significant negative effect of a small root bed on both parameters, especially under high CO2. Small pots inhibit root growth and thus reduce its sink strength for biomass allocation. This effect was taken as evidence of a feedback regulation of a plant's photosynthetic rate by the demand of its sinks (Thomas and Strain 1991). On that background, root-bed restriction and elevated CO2 should result in an additive negative effect, as was observed by Arp (1991) as well as in our study by a stronger inhibition of biomass production at elevated than under ambient CO2 (steeper slope at elevated CO2 in Fig. 1D). Because our tobacco plants were grown from the very beginning, i.e. seed germination, under the two CO2 concentrations, the so-called acclimation at the transition from ambient to high CO2 could neither be expected nor was it observed during plant development (Fig. 4).
Our experiments showed that irrespective of the CO2 concentration, biomass allocation to the root increased with decreasing restriction of root growth by the pot (Fig. 1E). While this observation could be expected, another finding was at first glance surprising: the allocation of biomass to the root was higher under elevated than under ambient CO2. With one exception: the smallest pot, where the high-CO2 plants had a higher shoot-to-root biomass ratio. A lower ratio of shoot-to-root biomass in high-CO2 plants than in ambient-CO2 plants has been observed with a variety of plant species (for reviews, see Pritchard et al. 1999; Stitt and Krapp 1999; Yong et al. 2000; Yang et al. 2010; Wang et al. 2013) and appears to be a general phenomenon irrespective of root-bed size. Data from forested ecosystems revealed that elevated CO2 leads to an increased fine root production (Norby et al. 2004; Stiling et al. 2013) and to deeper rooting (for a review, see Iversen 2010). Consequently, the negative impact of a limiting root-bed size on root growth should be more pronounced at elevated CO2, which was indeed observed. But the increase of limitation under high CO2 was not as strong as supposed because the rates of net CO2 uptake of older, fully expanded leaves did not respond to the CO2 concentration [see Supporting Information]. A reason for that might be seen in a partial closure of the stomates of the high-CO2 leaves as a response to the elevated CO2 concentration. This explanation was corroborated by the transpiration rates of those leaves that were significantly higher under ambient than under high CO2 (Table 2). The stronger promotion of shoot than root growth of tobacco plants in 1-L pots at elevated CO2 was linked to a changed allocation pattern of the assimilates. One-litre pots were completely packed with roots, especially under elevated CO2. Root growth was more or less completely inhibited and the shoot received an excessive share of assimilates as indicated by the high C/N ratios of these plants (Fig. 2C).
A sequence of processes that take part in the regulation of growth have their origin in the uptake of macronutrients, in particular of nitrogen (McConnaughay et al. 1993; Stitt and Krapp 1999; Yang et al. 2007). Nutrient uptake is dependent on nutrient supply as well as on nutrient uptake associated with growth of the roots. Restriction of root growth results not only in an enhanced allocation of biomass to the root (decreasing S/R ratio) but also in a drop of the plant's nitrogen status (Ronchi et al. 2006; Yang et al. 2007). Both phenomena have been interpreted to reflect a decline of specific root functions in small pots (Yang et al. 2010). They were also observed in our experiments, in which the nitrogen content of the leaves increased with pot size (Fig. 2B). As expected, the nitrogen content of the youngest leaves (no. 20, as counted from the base) was much higher than that of leaves at the end of the expansion process (no. 10) or the oldest leaves at the bottom of the stem (no. 20). An age-dependent decline of the nitrogen content of the leaves is quite normal, and in tobacco the carbon content also decreases, due to the deposition of calcium oxalate as sand especially in the older leaves. The nitrogen contents of the leaves of plants grown at ambient CO2 were significantly higher than those of the leaves of high-CO2 plants irrespective of the position of the leaves. This finding reflects the increased demand for nutrients of the plants when grown under elevated CO2. Under that condition, the observed C/N ratios are understandable by assuming carbon limitation of growth under ambient CO2 and nitrogen limitation under enhanced CO2. Similar effects have been reported for a variety of other plant species (Yin 2002; for a review, see Taub and Wang 2008), and in more detail recently for wheat (Gutiérrez et al. 2013; Wang et al. 2013). Leaves of tobacco plants grown under high CO2 were consistently bigger than corresponding leaves of plants growing at ambient CO2 (Table 1), and if nutrient uptake by the roots does not match plant growth a reduced nutrient content must result. Another consequence of a reduced nutrient—especially nitrogen—availability under elevated CO2 is the enhanced formation of axial tissue, whose structural elements contain less nitrogen compared with leaf tissue (Fig. 5). In that context, it must be underlined that in contrast to all variants of nutrient supply described in the literature, our plants grew in purified quartz sand, which was continuously (day and night) flushed with a nutrient solution that had been optimized for the growth of tobacco. Therefore, restricted uptake rather than availability was the reason for nutrient limitation by the size of the root bed. Assuming the 15-L root-bed volume as sufficient for optimal root growth and maximal nutrient acquisition, the C/N ratios of plants grown in 15-L pots would reflect a kind of standard for optimal plant growth under the various CO2 concentrations. For the high-CO2 variant, this could even be the maximal achievable biomass production and growth of N. tabacum cv. Samsun.
Cytokinins as potential signals in the realization of the effects of a restricted root bed and of growth under elevated CO2
Cytokinins as one group of phytohormones are associated with regulation of the size and activity of the shoot and root apical meristem (for a review, see Skylar and Wu 2011). The effective concentrations to promote root growth are low whereas shoot growth is favoured by relatively high concentrations (Skoog and Miller 1957; Cary et al. 1995; Werner et al. 2001, 2003, 2010).
Our experiments showed a clear positive correlation between root-bed size, cytokinin concentrations in the apices and developing leaves and growth of the shoot. They also showed, irrespective of root-bed volume, much lower concentrations in the roots than in the growth regions of the shoot. While cytokinin concentrations in the roots of plants from the 15-L pots were only slightly higher than those in the roots of corresponding plants from the 1-L pots, the concentration gradients between the roots and the shoots were much lower in the small plants from the 1-L pots. With Urtica, a close correspondence of the daily cytokinin export from the root to the shoot and the nutrient (nitrogen) status of the roots has been reported (Beck 1999), and molecular models for translation of the nitrogen status of the root into the cytokinin signal were presented (Takei et al. 2004; Sakakibara et al. 2006). However, a recent study with transgenic tobacco and Arabidopsis plants with a reduced cytokinin concentration in the roots showed stimulation of root growth by the lowered cytokinin concentration, but no change of the shoot phenotype. This was interpreted as an indication that the shoot growth is not directly controlled by the cytokinin supply from the roots. Instead, shoot meristems themselves seem to produce cytokinins in sufficient amounts to maintain growth (Werner et al. 2010).
At first glance, the identity of the cytokinin patterns and flow rates of the xylem fluids of ambient- and high-CO2 plants as measured at the base of the stems seems to corroborate the conclusions from studies with transgenic tobacco. In addition, the identity of the cytokinins in the xylem fluids of the petioles of mature leaves of plants grown under 360 and 700 ppm CO2, respectively, underlines this notion. In that case, however, the distinct differences in the qualitative and quantitative patterns of cytokinins between the xylem fluids of the stems and petioles require further explanation because both sampling positions were at most 10 cm apart. The fact that tobacco as a member of the Solanaceae family has a bicollateral vascular system with an interior and an exterior phloem might provide an explanation. Studies with tomato (Houngbossa and Bonnemain 1985) and Nicotiana benthamiana (Cheng et al. 2000) have suggested that the meristems of the shoots are supplied by the internal phloem, while export from source leaves to the roots is mainly by the external phloem. Thus the ‘xylem fluid’ collected at the bottom of the stem from an adequately pressurized root system is composed of true xylem fluid and the likewise upwards flowing content of the internal phloem. In contrast, xylem fluid collected from a petiole may only contain negligible amounts of (internal) phloem sap due to the comparably few internal phloem elements. The bulk of the content of the internal phloem of the shoot obviously bypasses the petioles of mature leaves and hence the exudates from the petiole rather reflect true xylem fluid. Transport from the external to the internal phloem via ray parenchyma cells is possible but slow as observed with transport of viruses (Cheng et al. 2000). It is also known that assimilates can move from the external phloem via stem ray cells to the xylem. The cytokinin content of that sap from high-CO2 plants was higher than from ambient-CO2 plants, irrespective of the shares at which the xylem and the internal phloem contributed to the exudates from pressurized roots. This finding could reflect the stronger root signal hypothesized for plants grown under elevated CO2 (Hypothesis iii; see also Yong et al. 2000). Because the patterns of the cytokinin species in the root exudates did not match those in the apical meristems or sink leaves, a direct contribution of the cytokinin root signal to the cytokinin content of the meristems is unlikely. Rather, this signal could trigger cell division associated with endogenous cytokinin production in the meristems.
Elevated CO2 concentration already accelerated growth during germination and seedling development
Contrary to some reports in the literature (e.g. Miller et al. 1997; Ludewig and Sonnewald 2000), an accelerated ontogeny under elevated CO2 could only be observed at the very early stage when the cotyledons opened. They unfolded 1 day earlier under high than under ambient CO2. This advance turned out to be the basis for the persistently higher biomass production of plants grown at 700 ppm CO2 (Fig. 4B). Also, leaf formation was faster under high CO2, but the total leaf number per plant was equal under both CO2 concentrations until onset of flower bud formation (Table 1).
Conclusions
Keeping several environmental factors constant, in particular nutrient concentration in the root bed, the effects of two variables, size of the root bed and atmospheric CO2 concentration, on the growth of tobacco plants could be compared. Elevated CO2 consistently stimulated growth but the effect of root-bed volume still overrode that effect (Hypothesis i). Limiting the production of new fine roots, spatial restriction of root growth in turn curtails nutrient uptake, as indicated by a higher C/N ratio of high-CO2 plants. Our experiments did not comprise a transfer of plants from an atmosphere of ambient CO2 into one with elevated CO2; thus the classical acclimation effect was not in the scope of this work. Nevertheless, higher RGRs could be observed during germination and seedling development, which after 3 weeks declined and subsequently equalled those of ambient-CO2 plants. In spite of this decrease in RGR, biomass production was consistently higher under elevated CO2 and thus acclimation did not take place (Hypothesis ii). The effect of root-bed volume was strongly mirrored by the cytokinin concentrations of the meristems of the shoot, but less so of the stem, mature leaves and roots. A similar but less pronounced effect on the cytokinin concentrations was seen from the CO2 concentration. In spite of the overall low cytokinin concentration in roots, the amounts of cytokinins moving from the root to the shoot were substantially higher in high-CO2 plants (Hypothesis iii). Part of this root signal most probably migrates via the internal phloem of the bicollateral vascular system of the tobacco plant. The composition of the cytokinin patterns appears to be one of the major control points in which various environmental cues are integrated into one signal for optimized growth of the (tobacco) plants.
Sources of Funding
Our work was funded by the German Research Foundation with grant BE 473/23-4 to E.B.
Contributions by the Authors
U.S. and B.D. conducted research, C.R. analysed data and wrote the manuscript, and E.B. designed the experiments, is the senior author and finalized the manuscript.
Conflicts of Interest Statement
None declared.
Supporting Information
The following Supporting Information is available in the online version of this article –
Figure SI 1. Regression lines resulting by plotting the products of length and width of the leaves of 42-day-old plants grown at 360 or 700 ppm CO2 against their areas determined with an area meter (means of n = 3 and standard deviations).
Figure SI 2. Concentrations of cytokinins in the xylem sap taken from the shoot base (representing the location of loading from the root into the shoot) or the petioles of source leaves (representing the location of unloading into the source leaf) of 35-day-old tobacco plants.
Table SI 1. Reactivities (‘cross-reactivities’) of the antibodies against DHZR, ZR and 2iPA with various cytokinin standards. The intensity of the reaction in ELISA with the immediate antigen was set at 100 %.
Table SI 2. Minimum amounts of fresh material used for cytokinin determination.
Table SI 3. CO2 net assimilation rates of a typical source (leaf no. 10) and a still growing leaf (leaf no. 15) of 42-day-old tobacco plants grown at ambient and 700 ppm CO2, respectively, in 15-L pots. Carbon dioxide gas exchange of the leaves was measured in situ. Measurements were performed with a portable porometer (HCM 1000; Heinz Walz GmbH, Effeltrich, Germany), which was placed in the climate cabinets. Since leaf no. 15 was ∼25 cm above leaf no. 10, it received a higher quantum flux density. The rates were means of five plants each with SE.
Acknowledgements
The authors thank Mrs Tina Leistner and Mr Jörg Kastner for skilful technical assistance.
Literature Cited
- Ainsworth EA, Long SP. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist. 2005;165:351–372. doi: 10.1111/j.1469-8137.2004.01224.x. [DOI] [PubMed] [Google Scholar]
- Ainsworth EA, Leakey ADB, Ort DR, Long SP. FACE-ing the facts: inconsistencies and interdependence among field, chamber and modeling studies of elevated [CO2] impacts on crop yield and food supply. New Phytologist. 2008;179:5–9. doi: 10.1111/j.1469-8137.2008.02500.x. [DOI] [PubMed] [Google Scholar]
- Arp WJ. Effects of source–sink relations on photosynthetic acclimation to elevated CO2. Plant, Cell and Environment. 1991;14:869–875. [Google Scholar]
- Beck E. Towards an understanding of plant growth regulation: cytokinins as major signals for biomass distribution. In: Strnad M, Pec P, Beck E, eds. Advances in regulation of plant growth and development. 1999:97–110. Prague: Peres Publishers, [Google Scholar]
- Beck E, Wagner BM. Quantification of the daily cytokinin transport from the root to the shoot of Urtica dioica. Botanica Acta. 1994;107:342–348. [Google Scholar]
- Berntson GM, McConnaughay KDM, Bazzaz FA. Elevated CO2 alters deployment of roots in ‘small’ growth containers. Oecologia. 1993;94:558–564. doi: 10.1007/BF00566972. [DOI] [PubMed] [Google Scholar]
- Bishopp A, Lehesranta S, Vatén A, Help H, El-Showk S, Scheres B, Helariutta K, Mähönen AP, Sakakibara H, Helariutta Y. Phloem-transported cytokinin regulates polar auxin transport and maintains vascular pattern in the root meristem. Current Biology. 2011;21:927–932. doi: 10.1016/j.cub.2011.04.049. [DOI] [PubMed] [Google Scholar]
- Bürkle L, Cedzich A, Döpke C, Stransky H, Okumoto S, Gillissen B, Kühn C, Frommer WB. Transport of cytokinins mediated by purine transporters of the PUP family expressed in phloem, hydathodes, and pollen of Arabidopsis. The Plant Journal. 2003;34:13–26. doi: 10.1046/j.1365-313x.2003.01700.x. [DOI] [PubMed] [Google Scholar]
- Cary AJ, Liu W, Howell SH. Cytokinin action is coupled to ethylene in its effects on the inhibition of root and hypocotyls elongation in Arabidopsis thaliana seedlings. Plant Physiology. 1995;107:1075–1082. doi: 10.1104/pp.107.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng N-H, Su C-L, Carter SA, Nelson RS. Vascular invasion routes and systemic accumulation patterns of tobacco mosaic virus in Nicotiana benthamiana. The Plant Journal. 2000;23:349–362. doi: 10.1046/j.1365-313x.2000.00788.x. [DOI] [PubMed] [Google Scholar]
- Crozier A, Sandberg G, Monteiro AM, Sundberg B. The use of immunological techniques in plant hormone analysis. In: Bopp M, editor. Plant growth substances 1985. Berlin, Heidelberg, New York, Tokyo: Springer; 1986. pp. 13–21. [Google Scholar]
- Curtis PS, Vogel CS, Wang XZ, Pregitzer KS, Zak DR, Lussenhop J, Kubiske M, Teeri JA. Gas exchange, leaf nitrogen, and growth efficiency of Populus tremuloides in a CO2 enriched atmosphere. Ecological Applications. 2000;10:3–17. [Google Scholar]
- Dertinger U, Schaz U, Schulze E-D. Age-dependence of the antioxidative system in tobacco with enhanced glutathione reductase activity or senescence-induced production of cytokinin. Physiologia Plantarum. 2003;119:19–29. [Google Scholar]
- Drake BG, Gonzàlez-Mehler MA, Long SP. More efficient plants: a consequence of rising atmospheric CO2? Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:609–639. doi: 10.1146/annurev.arplant.48.1.609. [DOI] [PubMed] [Google Scholar]
- Dudits D, Ábraham E, Miskolci P, Ayaydin F, Bilgin M, Horváth G. Cell-cycle control as a target for calcium, hormonal and developmental signals: the role of phosphorylation in the retinoblastoma-centred pathway. Annals of Botany. 2011;107:1193–1202. doi: 10.1093/aob/mcr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis D, Sorrell DA. The interface between the cell cycle and plant growth regulators: a mini review. Plant Growth Regulation. 2001;33:1–12. [Google Scholar]
- Geiger M, Haake V, Ludewig F, Sonnewald U, Stitt M. The nitrate and ammonium nitrate supply have a major influence on the response of photosynthesis, carbon metabolism, nitrogen metabolism and growth to elevated carbon dioxide in tobacco. Plant, Cell and Environment. 1999;22:1177–1199. [Google Scholar]
- Gutiérrez D, Morcuende R, Del Pozo A, Martínez-Carrasco R, Pérez P. Involvement of nitrogen and cytokinins in photosynthetic acclimation to elevated CO2 of spring wheat. Journal of Plant Physiology. 2013;170:1337–1343. doi: 10.1016/j.jplph.2013.05.006. [DOI] [PubMed] [Google Scholar]
- Harashima H, Schnittger A. The integration of cell division, growth and differentiation. Current Opinion in Plant Biology. 2010;13:66–74. doi: 10.1016/j.pbi.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Hartig K, Beck E. Endogenous cytokinin oscillations control cell cycle progression of tobacco BY-2 cells. Plant Biology. 2005;7:1–8. doi: 10.1055/s-2004-830474. [DOI] [PubMed] [Google Scholar]
- Hikosaka K, Kinugasa T, Oikawa S, Onada Y, Hirose T. Effects of elevated CO2 concentration on seed production in C3 annual plants. Journal of Experimental Botany. 2011;62:1523–1530. doi: 10.1093/jxb/erq401. [DOI] [PubMed] [Google Scholar]
- Houngbossa S, Bonnemain J-L. Réexportation d'úne fraction du carbone importé lors de la phase de transition importation-exportation chez la feuille de tomate (Lycopersicon esculentum) Comptes Rendus de l'Académie des Sciences- Paris Séries. 1985;3300:131–136. [Google Scholar]
- Hurlbert SH. Pseudoreplication and the design of ecological field experiments. Ecological Monographs (Ecological Society of America) 1984;54:187–211. [Google Scholar]
- IPCC. Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 2013 http://www.ipcc.ch/report/ar5/wg2/ [Google Scholar]
- Iversen CM. Digging deeper: fine-root responses to rising atmosphere CO2 concentration in forested ecosystems. New Phytologist. 2010;186:346–357. doi: 10.1111/j.1469-8137.2009.03122.x. [DOI] [PubMed] [Google Scholar]
- Johnsen DW. Progressive N limitation in forests: review and implications for long-term responses to elevated CO2. Ecology. 2006;87:64–75. doi: 10.1890/04-1781. [DOI] [PubMed] [Google Scholar]
- Kant S, Seneweera S, Rodin J, Materne M, Burch D, Rothstein SJ, Spangenberg G. Improving yield potential in crops under elevated CO2: integrating the photosynthetic and nitrogen utilization efficiencies. Frontiers in Plant Science. 2012;3:162. doi: 10.3389/fpls.2012.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Körner C. Plant CO2 responses; an issue of definition, time and resource supply. New Phytologist. 2006;172:393–411. doi: 10.1111/j.1469-8137.2006.01886.x. [DOI] [PubMed] [Google Scholar]
- Krupa S. Atmosphere and agriculture in the new millennium. Environmental Pollution. 2003;126:293–300. doi: 10.1016/s0269-7491(03)00242-2. [DOI] [PubMed] [Google Scholar]
- Lindroth RL. Impacts of elevated CO2 and O3 on forests: phytochemistry, trophic interactions, and ecosystem dynamics. Journal of Chemical Ecology. 2010;36:2–21. doi: 10.1007/s10886-009-9731-4. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Rogers A, Ort DR. Rising atmospheric carbon dioxide: plants face the future. Annual Review of Plant Physiology and Plant Molecular Biology. 2004;55:591–628. doi: 10.1146/annurev.arplant.55.031903.141610. [DOI] [PubMed] [Google Scholar]
- Long SP, Ainsworth EA, Leakey ADB, Nösberger J, Ort DR. Food for thought: lower-than-expected crop yield stimulation with rising CO2 concentrations. Science. 2006;312:1918–1921. doi: 10.1126/science.1114722. [DOI] [PubMed] [Google Scholar]
- Ludewig F, Sonnewald U. High CO2-mediated down-regulation of photosynthesis gene transcripts is caused by accelerated leaf senescence rather than sugar accumulation. FEBS Letters. 2000;479:19–24. doi: 10.1016/s0014-5793(00)01873-1. [DOI] [PubMed] [Google Scholar]
- Makino A, Mae T. Photosynthesis and plant growth at elevated CO2. Plant and Cell Physiology. 1999;40:999–1006. [Google Scholar]
- Makino A, Harada M, Sato T, Nakano H, Mae T. Growth and N-allocation in rice plants under CO2 enrichment. Plant Physiology. 1997;115:199–203. doi: 10.1104/pp.115.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnaughay KDM, Berntson GM, Bazzaz FA. Limitations to CO2-induced growth enhancement in pot studies. Oecologia. 1993;94:550–557. doi: 10.1007/BF00566971. [DOI] [PubMed] [Google Scholar]
- Miller A, Tsai C-H, Hemphill D, Endress M, Rodermel S, Spalding M. Elevated CO2 effects during leaf ontogeny. Plant Physiology. 1997;115:1195–1200. doi: 10.1104/pp.115.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate. The Plant Journal. 2004;37:128–138. doi: 10.1046/j.1365-313x.2003.01945.x. [DOI] [PubMed] [Google Scholar]
- Mok DWW, Mok MC. Cytokinin metabolism and action. Annual Review of Plant Physiology. 2001;52:89–118. doi: 10.1146/annurev.arplant.52.1.89. [DOI] [PubMed] [Google Scholar]
- Motyka V, Vankova R, Capkova V, Petrasek J, Kaminek M, Schmülling T. Cytokinin-induced upregulation of cytokinin oxidase activity in tobacco includes changes in enzyme glycosylation and secretion. Physiologia Plantarum. 2003;117:11–21. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Journal of Plant Physiology. 1962;15:473–479. [Google Scholar]
- Nakano H, Makino A, Mae T. The effect of elevated partial pressures of CO2 on the relationship between photosynthetic capacity and N content in rice leaves. Plant Physiology. 1997;115:191–198. doi: 10.1104/pp.115.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norby RJ, Ledford J, Reilly CD, Miller NE, O'Neill G. Fine-root production response of a decidious forest to atmospheric CO2 enrichment. Proceedings of the National Academy of Sciences of the USA. 2004;101:9689–9693. doi: 10.1073/pnas.0403491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB. The transport of water from soil to shoot in wheat seedlings. Journal of Experimental Botany. 1980;31:333–345. [Google Scholar]
- Paul M, Foyer C. Sink regulation of photosynthesis. Journal of Experimental Botany. 2001;52:1383–1400. doi: 10.1093/jexbot/52.360.1383. [DOI] [PubMed] [Google Scholar]
- Pengelly WL. Validation of immunoassays. In: Bopp M, editor. Plant growth substances 1985. Berlin: Springer; 1986. pp. 35–43. [Google Scholar]
- Poorter H, Navas M-L. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist. 2003;157:175–198. doi: 10.1046/j.1469-8137.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- Prentice IC, Farquhar GD, Fasham MJR, Goulden ML, Heimann M, Jaramillo VJ, Kheshgi HS, LeQuere C, Scholes RJ, Wallace DWR. The carbon cycle and atmospheric carbon dioxide. In: Houghton JT, Ding Y, Griggs DJ, Noguer M, Van der Linder PJ, Dai X, Maskell K, Johnson CA, editors. Climate change 2001: the scientific basis. Contributions of Working Group I to the Third Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge University Press; 2001. pp. 183–238. [Google Scholar]
- Pritchard SG, Rogers HH, Prior SA, Peterson CM. Elevated CO2 and plant structure: a review. Global Change Biology. 1999;5:807–837. [Google Scholar]
- Rabha BK, Uprety DC. Effects of elevated CO2 and moisture stress on Brassica juncea. Photosynthetica. 1998;34:597–602. [Google Scholar]
- Reekie ED, MacDougall G, Wong I, Hicklenton PR. Effect of sink size on growth response to elevated atmospheric CO2 within the genus Brassica. Canadian Journal of Botany. 1998;76:826–835. [Google Scholar]
- Reich PB, Hobbie SE, Lee T, Ellsworth DS, West JB, Tilman D, Knops JMH, Naeem S, Trost J. Nitrogen limitation constrains sustainability of ecosystem responses to CO2. Nature. 2006;440:922–925. doi: 10.1038/nature04486. [DOI] [PubMed] [Google Scholar]
- Ronchi CP, DaMatta FM, Batista KD, Moraes G, Loureiro ME, Ducatti C. Growth and photosynthetic down-regulation in Coffea arabica in response to restricted root volume. Functional Plant Biology. 2006;33:1013–1023. doi: 10.1071/FP06147. [DOI] [PubMed] [Google Scholar]
- Sakakibara H, Takei K, Hirose N. Interactions between nitrogen and cytokinin in the regulation of metabolism and development. Trends in Plant Science. 2006;11:440–448. doi: 10.1016/j.tplants.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Sicher RC, Bunce JA. Relationship of photosynthetic acclimation to changes of Rubisco activity in field-grown winter wheat and barley during growth in elevated carbon dioxide. Photosynthesis Research. 1997;52:27–38. [Google Scholar]
- Skoog F, Miller CO. Chemical regulation of growth and organ formation in plant tissue cultures in vitro. Symposia of the Society for Experimental Biology. 1957;11:118–131. [PubMed] [Google Scholar]
- Skylar A, Wu X. Regulation of the meristem size by cytokinin signaling. Journal of Integrative Plant Biology. 2011;53:446–454. doi: 10.1111/j.1744-7909.2011.01045.x. [DOI] [PubMed] [Google Scholar]
- Stiling P, Moon D, Rossi A, Forkner R, Hungate BA, Day FP, Schroeder RE, Drake B. Direct and legacy effects of long-term elevated CO2 on fine root growth and plant insect interactions. New Phytologist. 2013;200:788–795. doi: 10.1111/nph.12295. doi:10.1111/nph.12295. [DOI] [PubMed] [Google Scholar]
- Stitt M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant, Cell and Environment. 1991;14:741–762. [Google Scholar]
- Stitt M, Krapp A. The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant, Cell and Environment. 1999;22:583–621. [Google Scholar]
- Takei K, Ueda N, Aoki K, Kuromori T, Hirayama T, Shinozaki K, Yamaya T, Sakakibara H. AtIPT3 is a key determinant of nitrate-dependent cytokinin biosynthesis in Arabidopsis. Plant and Cell Physiology. 2004;45:1053–1062. doi: 10.1093/pcp/pch119. [DOI] [PubMed] [Google Scholar]
- Taub DR, Wang F. Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. Journal of Integrative Plant Biology. 2008;50:1365–1374. doi: 10.1111/j.1744-7909.2008.00754.x. [DOI] [PubMed] [Google Scholar]
- Thomas RB, Strain BR. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiology. 1991;96:627–634. doi: 10.1104/pp.96.2.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner BM, Beck E. Cytokinins in the perennial herb Urtica dioica as influenced by its nitrogen status. Planta. 1993;190:511–518. [Google Scholar]
- Waller BM, Warmelink L, Liebal K, Micheletta J, Slocombe KE. Pseudoreplication: a widespread problem in primate communication research. Animal Behaviour. 2013;86:483–486. [Google Scholar]
- Wang L, Feng Z, Schjoerring JK. Effects of elevated atmospheric CO2 on physiology and yield of wheat (Triticum aestivum): a meta-analytic test of current hypotheses. Agriculture, Ecosystems and Environment. 2013;178:57–63. [Google Scholar]
- Wang TL, Thompson AG, Horgan R. A cytokinin glucoside from the leaves of Phaseolus vulgaris. Planta. 1977;135:285–288. doi: 10.1007/BF00384901. [DOI] [PubMed] [Google Scholar]
- Weiler EW, Zenk MH. Radioimmunoassay for the determination of digoxin and related compounds in Digitalis lanata. Phytochemistry. 1976;15:1537–1545. [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences of the USA. 2001;28:10487–10492. doi: 10.1073/pnas.171304098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell. 2003;15:2532–2550. doi: 10.1105/tpc.014928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Holst K, Pörs Y, Giuvarc'h A, Mustroph A, Chriqui D, Grimm B, Schmülling T. Cytokinin deficiency causes distinct changes of sink and root source parameters in tobacco shoots and roots. Journal of Experimental Botany. 2008;59:2659–2672. doi: 10.1093/jxb/ern134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Köllmer I, Novák O, Strnad M, Krämer U, Schmülling T. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco. The Plant Cell. 2010;22:3905–3920. doi: 10.1105/tpc.109.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Yu H, Xu Y, Goh CJ. Investigation of cytokinin-deficient phenotypes in Arabidopsis by ectopic expression of orchid DSCKX1. FEBS Letters. 2003;555:291–296. doi: 10.1016/s0014-5793(03)01259-6. [DOI] [PubMed] [Google Scholar]
- Yang TZ, Zhu LN, Wang SP, Gu WJ, Huang DF, Xu WP, Jiang AL, Li SC. Nitrate uptake kinetics of grapevine under root restriction. Scientia Horticulturae. 2007;111:358–364. [Google Scholar]
- Yang Z, Hammer G, van Oosterom E, Rochais D, Deifel K. Effects of the pot size on growth of maize and sorghum plants. Proceedings of the First Australian Summer Grains Conference; 21–24 June 2010; Gold Coast, Australia. 2010. [Google Scholar]
- Yin X. Responses of leaf nitrogen and specific leaf area to atmospheric CO2 enrichment: a retrospective synthesis across 62 species. Global Change Biology. 2002;8:631–642. [Google Scholar]
- Yong JWH, Wong SC, Letham DS, Hocart CH, Farquhar GD. Effects of elevated [CO2] and nitrogen nutrition on cytokinins in the xylem sap and leaves of cotton. Plant Physiology. 2000;124:767–779. doi: 10.1104/pp.124.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu LN, Wang SP, Yang TY, Zhang CX, Xu WP. Vine growth and nitrogen metabolism of ‘Fujiminori’ grapevines in response to root restriction. Scientia Horticulturae. 2006;107:143–149. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.