Abstract
Objective
To determine the longitudinal trends in the probability of blindness due to open-angle glaucoma (OAG) in Olmsted County, Minnesota from 1965 to 2009.
Design
Retrospective, population-based cohort study.
Participants
All residents of Olmsted County, Minnesota (40 years of age and over) who were diagnosed with OAG between January 1, 1965 to December 31, 2000.
Methods
All available medical records of every incident case of OAG were reviewed until December 31, 2009 to identify progression to blindness, defined as visual acuity of 20/200 or worse, and/or visual field constriction to 20° or less. Kaplan–Meier analysis was used to estimate the cumulative probability of glaucoma-related blindness. Population incidence of blindness within 10 years of diagnosis was calculated using United States Census data. Rates for subjects diagnosed in the period 1965–1980 were compared with rates for subjects diagnosed in the period 1981–2000 using logrank tests and Poisson regression models.
Main Outcome Measures
Cumulative probability of OAG-related blindness, and population incidence of blindness within 10 years of diagnosis.
Results
Probability of glaucoma-related blindness in at least one eye at 20 years decreased from 25.8 % (95% Confidence interval [CI]: 18.5–32.5) for subjects diagnosed in 1965–1980, to 13.5% (95% CI: 8.8–17.9) for subjects diagnosed in 1981–2000 (P=0.01). The population incidence of blindness within 10 years of the diagnosis decreased from 8.7 per 100,000 (95% CI: 5.9–11.5) for subjects diagnosed in 1965–1980, to 5.5 per 100,000 (95% CI: 3.9–7.2) for subjects diagnosed in 1981–2000 (P=0.02). Higher age at diagnosis was associated with increased risk of progression to blindness (P< 0.001).
Conclusions
The 20-year probability and the population incidence of blindness due to OAG in at least one eye have decreased over a 45 year period from 1965 to 2009. However, a significant proportion of patients still progress to blindness despite recent diagnostic and therapeutic advancements.
Introduction
Glaucoma is a leading cause of irreversible blindness worldwide. It has been estimated that 60.5 million people were affected with open angle glaucoma (OAG) and angle closure glaucoma (ACG) in 2010, increasing to 79.6 million by 2020, and of these, 74% will have OAG.1 Glaucoma affects more than 2.7 million individuals in the United States age 40 and older, or about 1.9% of this population.2 It is the second leading cause of blindness among blacks, after cataract, and the third leading cause of blindness in whites, after age related macular degeneration and cataract.3–5
Diagnostic criteria for glaucoma have undergone significant modifications over the last 40 years with greater importance placed on characteristic changes in the optic disc and retinal nerve fiber layer, and decreased reliance on elevated intraocular pressure (IOP).6 Nevertheless, reduction of IOP remains the only treatment for glaucoma.7, 8 New therapies for IOP reduction, as well as new diagnostic and progression analysis tools continue to be developed with significant advances occurring over the last 4 decades. These improvements in glaucoma management techniques have undoubtedly benefited individual patients. However, their effect on the rates of visual impairment in populations is poorly understood.
Although several studies have addressed the probability of progression to blindness,9–12 none have assessed longitudinal changes in the risk of progression to blindness or the population incidence of glaucoma-related blindness. A better understanding of epidemiological trends in glaucoma can help optimize the distribution of health and medical resources, and provide feedback on the efficacy of novel management approaches on a population basis. The purpose of this study was to determine the population incidence of OAG-related blindness and the probability of progression to blindness for newly diagnosed OAG patients, and to assess longitudinal changes in these metrics over a 45 year time period.
Methods
Data Collection
This is a population-based study of all residents of Olmsted County, Minnesota, who were newly diagnosed with OAG between 1965 and 2000. As a result of a unique resource known as the Rochester Epidemiology Project (REP), Olmsted County is one of the few places in the world where longitudinal population-based studies are conducted. REP13–15 is a surveillance and medical records linkage system established to study the occurrence and natural history of disease among the residents of Olmsted County, Minnesota. Population-based studies are possible since the county is isolated from other urban centers, with virtually all medical care to area residents provided by the Mayo Clinic and its affiliated hospitals (St. Mary’s Hospital and Rochester Methodist Hospital), or the Olmsted Medical Group with its affiliated Olmsted Community Hospital. All providers in Olmsted County use a medical record system whereby all medical information on each resident is accumulated within a single dossier.13 Indices that contain all clinical and pathologic diagnoses and surgical procedures have been created and can be used to retrieve records for various study populations. The University of Minnesota Hospitals, the Veterans Affairs Medical Center in Minneapolis, and other medical facilities in the region are contacted periodically to maintain the completeness of the system.13
Data from a previously reported study examining glaucoma incidence and blindness in Olmsted County residents diagnosed with OAG during the period 1965–1980,16, 17 with follow up through the end of 1995, were pooled with newly collected data for subjects diagnosed with OAG during the period from 1981–2000, with follow-up through the end of 2009. For this study, we defined OAG as primary open angle glaucoma, exfoliation glaucoma, pigmentary glaucoma, and treated ocular hypertension (OHT). The inclusion of treated OHT was necessary to harmonize the data sets, allowing comparison of the previously collected data with our newly collected data. For the new data, study subjects were identified by performing a computerized search of the REP database to identify all the residents of Olmsted County, Minnesota with a coded diagnosis of OAG during the period January 1, 1981 to December 31, 2000. All charts of subjects with a new diagnosis of OAG, glaucoma suspect, and OHT between 1981 and 2000 were reviewed to verify a new diagnosis of OAG or treated ocular hypertension during this period. Subjects with pre-existing glaucoma, glaucoma due to secondary causes (e.g., neovascular, uveitic, congenital, closed angle) or angle closure were excluded. Glaucoma was defined using the American Academy of Ophthalmology Preferred Practice Pattern criteria18 as the presence of either one or both of the following characteristics documented in the medical records: 1) Optic nerve damage consistent with glaucoma (diffuse thinning, focal narrowing, or notching of the optic disc rim, documented progression of cupping of the optic disc, diffuse or localized abnormalities of the peripapillary retinal nerve fiber layer, disc rim or peripapillary retinal nerve fiber layer hemorrhages or optic disc neural rim asymmetry of the two eyes); 2) Visual field damage consistent with retinal nerve fiber layer damage (nasal step, arcuate field defect, or paracentral depression in clusters of test sites). Treated OHT was defined as subjects who were treated with ocular hypotensive medications due to increased IOP without evidence of optic disc damage or visual field defects at diagnosis. This study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center.
For the 1981–2000 dataset, all available medical records of every incident case were reviewed through December 31, 2009. Demographic and clinical information including age at diagnosis, gender, date of initiation and type of therapy, type of OAG, visual acuity, IOP, visual field test results, and optic disc appearance were reviewed at initial diagnosis and during the entire period of follow up. If blindness occurred, the earliest date of documented occurrence, affected eye, and etiology of the blindness were recorded. Blindness was defined as best-corrected visual acuity of 20/200 or worse as measured by Snellen acuity, and/or visual field constriction to 20° or less in its widest diameter using the Goldmann III4e test object, or its equivalents on automated perimetry (size-III target at 10 dB with the Humphrey automated perimeter or size-III target at 7 dB with the Octopus automated perimeter) or tangent screen (10-mm target at 1 m). Each eye was evaluated individually for blindness. Cases in which blindness was not primarily caused by glaucoma were not included in calculations of blindness risk or incidence. The distinction was almost always clear by careful review of the charts and the sequence of eye disease progression. Any ambiguous cases were not included as incident cases. Olmsted County residency for all incident cases at the time of diagnosis was verified using the REP database.
Similar methods were used for the collection of the previous 1965–1980 data set16, 17 and the new 1980–2000 data set. The only significant difference was the definition of treated OHT utilized. For the 1965–1980 data set, subjects were classified as treated OHT if there was no documented evidence of visual field or disc damage at diagnosis, even if damage was documented at a later date. This may have resulted in selection bias and a very high reported probability of blindness at 20-years of 27%.16 However, for the 1981–2000 data set, we assumed that OHT patients who later developed visual field or disc damage were actually patients with pre-perimetric glaucoma, and therefore classified them as OAG. In order to allow comparison of the two datasets, we pooled OAG and treated OHT patients for combined analyses.
Probability of glaucoma-related blindness
Kaplan–Meier analysis was used to estimate the 10, 15 and 20-year cumulative probability of glaucoma-related blindness in at least one eye, as well as in both eyes, by either visual acuity or visual field criteria adjusted for follow-up time. Statistical analyses were performed on both current and previous data sets, and Kaplan–Meier cumulative probability estimates of blindness for the two studies were compared using logrank tests. Statistical significance was assumed for P<0.05. Cox proportional hazards models were used to analyze age at diagnosis and gender as risk factors associated with the development of blindness.
Population incidence of glaucoma-related blindness
Calculation of the population incidence of glaucoma-related blindness was necessary to address the potential confounding effect of earlier diagnosis. Subjects who are diagnosed with glaucoma at an earlier stage of disease would be expected to have a longer period of time until blindness, assuming no change in management, and thus a lower probability of blindness for a given time frame. However, calculation of the true population incidence of blindness due to glaucoma requires following all subjects who have ever been diagnosed with glaucoma for their entire lifetimes. Besides being impractical, this approach is unreliable as a measure of shorter term improvements in management strategies since the efficacies of all the different medical and surgical treatment modalities ever introduced during a lifetime of follow up contribute to the blindness incidence rates. As well, increasing longevity may result in an increase in the lifetime risk for glaucoma blindness.
To address these issues, we utilized two models to calculate and assess trends in population incidence of blindness due to glaucoma. In the first model, we calculated the OAG-related annual population incidence of blindness for the new dataset (1981–2000) based on the development of blindness anytime during the follow-up period of the study (1981–2009). For the previous dataset (1965–1980), we used the same model to calculate annual population incidence of blindness and the development of blindness anytime during the follow up period of that study (1965–1995). Annual population incidence of OAG-related blindness was also calculated and compared for 5-year intervals from 1965 to 2000. The second model for assessing longitudinal trends in population incidence of glaucoma-related blindness was based on the population incidence of blindness in at least one eye within 10 years of initial diagnosis of glaucoma. Since the follow-up period for our study continued until end of 2009, this portion of the study included cases that were diagnosed from 1981 to 1999, instead of 1981 to 2000, in order to ensure that a full 10 years of follow up was available for each newly diagnosed glaucoma patient.
Population data for blindness incidence calculations were drawn from United States Census data for Olmsted County. The annual blindness incidence was determined by dividing the number of incident cases by the estimated Olmsted County population. Incidences were then age- and gender- adjusted to the U.S. white population age 40 years and above in 2000 (Olmsted County population was 90.3 % Caucasian in 2000).19 Poisson distributions were used to calculate the 95% confidence intervals (CI) for incidences. Incidences for different time period were compared using Poisson regression models. Statistical significance was assumed for P<0.05 for all tests.
Results
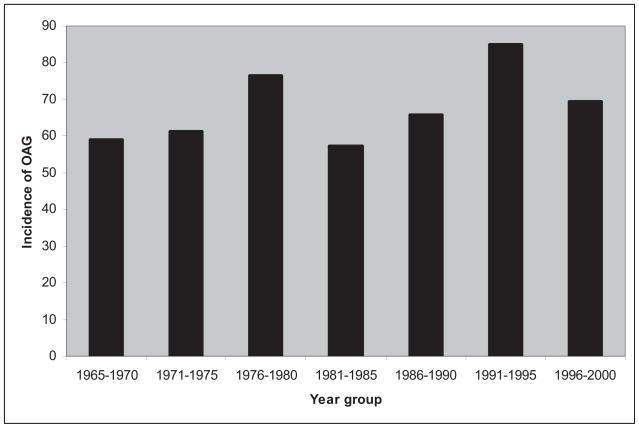
Five hundred and sixty three new cases of OAG were diagnosed during the period from 1981 to 2000. There were 235 male and 328 female subjects, with the mean age of 65.2 ± 15.3 (mean ± standard deviation [SD]) years. The follow up time extended to the end of 2009 and ranged from less than a month to 28.2 years with a mean follow up time of 11.2 ± 6.7 years. Table 1 summarizes the characteristics of incident cases from this period, as well as the previously collected study data set for the period 1965–1980.16, 17 The follow up period of that study was from 1965 to 1995, with a mean follow up time of 15.0 ± 8.0 years. Figure 1 depicts the age and gender adjusted annual incidence of OAG and treated OHT for the period of 1965–2000 which encompasses both the current and previous studies.
Table 1.
Characteristics of patients for the current and previous study periods. Numbers in parentheses indicate number of subjects.
| Current Study Period (1981–2000) | Previous Study Period (1965–1980) | |
|---|---|---|
| Number of cases | 563 | 294 |
| Age at Diagnosis (Years, mean ± SD) | 65.2 ± 15.3 | 66.7 ± 13.1 |
| Gender (%) | ||
| Male | 41.7% (235) | 37.1% (109) |
| Female | 58.3% (328) | 62.9% (185) |
| Type of Glaucoma | ||
| Primary Open Angle | 65.0% (366) | 29.6% (87) |
| Pseudoexfoliation | 14.7% (83) | 8.5% (25) |
| Pigmentary | 5.7% (32) | 1.7% (5) |
| Treated Ocular Hypertension | 14.6% (82) | 60.2% (177) |
Definitions for treated ocular hypertension differed between the current and previous studies, as explained in the Methods section.
SD – standard deviation
Figure 1.
Annual incidence of open angle glaucoma (including treated ocular hypertension) in 5 year time intervals per 100,000 population (>40 years) from 1965–2000. OAG = open-angle glaucoma
Table 2 describes the characteristics of the OAG cases who developed blindness due to glaucoma. Nine subjects were blind in at least one eye at the time of diagnosis. One of these subjects was bilaterally blind and was excluded from the blindness incidence calculations. The other 8 subjects were blind in one eye and were included in the calculations for the bilateral blindness incidence but not for calculation of unilateral blindness incidence. The same criteria were applied to the data set from the previous study period,16, 17 with 9 subjects unilaterally blind and 2 two subjects bilaterally blind at diagnosis. There was a significant difference in the average time from glaucoma diagnosis to blindness between the previous and current study periods (8.7 ± 6.9 years and 5.8 ± 5.6 years, respectively, P= 0.02). Age at blindness was not significantly different between the two periods (P=0.36). Linear regression analysis of age at diagnosis indicated a marginally significant trend towards increasing age at first diagnosis (P=0.0506, Table 2).
Table 2.
Characteristics of patients who developed blindness due to open angle glaucoma.
| Diagnosis Period | Blindness at Diagnosis | Blindness During Follow Up | Age at Diagnosis (Years) | Age at Blindness (Years)* | Follow Up Time to Blindness (Years)§ | ||
|---|---|---|---|---|---|---|---|
| Monocular | Binocular | Monocular | Binocular | ||||
| 1965–1970 | 1 | 0 | 8 | 3 | 69.3 ± 8.1 | 80.2 ± 5.6 | 10.9 ± 6.6 |
| 1971–1975 | 4 | 1 | 13 | 7‡ | 68.3 ± 14.4 | 77.9 ± 13.5 | 9.6 ± 8.3 |
| 1976–1980 | 4 | 1 | 19 | 9‡ | 73.2 ± 9.7 | 80.4 ± 9.0 | 7.3 ± 5.5 |
| 1965–1980 | 9 | 2 | 40 | 19 | 70.7 ± 11.4 | 79.5 ± 10.4 | 8.7 ± 6.9 |
| 1981–1985 | 3 | 0 | 9 | 4 | 78.1 ± 9.4 | 83.9 ± 6.2 | 5.9 ± 7.0 |
| 1986–1990 | 1 | 0 | 5 | 7† | 76.4 ± 11.0 | 81.5 ± 7.2 | 5.2 ± 6.2 |
| 1991–1995 | 2 | 0 | 10 | 3 | 70.5 ± 14.9 | 78.2 ± 15.8 | 7.7 ± 4.5 |
| 1996–2000 | 2 | 1 | 8 | 1 | 77.2 ± 8.4 | 81.0 ± 9.1 | 3.8 ± 3.4 |
| 1981–2000 | 8 | 1 | 32 | 15 | 75.4 ± 11.4 | 81.2 ± 10.3 | 5.8 ± 5.6 |
Patients who were binocularly blind at diagnosis were excluded from blindness incidence calculations. Patients who were blind in one eye at diagnosis were included in the calculations for bilateral blindness incidence but not for unilateral blindness incidence.
Age at first blindness
Years to first blindness
Two patients in this group were blind in one eye at diagnosis and became blind in both eyes during follow-up.
One patient in this group was blind in one eye at diagnosis and became blind in both eyes during follow-up.
Probability of glaucoma-related blindness
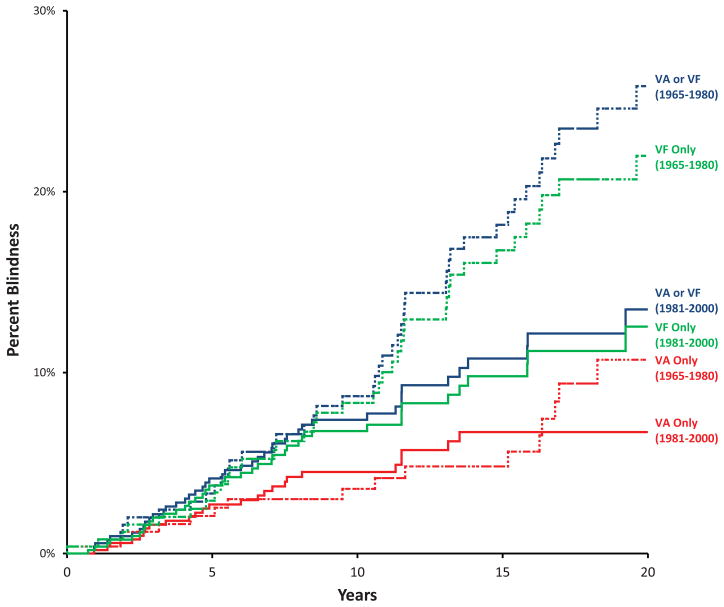
Kaplan–Meier cumulative probability of OAG-related blindness by visual acuity and/or visual field definition at 20 years in at least one eye was estimated to be 13.5 % (8.8–17.9) for the cases diagnosed during 1981–2000 and 25.8 % (18.5–32.5) for the cases diagnosed during 1965–1980 (P = 0.01) (Figure 2). Table 3 (available at http://aaojournal.org) lists the 10, 15 and 20 year Kaplan-Meier probabilities of blindness in at least one eye by either visual acuity (VA) or visual field (VF) definitions in 5 year intervals. Since case follow-up extended only until the end of 2009, Kaplan–Meier estimates of probability of OAG-related blindness were not possible for the 20-year timeframe for cases diagnosed after 1990, or for the 15-year timeframe after 1996.
Figure 2.
Kaplan–Meier cumulative probability of open angle glaucoma-related blindness (including treated ocular hypertension). Red lines: blindness in at least one eye by visual acuity (VA) criteria (≤20/200). Green lines: blindness in at least one eye by visual field (VF) criteria (constriction to 20° or less). Blue lines: blindness in at least one eye by either visual acuity criteria or visual field criteria. Solid lines: Current Study Period: 1981–2000; Dashed lines: Previous Study Period: 1965–1980.
Kaplan–Meier cumulative probability of OAG-related blindness was also calculated separately for VA criteria (Table 4 - available at http://aaojournal.org, Figure 2) and VF criteria (Table 5 - available at http://aaojournal.org, Figure 2). There was no statistically significant difference between 20-year estimates of blindness probability based on VA criteria alone for the 1981–2000 period (6.7 %; 95% CI: 4.0–9.4) compared with the 1965–1980 period (10.7 %; 95% CI: 5.1–16.0) (P= 0.85). However, there was a statistically significant difference in the 20-year estimate of blindness probability by VF definition alone for the 1981–2000 period (12.5 %; 95% CI: 8.0–16.9) compared with the 1965–1980 period (22.0 %; 95% CI: 15.1–28.1) (P=0.03).
The 20-year Kaplan–Meier Cumulative probability of bilateral glaucoma-related blindness by VA or VF was 4.3 % (95% CI: 1.9–6.6%) for cases diagnosed during the period from 1981–2000 (Table 6). This was not significantly different from the 20-year probability of bilateral glaucoma-related blindness for subjects diagnosed during the period from 1965–1980 (9.0 %; 95% CI: 4.2–13.5%) (P=0.33).
Table 6.
Kaplan–Meier cumulative probability of open angle glaucoma-related blindness in both eyes by either visual field or visual acuity criteria at different time intervals.
| Diagnosis year | Probability of Blindness In Both Eyes (95% CI) | ||
|---|---|---|---|
| 10-Year | 15-Year | 20-Year | |
| 1965–1970 | 0.0 % (0.0-0.0) | 0.0 % (0.0-0.0) | 4.4 % (0.0–11.1) |
| 1971–1975 | 5.0 % (0.0–10.4) | 8.8 % (1.1–15.9) | 8.8 % (1.1–15.9) |
| 1976–1980 | 2.3 % (0.0–5.4) | 10.3 % (2.6–17.3) | 12.6 % (3.8–20.6) |
| Overall: 1965–1980 | 2.2 % (0.3–4.1) | 6.2 % (2.7–9.6) | 9.0 % (4.2–13.5) |
| 1981–1985 | 5.2 % (0.1–10.1) | 5.2 % (0.1–10.1) | 5.2 % (0.1–10.1) |
| 1986–1990 | 6.0 % (1.2–10.6) | 6.0 % (1.2–10.6) | 7.7 % (1.9–13.1) |
| 1991–1995 | 2.3 % (0.0–4.9) | 2.3 % (0.0–4.9) | * |
| 1996–2000 | 1.7 % (0.0–3.3) | * | * |
| Overall: 1981–2000 | 3.4 % (1.7–5.1) | 3.4 % (1.7–5.1) | 4.3 % (1.9–6.6) |
There was no significant difference in the probability of blindness by visual field criteria for patients diagnosed in the 1965–1980 period compared with patients diagnosed in the 1981–2000 period (P= 0.33, 0.49, and 0.33 for the 20-year, 15-year, and 10-year time periods respectively, logrank test).
Kaplan-Meier rate could not be calculated due to inadequate follow up time
CI – confidence interval
Population incidence of glaucoma-related blindness
Table 7 summarizes the annual population incidence of glaucoma-related blindness, in at least one eye by either VA or VF criteria, occurring any time during the study follow-up period for both the current and previous studies. For the current study, the annual population incidence for 5-year intervals showed a trend towards a decrease from 1986–1990 to 1996–2000 (P=0.10). For the entire period 1981–2000, the annual population incidence of glaucoma-related blindness was 7.1 (5.2–9.0) per 100,000 population (>40 years). For the previous study period, the annual population incidence increased from the period 1965–1970 until 1976–1980 (P= 0.007). For the entire study period 1965–1980, the annual population incidence was 16.6 (12.6–20.5) per 100,000 population, which was significantly greater than the incidence for the current study period (P<0.001).
Table 7.
Annualized population incidence per 100,000 (40 years age and above) of open angle glaucoma related blindness in at least one eye by visual acuity or visual field.
| Diagnosis year | Annualized Population Incidence Of Open Angle Glaucoma Related Blindness Per 100,000 (95% CI) |
|---|---|
| 1965–1970 | 8.4 (3.7–13.2) |
| 1971–1975 | 17.8 (10.5–25.1) |
| 1976–1980 | 23.6 (15.6–31.7) |
| Overall: 1965–1980 | 16.6 (12.6–20.5) |
| 1981–1985 | 6.7 (3.4–10.1) |
| 1986–1990 | 7.5 (3.4–11.5) |
| 1991–1995 | 6.8 (3.3–10.4) |
| 1996–2000 | 5.0 (2.2–7.8) |
| Overall: 1981–2000 | 7.1 (5.2–9.0) |
The incidence during the period 1981–2000 was significantly lower than during the period 1965–1980 (Poisson regression, P < 0.001).
CI – confidence interval
Table 8 summarizes the annual population incidence of blindness due to glaucoma in at least one eye by VA or VF criteria within the first 10 years after diagnosis. The incidence of blindness within 10 years of diagnosis, in 5-year intervals, increased significantly during the period of the previous study period from 1965–1980 (P<0.001). However, the current study period from 1981–1999 had a significantly lower population incidence of glaucoma-related blindness within 10 years of diagnosis (5.5 per 100,000; 95% CI: 3.9–7.2 per 100,000 over 40 years old) than the previous study period from 1965–1980 (8.7 per 100,000; 95% CI: 5.9–11.5 per 100,000 over 40 years old) using a Poisson regression model (P=0.02).
Table 8.
Annualized population incidence per 100,000 (above 40 years age) of open angle glaucoma that leads to blindness in at least one eye within 10 years of initial diagnosis, by visual acuity or visual field criteria.
| Diagnosis year | Annualized Population Incidence Of Open Angle Glaucoma Related Blindness Within 10 Years of Diagnosis Per 100,000 (95% CI) |
|---|---|
| 1965–1970 | 3.5 (0.4–6.6) |
| 1971–1975 | 8.4 (3.4–13.4) |
| 1976–1980 | 14.2 (8.0–20.5) |
| Overall: 1965–1980 | 8.7 (5.9–11.5) |
| 1981–1985 | 5.5 (2.5–8.4) |
| 1986–1990 | 6.3 (2.6–10.0) |
| 1991–1995 | 3.9 (1.2–6.6) |
| 1996–1999 | 4.6 (1.9–7.3) |
| Overall: 1981–1999 | 5.5 (3.9–7.2) |
The incidence during the period 1981–2000 was significantly lower than during the period 1965–1980 (Poisson regression, P=0.02).
CI – confidence interval
Age and gender as risk factors for the progression to blindness
Cox proportional hazards models were used to analyze age at diagnosis and gender as risk factors associated with the development of blindness. Gender was not a risk factor for glaucoma-related unilateral blindness by VA or VF criteria (P=0.49 and 0.93, respectively), or bilateral blindness by VA or VF criteria (P=0.24 and 0.40, respectively) during the period 1965–2000. However, older age at diagnosis was a significant risk factor for unilateral and bilateral blindness, using either VA or VF criteria (P<0.001).
Discussion
The Rochester Epidemiology Project tracks the entire population of Olmsted County over multiple decades, and is particularly well-suited for assessing longitudinal trends in OAG-related blindness rates. In contrast, prospective population-based eye studies are primarily designed to assess prevalence instead of incidence, and are unable to assess long-term trends in a relatively rare condition like OAG-related blindness. Even if serial ophthalmic examinations could be performed for the study populations, these studies typically have sample sizes between 3000–6000 participants.20–24 As a result, they would only detect a single case of OAG-related blindness every 3 to 5 years, based on our annual incidence of 7.1 cases per 100,000 population for patients diagnosed between 1981–2000. Assessment of long-term trends in OAG-related blindness would therefore be difficult with prospective population-based studies.
The probability of glaucoma-related blindness in our current study period (1981–2000) is consistent with the results of Broman et al9, who used duration of follow up and rate of change in Mean Deviation (MD) in standard automated perimetry to estimate that 15% of OAG patients would be judged blind in at least one eye by visual field criteria in their lifetime. Community or population based studies like our study often report higher progression rates than those generally reported by clinical trials or clinical based studies.10–12, 25–28 There are several reasons for this notable difference.9 First, the study subjects of clinical studies do not represent the natural history of all persons affected by OAG in a population due to selection bias. Second, study participation may result in improved follow-up and compliance with therapy compared with the general population. Third, OAG is a chronic disease with patients being affected for an average of 13 to 16 years.29 The relatively short term follow-up that is feasible for most clinical trials may lead to an underestimate of the blindness rate of glaucoma. In our current study, the mean time from OAG diagnosis to blindness was 7.2 years with a median time of 6.0 years, which is similar to the maximum follow-up duration for large clinical trials. This suggests that as many as half of the expected cases of blindness due to glaucoma may be missed due to limited follow-up in clinical trials.
Our results indicate that the 20-year probability of progression to blindness in at least one eye has decreased significantly from the previous study period of 1965–1980, originally reported by Hattenhauer et al,16 to the current study period of 1981–2000. There was also a trend towards decreasing probability of progression to bilateral blindness, although the difference was not statistically significant. This is likely due to the relatively small number of patients progressing to bilateral blindness in either time period, which is consistent with the results of population-based surveys.1
There are numerous possible reasons why the probability of OAG-related blindness in Olmsted County may be decreasing. First, improvements in glaucoma management techniques from 1965 to the end of the follow-up period in 2009 may have resulted in a real decrease in the risk of OAG-related blindness. Numerous changes in glaucoma management have occurred during this timeframe, including improvements in medical and surgical therapy, the development of laser trabeculoplasty, automated perimetry, optic disc and nerve fiber layer imaging, and a better understanding of glaucoma pathophysiology and the risk factors for progression. However, it is also possible that changes in diagnostic criteria for glaucoma have resulted in earlier diagnosis of glaucoma, which could manifest as an increased duration until blindness, and a lower probability of blindness over 20-years. As well, better public awareness of glaucoma may result in a higher diagnosis rate of asymptomatic glaucoma, which would also reduce the risk of progressing to blindness in a fixed time period. However, there was no statistically significant difference in the annual incidence of OAG in the current study period (Figure 1) compared to the previous study period, despite the technological advances and improved awareness of the disease that may have caused glaucoma to be diagnosed at earlier stages.
To further assess the possible changes in OAG-related blindness rates, we investigated the annual population incidence of OAG-related blindness. Population incidence can be calculated using several different methods. As discussed previously, determination of true population incidence of OAG-related blindness by using a medical record based study requires identification of every case ever diagnosed with OAG and life long follow up. This approach is impractical, and also does not reflect more recent changes in disease management or changing life expectancy. To address this issue, we used two models to test for possible recent changes in the population incidence of blindness. Our first model assessed the annual population incidence of OAG-related blindness that occurred during the study period. This method has the advantage that virtually all cases of blindness would be captured given a long enough follow-up time. For our current study period, including cases diagnosed from 1981–2000, the mean follow-up period was 11.2 ± 6.7 years (mean ± SD). For the previous study period, including cases diagnosed from 1965–1980, the mean follow-up period was 15.0 ± 8.0 years (mean ± SD). We found that the incidence of glaucoma-related blindness was significantly lower in the current period compared with the previous period. It is possible the differences in average follow-up time may contribute to the lower rates in the current study period. However, patients in the 1981–2000 cohort tended to be diagnosed at a later age than those in the 1965–1980 cohort. As well, most of the cases of blindness in the previous study belong to those diagnosed during 1976–1980, with a maximum follow-up time of 15–20 years. In comparison, the current study, the maximum follow-up time for the period 1981–1990 was 14–29 years. Despite the similar follow-up times, the population incidence of blindness was significantly less in the 1981–1990 time period.
To address the issue of variable follow-up times, we calculated the incidence of glaucoma-related blindness within 10 years of diagnosis. Using this fixed follow-up period, we found that annual population incidence of glaucoma-related blindness within 10 years of diagnosis was lower in the current study (1981–2000) than with the previous study (1965–1980). One possible confounding factor is that, similar to the calculation of blindness probability, using a fixed follow-up period may be affected by earlier diagnosis of glaucoma. However, the lack of change in the annual population incidence of OAG indicates that there has been little change in the stage of disease at diagnosis.30 The similar trends in all our models of glaucoma-related blindness incidence and probability are suggestive of a real decrease in blindness rates, rather than an artifact of follow up time or earlier diagnosis.
To address the issue of changing definitions of disease, it was necessary to combine OAG and treated OHT patients. There was a marked difference in the percentage of patients with OHT in the 1965 to 1980 period compared with the 1980 to 2000 period. Since diagnostic criteria change over time, there is no ideal way of separating OAG patients from OHT patients who later convert to OAG. For the earlier time period, it is possible that some of these patients had pre-perimetric disease but were classified as OHT due to prevailing definitions of glaucoma at the time. If only patients who had definite OAG at the time of diagnosis were included, this would tend to bias the results as the patients from the later time period would tend to have an earlier stage of disease and therefore would have a lower risk of developing blindness over time. Although not ideal, combining treated OHT and OAG provided the best possibility for minimizing this bias. Regardless of any potential bias in disease definition, the population incidence of blindness would not be affected.
Even though blindness rates due to glaucoma appear to be decreasing, it is noteworthy that a significant percentage of patients (15%) still progress to blindness within 20 years in at least one eye, despite advances in diagnosis and therapy. This suggests that a subset of glaucoma patients may have more aggressive disease and may be particularly susceptible to progression, possibly due to non-IOP related factors that contribute to retinal ganglion cell death and vision loss.
Our study did not show any difference in the risk of blindness based on gender but did indicate that increasing age at diagnosis was a significant risk factor for progression to blindness. These findings are in agreement with most published data,10, 11, 26, 31 and are indicative of late diagnosis of disease. As well, the age of first diagnosis of glaucoma appeared to increasing over time. This would tend to increase the probability of glaucoma-related blindness in the later time periods, as older age at diagnosis was a significant risk factor for blindness. Despite this trend, the probability of glaucoma-related blindness decreased over time.
There are several disadvantages to a community based study. In particular, our study relied on medical records filed by multiple examiners. Although glaucoma is a clinical diagnosis, there was no unique protocol to assess the optic discs and visual fields used to diagnose glaucoma and assess its progression. Several different perimetry techniques had been used during the long course of study (1965–2009), ranging from tangent screen and Goldmann perimetry, to standard automated perimetry. If changes in perimetry resulted in decreasing sensitivity over time, the blindness rate could have been artificially lowered in the later time periods. However, automated perimetry tends to detect glaucomatous defects earlier, potentially leading to earlier appearance of blindness based on visual field criteria.32, 33 In contrast, our study found that the risk of blindness based on visual field criteria decreased over time.
Another possible limitation to this study is that 90.8% of population of Olmsted County was white in 2000, and the outcome of glaucoma may be different in communities with different racial demographics. Race has been reported to be a risk factor for glaucoma-related blindness, with a significantly higher probability of blindness in non-Caucasian populations. 34, 35 Chen reported 14.6% unilateral and 1.7% bilateral blindness at 15 years in a study on a predominantly Caucasian (82%) clinic-based population,36 consistent with the results of our study. Similarly, in a study on 423 subjects with an average glaucoma duration of 11.2 ± 6.6 years, Peters et al37 reported a 15.2% lifetime risk of bilateral blindness in an almost entirely Caucasian population in Sweden. In contrast, Kooner et al.38 reported a much higher rate of blindness rate of 42.1% in either eye in a more racially diverse (44.0% Caucasian, 47.4% African American) study of 487 clinic and hospital patients over 5.5 ± 3.6 years of follow-up. However, only 195 of these patients were followed from the time of their initial diagnosis. Furtado et al.39 reported on the risk of blindness from primary open angle glaucoma in a tertiary care hospital in Brazil in patients with at least 15 years of follow-up. Their patients had a 24.5% risk of unilateral and 34% risk of bilateral blindness at the end of their follow-up period. However, this was a small study of 53 patients, and the time from diagnosis was not reported. Although the results of our study are based on a predominantly Caucasian population with reasonably good access to care, and should be cautiously interpreted in different demographics, little other population-based data concerning the risk of blindness from glaucoma exists.
In conclusion, the 20-year probability of progression to blindness in at least one eye due to OAG, and the annual population incidence of OAG-related blindness, were both lower for patients diagnosed between 1980 and 2000 compared with patients diagnosed between 1965 and 1980 in the same geographic location. However, a significant proportion of patients still progress to blindness despite diagnostic and therapeutic advancements. Future studies will be required to determine if these trends continue and if current advances in glaucoma management result in further decreases in blindness rates.
Supplementary Material
Acknowledgments
Financial Support: Mayo Foundation for Medical Education and Research, a Research to Prevent Blindness Special Scholar Award (AJS), an unrestricted departmental grant from Research to Prevent Blindness, and the Rochester Epidemiology Project (Grant Number R01 AG034676 from the National Institute on Aging). The sponsors or funding organizations had no role in the design or conduct of this research.
Footnotes
Meeting Presentation: Presented in part at the Annual Meeting of the American Glaucoma Society, Naples, FL, March 2010
Conflict of Interest: No conflicting relationship exists for any author
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prevent Blindness America. [Accessed July 24, 2013];Vision Problems in the US. Available at: http://www.visionproblemsus.org/
- 3.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–85. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 4.Eye Diseases Prevalence Research Group. Prevalence of open-angle glaucoma among adults in the United States. Arch Ophthalmol. 2004;122:532–8. doi: 10.1001/archopht.122.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hennis AJ, Wu SY, Nemesure B, et al. Barbados Eye Studies Group. Nine-year incidence of visual impairment in the Barbados Eye Studies. Ophthalmology. 2009;116:1461–8. doi: 10.1016/j.ophtha.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weinreb RN, Friedman DS, Fechtner RD, et al. Risk assessment in the management of patients with ocular hypertension. Am J Ophthalmol. 2004;138:458–67. doi: 10.1016/j.ajo.2004.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol. 2002;120:1268–79. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 8.Gordon MO, Kass MA Ocular Hypertension Treatment Study Group. The Ocular Hypertension Treatment Study: design and baseline description of the participants. Arch Ophthalmol. 1999;117:573–83. doi: 10.1001/archopht.117.5.573. [DOI] [PubMed] [Google Scholar]
- 9.Broman AT, Quigley HA, West SK, et al. Estimating the rate of progressive visual field damage in those with open-angle glaucoma, from cross-sectional data. Invest Ophthalmol Vis Sci. 2008;49:66–76. doi: 10.1167/iovs.07-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eid TM, Spaeth GL, Bitterman A, Steinmann WC. Rate and amount of visual loss in 102 patients with open-angle glaucoma followed up for at least 15 years. Ophthalmology. 2003;110:900–7. doi: 10.1016/S0161-6420(03)00076-9. [DOI] [PubMed] [Google Scholar]
- 11.Kwon YH, Kim CS, Zimmerman MB, et al. Rate of visual field loss and long-term visual outcome in primary open-angle glaucoma. Am J Ophthalmol. 2001;132:47–56. doi: 10.1016/s0002-9394(01)00912-6. [DOI] [PubMed] [Google Scholar]
- 12.Lee AC, Sample PA, Blumenthal EZ, et al. Infrequent confirmation of visual field progression. Ophthalmology. 2002;109:1059–65. doi: 10.1016/s0161-6420(02)01043-6. [DOI] [PubMed] [Google Scholar]
- 13.Maradit Kremers H, Crowson CS, Gabriel SE. Rochester Epidemiology Project: a unique resource for research in the rheumatic diseases. Rheum Dis Clin North Am. 2004;30:819–34. vii. doi: 10.1016/j.rdc.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 14.Gray DT, Hodge DO, Ilstrup DM, et al. Concordance of Medicare data and population-based clinical data on cataract surgery utilization in Olmsted County, Minnesota. Am J Epidemiol. 1997;145:1123–6. doi: 10.1093/oxfordjournals.aje.a009075. [DOI] [PubMed] [Google Scholar]
- 15.Melton LJ., III History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 16.Hattenhauer MG, Johnson DH, Ing HH, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- 17.Schoff EO, Hattenhauer MG, Ing HH, et al. Estimated incidence of open-angle glaucoma in Olmsted County, Minnesota. Ophthalmology. 2001;108:882–6. doi: 10.1016/s0161-6420(01)00550-4. [DOI] [PubMed] [Google Scholar]
- 18.American Academy of Ophthalmology Glaucoma Panel. Primary open-angle glaucoma. San Francisco, CA: American Academy of Ophthalmology; 2010. [Accessed August 30, 2013]. Preferred Practice Pattern Guidelines. Available at: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx. [DOI] [PubMed] [Google Scholar]
- 19.U.S. Census Bureau. Profile of General Demographic Characteristics: 2000. Geographic Area; Olmsted County, MN: [Accessed August 30, 2013]. Census 2000. Table DP-1. Available at: http://censtats.census.gov/data/MN/05027109.pdf. [Google Scholar]
- 20.Francis BA, Varma R, Vigen C, et al. Los Angeles Latino Eye Study Group. Population and high-risk group screening for glaucoma: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2011;52:6257–64. doi: 10.1167/iovs.09-5126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitchell P, Smith W, Attebo K, Healey PR. Prevalence of open-angle glaucoma in Australia: the Blue Mountains Eye Study. Ophthalmology. 1996;103:1661–9. doi: 10.1016/s0161-6420(96)30449-1. [DOI] [PubMed] [Google Scholar]
- 22.Shen SY, Wong TY, Foster PJ, et al. The prevalence and types of glaucoma in Malay people: the Singapore Malay Eye Study. Invest Ophthalmol Vis Sci. 2008;49:3846–51. doi: 10.1167/iovs.08-1759. [DOI] [PubMed] [Google Scholar]
- 23.Klein BE, Klein R, Sponsel WE, et al. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology. 1992;99:1499–504. doi: 10.1016/s0161-6420(92)31774-9. [DOI] [PubMed] [Google Scholar]
- 24.Tielsch JM, Sommer A, Witt K, et al. Blindness and visual impairment in an American urban population. The Baltimore Eye Survey. Arch Ophthalmol. 1990;108:286–90. doi: 10.1001/archopht.1990.01070040138048. [DOI] [PubMed] [Google Scholar]
- 25.Rasker MT, van den Enden A, Bakker D, Hoyng PF. Rate of visual field loss in progressive glaucoma. Arch Ophthalmol. 2000;118:481–8. doi: 10.1001/archopht.118.4.481. [DOI] [PubMed] [Google Scholar]
- 26.Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT): 6. Treatment group differences in visual field changes. Am J Ophthalmol. 1995;120:10–22. doi: 10.1016/s0002-9394(14)73754-7. [DOI] [PubMed] [Google Scholar]
- 27.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 4. Comparison of treatment outcomes within race. Seven-year results. Ophthalmology. 1998;105:1146–64. doi: 10.1016/s0161-6420(98)97013-0. [DOI] [PubMed] [Google Scholar]
- 28.Lichter PR, Musch DC, Gillespie BW, et al. CIGTS Study Group. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology. 2001;108:1943–53. doi: 10.1016/s0161-6420(01)00873-9. [DOI] [PubMed] [Google Scholar]
- 29.Quigley HA, Vitale S. Models of open-angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- 30.AQ: meeting presentations are cited parenthetically within the text; delete here, renumber remaining refs and correct callouts
- 31.AGIS Investigators. The Advanced Glaucoma Intervention Study (AGIS): 1. Study design and methods and baseline characteristics of study patients. Control Clin Trials. 1994;15:299–325. doi: 10.1016/0197-2456(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 32.Beck RW, Bergstrom TJ, Lichter PR. A clinical comparison of visual field testing with a new automated perimeter, the Humphrey Field Analyzer, and the Goldmann perimeter. Ophthalmology. 1985;92:77–82. doi: 10.1016/s0161-6420(85)34065-4. [DOI] [PubMed] [Google Scholar]
- 33.Katz J, Tielsch JM, Quigley HA, Sommer A. Automated perimetry detects visual field loss before manual Goldmann perimetry. Ophthalmology. 1995;102:21–6. doi: 10.1016/s0161-6420(95)31060-3. [DOI] [PubMed] [Google Scholar]
- 34.Hiller R, Kahn HA. Blindness from glaucoma. Am J Ophthalmol. 1975;80:62–9. doi: 10.1016/0002-9394(75)90870-3. [DOI] [PubMed] [Google Scholar]
- 35.Sommer A, Tielsch JM, Katz J, et al. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 36.Chen PP. Blindness in patients with treated open-angle glaucoma. Ophthalmology. 2003;110:726–33. doi: 10.1016/S0161-6420(02)01974-7. [DOI] [PubMed] [Google Scholar]
- 37.Peters D, Bengtsson B, Heijl A. Factors associated with lifetime risk of open-angle glaucoma blindness. Acta Ophthalmol. doi: 10.1111/aos.12203. In press. [DOI] [PubMed] [Google Scholar]
- 38.Kooner KS, AlBdoor M, Cho BJ, Adams-Huet B. Risk factors for progression to blindness in high tension primary open angle glaucoma: comparison of blind and nonblind subjects. Clin Ophthalmol. 2008;2:757–62. doi: 10.2147/opth.s3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paula JS, Furtado JM, Santos AS, et al. Risk factors for blindness in patients with open-angle glaucoma followed-up for at least 15 years. Arq Bras Oftalmol. 2012;75:243–6. doi: 10.1590/s0004-27492012000400004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.