Abstract
Objective
This study was conducted to determine whether a low dose application of Difluoromethylornithine (DFMO) to moderately sun-damaged skin with actinic skin keratoses is efficacious.
Materials and Methods
High resolution digitized imagery of nuclei from histologic sections of 4mm punch biopsies from sun-damaged skin on the posterolateral forearms were recorded, at baseline and at the end of six months of study. There were four treatments, DFMO + Eucerin®, DFMO + Triamcinolone, Triamcinolone + Eucerin®, and Eucerin® + Eucerin® to serve as placebo.
Results
With 102 participants and 185 skin biopsies, a total of 16,395 nuclei were recorded. The nuclei were analyzed to assess the changes in the pattern of the nuclear chromatin. Two specific measures of endpoint evaluation were computed, including the percentage of nuclei with high values of nuclear abnormality, and the reduction of the percentage of nuclei assigned by a discriminant function to the baseline data set.
All three, low dose topical DFMO, Triamcinolone, and DFMO plus Triamcinolone interventions led to statistically significant reductions of both the number of nuclei with high nuclear abnormality, as well as the number of nuclei assigned to the baseline data set. These reductions were found for all three treatments involving DFMO or Triamcinolone. For the Placebo data sets only small, statistically insignificant increases or decreases of these percentages were observed.
Conclusions
The low-dose, topical drug interventions were all effective in reducing skin biopsy nuclear abnormality by a statistically significant 15% to 20%, just above the limit of detection by karyometric assessment; however, these effects were clearly greater than the case-to-case sampling error.
Keywords: Chemoprevention, DFMO, low dose intervention, sampling error
Introduction
The effects of a chemopreventive intervention can prove to be extremely difficult to measure quantitatively. Karyometry has been shown to offer a quantitative and statistically secured assessment of chemopreventive agent efficacy, serving as an integrating biomarker. The nuclear chromatin pattern responds in a sensitive and timely fashion to an intervention (1,2,3,4). The analysis of karyometric data, though, in the setting of a low dose clinical chemoprevention trial faces a number of challenges.
Skin biopsy site sampling variability may enter at several levels. The groups of baseline posterolateral forearm biopsies assigned to different treatments in an intervention trial are visually assessed as closely comparable, e.g., as moderate to severe sun damage in the present study. Such subjective evaluation naturally will involve some differences between trial participants, and even between the groups of participants assigned to different treatments, notwithstanding blinded random assignments.
The karyometric measurements utilized in the present study with their precision and high sensitivity of detection are expected to reveal those differences. They may be small, but when the effects expected from the chemopreventive intervention are subtle, they have to be taken into proper account.
Bias between groups of participants assigned to different treatments in a trial introduces a systematic difference and mandates a comparison of matched data from the baseline and the end of study biopsies obtained from each participant in a given treatment group.
Sampling variability, furthermore, may result in biopsy to biopsy variability within a given case. The extent of such variability within a case has recently been evaluated (5).
Finally, there could be variability due to the random selection of biopsy sample nuclei.
In a situation as encountered in the present study (i.e., a low dose intervention trial) it is therefore necessary to make appropriate provisions to separate efficacy of the intervention from sampling variability.
Karyometric data sets recorded in a chemopreventive clinical trial rarely involve a homogeneous set of nuclei. Rather, only a certain proportion of the nuclei exhibit a deviation from normal. The majority of randomly sampled nuclei usually have a nuclear chromatin pattern that is either normal or almost so. Only the nuclei with deviations from normal, though, can be expected to respond to the intervention. The analysis thus may have to identify this subset of nuclei first (6); however, the first order of the “day” is to establish efficacy based on an assessment of the full data set.
Materials and Methods
This phase II study was conducted to determine if topical administration of 10% difluoromethylornithine cream can reduce the severity of moderately sun-damaged skin as assessed by histopathology, clinical evaluation, and biomarkers of response. Additionally, we wanted to determine if topical administration of Triamcinolone cream applied once daily would reduce DFMO-induced skin irritation. Subjects were recruited for an initial two-week sunscreen run-in to assess baseline adherence. Informed consent under a University of Arizona IRB- approved protocol was obtained from all subjects. Non-adherent subjects were excluded from the study before randomization.
After sunscreen run-in was completed 4-mm skin punch biopsies were taken from the dorsal forearms for assessment of various biomarkers. One biopsy from an actinic keratosis (AK) on the right posterolateral forearm was reserved for karyometric analysis. Qualified subjects were randomized in a double-blinded fashion to one of four treatment groups.
The four treatments of the study consisted of application of 10% DFMO + Eucerin®, DFMO + 1% Triamcinolone, Triamcinolone + Eucerin®, and Eucerin® + Eucerin® as placebo. Subjects were instructed to administer a dose (1-inch) of the test article or placebo to each posterolateral forearm once daily. At each visit they brought their tubes to the clinic to be weighed in order to monitor compliance rate. Subjects were provided with Solbar sunscreen (SPF 50) to apply to forearms daily.
Clinical biopsies were fixed in 10% neutral buffered formalin and embedded in paraffin, resulting in one tissue block for each specimen. Blocks were sectioned to 5 microns, mounted on slides and batch stained in hematoxylin and eosin ( H&E ) according to standard operating procedures and knowledge of tissue staining for karyometry. Staining bath time was optimized to keep pixel optical density values within a range for acccurate microphotometry.
Data recording involved a two step process. Sections were first imaged at 20:1 objective magnification, laser printed and submitted to histopathology for review to confirm sampling of the AK lesions.
Slides were then transferred to a video-microphotometer equipped with a 100:1, N.A. 1.40 planapochromatic, oil immersion objective (Zeiss, Oberkochen, Germany). Relay optics adjusted the sampling density to 6 pixels/linear micron. A DXC9000 three CCD video camera (SONY, Melville, N.Y.) was used to digitize the imagery. A narrow band (610nm) interference filter was used to enhance contrast.
From each recorded image 100 nuclei were randomly selected and segmented. Each was labeled for later reference. Segmentation was based on a semi-automated method with visual/manual control and correction where needed.
For each nucleus 95 karyometric features were computed, as described earlier (7,8,9). Nuclear signatures and lesion signatures were computed (10). When, as a measure of deviation from normal, in connection with a nuclear abnormality value (ANA) the term “averaged standard deviation” is used, it refers to the standard deviation averaged over all 95 karyometric features. It serves as a distance measure indicating deviation from a reference population of nuclei. As such from normal skin might be used or another well defined data set. In this study a data set of 10 cases of normal skin with no visible sun damage (11) was used as a reference for the computation of the average nuclear abnormality. In addition, the data set of placebo treated cases at baseline was used as a reference profile to derive a distance measure.
The sample sizes listed in Table I were available
Table I.
Participant and skin biopsy nuclei sample sizes
| Cases | Nuclei | Nuclei with ANA > 1.0 | ||||
|---|---|---|---|---|---|---|
| Treatment | B1* | Es+ | B1 | Es | B1 | Es |
| DFMO§+Triamcinolone | 29 | 22 | 2,768 | 2,125 | 947 | 592 |
| DFMO§+Eucerin | 29 | 23 | 2,679 | 1,987 | 528 | 240 |
| Triamcinolone+Eucerin | 22 | 15 | 1,998 | 1,506 | 725 | 599 |
B1 = Baseline
Es=End of Study
Difluoromethylornithine
Two measures for efficacy of the interventions were used. The first measure was based on the nuclear abnormality values, expressed in units of normalized, averaged standard deviations. The second are differences in proportions of nuclei assigned by a discriminant algorithm to the baseline category. The underlying assumption is that the baseline biopsy represents the greater deviation from normal. Significance of differences in proportions, and required sample sizes were computed using the formulas given by Fleiss (12).
In addition to the probe of the overall efficacy of the intervention, the response in individual cases was determined. The results are presented for each treatment group as a distribution of number of cases with a certain reduction in the proportion of nuclei assigned to the baseline category, i.e. the group with nuclei more deviant from normal. Since for the individual cases the available sample size was limited to 100 nuclei, the significance level for an individual case was set to p = 0.05 and the power of the test to 0.90. For this, a reduction in the proportion of the nuclei by 25% is required.
Results
One-hundred and twenty-four participants were randomized to one of four treatment groups, balanced by age, gender and average AK. There were no statistically significant baseline differences by treatment group with respect to age (p=0.88), AK count (p=0.86) or gender (0.39). Shown on Table 1 are the participant and skin cell nuclei sample sizes for the 102 participants who completed the study successfully with adequate baseline and end of study AK biopsies from the posterolateral forearms.
As a first step in this analysis the distributions of average nuclear abnormality values (ANA), i.e. the lesion signatures, were computed for all data sets. The distributions repeated a pattern seen in earlier studies: the bulk of nuclei, in samples taken at baseline (Bl) and at the end of study (Es), show no or only modest deviations from normal. The distributions show a peak around an average nuclear abnormality (ANA) value of 0.70, very close to a value expected for a normal population of nuclei (ANA = 0.65). All distributions showed a high degree of skew, with a long tail, extending in some cases to average deviations from normal by three standard deviations. Overall, the lesions sampled (moderate AK) for this study represent low grade keratoses, with values of most key karyometric features placing them at the low end of the progression curve (13).
It is the nuclei with high ANA values which reflect the effects of solar actinic damage. It is also those nuclei which can be expected to reflect best any efficacy of a chemopreventive intervention.
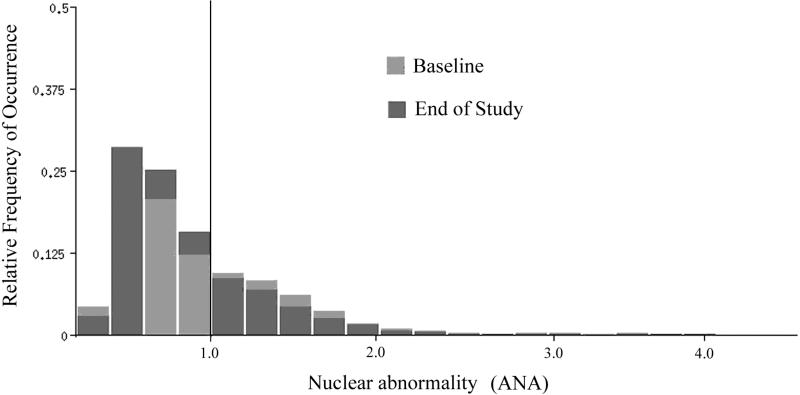
As an example, results obtained for the DFMO+Triamcinolone intervention are shown. Fig. 1 shows the lesion signatures (i.e. ANA). At the end of study a shift towards lower deviations from normal was seen. The ANA reduced from 0.939 at baseline to 0.884 at the end of study. The two distributions are shown by the Kolmogorov-Smirnov statistic to be statistically significantly different. The Dmax value was 0.0635, exceeding D alpha, the critical value of 0.0423, for a p-value of <0.001 Fig. 1 also shows a threshold, arbitrarily set by visual inspection at ANA = 1.0, i.e., one averaged standard deviation from the mean in a normal population of nuclei.
Fig.1.
Distribution of nuclear abnormality values for the DFMO + Triamcinolone treatment arm for baseline and end of study. Note the shift towards lower ANA values at end of study.
The effects of a chemopreventive intervention may be expected to be expressed most clearly by the nuclei with high ANA values at baseline. Table II lists the sample sizes and proportions of nuclei with ANA >1.0 in the four treatment groups, the differences in proportion of the ANA >1.0 nuclei from baseline to end of study, and the statistical significance of these differences in proportion, with the corresponding power of the test.
Table II.
Proportions of nuclei with ANA >1.0
| Proportion | ||||||
|---|---|---|---|---|---|---|
| Treatment | Bl* | Es+ | Diff. | % | alpha | power |
| DFMO§+Triamcinolone | .342 | .279 | – | 6.3 | <0.01 | 0.95 |
| DFMO§+Eucerin | .296 | .267 | – | 2.9 | n.s. | |
| Triamcinolone+Eucerin | .423 | .237 | – | 18.7 | <0.01 | 0.95 |
| Double Placebo (Eucerin) | .353 | .398 | + | 4.5 | n.s. | |
Bl = Baseline
Es=End of Study
Difluoromethylornithine
There was a reduction in the percentage of nuclei exceeding the threshold of ANA = 1.0 in all data sets representing an intervention. In contrast, the placebo treated group showed a small increase in nuclear abnormality, which for the available sample size is not statistically significant.
Following the rationale that the nuclei with the highest ANA-values should show the effects of an intervention most clearly, data sets were formed of only the 10% of most deviant nuclei from each case. This produced a data set of 960 nuclei, in total, at baseline, and 780 nuclei at end of study. Table III shows the decrease in the computed ANA values.
Table III.
Decrease in ANA values for 10% most deviant AK biopsy nuclei
| ANA values | Difference in | Avg Standard Dev. | ||||
|---|---|---|---|---|---|---|
| Treatment | Bl* | Es+ | % | |||
| DFMO§+Triamcinolone | 1.95 | 1.83 | – | 0.12 | – | 6.1 |
| DFMO§+Eucerin | 2.114 | 1.78 | – | 0.12 | – | 15.8 |
| Triamcinolone+Eucerin | 2.35 | 1.74 | – | 0.334 | – | 25.9 |
| Double Placebo (Eucerin) | 2.06 | 2.01 | – | 0.05 | – | 2.4 |
Bl = Baseline
Es=End of Study
Difluoromethylornithine
There appears to be a decrease in the average nuclear abnormality in those nuclei most expected to respond to an intervention, especially in the DFMO and Triamcinolone single agent groups. The smallest decrease in ANA values from baseline to end of study was observed for the double placebo intervention.
Given the small intervention effects expected in this study, we computed a distance measure based on the normalized deviations from values recorded for the placebo baseline data set.
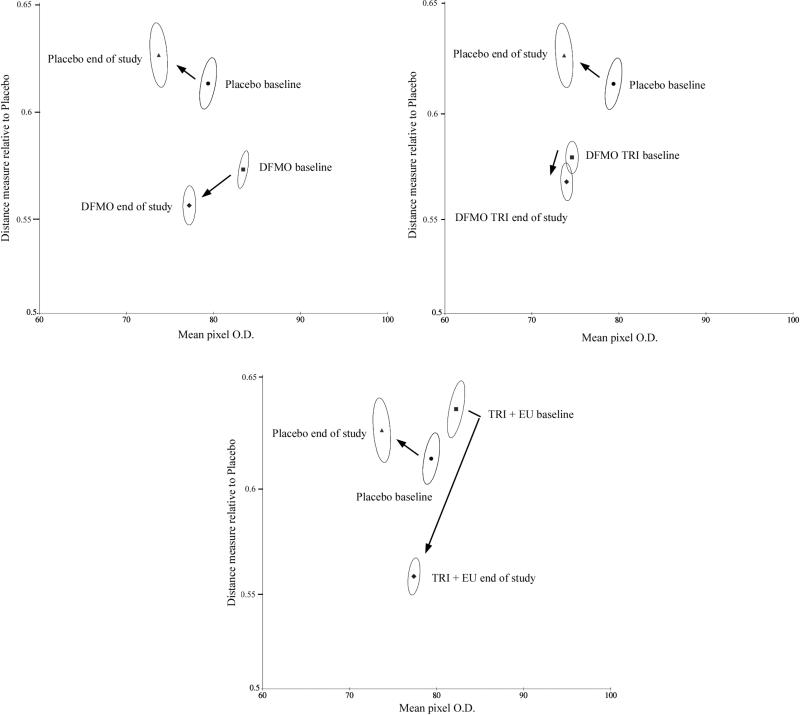
Figs. 2 a,b,c show such relative displacement values plotted on the ordinate, and the average staining density of the nuclei on the abscissa. This is shown for double placebo baseline and end of study the DFMO + Eucerin and Triamcinolone + Eucerin treated groups (Fig. 2a. Fig. 2b).
Fig.2.
Bivariate plots of mean staining density versus a distance measure to the feature distributions in the placebo data set. Shown are the bivariate mean values and the 95% confidence ellipses for nuclei, indicating statistically significant shifts.
Shown are the mean values for the full set of nuclei, and the 95% confidence ellipses. The double placebo data set demonstrates a change towards greater deviation from the baseline values, i.e. away from “normal”, whereas the DFMO + Eucerin data set, the DFMO + Triamcinolone data set and the Triamcinolone + Eucerin data sets all show a decrease from the baseline values computed for the double placebo data. The 95% confidence ellipses do not overlap, providing statistical significance of differences between the value distributions for these nuclei.
The range of values along the abscissa in Figure 2 shows difference in average staining density for these nuclei all within a range of +/– 10%.
The differences in displacement are small for each of the three topical intervention groups, the reductions in % from baseline are in contrast to the positive displacement value associated with the double placebo group. Table IV lists these values.
Table IV.
Relative displacement to placebo baseline data
| Displacement Values | ||||
|---|---|---|---|---|
| Treatment | Bl* | Es+ | % | |
| DFMO§+Triamcinolone | 0.579 | 0.5666 | – | 2.2 |
| DFMO§+Eucerin | 0.5726 | 0.5545 | – | 3.0 |
| Triamcinolone+Eucerin | 0.6411 | 0.5566 | – | 13.2 |
| Double Placebo (Eucerin) | 0.6162 | 0.6305 | + | 2.3 |
Bl = Baseline
Es=End of Study
Difluoromethylornithine
The proportions reported in Table I represent the relative proportion of nuclei with an ANA >1.0. The ANA is a rather robust measure of efficacy, being based on an average over the values of 95 different features. More sensitive and specific measures are offered by discriminant analysis, e.g., the proportion of nuclei assigned to the baseline category, for the baseline biopsies and the end of study biopsies. This difference of proportions has been used effectively as a measure of intervention efficacy.
The discriminant analysis to probe the effects of the topical, low dose chemopreventive interventions served as a dimensionality reduction, and facilitated the derivation of a numeric measure of lesion regression, and a determination of which nuclear chromatin features were affected. Thus, the baseline and end of study data sets for the DFMO + Triamcinolone were randomly divided into two halves, one to serve as a training set with 1396 and 1063 nuclei, respectively, the other as a test set with 1372 and 1062 nuclei, respectively.
Feature selection by a Kruskal Wallis test identified more than 20 nuclear chromatin features with differences of statistical significance exceeding p <0.005. Of these, seven features were submitted to the discriminant algorithm, which retained only three.
For the training set, the proportion of nuclei assigned to the baseline data set (i.e., to the data set with greater deviation from normal) was reduced from 56.7% to 42.3%, or by 14.4%.
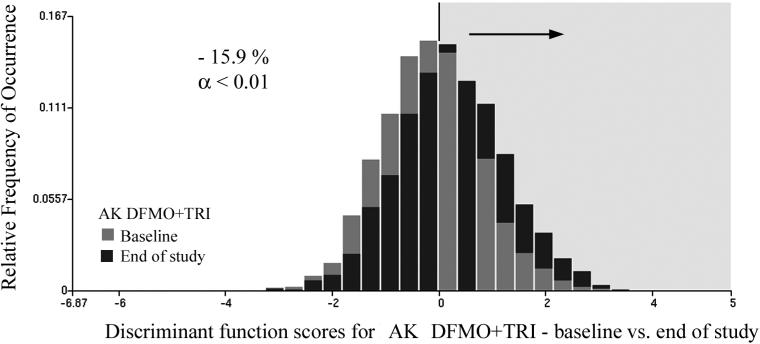
The function was then applied to the test set. Here, the reduction of the proportion of nuclei was from 57.8% to 40.0%, i.e., by 17.8%. When applied to the combined data set, the proportion of nuclei assigned to the baseline data set was reduced from 58.7% to 42.8%, i.e. by 15.9%. For the sample sizes involved, these reductions are statistically significant at p <0.01, and power of the test was 0.95.
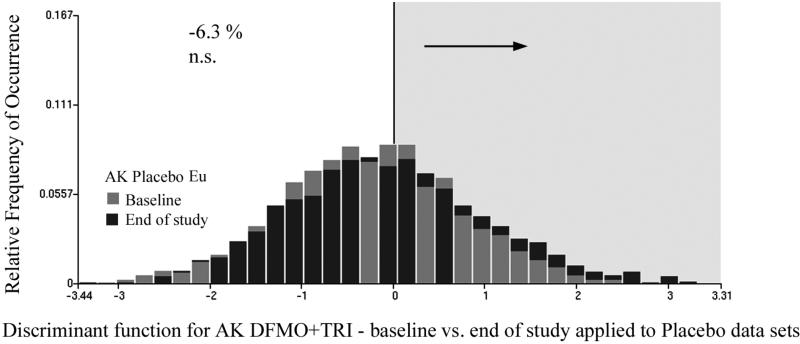
Fig. 3 shows the distribution of discriminant function scores for the baseline and the end of study data related to the DFMO + Triamcinolone intervention. The discriminant function algorithm assigned positive score values to the end of study data sets (i.e., a shift in the distribution to the right, exemplified by the black bars) indicates a trend towards less deviation from normal. The same discriminant function was next applied to the placebo baseline and end of study data sets. It resulted in a reduction of the nuclei assigned to the baseline category by 6.3%. This reduction was not statistically significant (p-value = 0.20). Fig.4 shows the score distributions.
Fig.3.
Distribution of discriminant function scores for the DFMO + Triamcinolone treatment regimen, baseline versus end of study. The proportion of nuclei assigned to baseline at the end of study is reduced by 15.4%.
Fig.4.
The discriminant function shown in Fig.3 applied to the placebo data set, at baseline and end of study. The placebo treatment results in only an insignificant reduction of the proportion of nuclei assigned to the baseline data set, at end of study.
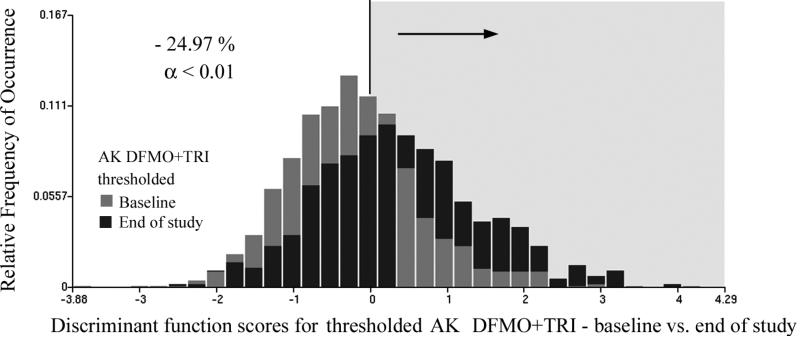
On the treatment group DFMO + Triamcinolone, the sample sizes available for the data sets thresholded at ANA = 1.0 allowed the formation of both training and test sets and the derivation of a discriminant function. The data sets were randomly divided into two halves, with 483 and 304 nuclei, respectively, available for the training set, and 464 and 288 nuclei available for the test set, respectively. The discriminant function used five features and reduced Wilks Lambda to 0.91.
For these data sets with ANA > 1.0, the intervention reduced the proportion of the epidermal nuclei assigned to the baseline data set for the training set from 67.5% to 41.8%, i.e., by 25.7%. For the test set the reduction was from 64.7% to 38.5%, i.e., by 26.2%. Applied to the combined data sets the reduction was from 61.8% to 36.8%, i.e., by 24.9%. This reduction in proportions is significant at p <0.01 and with power of the test of 0.95.
Fig. 5 shows the score distributions (i.e., gray bars represent baseline values and black bars represent end-of-study values). There is a strong shift towards more positive score values (i.e., reduced nuclear abnormality) in the end of study data.
Fig.5.
Distribution of discriminant function scores for baseline and end of study data sets of the DFMO + Triamcinolone treatment, thresholded so that only nuclei with high nuclear abnormality > 1.0 are included.
Applying the same discriminant algorithm to the placebo data set of epidermal nuclei with an ANA >1.0 resulted in an increase in the number of nuclei with greater deviation from normal (i.e. from 50.1% to 57.1% assigned to the baseline category). This change was not statistically significant.
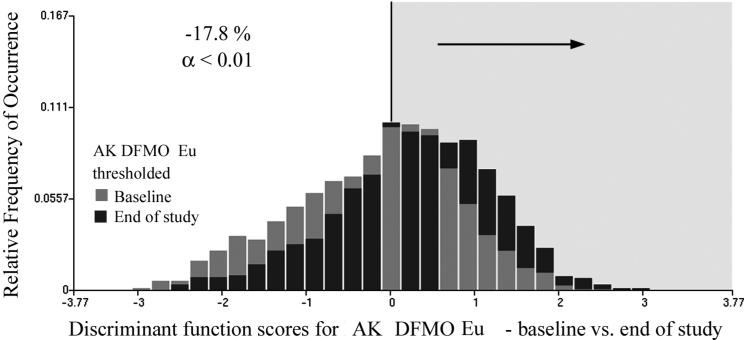
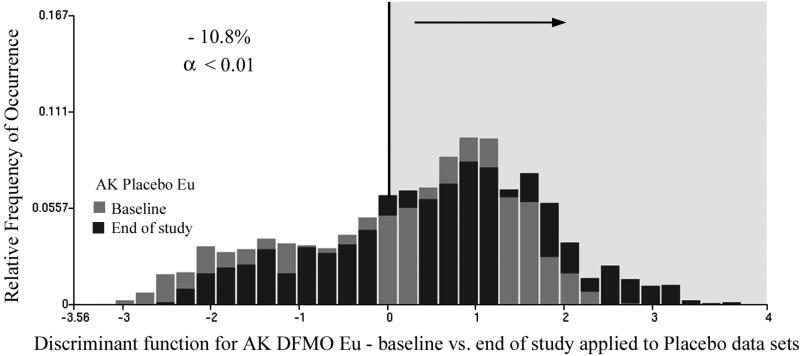
The same analytical procedure was followed for the DFMO + Eucerin topical intervention data sets. There were 1346 and 990 nuclei available for the training set, and 1333 and 997 nuclei for the test set. The discriminant algorithm selected four nuclear chromatin features, and reduced Wilks Lambda to 0.94. For the training set the proportion of nuclei assigned to the baseline category was reduced from 55.5% to 36.8%, i.e., by 18.7%. Applied to the test set, the function reduced the proportion of nuclei assigned to the baseline data set from 56.6% to 38.4% (i.e. by 18.4%). For the combined data sets the reduction was from 55.1% to 37.3%, i.e. by 17.8%. This is a statistically significant reduction at p <0.01 and power of 0.95. Fig. 6 shows the score distributions, again demonstrating the statistically significant reduction in nuclear abnormality (i.e., gray bars). Application of the same discriminant function to the placebo data resulted in a reduction of the proportion of nuclei assigned to the baseline category by 10.8%, which is statistically significant. The score distributions are shown in Fig. 7.
Fig.6.
Same function as shown in Fig.5 applied to the placebo data sets at baseline and end of study. The proportion of nuclei assigned to the baseline data set is increased by 4.6%.
Fig.7.
Discriminant function score distributions for the DFMO + Eucerin treatment, at baseline and end of study. The proportion of nuclei assigned to the baseline data set is reduced by 17.8%.
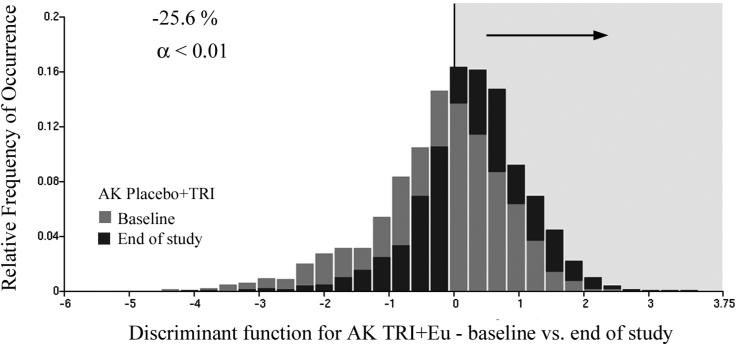
For the Triamcinolone + Eucerin data sets there were 1082 and 1089 nuclei available for the training set, and 1075 and 1086 nuclei for the test set. The discriminant algorithm selected just two nuclear chromatin features. It reduced Wilks Lambda to 0.91. For the training set the function resulted in a reduction of the proportion of nuclei assigned to the baseline category from 56.5% to 32.0% (i.e., reduction of 24.5%). For the test set the reduction was from 57.8% to 31.8% (i.e., reduction of 26.0%). For the combined data set the reduction was from 58.2% to 32.6% (i.e., reduction of 25.6%). This is a statistically significant result, at p <0.01 and power of 0.95. Fig. 8 shows the score distributions with the black bars representing end-of-study results.
Fig.8.
Distribution of discriminant function scores for the Triamcinolone + Eucerin treatment arm. The proportion of nuclei assigned to the baseline data set is reduced by 25.6 %.
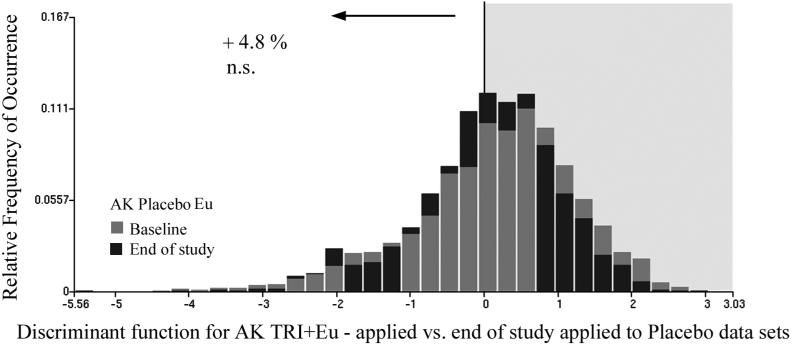
Applying the function to the placebo data sets resulted in an increase in the proportion of nuclei assigned to the baseline category by 4.8%, which is not significant but suggests a trend toward greater epidermal nuclear abnormality. The distributions are shown in Fig.9.
Fig.9.
Same function as shown in Fig. 8 applied to the placebo data sets at baseline and end of study. The proportion of nuclei assigned to the baseline data set is increased by 4.8 %.
Efficacy in individual cases
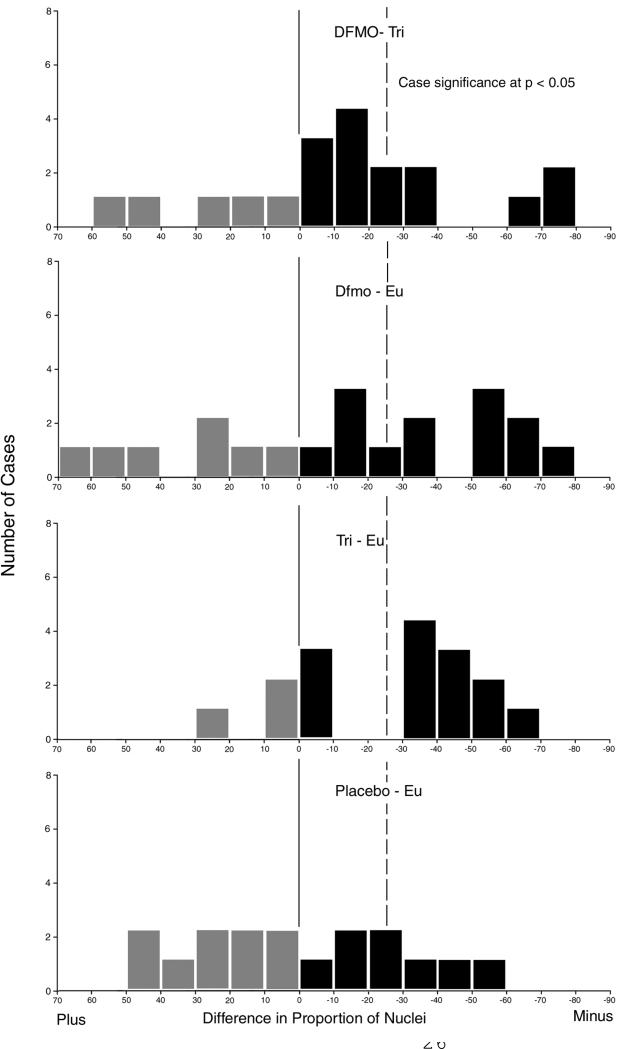
Fig. 10 shows the distribution of proportions of reduction for individual cases in each treatment group and in the placebo data set. For the DFMO + Triamcinolone treatment, baseline to end of study data pairs, 14/19 show a reduction. The 25% level of reduction required for significance is reached by 7/19 pairs. For the DFMO + Eucerin data sets 13/20 pairs show a reduction, with 9/20 reaching the 25 % level. In the Triamcinolone + Eucerin treatment, group 13/16 cases show a reduction with 10/16 reaching the 25% level. In the Placebo + Eucerin treatment, 8/17 show a reduction with 4/17 reaching the 25% level.
Fig. 10.
Distributions of response to chemopreventive intervention in individual cases.
Effects of sampling variability
In a clinical trial with a low dose topical drug intervention one can expect only modest changes in the end of study data. The question invariably arises to what extent any observed effects might have been due to sampling errors. The effect of such random fluctuations might be evaluated at the level of nuclear variability and of case to case variability.
The high sensitivity of discriminant analysis, with its capability to search out and capitalize on small differences, might lead to spurious results possibly due to some heterogeneity in the sampling of nuclei. It might result in the detection of differences in two theoretically identical data sets.
To probe the potential risk for spurious results due to nuclear sampling, the baseline data set of associated DFMO + Eucerin was divided into two subsets, assigning alternate nuclei to subsets A and B. Applying the discriminant function algorithm to the two subsets, A and B, led to a 1% difference in the proportion of nuclei assigned to subsets A (994 nuclei) versus B (993 nuclei). Thus, random fluctuations in nuclear features led to a very small and statistically insignificant difference in proportions.
The second cause of variability, case to case differences, is more serious and could lead to larger sampling errors. To develop an estimate of the magnitude of such differences the baseline data set for DFMO + Eucerin was divided into two subsets, C and D, by assigning alternate cases to either subset. The rationale was that one of the two subsets could serve as a “sham end of study” set, with no treatment effects, and to probe whether the discriminant function DFMO + Eucerin versus DFMO + Eucerin. Sham end-of-baseline study could lead to a significant difference in the proportion of nuclei assigned to one of the subsets (i.e., due to case-to-case sampling variability).
Applying the discriminant function to the two subsets, which are presumed to be identical, resulted in a 1.9% difference in the proportion of nuclei assigned to either one (i.e., 54.2 to 56.1% respectively). There were 1440 and 1239 nuclei in the two data sets, respectively. For these sample sizes the resulting difference is not statistically significant. Thus, case to case sampling variability also led to only modest differences in the proportions of nuclei assigned. A similar result was obtained, using the double placebo baseline data set.
To further probe the potential of a statistically significant difference in the proportion of nuclei assigned, the DFMO + Triamcinolone baseline data set and the DFMO + Triamcinolone end of study data set were scrambled to form two new data sets, consisting of 50% DFMO + Triamcinolone baseline and 50% of DFMO + Triamcinolone end-of-study cases each. Then the discriminant function for DFMO + Triamcinolone baseline versus DFMO + Triamcinolone end-of-study was applied. The rationale was that in this situation, case to case variability due to treatment effects was considered. The result was a modest difference in the proportion of assigned nuclei of 3.9%. Application of the discriminant function to the similarly scrambled placebo data sets resulted in a difference in proportion of 7.1%. For the sample sizes involved, both differences in proportion are statistically insignificant.
Case to case variability thus does not appear to produce a spuriously significant difference in proportion of assigned nuclei, as attained in the three treatment arms.
Discussion
In this phase IIb chemoprevention trial, the observed effects on lateral forearm sun-damaged skin were small for each of the three treatments, but clearly statistically significant, and in excess of what case to case sampling error produces. It should be considered that averaging over all cases in each treatment arm results in a conservative estimate since there were cases included with response only in the range of the case to case variability.
Also, the discriminant functions for each of the treatments were developed to optimize a separation for the baseline versus the end of study data sets. These functions could be expected to reflect efficacy of intervention, e.g., by a reduction of the proportion of nuclei assigned to the baseline data sets. They could also be expected to reflect some further progression, as was indeed found in some applications to the placebo data sets. However, the functions were not optimized to reflect changes in features affected by further progression towards high grade AK. Consequently an observed increase in the proportion of nuclei assigned to the baseline data sets might be an underestimate.
The observed decreases in the number of nuclei assigned to the baseline study sets are to be interpreted with some deliberation. In the DFMO + Eucerin treatment arm one may attribute the change to a reduction of progression in the AK lesion. Some key variables, such as the total optical density, decrease in value. In the Triamcinolone + Eucerin treatment arm, we observed the effects of a reduction in inflammation. It is not clear whether the degree of progression of the AK lesion is affected. It becomes equally equivocal in the interpretation of the treatment with DFMO + Triamcinolone.
In summary, with averaging over all nuclei from all cases in each treatment trial statistically significant changes can be demonstrated, for each of the three different treatment regimens. The changes observed in the placebo -treated groups were statistically insignificant and indicate no change in lesion progression. The low dose intervention can be shown to result in an improvement that is greater than could be expected from case to case sampling errors. On the other hand, the improvements are only small, enough to just exceed the limits of detection of chemopreventive efficacy by karyometric analysis (5).
References
- 1.Boone CW, Lieberman R, Mairinger T, Palcic B, Bacus J, Bartels PH. Computer-assisted image analysis-derived intermediate endpoints. Urology. 2001;57(Supplement 4A):129–131. doi: 10.1016/s0090-4295(00)00956-0. [DOI] [PubMed] [Google Scholar]
- 2.Bozzo P, Alberts DS, Vaught L, da Silva VD, Thompson D, Warnecke J, Miller RC, Einspahr J, Bartels PH. Measurement of chemopreventive efficacy in skin biopsies. Anal Quant Cytol Histol. 2001;23:300–312. [PubMed] [Google Scholar]
- 3.Bartels PH, Ranger-Moore J, Stratton MS, Bozzo P, Einspahr J, Liu Y, Thompson D, Alberts DS. Statistical analysis of chemopreventive efficacy of vitamin A in sun-exposed, normal skin. Anal Quant Cytol Histol. Aug. 2002;24(4):185–97. [PubMed] [Google Scholar]
- 4.Alberts D, Ranger-Moore J, Einspahr J, Saboda K, Bozzo P, Liu Y, Xu XC, Lotan R, Warneke J, Salasche S, Stratton S, Levine N, Goldman R, Islas M, Duckett L, Thompson D, Bartels P. Safety and efficacy of dose intensive oral vitamin A in subjects with sun-damaged skin. Clin Cancer Res. 2004;10:1875–80. doi: 10.1158/1078-0432.ccr-03-0188. [DOI] [PubMed] [Google Scholar]
- 5.Bartels PH, Yozwiak M, Bartels HG, Liu Y, Hess LM, Alberts DS. Limits of detection of chemopreventive efficacy: Karyometry of skin biopsies. Cancer Epidemiol.Biomarkers Prev. 2008;17:1689–1695. doi: 10.1158/1055-9965.EPI-08-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartels PH, Fabian CJ, Kimler BF, Ranger-Moore J, Frank DH, Yozwiak M, Alberts DS. Karyometry of breast epithelial cells acquired by random periareolar fine needle aspiration in women at high risk for breast cancer. Anal.Quant.Cytol. Histol. 2007;29:63–70. [PubMed] [Google Scholar]
- 7.Bartels PH, Wied GL. Extraction and evaluation of information from digitized cell images. In: Richmond CR, et al., editors. Mammalian cells: probes and problems. Tenn., Technical Information Center; Oak Ridge: 1975. pp. 15–28. [Google Scholar]
- 8.Bartels PH, Olson GB. Computer analysis of lymphocyte images. In: Catsimpoolas N, editor. Methods of Cell separation. Vol. 3. Plenum Press; New York: 1980. pp. 1–99. [Google Scholar]
- 9.Young T, Verbeek PW, Mayall B. Characterization of chromatin distributions in cell nuclei. Cytometry. 1986;7:467–474. doi: 10.1002/cyto.990070513. [DOI] [PubMed] [Google Scholar]
- 10.Bartels PH, da Silva VD, Montironi R, Hamilton PW, Thompson D, Vaught L, Bartels HG. Chromatin texture signatures in nuclei from prostate lesions. Anal Quant Cytol Histol. 1998;20(5):407–416. [PubMed] [Google Scholar]
- 11.Bartels PH, Alberts DS, Yozwiak M, Liu Y, Bartels HG. Actinic damage in histopathologically normal skin. Anal.Quant.Cytol.Histol. 2008 in press. [PMC free article] [PubMed] [Google Scholar]
- 12.Fleiss J. Statistical methods for rates and proportions. Wiley; New York: 1981. [Google Scholar]
- 13.Krouse R, Alberts DS, Bartels HG, Yozwiak M, Liu Y, Bartels PH. Progression of skin lesions from normal skin to squamous cell carcinoma. Anal.Quant.Cytol. Histol. 2008 in press. [PMC free article] [PubMed] [Google Scholar]