We reviewed all available studies on Phragmites australis management in the United States. Our results show that there is a heavy emphasis on herbicides to manage Phragmites, relative to other methods, and a lack of information on what types of plant communities establish once Phragmites is removed. Our model of Phragmites establishment and reproduction describes the invasion as a symptom of watershed-scale land use and disturbance. We advocate more holistic approaches to control and management that focus on improving water quality and minimizing human disturbance to deter future invasion and improve resilience of native plant communities.
Keywords: Common reed, ecological restoration, herbicide, invasive plant, invasive species, management, Phragmites australis, watershed restoration.
Abstract
Studies on invasive plant management are often short in duration and limited in the methods tested, and lack an adequate description of plant communities that replace the invader following removal. Here we present a comprehensive review of management studies on a single species, in an effort to elucidate future directions for research in invasive plant management. We reviewed the literature on Phragmites management in North America in an effort to synthesize our understanding of management efforts, identify gaps in knowledge and improve the efficacy of management. Additionally, we assessed recent ecological findings concerning Phragmites mechanisms of invasion and integrated these findings into our recommendations for more effective management. Our overall goal is to examine whether or not current management approaches can be improved and whether they promote reestablishment of native plant communities. We found: (i) little information on community-level recovery of vegetation following removal of Phragmites; and (ii) most management approaches focus on the removal of Phragmites from individual stands or groups of stands over a relatively small area. With a few exceptions, recovery studies did not monitor vegetation for substantial durations, thus limiting adequate evaluation of the recovery trajectory. We also found that none of the recovery studies were conducted in a landscape context, even though it is now well documented that land-use patterns on adjacent habitats influence the structure and function of wetlands, including the expansion of Phragmites. We suggest that Phragmites management needs to shift to watershed-scale efforts in coastal regions, or larger management units inland. In addition, management efforts should focus on restoring native plant communities, rather than simply eradicating Phragmites stands. Wetlands and watersheds should be prioritized to identify ecosystems that would benefit most from Phragmites management and those where the negative impact of management would be minimal.
Introduction
Wetlands are landscape sinks for nutrients and propagules, making them especially vulnerable to plant invasions as they are downstream from most disturbances (Zedler and Kercher 2004). One such invader, a Eurasian lineage of the common reed, Phragmites australis (hereafter referred to as Phragmites), is increasingly dominant in wetlands across North America (Marks et al. 1994; Chambers et al. 1999; Saltonstall 2003; Kettenring et al. 2012b in this issue). Phragmites invasions are often associated with decreases in plant biodiversity (Chambers et al. 1999; Keller 2000; Bertness et al. 2002), declines in habitat quality for fish and wildlife (Fell et al. 2003, 2006; Gratton and Denno 2006; Chambers et al. 2012), disruptions to biogeochemical cycles (Meyerson et al. 1999, 2000; Findlay et al. 2003) and other ecosystem services (but see Kiviat 2013 and Kettenring et al. 2012b in this special issue, which highlight Phragmites benefit to wildlife or lack/weaknesses of data on actual impacts). Phragmites invasion is becoming an increasingly large management concern in a variety of systems: tidal marshes along the Atlantic Coast (Chambers et al. 1999; Warren et al. 2001; Bertness et al. 2002); the Great Lakes (Tulbure et al. 2007; Carlson et al. 2009; Uzarski et al. 2009; Willcox 2013); inland brackish wetlands of the Great Basin (Kettenring and Mock 2012; Kettenring et al. 2012a) and the Gulf Coast (Kettenring et al. 2012b in this special issue).
Phragmites is a clonal, rhizomatous grass with a cosmopolitan distribution (Haslam 1972). Several genetic lineages, including some native lineages, are present in North America (Saltonstall 2002, 2003; Meyerson et al. 2012 in this special issue; Lambertini et al. 2012a, b in this special issue). However, the invasion by the Eurasian genetic lineage in wetlands across North America has been striking due to its rapid spread, abundance and impacts. Eurasian Phragmites' dominance at the landscape scale has been attributed to anthropogenic factors, including hydrologic alteration, increased nutrients and global change (Minchinton 2002a; Burdick and Konisky 2003; Silliman and Bertness 2004; Bart et al. 2006; King et al. 2007; Brisson et al. 2008; Mozdzer et al. 2010; Kettenring et al. 2011; Mozdzer and Megonigal 2012; Mozdzer et al. 2013 in this special issue). Since the turn of the 20th century, non-native Phragmites in North America has been associated with denuded soil and anthropogenic disturbance (Taylor 1938), but natural disturbances also produce favourable conditions for Phragmites establishment (Minchinton and Bertness 2003; Baldwin et al. 2010). Phragmites thrives in freshwater and brackish wetlands (Meyerson et al. 2000; Wilcox et al. 2003), and is expanding in managed systems like highway ditches (Lelong et al. 2007; Jodoin et al. 2008) and constructed wetlands (Havens et al. 2003).
Phragmites management strategies typically focus on the use of a limited number of techniques (described later) applied to individual patches or groups of patches. To critically and effectively evaluate restoration after an invasive species has been removed, data need to be collected to assess the initial wetland state, monitor the system through treatment (to inform adaptive management) and monitor for multiple years after treatment (see discussion in Blossey 1999). However, studies on the management of invasive plants (not just those investigating Phragmites) rarely report data beyond the response of the invader (reviewed in Reid et al. 2009), and monitoring for treatment effectiveness seldom lasts more than 2 years (reviewed in Kettenring and Reinhardt Adams 2011). A lack of long-term monitoring is likely due to: (i) the cultural mindset of land management agencies; and (ii) financial considerations and logistical constraints. Phragmites management in the USA has been occurring for over 35 years (Riemer 1976; Marks et al. 1994). Yet, while monitoring appears prohibitively expensive for specific projects, land managers spent over $4.6 million per year on Phragmites management across North America over a 5-year period (Martin and Blossey 2013), with no published data to justify the effectiveness of these management efforts to restore native plant communities. Given that eradication of Phragmites is rare, and is not likely without many years of follow-up treatments (Warren et al. 2002; Getsinger et al. 2006; Kettenring et al. 2012a; Lombard et al. 2012), monitoring of treatment effectiveness should be an essential component of any management programme.
Here we review current strategies for Phragmites management in North America and identify the factors that have the potential to transform future management. We begin with a literature review that addresses two central questions: (i) are current management practices successful? and (ii) do current Phragmites management practices allow for the restoration of native species assemblages? We address these questions by building upon earlier comprehensive reviews of Phragmites management (Marks et al. 1994; Kiviat 2006) in light of recent findings on the relationships among Phragmites invasion, land use and reproductive strategies within and among Phragmites patches. We also present a conceptual model of Phragmites invasion that integrates recent research findings. We argue that Phragmites management is best approached from a holistic perspective that integrates nutrient and disturbance management at landscape scales while addressing modes of reproduction and spread.
Review of Existing Control Measures
Methods
We reviewed the available literature on Phragmites management in the USA to determine: (i) which practices have been tested, (ii) where deficiencies in our knowledge exist, and (iii) what is known about recovery of native communities following attempts to eradicated Phragmites. We queried Google Scholar® and ISI Web of Science® for the technical and grey literature on Phragmites removal. We used the key words ‘Phragmites removal’ and ‘Phragmites management’ for all available dates. Articles, reports and theses from North America were included in our review (34 in total), along with reference to conclusions from previous reviews of the same topics. Only field studies that are applicable to management actions were included; meso- and microcosm studies are omitted. While our review focuses on non-native Phragmites in North America, they are presented in context with findings from other parts of the world. We did not consider Phragmites removal by hydrologic restoration in our quantitative review as that topic has recently been evaluated (Chambers et al. 2012); however, this approach is dealt with contextually when tied to another management method.
Results and discussion
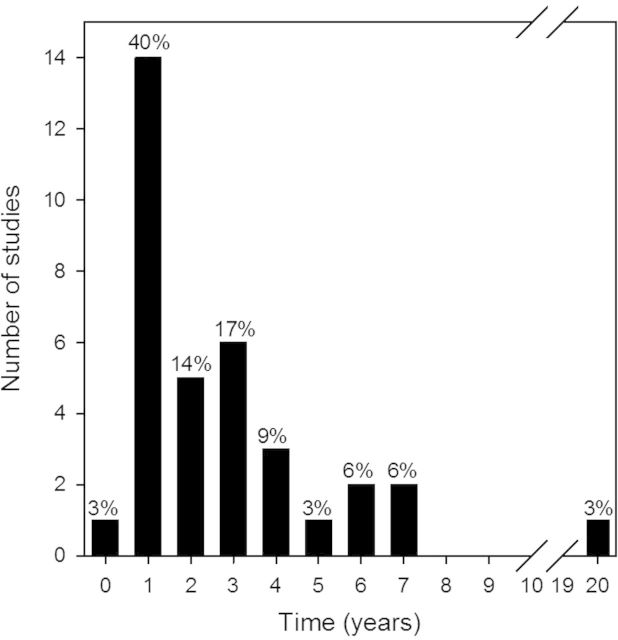
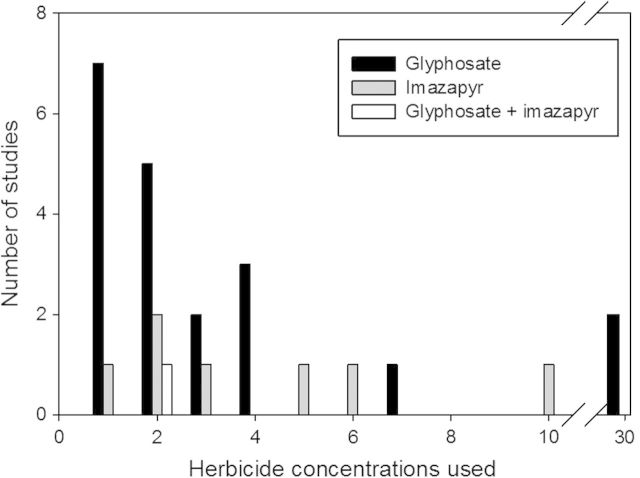
The most common response variables measured in our review were Phragmites-only metrics or functional vegetation (vegetation type, diversity, etc.) (21/34 studies; Fig. 3). Several studies (5) quantified plant species composition following Phragmites management, although none performed any analysis that compared plant community composition. Additionally, only one study (Moore et al. 2012) compared recovering vegetation to reference sites, which is often critical in restoration and management (Neckles et al. 2002). Notably, two studies reported seed bank changes in response to Phragmites management and recorded ample seedbank for passive revegetation. Most studies (14) reported a single year of data and only 5 report >5 years of follow-up data, the most notable of which was a study that reported a 20-year follow-up observation (Fig. 1). The most commonly tested management technique was the use of herbicides (Fig. 2). Of the 34 studies, 27 reported results of the use of herbicides alone or in combination with other methods. A combination of cutting or mowing Phragmites, often in combination with flooding or herbicide use was studied in 15 instances (Fig. 2; Table 1).
Figure 3.
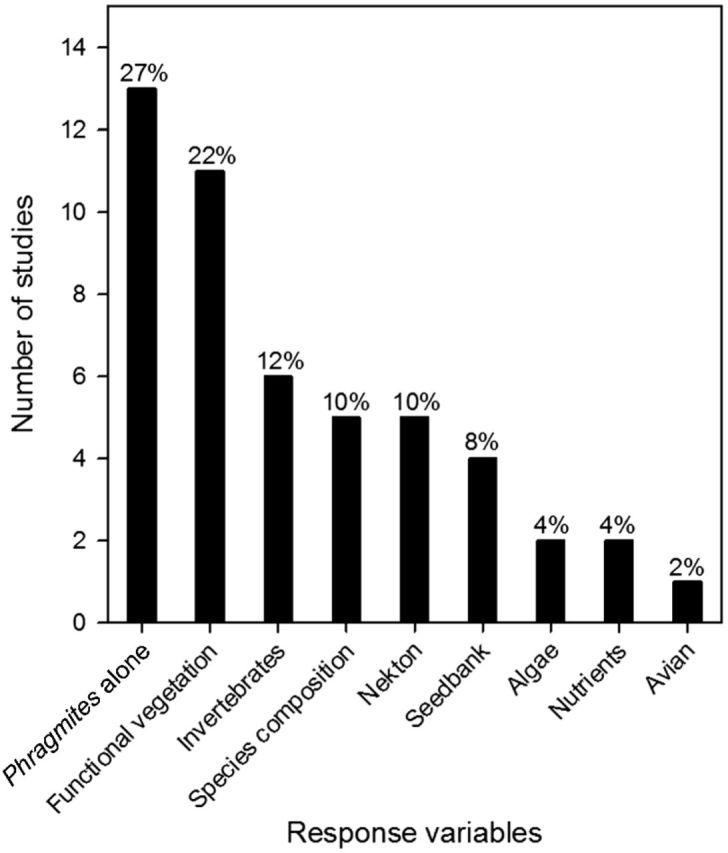
Response variables measured in reviewed studies. Functional vegetation represents only diversity, functional groups or species of interest, but not plant communities. Seedbank represents studies where germination trials were conducted.
Figure 1.
Duration of studies included in review. One study conducted a single survey and is denoted with the time = 0 bar.
Figure 2.
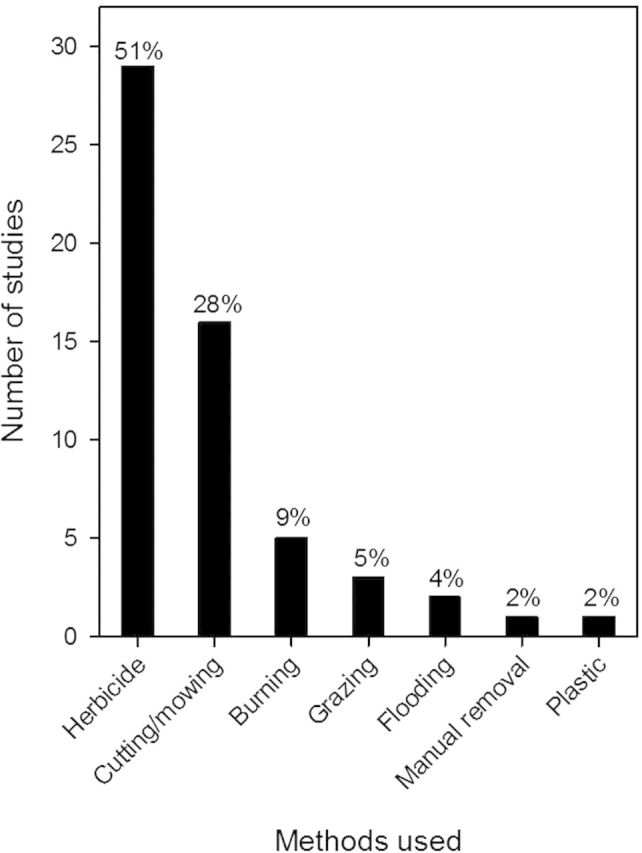
Management methods used in reviewed articles. Methods used in combination are counted individually.
Table 1.
Studies included in quantitative review. Herbicide concentrations (rounded to 0.25 %) are reported for spray techniques alone and are reported as percent solution of commercial herbicide product in water. aSB, seed bank composition; NU, nutrients; NK, nekton; AG, algae; IV, invertebrates; SC, species composition of nontarget plants; FV, functional vegetation (diversity, species of interest, native cover, etc.). b‘G’ for glyphosate, ‘I’ for Imazapyr, ‘G + I’ for combined, ‘Varied’ if concentrations varied by site, ‘NR’ for studies that did not report concentrations, and ‘NA’ for studies that did not use herbicide. cIndicates that study reported herbicide in mass of dry active ingredient, these values were converted to % solution based on the standard concentration of 58.3 % active ingredient in commercial herbicide blends (URS 2005). dCombination of results from multiple studies.
| Study | Location | Response variablesa | Method | Duration (years) | Herbicide concentrationb |
|---|---|---|---|---|---|
| Ailstock et al. (2001) | MD | SB, SC | Herbicide, mow, burn | 4 | G: 1.5 % |
| Back et al. (2012) | OH | PA, AG, IV | Herbicide | 1 | G: 30 % |
| I: 5 % | |||||
| Back and Holomuzki (2008) | OH | PA | Herbicide | 7 | G: 30 % |
| I: 5 % | |||||
| Brundage (2010) | MD | FV | Grazing (goats) | 1 | NA |
| Carlson et al. (2009) | Great Lakes | SB, SC, NU | Herbicide, cutting | 2 | G: NR |
| Derr (2008a, 12–16) | NJ | PA | Herbicide, mow | 1 | *G: 1.75 % |
| Derr (2008b, 153–157) | NJ | PA | Herbicide | 1 | G: 3 % |
| I: 1 % | |||||
| Farnsworth and Meyerson (1999) | CT | SC | Herbicide, mow | 3 | G: 1 % |
| Fell et al. (2003) | CT | FV, NK, IV | Herbicide, mow | 1 | G: 1.25 % |
| Fell et al. (2006) | CT | FV, IV, NK | Herbicide, mow | 1 | G: 1.25 % |
| Findlay et al. (2003) | CT | NU | Herbicide, mowing | 3 | G: 1 % |
| Getsinger et al. (2006) | MI | SC | Herbicide, burn, mow, flood | 3/4 | G: 3 % |
| I: 1.5 % | |||||
| G + I: 2 % + 2 % | |||||
| Gratton and Denno (2005) | NJ | IV | Herbicide | 5 | G: Varied |
| Hellings and Gallagher (1992) | DE | PA | Mow, flood | 1 | NA |
| Hallinger and Shisler (2009) | NJ | SB | Herbicide, cutting | 5 | G: 4 % |
| Kay (1995) | NC | PA | Herbicide (wipe on), Mow | 2 | NA |
| Kimball and Able (2007) | DE | NK | Herbicide, burn | 1 | G: Varied |
| Knezevic et al. (2013) | NE | PA | Herbicide | 1 | G: Varied |
| I: Varied | |||||
| G + I: Varied | |||||
| Kulesza et al. (2008) | OH | AG, NK, IV | Herbicide (wipe on) | 2 | NA |
| Lazaran et al. (2013) | OH | FV, AV | Herbicide | 1 | NR |
| Lombard et al. (2012) | MA | PA | Herbicide (clip and drip, wipe on, spray) | 7 | G: 2 % (spray) |
| Mozdzer et al. (2008) | VA | FV | Herbicide | 1 | G: 2 % |
| I: 2, 5 % | |||||
| Myers et al. (2009) | VA | SB, FV | Herbicide | 6 | I: 6 % |
| Myers et al. (2007) | VA | PA | Herbicide | 4 | I: 10 % |
| Plentovich (2008) | WI | FV | Herbicide, burn, mow | 1 | I: 2.5 % |
| URS (2005) | DE | FV | Grazing, mowing, herbicide (wipe on, spray) excavation | 6 | G: Varied |
| Rapp et al. (2012) | NE, WY | PA | Herbicide, mowing, disking | 3 | G: 4 % |
| I: 4 % | |||||
| Riemer (1976) | NJ | PA | Herbicide | 3 | cG: 2.25, 4.25, 6.5 % |
| Smith (2005) | MA | PA | Manual | 1 | NA |
| Tesauro and Ehrenfeld (2007) | NJ | FV | Grazing (cattle) | Single survey | NA |
| Turner and Warren (2003)d | CT | FV | Herbicide | 20 | 1G: 1.25 % |
| 1G: Varied | |||||
| Warren et al. (2001) | CT | NK, SC, IV | Herbicide, mow | 2 | G: 1.25 % |
| Wang et al. (2006) | NJ | FV | Herbicide, planting | 3 | NR |
| Willcox (2013) | CT | PA | Plastic | 1 | NA |
Our review focused on four main categories of methods for controlling Phragmites: mechanical, chemical, biological and novel methods. Here we review these methods to discuss their effectiveness and to highlight research needs.
Mechanical control
Mechanical control is perhaps the first human reaction to remove an unwanted plant, and the methods vary in efficacy and degree of effort. It is largely achieved with mechanical mowing or cutting with hand tools, hand-pulling, crushing, excavation of entire plants, burning or cutting, often followed by covering the area with soil or plastic.
Mowing and cutting
For a perennial rhizomatous grass, mowing does little to reduce its dominance. Mowing actually stimulated shoot production and resulted in increased density of Phragmites shoots (but decreased shoot height and biomass) in both non-tidal (Güsewell et al. 1998; Güsewell 2003; Asaeda et al. 2006; Derr 2008a) and tidal wetlands (Warren et al. 2001).
Variable results following cutting were likely due to a combination of phenology, abiotic conditions and patch size. Impacts from cutting vary relative to the phenology of the plant, due to shoot/rhizome interactions, as reserves are mobilized and stored differently according to season (Weisner and Granéli 1989; Asaeda et al. 2006 and references therein). For example, cutting in June showed significant impacts to aboveground and rhizome biomass the following growing season, whereas cutting in July showed no significant impacts compared with controls (Asaeda et al. 2006) and open wetlands to pelagic flushing (Uzarski et al. 2009). External environmental factors (e.g. temperature and salinity) can influence success; cutting just before the flooding season has been reported to improve control (Marks et al. 1994; Kiviat 2006). Some researchers report cutting treatments are less effective when soils are sandy or aerated (Weisner and Granéli 1989). One primitive approach broke shoots and removed them by hand (several shoots were held tight and broken below the waterline as the bases were kicked) along shorelines of five fresh water ponds (Smith 2005). High water levels in all ponds resulted in broken/crushed shoots remaining underwater for an extended period and mortality ranged from 41 to 99 % after 1 year (Smith 2005).
On a large scale, hand cutting will largely be ineffective due to time and resources, but may be an important strategy of rapid response efforts. Overall, simply cutting will be ineffective in eliminating Phragmites, but with proper timing, cutting may help reduce dominance (through depletion of underground reserves) and control expansion.
The most effective means of Phragmites mechanical control is a combination of cutting or mowing (usually in the spring) and covering stubble with plastic (for one growing season). However, there are limitations to this application; it is usually applied to small areas, as it is labour-intensive (Dawson and Hallows 1983; Boone et al. 1988; Marks et al. 1994; Kiviat 2006; Willcox 2013). In one removal experiment, Phragmites shoot density averaged 0.1 m−2 beneath the plastic compared with 20.7 m−2 in plots without plastic (Burdick et al. 2010). Thus, unless cutting is combined with plastic sheeting or herbicide, mowing alone will have little effect on Phragmites management other than containment.
Burning
Burning of Phragmites provides an alternative mechanism for physical removal, similar to mowing, but burning has not been effective unless coupled with either hydrological restoration or herbicide application (Marks et al. 1994). Burning alone has produced variable results and even stimulated Phragmites growth and stand development (van der Toorn and Mook 1982; Thompson and Shay 1985; Cross and Fleming 1989; Granéli 1989).
Cutting and burning appear to enhance control efforts if used as secondary treatments. For example, mechanical control efforts improved significantly following either herbicide use (Carlson et al. 2009) or the reintroduction of flood waters in tidal wetlands (Hellings and Gallagher 1992; Teal and Peterson 2005; Getsinger et al. 2006). In some instances, burning to remove standing dead biomass in winter was found to enhance control following restoration of tidal exchange (Sun et al. 2007). Burning aboveground shoots (or other methods like cutting or crushing) followed by flooding can be used to cut off the oxygen flow to the rhizomes (Weisner and Granéli 1989; Rolletschek et al. 2000).
Removal or mulching of the aboveground material following cutting has been recommended (Marks et al. 1994; Kiviat 2006), even though removal and disposal involves more effort to prevent recolonization from rhizomes. Burning removes the dead thatch and aids in the regeneration of native plants (Ailstock et al. 2001)—typically a primary goal where managers wish to control Phragmites. Removal by either mechanism also increases light availability that warms exposed soils. Such conditions enhance germination and recruitment of native plants from seed banks, which is critical for wetland recovery (Marks et al. 1994; Farnsworth and Meyerson 1999; Ailstock et al. 2001; Kiviat 2006; Carlson et al. 2009).
Excavation
Excavation provides complete Phragmites control, and is likely the only landscape-scale option for mechanical removal, but requires disproportionally greater costs in both time and resources. Land managers have successfully restored Phragmites-dominated dredge spoil sites to highly valued salt marshes in New England (Moore et al. 2009). In such cases, excavation to elevations at or below mean high water (i.e. coupling removal with restoration of hydrology) results in daily tidal flooding, increased salinity and sulfide, and resulted in restoration of native plant communities and associated faunal species in Connecticut and New Hampshire (Moore et al. 2009).
Chemical control
Herbicide
Herbicides are currently the primary tool used by land managers to control or eliminate Phragmites in North America (94 % in a recent national survey; Martin and Blossey 2013; and 97 % in Utah alone, Kettenring et al. 2012a). There are several application methods and two main herbicide active ingredients (glyphosate and imazapyr) that have been used with varying levels of success (see recent herbicide comparison by Cheshier et al. 2012). Perhaps one of the greatest challenges in understanding the efficacy of herbicides on Phragmites management is the lack of data on the long-term impacts of herbicide application on Phragmites and non-target species (Figs 1 and 3). In addition, few studies have specifically addressed different application rates and/or application time (Back and Holomuzki 2008; Derr 2008b; Mozdzer et al. 2008; Back et al. 2012; Cheshier et al. 2012). The majority of the data that we found were not reported in peer-reviewed publications but in technical reports and bulletins in the ‘grey literature’ which are rarely readily available. We divide information on the use of herbicides into (i) herbicide types and their effects on ecosystem recovery, and (ii) a comparison of herbicide efficacy and potential effects on non-target vegetation.
Glyphosate
The most commonly used herbicides contain the active ingredient glyphosate; this is likely attributed to the fact that glyphosate herbicides were the only Environmental Protection Agency (EPA)-approved herbicides for application in aquatic environments until 2003. Common trade names approved for aquatic application of glyphosate to control Phragmites include Rodeo™, GlyPro™ and Aqua Neat™. As a broad-spectrum systemic herbicide, glyphosate is non-selective and also kills non-target plants including woody and herbaceous plants. According to the Rodeo™ label, glyphosate is taken up through the plant epidermis and subsequently moves into the root system through the vascular tissue. In the plant, it interferes with amino acid synthesis specifically found in plants and microorganisms. Degradation of glyphosate is reported to occur through microbial pathways in <7 days; however, greenhouse studies have reported the persistence of glyphosate or glyphosate-related products for up to 79 days (Meyerson et al. 1997), suggesting that any subsequent replanting should occur several weeks after replanting dates given by the label instructions, due to potential negative effects on non-target native plants. A surfactant must be added to aid in foliar uptake, and reported toxicity in fauna has been attributed to surfactants in the various formulations (Tu et al. 2001), and not the herbicide itself.
Historically, glyphosate was applied at the end of the growing season (per label instructions) when plants were translocating resources to belowground rhizomes. Owing to the extremely long growing season of non-native Phragmites (Farnsworth and Meyerson 2003; League et al. 2006), it was possible to apply glyphosate after native plant senescence with minimal effect on native vegetation. Two recent studies have found that, contrary to label instructions, earlier application of glyphosate (June vs. September) is more effective at controlling Phragmites (Derr 2008b; Mozdzer et al. 2008). However, earlier application also has the potential to negatively impact native plants (Mozdzer et al. 2008), which is often at odds with management goals.
The use of glyphosate-containing herbicides usually requires multiple applications over successive years to be effective. Unfortunately, no published studies exist that have evaluated how many applications of glyphosate are necessary for complete Phragmites control. We speculate that the effectiveness of any herbicide is likely related to the amount of belowground reserves, abiotic conditions and applicator error. However, there is an urgent need to understand the appropriate control application methods to reduce excess herbicides from entering wetland systems (see concentrations tested in Fig. 4).
Figure 4.
Herbicide concentrations (as percent solution of active ingredient in water) used by herbicide removal studies.
Imazapyr
The active ingredient imazapyr was approved in 2003 by the US EPA for application in wetland habitats labelled as Habitat™, Eagre™ and EcoImazapyr™. Since then, land managers have been using this herbicide (Marris 2005; Clarke 2006) to control Phragmites. According to the label, imazapyr works by a mechanism targeting broad-chained plant-specific amino acids in meristematic regions, and is translocated belowground to kill rhizomes. Unlike glyphosate, imazapyr is taken up by the plants’ leaves as well as by its roots. In solution, imazapyr is broken down through photodegradation with an average half-life of 2 days. However, in soils where ultraviolet breakdown does not occur, microbial breakdown of imazapyr is the primary mechanism of degradation with half-lives ranging from 1 month to over 4 years (Tu et al. 2001) with soil moisture, soil depth, pH and temperature affecting the rates of microbial degradation (Vizantinopoulos and Lolos 1994). Toxicity is described as low to birds and mammals; however, non-ionic surfactants may have detrimental effects on invertebrates (Tu et al. 2001).
Controlled comparative studies have found that imazapyr is more effective than glyphosate in controlling Phragmites (Kay 1995; Getsinger et al. 2006; Derr 2008b; Mozdzer et al. 2008), but not without serious negative consequences to native plants including recolonization following the death of Phragmites (Mozdzer et al. 2008). The only studies that reported glyphosate exhibiting a greater impact on Phragmites under field conditions were two that used higher concentrations than recommended by the manufacturer (30 % in study vs. <6 % recommended) and were not comparable to the rate of imazapyr used (5 %) (Back and Holomuzki 2008; Back et al. 2012) (Fig. 4). Other studies have demonstrated that there is no need to use glyphosate in concentrations higher than those listed on the product label (Cheshier et al. 2012), and label instructions should not be exceeded due to potential negative consequences on flora and fauna. Land managers have noted that wetlands are slower to recover when imazapyr is used when compared with glyphosate herbicides (Mozdzer et al. 2008), which may be attributed to greater persistence in the soil. Given the potential for non-selective root uptake of imazapyr by all plants, the presence of imazapyr or imazapyr residues may be affecting the seed banks of native plants. Research is critically needed to understand whether imazapyr has negative impacts on the seed bank, or if the delayed recovery can be attributed to the persistence of the herbicide in the soils impairing growth of seedlings.
Landscape-scale Phragmites control programmes using herbicides
Few have investigated or attempted to control Phragmites at the landscape level, and even fewer have made the results available to the scientific community. Perhaps one of the largest restoration projects occurred on the Delaware River as part of the Public Service Electric and Gas restoration. Several papers (Turner and Warren 2003; Gratton and Denno 2005; Teal and Peterson 2005; URS 2005; Kimball and Able 2007) were published midway through the restoration process, reporting on the management approach, but the final results assessing if the management objectives to restore vegetatively diverse, functioning wetlands were achieved have never been published as a peer reviewed study.
In Virginia, USA, land managers have established one of the most thorough management and coordination programmes that we are aware of by combining efforts with private, state and federal stakeholders (Myers et al. 2009). Partnering with numerous public and private entities, state staff targeted priority conservation areas (the coastal habitats of Virginia around Chesapeake Bay) to reduce the cover and rate of Phragmites spread. These efforts spanned 6 years and often included an initial aerial application that was followed by ground-based applications in subsequent years to control any re-sprouting. Most of the sites that were treated were surveyed by helicopters in 2004 and 2008. Given that the treatments and surveys were coordinated at the landscape level (Myers et al. 2009), the effort enabled land managers to share resources, resulting in one of the few examples of landscape-scale management and control.
The coordinated work in Virginia (Myers et al. 2009) revealed several patterns, which provided insights for future management. In treated areas, land managers were able to reduce Phragmites abundance by 34 % from 706 acres to 468 acres. However, where aerial control was not applied, there was a 22 % increase in Phragmites abundance from 657 to 805 acres. Cumulatively over a 4-year period, Phragmites abundance was only reduced by 4 % total since management focused primarily on large stands (>5 acres). However, during this same period, the small (<0.25 acres) and medium (>0.25 and <5.0 acres) sized class populations increased in abundance by 22 and 87 %, respectively, accounting for almost all the gains in habitat from controlling the large stands. These findings suggest that targeting large stands may not be appropriate for controlling Phragmites at the landscape level. Instead, priority should be given to small patches that are likely to expand in the future and may contribute to future expansion by sexual reproduction (Myers et al. 2009), which is an approach supported in general recommendations for invasive species control (Moody and Mack 1988).
Regardless of the herbicide used, one-time applications are never 100 % effective (Kettenring and Reinhardt Adams 2011). In order for a control and restoration programme to be successful, land managers must commit to multi-year applications (e.g. Riemer 1976; Kay 1995; Warren et al. 2001; Cheshier et al. 2012; Lombard et al. 2012) in addition to a long-term commitment from land managers and stakeholders (Teal and Peterson 2005).
Biological control
Plant competition
Plant competition by native plants can alter the restoration trajectory. Unmanaged areas where Phragmites has been controlled effectively, but not replanted with native species, are often reinvaded by Phragmites immediately either by seeds or regrowth from rhizomes that were not killed. The importance of Phragmites seed banks in reinvasion varies. Earlier studies reported that Phragmites was not present in the seed bank (Van der Valk and Davis 1979; Wilson et al. 1993; Baldwin and DeRico 1999); however, more recent studies have found ample Phragmites seed in the seed bank (Smith and Kadlec 1983; Welling et al. 1988a, b; Leck 2003; Baldwin et al. 2010). As a grass, Phragmites seeds do not remain viable in the seed bank for very long. Where germination of Phragmites seeds has been reported, the density of the germinated seeds can be almost as high as the number of viable seeds produced (∼700 seeds m−2, Baldwin et al. 2010). If this scenario is typical, it suggests that revegetation of areas from which Phragmites has been killed should be planted or seeded with native plants as soon as possible, under the theory that native plants will competitively exclude Phragmites seedlings (Farnsworth and Meyerson 1999; Wang et al. 2006; Carlson et al. 2009; Byun et al. 2013). Field experiments in tidal marshes have shown that native plants, though smaller, can slow the recolonization of Phragmites seedlings (Minchinton 2002b; Minchinton and Bertness 2003) and reduce the success of resprouting from rhizomes (Amsberry et al. 2000; Konisky and Burdick 2004; Wang et al. 2006; Peter and Burdick 2010).
Greater species richness in resident plant communities may reduce the ability of Phragmites to colonize and expand. A wetland with intact vegetation will have fewer opportunities for Phragmites colonization (Kennedy et al. 2002). The potential of native species to successfully compete with Phragmites was demonstrated in a field experiment in which one or four native species were planted with Phragmites shoots that were grown from rhizomes. Plots with greater species richness had the most dramatic effects, reducing Phragmites shoot density >50 %, biomass >90 % and survival >65 % compared with unplanted controls (Peter and Burdick 2010). A Canadian competition study evaluated plant functional diversity as a factor in Phragmites competition. Byun et al. (2013) found that biotic resistance in plant communities increased by niche preemption (native plants germinated before Phragmites seeds) and niche partitioning (functional diversity). These two experiments demonstrate the importance of plant communities and post-control revegetation in resisting Phragmites invasion.
Accelerated development or succession provides an alternative management strategy. This strategy can be successful where the vegetation of forested wetlands or upland edges of wetlands has been disturbed and replaced by Phragmites. Here, removal could be coupled with planting trees and shrubs to shade out Phragmites (Kiviat 2006; Geoff Wilson, Northeast Wetland Restoration, pers. comm.). A survey of Phragmites invasion of 15 created tidal wetlands found Phragmites stands decreased cover where shrub/scrub habitat developed (Havens et al. 2003). This approach may prevent Phragmites reestablishment over the long term, or may allow only scattered Phragmites plants to survive.
Native seed banks are critical for successful revegetation after Phragmites removal. The literature is full of conflicting results, but overall, wetlands tend to have diverse persistent seed banks (Leck and Simpson 1995; Ungar 2001; Leck 2003; Leck and Leck 2005) and seed bank studies have not resulted in any clear relationship between the diversity of species in the seed bank and Phragmites invasion. In a Great Lakes study, Carlson et al. (2009) found that the diversity of vegetation after Phragmites removal depended upon the diversity of the native seed bank. It has also been shown that a diverse native seed bank can persist in monocultures of Phragmites (Baldwin et al. 2010). In fact, the diversity of herbaceous species in the seed bank has been found to be greater in stands dominated by Phragmites compared with surrounding areas dominated by native vegetation (Minchinton et al. 2006). Minchinton and colleagues concluded that the high cover of Phragmites and the thick litter layer inhibited the germination of non-Phragmites seeds in the seed bank. In a tidal freshwater system, Ailstock et al. (2001) found that the seed bank under Phragmites and after Phragmites removal both had a high diversity of species. These authors concluded that the type of Phragmites management will alter the seed bank, with herbicide-burn treatments having a different seed bank species composition compared with herbicide alone which impacts the outcome of passive revegetation. Hallinger and Shisler (2009) reported successful recolonization of native vegetation from the seed bank alone (with minor reseeding) in a New Jersey salt marsh following Phragmites removal. In New England, greater plant diversity was found in treated areas compared with both invaded and uninvaded controls (Moore et al. 2012). These studies indicate that the seed bank can play an important role in any wetland restoration effort following Phragmites removal.
Herbivory
Grazing has long been used to manage Phragmites stands, primarily in Europe (Marks et al. 1994), yet there are very few empirical studies evaluating grazing in North America (reviewed in Kiviat 2006). Tesauro and Ehrenfeld (2007) used grazing to manage Phragmites and other invasive species in a New Jersey wetland and found the method beneficial to plant species diversity and animal habitat, but the study lacked replication. Brundage (2010) showed that in Maryland, goats can significantly decrease Phragmites density, height and biomass while concurrently increasing species diversity in grazed plots. Around the Great Salt Lake in Utah, several agencies use grazing to manage Phragmites, primarily using cattle (49 % of surveyed land managers in Kettenring et al. 2012a). Although there are no formal monitoring data available, wetlands in Utah that receive high-intensity, short-duration grazing appear to respond best, with Distichlis spicata replacing Phragmites after 3 years of grazing rotation (Rich Hansen, Utah Department of Wildlife Resources, pers. comm.), and increases in shorebirds and waterfowl as well (Chad Cranney, Utah Department of Wildlife Resources, pers. comm.). In contrast, a study that tested goat grazing in New Jersey marshes in low densities (∼1 goat per acre) found that goats preferentially ate all vegetation except Phragmites, only consuming Phragmites when all other options were exhausted (Teal and Peterson 2005; John Teal, J. M. Teal Associates, pers. comm.; URS 2005). Forced grazing in small plots, where grazing mammals do not have an alternative food source, can be successful in controlling Phragmites if applied appropriately (B. R. Silliman et al., in review). However, there are obvious tradeoffs associated with high-intensity grazing, such as soil compaction, trampling and/or nutrient enrichment that may prevent it from being a suitable method in many areas. Diverse communities of natural herbivores also help suppress Phragmites expansion. Small mammals appear to decrease establishment of Phragmites in lower salinity tidal marshes (Gedan et al. 2009). Muskrats graze Phragmites in freshwater systems in the western United States (E.L.G.H., pers. observ.) and brackish wetlands (T.J.M., pers. observ.), indicating that natural herbivory will influence species assemblages in wetlands that contain Phragmites. Natural grazing by small mammals may be fostered in brackish marshes by providing muskrat platforms and enhancing habitat for natural herbivores (see Kiviat 2006). Other natural herbivores seem deterred by Phragmites (Litorina irrorata; Hendricks et al. 2011). There is little information on how either natural herbivory or targeted grazing allow for the reassembly of native plant communities.
Classical biocontrol organisms
Biocontrol organisms are currently highly prioritized by land management agencies as a low-cost management strategy alternative. Traditional biocontrol agents are insect herbivores found in the invasive plant's native range that can have strong impacts on its growth and reproduction (Tscharntke 1999; Van Driesche et al. 2010). Planned introductions of invertebrates are often controversial as there is a potential for unintended effects on non-target organisms or even across trophic levels (Thomas and Reid 2007), with only 27 % of studies reporting complete success in eliminating invasive plants (Van Driesche et al. 2010). A recent survey of land managers found that 91 % would release biocontrol organisms for Phragmites, indicating that there is a strong desire for new techniques to control this grass (Martin and Blossey 2013). Some land managers expressly prohibit the use of biocontrols due to the potential for unintended impacts and the risks to non-target organisms (Tu et al. 2001). The search for a biocontrol for Phragmites in North America has been going on for over a decade (Tscharntke 1999; Tewksbury et al. 2002; Blossey 2003; Häfliger et al. 2005), and several potential insect biocontrols have been identified and are currently undergoing host-specificity testing with potential releases in 2–3 years from time of writing (B. Blossey, Cornell University, pers. comm.).
In the native range of Eurasian Phragmites, there are several dozen invertebrate herbivores in reed stands (Tscharntke 1999) and many of the natural enemies are also found in North America (see Tewksbury et al. 2002 for a comprehensive review). Indeed, Phragmites herbivores are still being discovered in North America (Eichiner et al. 2011). Several herbivores prefer native conspecific Phragmites to the non-native lineage (Lambert et al. 2007), findings that are troubling given the potential impacts on the widely distributed native Phragmites in North America. The herbivores currently present in North America are not considered effective at controlling the spread of the invasive form of Phragmites, though some can prevent flowering (e.g. Lipara spp., Lambert et al. 2007). An ongoing study in the Chesapeake Bay has found stem infection rates by insects of over 50 % (E. L. G. Hazelton et al., in review), yet the degree of impact on competitive dominance and reproductive output is yet to be studied.
Novel methods in Phragmites management
Several new management methods are currently in development, ranging from hydrologic restoration to alteration of rhizosphere conditions, novel molecular tools and fungal pathogens. Multiple research groups are investigating pathogens as potential biocontrols. A group at Cornell University is looking at oomycetes as a potential Phragmites management tool (Nelson 2009). Shearer and Harms (2012) attempted to isolate fungal pathogens that will preferentially attack non-native Phragmites in North America. In a converse approach, another group is using fungal inhibitors to eliminate endophytes in Phragmites and then assess reductions in performance (USGS Great Lakes Science Center 2012, 2013). Gene silencing techniques are in development with a goal of identifying knock out genes associated with Phragmites growth and photosynthesis (USGS Great Lakes Science Center 2012).
In tidal wetlands, restoring hydrology often results in increased porewater sulfide shifting the competitive advantage to native vegetation over Phragmites (Warren et al. 2001; Chambers et al. 2003; Moore et al. 2012). High concentrations of sulfide impede nutrient uptake (Chambers et al. 1999) and also decrease Phragmites growth (Howes et al. 2005). Observations of lower sulfide levels in tidal marsh soils with Phragmites stands suggest that high sulfide levels may limit Phragmites distribution (Chambers et al. 1999, 2002). Seeds, seedlings and cuttings can tolerate sulfide concentrations of up to ∼1.5 mM sulfide (reviewed in Chambers et al. 2003), but mature culms were able to survive consistent sulfide levels of 1.5 mM (Howes et al. 2005). These findings suggest that mature stands with clonal connections may be tolerant of high sulfide concentrations. Therefore, hydrologic control might work best following mechanical actions to eliminate aboveground portions of mature shoots, preventing Phragmites from oxygenating the rhizosphere.
Other invasive grasses have been successfully managed by nitrogen control including Bromus tectorum (Kulmatiski and Beard 2006; Vasquez et al. 2008) and Phalaris arundinacea (Ianone et al. 2008). Vasquez et al. (2008) found that more holistic management practices consisting of controlled grazing, microbial change (through carbon amendment) and native planting helped control nitrogen and make sites less invasible by B. tectorum in semi-arid systems. In other systems, addition of sawdust to promote microbial nitrogen immobilization, combined with planting diverse plant assemblages allowed native species to recover following management for P. arundinacea (Ianone et al. 2008). Sawdust addition impacts non-native grasses more than non-native and native forbs and native grasses (Alpert and Maron 2000). Sugar amendment decreased the success of multiple invasive plants greater than adding activated charcoal (Mitchell and Bakker 2011). Even carbon amendment will likely require watershed-scale restoration to permanently decrease plant-available nitrogen (Perry et al. 2010) and future studies will need to determine the efficacy of such approaches on Phragmites.
Based on this review, we see the need for more research that investigates comprehensive, landscape-scale, integrative management strategies. There is a clear bias in the literature to herbicide use and mowing or cutting, which is reflected in recent surveys of land managers (Kettenring et al. 2012a; Martin and Blossey 2013). These methods may be effective on a site-by-site basis, but they do not address the factors that contribute to Phragmites invasion. Whether the management goal is to eliminate Phragmites or merely reduce its dominance, control measures will be more successful if linked with establishment of native plants to occupy the site and periodic monitoring to identify, mark and treat invasive plants. Regardless of the control method and initial success of native plants, non-native Phragmites will recolonize in most cases (unless salinities are high, as in Sun et al. 2007) and will be difficult to eliminate from invaded wetlands (Farnsworth and Meyerson 1999; Warren et al. 2001).
Integrating Recent Insights about Phragmites Ecology into Management: A Conceptual Model
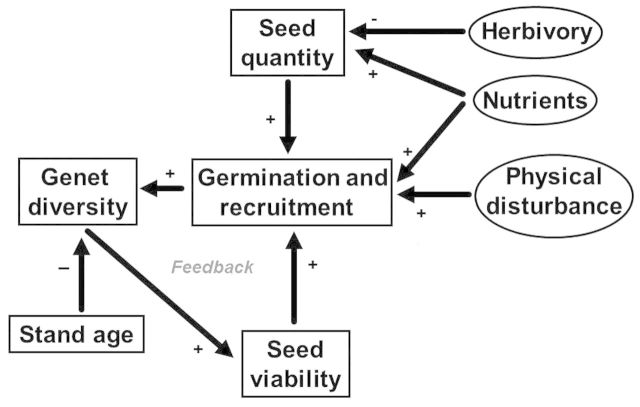
Plant invasions, including that of Phragmites, are triggered by both intrinsic and extrinsic factors and are typically interactions between nutrients, disturbance and propagule pressure (Colautti et al. 2006). Intrinsic factors are aspects of a species' biology that drive its establishment and spread. Extrinsic factors include anthropogenic disturbances, nutrient enrichment and herbivory. We developed a conceptual model of Phragmites spread that is driven by interactions between intrinsic and extrinsic factors (Fig. 5). This model can be used to guide future efforts to manage Phragmites. The model is comprised of four intrinsic components that positively affect spread: (i) seed quantity; (ii) seed viability; (iii) germination and recruitment; and (iv) genet diversity. In our model, germination and recruitment are central to increasing genet diversity (outcrossing potential). Increased genet diversity through outcrossing potential leads to an increase in seed viability (Kettenring et al. 2010, 2011; McCormick et al. 2010a, b). Increases in seed quantity or seed viability will result in higher recruitment rates (new clonally diverse Phragmites stands), feeding the cycle. Stand age is an intrinsic factor that slows this feedback loop. Three extrinsic factors are nutrients, disturbances and herbivory; the first two of which positively impact spread while herbivory has a negative effect through reductions in seed production. Nutrients and physical disturbance also fuel the cycle by increasing seed quantity and recruitment (nutrients), and creating microsites for germination (disturbance). We describe each of these components in greater detail below.
Figure 5.
Conceptual model of Phragmites spread. Intrinsic factors are shown in boxes; extrinsic factors are in ovals. Genet diversity has a positive effect on viable seed production due to increased out-crossing potential. There is a positive feedback between the intrinsic factors affecting sexual reproduction and spread that are further enhanced by physical disturbances and nutrients.
Phragmites invasions were long thought to originate primarily from vegetative propagules (e.g. rhizomes) on the upland edge of wetlands (Bart et al. 2006), despite the fact that Phragmites is capable of sexual reproduction and spread from seed. Seed is dispersed by wind or birds (Haslam 1969; Soons 2006) and new molecular evidence has made it increasingly clear that seeds, rather than vegetative propagules, are the primary means of reproduction for colonization by Phragmites (Campbell 2007; Brisson et al. 2008; Baldwin et al. 2010; Belzile et al. 2010; Kettenring et al. 2010, 2011; McCormick et al. 2010a, b; Kirk et al. 2011; Kettenring and Mock 2012).
Viable seed production in Phragmites is driven by outcrossing potential, a phenomenon that is enhanced in polyclonal patches (Kettenring et al. 2010, 2011). Viable seeds will lead to the production of new clones, thereby increasing outcrossing potential in a positive feedback that is further enhanced by the presence of disturbances and nutrients (Kettenring et al. 2010, 2011; McCormick et al. 2010a, b). In particular, inflorescence size and seed quantity increase with elevated nutrients (Kettenring et al. 2011), and Phragmites in watersheds with a greater degree of anthropogenic development produce more seeds than those with less human impact (King et al. 2007; Kettenring and Whigham 2009; Baldwin et al. 2010; Kettenring et al. 2010; McCormick et al. 2010a). Phragmites seedlings then can exhibit ‘explosive growth’ in response to elevated nutrients (Saltonstall and Stevenson 2007).
Phragmites is a disturbance specialist and its seeds require light and large diurnal temperature fluctuation to break dormancy; conditions typically found on bare, non-inundated soils (Armstrong et al. 1999; Ekstam and Foresby 1999; Ekstam et al. 1999). Bare soils can be the result of anthropogenic or natural events such as burial by wrack (Minchinton 2002a; Minchinton and Bertness 2003), a water level drawdown (Smith and Kadlec 1983; Galinato and Van der Valk 1986; Welling et al. 1988a, b; Tulbure et al. 2007; Whyte et al. 2008; Tulbure and Johnston 2010; Wilcox 2012), or removal of litter and vegetation by wave action (Baldwin et al. 2010). Specific conditions for seed germination are found in the upper edge of wetlands where there is ample oxygen (Wijte and Gallagher 1996a) and salinities are typically low (Wijte and Gallagher 1996a, b; Greenwood and MacFarlane 2006). Then the plant expands primarily through vegetative means via rhizome or stolon extension (Amsberry et al. 2000; Bart et al. 2006). Although susceptible to flooding during early stages, seedling tolerance to flooding increases with age (Wijte and Gallagher 1996b; Mauchamp et al. 2001; Baldwin et al. 2010; also see review in Weisner and Granéli 1989; Clevering 1999; Engloner 2010).
Clonal diversity decreases with stand age (Koppitz et al. 1997; Koppitz and Kuhl 2000; Curn et al. 2007; Krivackova-Sucha et al. 2007), potentially decreasing future sexual reproduction by decreasing outcrossing potential. Thus, older stands may decrease in management priority as their clonal diversity decreases. Hyper-adapted clones will be able to prevent seeding establishment by shading the underlying substrate. The outcome of these interactions is that a single clone may eventually competitively exclude other clones, potentially decreasing future sexual reproduction by decreasing outcrossing potential. Many of the oldest stands in Chesapeake Bay appear to have decreased their rate of spread (Rice et al. 2000), perhaps as a wetland reaches carrying capacity.
In addition to stand age effects on sexual reproduction, several obligate Phragmites endophagous herbivores eliminate Phragmites apical dominance, thus destroying flowering potential on attacked culms (e.g. Lipara spp., Giraudiella spp., Calamomyia spp., Lasioptera spp., Tetramesa spp. in Tscharntke 1999; Tewksbury et al. 2002; Lambert et al. 2007). While the total impact of herbivory on seed production at the stand or population level is not clear, rates of attack can reach levels likely to decrease seed production substantially (often >50 % of stems attacked, Lambert et al. 2007; >90 % E. L. G. Hazelton et al., in review).
Watershed-scale changes in land use resulting from development, and associated increases in disturbances and the availability of limiting nutrients such as nitrogen, contribute to Phragmites invasion (Bertness et al. 2002; Silliman and Bertness 2004; King et al. 2007). Phragmites presence is linked to development at or near the shoreline (Bertness et al. 2002; King et al. 2007). The absence or disruption of forested buffers at the upland–wetland–estuarine ecotone edge has been shown to result in expansion of Phragmites in New England (Burdick and Konisky 2003; Silliman and Bertness 2004) and the Chesapeake Bay (King et al. 2007; Chambers et al. 2008). Greater wave energy and watershed-scale nutrient loading interact to increase sexual reproduction and clonal diversity in Phragmites stands (Baldwin et al. 2010; Kettenring et al. 2011). Once wetlands within nutrient enriched watersheds have been invaded, Phragmites can spread rapidly through sexual reproduction and the subsequent dispersal of seeds (McCormick et al. 2010a; Kettenring et al. 2011). Anthropogenic vectors (highways and boat transport) promote the transport and expansion of Phragmites between watersheds and across the landscape (Lelong et al. 2007; Jodoin et al. 2008; Kettenring et al. 2012a, b in this special issue).
Our model of Phragmites spread and reproduction is consistent with observations in other species, where increasing nutrient availability and physical disturbance make ecosystems more susceptible to invasion (Alpert et al. 2000; Richardson and Pysek 2012). In order to truly manage Phragmites, we will need to work at the watershed scale to make sites less able to be invaded through nutrient management and decreased anthropogenic disturbance (Alpert et al. 2000) and create conditions that do not favour seed production. Nitrogen management may become the most effective means to control Phragmites in the future (Kettenring et al. 2011), especially with climate change and increasing CO2 (Mozdzer and Megonigal 2012). Efforts at the watershed scale to promote ‘restoration to ensure resilience’ (Suding 2011) are needed to combat spread from seed. In addition, addressing sexual reproduction as part of management efforts will be critical (Kettenring et al. 2011), especially given that the common practice to control Phragmites in the fall with glyphosate often occurs after seeds have been produced (Marks et al. 1994; Kettenring et al. 2011).
Conclusions
Critiques of Phragmites management are not new, and some authors have called for revaluation of Phragmites and the tradeoffs associated with management. Several authors have demonstrated that non-native Phragmites provides valuable ecosystem services, especially in the context of increasing anthropogenic stressors and climate change. The services include providing resilient vegetation (Ludwig et al. 2003), accretion rates that can keep pace with sea level rise (Rooth et al. 2003), habitat quality (Meyerson et al. 2010), nutrient removal (Mozdzer et al. 2010) and other ecosystem services (Rooth and Windham 2000; Kiviat 2006, 2013 in this special issue; Hershner and Havens 2008). The potential ecosystem services provided by Phragmites must be weighed against the desired management outcomes (such as waterfowl management; Cross and Fleming 1989) associated with Phragmites removal. Since we still know little about the composition of vegetation communities after Phragmites is removed, we should weigh the costs of management heavily against the assumed benefits. Phragmites management has a great economic cost (Martin and Blossey 2013) and could be met with public backlash due to the use of herbicide and other cultural perceptions (Teal and Peterson 2005). It is unlikely that a single strategy will work at all sites; and all management actions should be conducted in a case-specific manner with considerations for the likelihood of success and the costs of management in each watershed.
Managers may decide that certain landscapes have been altered too far from a natural state to successfully control Phragmites and have reached an alternate stable state that includes non-native Phragmites monocultures. Choosing to restore sites that are less degraded and facilitating native plant communities are critical steps toward successful management of invasive plants (Reid et al. 2009). Research and land managers should focus on identifying and restoring sites that are likely to recover and remain Phragmites free (sensu: Ailstock et al. 2001; Reid et al. 2009). Restoration efforts may not succeed at all unless they are conducted at the watershed scale in order to address the initial cause (or source) of the invasion (Palmer 2009). Based on our model of Phragmites invasion, sites that are in low nutrient watersheds where physical anthropogenic disturbances are unlikely should resist invasion (also see discussion in Kettenring et al. 2010). Large-scale comparative studies that manage Phragmites across multiple watersheds will help us determine the factors that contribute to success and failure in Phragmites restoration efforts (sensu Suding 2011). Once established, Phragmites is difficult to remove; preventing invasion may be more efficient than control. Phragmites control programmes that focus on protection of non-invaded wetlands through prioritization will likely be more successful than those aiming to reduce or eliminate Phragmites in heavily invaded watersheds.
The actual outcomes of Phragmites removal are still largely unclear. In perhaps the most comprehensive study to date, Ailstock et al. (2001) recommended site-specific management with clearly defined restoration objectives. Restoration and management efforts that remove an invasive species often do not result in colonization by desirable native species (Kettenring and Reinhardt Adams 2011; Suding 2011). Changes are temporary and do not necessarily lead to habitat improvement. We advocate increased research into the outcomes of Phragmites management, the efficacy of management strategies and preplanning to assess which sites to manage (i.e. tradeoffs between management efforts and potential gains). Research can be used to guide landscape-scale multi-year removals that are structured to allow monitoring and adaptive responses to address challenges and meet management outcomes. Programmes should also consider possible underlying causes for Phragmites invasion (shoreline buffers to prevent disturbance from development and excess nutrient inputs) and broadening partnerships between ecologists, managers and policy makers (sensu Suding 2011) to manage Phragmites in a more holistic manner.
Sources of Funding
This manuscript is the direct outcome of the ‘Phragmites australis in North America and Europe’ symposium at the 2011 meeting of the Society of Wetland Scientists and was sponsored by AoB PLANTS. E.L.G.H., K.M.K. and D.F.W. are partially funded by NOAA (grant #NA09NOS4780214). Funding support for T.J.M. was provided from MD Sea Grant Award SA7528114-WW and NSF DEB-0950080. E.L.G.H. is supported by the Utah State University Ecology Center, Delta Waterfowl, and a Smithsonian Institution Predoctoral Fellowship. D.M.B. was supported by the Class of 1937 Professorship in Marine Science; Jackson Estuarine Laboratory Contribution #514. This is publication #14-001 of the NOAA/CSCOR Mid-Atlantic Shorelines project.
Contributions by the Authors
E.L.G.H. and T.J.M. are equal contributors in structuring and researching the manuscript. Other authors contributed equally to their respective expertise.
Conflicts of Interest Statement
None declared.
Acknowledgements
Amanda Sweetman, Susan Adamowicz (USFWS), Geoff Wilson (Northeast Wetland Restoration), Paul Clarke (formerly of Virginia Natural Heritage Program) and the Kettenring Lab provided valuable suggestions and insights on the manuscript. Christine Rohal provided the cover imagery.
Literature Cited
- Ailstock MS, Norman CM, Bushmann PJ. Common reed Phragmites australis: control and effects upon biodiversity in freshwater nontidal wetlands. Restoration Ecology. 2001;9:49–59. [Google Scholar]
- Alpert P, Maron JL. Carbon addition as a countermeasure against biological invasion. Biological Invasions. 2000;2:33. [Google Scholar]
- Alpert P, Bone E, Holzpfel C. Invasiveness, invisibility and the role of environmental stress in the spread of non-native plants. Perspectives in Plant Ecology, Evolution and Systematics. 2000;3:52–66. [Google Scholar]
- Amsberry L, Baker MA, Ewanchuk PJ, Bertness MD. Clonal integration and the expansion of Phragmites australis. Ecological Applications. 2000;10:1110–1118. [Google Scholar]
- Armstrong J, Afreen-Zobayed F, Blyth S, Armstrong W. Phragmites australis: effects of shoot submergence on seedling growth and survival and radial oxygen loss from roots. Aquatic Botany. 1999;64:275–289. [Google Scholar]
- Asaeda T, Rajapakse L, Manatunge J, Sahara N. The effect of summer harvesting of Phragmites australis on growth characteristics and rhizome resource storage. Hydrobiologia. 2006;553:327–335. [Google Scholar]
- Back CL, Holomuzki JR. Long term spread and control of invasive, common reed (Phragmites australis) in Sheldon Marsh, Lake Erie. Ohio Journal of Science. 2008;108:108–112. [Google Scholar]
- Back CL, Holomuzki JR, Klarer DM, Whyte RS. Herbiciding invasive reed: indirect effects on habitat conditions and snail-algal assemblages one year post application. Wetlands Ecology and Management. 2012;20:419–431. [Google Scholar]
- Baldwin AH, DeRico EF. The seed bank of a restored tidal freshwater wetland in Washington, D.C. Urban Ecosystems. 1999;3:5–20. [Google Scholar]
- Baldwin AH, Kettenring KM, Whigham DF. Seed banks of Phragmites australis-dominated brackish wetlands: relationships to seed viability, inundation, and land cover. Aquatic Botany. 2010;93:163–169. [Google Scholar]
- Bart D, Burdick D, Chambers R, Hartman JM. Human facilitation of Phragmites australis invasions in tidal marshes: a review and synthesis. Wetlands Ecology and Management. 2006;14:53–65. [Google Scholar]
- Belzile F, Labbé J, LeBlanc M-C, Lavoie C. Seeds contribute strongly to the spread of the invasive genotype of the common reed (Phragmites australis) Biological Invasions. 2010;12:2243–2250. [Google Scholar]
- Bertness MD, Ewanchuk PJ, Silliman BR. Anthropogenic modification of New England salt marsh landscapes. Proceedings of the National Academy of Sciences of the USA. 2002;99:1395–1398. doi: 10.1073/pnas.022447299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blossey B. Before, during and after: the need for long-term monitoring in invasive plants species management. Biological Invasions. 1999;1:301–311. [Google Scholar]
- Blossey B. 2003. http://www.invasiveplants.net/
- Boone J, Furbish E, Turner K, Bratton S. Clear plastic: a non-chemical herbicide. Restoration and Management Notes. 1988;6:94–95. [Google Scholar]
- Brisson J, Paradis E, Bellevance M-E. Evidence of sexual reproduction in the invasive common reed (Phragmites australis subsp. australis; Poaceae) in Eastern Canada: a possible consequence of global warming. Rhodora. 2008;110:225–230. [Google Scholar]
- Brundage A. College Park, MD, USA: 2010. Grazing as a management tool for controlling Phragmites australis and restoring native plant biodiversity in wetlands. MS Thesis. [Google Scholar]
- Burdick D, Konisky R. Determinants of expansion for Phragmites australis, common reed, in natural and impacted coastal marshes. Estuaries and Coasts. 2003;26:407–416. [Google Scholar]
- Burdick DM, Peter CR, Moore GE, Wilson G. Portsmouth, NH: 2010. Comparison of restoration techniques to reduce dominance of Phragmites australis at Meadow Pond, Hampton New Hampshire; p. 73. Final Report. [Google Scholar]
- Byun C, deBlois S, Brisson J. Plant functional group identity and diversity determine biotic resistance to invasion by an exotic grass. Journal of Ecology. 2013;101:128–139. [Google Scholar]
- Campbell AL. Columbus, OH, USA: 2007. Sexual reproduction in non-native common reed, Phragmites australis’. MS Thesis. [Google Scholar]
- Carlson ML, Kowalski KP, Wilcox DA. Promoting species establishment in a Phragmites-dominated Great Lakes coastal wetland. Natural Areas Journal. 2009;29:263–280. [Google Scholar]
- Chambers RM, Meyerson LA, Saltonstall K. Expansion of Phragmites australis into tidal wetlands of North America. Aquatic Botany. 1999;64:261–273. [Google Scholar]
- Chambers RM, Osgood DT, Kalapasev N. Hydrologic and chemical control of Phragmites growth in tidal marshes of SW Connecticut, USA. Marine Ecology Progress Series. 2002;239:83–91. [Google Scholar]
- Chambers RM, Osgood DT, Bart DJ, Montalto F. Phragmites australis invasion and expansion in tidal wetlands: interactions among salinity sulfide, and hydrology. Estuaries. 2003;26:398–406. [Google Scholar]
- Chambers RM, Havens KJ, Killeen S, Berman M. Common Reed Phragmites australis occurrence and adjacent land use along estuarine shoreline in Chesapeake Bay. Wetlands. 2008;28:1097–1103. [Google Scholar]
- Chambers RM, Meyerson LA, Dibble KL. Ecology of Phragmites australis and responses to tidal restoration. In: Roman CT, Burdick DM, editors. Tidal marsh restoration. Washington, DC: Island Press/Center for Resource Economics, 81–96; 2012. [Google Scholar]
- Cheshier JC, Madsen JD, Wersal RM, Gerard PD, Welch ME. Evaluating the potential for differential susceptibility of common reed (Phragmites australis) haplotypes I and M to aquatic herbicides. Invasive Plant Science and Management. 2012;5:101–105. [Google Scholar]
- Clarke PA. Richmond, VA: Virginia Department of Conservation and Recreation; 2006. Aquatic resources trust fund Phragmites australis control in Eastern Virginia; Year 3 final report; p. 16. [Google Scholar]
- Clevering O. Between- and within-population differences in Phragmites australis 1. The effects of nutrients on seedling growth. Oecologia. 1999;121:447–457. doi: 10.1007/s004420050951. [DOI] [PubMed] [Google Scholar]
- Colautti RI, Grigoravich IA, MacIsaac HJ. Propagule pressure: a null model for biological invasions. Biological Invasions. 2006;8:1023–1037. [Google Scholar]
- Cross DH, Fleming KL. Control of Phragmites or common reed. Washington, DC: Waterfowl Management Handbook Leaflet 13.4.12, U.S. Fish and Wildlife Service; 1989. [Google Scholar]
- Curn V, Kubatova B, Vavrova P, Krivackova-Sucha O, Cizkova H. Phenotypic and genotypic variation of Phragmites australis: comparison of populations in two human-made lakes of different age and history. Aquatic Botany. 2007;86:321–330. [Google Scholar]
- Dawson FH, Hallows HB. Practical applications of a shading material for macrophytes control in watercourses. Aquatic Botany. 1983;17:301–308. [Google Scholar]
- Derr JF. Common reed (Phragmites australis) response to mowing and herbicide application. Invasive Plant Science and Management. 2008a;1:12–16. [Google Scholar]
- Derr JF. Common reed (Phragmites australis) response to postemergence herbicides. Invasive Plant Science and Management. 2008b;1:153–157. [Google Scholar]
- Eichiner FB, Hoebeke ER, Nartshuk EP. A new species of Calamoncosis Enderlein (Diptera: Chloropidae) associated with common reed, Phragmites australis (Poaceae), in Eastern North America. Proceedings of the Entomological Society of Washington. 2011;113:109–118. [Google Scholar]
- Ekstam B, Foresby A. Germination response of Phragmites australis and Typha latifolia to diurnal fluctuations in temperature. Seed Science Research. 1999;9:167–163. [Google Scholar]
- Ekstam B, Johannesson R, Milberg P. The effect of light and number of diurnal temperature fluctuations on germination of Phragmites australis. Seed Science Research. 1999;9:165–170. [Google Scholar]
- Engloner AI. Structure, growth dynamics and biomass of reed (Phragmites australis)—a review. Flora. 2010;204:331–346. [Google Scholar]
- Farnsworth EJ, Meyerson LA. Species composition and inter-annual dynamics of a freshwater tidal plant community following removal of the invasive grass, Phragmites australis. Biological Invasions. 1999;1:115–127. [Google Scholar]
- Farnsworth EJ, Meyerson LA. Comparative ecophysiology of four wetland plant species along a continuum of invasiveness. Wetlands. 2003;23:750–762. [Google Scholar]
- Fell PE, Warren RS, Light JK, Rawson RL, Fairley SM. Comparison of fish and macroinvertebrate use of Typha latifolia, Phragmites australis, and treated Phragmites australis marshes along the Lower Connecticut River. Estuaries. 2003;26:534–551. [Google Scholar]
- Fell PE, Warren RS, Curtis AE, Steiner EM. Short-term effects on macroinvertebrates and fishes of herbiciding and mowing Phragmites australis dominated tidal marsh. Northeast Naturalist. 2006;13:191–212. [Google Scholar]
- Findlay S, Groffman P, Dye S. Effects of Phragmites australis removal on marsh nutrient cycling. Wetlands Ecology and Management. 2003;11:157–165. [Google Scholar]
- Galinato MI, Van der Valk AG. Seed germination traits of annuals and emergents recruited during drawdowns in the Delta Marsh, Manitoba, Canada. Aquatic Botany. 1986;26:89–102. [Google Scholar]
- Gedan KB, Crain CM, Bertness MD. Small mammal herbivore control of secondary succession in New England tidal marshes. Ecology. 2009;90:430–440. doi: 10.1890/08-0417.1. [DOI] [PubMed] [Google Scholar]
- Getsinger KD, Nelson LS, Glomski LM, Kafcas E, Schafer J, Kogge S, Nurse M. Lansing, MI: 2006. Control of Phragmites in a Michigan Great Lakes marsh. [Google Scholar]
- Granéli W. Influence of standing litter on shoot production in reed, Phragmites australis (Cav.) Trin. Ex. Steudel. Aquatic Botany. 1989;35:99–109. [Google Scholar]
- Gratton C, Denno RF. Restoration of arthropod assemblages in a Spartina salt marsh following removal of the invasive plant Phragmites australis. Restoration Ecology. 2005;13:358–372. [Google Scholar]
- Gratton C, Denno RF. Arthropod food web restoration following removal of an invasive wetland plant. Ecological Applications. 2006;16:622–631. doi: 10.1890/1051-0761(2006)016[0622:afwrfr]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Greenwood MA, MacFarlane GR. Effects of salinity and temperature on the germination of Phragmites australis, Juncus krausii and Juncus acutus: implications for estuarine restoration initiatives. Wetlands. 2006;26:854–861. [Google Scholar]
- Güsewell S. Management of Phragmites australis in Swiss fen meadows by mowing in early summer. Wetlands Ecology and Management. 2003;11:433–445. [Google Scholar]
- Güsewell S, Buttler A, Klötzli F. Short-term and long-term effects of mowing on the vegetation of two calcareous fens. Journal of Vegetation Science. 1998;9:861–872. [Google Scholar]
- Häfliger P, Schwarzländer M, Blossey B. Biology of Platycephela planifrons (Diptera: Chloropidae) and its potential effectiveness as biological control agent for invasive Phragmites australis in North America. Biological Control. 2005;34:302–311. [Google Scholar]
- Hallinger KD, Shisler JK. Seed bank colonization in tidal wetlands following Phragmites control (New Jersey) Ecological Restoration. 2009;27:16–18. [Google Scholar]
- Haslam SM. Development and emergence of buds in Phragmites communis Trin. Annals of Botany. 1969;33:289–301. [Google Scholar]
- Haslam SM. Phragmites australis Trin. (Arundo Phragmites L.? Phragmites australis (Cav.) Trin. Ex Steud) Journal of Ecology. 1972;60:585–610. [Google Scholar]
- Havens KJ, Berquist H, Priest WI., III Common reed grass, Phragmites australis, expansion into constructed wetlands: are we mortgaging our wetland future? Estuaries and Coasts. 2003;26:417–422. [Google Scholar]
- Hellings SE, Gallagher JL. The effects of salinity and flooding on Phragmites australis. Journal of Applied Ecology. 1992;29:41–49. [Google Scholar]
- Hendricks LG, Mossop HE, Kicklighter CE. Palatability and chemical defense in Phragmites australis to the marsh periwinkle Litorina irrorata. Journal of Chemical Ecology. 2011;37:838–845. doi: 10.1007/s10886-011-9990-8. [DOI] [PubMed] [Google Scholar]
- Hershner C, Havens KJ. Managing invasive aquatic plants in a changing system: strategic consideration of ecosystem services. Conservation Biology. 2008;22:544–550. doi: 10.1111/j.1523-1739.2008.00957.x. [DOI] [PubMed] [Google Scholar]
- Howes BL, Teal JM, Peterson S. Experimental Phragmites control through enhanced sediment sulfur cycling. Ecological Engineering. 2005;25:292–303. [Google Scholar]
- Ianone BV, Galatowitsch SM, Rosen CJ. Evaluation of resource-limiting strategies intended to prevent Phalaris aruninacea (reed canarygrass) invasions in restored sedge meadows. Ecoscience. 2008;15:508–518. [Google Scholar]
- Jodoin Y, Lavoie CL, Villeneuve P, Theriault M, Beaulieu J, Belzile F. Highways as corridors and habitats for the invasive common reed Phragmites australis in Quebec, Canada. Journal of Applied Ecology. 2008;45:459–466. [Google Scholar]
- Kay SH. Efficacy of wipe-on applications of glyphosate and imazapyr on common reed in aquatic sites. Journal of Aquatic Plant Management. 1995;33:25–26. [Google Scholar]
- Keller BEM. Plant diversity in Lythrum, Phragmites and Typha marshes, Massachusetts, USA. Wetlands Ecology and Management. 2000;8:391–401. [Google Scholar]
- Kennedy T, Naeem S, Howe KM, Knops JMH, Tilman D, Reich P. Biodiversity as a barrier to ecological invasion. Nature. 2002;417:636–638. doi: 10.1038/nature00776. [DOI] [PubMed] [Google Scholar]
- Kettenring KM, Mock KE. Genetic diversity, reproductive mode, and dispersal differ between the cryptic invader, Phragmites australis, and its native conspecific. Biological Invasions. 2012;14:2489–2504. [Google Scholar]
- Kettenring KM, Reinhart Adams C. Lessons learned from invasive plant control experiments: a systematic review and meta-analysis. Journal of Applied Ecology. 2011;48:970–979. [Google Scholar]
- Kettenring KM, Whigham DF. Seed viability and seed dormancy of non-native Phragmites australis in suburbanized and forested watersheds of the Chesapeake Bay, USA. Aquatic Botany. 2009;91:199–204. [Google Scholar]
- Kettenring KM, McCormick MK, Baron HM, Whigham DF. Phragmites australis (common reed) invasion in the Rhode River subestuary of the Chesapeake Bay: disentangling the effects of foliar nutrients, genetic diversity, patch size, and seed viability. Estuaries and Coasts. 2010;33:118–126. [Google Scholar]
- Kettenring KM, McCormick MK, Baron HM, Whigham DF. Mechanisms of Phragmites australis invasion: feedbacks among genetic diversity, nutrients, and sexual reproduction. Journal of Applied Ecology. 2011;48:1305–1313. [Google Scholar]
- Kettenring KM, Garvie K, Hazelton ELG, Hough-Snee N, Ma Z. Phragmites invasion and control in the Great Salt Lake: 2012 managers’ survey. 2012a.
- Kettenring KM, de Blois S, Hauber DP. Moving from a regional to a continental perspective of Phragmites australis invasion in North America. AoB PLANTS. 2012b;2012:pls040. doi: 10.1093/aobpla/pls040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimball ME, Able KW. Nekton utilization of intertidal salt marsh creeks: tidal influences in natural Spartina, invasive Phragmites, and marshes treated for Phragmites removal. Journal of Experimental Marine Biology and Ecology. 2007;346:87–101. [Google Scholar]
- King RS, Deluca WV, Whigham DF, Marra PP. Threshold effects of coastal urbanization on Phragmites australis (common reed) abundance and foliar nitrogen in Chesapeake Bay. Estuaries and Coasts. 2007;30:469–481. [Google Scholar]
- Kirk H, Paul J, Straka J, Freeland JR. Long distance dispersal and genetic diversity are implicated in the invasive spread of the common reed Phragmites australis (Poaceae) in Eastern North America. American Journal of Botany. 2011;98:1180–1190. doi: 10.3732/ajb.1000278. [DOI] [PubMed] [Google Scholar]
- Kiviat E. Annandale, NY:: Hudsonia Ltd.; 2006. Phragmites management sourcebook for the Tidal Hudson River; p. 74. Report to the Hudson River Foundation, New York. [Google Scholar]
- Kiviat E. Ecosystem services of Phragmites in North America with emphasis on habitat functions. AoB PLANTS. 2013;5:plt008. doi: 10.1093/aobpla/plt008. [DOI] [Google Scholar]
- Knezevic SZ, Rapp RE, Datta A, Irmak S. Common reed (Phragmites australis) control is influenced by the timing of herbicide application. International Journal of Pest Management. 2013;59:224–228. [Google Scholar]
- Konisky R, Burdick DM. Effects of stressors on invasive and halophytic plants of New England salt marshes: a framework for predicting response to tidal restoration. Wetlands. 2004;24:434–447. [Google Scholar]
- Koppitz H, Kuhl H. To the importance of genetic diversity of Phragmites australis in the development of reed stands. Wetlands Ecology and Management. 2000;8:403–414. [Google Scholar]
- Koppitz H, Kuhl H, Hesse K, Kohl J-G. Some aspects of the importance of genetic diversity in Phragmites australis (Cav.) Trin. Ex Steudel for the development of reed stands. Botanica Acta. 1997;110:217–223. [Google Scholar]
- Krivackova-Sucha O, Vavrova P, Cizkova H, Curn V, Kubotova B. Phenotypic and genotypic variation of Phragmites australis: a comparative study of clones originating from tow populations of different age. Aquatic Botany. 2007;86:361–368. [Google Scholar]
- Kulesza AE, Holomuzki JR, Klarer DM. Benthic community structure in stands of Typha angustifolia and herbicide-treated and untreated Phragmites australis. Ohio Journal of Science. 2008;28:40–56. [Google Scholar]
- Kulmatiski A, Beard KH. Activated carbon as a restoration tool: potential for control of invasive plants in abandoned agricultural fields. Restoration Ecology. 2006;14:251–257. [Google Scholar]
- Lambert AM, Winiarski K, Casagrande RA. Distribution and impact of exotic fall flies (Lipara spp.) on native and exotic Phragmites australis. Aquatic Botany. 2007;86:163–170. [Google Scholar]
- Lambertini C, Mendelssohn IA, Gustafsson MHG, Oleson B, Riis T, Sorrell BK, Brix H. Tracing the origin of Gulf Coast Phragmites (Poaceae): a story of long-distance dispersal and hybridization. American Journal of Botany. 2012a;90:1–14. doi: 10.3732/ajb.1100396. [DOI] [PubMed] [Google Scholar]
- Lambertini C, Sorrell BK, Riis T, Olesen B, Brix H. Exploring the borders of European Phragmites within a cosmopolitan genus. AoB PLANTS. 2012b;2012:pls020. doi: 10.1093/aobpla/pls020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaran MA, Bocetti CI, Whyte RS. Impacts of Phragmites management on Marsh Wren nesting behavior. The Wilson Journal of Ornithology. 2013;125:184–187. [Google Scholar]
- League M, Colbert EP, Seliskar DM, Gallagher JL. Rhizome growth dynamics of native and exotic haplotypes of Phragmites australis (common reed) Estuaries and Coasts. 2006;29:269–276. [Google Scholar]
- Leck MA. Seed bank and vegetation development in a created tidal freshwater wetland on the Delaware River, Trenton, New Jersey, USA. Wetlands. 2003;23:310–343. [Google Scholar]
- Leck MA, Leck CF. Vascular plants of a Delaware River tidal freshwater wetland and adjacent terrestrial areas: seed bank vegetation comparisons of reference and constructed marshes and annotated species list. Journal of the Torrey Botanical Society. 2005;132:323–354. [Google Scholar]
- Leck MA, Simpson RL. Ten-year seed bank and vegetation dynamics of a tidal freshwater marsh. American Journal of Botany. 1995;82:1547–1557. [Google Scholar]
- Lelong B, Lavoie C, Jodoin Y, Belzile F. Expansion pathways of the exotic common reed (Phragmites australis): a historical and genetic analysis. Diversity and Distributions. 2007;13:430–437. [Google Scholar]
- Lombard KB, Tomasi D, Ebersole J. Long term management of an invasive plant: lessons from seven years of Phragmites australis control. Northeast Naturalist. 2012;19:181–193. [Google Scholar]
- Ludwig DF, Iannuzzi TJ, Esposito AN. Phragmites and environmental management: a question of values. Estuaries. 2003;26:624–630. [Google Scholar]
- Marks M, Lapin B, Randall J. Phragmites australis (P. communis): threats, management and monitoring. Natural Areas Journal. 1994;14:285–294. [Google Scholar]
- Marris E. Invasive species: shoot to kill. Nature. 2005;438:272–273. doi: 10.1038/438272a. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Blossey B. The runaway weed: costs and failures of Phragmites australis management in the USA. Estuaries and Coasts. 2013;36:626–632. [Google Scholar]
- Mauchamp A, Blanch S, Grillas P. Effects of submergence on the growth of Phragmites australis seedlings. Aquatic Botany. 2001;69:147–164. [Google Scholar]
- McCormick MK, Kettenring KM, Baron HM, Whigham DF. Spread of invasive Phragmites australis in estuaries with differing degrees of development: genetic patterns, allee effects and interpretation. Journal of Ecology. 2010a;98:1369–1378. [Google Scholar]
- McCormick MK, Kettenring KM, Baron HM, Whigham DF. Extent and reproductive mechanisms of Phragmites australis spread in brackish wetlands in Chesapeake Bay, Maryland (USA) Wetlands. 2010b;30:67–74. [Google Scholar]
- Meyerson LA, McLain JET, Mayfield AE, III, Berlyn GP. Three greenhouse plant bioassays to determine the persistence of the herbicide glyphosate in soil. Restoration and Management Notes. 1997;15:200–201. [Google Scholar]
- Meyerson LA, Chambers RM, Vogt KA. The effects of Phragmites removal on nutrient pools in a freshwater tidal marsh ecosystem. Biological Invasions. 1999;1:129–136. [Google Scholar]
- Meyerson LA, Saltonstall K, Windham L, Kiviat E, Findlay S. A comparison of Phragmites australis in freshwater and brackish marsh environments in North America. Wetlands Ecology and Management. 2000;8:89–103. [Google Scholar]
- Meyerson LA, Lambert AM, Saltonstall K. A tale of three lineages: expansion of common reed (Phragmites australis) in the U.S. Southwest and Gulf Coast. Invasive Plant Science and Management. 2010;3:515–520. [Google Scholar]
- Meyerson LA, Lambertini C, McCormick MK, Whigham DF. Hybridization of common reed in North America? The answer is blowing in the wind. AoB PLANTS. 2012;2012:pls022. doi: 10.1093/aobpla/pls022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchinton T. Precipitation during El Nino correlates with increasing spread of Phragmites australis in New England, USA, coastal marshes. Marine Ecology. 2002a;242:305–309. [Google Scholar]
- Minchinton TE. Disturbance by wrack facilitates spread of Phragmites australis in a coastal marsh. Journal of Experimental Marine Biology and Ecology. 2002b;281:89–107. [Google Scholar]
- Minchinton TE, Bertness MD. Disturbance-mediated competition and the spread of Phragmites australis in a coastal marsh. Ecological Applications. 2003;13:1400–1416. [Google Scholar]
- Minchinton TE, Simpson JC, Bertness MD. Mechanisms of exclusion of native coastal marsh plants by an invasive grass. Journal of Ecology. 2006;94:342–354. [Google Scholar]
- Mitchell RM, Bakker JD. Carbon addition as a technique for controlling invasive species in Pacific Northwest prairies. Northwest Science. 2011;85:247–254. [Google Scholar]
- Moody ME, Mack RN. Controlling the spread of plant invasions: the importance of nascent foci. Journal of Applied Ecology. 1988;25:1009–1021. [Google Scholar]
- Moore GE, Burdick DM, Peter CR, Leonard-Duarte A, Dionne M. Gloucester, MA: 2009. Regional assessment of tidal marsh restoration in New England using the restoration performance index; p. 230. Final Report. [Google Scholar]
- Moore GE, Burdick DM, Buchsbaum R, Peter CR. Boston, MA: 2012. Investigating causes of Phragmites australis colonization in Great Marsh, Parker River National Wildlife Refuge; p. 36. [Google Scholar]
- Mozdzer TJ, Megonigal JP. Jack-and-Master trait responses to elevated CO2 and N: a comparison of native and introduced Phragmites australis. PlosONE. 2012;7:e42794. doi: 10.1371/journal.pone.0042794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozdzer TJ, Hutto CJ, Clarke PA, Field DP. Efficacy of imazapyr and glyphosate in the control of non-native Phragmites australis. Restoration Ecology. 2008;16:221–224. [Google Scholar]
- Mozdzer TJ, Zieman JC, McGlathery KJ. Nitrogen uptake by native and invasive temperate coastal macrophytes: importance of dissolved organic nitrogen. Estuaries and Coasts. 2010;33:784–797. [Google Scholar]
- Mozdzer TJ, Brisson J, Hazelton EL. Physiological ecology and functional traits of North American native and Eurasian introduced Phragmites australis lineages. AoB PLANTS. 2013;5 plt048; doi:10.1093/aobpla/plt048. [Google Scholar]
- Myers RK, Field DP, Heffernan KE, Weber JT, Ayers RA, Clarke PA, Shuart W, Garman G. Management and education to control Phragmites on the seaside of Virginia's Eastern Shore. 2007. Virginia Department of Conservation and Recreation, Division of Natural Heritage, Richmond, Virginia. Final report for Year Four of the Seaside Heritage Program submitted to USDC National Oceanic and Atmospheric Administration. Natural Heritage Technical Report # 07-06, February 2007, 40 pp.
- Myers RK, Heffernan KE, Clarke PA, Field DP. Management and education to control Phragmites on the seaside of Virginia's Eastern Shore. 2009. Virginia Department of Conservation and Recreation, Division of Natural Heritage, Richmond, Virginia. Final report for Year Six of the Seaside Heritage Program submitted to USDC National Oceanic and Atmospheric Administration. Natural Heritage Technical Report # 09-05, February 2009, 45 pp.
- Neckles HA, Dionne M, Burdick DM, Roman CT, Buchsbaum R, Hutchins E. A monitoring protocol to assess tidal restoration of salt marshes on local and regional scales. Restoration Ecology. 2002;10:556–563. [Google Scholar]
- Nelson E. 2009. http://www.plantpath.cornell.edu/labs/enelson/Research.html .
- Palmer MA. Reforming watershed restoration: science in need of application and application in need of science. Estuaries and Coasts. 2009;32:1–17. [Google Scholar]
- Perry LG, Blumenthal DM, Monaco TA, Paschke MW, Redente EF. Immobilizing nitrogen to control plant invasions. Oecologia. 2010;163:13. doi: 10.1007/s00442-010-1580-x. [DOI] [PubMed] [Google Scholar]
- Peter CR, Burdick DM. Can plant competition and diversity reduce the growth and survival of exotic Phragmites australis invading a tidal marsh? Estuaries and Coasts. 2010;33:1225–1236. [Google Scholar]
- Plentovich D. Green Bay: University of Wisconsin; 2008. A comparison of Phragmites australis control measures in Wisconsin coastal wetlands. MS Thesis. [Google Scholar]
- Rapp RE, Datta A, Irmak S, Arkebauer TJ, Knevic SZ. Integrated management of common reed (Phragmites australis) along the Platte River in Nebraska. Weed Technology. 2012;26:326–333. [Google Scholar]
- Reid AM, Morin L, Downey PO, French K, Virtue JG. Does invasive plant management aid in the restoration of natural ecosystems? Biological Conservation. 2009;142:2342–2349. [Google Scholar]
- Rice D, Rooth J, Stevenson JC. Colonization and expansion of Phragmites australis in upper Chesapeake Bay tidal marshes. Wetlands. 2000;20:280–299. [Google Scholar]
- Richardson DM, Pysek P. Naturalization of introduced plants: ecological drivers and biogeographical patterns. New Phytologist. 2012;196:383–396. doi: 10.1111/j.1469-8137.2012.04292.x. [DOI] [PubMed] [Google Scholar]
- Riemer DN. Long term effects of glyphosate application to Phragmites. Journal of Aquatic Plant Management. 1976;13:39–43. [Google Scholar]
- Rolletschek H, Rolletschek A, Hartzendorf T, Kohl J. Physiological consequences of mowing and burning of Phragmites australis stands for rhizome ventilation and amino acid metabolism. Wetlands Ecology and Management. 2000;8:425–433. [Google Scholar]
- Rooth JE, Windham L. Phragmites on death row: is biocontrol really warranted? Wetland Journal. 2000;12:29. [Google Scholar]
- Rooth JE, Stevenson JC, Cornwell JC. Increased sediment accretion rates following invasion by Phragmites australis: the role of litter. Estuaries and Coasts. 2003;26:475–483. [Google Scholar]
- Saltonstall K. Cryptic invasion by a non-native genotype of the common reed, Phragmites australis, into North America. Proceedings of the National Academy of Sciences of the USA. 2002;99:2445–2449. doi: 10.1073/pnas.032477999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltonstall K. Genetic variation among North American populations of Phragmites australis: implications for management. Estuaries. 2003;26:444–451. [Google Scholar]
- Saltonstall K, Stevenson JC. The effect of nutrients on seedling growth of native and introduced Phragmites australis. Aquatic Botany. 2007;86:331–336. [Google Scholar]
- Shearer JF, Harms NE. 2012. USACE. Survey for pathogens of Phragmites in New York. ERDC/EL TN-12-1.
- Silliman BR, Bertness MD. Shoreline development drives invasion of Phragmites australis and the loss of plant diversity on New England salt marshes. Conservation Biology. 2004;18:1424–1434. [Google Scholar]
- Smith LM, Kadlec JA. Seed banks and their role during drawdown of a North American marsh. Journal of Applied Ecology. 1983;20:673–684. [Google Scholar]
- Smith SM. Manual control of Phragmites australis on pond shores of Cape Cod National Seashore, Massachusetts, USA. Journal of Aquatic Plant Management. 2005;43:50–53. [Google Scholar]
- Soons MB. Wind dispersal in freshwater wetlands: knowledge for conservation and restoration. Applied Vegetation Science. 2006;9:271. [Google Scholar]
- Suding KN. Toward an era of restoration in ecology: successes, failures, and opportunities ahead. Annual Review of Ecology and Systematics. 2011;42:465–487. [Google Scholar]
- Sun H, Brown A, Coppen J, Steblein P. Response of Phragmites to environmental parameters associated with treatments. Wetlands Ecology and Management. 2007;15:63–79. [Google Scholar]
- Taylor N. Vol. 316. New York State Museum Bulletin; 1938. A preliminary report on the salt marsh vegetation of Long Island, New York; pp. 21–84. [Google Scholar]
- Teal JM, Peterson S. The interaction between science and policy in the control of Phragmites in oligohaline marshes of Delaware Bay. Restoration Ecology. 2005;13:223–227. [Google Scholar]
- Tesauro J, Ehrenfeld D. The effects of livestock grazing on the bog turtle Clyptemys (=Clemmys) muhlenbergii. Herpetologica. 2007;63:293–300. [Google Scholar]
- Tewksbury L, Casagrande R, Blossey B, Hafliger P, Schwarzlander M. Potential for biological control of Phragmites australis in North America. Biological Control. 2002;23:191–212. [Google Scholar]
- Thompson DJ, Shay JM. The effects of fire on Phragmites australis in the Delta Marsh, Manitoba. Canadian Journal of Botany. 1985;63:1864–1869. [Google Scholar]
- Thomas MB, Reid AM. Are natural enemies an effective way of controlling invasive plants? Trends in Ecology and Evolution. 2007;22:447–454. doi: 10.1016/j.tree.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Tscharntke T. Insects on common reed (Phragmites australis): community structure and the impact of herbivory on shoot growth. Aquatic Botany. 1999;64:399–410. [Google Scholar]
- Tu M, Hurd C, Randall JM The Nature Conservancy. Weed control methods handbook: tools and techniques for use in natural areas. 2001. All U.S. Government Documents (Utah Regional Depository). Paper 533. http://digitalcommons.usu.edu/govdocs/533 . [Google Scholar]
- Tulbure MG, Johnston CA. Environmental conditions promoting non-native Phragmites australis expansion in Great Lakes Coastal Wetlands. Wetlands. 2010;30:577–587. [Google Scholar]
- Tulbure MG, Johnston CA, Auger DL. Rapid invasion of a Great Lakes coastal wetland by non-native Phragmites australis and Typha. Journal of Great Lakes Research. 2007;33:269–279. [Google Scholar]
- Turner RE, Warren RS. Valuation of continuous and intermittent Phragmites control. Estuaries. 2003;26:618–623. [Google Scholar]
- Ungar IA. Seed banks and seed population dynamics of halophytes. Wetlands Ecology and Management. 2001;9:499–510. [Google Scholar]
- URS. Phragmites control alternatives assessment report. 2005.
- USGS Great Lakes Science Center. 2012. Novel Tools for Controlling Phragmites australis. Fact sheet http://www.glsc.usgs.gov/_files/factsheets/2012-1%20Controlling%20Phragmites.pdf .
- USGS Great Lakes Science Center. 2013. Innovative Phragmites Control Strategies for the Great Lakes Restoration Initiative. Fact Sheet GLRI 67-2013.
- Uzarski DG, Burton TM, Kolar RE, Cooper MJ. The ecological impacts of fragmentation and vegetation removal in Lake Huron's coastal wetlands. Aquatic Ecosystem Health and Management. 2009;12:45–69. [Google Scholar]
- Van der Toorn J, Mook JH. The influence of environmental factors and management on stands of Phragmites australis I. Effects of burning, frost and insect damage on shoot density and shoot size. Journal of Applied Ecology. 1982;19:477–499. [Google Scholar]
- Van der Valk AG, Davis CB. A reconstruction of the recent vegetational history of a prairie marsh, Eagle Lake, Iowa, from its seed bank. Aquatic Botany. 1979;6:29–51. [Google Scholar]
- Van Driesche RG, Carruthers RI, Center T, Hoddle MS, Hough-Goldstein J, Morin L, Van Klinken RD. Classical biological control for the protection of natural ecosystems. Biological Control. 2010;54:S2–S33. [Google Scholar]
- Vasquez E, Sheley R, Svejcar T. Creating invasion resistant soils via nitrogen management. Invasive Plant Science and Management. 2008;1:304–314. [Google Scholar]
- Vizantinopoulos S, Lolos P. Persisence and leaching of the herbicide imazapry in soil. Bulletin of Environmental Contamination and Toxicology. 1994;52:404–410. doi: 10.1007/BF00197829. [DOI] [PubMed] [Google Scholar]
- Wang J, Seliskar DM, Gallagher JL, League MT. Blocking Phragmites australis reinvasion of restored marshes using plants selected from wild populations and tissue culture. Wetlands Ecology and Management. 2006;14:539–547. [Google Scholar]
- Warren RS, Fell PE, Grimsby JL, Buck EL, Rilling GC, Fertik RA. Rates, patterns, and impacts of Phragmites australis expansion and effects of experimental Phragmites control on vegetation, macroinvertebrates, and fish within tidelands of the lower Connecticut River. Estuaries and Coasts. 2001;24:90–107. [Google Scholar]
- Warren RS, Fell PE, Rozsa R, Brawley AH, Orsted AC, Olson ET, Swamy V, Niering WA. Salt marsh restoration in Connecticut: 20 years of science and management. Restoration Ecology. 2002;10:497–513. [Google Scholar]
- Weisner SEB, Granéli W. Influence of substrate conditions on the growth of Phragmites australis after a reduction in oxygen transport to below-ground parts. Aquatic Botany. 1989;35:71–80. [Google Scholar]
- Welling CH, Pederson RL, Van der Valk AG. Recruitment from the seed bank and the development of zonation of emergent vegetation during a drawdown in a prairie wetland. The Journal of Ecology. 1988a;76:483–496. [Google Scholar]
- Welling CH, Pederson RL, Van der Valk AG. Temporal patterns in recruitment from the seed bank during drawdowns in a prairie wetland. Journal of Applied Ecology. 1988b;25:999–1007. [Google Scholar]
- Whyte RS, Trexel-Kroll D, Klarer DM, Shields R, Francko DA. The invasion and spread of Phragmites australis during a period of low water in a Lake Erie coastal wetland. Journal of Coastal Research. 2008;55:111–120. [Google Scholar]
- Wijte AHBM, Gallagher JL. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. I. Different germination strategies of Spartina alterniflora and Phragmites australis (Poaceae) American Journal of Botany. 1996a;83:1337–1342. [Google Scholar]
- Wijte AHBM, Gallagher JL. Effect of oxygen availability and salinity on early life history stages of salt marsh plants. II. Early seedling development advantage of Spartina alterniflora over Phragmites australis (Poaceae) American Journal of Botany. 1996b;83:1343–1350. [Google Scholar]
- Wilcox DA. Response of wetland vegetation to the post-1986 decrease in Lake St. Clair water levels: seed-bank emergence and beginnings of the Phragmites australis invasion. Journal of Great Lakes Research. 2012;38:270–277. [Google Scholar]
- Wilcox KL, Petrie SA, Maynard LA, Meyer SW. Historical distribution and abundance of Phragmites australis at Long Point, Lake Erie, Ontario. Journal of Great Lakes Research. 2003;29:664–680. [Google Scholar]
- Willcox JD. 2013. Response of Phragmites australis to black plastic treatment. Master's Theses Paper 444 http://digitalcommons.uconn.edu/gs_theses/444 . [Google Scholar]
- Wilson SD, Moore DRJ, Keddy PA. Relationship of marsh seed banks to vegetation patterns along environmental gradients. Freshwater Biology. 1993;29:361–370. [Google Scholar]
- Zedler JB, Kercher S. Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences. 2004;23:431–452. [Google Scholar]