It may not be fair, but it's true. Women are automatically at greater risk of developing osteoporosis than men. Why are there gender differences? First, women start with lower bone mineral density (BMD) than their male peers. Heritability of skeletal traits has been demonstrated and many common single nucleotide polymorphisms (SNP) have pointed to genes accounting for variability in BMD and fracture risk (see Commentary by Nielson et al.) (1). These observations led to an investigation whether gene variation affects the female skeleton differently than it does the male. The gene-by-gender interactions could identify new signals not detected in the sex-combined analyses and indicate important gender-dependent skeletal biology. Yet, a recent report shows that no gender differences in the effects of autosomal SNPs on BMD were found in analyses of more than 50,000 men and women. (2) Although other genetic features beyond SNPs and BMD can be explored, (1) postnatal factors are important to be considered.
Indeed, women lose bone mass more quickly than men as they age. Estrogen deficiency has been regarded as the main causative factor in postmenopausal osteoporosis, which is characterized by an increase in bone turnover rate and a remodeling imbalance of bone resorption exceeding bone formation. If estrogen deficiency is the sole factor responsible for postmenopausal bone loss, the exact mechanism as why bone resorption outpaces bone formation remains unknown.
One factor that has long been overlooked is that while estrogen decreases by 90% during menopausal transition, levels of serum ferritin are increased 2 to 3-fold from premenopause to postmenopause. Based on this observation, it has been hypothesized that in addition to estrogen deficiency, increased iron as a result of menopause could contribute to bone loss in postmenopausal women. (3) It is known that individuals with pathological iron overload, such as those with hereditary hemochromatosis and β-thalassemia, have decreased BMD. Yet, one cannot rule out the possibility that the disease itself, e.g., HFE (hemochromatosis Fe) mutation but not iron overload had an effect on bone metabolisms. Although extensive animal evidence about the detrimental effects of iron on bone metabolisms emerged, (4–8) association of iron with bone loss in healthy adults has not been demonstrated until recently.
Thanks for a team of scientists in Seoul. They have used a 3-year longitudinal health promotion center-based study, which included 789 men and 940 women who were aged 40 years or older. Individuals who might have had inflammatory diseases were excluded, as inflammation has been suggested to play a role in the pathogenesis of osteopenia and osteoporosis and serum ferritin levels are known to be elevated in the inflammatory state. Serum ferritin levels and BMD of the total femur, femur neck and trochanter were measured at baseline and at follow-up in all study participants. It has been found that serum ferritin levels were positively correlated with accelerated bone loss at all three sites in both men and women in a dose-dependent manner. (9) This is a first large population study which shows an association of high body iron stores with bone loss in healthy individuals. Yet, the finding of the study poses another question about the role of iron in bone metabolisms. In spite of lower ferritin levels, the effect of body iron stores on bone loss appears more striking in postmenopausal women than in middle-aged men. Moreover, the researchers did not find a significant association between fracture risk and serum ferritin levels in men; however, in women, a dose-dependent increase in the risk of fracture was evident. Why does a lower iron level have a greater impact on the bone of postmenopausal women than that of middle-aged men? Here we looked at changes of iron with sex hormones over time. The difference in timing and pattern of changes may explain the more damaging effects of iron on bone in women and the resulting gender difference in osteoporosis incidence.
Iron tangoes with estrogen in women
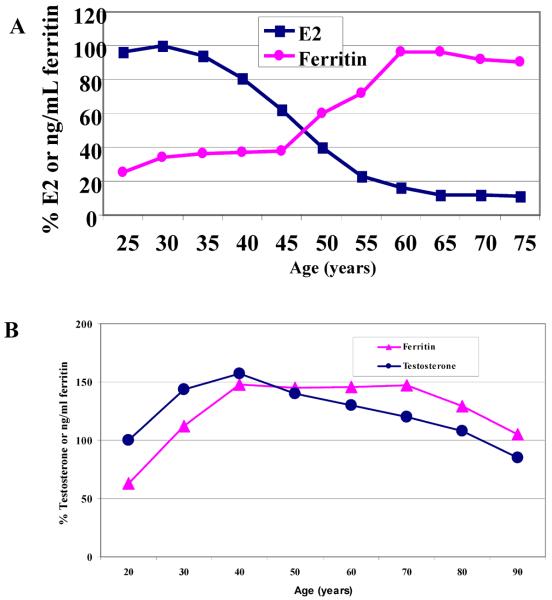
Menstruation is a unique physiological phenomenon in women, characterized by the periodic high levels of estrogen and endometrium shedding in the form of blood. As a result of this monthly blood loss, iron deficiency in young premenopausal women is highly prevalent.(10) As women get older, iron is no longer lost through menstruation, body iron level is significantly increased in postmenopausal women.(11, 12) By compiling separate large population studies on iron and estrogen, we found a concurrent but inverse change in iron and estrogen during menopausal transition (Figure 1a).(3) If they are viewed as dancing partners over the lifespan of a woman, they are mostly apart and seem doing tango dancing.
Figure 1.
Changes of iron and sex hormones over the lifespan of a woman (A) and a man (B).
Iron waltzes with androgen in men
It is known that iron also increases in men during their adolescent years. When we searched changes in iron and testosterone (T) levels in men, we found that significant differences exist. The timing and pattern of changes in sex hormones and iron levels are not the same at all. Iron levels rise in men in conjunction with the increase of T from 20 to 40 years old, then reverse between ages 40–50 years old, and gradually decrease in tandem thereafter (Figure 1b).(12, 13) If they are viewed as dancing partners, they are always in close contact and seem doing waltz dancing.
Does the difference in dancing patterns contribute to the gender difference in osteoporosis incidence?
It is well established that E2 deficiency enhances bone resorption and T increases bone mass by promoting bone formation.(14–16) According to our previous results, (7) increased iron could be a risk factor for osteoporosis by mainly inhibiting bone formation. Therefore, simultaneous increases in T and Fe could neutralize each other's effects on bone formation. In young men, the promoting effects of T slightly supersede the inhibiting effects of iron on bone and, in older men, the reverse occurs, resulting in somewhat slower bone loss. Possibly because of the presence of the two antagonizing factors, the overall incidence of osteoporosis in older men is low. In women, the gap between Fe and E2 after 45 years of age is much larger than the gap between Fe and T in men at the same age. The decreased bone formation by iron, coupled with enhanced bone resorption by E2 deficiency, could accelerate bone loss. Thus, the overall incidence of osteoporosis in older women is high. Together, all data from animal and human studies support a hypothesis that it is the combined effects of decreased sex hormones and increased iron that orchestrate bone loss in both postmenopausal women and middle-aged men.
Systemic interaction of estrogen with iron
Before testing the above hypothesis, one important question must be answered. Is increased iron a downstream effect of estrogen deficiency? If it is, estrogen replacement therapy should ease the iron loading concern. Thus, clarification of the mechanism may have important clinical implications. By examining the effects of E2 on hepcidin, a key negative regulator of iron absorption (17), it has been found that transcription of hepcidin was suppressed by E2 treatment, suggesting that hepcidin inhibition in young women by high E2 is to increase iron uptake, a mechanism to compensate iron loss during menstruation (18). This mechanism may also contribute to increased iron stores in young women who use oral contraceptives. Although more rigorous studies are needed, we could anticipate that estrogen deficiency in postmenopausal women might upregulate hepcidin, leading to lower iron uptake and lower body iron stores. Indeed, estrogen deficiency by OVX decreased serum iron levels by 37% in OVX rats compared to control rats (sham operated). (19) Together, these results suggest that estrogen deficiency could not lead to iron increase, supporting the study by Kim et al. that increased iron is an independent risk factor for accelerated bone loss in postmenopausal women.
Perspective for future research
It has been well established that estrogen deficiency mainly promotes bone resorption and testosterone deficiency inhibits bone formation. (14–16) The mode of action of iron accumulation needs to be determined precisely. Although it has been shown that iron could affect both bone resorption and bone formation, (4–8) the inhibitory effects of iron on bone formation may be detrimental in postmenopausal women. In conjunction with estrogen deficiency, it may orchestrate the underlying mechanism of bone resorption outpacing bone formation. This has to come with more evidence. Second, a large gap between iron accumulation and deficiencies in sex hormones may have coordinated an accelerated bone loss in postmenopausal women and middle-aged men. Thus, future epidemiological studies should determine whether a larger difference between iron and sex hormones may contribute to greater bone loss. By doing so, we may gain knowledge as why a lower iron level has a greater impact on the bone of postmenopausal women. Once the detrimental effect of iron on bone is confirmed, modulating iron level may present a novel therapeutic solution for osteoporosis treatment.
Footnotes
Disclosure: All authors state that they have no conflict of interest.
References
- 1.Nielson CM, Klein RF, Orwoll ES. Sex and the single nucleotide polymorphism: Exploring the genetic causes of skeletal sex differences. J Bone Miner Res. 2012;27:2047–2050. doi: 10.1002/jbmr.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu CT, Estrada K, Yerges-Armstrong LM, Amin N, Evangelou E, Li G, Minster RL, Carless MA, Kammerer CM, Oei L, Zhou Y, Alonso N, Dailiana Z, Eriksson J, Garcia-Giralt N, Giroux S, Husted LB, Khusainova RI, Koromila T, Kung AW, Lewis JR, Masi L, Mencej-Bedrac S, Nogues X, Patel MS, Prezelj J, Richards JB, Sham PC, Spector T, Vandenput L, Xiao SM, Zheng HF, Zhu K, Balcells S, Brandi ML, Frost M, Goltzman D, Gonzalez-Macias J, Karlsson M, Khusnutdinova EK, Kollia P, Langdahl BL, Ljunggren O, Lorentzon M, Marc J, Mellstrom D, Ohlsson C, Olmos JM, Ralston SH, Riancho JA, Rousseau F, Urreizti R, Van Hul W, Zarrabeitia MT, Castano-Betancourt M, Demissie S, Grundberg E, Herrera L, Kwan T, Medina-Gomez C, Pastinen T, Sigurdsson G, Thorleifsson G, Vanmeurs JB, Blangero J, Hofman A, Liu Y, Mitchell BD, O'Connell JR, Oostra BA, Rotter JI, Stefansson K, Streeten EA, Styrkarsdottir U, Thorsteinsdottir U, Tylavsky FA, Uitterlinden A, Cauley JA, Harris TB, Ioannidis JP, Psaty BM, Robbins JA, Zillikens MC, Vanduijn CM, Prince RL, Karasik D, Rivadeneira F, Kiel DP, Cupples LA, Hsu YH. Assessment of gene-by-sex interaction effect on bone mineral density. J Bone Miner Res. 2012;27:2051–2064. doi: 10.1002/jbmr.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jian J, Pelle E, Huang X. Iron and menopause: does increased iron affect the health of postmenopausal women? Antioxid Redox Signal. 2009;11:2939–2943. doi: 10.1089/ars.2009.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guggenbuhl P, Fergelot P, Doyard M, Libouban H, Roth MP, Gallois Y, Chales G, Loreal O, Chappard D. Bone status in a mouse model of genetic hemochromatosis. Osteoporos Int. 2011;22:2313–2319. doi: 10.1007/s00198-010-1456-2. [DOI] [PubMed] [Google Scholar]
- 5.Ishii KA, Fumoto T, Iwai K, Takeshita S, Ito M, Shimohata N, Aburatani H, Taketani S, Lelliott CJ, Vidal-Puig A, Ikeda K. Coordination of PGC-1beta and iron uptake in mitochondrial biogenesis and osteoclast activation. Nat Med. 2009;15:259–266. doi: 10.1038/nm.1910. [DOI] [PubMed] [Google Scholar]
- 6.Tsay J, Yang Z, Ross FP, Cunningham-Rundles S, Lin H, Coleman R, Mayer-Kuckuk P, Doty SB, Grady RW, Giardina PJ, Boskey AL, Vogiatzi MG. Bone loss caused by iron overload in a murine model: importance of oxidative stress. Blood. 2010;116:2582–2589. doi: 10.1182/blood-2009-12-260083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang Q, Jian J, Abramson SB, Huang X. Inhibitory effects of iron on bone morphogenetic protein 2-induced osteoblastogenesis. J Bone Miner Res. 2011;26:1188–1196. doi: 10.1002/jbmr.337. [DOI] [PubMed] [Google Scholar]
- 8.Zarjou A, Jeney V, Arosio P, Poli M, Zavaczki E, Balla G, Balla J. Ferritin ferroxidase activity: a potent inhibitor of osteogenesis. J Bone Miner Res. 2010;25:164–172. doi: 10.1359/jbmr.091002. [DOI] [PubMed] [Google Scholar]
- 9.Kim BJ, Ahn SH, Bae SJ, Kim EH, Lee SH, Kim HK, Choe JW, Koh JM, Kim GS. Iron overload accelerates bone loss in healthy postmenopausal women and middle-aged men: a 3-year retrospective longitudinal study. J Bone Miner Res. 2012;27:2279–2290. doi: 10.1002/jbmr.1692. [DOI] [PubMed] [Google Scholar]
- 10.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370:511–520. doi: 10.1016/S0140-6736(07)61235-5. [DOI] [PubMed] [Google Scholar]
- 11.Milman N, Kirchhoff M. Iron stores in 1359, 30- to 60-year-old Danish women: evaluation by serum ferritin and hemoglobin. Ann Hematol. 1992;64:22–27. doi: 10.1007/BF01811467. [DOI] [PubMed] [Google Scholar]
- 12.Zacharski LR, Ornstein DL, Woloshin S, Schwartz LM. Association of age, sex, and race with body iron stores in adults: analysis of NHANES III data. Am Heart J. 2000;140:98–104. doi: 10.1067/mhj.2000.106646. [DOI] [PubMed] [Google Scholar]
- 13.Milman N. Serum ferritin in Danes: studies of iron status from infancy to old age, during blood donation and pregnancy. Int J Hematol. 1996;63:103–135. doi: 10.1016/0925-5710(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 14.Riggs BL, Khosla S, Melton LJ., III . In: Estrogen, bone homeostasis, and osteoporosis. Osteoporosis R, Marcus, Feldman D, Nelson D, Rosen CJ, editors. Elsevier; New York: pp. 1011–1039. Yea. [Google Scholar]
- 15.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 16.Khosla S. The effects of androgens on osteoblast function in vitro. Mayo Clin Proc. 2000;75(Suppl):S51–54. [PubMed] [Google Scholar]
- 17.Ganz T, Nemeth E. Hepcidin and Disorders of Iron Metabolism. Annu Rev Med. 2010 doi: 10.1146/annurev-med-050109-142444. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Jian J, Katz S, Abramson SA, Huang X. Estrogen inhibits iron hormone hepcidin through an estrogen responsive element half-site ENdocrinology. 2012. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattace Raso G, Irace C, Esposito E, Maffettone C, Iacono A, Di Pascale A, Santamaria R, Colonna A, Meli R. Ovariectomy and estrogen treatment modulate iron metabolism in rat adipose tissue. Biochem Pharmacol. 2009;78:1001–1007. doi: 10.1016/j.bcp.2009.05.034. [DOI] [PubMed] [Google Scholar]