Abstract
Organisms have evolved under stable natural lighting regimes, employing cues from these to govern key ecological processes. However, the extent and density of artificial lighting within the environment has increased recently, causing widespread alteration of these regimes. Indeed, night-time electric lighting is known significantly to disrupt phenology, behaviour, and reproductive success, and thence community composition and ecosystem functioning. Until now, most attention has focussed on effects of the occurrence, timing, and spectral composition of artificial lighting. Little considered is that many types of lamp do not produce a constant stream of light but a series of pulses. This flickering light has been shown to have detrimental effects in humans and other species. Whether a species is likely to be affected will largely be determined by its visual temporal resolution, measured as the critical fusion frequency. That is the frequency at which a series of light pulses are perceived as a constant stream. Here we use the largest collation to date of critical fusion frequencies, across a broad range of taxa, to demonstrate that a significant proportion of species can detect such flicker in widely used lamps. Flickering artificial light thus has marked potential to produce ecological effects that have not previously been considered.
Introduction
For many animals perception of light reflected from objects is the primary method used to gain information about their environment, and is critical for finding food and mates and for avoiding predation. A species' visual system must therefore be well adapted to its habitat and activity patterns in terms of spectral sensitivity, detectable light levels and the temporal resolution at which it can sample its environment. It has been known for some time that an evolutionary trade off between light sensitivity and temporal resolution exists, such that photoreceptors capable of detecting low light levels cannot sample the environment as frequently as can those operating at higher light intensities. Indeed, this trade-off was first recognised by Autrum [1], who demonstrated how the eyes of fast moving diurnal insects such as honey bees and dragonflies have much higher temporal resolution than do those of slow moving nocturnal species such as crickets and stick insects. This is also true for higher vertebrates, with, for example, fish living in clear waters with high light levels tending to have higher temporal visual resolution than do those found in turbid waters [2], and diurnal sharks found in shallower waters having higher temporal resolution than species found in deeper waters [3].
Animal visual systems, including their temporal resolution capabilities, have evolved across vast geological timescales with stable light regimes, provided almost exclusively by variation in sunlight (including that reflected by the moon). It is therefore unsurprising that there has been growing concern over the environmental implications of increasing artificial light levels, which typically differ significantly from natural light, and which have characteristics not necessarily optimal for highly adapted animal eyes. These differences have been shown to have implications for animal behaviour [4], [5], reproduction and mortality [6], [7], and community composition [8]. Such studies have, however, concentrated almost exclusively on the biological effects of the intensity, duration and, to a lesser extent, spectral signature of artificial light [9]. Another characteristic of many forms of artificial light has largely been overlooked. This is that the outputs of electrical lamps often flicker. The majority of modern artificial light sources connected to an alternating current (AC) will fluctuate or flicker, although the frequency and intensity of flicker will vary with the lighting technology. Incandescent bulbs flicker at the frequency of the electrical supply (50–60 Hz), although the intensity of flicker is low (low flicker index), whereas fluorescent lighting extinguishes and returns to full brightness twice over each voltage cycle (100–120 Hz), leading to a pronounced flicker effect (high flicker index). Light emitting diode (LED) technology is also increasing in popularity particularly for street lighting and these lamps also have a high flicker index. Indeed the perceived brightness of many LED lamps is controlled by regulating the flicker frequency.
The potential for widespread biological implications of flicker as a characteristic of artificial nighttime light is suggested by studies of the effects on humans. Major concerns have been raised about the impacts on human health and wellbeing of living and working under artificial lights, both for typical behavioural patterns in developed countries (e.g. regular periods of evening/night spent under artificial light), and for more acute exposures (e.g. night-shift workers). Documented effects of flicker include headaches, visual effects, and both neurological and physiological symptoms (Table 1). In some cases these arise under flicker rates that individuals can visually perceive. In others cases however they occur at higher rates which cannot be perceived by the individual but have been demonstrated to produce detectable physiological and neurological effects. In addition, there is some evidence, in a limited number of species, that flickering lamps can have a range of effects on non-human animals (Table 2) and that these effects can be produced by flicker that may or may not be perceivable by the animal.
Table 1. Documented effects of flickering artificial lights on humans.
| Effects | Source of flicker | Frequency (Hz) | Reference |
| Headaches/Visual Effects | Low frequency fluorescent | 100 | [10] |
| Neurological Effects | Malfunctioning fluorescent | 50 | [11] |
| Neurological Effects | Amplitude-moderated flickering light | 20–75 | [12] |
| Neurological effects in photosensitive epileptics | Xenon gas discharge photo-stimulator | 3–60 | [13] |
| Physiological effects in agoraphobics | Low frequency fluorescent | 100 | [14], [15] |
| Seizures in photosensitive epileptics | Various | Various | [13] |
| Unperceived neurological effects | Light-emitting diode | Up to 200 Hz | [16] |
| Unperceived neurological effects | Computer monitor | 42.5–75 | [17] |
| Unperceived retinal effects | Various | 76–162 | [18] |
| Unperceived retinal effects | Cathode ray tube | 76 | [18] |
| Visual Effects | Low frequency fluorescent | 100 | [19] |
| Visual Effects | Cathode ray tube | 50 & 100 | [19] |
| Visual Effects | Low frequency fluorescent | 120 | [20] |
| Visual Effects | Computer monitor | 70–110 | [21] |
Table 2. Documented effects of flickering artificial lights on animals.
| Species common name | Species scientific name | Effects | Reference |
| Honeybee | Apis mellifera | Behavioural | [22] |
| Minute Pirate Bug | Orius tristicolor | Behavioural | [23] |
| White Fly | Aleyrodidae | Behavioural | [24] |
| Southern House Mosquito | Culex quinquefasciatus | Behavioural | [25] |
| Housefly | Musca domestica | Behavioural | [25] |
| Pink boll worm | Pectinophora gossypiela | Behavioural | [25] |
| House cricket | Acheta domesticcus | Behavioural | [25] |
| Housefly | Musca domestica | Behavioural | [26] |
| European Starling | Sturnus vulgaris | Physiological | [27] |
| European Starling | Sturnus vulgaris | Behavioural | [28] |
| European Starling | Sturnus vulgaris | Possible Physiological stress | [29] |
| European Starling | Sturnus vulgaris | Physiological stress & behavioral | [27] |
| European Starling | Sturnus vulgaris | Behavioural | [30] |
| European Starling | Sturnus vulgaris | Physiological stress & behavioral | [31] |
| Albino Rat | Rattus norvegicus | Physiological stress | [32] |
| Laboratory Mouse | Mus musculus | Visual | [33] |
A key measure of the temporal resolution of vision systems is the critical fusion frequency (CFF), the threshold at which an animal ceases to perceive a flickering light source as a series of flashes, but rather as a continuous stream of light. An obvious first step towards assessing the likely effects of the flickering of artificial lights on animals other than humans is to determine in which species the CFF is higher than the flicker rates of widely used electrical lamps, and hence those that will perceive the light source as flickering. In addition, we explore whether the perception of flicker is segregated taxonomically or towards animals adapted to light, dark or variable natural light environments. Here we do this, building on the extensive, but scattered, literature in which CFF values have been reported.
Materials and Methods
A literature search was conducted using ISI Web of Science, Google Scholar and Google. The ISI Web of Science search used the terms “flicker fusion frequency” and “critical flicker fusion” as topic fields spanning all years with no filters. The same terms were entered into Google Scholar and Google with no filters. Searches were conducted in September 2012. The Web of Science search produced 577 and 240 results for each term respectively. The Google Scholar search produced 4810 and 6150 results respectively whilst the Google search produced 23300 and 26400 results respectively. All Web of Science results were scanned for the use of non-human animals in the title and the resulting papers were downloaded from the University of Exeter electronic library for subsequent analysis. The first 1000 results for both search terms from Google Scholar and Google were also scanned for papers containing non-human animals in the title and these papers were then obtained either directly from the Internet or via the University of Exeter electronic library. All the resulting papers were then searched for data on the measurements of CFFs, which, when present, where added to the dataset along with the species name. Where CFFs were measured over a range of light intensities we took the mean measured value (CFFmean) under photopic conditions for use in the subsequent analysis. Where data were only presented graphically we extracted data from papers using Graph Click (Arizona Software). We also searched all papers for references to other papers reporting CFFs for non-human animals. Only data from peer-reviewed papers were used in the subsequent analysis. We also only included data from papers containing a full description of the methodology involved. CFF values are determined either by using electroretinography (ERG) to measure the electrophysiological response of the retina to flickering light of various frequencies, or by examining the spontaneous or taught behavioural response to flickering light. Using either method the CFF is taken as the frequency at which the subject ceases to respond to an increase in flicker frequency [34]. As behavioural methods often produce lower values than those of ERG, the method used to calculate CFF was included as a fixed factor in the analysis. In addition as different light sources with differing spectra characteristics may affect CFF values above and beyond the intrinsic CFFs of the species being measured we including light source (Incandescent, luminescent, gas discharge or monochromator) used to make the measurement within the analysis as a fixed factor.
It has been known for some time that animals adapted for low light environments tend to have lower CFFs than animals found in more intense light environments [3]. Hence we also included within the analysis a measure of the light intensity each species is likely to experience in natural situations. All diurnal animals were considered to be exposed to high light levels. Crepuscular and cathemeral animals were considered to be exposed to a variable light regime. Nocturnal and aquatic species inhabiting deep waters were considered as being exposed to low light levels.
To explore differences in CFF between taxonomic classes, natural lighting regimes and CCF calculation method we used linear mixed effects models (Gaussian error structure) fitted with the R (v3.0.2) language and environment [35], using the package ‘lme4’ [36]. Within the models CFFmean was the dependent variable, taxonomic class, light level exposure, light source type and CCF calculation method were fixed factors, and species was a random factor (intercept only) to account for multiple data for some species. As the purpose of this study was only to identify groups of animals most likely to be affected by flicker we did not include a phylogenetic component to the model. To evaluate the variance explained by the model we calculated R2GLMM(m), the marginal R2 which describes the variance explained by the fixed factors and R2GLMM(c), the conditional R2 which is concerned with the variance explained by both the fixed and random factors [37]. Additional metrics of model fit were also calculated, these being the pseudo R2(N) of the models using the methods of Nagelkerke [38] and the % deviance explained by the fixed effects component of the model. This was achieved by comparing the deviance to a null (intercept only) model such that % deviance = (deviancenull–deviancemodel)/deviancenull. Package ‘MuMIn’ [39] was used for R2 calculations. Package ‘lmerTest’ [40] was used to calculate F and p values using Satterthwaite [41] approximations to determine denominator degrees of freedom.
Results
We located 56 studies containing 93 measurements (19 behavioural & 74 using ERG) of CFFmean for 81 species from 66 genera and 9 classes (Table 3, Table S1). We found a considerable range in CFFmean values amongst species, with the lowest value of 6.7 Hz being obtained for the cane toad (Bufo marinus), and the highest value of 400 Hz for the black fire beetle (Melanophila acuminate) (Fig. 1A). In a considerable number of cases (16.1% of measurements, 13.5% of species, 16.6% of genera and 44.4% of classes), CFFmean values were above the flicker frequency of fluorescent lamps on a 50 Hz electrical supply; i.e. 100 Hz. It should be noted that a number of countries utilise a 60 Hz electrical supply and hence the flicker frequency with be higher at 120 Hz.
Table 3. Species for which critical fusion frequencies have been measured.
| Species common name | Species scientific name | Genus | Class | Light levels | Method | Mean CFF | Reference |
| Cane toad | Bufo marinus | Bufo | Amphibia | Low | ERG | 6.7 | [42] |
| Wolf spider | Lycosa baltimoriana | Lycosa | Arachnida | Low | ERG | 10.0 | [43] |
| Isopod | Glyptonotus antarcticus | Glyptonoyus | Malacostraca | Low | ERG | 11.0 | [44] |
| European eel | Anguilla anguilla | Anguilla | Actinopterygii | Low | Behavioural | 14.0 | [45] |
| Pandalid shrimp | Plesionika rossignoli | Plesionika | Malacostraca | Low | ERG | 14.0 | [46] |
| Little skate | Leucoraja erinacea | Leucoraja | Chondrichthyes | Low | ERG | 17.5 | [47] |
| Oplophorid shrimp | Acanthephyra purpurea | Acanthephyra | Malacostraca | Low | ERG | 18.0 | [46] |
| Blacknosed shark | Carcharhinus acronotus | Carcharhinus | Chondrichthyes | Variable | ERG | 18.0 | [3] |
| Penaeid shrimp | Funchalia villosa | Funchalia | Malacostraca | Low | ERG | 21.0 | [45] |
| Glass shrimp | Pasiphaea multidentata | Pasiphaea | Malacostraca | Low | ERG | 21.0 | [46] |
| Green frog | Rana clamitans | Rana | Amphibia | Low | ERG | 21.0 | [48] |
| Sergestid shrimp | Sergestes arcticus | Sergestes | Malacostraca | Low | ERG | 21.0 | [46] |
| Oplophorid shrimp | Systellaspis debilis | Systellaspis | Malacostraca | Low | ERG | 21.0 | [49] |
| Oplophorid shrimp | Systellaspis debilis | Systellaspis | Malacostraca | Low | ERG | 21.0 | [45] |
| Swordfish | Xiphias gladius | Xiphias | Actinopterygii | High | ERG | 22.0 | [50] |
| Harp seal | Pagophilus groenlandicus | Pagophilus | Mammalia | High | Behavioural | 22.5 | [51] |
| Oplophorid shrimp | Janicella spinicauda | Janicella | Malacostraca | Low | ERG | 23.0 | [46] |
| Euphausiid shrimp | Meganyctiphanes norvegica | Meganyctiphanes | Malacostraca | Low | ERG | 23.0 | [45] |
| Euphausiid shrimp | Euphausia gibboides | Euphausia | Malacostraca | Low | ERG | 24.0 | [46] |
| Penaeid shrimp | Funchalia villosa | Funchalia | Malacostraca | Low | ERG | 24.0 | [49] |
| Brown rat | Rattus norvegicus | Rattus | Mammalia | Low | ERG | 25.0 | [52] |
| Sergestid shrimp | Sergia filictum | Sergia | Malacostraca | Low | ERG | 25.0 | [49] |
| Anole lizard | Anolis neckeri | Anolis | Reptilia | High | ERG | 26.1 | [53] |
| Hammerhead shark | Sphyrna lewini | Sphyrna | Chondrichthyes | Variable | ERG | 27.0 | [3] |
| Euphausiid shrimp | Nematoscelis megalops | Nematoscelis | Malacostraca | Low | ERG | 28.0 | [46] |
| Anole lizard | Anolis limifrons | Anolis | Reptilia | High | ERG | 28.5 | [53] |
| Tiger salamander | Ambystoma tigrinum | Ambystoma | Amphibia | High | ERG | 30.0 | [54] |
| Oplophorid shrimp | Janicella spinacaud | Janicella | Malacostraca | Low | ERG | 31.0 | [49] |
| Bonnethead shark | Sphyrna tiburo | Sphyrna | Chondrichthyes | Variable | ERG | 31.0 | [3] |
| Oplophorid shrimp | Janicella spinacauda | Jancella | Malacostraca | Low | ERG | 32.0 | [49] |
| Oplophorid shrimp | Oplophorus gracilirostris | Oplophorus | Malacostraca | Low | ERG | 32.0 | [49] |
| Euphausiid shrimp | Nematobrachion boopis | Nematobrachion | Malacostraca | Low | ERG | 33.0 | [46] |
| Anole lizard | Anolis valencienni | Anolis | Reptilia | High | ERG | 33.4 | [53] |
| Euphausiid shrimp | Stylocheiron maximum | Stylocheiron | Malacostraca | Low | ERG | 34.0 | [46] |
| Anole lizard | Anolis sagrei | Anolis | Reptilia | High | ERG | 34.4 | [53] |
| Anole lizard | Anolis carolinensis | Anolis | Reptilia | High | ERG | 34.6 | [53] |
| Anole lizard | Anolis grahami | Anolis | Reptilia | High | ERG | 34.7 | [53] |
| Euphausiid shrimp | Nematobrachion sexspinosus | Nematobrachion | Malacostraca | Low | ERG | 36.0 | [46] |
| Euphausiid shrimp | Stylocheiron maximus | Stylocheiron | Malacostraca | Low | ERG | 36.0 | [49] |
| Lemon shark | Negaprion brevirostris | Negaprion | Chondrichthyes | Variable | ERG | 37.0 | [55] |
| Japanese rice fish | Oryzias latipes | Oryzias | Actinopterygii | Variable | ERG | 37.2 | [56] |
| Great horned owl | Bubo virginianus | Bubo | Aves | Low | ERG | 40.0 | [57] |
| Jumping spider | Maevia inclemens | Maevia | Arachnida | High | ERG | 40.0 | [58] |
| Anole lizard | Anolis auratus | Anolis | Reptilia | High | ERG | 41.5 | [53] |
| American cockroach | Periplaneta americana | Priplaneta | Insecta | Low | ERG | 42.5 | [59] |
| Green swordtail | Xiphophorus helleri | Xiphophorus | Actinopterygii | Variable | ERG | 43.0 | [60] |
| Euphausiid shrimp | Nematobranchion flexipes | Nematobranchion | Malacostraca | Low | ERG | 44.0 | [49] |
| Siamese fighting fish | Betta splendens | Betta | Actinopterygii | Variable | Behavioural | 45.1 | [61] |
| Domestic cat | Felis domesticus | Felis | Mammalia | High | ERG | 47.5 | [62] |
| Anole lizard | Anolis gundlachi | Anolis | Reptilia | High | ERG | 50.0 | [63] |
| Little owl | Athene noctua | Athene | Aves | Low | ERG | 50.0 | [64] |
| American crayfish | Cambarus spp | Cambarus | Malacostraca | Low | ERG | 53.0 | [65] |
| Hermit crabs | Pagurus spp | Pagursu | Malacostraca | Variable | ERG | 53.0 | [65] |
| Human | Homo sapiens | Homo | Mammalia | High | ERG | 55.0 | [66] |
| Decapod | Jasus edwardsii | Jasus | Malacostraca | Low | ERG | 55.0 | [67] |
| Tuatara | Sphenodon punctatus | Spenodon | Reptilia | Low | Behavioural | 55.4 | [68] |
| Tuatara | Sphenodon punctatus | Spenodon | Reptilia | Low | Behavioural | 55.4 | [69] |
| Euphausiid shrimp | Nematobranchion sexspinosus | Nematobranchion | Malacostraca | Low | ERG | 56.0 | [49] |
| Anole | Anolis cristatellus | Anolis | Reptilia | High | ERG | 58.5 | [63] |
| Human | Homo sapiens | Homo | Mammalia | High | Behavioural | 60.0 | [70] |
| Human | Homo sapiens | Homo | Mammalia | High | Behavioural | 60.0 | [71] |
| Tree shrew | Tupaia belangeri | Tupaia | Mammalia | High | Behavioural | 60.0 | [72] |
| Rhesus macaque | Macaca mulatta | Macaca | Mammalia | High | Behavioural | 61.0 | [73] |
| Anole lizard | Anolis pulchellus | Anolis | Reptilia | High | ERG | 63.0 | [63] |
| Domestic chicken | Gallus domesticus | Gallus | Aves | High | Behavioural | 63.5 | [74] |
| Migratory locust | Locusta migratoria | Locusta | Insecta | High | ERG | 65.0 | [59] |
| American red squirrel | Tamiasciurus hudsonicus | Tamiasciurus | Mammalia | High | ERG | 65.0 | [75] |
| Threespined stickleback | Gasterosteus aculeatus | Gasterosteus | Actinopterygii | Variable | Behavioural | 67.0 | [76] |
| Guppy | Poecilia reticulata | Poecilia | Actinopterygii | High | Behavioural | 67.0 | [76] |
| Short-eared owl | Asio fammeus | Asio | Aves | Variable | ERG | 67.5 | [77] |
| Chinese tussah moth | Antheraea pernyi | Antheraea | Insecta | Low | ERG | 70.0 | [59] |
| Domestic chicken | Gallus domesticus | Gallus | Aves | High | Behavioural | 71.5 | [78] |
| Salmon | Salmo salar | Salmo | Actinopterygii | Variable | ERG | 72.0 | [79] |
| Domestic dog | Canis familiaris | Canis | Mammalia | High | Behavioural | 75.0 | [80] |
| Emperor moth | Saturnia pavonia | Saturnia | Insecta | Low | ERG | 75.0 | [59] |
| Rock Pigeon | Columba livia | Columba | Aves | High | Behavioural | 77.0 | [81] |
| Fruit fly | Drosophila hydei | Drosophila | Insecta | High | ERG | 80.0 | [59] |
| Domestic Chicken | Gallus domesticus | Gallus | Aves | High | Behavioural | 87.0 | [82] |
| Rhesus Monkey | Macaca mulatta | Macaca | Mammalia | High | ERG | 95.0 | [83] |
| Rock Pigeon | Columba livia | Columba | Aves | High | ERG | 100.0 | [77] |
| Starling * | Sturnus vulgaris | Sturnus | Aves | High | ERG | 100.0 | [84] |
| Domestic Chicken | Gallus domesticus | Gallus | Aves | High | ERG | 104.0 | [85] |
| Domestic Chicken | Gallus domesticus | Gallus | Aves | High | Behavioural | 105.0 | [86] |
| Yellow-pine Chipmunk | Neotamias amoenus | Neotamis | Mammalia | High | ERG | 108.0 | [75] |
| Ground squirrel | Spermophilus laterali | Citellus | Mammalia | High | ERG | 108.0 | [75] |
| Rock louse | Ligia occidentalis | Ligia | Malacostraca | High | ERG | 120.0 | [87] |
| Rock Pigeon | Columba livia | Columba | Aves | High | ERG | 143.0 | [88] |
| Tsetse fly | Glossina morsitans | Glossina | Insecta | High | ERG | 145.0 | [59] |
| Honeybee | Apis mellifera | Apis | Insecta | High | Behavioural | 200.0 | [89] |
| Honeybee | Apis mellifera | Apis | Insecta | High | Behavioural | 200.0 | [90] |
| Dragonflies | Anisoptera | Anisoptera | Insecta | High | ERG | 240.0 | [91] |
| Honeybee | Apis mellifera | Apis | Insecta | High | ERG | 240.0 | [92] |
| Blow-fly | Calliphoridae | Calliphora | Insecta | High | ERG | 240.0 | [1] |
| Black Fire Beetle | Melanophila acuminata | Melanophila | Insecta | High | ERG | 400.0 | [93] |
*Indirect evidence suggesting CFF<100 Hz.
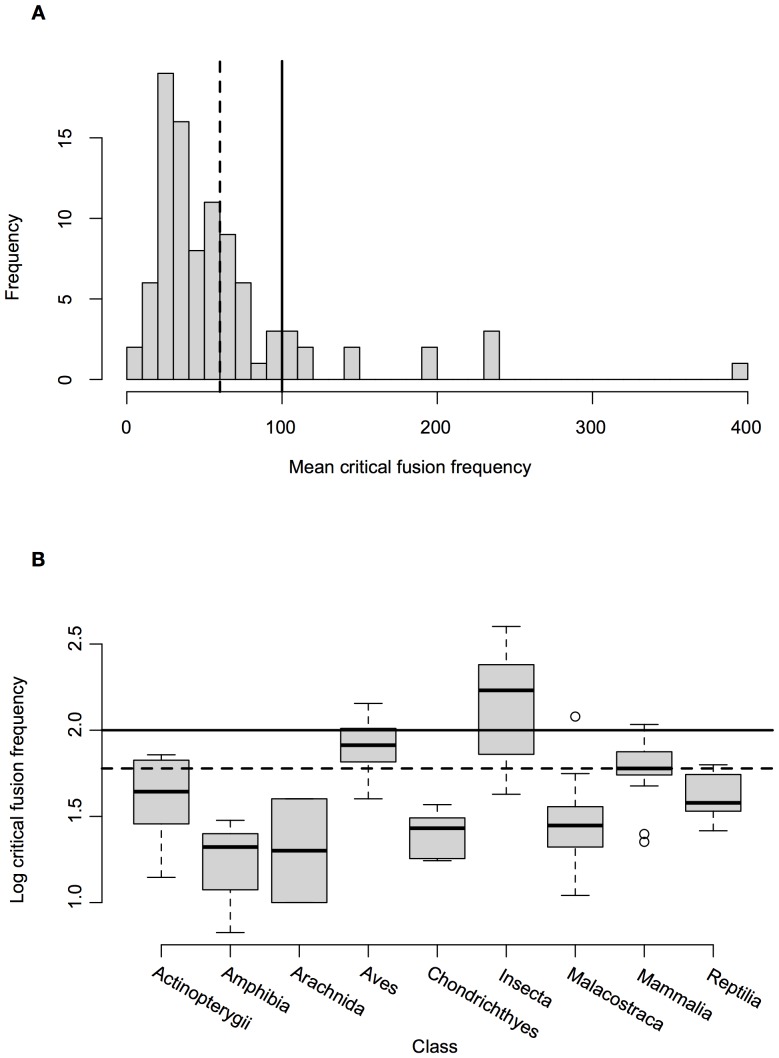
Figure 1. Distribution of critical fusion frequencies.
(a) Histogram of the mean critical fusion frequency for all taxa; and (b) Boxplot of log10 critical fusion frequency (CFF) by class. The solid line indicates the flicker frequency of lamps on a 50 Hz electrical supply. The dotted line indicates mean CFF for humans. CFFs for Insecta are significantly higher and Amphibia significantly lower than for other classes.
The mixed effects model explained a substantial proportion of the variation in CFFmean (R2GLMM(m) = 0.70, R2GLMM(c) = 0.94, R2(N) = 0.76, % deviance = 0.55) and highlighted significant differences between the taxonomic classes (F[8,81] = 9.47, p<0.0001; Fig 1B) and between levels of light exposure (F[2,80] = 17.63, p<0.0001) but no differences were found between the methods used to calculate CFF (F[2,22] = 1.10, p = 0.30) and the lighting source used (F[2,40] = 0.54, p = 0.70, data were available for 71 of the 93 studies). To further ensure that these differences were not related to the methodology used to obtain the CFF measurements we conducted the analysis on a subset of data where CFF values had been measured only by ERG, which retained significant differences between taxonomic classes (F[8,71] = 14.37, p<0.0001) and levels of light exposure (F[2,71] = 25.15, p<0.0001).
Nocturnal animals had the lowest CFF values (mean = 32.09, SD = 16.73) compared to animals exposed to variable lighting regimes (mean = 45.25, SD = 17.77) and diurnal animals (mean = 92.21, SD = 75.72), which had significantly higher CFF values.
The lowest CFF values were found within the amphibians (mean = 19.23, SD = 11.75), followed by arachnids (mean = 25,SD = 21.21), Chondrichthyes (mean = 26.1, SD = 8.41), and malacostracans (mean = 33.7,SD = 21.26), although one intertidal species, the tock louse (Ligia occidentalis), had a CFFmean of 120 Hz. All the reptiles (mean = 42.94, SD = 12.79) and actinopterygians (mean = 45.9,SD = 21.53) had CFF values below 100 Hz, as did most mammals (mean = 64.77, SD = 26.77) with the exception of two species, the yellow-pine chipmunk (Neotamias amoenus) and the golden-mantled ground squirrel (Spermophilus laterali) which both had CFFmean values of 108 Hz. Birds (mean = 82.23, SD = 28.97) had the second highest CFFmean, although two of the six species of birds within the dataset were nocturnal owls. When the diurnal species were considered independently CFFmean rose to 93.38, with domestic chickens (Gallus domesticus) (one out of five measurements), pigeons (Columba livia) (two out of three measurements), and the common starling (Sturnus vulgaris) all having CFFmean values above 100 Hz. Insects (mean = 166.46, SD = 106.29) had by far the highest CFFmean values, although there was a distinct difference between nocturnal (mean = 70) and diurnal species (mean = 201.1), a pattern first noted by Autrum [1]. Most diurnal insects, including dragonflies Anisoptera spp., honeybees (Apis mellifera), blow-flies Calliphoridae spp., tsetse flies (Glossina morsitans) and black fire beetles had CFF values greater than 100 Hz, apart from the fruit fly (Drosophila hyde)i and migratory locust (Locusta migratoria).
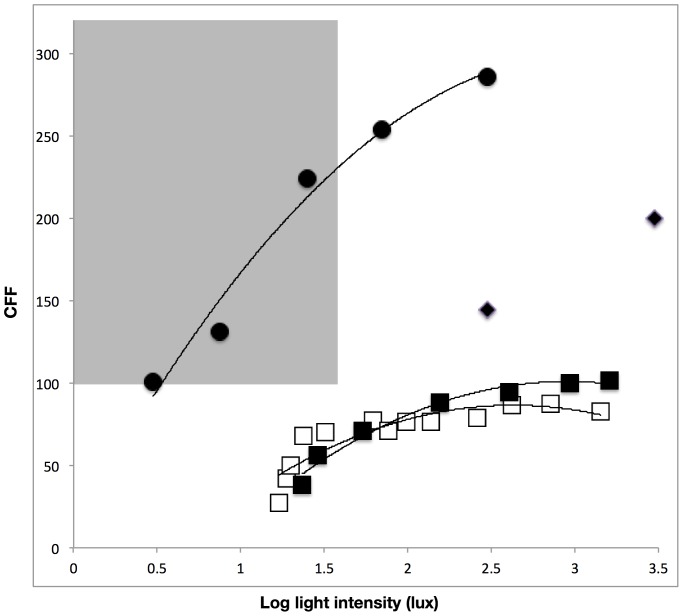
A subset of the dataset of those species with CFFs of over 100 Hz also measured CFFs over a range of light intensities, allowing us to estimate the minimal light intensity required to produce such values. Tsetse flies produced a CFF of 286 at 300 lux, which dropped to 101 Hz at 3 lux. For domestic chickens, CFFs of greater than 100 Hz can be achieved at around 250 cd/m2 (approximately 150 lux) [85]. Srinivasan & Lehrer [89] measured CFF for honeybees at two light intensities, 3000 lux, producing a CFF of 200 Hz, and 300 lux, producing a value of 144 Hz. Interpolating from these data we predict a light level of around 138 lux would be needed to produce a CFF of 100 Hz.
Discussion
Our results clearly demonstrate how a significant proportion of animals, particularly fast moving diurnal birds and insects have the potential to perceive the flicker of electric lamps, which has been demonstrated to have detrimental effects on both human and non-human species. When we also consider that in the case of humans, flicker can produce symptoms when it cannot be perceived (but can invoke measurable physiological changes [16], [17], [18]) we suggest that, in addition, any species with a CFF of 60 Hz (as in humans) or higher, including many other mammals, and some crustaceans, reptiles and fish, have the potential to be affected by flicker.
This said, the perception of flicker is likely to be limited in many natural situations for a number of reasons. First, all animals able to detect artificial flicker are naturally diurnal hence only species for which artificial light serves effectively to increase day length, or which are facultatively nocturnal, are likely to be affected [9]. Second, CFF decreases with light intensity, which itself decays with distance from the light source. Hence lamps need to produce light that, once it reaches the animal, is still sufficiently intense to induce CFFs that are high enough for animals to perceive the flicker. For example, CFFs in Tsetse flies remain relatively high at low light intensities, such that individuals will still be able to perceive flickering street lamps at 3 lux, which is approximately the lowest intensity light found at ground level between neighbouring metal halide lamps [94]. Whereas in honeybees and domestic chickens a much greater light intensity is required, such that, based on the output from a typical metal halide street lamp (Phillips Cosmopolis 80 W, measured by JB), a subject would need to be closer than three metres to the light source in order to perceive the flicker.
The evidence presented here suggests that impacts from the perception of the flicker of artificial light may be rather limited for most species studied thus far. There remains, however, a scarcity of data on CFFs for many taxa that are most likely to be affected, hence we suggest that the potential ecological effects should not be wholly dismissed. Considering the case of insects, CFFs have only been characterised for nine diurnal and three nocturnal species, producing a wide range of values (42.5–400 Hz). Given the vital role insects play in ecosystem functioning (e.g. pollination, decomposition), and as nutrition for other organisms, this depauperate knowledge base should give cause for concern. Those species for which we have data suggest that a wide range of insects have the potential to be affected by flicker and as such should be a key target for further research. To the best of our knowledge, quantification of CFFs has only been carried out in five species of birds (although CFFs of greater than 100 Hz have been inferred for starlings), with considerable variation within the range of values being reported (40–143 Hz). More importantly, we only have direct measurement of CFFs for two diurnal species. One of these is the domestic chicken, whose wild counterparts are weakly flying, mostly ground dwelling birds, adapted to living under the forest canopy which are unlikely to move rapidly enough to have adapted a particularly high temporal sensitivity. The only other diurnal species of bird for which CFFs have been quantified is the pigeon, a very common urban bird that is likely to encounter high levels of artificial light. Pigeons have the highest CFF values recorded (143 Hz) of any vertebrate species and will easily be able to perceive flicker from artificial lights. From an evolutionary perspective, it is likely that other birds capable of rapid flight have high CFFs, and are able to detect flickering artificial light.
For both birds and insects there is substantial variation in the range of CFFs recorded. A component of this variation may be explained by the method by which CFF measurements were made, as behavioural mechanisms are generally considered to produce lower values than those obtained via ERG, because of post-retina neurological processing [34], though this study found no effect of experimental method. There remains however, considerable variation within measurements for each method, suggesting other causes. Understanding of what drives variation in temporal sensitivity within and between taxa remains poor, beyond the fact that CFFs are related to the light levels to which animals are exposed and tend to scale with metabolic rate and body size [95]. At the level of the individual a number of factors have been found to influence CFF, most importantly light intensity, with CFF increasing linearly with the logarithm of light intensity (within certain limits) for humans [96], [97]. The shape of this relationship and how it varies within and between taxa are key to understanding the potential impacts of flicker from artificial light, but have only been determined for a limited number of non-human species [82]. For example, tsetse flies will be able to perceive flicker within a range of light intensities likely to be produced by artificial lighting sources within the environment (Fig. 2), where as for domestic chickens their ability to perceive flicker is at the very limits of their visual capabilities. As such chickens will only be able to perceive flicker at light intensities exceeding the range likely to be produced by artificial lamps.
Figure 2. CFF responses to light intensity.
How CFF decreases with light intensities for different species. Squares indicate data collected from domestic chickens, with empty squares being from behavioural measurements (y = −21.5x2+113.1x−62.6, R2 = 0.8, data from Linsey et al. 2011) and filled squares from ERG measurements (y = −22.4x2+132.2x−94.1, R2 = 0.97, data from [82]). Solid circles indicate data collected from tsetse flies (y = −30.6x2+118.4x−8.9, R2 = 0.98, data from [59]). Two data points were available for honey bees, taken from [89]; shown as filled diamonds). Shaded area highlights the parameter space within which flicker from 50 Hz supply lamps will be perceptible under light intensities commonly produced by artificial lamps within the environments (0–40 lux [9], [94]).
Our results suggest that diurnal species are most likely to be affected by flicker as nocturnal species studied up until now have CFFs too low to perceive flicker, and many nocturnal species tend to shun artificially lit areas. There remains however a number of nocturnal animals groups likely to be affected by flickering lights, these being animals which move rapidly in light environments. This presents a particular challenge for visual systems, as generally there is a trade-off between detectable light levels and the temporal resolution at which they can sample the environment. Potential groups prone to being affected by flicker include, but are not limited to, nocturnal flying insects, for example hawkmoths, nocturnal flying birds (including some Charadriiformes, Caprimulgiformes and Strigiformes) and bats. Whilst bats primary mechanism of navigation is via echolocation, there exists increasing evidence that many species rely to a varying extent on vision [98]. In addition, it is becoming increasingly apparent that some species of bats often utilise artificially lit areas as they act to concentrate their food resources, which often aggregate around the lamps [99], [100]. Given these factors it seems prudent further to investigate the visual systems of these groups in order to identify those with the potential to be affected by the flickering of artificial lamps.
Explicit studies of the biological effects of light flicker on animals remain scarce, and have focused on the efficacy of trapping devices for insects and on welfare issues for captive vertebrates. Nonetheless, those that have been conducted provide further support for a potentially widespread impact (Table 2), as flicker has been found to influence behavioural and movement patterns, visual systems and levels of stress.
A wide range of taxa are likely to be able to detect flicker, and it has been found to produce detrimental effects in both human and non-human animals. We suggest that flickering from electric lamps represents a potential environmental impact from artificial light pollution which has not previously been considered. Given the precautionary principle and that it is likely that in the future an increasing level of artificial light will come from lamps with a high flicker index, as incandescent bulbs are phased out, and replaced with fluorescent (including compact fluorescent bulbs, generally referred to as ‘low energy bulbs’) and LED lamps, we suggest the impacts of flickering light on natural systems are given urgent attention.
Supporting Information
Species for which critical fusion frequencies have been measured. Includes the light source type used in the measurement of CFF values. Species are ordered by class.
(XLSX)
Acknowledgments
We greatly appreciate the input from two anonymous reviewers whose insights have greatly improved this manuscript.
Funding Statement
The research leading to this paper received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007–2013)/ERC grant agreement no. 268504 to K.J.G. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Autrum H (1950) Die Belichtungspotentiale und das Salen der Insekten. Z. Vergleich. Physiol 32: 176–227. [PubMed] [Google Scholar]
- 2.Ali MA, Klyne MA (1985) Vision in Vertebrates. New York: Plenum Press. [Google Scholar]
- 3. McComb DM, Tamara DM, Frank TM, Hueter RE, Kajiura SM (2010) Temporal resolution and spectral sensitivity of the visual system of three coastal shark species from different light environments. Physio Biochem Zool 83: 299–307. [DOI] [PubMed] [Google Scholar]
- 4. Bird BL, Branch LC, Miller DL (2004) Effects of coastal lighting on foraging behavior of beach mice. Conserv Biol 18: 1435–1439. [Google Scholar]
- 5. Buchanan BW (1993) Effects of enhanced lighting on the behaviour of nocturnal frogs. Anim Behav 45: 893–899. [Google Scholar]
- 6. Kempenaers B, Borgström P, Loës P, Schlicht E, Valcu M (2010) Artificial night lighting affects dawn song, extra-pair siring success, and lay date in songbirds. Curr Biol 20: 1735–1739. [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez A, Rodriguez B, Curbelo ÁJ, Pérez A, Marrero S, et al. (2012) Factors affecting mortality of shearwaters stranded by light pollution. Anim Conserv 15: 519–526. [Google Scholar]
- 8. Davies TW, Bennie J, Gaston KG (2012) Street lighting changes the composition of invertebrate communities. Biol Lett 8: 764–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gaston KJ, Bennie J, Davies TW, Hopkins J (2013) The ecological impacts of nighttime light pollution: a mechanistic appraisal. Biol Rev 88: 912–927. [DOI] [PubMed] [Google Scholar]
- 10. Wilkins AJ, Nimmo-Smith IM, Slater A, Bedocs L (1989) Fluorescent lighting, headaches and eye-strain. Lighting Res Technol 21: 11–18. [Google Scholar]
- 11. Binnie CD, de Korte RA, Wisman T (1979) Fluorescent lighting and epilepsy. Epilepsia 20: 725–727. [DOI] [PubMed] [Google Scholar]
- 12. Sandström M, Lyskov E, Berglund A, Medvedev S, Mild KH (1997) Neurophysiological effects of flickering light in patients with perceived electrical hypersensitivity. J Occup Environ Med 39: 15–22. [DOI] [PubMed] [Google Scholar]
- 13.Harding GFA, Jeavons P (1994) Photosensitive epilepsy. London: Mac Keith Press. [Google Scholar]
- 14. Hazell J, Wilkins AJ (1990) A contribution of fluorescent lighting to agoraphobia. Psychol Med 20: 591–596. [DOI] [PubMed] [Google Scholar]
- 15. Watts FN, Wilkins AJ (1989) The role of provocative visual stimuli in agoraphobia. Psychol Med 19: 875–885. [DOI] [PubMed] [Google Scholar]
- 16. Burns SA, Elsner AE, Kreitz MR (1992) Analysis of nonlinearities in the flicker ERG. Optom Vis Sci 69: 95–105. [DOI] [PubMed] [Google Scholar]
- 17. Lu S, Cai Y, Shen M, Zhou Y, Han S (2012) Alerting and orienting of attention without visual awareness. Conscious Cogn 21: 928–938. [DOI] [PubMed] [Google Scholar]
- 18. Berman SM, Greenhouse DS, Bailey IL, Clear R, Raasch TW (1991) Human electroretinogram responses to video displays, fluorescent lighting and other high frequency sources. Optometry Vision Sci 68: 645–662. [DOI] [PubMed] [Google Scholar]
- 19. Wilkins AJ (1986) Intermittent illumination from visual display units and fluorescent lighting affects movements of the eyes across text. Hum Factors 28: 75–81. [DOI] [PubMed] [Google Scholar]
- 20. Veitch JA, McColl SL (1995) Modulation of fluorescent light: flicker rate and light source effects on visual performance and visual comfort. Lighting Res Tech 27: 243–256. [Google Scholar]
- 21.Kennedy A, Brysbaert M, Murray WS (1998) The effects of intermittent illumination on a visual inspection task. Q. J. Exp. Psychol. A 51, 135–51. [DOI] [PubMed]
- 22. Renner M (1957) Neue versuche über den zeitsinn der honigbiene. Z Vgl Physiol 40: 85–118. [Google Scholar]
- 23. Shields EJ (1980) Locomotory activity of Orius tristicolor under various intensities of flickering and non-flickering light. Ann Entomol Soc Am 73: 74–77. [Google Scholar]
- 24. Chu CC, Chen TY, Henneberry TJ (2004) Adult whiteflies (Homoptera: Aleyrodidae), and whitefly parasitoids (Hymenoptera: Aphelinidae) response to cool white fluorescent light powered by alternating or direct current. Southwest Entomol 29: 111–116. [Google Scholar]
- 25. Chu C, Chen T, Henneberry TJ (2006) Attractiveness of flickering and non-flickering cool white fluorescent light to Culex quinquefasciatus, Musca domestica and Pectinophora gossypiela adults, and Acheta domesticus and Periplaneta americana nymphs. Southwest Entomol 31: 77–81. [Google Scholar]
- 26. Syms PR, Goodman LJ (1987) The effect of flickering U-V light output on the attractiveness of an insect electrocutor trap to the house-fly Musca domestica . Entomol Exp Appl 43: 81–85. [Google Scholar]
- 27. Smith EL, Evans JEE, Parraga CA (2005) Myoclonus induced by cathode ray tube screens and low-frequency lighting in the European starling (Sturnus vulgaris), The Vet Rec. 157: 148–150. [DOI] [PubMed] [Google Scholar]
- 28. Greenwood VJ, Smith EL, Goldsmith AR, Cuthill IC, Crisp LH, et al. (2004) Does the flicker frequency of fluorescent lighting affect the welfare of captive European starlings? Appl Anim Behav Sci 86: 145–159. [Google Scholar]
- 29. Maddocks SA, Goldsmith AR, Cuthill IC (2001) The influence of flicker rate on plasma corticosterone levels of European starlings, Sturnus vulgaris . Gen Comp Endocr 124: 315–320. [DOI] [PubMed] [Google Scholar]
- 30. Evans JE, Cuthill IC, Bennett ATD (2006) The effect of flicker from fluorescent light on mate choice in captive birds. Anim Behav 72: 393–400. [Google Scholar]
- 31. Evans JE, Smith EL, Bennett ATD, Cuthill IC, Buchanan KL (2012) Short-term physiological and behavioural effects of high- versus low-frequency fluorescent light on captive birds. Anim Behav 83: 25–33. [Google Scholar]
- 32. Lalitha R, Suthanthirarajan N, Namasivayam A (1988) Effect of flickering light stress on certain biochemical parameters in rats. Indian J. Physiol Pharmacol 32: 182–6. [PubMed] [Google Scholar]
- 33. Yu Y, Chen H, Tuo J, Zhu Y (2011) Effects of flickering light on refraction and changes in eye axial length of C57BL/6 mice. Ophthal Res 46: 80–87. [DOI] [PubMed] [Google Scholar]
- 34. D'Earth RB (1998) Can video images imitate real stimuli in animal behaviour experiments? Biol Rev 73: 267–292. [Google Scholar]
- 35.R Core Team (2012) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0, URL http://www.R-project.org/.
- 36.Bates D, Bolker B, Maechler M, Walker S (2013) Lme4: linear mixed-effect models using Eigen and S4. R package, version 1.0-4. http://cran.r-project.org/web/packages/lme4/index.html.
- 37. Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods in Ecol and Evol 4: 133–142. [Google Scholar]
- 38. Nagelkerke NJD (1991) A note on a general definition of the coefficient of determination. Biometrika 3: 691–2. [Google Scholar]
- 39.Bartoń K (2011) Model selection and model averaging based on information criteria (AICc and alike). R package, version 1.9.5. Available: http://cran.r-project.org/web/packages/MuMIn/index.html.
- 40.Kuznetsova A, Brockhoff PB, Christensen RHB (2013) lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package). R package, version, 2.0-0. Available: http://cran.r-project.org/web/packages/lmerTest/index.html.
- 41. Satterthwaite FE (1946) An approximate distribution of estimates of variance components, Biometrics Bulletin. 2: 110–114. [PubMed] [Google Scholar]
- 42. Nowak LM, Green DG (1983) Flicker fusion characteristics of rod receptors in the toad. Vis Res 23: 845–849. [DOI] [PubMed] [Google Scholar]
- 43. Devoe RD (1963) Linear relations between stimulus amplitudes and amplitudes of retinal action potentials from the eye of the wolf spider. J Gen Physiol 47: 13–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer-Rochow VB, Laughlin SB (1997) Intracellular recordings from photoreceptors of the Antarctic isopod Glyptonotus antarcticus. In: 3rd Int Congr Physiol Sci (St Petersburg).
- 45. Adrian ED, Matthews R (1928) The action of light on the eye: Part III. The interaction of retinal neurons. J Physiol 65: 273–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Frank TM (2003) Effects of Light adaptation on the temporal resolution of deep-sea crustaceans. Integr Comp Biol 43: 559–570. [DOI] [PubMed] [Google Scholar]
- 47. Green DG, Siegel IM (1975) Double branched flicker fusion curves from the all-rod skate retina. Science 188: 1120–1122. [DOI] [PubMed] [Google Scholar]
- 48. Granit R, Riddell HA (1934) The electrical responses of light- and dark-adapted frog's eyes to rhythmic and continuous stimuli,. J. Physiol 81: 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Frank TM (1999) Comparative study of temporal resolution in the visual systems of mesopelagic crustaceans. Biol Bull 196: 137–144. [DOI] [PubMed] [Google Scholar]
- 50. Fritsches KA, Brill RW, Warrant EJ (2005) Warm eyes provide superior vision in swordfishes. Curr Biol 15: 55–58. [DOI] [PubMed] [Google Scholar]
- 51. Bernholz D, Matthews ML (1975) Critical flicker fusion frequency in a harp seal: evidence for duplex retinal organization. Vision Res 15: 733–736. [DOI] [PubMed] [Google Scholar]
- 52. Williams RA, Pollitz CH, Smith JC, Williams TP (1985) Flicker detection in the albino rat following light-induced retinal damage. Physiol Behav 34: 259–266. [DOI] [PubMed] [Google Scholar]
- 53. Jenssen TA, Swenson B (1974) An ecological correlate of critical flicker fusion frequencies for some anolis lizards. Vision Res 14: 965–970. [DOI] [PubMed] [Google Scholar]
- 54. Creiver DW, Meister M (1998) Synchronous period-doubling in flicker vision of salamander and man. J Neurophysiol 79: 1869–1878. [DOI] [PubMed] [Google Scholar]
- 55.Gruber SH (1969) The Physiology of Vision in the Lemon Shark, Negaprion brevirostris (Poey): A Behavioral Analysis. PhD diss. University of Miami, Coral Gables, FL.
- 56. Carvalho PSM, Noltie DB, Tillitt DE (2002) Ontogenetic improvement of visual function in the medaka Oryzias latipes based on optomotor testing system for larval and adult fish. Anim Behav 64: 1–10. [Google Scholar]
- 57. Ault SJ, House EW (1987) Electroretinographic responses of the great horned owl (Bubo virginianus). J Raptor Res 21: 147–152. [Google Scholar]
- 58.Forster LM (1985) Target discrimination in jumping spiders. In: Barth FG (ed) Neurobiology of animals. New York: Springer. pp. 250–274.
- 59. Miall RC (1978) The flicker fusion frequencies of six laboratory insects, and the response of the compound eye to mains fluorescent ‘ripple’. Physiol Entomol 3: 99–106. [Google Scholar]
- 60. Crozier WJ, Wolf E (1939) The flicker response contours for genetically related fishes. II. Journal of Gen Physiol 22: 463–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Douglas RH, Hawryshyn CW (1990) Behavioural studies of fish vision: an analysis of feature-positive effect. In: Douglas RH, Djamgoz MBA (eds) The visual system of fish. London: Chapman Hall. pp. 279–343.
- 62. Loop MS, Berkeley MS (1975) Temporal modulation sensitivity of the cat: I. Behavioral methods. Vision Res 15: 555–561. [DOI] [PubMed] [Google Scholar]
- 63. Fleishman LJ, Marshall CJ, Hertz PE (1995) Comparative study of temporal response properties of the visual system of three species of anoline lizards. Copeia 1995: 422–431. [Google Scholar]
- 64. Porciatti V, Fontanesi G, Bagnoli P (1989) The electroretinogram of the little owl (Athene noctua). Vision Res 29: 1693–1698. [DOI] [PubMed] [Google Scholar]
- 65.Waterman TH (1981) Polarization sensitivity. In: Autrum H (ed) Handbook of sensory physiology, vol VII/6C. Berlin: Springer. pp. 281–469.
- 66.Sekular R, Blake R (2002) Perception. New York: McGraw-Hill.
- 67. Meyer-Rochow VB, Tiang KM (1984) The eye of Jasus edwardsii (Crustacea, Decapoda): electrophysiology, histology, and behaviour. Zoologica 45: 1–85. [Google Scholar]
- 68. Walls GL (1934) The reptilian eye. Am J Ophthalmol 17: 892–915. [Google Scholar]
- 69. Woo KL, Hunt M, Harper D, Nelson NJ, Daugherty CH, et al. (2008) Discrimination of flicker frequency rates in the reptile tuatara (Spenodon). Naturwissenschaften 96: 415–419. [DOI] [PubMed] [Google Scholar]
- 70. Hecht S, Verrijp CD (1933) Intermittent stimulation by light. J Gen Physiol 17: 237–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Landis C (1954) Determinants of the critical flicker-fusion threshold. Physiol Rev 34: 259–286. [DOI] [PubMed] [Google Scholar]
- 72. Callahan TL, Petry HM (1999) Psychophysical measurement of temporal modulation sensitivity in the tree shrew (Tupaia belangeri). Vision Res 40: 455–458. [DOI] [PubMed] [Google Scholar]
- 73. Symmes (1962) Self-determination of critical flicker frequencies in monkeys. Science 136: 714–715. [DOI] [PubMed] [Google Scholar]
- 74. Rubene D, Hastad O, Tauson R, Wall H, Odeen A (2010) The presence of UV wavelengths improves the temporal resolution of the avian visual system. J Exp Biol 213: 3357–3363. [DOI] [PubMed] [Google Scholar]
- 75. Tansley K, Copenhaver RM, Gunkel RD (1961) Some aspects of the electroretinographic response of the American red squirrel, Tamiasciurus hudsonicus loquax . J Cell Compar Physl 57: 11–19. [DOI] [PubMed] [Google Scholar]
- 76.Lythgoe JN (1979) The ecology of vision. Oxford: Claredon Press. [Google Scholar]
- 77. Bornshein H, Tansley K (1961) Elektroretinogramm und netzhautstrukturder sumpfohreule (Asio flammeus). Experientia 17: 185–187.13776728 [Google Scholar]
- 78. Jarvis JR, Taylor NR, Prescott NB, Meeks I, Wathes CM (2002) Measuring and modeling the photopic flicker sensitivity of the chicken (Gallus g. domesticus). Vision Res 42: 99–106. [DOI] [PubMed] [Google Scholar]
- 79. Hanyu I, Ali MA (1964) Electroretinogram and its flicker fusion frequency at different tempretures in light adapted salmon (Salmo salar). J Cell Comp Physiol 63: 309–321. [DOI] [PubMed] [Google Scholar]
- 80. Coile DC, Pollitz CH, Smith JC (1989) Behavioral determination of critical flicker fusion in dogs. Physiol Behav 45: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 81. Hendricks J (1966) Flicker thresholds as determined by a modified conditioned suppression procedure. J Exp Anal Behav 9: 501–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lisney TJ, Rubene D, Rózsa J, Løvlie H, Håstad O, et al. (2011) Behavioural assessment of flicker fusion frequency in chicken Gallus gallus domesticus . Vision Res 51: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 83. Shumake SA, Smith JC, Taylor HL (1968) Critical fusion frequency in rhesus monkeys. Psychol Rec 18: 537–542. [Google Scholar]
- 84. Maddocks SA, Goldsmith AR, Cuthil IC (2001) The influence of flicker rate on plasma corticosterone levels of European starlings, Sturnus vulgaris . Gen Comp Endocr 124: 315–320. [DOI] [PubMed] [Google Scholar]
- 85. Lisney TJ, Ekesten B, Tauson R, Håstad O, Ödeen A (2012) Using electroretinograms to assess flicker fusion frequency in domestic hens Gallus gallus domesticus . Vision Res 62: 125–133. [DOI] [PubMed] [Google Scholar]
- 86. Nuboer JK, Coemans MAJM, Vos JJ (1992) Artificial lighting in poultry houses: do hens perceive the modulation of fluorescent lamps as flicker? Brit Poultry Sci 33: 123–133. [DOI] [PubMed] [Google Scholar]
- 87. Ruck P, Jahn TL (1954) Electrical studies on the compound eye of Ligia occidentalis . J Gen Physiol 37: 825–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dodt E, Wirth A (1953) Differentiation between rods and cones by flicker electroretinography in pigeon and guinea pig. Acta Physiol Scand 30: 80–89. [DOI] [PubMed] [Google Scholar]
- 89. Srinivasan MV, Lehrer M (1984) Temporal acuity of honeybee vision: behavioural studies using moving stimuli. J Comp Physiol A 155: 297–312. [Google Scholar]
- 90. Van Praagh JP (1975) Towards a controlled-environment room suitable for normal colony life of honeybees. J Apicult Res 11: 77–87. [Google Scholar]
- 91. Autrum H, Gallwitz U (1951) Zur analyse der Belichtungspotentiale des insecktenauges. Zeitschrift fur Vergleichende Physiologie 33: 407–435. [Google Scholar]
- 92. Autrum H, Stoecker M (1950) Die Verschmelzungsfrequenzen des bienenauges. Zeitschrift fur Naturforschung 5: 38–43. [Google Scholar]
- 93. Hammer DX, Schmitz H, Schmitz A, Rylander III HG, Welch AJ (2001) Sensitivity threshold and response characteristics of infrared detection in the beetle Melanophila acuminata (Coleoptera: Buprestidae). Comp Biochem Phys A 128: 805–819. [DOI] [PubMed] [Google Scholar]
- 94. Gaston KJ, Davies TW, Bennie J, Hopkins J (2012) Reducing the ecological consequences of night-time light pollution: options and developments. J App Ecol 49: 1256–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Healy K, McNally L, Ruxton GD, Cooper N, Jackson AL (2013) Metabolic rate and body size are linked with perception of temporal information. Anim Biol 86: 685–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ferry ES (1892) Persistence of vision. Am J Sci 44: 192–207. [Google Scholar]
- 97. Porter TC (1902) Contributions to the study of flicker. P R Soc London 70: 313–329. [Google Scholar]
- 98.Orbach DN, Fenton B (2010) Vision impairs the abilities of bats to avoid colliding with stationary obstacles. Plos One, 5, e13912. [DOI] [PMC free article] [PubMed]
- 99. Rydell J (1992) Exploitation of insects around street-lamps by bats in Sweeden. Funct Ecol 6: 744–750. [Google Scholar]
- 100. Rydell J (1991) Seasonal use of illuminated areas by foraging northern bats Eptesicus nilssoni . Holarctic Ecol 14: 203–207. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Species for which critical fusion frequencies have been measured. Includes the light source type used in the measurement of CFF values. Species are ordered by class.
(XLSX)