Abstract
Leishmaniasis is a major health problem in some endemic areas and yet, no vaccine is available against any form of the disease. Historically, leishmanization (LZ) which is an inoculation of individual with live Leishmania, is the most effective control measure at least against cutaneous leishmaniasis (CL). Due to various reasons, LZ is not used today. Several live attenuated Leishmania have been developed but their use is limited. Previously, we developed a transgenic strain of L. major that harbors two suicide genes tk and cd genes (lmtkcd+/+) for use as a challenge strain in vaccine studies. These genes render the parasite susceptible to Ganciclovir (GCV) and 5-flurocytosine (5-FC). The dual drug sensitive strain of L. major was developed using gene targeting technology using a modified Herpes Simplex Virus thymidine kinase gene (hsv-tk) sensitive to Ganciclovir antibiotic and Saccharomyces cerevisae cytosine deaminase gene (cd sensitive to 5-flurocytosine) that were stably introduced into L. major chromosome. BALB/c mice inoculated with lmtkcd+/+ developed lesions which upon treatment with GCV and 5-FC completely healed. In the current study, the transgenic lmtkcd+/+strain was assessed as a live vaccine model to determine the time necessary to develop a protective immune response. C57BL/6 mice were inoculated with the transgenic lmtkcd+/+strain, and treated at the time of inoculation (day0) or at day 8 after inoculation. Immunized animals were challenged with wild-type L. major, and complete protection was induced in mice that were treated at day 8. The results show that in contrast to leishmanization, in group of mice inoculated with a dual sensitive L. major development and persistence of lesion is not necessary to induce Th1 response and protection.
Author Summary
Leishmaniasis is still a major health problem in some endemic foci, yet no vaccine is available against any form of leishmaniasis. It is a general belief that recovery from cutaneous leishmaniasis (CL) is accompanied with long life protection. An inoculation of live pathogenic L. major into healthy individuals to induce lesion similar to CL is called Leishmanization (LZ). Historically LZ showed to be the most effective control tool against CL. One of the drawbacks and reason for discontinuation of LZ was lesion development, which rarely lasts long. Treatment of CL is not an easy task. One line of development of an effective vaccine against leishmaniasis, a transgenic strain of L. major harboring two suicide genes tk and cd genes (lmtkcd+/+), was developed and previously checked in BALB/c mice. In this study, C57BL/6 mice were inoculated with transgenic lmtkcd +/+strain; the rate of protection, parasite burden and the type of immune response were checked, and the results showed that complete protection induced by inoculation of lmtkcd +/+strain if treatment is initiated on day 8 post inoculation.
Introduction
Cutaneous leishmaniasis (CL) manifests as a localized self-healing lesion(s) that in rare cases develops to a non-healing lesion. If non-healing lesions develop, they are extremely difficult to treat with current therapies [1]. Control measures for leishmaniasis such as vector and/or réservoir control are not always practical, especially in remote endemic areas with limited resources. Efficacy of available drugs for leishmaniasis especially for CL is not acceptable and resistant is emerging [2], [3], [4], [5], [6]. Leishmanization (LZ) involves inoculating of individuals with live virulent Leishmania major to induce a single lesion that mimics a natural infection but with the lesion located at a predetermined site. Upon healing, the leishmanized individuals are protected against natural infection. LZ has been shown to be the most effective control measure at least against CL but the practice has been discontinued except on a limited scale in Uzbekistan. Primarily this is due to the development of chronic lesions that require medical intervention [7], [8], [9]. Despite ample evidence that development of an effective vaccine against leishmaniasis is possible there is still no vaccine available against any form of human leishmaniasis [10], [11], [12], [13]. One approach is to derive attenuated live vaccine strains of Leishmania through genetic manipulation to develop a parasite strain which has no virulence or a limited pathogenicity. A number of genetically manipulated Leishmania strains have been developed and studied in animal models with controversial results [14], [15], [16], [17], [18].
Previously, we developed a transgenic strain of L. major (tk +/+ –cd +/+)[lmtkcd+/+] harboring two suicide genes tk and cd genes that confer susceptibility to GCV and 5-FC,.as a challenge strain for vaccine studies. When BALB/c mice were inoculated in the flank with lmtkcd+/+, lesions developed at the site of inoculation, upon treatment with GCV and 5-FC complete healing occurred [16]. To extend these studies lmtkcd+/+ was used to determine whether persistent infection is required for induction of a protective immune response against subsequent L major infection. The lmtkcd+/+ promastigotes were inoculated into C57BL/6 mice and the inoculated mice were treated at set times with GCV to clear the infection. The mice were then challenged with wild type L major. Long term (3 months) complete protection against challenge with wild type L major was achieved with as little as 8 days vaccination time demonstrating that persistent infection is not required for complete protection.
Materials and Methods
Ethics statement
The ethical committee; Institutional Animal Care and Research Advisory Committee of Pasteur Institute of Iran, Education Office dated January, 2008, based on the Specific National Ethical Guidelines for Biomedical Research issued by the Research and Technology Deputy of Ministry of Health and Medicinal Education of Iran, issued in 2005, approved the protocol.
Parasites
The L. major promastigotes (MHOM/IR/76/ER) used and from which the transgenic lmtkcd+/+parasites were derived, this L. major is the same isolate which was used for mass leishmanization, preparation of old world experimental vaccine and the Leishmania used for the skin test. Promastigotes were cultured in M199 medium (Life Technologies, Inc.) supplemented with 10% heat inactivated fetal calf serum (Gibco BRL) and 25 mM HEPES (Gibco BRL), pH 7 at 26°C. The parasite virulence was maintained by passage in BALB/c mouse.
Mice
Female C57BL/6 mice, 6–8 week-old were purchased from the Animal Breeding Facility Centre (ABFC) of Pasteur Institute, Karaj, Iran. The animals were maintained in the animal facility of the Pasteur Institute of Tehran. The experiments were carried out according to the guidelines of Ethic Committee for Human use of Laboratory Animals, Pasteur Institute, Tehran, Iran.
Infection, treatment and challenge
Mice were inoculated subcutaneously (SC) at the right hind footpad with 2×106 stationary phase promastigotes of either L. major (MHOM/IR/76/ER) wild type (WT) or the transgenic lmtkcd+/+parasites in 50 µl PBS. The mice inoculated with lmtkcd+/+were divided into 3 groups and treated with a combination of GCV and 5Fcyt, 100 mg/Kg, intra-peritoneally (IP) either at the time of parasite inoculation (day 0), at day 8 after inoculation or for the control group which was left untreated. The dosage of the drugs used in this study was based on our previous study (17). The lmtkcd+/+ inoculated groups were challenged in the left footpads with 2×106 virulent WT L. major SC at 3 weeks after the end of the treatment period.
Lesion development
The lesion development was recorded by weekly measurement of the footpad thickness at the site of inoculation using a metric caliper up to 12 weeks after inoculation.
Parasite burden assay
Parasite burden was quantified once at week 10 after inoculation of the mice with either L. major wild type or with lmtkcd+/+ and again 5 weeks after the challenge with wild type L. major (2–5 mice per group). The parasite burden in the spleen and draining lymph nodes were determined using limiting dilution analysis. To enhance sensitivity, 2-fold dilutions of the samples (up to 1/100) were used.
DTH response
Delayed-type hypersensitivity (DTH) reaction was checked prior to challenge by injection of freeze-thawed (FT) Leishmania major (2×106 promastigotes in 50 µl per injection) into the contralateral uninfected hind footpad. FT L. major promastigotes were prepared by repeating a freeze (−196°C)/thaw (37°C) cycle ten times. Footpad swelling was measured using a metric caliper at 24, 48 and 72 h after injection.
Lymphocyte proliferation assay
Three mice from each group were sacrificed before and at 5 weeks after challenge inoculation, spleens were removed and cells cultured in complete RPMI-1640 medium in the presence or absence of 20 µg/well of Soluble Leishmania Antigens (SLA, 107 Leishmania promastigotes/ml equal to 100 µg/ml) or Concavalin A (ConA;10 µg/ml) or without stimulation as a control.
Cytokine assay
The levels of IFN-γ and IL-4 at weeks 5 and 10 post inoculation with lmtkcd +/+ or WT L.major and 5 weeks after challenge were determined in the supernatant collected from spleen cell culture (5 mice per group). Briefly, single spleen cell suspension was prepared, cultured and re-stimulated either with SLA (100 µg/ml) or Con A (10 µg/ml). The supernatant was collected at 72 h. Then, the levels of IFN-γ and IL-4 were titrated using ELISA method according to the manufacturer's instruction (Bender Medsystems, Gmbh, Austria). The sensitivity of the ELISA kits was 3 pg/ml for IL-4 and 7.5 pg/ml for IFN- γ.
Antibody response (IgG1 and IgG2a)
At week 5 after challenge, different groups of mice were tail bled and the levels of anti-Leishmania IgG1 and IgG2a Abs were checked by ELISA.
Statistical analysis
All experiments were done in triplicates and the data was expressed as means ± S.E.M. The data was analyzed by one-way ANOVA followed by Tukey's test using SPSS V.13 software. P value<0.05 was considered as statistically significant.
Results
Footpad thickness after infection with WT L. major or lmtkcd+/+
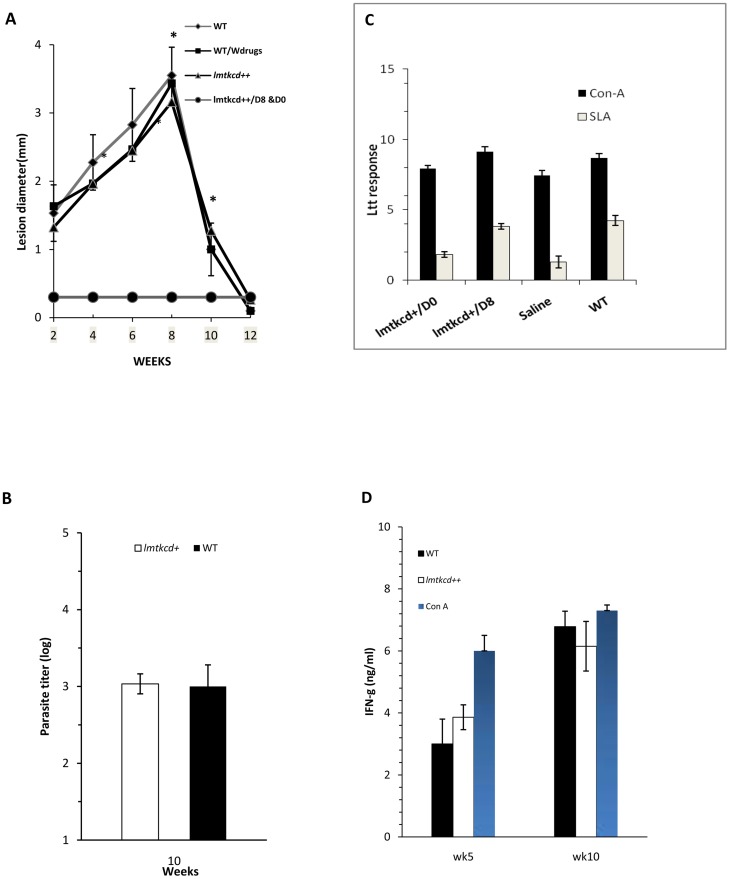
C57BL/6 mice were inoculated SC with live wild type (WT) L. major parasites or lmtkcd +/+ parasites and were either left untreated or treated with GCV/5-FCy at day 0 or day 8. Lesion development was followed by the measurement of footpad thickness. Following challenge with L. major, the protection rate and the immune responses generated were assessed. C57BL/6 mice inoculated with lmtkcd+/+ or WT parasites and left untreated developed a similar lesion size which was cured around week 8–9. In contrast, no lesion was developed in the group of mice which was inoculated with lmtkcd+/+ and received GCV/5-FCyt treatment at day 0 or day 8. The group of mice inoculated with WT L. major which was treated with GCV/5-FCyt developed a lesion similar to the untreated group of mice (Fig. 1A).
Figure 1. C57BL/6 mice were subcutaneously (SC) inoculated with either 2×106 WT L. major or with lmtkcd+/+ parasites and were treated with GCV/5-FCyt on day 0 (lmtkcd+/+/D0) or day 8 (lmtkcd+/+/D8) or left untreated.
A. Lesion development was assessed by weekly measurement of footpad swelling. B. Parasite burden in groups of mice inoculated either with WT L. major or lmtkcd+/+ was assessed at week 10. C. Lymphocyte transformation test was done on spleen cells. D. production of IFN-γ using ELISA method. Presented data are representative of 2 independent experiments.
Parasite burden after infection with WT L. major or lmtkcd+/+
The draining lymph nodes (LN) and spleen parasite burden was measured at week 10 post-inoculation (5 mice/group). The results showed no difference in the number of parasite in spleen and LN's in groups of mice inoculated with WT L. major and the group which was inoculated with lmtkcd +/+ and received no treatment, the parasite burden of spleen at week 10 after inoculation is presented in Fig. 1B and only parasite burden of spleen at week 5 after challenge with WT L. major is presented in Fig. 2B.
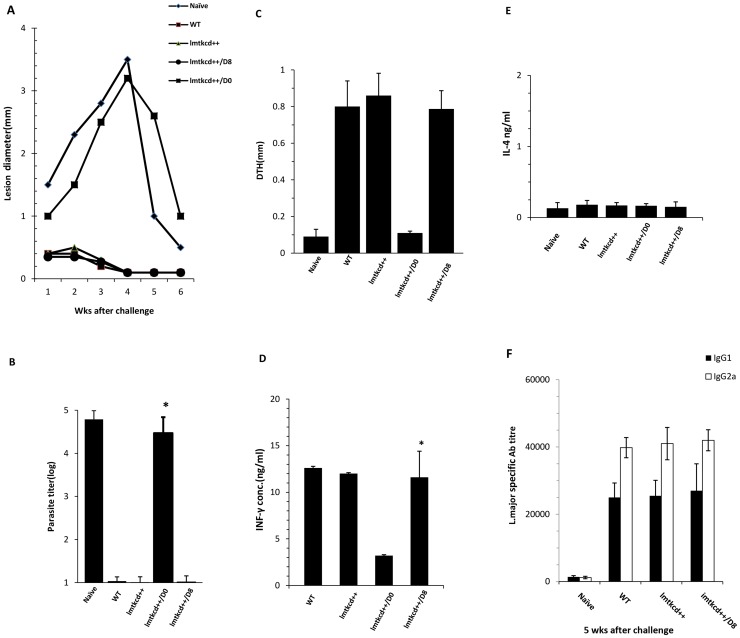
Figure 2. C57BL/6 mice with history of L. major infection or group of mice which were inoculated with lmtkcd+/+ L. major promastigotes and treated with GCV/5-Fcyt on day 0 or day 8 were subcutaneously (SC) challenged with 2×106 wild type L. major along with a group of naive mice.
A. Lesion development was assessed by weekly measurement of footpad swelling. B. Parasite burden was quantified in spleen on week 5 post challenge. C. DTH reaction was checked by measurement of footpad swelling at 72 hours after injection of freeze-thawed L. major into the contralateral uninfected hind footpad. Five weeks after challenge, the splenocytes were cultured and stimulated in vitro with SLA (100 µg/ml), Con A (10 µg/ml), or with no stimulation for 72 hrs. D & E The supernatants were collected and the levels of IFN-γ (D) and IL-4 (E) were titrated using ELISA, F. Anti Leishmania IgG1 and IgG2a at 5 weeks post challenge. Presented data are representative of 2 independent experiments.
Immune response assay after infection with WT L. major or lmtkcd+/+
At weeks 5, 10 post-inoculation and week 5 post challenge mice (5 per group) were sacrificed and spleens were removed. A single cell suspension of spleen was prepared and cultured in the presence of either SLA (100 µg/ml), Con A (10 µg/ml) or without additional stimulation, lymphocyte transformation test (LTT) was done at 72 hours and the results showed a significantly (p<0.05) stronger LTT in group of mice with history of L. major infection and the group which was inoculated with lmtkcd+/+parasites and treated on day 8 than the group of mice inoculated with lmtkcd+/+parasites and treated on day 0 (Fig. 1C). The supernatants were collected and the levels of IFN-γ were titrated (Fig. 1D). Similar levels of IFN-γ were produced in spleen cells of group of mice inoculated with WT L. major and the group of mice inoculated with lmtkcd+/+. The level of IL-4 production was low and similar in group of mice inoculated with wild-type L. major or inoculated with lmtkcd +/+ at week 16 post infection (data not shown).
Challenge with WT L. major
To assess whether groups of C57BL/6 mice inoculated with lmtkcd+/+ parasites are protected against WT L. major challenge, at week 5–6 post inoculation (3 weeks after the end of treatment upon commencing time), the groups of mice which received lmtkcd+/+ and were treated on day 0 or 8 were challenged with L. major. As well, a group of mice which had healed spontaneously after L. major infection and a group of naïve mice were inoculated with L. major as controls. The results showed that the group of mice which was inoculated with lmtkcd+/+ parasites and treated with GCV/5-Fcyt on day 8 and then challenged with WT at week 6, did not develop any lesion or swelling similar to the group of mice challenged with L. major after previously self-healing lesion. In contrast, the group of mice which was inoculated with lmtkcd+/+ and treated at the same time (Day 0) with GCV/5-Fcyt and the group of naïve mice inoculated with L. major developed lesions (Fig. 2A).
Parasite burden post-challenge with wild L. major
The parasite burden was quantified in draining LN at week 5 post-challenge with L. major, as shown in Fig. 2B. The number of parasites isolated from the group of mice which was inoculated with lmtkcd+/+ and treated at day 8 with GCV/5-FCyt and the group of mice which had previously self-healed following L. major infection was significantly (p<0.05) lower than the group which was inoculated with lmtkcd+/+ and treated at the same time (day 0) and the group of naïve mice which were inoculated with L. major for the first time. The number of parasites was very low in the groups of mice inoculated with either lmtkcd+/+ and treated at day 8 or inoculated with lmtkcd+/+ and not treated or the group of mice with history of L. major infection or the group of mice which were inoculated with lmtkcd+/+ and treated at day 0, no significant difference was seen between the number of parasite in these groups.
Immune response evaluation (DTH and cytokine assay)
DTH was done in different group of mice by injection of freeze-thawed (FT) Leishmania major (2×106 promastigotes in 50 µl per injection) into the contra lateral uninfected hind footpad. The results are presented in Fig. 2C, a similar strong DTH response is seen in group of mice inoculated with WT L. major, or inoculated with lmtkcd+/+ and treated with GCV/5-FCy on day 8 or left untreated, a low DTH response was seen in groups of mice inoculated with lmtkcd+/+ and treated with GCV/5-FCy on day 0 or uninfected naïve mice. At week 10 after inoculation (before challenge) and 5 weeks after challenge, the splenocytes were cultured, stimulated in vitro with either SLA (100 µg/ml), or Con A (105 µg/ml), or left unstimulated. LTT was done and the culture supernatants were collected at 72 hours and the level of IFN-γ and IL-4 was titrated using ELISA method. A significantly (p<0.05) stronger LTT was seen in mice with history of L. major infection and the group which was inoculated with lmtkcd+/+ parasites and treated on day 8 than the group of mice inoculated with lmtkcd+/+ parasites and treated on day 0 (data not shown). The level of IFN-γ was significantly higher in groups of mice inoculated with WT L. major or inoculated with lmtkcd+/+ and treated with GCV/5-FCy on day 8 or left untreated (Fig. 2D). The level of IL-4 was similar in all the groups (Fig. 2E).
IgG response
Serum samples were collected at 5 weeks after challenge, the results are presented in Fig. 2F, as shown a significantly (P = 0.002) higher anti-L.major IgG antibodies were seen in the group of mice with history of L. major lesion or group of mice inoculated with lmtkcd++ and treated with GCV/5-FCy on day 8, in comparison with the group of naïve mice or group of mice inoculated with lmtkcd+/+ and treated with GCV/5-FCy on day 0. IgG1 and IgG2a showed a significant (P = 0.001) increase after challenge compared to before challenge in all the groups and no significant difference was seen between the groups.
Discussion
Cutaneous leishmaniasis manifests as a self-healing skin lesion(s) in exposed parts of the body, the healing process for lesions depends upon the Leishmania species involved and the host immune response. Usually healing takes up to 2 years, but CL might not be cured for several years with currently available treatments. Choices of therapeutic treatments for CL are limited and not always effective, often requiring multiple injections, introduce side effects and control measure tools are not always practical and successful [1], [2], [3], [6], [19], [20], [21]. It is well established that individuals with a history of CL are protected against development of further CL lesion. CL lesion(s) development is accompanied by the induction of strong immune response shown by in vivo and in vitro tests (9, 21). Despite many studies on leishmaniasis, immunological surrogate marker(s) of protection is not well defined in human leishmaniasis [9], [10], [22]. There is ample evidence to suggest that development of an effective vaccine against leishmaniasis is possible, but so far no vaccine is available against any form of leishmaniasis. The results of phase 3 clinical trials using crude Leishmania as vaccine were not promising [4], [12], [23], [24]. It has been shown that in vitro CD4+/CD8+ T-cell responses to live Leishmania major are significantly stronger than responses to dead parasites [25]. The only successful protective measure against CL has been shown to be leishmanization. One of the major drawbacks of LZ is the development of a lesion which might not heal during the expected time period and not respond to treatment [7], [9], [10]. Research have therefore focused on developing a Leishmania strain which upon inoculation does not induce a lesion or induces a lesion with limited pathogenicity, but at the same time maintains immunogenicity and as such induce protection in which the leishmanized individuals upon natural infection induce no lesion or even a limited fast healing lesion. In this regard attenuated and genetically manipulated Leishmania were developed and showed to induce protection in murine model of leishmaniasis [4], [15], [16], [26], [27]. Co-inoculation of Leishmania with CpG ODN showed to reduce the pathogenicity, but yet no Leishmania preparation reached to human use [28], [29].
Previously, the same group developed a recombinant double drug sensitive strain of lmtkcd+/+ by integration of a genetically engineered HSV tk gene to confer sensitivity to GCV, and the S. cerevisiae cd gene to induce sensitivity to 5-fluorocytosine. Inoculation of BALB/c mice with lmtkcd+/+ induces lesion similar to WT L. major, but the lesion was controllable by treatment with GCV/5-FCyt [16]. BALB/c mice does not mimic human CL so in the current study, C57BL/6 strain which is not a perfect model of human CL but more mimic the disease is used. Leishmanization which is an inoculation of virulent L. major in a predetermined part of the susceptible individuals, LZ induces a lesion similar to natural infection, protection against further multiple lesions is usually developed upon cure of the lesion caused by LZ and so far LZ showed to be the most effective preventive measure against CL. The main drawback of LZ is development of lesion [16].
Using drug sensitive Leishmania mimic natural infection similar to LZ and at the same time due to sensitivity of Leishmania to approved drugs assures a controllable lesion. As it is presented in Fig. 1, C57BL/6 mice inoculated with L. major lmtkcd+/+ showed a lesion similar to WT L. major (Fig. 1A, Fig. 2A) with no difference in parasite burden (Fig. 1B, Fig. 2B). A very low number of Leishmania parasite is detected in the group of mice inoculated with lmtkcd+/+ and treated with GCV/5-FCyt, A small number of Leishmania was detected in spleen of C57BL/6 mice long after recovery from L. major infection (unpublished data). A similar Th1 response was induced shown by LTT (Fig. 1C), DTH (Fig. 2C) and the cytokine levels of IFN-γ (Fig. 1D, Fig. 2D) and IL-4 (Fig. 2E) in groups of mice inoculated with WT and group of mice inoculated with lmtkcd+/+and treated with GCV and 5-FCyt on day 8 or left untreated, although in the group of mice inoculated with lmtkcd+/+and treated with GCV/5-FCyt on day 8, no lesion was developed at the site of inoculation but the reason for small increase in the size of footpad swelling is due to a slight inflammation which induced at the site of inoculation. Upon challenge with L. major, no lesion was developed and strong protection was seen similar to the group of mice cured from L. major infection (Fig. 2 A). The results showed that despite of no lesion development which was due to under control of recombinant L. major with ganciclovir and 5-Flourocytosin, strong Th1 immune response and protection against WT L. major was induced.
Acknowledgments
The authors would like to thank Ms. Khodayari (Department of Medical Biotechnology, Pasteur Institute of Iran) for her technical assistance.
Funding Statement
The Funder is Pasteur Institute of Iran with ID No:0217. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dowlati Y (1996) Cutaneous leishmaniasis: clinical aspect. Clin Dermatol 14: 425–431. [DOI] [PubMed] [Google Scholar]
- 2. Croft SL, Olliaro P (2011) Leishmaniasis chemotherapy–challenges and opportunities. Clin Microbiol Infect 17: 1478–1483. [DOI] [PubMed] [Google Scholar]
- 3. Shazad B, Abbaszadeh B, Khamesipour A (2005) Comparison of topical paromomycin sulfate (twice/day) with intralesional meglumine antimoniate for the treatment of cutaneous leishmaniasis caused by L. major. Eur J Dermatol 15: 85–87. [PubMed] [Google Scholar]
- 4. Alvar J, Velez ID, Bern C, Herrero M, Desjeux P, et al. (2012) Leishmaniasis worldwide and global estimates of its incidence. PLoS One 7: e35671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Khatami A, Firooz A, Nassiri-Kashani M, Dowlati Y (2011) Few comments on the treatment of Old World cutaneous leishmaniasis. Int J Dermatol 50: 754. [DOI] [PubMed] [Google Scholar]
- 6. Hadighi R, Boucher P, Khamesipour A, Meamar AR, Roy G, et al. (2007) Glucantime-resistant Leishmania tropica isolated from Iranian patients with cutaneous leishmaniasis are sensitive to alternative antileishmania drugs. Parasitol Res 101: 1319–1322. [DOI] [PubMed] [Google Scholar]
- 7. Nadim A, Javadian E, Tahvildar-Bidruni G, Ghorbani M (1983) Effectiveness of leishmanization in the control of cutaneous leishmaniasis. Bull Soc Pathol Exot Filiales 76: 377–383. [PubMed] [Google Scholar]
- 8. Gafurov IM (1999) [Experience in controlling and preventing zoonotic cutaneous leishmaniasis in Uzbekistan]. Med Parazitol (Mosk) 58–59. [PubMed] [Google Scholar]
- 9. Khamesipour A, Dowlati Y, Asilian A, Hashemi-Fesharki R, Javadi A, et al. (2005) Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 23: 3642–3648. [DOI] [PubMed] [Google Scholar]
- 10. Khamesipour A, Rafati S, Davoudi N, Maboudi F, Modabber F (2006) Leishmaniasis vaccine candidates for development: a global overview. Indian J Med Res 123: 423–438. [PubMed] [Google Scholar]
- 11. Costa CH, Peters NC, Maruyama SR, de Brito EC Jr, Santos IK (2011) Vaccines for the leishmaniases: proposals for a research agenda. PLoS Negl Trop Dis 5: e943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Noazin S, Khamesipour A, Moulton LH, Tanner M, Nasseri K, et al. (2009) Efficacy of killed whole-parasite vaccines in the prevention of leishmaniasis: a meta-analysis. Vaccine 27: 4747–4753. [DOI] [PubMed] [Google Scholar]
- 13. Modabber F (2010) Leishmaniasis vaccines: past, present and future. Int J Antimicrob Agents 36 Suppl 1: S58–61. [DOI] [PubMed] [Google Scholar]
- 14. Silvestre R, Cordeiro-da-Silva A, Ouaissi A (2008) Live attenuated Leishmania vaccines: a potential strategic alternative. Arch Immunol Ther Exp (Warsz) 56: 123–126. [DOI] [PubMed] [Google Scholar]
- 15. Muyombwe A, Olivier M, Harvie P, Bergeron MG, Ouellette M, et al. (1998) Protection against Leishmania major challenge infection in mice vaccinated with live recombinant parasites expressing a cytotoxic gene. J Infect Dis 177: 188–195. [DOI] [PubMed] [Google Scholar]
- 16. Davoudi N, Tate CA, Warburton C, Murray A, Mahboudi F, et al. (2005) Development of a recombinant Leishmania major strain sensitive to ganciclovir and 5-fluorocytosine for use as a live vaccine challenge in clinical trials. Vaccine 23: 1170–1177. [DOI] [PubMed] [Google Scholar]
- 17. Webb JR, McMaster WR (1994) Leishmania major HEXBP deletion mutants generated by double targeted gene replacement. Mol Biochem Parasitol 63: 231–242. [DOI] [PubMed] [Google Scholar]
- 18. Titus RG, Gueiros-Filho FJ, de Freitas LA, Beverley SM (1995) Development of a safe live Leishmania vaccine line by gene replacement. Proc Natl Acad Sci U S A 92: 10267–10271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asilian A, Jalayer T, Nilforooshzadeh M, Ghassemi RL, Peto R, et al. (2003) Treatment of cutaneous leishmaniasis with aminosidine (paromomycin) ointment: double-blind, randomized trial in the Islamic Republic of Iran. Bull World Health Organ 81: 353–359. [PMC free article] [PubMed] [Google Scholar]
- 20. Kashani MN, Firooz A, Eskandari SE, Ghoorchi MH, Khamesipour A, et al. (2007) Evaluation of meglumine antimoniate effects on liver, kidney and pancreas function tests in patients with cutaneous leishmaniasis. Eur J Dermatol 17: 513–515. [DOI] [PubMed] [Google Scholar]
- 21. Mahmoodi M, Khamesipour A, Dowlati Y, Rafati S, Momeni AZ, et al. (2003) Immune response measured in human volunteers vaccinated with autoclaved Leishmania major vaccine mixed with low dose of BCG. Clin Exp Immunol 134: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dogra N, Warburton C, McMaster WR (2007) Leishmania major abrogates gamma interferon-induced gene expression in human macrophages from a global perspective. Infect Immun 75: 3506–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Alvar J, Croft SL, Kaye P, Khamesipour A, Sundar S, et al. (2013) Case study for a vaccine against leishmaniasis. Vaccine 31 Suppl 2: B244–249. [DOI] [PubMed] [Google Scholar]
- 24. Noazin S, Modabber F, Khamesipour A, Smith PG, Moulton LH, et al. (2008) First generation leishmaniasis vaccines: a review of field efficacy trials. Vaccine 26: 6759–6767. [DOI] [PubMed] [Google Scholar]
- 25. Nateghi Rostami M, Keshavarz Valian H, Eskandari SE, Miramin Mohammadi A, Shahrestani ST, et al. (2010) Differential in vitro CD4+/CD8+ T-cell response to live vs. killed Leishmania major. Parasite Immunol 32: 101–110. [DOI] [PubMed] [Google Scholar]
- 26. Palatnik-de-Sousa CB (2008) Vaccines for leishmaniasis in the fore coming 25 years. Vaccine 26: 1709–1724. [DOI] [PubMed] [Google Scholar]
- 27. Daneshvar H, Molaei MM, Kamiabi H, Burchmore R, Hagan P, et al. (2010) Gentamicin-attenuated Leishmania infantum: cellular immunity production and protection of dogs against experimental canine leishmaniasis. Parasite Immunol 32: 722–730. [DOI] [PubMed] [Google Scholar]
- 28. Mendez S, Tabbara K, Belkaid Y, Bertholet S, Verthelyi D, et al. (2003) Coinjection with CpG-containing immunostimulatory oligodeoxynucleotides reduces the pathogenicity of a live vaccine against cutaneous Leishmaniasis but maintains its potency and durability. Infect Immun 71: 5121–5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hejazi H, Tasbihi M, Jaafari M, Badiee A, Pestechian N, et al. (2010) The Role of Liposomal CpG ODN on the Course of L. major Infection in BALB/C Mice. Iran J Parasitol 5: 47–54. [PMC free article] [PubMed] [Google Scholar]