Abstract
Background
It has been postulated that eye movement disorders in chronic progressive external ophthalmoplegia (CPEO) have a neurological as well as a myopathic component to them.
Aim
To investigate whether there is a supranuclear component to eye movement disorders in CPEO using eye movement recordings.
Methods
Saccade and smooth pursuit (SP) characteristics together with vestibulo-ocular reflex (VOR) gain and VOR suppression (VORS) gain in 18 patients with CPEO and 34 normal patients were measured using Eyelink II video-oculography.
Results
The asymptotic values of the peak velocity main sequence curves were reduced in the CPEO group compared to those of normal patients, with a mean of 161°/s (95% CI 126°/s to 197°/s) compared with 453°/s (95% CI 430 to 475°/s), respectively. Saccadic latency was longer in CPEO (263 ms; 95% CI 250 to 278), compared to controls (185 ms; 95% CI 181 to 189). Smooth pursuit and VOR gains were impaired in CPEO, although this could be explained by non-supranuclear causes. VORS gain was identical in the two groups.
Conclusions
This study does not support a supranuclear component to the ophthalmoplegia of CPEO, although the increased latencies observed may warrant further investigation.
Chronic progressive external ophthalmoplegia (CPEO) is a mitochondrial disorder characterised by progressive restriction of eye movements, ptosis and orbicularis weakness. Although myopathy accounts for a major part of the restriction of eye movements seen in CPEO, some authors have suggested that there may be a supranuclear component to the ophthalmoplegia.1 The distinction between myopathic and supranuclear components is not purely academic because the success of strategies to regenerate normal extraocular muscle, by stimulating endogenous satellite cells2 or by using embryonic stem cells, is dependent on CPEO being primarily a myopathy.
Vestibulo-ocular reflex suppression (VORS) measures central aspects of eye movement control because if VORS is complete, no actual eye movement has to be generated, thus isolating out the myopathic component of the eye movement problem. Impaired suppression of VOR has been cited as evidence of supranuclear involvement in myotonic dystrophy.3,4
METHODS
Ethical approval was gained from the local research ethics committee before starting the study. Patients were recruited into the study if they had a slowly progressive ophthalmoplegia, cytochrome c oxidasenegative fibres on muscle biopsy and had given written, informed consent.
Eye movement recordings
Eye movements were recorded using an EyelinkII video-oculography system (SR Research, Ottawa, Canada) at 500 samples per second. Calibration, saccadic and smooth pursuit stimuli were produced at a distance of approximately 1100 mm from the subject. The distance was measured and stimulus angle calculated individually for each subject.
All tests were done with the subject seated in a MiniTorque Barany chair (S.A. Instrumentation DIFRA, Welkenraedt, Belgium). A chinrest was mounted on the chair, which substantially reduced but did not completely eliminate head movements. Eyelink long-range infrared markers for head movement compensation were mounted on the fixation board and used during the Eyelink internal calibration. Eyelink head referenced data were used in the analysis, and the head movement markers were turned off during VOR testing. Saccades and smooth pursuit (SP) were recorded in low-level lighting, and VOR was measured in total darkness.
Eyelink calibration was done at an angle suitable for the range of movements of the subjects’ eyes. This ranged between ±7° and ±26° horizontally and ±5° and ±20° vertically, using a five-point calibration. Some subjects had ptosis, in which case the eyelids were taped up. Where the ptosis still interfered with calibration, a horizontal three-point calibration was used. The best possible Eyelink calibration was obtained, but in addition, up to nine horizontal fixations were used to derive a third-order polynomial that was retrospectively used as a secondary calibration and applied to the horizontal signals. Where the subject had limited eye movements, only those fixations within the range of valid movements were used for secondary calibration. Subjects with tropias were calibrated monocularly. Ideally, all subjects should have been calibrated monocularly. Calibration was difficult for some subjects in the CPEO group.
Saccades
Saccades were measured using an LED board. A zero-gap paradigm was used with stimulus amplitude up to a maximum of 26° left or right. Direction and timing were randomised. Leftward and rightward primary centrifugal saccades were used in the analysis. The start and end of saccades were defined at the points where velocity dropped below 20°/s. We excluded saccades contaminated by blinks, artefacts or with a latency <100 ms.
Smooth pursuit
Smooth pursuit was generated with a laser spot projected via a galvanometer mirror, with a sinusoidal profile of ±10° at 0.4, 0.6 and 0.8 Hz (peak velocities of 25°/s, 38°/s and 50°/s), the amplitude being chosen with the expectation that most patients with CPEO would have that degree of eye movement.
VOR and VOR suppression
VOR was generated using the Barany chair, using sinusoidal stimulation of ±80° at 0.1 and 0.2 Hz (peak velocities of 50°/s and 100°/s). Subjects were asked to imagine viewing a distant object and to perform mental arithmetic to maintain alertness. The VORS stimulus was an LED mounted on a moveable stalk, positioned in front of the subject at a distance of approximately 50–60 cm, depending on the size of the subject.
Statistical analysis
For each subject, only the “best eye” data were used, and this was determined from consideration of the Eyelink calibration reports and subjective examination of the quality of the signals.
Peak saccadic velocity main sequence curves were derived for each subject by fitting data to an equation of the form
where PVmax is the asymptotic value of the curve, A is the amplitude of the saccade and c is a constant relating to the slope. The mean and 95% confidence interval (CI) of the mean were then derived for PVmax.
Saccade latencies from all studies were pooled for the control and CPEO groups. Only the first 13 primary saccades for each study were used to ensure that the mean was not biased because of uneven numbers of saccades per study. Because the distribution of latencies was skewed, the reciprocal of the latencies was taken, mean and 95% CI were calculated, and the values transformed back.5
We hypothesised that VORS gain may be related to VOR gain such that subjects with a lower VOR gain would find it easier to suppress their VOR and therefore would be more likely to have a lower VORS gain. We plotted VORS gain against VOR gain to see if there was any relationship.
If the VOR was perfect, that is, the countermovement of the eyes perfectly matched the rotation of the chair, we would expect a VOR gain of 1. If VORS was perfect, we would expect a gain during chair rotation, while fixating the suppression LED, of zero.
RESULTS
Eighteen patients with CPEO were tested (median age 59 years). All patients had cytochrome c oxidase-negative fibres in skeletal muscle biopsies and slowly progressive ophthalmoplegia. Seven patients had multiple deletions, seven had single deletions, two had point mutations and in two cases no mutation could be identified. We could not reliably perform all of the measurements on all of these patients, and therefore the numbers for the different measurements differ slightly. Thirty-four control patients were tested (median age 36 years).
Saccades
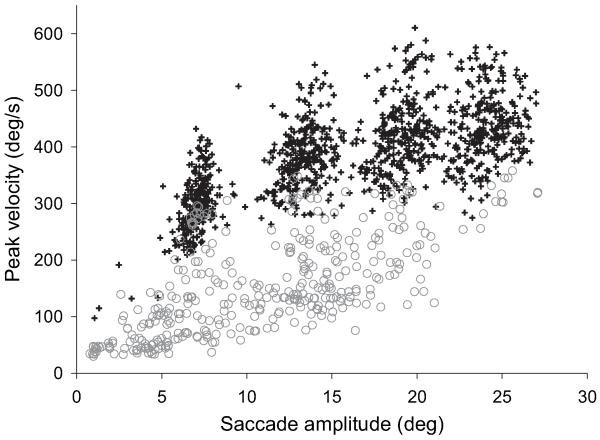
As expected, peak saccadic velocity was slow in CPEO in comparison with controls. Figure 1 shows the peak velocity for each saccade plotted against its amplitude (the peak velocity main sequence). The mean asymptotic peak velocity (PVmax) was 453°/s for controls (95% CI 430 to 475) and 161°/s for CPEO (95% CI 126 to 197).
Figure 1. Peak saccadic velocity of primary centrifugal saccades in controls (crosses, n=34) and patients with CPEO (circles, n=18).
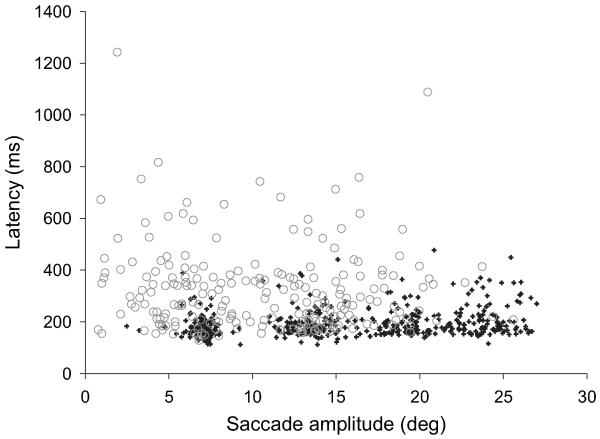
For analysis of latency, we used the same number of saccades from each subject to avoid biasing the results. Studies with a minimum of 13 primary centrifugal saccades suitable for measuring latency were used and the results are shown in figure 2.
Figure 2. Saccade latencies in controls (crosses, n=34) and patients with CPEO (circles, n=16).
Mean saccadic latency in CPEO (263 ms, 95% CI 250 to 278) was increased in relation to controls (185, 95% CI 181 to 189), although many saccades made by patients with CPEO had short latency times that overlapped with those of the control group.
Smooth pursuit
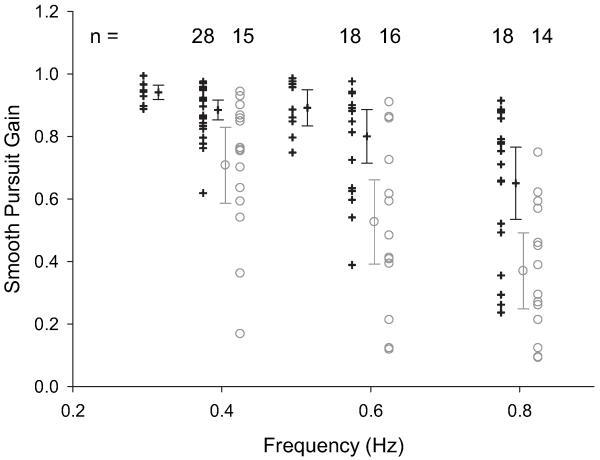
Participants who were unable to maintain smooth pursuit at all frequencies were excluded. Some controls had measurements made at different frequencies during the early stages of data collection and their data are excluded from the statistical calculations; however, the measurements at these frequencies are plotted on figure 3. There was some impairment of smooth pursuit in patients with CPEO compared to normal controls at all frequencies. At 0.4 Hz, mean gain for controls was 0.89 (0.85–0.92) compared with 0.71 (0.59–0.83) in the CPEO group. At 0.6 Hz, the means were 0.80 (0.72–0.89) in controls and 0.53 (0.39–0.66) for CPEO. Finally, at 0.8 Hz, the control group mean was 0.65 (0.54–0.77) compared with 0.37 (0.25–0.49) in CPEO.
Figure 3. Sinusoidal smooth pursuit gain at ±10° amplitude and 0.4, 0.6 and 0.8 Hz.
Means and 95% CI for the means are also shown. Control data are represented by crosses and CPEO by circles. Offsets were used when plotting for clarity.
VOR and VORS
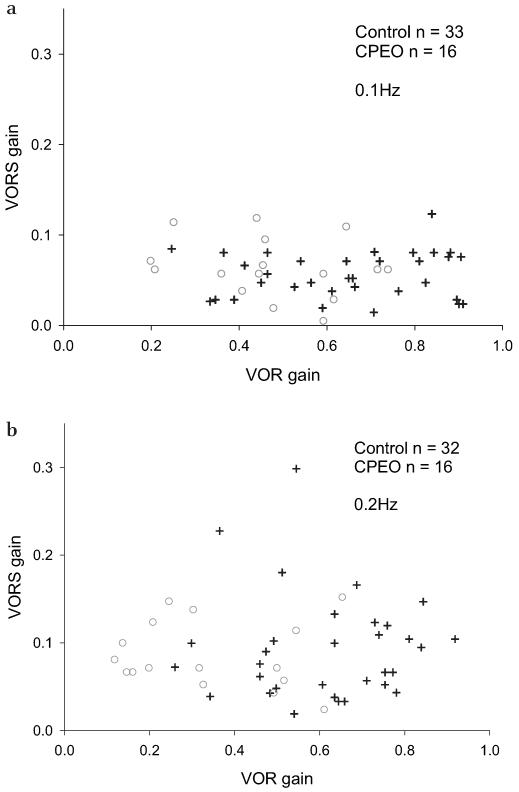
VOR gain was lower in the CPEO group than in controls. At 0.1 Hz, mean VOR gain was 0.65 (0.58–0.72) in the control group and 0.48 (0.39–0.57) in the CPEO group. At 0.2 Hz, mean VOR gain was 0.61 (0.55–0.67) in controls and 0.34 (0.25–0.44) in the CPEO group. Means and 95% CIs were almost identical for VORS gains. At 0.1 Hz, both groups had a mean of 0.06, but the CPEO group had a slightly larger CI of 0.05 to 0.08 compared to 0.05 to 0.07 for controls. At 0.2 Hz, both means were 0.09, with a control 95% CI of 0.07 to 0.12 and a CPEO 95% CI of 0.07 to 0.11. Figure 4 shows that there is no relationship between VOR gain and VORS gain.
Figure 4. VORS gain versus VOR gain at 0.1 Hz (A) and 0.2 Hz (B).
Controls are represented by crosses and CPEO by circles.
DISCUSSION
Interpretation of results should always take account of the limitations of the measurements. In our case, this mainly concerns the inherent inaccuracy of subject fixation, small head movements, and potential errors in calibration. These will introduce some variability into the measurements.
There is no doubt that there is a major myopathic component to CPEO. Evidence for this includes loss of muscle bulk on MRI scans,6 findings on muscle biopsy7 and the appearance of muscles at surgery. It has been proposed that eye muscles are uniquely vulnerable in mitochondrial myopathies because of their functional and ultrastructural properties.8 However, these findings do not preclude an underlying supranuclear component. Some previous studies point in this direction. There have been isolated case reports of patients with a greater range of eye movements to oculocephalic testing than ductions.1 Magnetic resonance imaging has shown abnormal metabolic profiles of the brain in patients with mitochondrial myopathies including CPEO,9 and brain stem reflexes have been found to be abnormal in patients with mitochondrial myopathies.10
The observed reduced saccadic velocities in CPEO are well documented11 and most likely muscular in origin. Increased latency might be taken as evidence of supranuclear pathway dysfunction so that it takes longer to generate the pulse required to move the eyes. Latency is easier to measure reliably in subjects with CPEO than other parameters because it is less influenced by calibration errors and ptosis. However, we hesitate to argue for abnormal supranuclear function from these data alone because other factors may be involved. One factor is the difference in age distribution of the two groups, although this is unlikely to account for all the difference. Irving et al12 found that subjects <50 years old had mean latencies of around 195 ms, and this rose in each decade to around 265 ms in those ≥80 years old. Using the mean values from their chart, we estimated the effect on our group means that each subject would have made due to the age difference with a nominal reference age. Depending on the reference age, we could account for 25–30 ms of the observed 78-ms difference in means between the groups.
VOR is responsible for maintaining images steady upon the retina while the head moves. It is a brain stem reflex mediated by the vestibular apparatus, vestibular nuclei and oculomotor nuclei. The maintenance of steady gaze while reading in a moving vehicle, for example, requires the ability to suppress the VOR. VORS is mediated by the pursuit pathway13,14 and the cerebellum.15 In the ophthalmoplegia of myotonic dystrophy, parallel impairment of SP and VORS has been taken as evidence of a supranuclear component to the ophthalmoplegia.3,4 We found impairment of SP (although there is large variability and considerable overlap with controls) and VOR,but no impairment of VORS. If the impaired VOR is due to peripheral ocular muscle weakness, then there would likely have been adaptive changes in the internal VOR gain, which nevertheless may have been insufficient to overcome the peripheral muscle weakness. The closed loop nature of the SP control system, along with parallel adaptation in the SP system,16,17 may enable continued suppression of the VOR. However, as with the VOR, this may still be insufficient to overcome the peripheral weakness so that SP is still impaired. This would explain our observations of impaired VOR and SP but preserved VORS. This suggests a peripheral rather than central pathology.
CONCLUSION
Although the observed increase in latency is consistent with a possible supranuclear component, there are other potential contributing factors. Although we found that the VOR gain was reduced in CPEO, VORS gain and CIs were almost identical in the two groups. Because VORS is mediated by the pursuit pathway, this supports our view that the reduction in smooth pursuit gain in CPEO is not supranuclear in origin. We did not observe any relationship between VORS gain and VOR gain. We considered VORS to be the more robust indicator in subjects with ophthalmoplegia, and it therefore seems unlikely from our data that supranuclear dysfunction is the primary event in CPEO. However, the increased latency observed may warrant further investigation.
Because of the small numbers, it not possible to make distinctions between CPEO due to single or multiple deletions.
Acknowledgements
AER received a bursary from the Wolfson Foundation. PFC is a Wellcome Trust Senior Fellow in Clinical Science who also receives funding from the Medical Research Council (UK), the UK Parkinson’s Disease Society and the UK NIHR Biomedical Research Centre for Ageing and Age-related disease award to the Newcastle upon Tyne Foundation Hospitals NHS Trust.
Funding: Newcastle upon Tyne Hospitals Special Trustee.
Footnotes
Competing interests: None.
Ethics approval: This study was conducted with the approval of the Newcastle upon Tyne Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
REFERENCES
- 1.Gupta SR, Brigell M, Gujrati M, et al. Supranuclear eye movement dysfunction in mitochondrial myopathy with tRNA(LEU) mutation. J Neuroophthalmol. 1995;15:20–5. [PubMed] [Google Scholar]
- 2.Andrews RM, Griffiths PG, Chinnery PF, et al. Evaluation of bupivacaine-induced muscle regeneration in the treatment of ptosis in patients with chronic progressive external ophthalmoplegia and Kearns-Sayre syndrome. Eye. 1999;13:769–72. doi: 10.1038/eye.1999.225. [DOI] [PubMed] [Google Scholar]
- 3.Anastasopoulos D, Kimmig H, Mergner T, et al. Abnormalities of ocular motility in myotonic dystrophy. Brain. 1996;119:1923–32. doi: 10.1093/brain/119.6.1923. [DOI] [PubMed] [Google Scholar]
- 4.Kimmig H, Petrick M, Orszagh M, et al. Role of anterior and occipital white matter lesions for smooth eye tracking in myotonic dystrophy. J Neurol Neurosurg Psychiatry. 2002;72:808–11. doi: 10.1136/jnnp.72.6.808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leigh RJ, Zee DS. The neurology of eye movements. Oxford University Press; USA: 2006. p. 113. [Google Scholar]
- 6.Carlow TJ, Depper MH, Orrison WW., Jr MR of extraocular muscles in chronic progressive external ophthalmoplegia. Am J Neuroradiol. 1998;19:95–9. [PMC free article] [PubMed] [Google Scholar]
- 7.Ringel SP, Wilson WB, Barden MT. Extraocular muscle biopsy in chronic progressive external ophthalmoplegia. Ann Neurol. 1979;6:326–39. doi: 10.1002/ana.410060406. [DOI] [PubMed] [Google Scholar]
- 8.Yu Wai Man CY, Chinnery PF, Griffiths PG. Extraocular muscles have fundamentally distinct properties that make them selectively vulnerable to certain disorders. Neuromuscul Disord. 2005;15:17–23. doi: 10.1016/j.nmd.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Salvan A, Vion-Dury J, Confort-Gouny S, et al. Brain metabolic profiles obtained by proton MRS in two forms of mitochondriopathies: Leber’s hereditary optic neuropathy and chronic progressive external ophthalmoplegia. Eur Neurol. 1998;40:46–9. doi: 10.1159/000007955. [DOI] [PubMed] [Google Scholar]
- 10.Koutroumanidis M, Papadimitriou A, Bouzas E, et al. Reduced brain stem excitability in mitochondrial myopathy: evidence for early detection with blink reflex habituation studies. Muscle Nerve. 1996;19:1586–95. doi: 10.1002/(SICI)1097-4598(199612)19:12<1586::AID-MUS8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 11.Yee RD, Whitcup SM, Williams IM, et al. Saccadic eye movements in myasthenia gravis. Ophthalmology. 1987;94:219–25. doi: 10.1016/s0161-6420(87)33470-0. [DOI] [PubMed] [Google Scholar]
- 12.Irving EL, Steinbach MJ, Lillakas L, et al. Horizontal saccade dynamics across the human life span. Invest Ophthalmol Vis Sci. 2006;47:2478–84. doi: 10.1167/iovs.05-1311. [DOI] [PubMed] [Google Scholar]
- 13.Bittencourt PR, Smith AT, Lloyd DS, et al. Determination of smooth pursuit eye movement velocity in humans by computer. Electroencephalogr Clin Neurophysiol. 1982;54:399–405. doi: 10.1016/0013-4694(82)90203-6. [DOI] [PubMed] [Google Scholar]
- 14.Chambers BR, Gresty MA. The relationship between disordered pursuit and vestibulo-ocular reflex suppression. J Neurol Neurosurg Psychiatry. 1983;46:61–6. doi: 10.1136/jnnp.46.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mira E, Mevio E, Zanocco P, et al. Impaired suppression of vestibular nystagmus by fixation of visual and acoustic targets in neurological patients. Ann N Y Acad Sci. 1981;374:706–21. doi: 10.1111/j.1749-6632.1981.tb30912.x. [DOI] [PubMed] [Google Scholar]
- 16.Optican LM, Zee DS, Chu FC. Adaptive response to ocular muscle weakness in human pursuit and saccadic eye movements. J Neurophysiol. 1985;54:110. doi: 10.1152/jn.1985.54.1.110. [DOI] [PubMed] [Google Scholar]
- 17.Carey MR, Lisberger SG. Signals that modulate gain control for smooth pursuit eye movements in monkeys. J Neurophysiol. 2004;91:623–31. doi: 10.1152/jn.00525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]