Abstract
The immune system uses much of the classic machinery of cell biology, but in ways that put a different spin on organization and function. Striking recent examples include the demonstration of intraflagellar transport protein and hedgehog contributions to the immune synapse, even though immune cells lack a primary cilium that would be the typical setting for this machinery. In a second example, lymphocytes have their own subfamily of integrins, the β2 subfamily, and only integrins in this family form a stable adhesion ring using freely mobile ligands, a key feature of the immunological synapse. Finally, we showed recently that T-cells use endosomal sorting complexes required for transport (ESCRTs) at the plasma membrane to generate T-cell antigen receptor–enriched microvesicles. It is unusual for the ESCRT pathway to operate at the plasma membrane, but this may allow a novel form of cell–cell communication by providing a multivalent ligand for major histocompatibility complex–peptide complexes and perhaps other receptors on the partnering B-cell. Immune cells are thus an exciting system for novel cell biology even with classical pathways that have been studied extensively in other cell types.
INTRODUCTION
Recent studies on T-cells in the immune system reveal new implementations of classic cell biology pathways in unique ways suited to the T-cell's “liquid” lifestyle. The concept of “liquid” and “solid” tissues is used in oncology to distinguish hematopoietic malignancies that are found in the blood and lymphoid tissues (liquid) from those that organize into tumors within tissues (solid). The liquidity of T-cells is not restricted to the blood and also manifests in lymphoid tissues, where these small, highly dynamic cells rapidly move about on a lacey stromal scaffold (Miller et al., 2002; Bajenoff et al., 2006). Lymphoid tissues (including lymph nodes, spleen, and Peyer's patches) have a stromal scaffold decorated with a network of dendritic cells (DCs) that display potential ligands for T-cells. The T-cells swarm around in the tissues like foraging ants (Miller et al., 2003; Lindquist et al., 2004; Bajenoff et al., 2006). At this stage, the level of adhesion between cells is low, making it very easy to release the cells from the tissue as a liquid. Activated antigen–bearing DCs use a combination of chemokine signals that increase the number of T-cells that make transient contacts. Only T-cells expressing appropriate T-cell antigen receptors (TCRs), as defined by binding with presented major histocompatibility complex (MHC)–peptide complexes, dwell longer with the DC or B-cells, both of which can present antigen (Castellino et al., 2006; Harris et al., 2012; Figure 1, A and B). It is important to note that the use of somatic gene rearrangement to generate the TCR (and the related B-cell antigen receptors) is a unique innovation of the immune system with no imitators (Hozumi and Tonegawa, 1976; Davis et al., 1984). The antigen-specific interface between T-cells and DCs can lead to a stable immunological synapse that lasts several hours (Iezzi et al., 1999; Lee et al., 2002; Huppa et al., 2003). The use of the term synapse is meant to convey a stable interface mediated by specific receptors across which chemical signals are relayed in a polarized manner (Dustin and Colman, 2002). Some unique cell biology takes place in or near the immunological synapse. This perspective will focus on how T-cells use three classic pathways—hedgehog, integrins, and endosomal sorting complexes required for transport (ESCRTs)—in the immunological synapse with a different “spin” compared with stromal models. T-cells seem to push these systems to extremes that are not observed in other cell types.
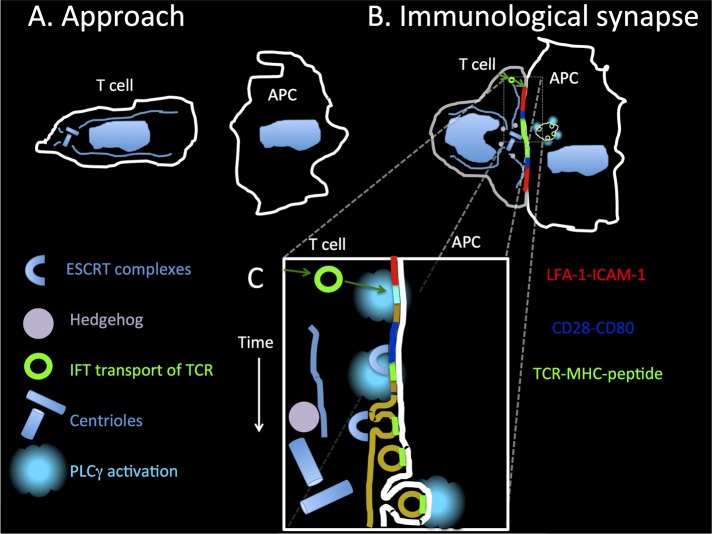
FIGURE 1:
(A) T-cells approach APCs using a combination of chemokinesis and chemotaxis (particularly for activated APCs). The white outline of the T-cell reflects a mix of TCR (green), CD28 (blue), and LFA-1 (red). (B) When the T-cell encounters the APC with appropriate MHC–peptide complexes, an immunological synapse forms, with coarse segregation of TCR and bound MHC–peptide into the center (green), an intermediate ring of CD28–CD80 interactions (blue), and an adhesion ring of LFA-1–ICAM-1 interaction (red). Hedgehog vesicles are formed (killer T-cells), and TCRs are delivered to the synapse by IFT20 vesicles. Microvesicles containing TCR–MHC–peptide interactions are internalized by B-cells and induce signaling. (C) Optical–electron microscopy correlation led to discovery of TCR-enriched microvesicles. The actin cytoskeleton moves the microclusters downward in the schematic, and this also serves as a time line for TCR microcluster and microvesicle formation. TCRs are delivered by IFT20 vesicles, a signaling microcluster is initiated, the ESCRT machinery recognizes ubiquitin added to TCR in microclusters and sorts the TCR into plasma membrane buds that are released into the synapse center, and then the APC takes up the TCR-enriched vesicle, which can trigger phospholipase Cγ in the APC even in the absence of the T-cell.
INTRAFLAGELLAR TRANSPORT AND THE T-CELL'S INNER CILIUM
Cytotoxic T-lymphocytes (CTLs) induce apoptosis of cells harboring intracellular pathogens (e.g., viruses) and some tumor cells. Early studies demonstrated that they dramatically change shape when they encounter targets with specific antigens and undergo a remarkable internal rearrangement to bring the centrioles to the immunological synapse with the target cell (Geiger et al., 1982). Similar events take place in helper T-cells (Kupfer et al., 1983). In both T-cell types, which share a similar antigen receptor (TCR) but have different functions, the immunological synapse acts as both a sensory structure and a site of delivery of soluble components into a protected synaptic cleft (Stinchcombe et al., 2001). In other cells, this sensory role is focused on a tiny primary cilium (Baldari and Rosenbaum, 2010). However, leukocytes lack a primary cilium. The volume within 500 nm of the immunological synapse and centrioles is abuzz with organelles and secretory vesicles. Baldari and Rosenbaum, and Griffiths and colleagues, converged on the concept that the T-cells might provisionally assemble a primary cilium–like structure at the immunological synapse.
The formation of a primary cilium requires a set of microtubule dependent transport processes that are organized by the intraflagellar transport (IFT) proteins. These form complexes with cargo that are needed to build and maintain cilia and flagella, but could also have other cargo delivery functions. Baldari, Rosenbaum, and coworkers investigated whether helper T-cells have IFTs and what they do (Finetti et al., 2009). They targeted IFT20, a protein that shuttles between the cilium and the Golgi apparatus and is necessary for motility of sperm flagella. IFT20 was expressed in T-cells and was concentrated near the immunological synapse and the Golgi apparatus. Knockdown of IFT20 resulted in depletion of TCR from the immunological synapse. This was due to failure of TCR intracellular transport to the synapse (Figure 1, B and C). Although T-cells are not large, the time required for diffusion of molecules from the far side of the cells is on the order of 20–40 min. Because T-cells form immunological synapses in a few minutes and may execute some functions and break off interactions in as little as 5–10 min, the time required for TCR to reach the immunological synapse is critical. This failure of TCR delivery significantly reduces T-cell responses. This observation supported a broader speculation that the immunological synapse contains the T-cell primary cilium equivalent.
Griffiths and colleagues have been leaders identifying molecular pathways in CTLs based on analysis of patients with primary immunodeficiencies and pigmentation defects, which can involve overlapping cytoplasmic transport mechanisms (Stinchcombe et al., 2000). They found that the centrioles actually appear to dock to the plasma membrane in CTLs in a manner reminiscent of centriole docking during cilium generation (Stinchcombe et al., 2006). This led them to ask similar questions to those of Baldari and Rosenbaum, but looking at the receptor level. The hedgehog pathway is obligatorily dependent on the primary cilium for function. However, no one had looked for a role of this pathway in T-cells previously. In a recently published study, they showed that CTLs express the receptor patched, the ligand indian hedgehog, and the receptor proximal signal transducer smoothened (de la Roche et al., 2013). They demonstrated that operation of the pathway early in activation of CTL leads to the generation of the transcription factor Gli and increased expression of Rac1, a key regulator of actin cytoskeleton (de la Roche et al., 2013). Hedgehog signaling is typically paracrine, with different nearby cells making ligands for responding cells. This is consistent with the importance of positioning smoothened and patched on the exposed primary cilium. T-cells take autocrine signaling to an extreme, in that they do not respond at all to extracellular indian hedgehog (de la Roche et al., 2013). Both the smoothened and patched receptors and the endogenously produced indian hedgehog ligand are present in cytoplasmic vesicles near the immunological synapse. The implication is that T-cells assemble an “inner” primary cilium at the immunological synapse. This feature of the immunological synapse allows T-cells to patch into the hedgehog-signaling pathway without being distracted by hedgehog ligands in the environment. In T-cells, it is possible that smoothened, patched, and indian hedgehog can all be expressed constitutively but may be assembled into an appropriate primary cilium–like configuration only upon immunological synapse formation. Whether this signaling process is entirely canonical Gli-mediated transcription of the actin regulator Rac1 or may also involve a component of noncanonical activation of existing Rac1 remains to be determined (Polizio et al., 2011a, b). A noncanonical role might also be supported by a recent report that patched1 is not required for immune function (Michel et al., 2013). In any case, there is compelling evidence that the immune system has incorporated intraflagellar transport and hedgehog into the immunological synapse.
REVERSIBLE SYMMETRY BREAKING
The immunological synapse has a characteristic bull's-eye structure that was first observed by Kupfer in cell–cell systems and then reconstituted in supported lipid bilayers (SLBs) using the integrin ligand ICAM-1 and the TCR ligand MHC–peptide complex (Monks et al., 1998; Grakoui et al., 1999). The reconstitution approach demonstrated that the TCR ligands move from the periphery to the center to form an MHC–peptide bull's eye surrounded by an adhesion ring (Grakoui et al., 1999). The adhesion ring is formed by the integrin LFA-1 and its ligand ICAM-1 (in the SLB). LFA-1 is a β2 subfamily, which is expressed only in leukocytes. Of interest, β1 integrins expressed on T- and B-cells cannot form a stable adhesion ring but end up in the synapse center in most cases (Carrasco and Batista, 2006). Studies using nanopatterned SLB reveal that β1 integrins of stromal cells are not activated to appropriate levels by freely mobile ligands (Yu et al., 2011), whereas LFA-1 is fully functional when interacting with freely mobile ICAM-1. Increasing the valency of ICAM-1 by clustering it in bilayers also leads to LFA-1 collapse into the center (Hartman et al., 2009). Thus the ability to form a stable adhesion ring seems to require reversibility of the LFA-1–ICAM-1 interaction, which is suppressed when the valency of the interaction is increased by local ligand clustering. A particularly interesting phenomenon is the ability of T-cells to maintain their position or move by manipulating the adhesion ring (Grakoui et al., 1999; Sims et al., 2007). When T-cells break symmetry of the adhesion ring, migration away from the opening in the ring immediately ensues. The symmetric adhesion ring can then reform some distance away and regenerate the immunological synapse pattern. Genetic studies show that symmetry breaking is promoted by protein kinase C-θ and countered by Wiskott–Aldrich syndrome protein (Sims et al., 2007). In this context, the adhesion ring can be compared with the lamella of motile stromal cells. Symmetry breaking is seen to arise from myosin II–dependent mechanical instabilities (Paluch et al., 2005; Yam et al., 2007). In this context, symmetry breaking is literally based on a fracture in the actin network due to increase myosin II–driven contraction. The stable immunological synapse displays centripetal F-actin flow starting from the outside edge of the synapse through the adhesion ring and to the outside of the central cluster (Kaizuka et al., 2007; Yi et al., 2012). This flowing network is slower and more visibly web-like in the myosin II– and integrin-rich peripheral supramolecular activation complex (pSMAC). When myosin IIA is decreased by small interfering RNA or inhibited with blebbistatin, the pSMAC structure lacks this web-like coherence, and the system also cannot maintain symmetry (Kumari et al., 2012). Therefore the formation of a stable immunological synapse seems to be a balancing act between having the correct mix of F-actin and myosin II activity in the pSMAC to generate a coherent adhesion ring without generating local mechanical instabilities that lead to symmetry breaking.
MICROCLUSTERS TO MICROVESICLES
The immunological synapse bull's eye can be resolved into three major compartments when the costimulatory ligand CD80 is added to the SLB. The outer ring is composed of integrin microclusters (pSMAC), and the central cluster splits into an outer central SMAC (cSMAC) ring rich in CD28–CD80 interactions and an inner cSMAC rich in TCR and MHC–peptide (Tseng et al., 2008; Yokosuka et al., 2008). The F-actin flow appears to effectively deliver TCR microclusters to the outer cSMAC. CD2-associated protein (CD2AP)–knockout mice displayed complex defects in signal termination, TCR down-regulation, and cSMAC formation (Dustin et al., 1998; Lee et al., 2003). A more extreme manifestation of this phenotype is elaborated when the ESCRT family member TSG-101 is knocked down (Vardhana et al., 2010). In this case, the TCRs are confined to the outer cSMAC ring even when no CD80 is included in the SLB (Vardhana et al., 2010). The interpretation of this ESCRT-dependent process was not clear until electron microscopy experiments were performed.
The SLB-based reconstitution of the immunological synapse has the advantage of offering laterally mobile physiological ligands with ideal optics. Although there are caveats to using a passive antigen-presenting system, there are also opportunities. For example, the SLB system might accumulate intermediates before the first active role of the antigen-presenting cell (APC). In this regard, the central cluster of MHC–peptide complexes has different characteristics in the T-cell–SLB and T-cell–B-cell systems. In the T-cell–SLB system the central accumulation of TCR is linear with MHC–peptide complexes, whereas the central accumulation of MHC–peptide in the T-cell–APC system seems to be maintained at a much lower level, although this is not immediately obvious from looking at early images (Monks et al., 1998; Grakoui et al., 1999). This suggests that TCRs are trapped at the T-cell–SLB interface, whereas they would be removed from the T-cell–APC interface, presumably by internalization in the T-cell. We used optical–electron correlation microscopy to examine the location of TCRs in the T-cell–SLB system (Choudhuri et al., 2014). To our surprise, we found that TCRs accumulated in many extracellular microvesicles trapped in the immune synaptic cleft. Further analysis demonstrated that the extracellular microvesicles were generated in an ESCRT-dependent manner at the plasma membrane (Figure 1, B and C). The vesicles are deposited on the SLB, allowing continued binding of MHC–peptide complexes, although the vesicles are separated from the cell, and no signaling in the T-cell is possible. When T-cells eventually break symmetry, they leave a patch of TCR-enriched material with bound MHC–peptide complexes on the SLB (Dustin et al., 1996; Grakoui et al., 1999). The optical–electron correlation analysis shows that these are patches of microvesicles (Choudhuri et al., 2014) that disperse by diffusion on the SLB because they are bound to laterally mobile MHC–peptide complexes. When live B-cells bearing the appropriate MHC–peptide complex come into contact with the patches of microvesicles, they respond by increasing cytoplasmic Ca2+ through activation of phospholipase Cγ (Figure 1, B and C). Furthermore, in helper T-cell–B-cell conjugates, TCRs are transferred into the B-cells in an ESCRT-dependent manner. Thus TCR-enriched microvesicles are trapped intermediates on SLBs but are taken into antigen-presenting B-cells, where they can continue to activate the B-cell even when the T-cell is gone. This is a new form of signaling between T-cells and APCs and reflects an atypical ESCRT-dependent sorting process at the plasma membrane. Along these lines, it is important to note that cell-free, TCR-dependent activities were invoked in studies on CTL-mediated killing and T-cell–B-cell collaboration that might be accounted for by TCR-enriched microvesicles (Guy et al., 1989; Peters et al., 1990). An important characteristic of ESCRT-dependent sorting at the immunological synapse is that it moves TCRs into microvesicles while retaining the cosignaling receptor CD28 in the plasma membrane for sustained signaling.
VIRAL INFORMATION TRANSFER
The generation of TCR-enriched microvesicles by T-cells and their transfer to APCs is reminiscent of the virological synapse, where HTLV1 or HIV-infected T-cells transfer viral particles to noninfected cells through a synapse-like interface (Igakura et al., 2003; Jolly et al., 2004). Retroviral budding is ESCRT dependent. Whereas the TCR is ubiquitinated and ubiquitin recognition is essential for sorting into the immunological synapse center, HIV Gag directly interacts with TSG-101 and ALIX to access the ESCRT machinery at the plasma membrane. It has also been noted in the context of HIV budding that T-leukemic cells possess polarized domains of ESCRT activity at the plasma membrane (Booth et al., 2006). There are various models for how virological synapses are triggered, but antigen recognition had not been evaluated as a polarizing signal. Of interest, HIV Gag–green fluorescent protein (GFP) expression in human T-cells excluded TCRs from the synapse center and replaced the TCRs by Gag-GFP in the inner cSMAC (Choudhuri et al., 2014). The optical–electron microscopy correlation analysis revealed that the Gag-GFP–positive regions correspond to virus-like particles—viral envelopes without genetic material, or, in this case, any HIV protein other than Gag. On the basis of these observations, we can entertain the possibility that antigen-dependent virological synapses could play an important role in infection of macrophages (Duncan et al., 2014) and generation of viral depots in DCs (McDonald et al., 2003). Although the TCR-enriched microvesicles that are normally released into the immunological synapse lack anything resembling a viral genome, they may contain nucleic acids than can influence the APC. For example, it has been demonstrated that microRNAs in T-cell–derived exosomes can reprogram B-cells that receive them through an immunological synapse (Mittelbrunn et al., 2011).
CONCLUSIONS
T-cells have come up with some unique solutions to the need for interconverting between rapid scanning of antigen-presenting cells to provisional synapses that allow for prolonged and polarized information exchange. The adhesion ring of the immunological synapse is based on lamellar organization of integrin-dependent adhesion in stromal cells but using different integrins that bind and release faster to allow for rapid motility or aggressive antigen gathering into the immunological synapse center. Although blood cells lack a primary cilium, T-cells have come up with a way to simulate the confinement of the primary cilium in a cytoplasmic compartment that allows obligate autocrine hedgehog signaling to make CTLs better killers. Our own recent data also show how T-cells use ESCRTs at the plasma membrane to generate TCR-enriched microvesicles that can deliver signals related to T-cells to help B-cells and perhaps other types of APCs. In each case the T-cell uses a classic pathway in cell biology in a new way that is not observed in other cell types.
Abbreviations used:
- APC
antigen-presenting cell
- CTL
cytotoxic T-lymphocyte
- DCs
dendritic cells
- ESCRT
endosomal sorting complexes required for transport
- IFT
intraflagellar transport
- MHC
major histocompatibility complex
- SLB
supported lipid bilayer
- SMAC
supramolecular activation cluster
- TCR
T-cell receptor
Footnotes
REFERENCES
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldari CT, Rosenbaum J. Intraflagellar transport: it's not just for cilia anymore. Curr Opin Cell Biol. 2010;22:75–80. doi: 10.1016/j.ceb.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol. 2006;172:923–935. doi: 10.1083/jcb.200508014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrasco YR, Batista FD. B-cell activation by membrane-bound antigens is facilitated by the interaction of VLA-4 with VCAM-1. EMBO J. 2006;25:889–899. doi: 10.1038/sj.emboj.7600944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature. 2006;440:890–895. doi: 10.1038/nature04651. [DOI] [PubMed] [Google Scholar]
- Choudhuri K, Llodra J, Roth EW, Tsai J, Gordo S, Wucherpfennig KW, Kam LC, Stokes DL, Dustin ML. Polarized release of T-cell-receptor-enriched microvesicles at the immunological synapse. Nature. 2014;507:118–123. doi: 10.1038/nature12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MM, Chien YH, Gascoigne NR, Hedrick SM. A murine T cell receptor gene complex: isolation, structure and rearrangement. Immunol Rev. 1984;81:235–258. doi: 10.1111/j.1600-065x.1984.tb01113.x. [DOI] [PubMed] [Google Scholar]
- de la Roche M, Ritter AT, Angus KL, Dinsmore C, Earnshaw CH, Reiter JF, Griffiths GM. Hedgehog signaling controls T cell killing at the immunological synapse. Science. 2013;342:1247–1250. doi: 10.1126/science.1244689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan CJ, Williams JP, Schiffner T, Gartner K, Ochsenbauer C, Kappes J, Russell RA, Frater J, Sattentau QJ. High multiplicity HIV-1 infection and neutralizing antibody evasion mediated by the macrophage-T cell virological synapse. J Virol 88, 2025–2034. 2014 doi: 10.1128/JVI.03245-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dustin ML, Colman DR. Neural and immunological synaptic relations. Science. 2002;298:785–789. doi: 10.1126/science.1076386. [DOI] [PubMed] [Google Scholar]
- Dustin ML, Miller JM, Ranganath S, Vignali DA, Viner NJ, Nelson CA, Unanue ER. TCR-mediated adhesion of T cell hybridomas to planar bilayers containing purified MHC class II/peptide complexes and receptor shedding during detachment. J Immunol. 1996;157:2014–2021. [PubMed] [Google Scholar]
- Dustin ML, et al. A novel adapter protein orchestrates receptor patterning and cytoskeletal polarity in T cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- Finetti F, Paccani SR, Riparbelli MG, Giacomello E, Perinetti G, Pazour GJ, Rosenbaum JL, Baldari CT. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol. 2009;11:1332–1339. doi: 10.1038/ncb1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Rosen D, Berke G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J Cell Biol. 1982;95:137–143. doi: 10.1083/jcb.95.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. The immunological synapse: a molecular machine controlling T cell activation. Science. 1999;285:221–227. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- Guy R, Ullrich SJ, Foo-Philips M, Hathcock KS, Appella E, Hodes RJ. Antigen-specific helper function of cell-free T cell products bearing TCR V beta 8 determinants. Science. 1989;244:1477–1480. doi: 10.1126/science.2472009. [DOI] [PubMed] [Google Scholar]
- Harris TH, et al. Generalized Levy walks and the role of chemokines in migration of effector CD8+ T cells. Nature. 2012;486:545–548. doi: 10.1038/nature11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci USA. 2009;106:12729–12734. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hozumi N, Tonegawa S. Evidence for somatic rearrangement of immunoglobulin genes coding for variable and constant regions. Proc Natl Acad Sci USA. 1976;73:3628–3632. doi: 10.1073/pnas.73.10.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppa JB, Gleimer M, Sumen C, Davis MM. Continuous T cell receptor signaling required for synapse maintenance and full effector potential. Nat Immunol. 2003;4:749–755. doi: 10.1038/ni951. [DOI] [PubMed] [Google Scholar]
- Iezzi G, Scotet E, Scheidegger D, Lanzavecchia A. The interplay between the duration of TCR and cytokine signaling determines T cell polarization. Eur J Immunol. 1999;29:4092–4101. doi: 10.1002/(SICI)1521-4141(199912)29:12<4092::AID-IMMU4092>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Igakura T, Stinchcombe JC, Goon PK, Taylor GP, Weber JN, Griffiths GM, Tanaka Y, Osame M, Bangham CR. Spread of HTLV-I between lymphocytes by virus-induced polarization of the cytoskeleton. Science. 2003;299:1713–1716. doi: 10.1126/science.1080115. [DOI] [PubMed] [Google Scholar]
- Jolly C, Kashefi K, Hollinshead M, Sattentau QJ. HIV-1 cell to cell transfer across an Env-induced, actin-dependent synapse. J Exp Med. 2004;199:283–293. doi: 10.1084/jem.20030648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci USA. 2007;104:20296–20301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari S, Vardhana S, Cammer M, Curado S, Santos L, Sheetz MP, Dustin ML. T lymphocyte myosin IIA is required for maturation of the immunological synapse. Front Immunol. 2012;3:230. doi: 10.3389/fimmu.2012.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfer A, Dennert G, Singer SJ. Polarization of the Golgi apparatus and the microtubule-organizing center within cloned natural killer cells bound to their targets. Proc Natl Acad Sci USA. 1983;80:7224–7228. doi: 10.1073/pnas.80.23.7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–1222. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T cell receptor signaling precedes immunological synapse formation. Science. 2002;295:1539–1542. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, Nussenzweig MC. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–1250. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]
- McDonald D, Wu L, Bohks SM, KewalRamani VN, Unutmaz D, Hope TJ. Recruitment of HIV and its receptors to dendritic cell-T cell junctions. Science. 2003;300:1295–1297. doi: 10.1126/science.1084238. [DOI] [PubMed] [Google Scholar]
- Michel KD, Uhmann A, Dressel R, van den Brandt J, Hahn H, Reichardt HM. The hedgehog receptor patched1 in T cells is dispensable for adaptive immunity in mice. PLoS One. 2013;8:e61034. doi: 10.1371/journal.pone.0061034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Cahalan MD, Parker I. Autonomous T cell trafficking examined in vivo with intravital two-photon microscopy. Proc Natl Acad Sci USA. 2003;100:2604–2609. doi: 10.1073/pnas.2628040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MJ, Wei SH, Parker I, Cahalan MD. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Science. 2002;296:1869–1873. doi: 10.1126/science.1070051. [DOI] [PubMed] [Google Scholar]
- Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, Bernad A, Sanchez-Madrid F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat Commun. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks CR, Freiberg BA, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Paluch E, Piel M, Prost J, Bornens M, Sykes C. Cortical actomyosin breakage triggers shape oscillations in cells and cell fragments. Biophys J. 2005;89:724–733. doi: 10.1529/biophysj.105.060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters PJ, Geuze HJ, van der Donk HA, Borst J. A new model for lethal hit delivery by cytotoxic T lymphocytes. Immunol Today. 1990;11:28–32. doi: 10.1016/0167-5699(90)90008-w. [DOI] [PubMed] [Google Scholar]
- Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem. 2011a;286:19589–19596. doi: 10.1074/jbc.M110.197111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizio AH, Chinchilla P, Chen X, Manning DR, Riobo NA. Sonic Hedgehog activates the GTPases Rac1 and RhoA in a Gli-independent manner through coupling of smoothened to Gi proteins. Sci Signal. 2011b;4:pt7. doi: 10.1126/scisignal.2002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TN, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature. 2006;443:462–465. doi: 10.1038/nature05071. [DOI] [PubMed] [Google Scholar]
- Stinchcombe JC, Page LJ, Griffiths GM. Secretory lysosome biogenesis in cytotoxic T lymphocytes from normal and Chediak Higashi syndrome patients. Traffic. 2000;1:435–444. doi: 10.1034/j.1600-0854.2000.010508.x. [DOI] [PubMed] [Google Scholar]
- Tseng SY, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase Ctheta. J Immunol. 2008;181:4852–4863. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhana S, Choudhuri K, Varma R, Dustin ML. Essential role of ubiquitin and TSG101 protein in formation and function of the central supramolecular activation cluster. Immunity. 2010;32:531–540. doi: 10.1016/j.immuni.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam PT, Wilson CA, Ji L, Hebert B, Barnhart EL, Dye NA, Wiseman PW, Danuser G, Theriot JA. Actin-myosin network reorganization breaks symmetry at the cell rear to spontaneously initiate polarized cell motility. J Cell Biol. 2007;178:1207–1221. doi: 10.1083/jcb.200706012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi J, Wu XS, Crites T, Hammer JA., 3rd Actin retrograde flow and actomyosin II arc contraction drive receptor cluster dynamics at the immunological synapse in Jurkat T cells. Mol Biol Cell. 2012;23:834–852. doi: 10.1091/mbc.E11-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, Dustin ML, Tokunaga M, Saito T. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu CH, Law JB, Suryana M, Low HY, Sheetz MP. Early integrin binding to Arg-Gly-Asp peptide activates actin polymerization and contractile movement that stimulates outward translocation. Proc Natl Acad Sci USA. 2011;108:20585–20590. doi: 10.1073/pnas.1109485108. [DOI] [PMC free article] [PubMed] [Google Scholar]