Abstract
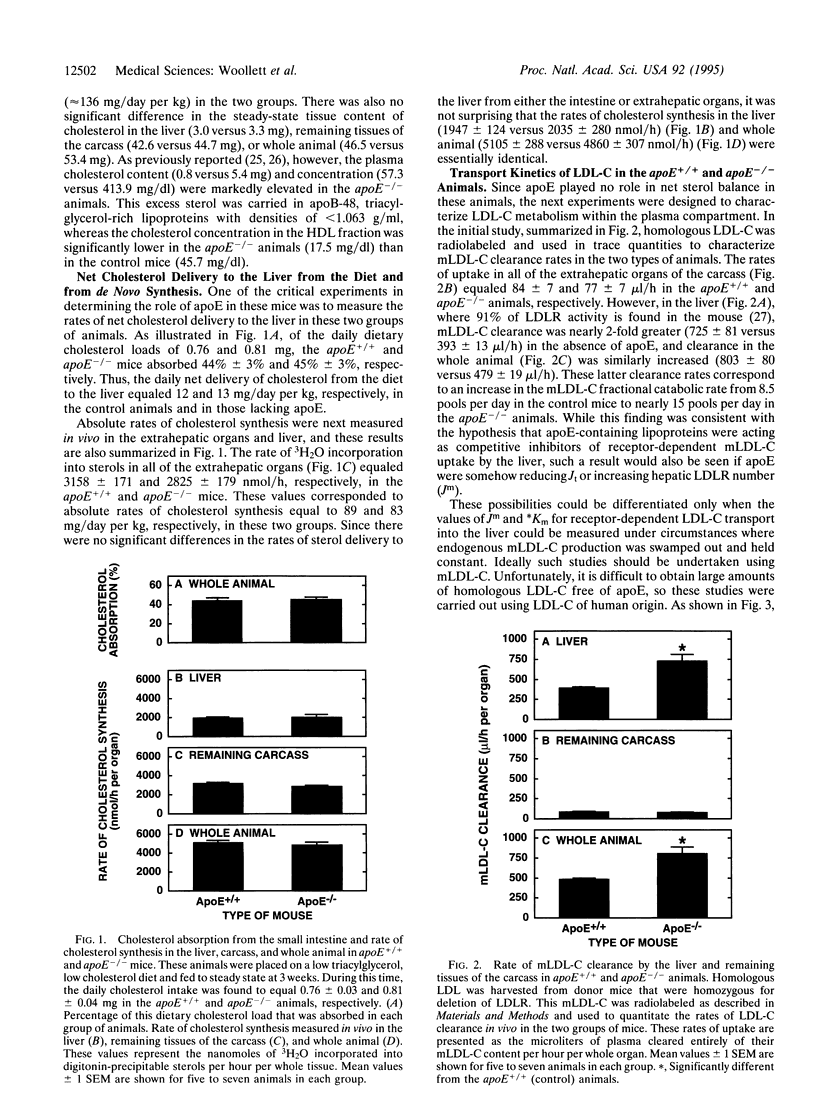
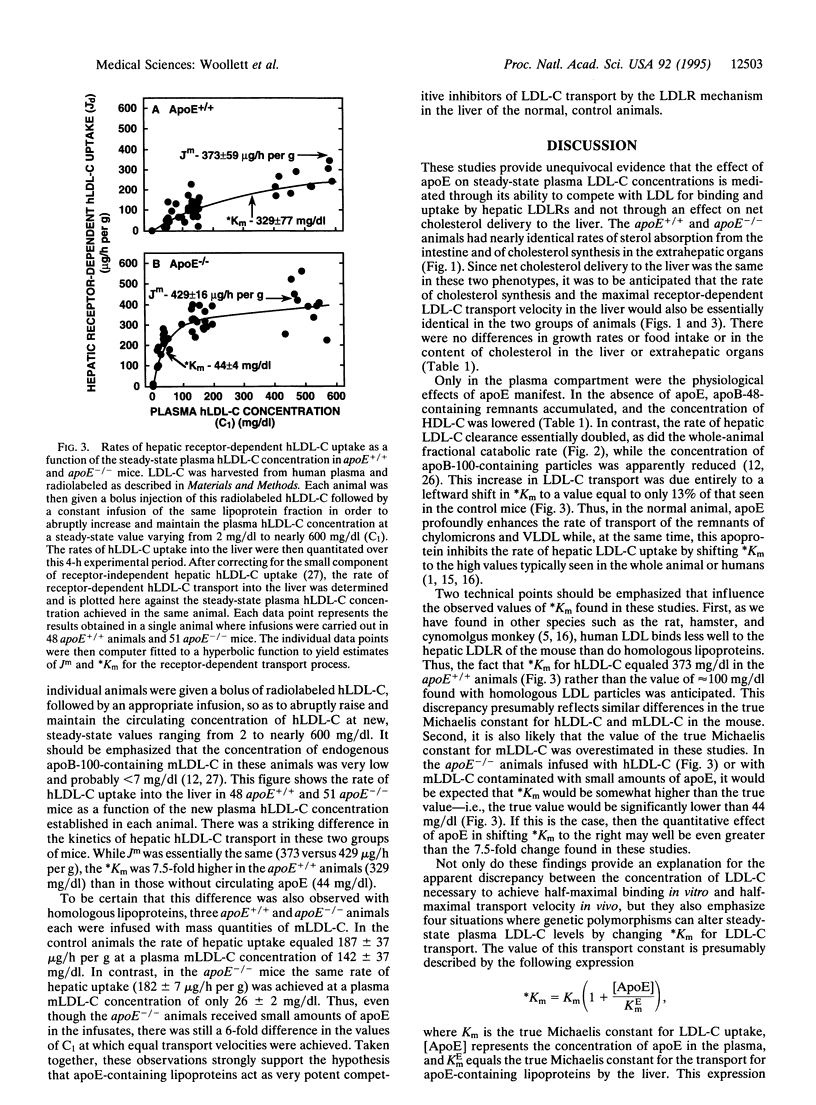
This study examines the question of whether apolipoprotein E (apoE) alters steady-state concentrations of plasma cholesterol carried in low density lipoproteins (LDL-C) by acting as a competitive inhibitor of hepatic LDL uptake or by altering the rate of net cholesterol delivery from the intestinal lumen to the liver. To differentiate between these two possibilities, rates of cholesterol absorption and synthesis and the kinetics of hepatic LDL-C transport were measured in vivo in mice with either normal (apoE+/+) or zero (apoE-/-) levels of circulating apoE. Rates of cholesterol absorption were essentially identical in both genotypes and equaled approximately 44% of the daily dietary load of cholesterol. This finding was consistent with the further observation that the rates of cholesterol synthesis in the liver (approximately 2,000 nmol/h) and extrahepatic tissues (approximately 3,000 nmol/h) were also essentially identical in the two groups of mice. However, the apparent Michaelis constant for receptor-dependent hepatic LDL-C uptake was markedly lower in the apoE-/- mice (44 +/- 4 mg/dl) than in the apoE+/+ animals (329 +/- 77 mg/dl) even though the maximal transport velocity for this uptake process was essentially the same (approximately 400 micrograms/h per g) in the two groups of mice. These studies, therefore, demonstrate that apoE-containing lipoproteins can act as potent competitive inhibitors of hepatic LDL-C transport and so can significantly increase steady-state plasma LDL-C levels. This apolipoprotein plays no role, however, in the regulation of cholesterol absorption, sterol biosynthesis, or hepatic LDL receptor number, at least in the mouse.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daumerie C. M., Woollett L. A., Dietschy J. M. Fatty acids regulate hepatic low density lipoprotein receptor activity through redistribution of intracellular cholesterol pools. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10797–10801. doi: 10.1073/pnas.89.22.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davignon J., Gregg R. E., Sing C. F. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988 Jan-Feb;8(1):1–21. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- Dietschy J. M., Turley S. D., Spady D. K. Role of liver in the maintenance of cholesterol and low density lipoprotein homeostasis in different animal species, including humans. J Lipid Res. 1993 Oct;34(10):1637–1659. [PubMed] [Google Scholar]
- Gylling H., Aalto-Setälä K., Kontula K., Miettinen T. A. Serum low density lipoprotein cholesterol level and cholesterol absorption efficiency are influenced by apolipoprotein B and E polymorphism and by the FH-Helsinki mutation of the low density lipoprotein receptor gene in familial hypercholesterolemia. Arterioscler Thromb. 1991 Sep-Oct;11(5):1368–1375. doi: 10.1161/01.atv.11.5.1368. [DOI] [PubMed] [Google Scholar]
- Gylling H., Kuusi T., Vanhanen H., Miettinen T. A. Apolipoprotein E phenotype and cholesterol metabolism in familial hypercholesterolemia. Atherosclerosis. 1989 Nov;80(1):27–32. doi: 10.1016/0021-9150(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Herz J., Maeda N., Goldstein J. L., Brown M. S. The two-receptor model of lipoprotein clearance: tests of the hypothesis in "knockout" mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeske D. J., Dietschy J. M. Regulation of rates of cholesterol synthesis in vivo in the liver and carcass of the rat measured using [3H]water. J Lipid Res. 1980 Mar;21(3):364–376. [PubMed] [Google Scholar]
- Kesäniemi Y. A., Ehnholm C., Miettinen T. A. Intestinal cholesterol absorption efficiency in man is related to apoprotein E phenotype. J Clin Invest. 1987 Aug;80(2):578–581. doi: 10.1172/JCI113107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita T., Goldstein J. L., Brown M. S., Watanabe Y., Hornick C. A., Havel R. J. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley R. W. Apolipoprotein E: cholesterol transport protein with expanding role in cell biology. Science. 1988 Apr 29;240(4852):622–630. doi: 10.1126/science.3283935. [DOI] [PubMed] [Google Scholar]
- Meddings J. B., Dietschy J. M. Regulation of plasma levels of low-density lipoprotein cholesterol: interpretation of data on low-density lipoprotein turnover in man. Circulation. 1986 Oct;74(4):805–814. doi: 10.1161/01.cir.74.4.805. [DOI] [PubMed] [Google Scholar]
- Miettinen T. A., Gylling H., Vanhanen H., Ollus A. Cholesterol absorption, elimination, and synthesis related to LDL kinetics during varying fat intake in men with different apoprotein E phenotypes. Arterioscler Thromb. 1992 Sep;12(9):1044–1052. doi: 10.1161/01.atv.12.9.1044. [DOI] [PubMed] [Google Scholar]
- Osono Y., Woollett L. A., Herz J., Dietschy J. M. Role of the low density lipoprotein receptor in the flux of cholesterol through the plasma and across the tissues of the mouse. J Clin Invest. 1995 Mar;95(3):1124–1132. doi: 10.1172/JCI117760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita J. A., Zhang S. H., Hagaman J. R., Oliver P. M., Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embryonic stem cells. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4471–4475. doi: 10.1073/pnas.89.10.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson F. W., Cumming A. M. Effects of apoprotein E polymorphism on serum lipoprotein concentration. Arteriosclerosis. 1985 May-Jun;5(3):283–292. doi: 10.1161/01.atv.5.3.283. [DOI] [PubMed] [Google Scholar]
- Soria L. F., Ludwig E. H., Clarke H. R., Vega G. L., Grundy S. M., McCarthy B. J. Association between a specific apolipoprotein B mutation and familial defective apolipoprotein B-100. Proc Natl Acad Sci U S A. 1989 Jan;86(2):587–591. doi: 10.1073/pnas.86.2.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Bilheimer D. W., Dietschy J. M. Rates of receptor-dependent and -independent low density lipoprotein uptake in the hamster. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3499–3503. doi: 10.1073/pnas.80.11.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Dietary saturated triacylglycerols suppress hepatic low density lipoprotein receptor activity in the hamster. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4526–4530. doi: 10.1073/pnas.82.13.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983 Mar;24(3):303–315. [PubMed] [Google Scholar]
- Spady D. K., Meddings J. B., Dietschy J. M. Kinetic constants for receptor-dependent and receptor-independent low density lipoprotein transport in the tissues of the rat and hamster. J Clin Invest. 1986 May;77(5):1474–1481. doi: 10.1172/JCI112460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spady D. K., Woollett L. A., Dietschy J. M. Regulation of plasma LDL-cholesterol levels by dietary cholesterol and fatty acids. Annu Rev Nutr. 1993;13:355–381. doi: 10.1146/annurev.nu.13.070193.002035. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Daggy B. P., Dietschy J. M. Cholesterol-lowering action of psyllium mucilloid in the hamster: sites and possible mechanisms of action. Metabolism. 1991 Oct;40(10):1063–1073. doi: 10.1016/0026-0495(91)90131-f. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Daggy B. P., Dietschy J. M. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology. 1994 Aug;107(2):444–452. doi: 10.1016/0016-5085(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Turley S. D., Spady D. K., Dietschy J. M. Role of liver in the synthesis of cholesterol and the clearance of low density lipoproteins in the cynomolgus monkey. J Lipid Res. 1995 Jan;36(1):67–79. [PubMed] [Google Scholar]
- Vega G. L., Grundy S. M. In vivo evidence for reduced binding of low density lipoproteins to receptors as a cause of primary moderate hypercholesterolemia. J Clin Invest. 1986 Nov;78(5):1410–1414. doi: 10.1172/JCI112729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Willnow T. E., Armstrong S. A., Hammer R. E., Herz J. Functional expression of low density lipoprotein receptor-related protein is controlled by receptor-associated protein in vivo. Proc Natl Acad Sci U S A. 1995 May 9;92(10):4537–4541. doi: 10.1073/pnas.92.10.4537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willnow T. E., Sheng Z., Ishibashi S., Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994 Jun 3;264(5164):1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- Woollett L. A., Spady D. K., Dietschy J. M. Saturated and unsaturated fatty acids independently regulate low density lipoprotein receptor activity and production rate. J Lipid Res. 1992 Jan;33(1):77–88. [PubMed] [Google Scholar]
- Zhang S. H., Reddick R. L., Piedrahita J. A., Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992 Oct 16;258(5081):468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]



