Abstract
The limb girdle muscular dystrophies (LGMDs) are a group of disorders with wide genetic and clinical heterogeneity. Recently, mutations in the ANO5 gene, which encodes a putative calcium-activated chloride channel belonging to the Anoctamin family of proteins, were identified in five families with one of two previously identified disorders, LGMD2L and non-dysferlin Miyoshi muscular dystrophy (MMD3). We screened a candidate group of 64 patients from 59 British and German kindreds and found the truncating mutation, c.191dupA in exon 5 of ANO5 in 20 patients, homozygously in 15 and in compound heterozygosity with other ANO5 variants in the rest. An intragenic SNP and an extragenic microsatellite marker are in linkage disequilibrium with the mutation, suggesting a founder effect in the Northern European population. We have further defined the clinical phenotype of ANO5-associated muscular dystrophy. Patients show adult onset proximal lower limb weakness with highly raised creatinine kinase (CK) values (average 4500 IU/l) and frequent muscle atrophy and asymmetry of muscle involvement. Onset varies from the early 20s to 50s and the weakness is generally slowly progressive, with most patients remaining ambulant for several decades. Distal presentation is much less common but a milder degree of distal lower limb weakness is often observed. Upper limb strength is only mildly affected and cardiac and respiratory function is normal. Females appear less frequently affected. In the North of England population we have identified eight patients with ANO5 mutations, suggesting a minimum prevalence of 0.27/100 000, twice as common as dysferlinopathy. We suggest that mutations in ANO5 represent a relatively common cause of adult onset muscular dystrophy with high CK and that mutation screening, particularly of the common mutation c.191dupA, should be an early step in the diagnostic algorithm of adult LGMD patients.
Keywords: Autosomal recessive, genetic or acquired neuromuscular disorders, muscle, muscular dystrophy
Introduction
The limb girdle muscular dystrophies (LGMDs) are a group of disorders with wide genetic and clinical heterogeneity, characterized by weakness of the pelvic and shoulder girdle musculature. There are 19 genes implicated in different forms of LGMD, and these encode a disparate collection of proteins involved in all aspects of muscle cell biology including sarcolemmal integrity [sarcoglycans (OMIM 253700, 608099, 604286, 601287)], sarcomeric integrity and function [Myotilin (OMIM 159000) and Titin (OMIM 608807)] and membrane repair [dysferlin (OMIM 253601)] (Guglieri et al., 2008).
While the LGMDs by definition involve predominantly the proximal musculature, mutations in some of the genes responsible for LGMD are associated with a broader phenotypic spectrum. For example, in dysferlinopathy mutations in the same gene cause LGMD type 2B (LGMD2B), Miyoshi myopathy (MM) (Nguyen et al., 2007), distal anterior compartment myopathy as well as rare congenital or late onset forms (Illa et al., 2001; Klinge et al., 2008; Paradas et al., 2009). While the elucidation of the large number of genes associated with LGMD and associated phenotypes has allowed many patients with LGMD to receive a precise molecular diagnosis, it is clear from population-based studies (Norwood et al., 2009) that not all patients with a diagnosis of LGMD can yet be assigned to one of these known groups, implying that full characterization of the genes responsible for LGMD has not yet been accomplished.
Recently, recessive mutations in ANO5 were identified as the cause of an autosomal recessive form of LGMD associated with asymmetric quadriceps femoris and biceps brachii atrophy (LGMD2L; OMIM 611307), which had been mapped to chromosome 11 in a group of French Canadian families, and a distal non-dysferlin Miyoshi muscular dystrophy (MMD3; OMIM 613319; Jarry et al., 2007; Bolduc et al., 2010). ANO5 encodes a member of the Anoctamin family of proteins which contain eight transmembrane domains [leading to their earlier classification as the TMEM16 (TransMEMbrane) family]. Dominant mutations in the ANO5 gene are associated with the skeletal disorder gnathodiaphyseal dysplasia (GDD; OMIM 166260; Tsutsumi et al., 2004). While the role of ANO5 is unknown, ANO1 and ANO2, which share significant sequence homology with ANO5, are known to be calcium-activated chloride channels (Schroeder et al., 2008; Yang et al., 2008; Almaca et al., 2009; Stephan et al., 2009; Stohr et al., 2009).
Recessive mutations in Anoctamin 5 have been found in three French Canadian LGMD2L families, one Finnish and one Dutch non-dysferlin MMD3 families (Bolduc et al., 2010). An exon 5 mutation, c.191dupA (p.Asn64LysfsX15), was found in a French Canadian LGMD2L family, as a compound heterozygous variant, and in one Dutch MMD3 family, in the homozygous state. This variant, located in exon 5 of the gene and the N-terminal tail of the Anoctamin 5 protein, introduces a shift in the reading frame and has been shown to induce nonsense-mediated decay of the transcript (Bolduc et al., 2010).
In light of the apparent phenotypic overlap of the patients with ANO5 mutations and dysferlinopathy, we undertook mutation screening in a candidate group of 64 patients from 59 British and German kindreds with a phenotype suggestive of LGMD2B/MM but with no dysferlin mutations. We show that the exon 5 mutation (referred to herein as the ‘common mutation’) of ANO5 represents a frequent cause of LGMD2L, found in 20 patients, and suggest that this mutation derives from a single founder event. Due to the prevalence of ANO5 mutations in this study, we suggest that ANO5 may represent a relatively frequent cause of adult onset limb girdle muscular dystrophy in Northern and Central Europe, and that mutation screening, particularly for the common mutation, should be an early step in the diagnostic algorithm of patients fitting this clinical description.
Materials and methods
Clinical assessment
Patients were recruited at the Newcastle Muscle Centre, at the Institute for Human Genetics, International Centre for Life, in Newcastle-upon-Tyne, as part of the National Commissioning Group (NCG) designated specialized diagnostic service for Limb Girdle Muscular dystrophies in the UK, and at the Friedrich Baur Institute, Ludwig-Maximilians University, in Munich, Germany. Patients were included in the study based on the presence of a combination of the following features: an LGMD or MM phenotype with absent dysferlin mutations; high creatinine kinase (CK) values; wasting of quadriceps femoris or calf muscles; adult age at onset and suggestive/putative autosomal recessive inheritance. Possible diagnoses of LGMD2A-F, 2I and 1C were excluded by muscle immunoanalysis, linkage analysis and/or mutation analysis (data not shown).
A total of 64 patients, 50 males and 14 females, from 59 unrelated kindreds were included. Consanguinity was not noted in any of the families. All participants provided appropriate consent. All were aged ≥18 years at time of the examination or DNA sample collection. All affected relatives and, where possible, first-degree unaffected relatives, were also assessed. At clinic visits complete histories and family histories were taken and the patients were asked about their muscle strength and performance before onset of symptoms. The patients were interviewed and examined by a neurologist or clinical geneticist experienced in neuromuscular disorders (K.B., V.S., H.L.). A detailed muscle assessment was undertaken by an experienced neuromuscular physiotherapist (M.E. and G.B.) or by the examiner and muscle strength was scored (Personius et al., 1994). Age ranged between 18 and 75 years at the time of the study, and all the patients have been on regular follow up in one of the two clinical centres for ~5 years. Serum CK levels were measured in all patients as well as in all available family members. Respiratory function was assessed with spirometry performed in sitting and lying. A formal cardiology assessment including electrocardiogram (ECG) and ECG and cardiac ultrasound was performed. Electromyography (EMG) was also performed in the majority of individuals.
Magnetic resonance imaging
Muscle MRI was performed in 7 patients. Axial and coronal planes of the pelvis and lower limbs were obtained using a conventional T1-weighted spin echo sequences. One patient (UK7A) had two MRI studies performed at age 47 and age 54 years, but images only from the second investigation were available for publication.
Histological examination
Muscle histology was assessed by H&E staining. All muscle biopsies were processed for immunohistochemistry (IHC) and multiplex western blot (WB). Immunostaining of unfixed frozen tissue for both procedures was performed using antibodies relating to diagnosis of LGMD as previously described (Pogue et al., 2001).
Sequence analysis
Genomic DNA was extracted from venous blood taken from patients and family members where available, by automated DNA extraction on the M48 BioRobot using the MagAttract DNA blood Mini M48 kit (Qiagen 951336) as part of the routine service performed by the Northern Region Genetics service molecular laboratory (for UK patients), or by use of a blood and tissue DNA extraction kit as per the manufacturers instructions (Qiagen, Hilden, Germany), for German patients. Primer sequences to the 22 exons of ANO5 were designed using the Primer-3 Plus program (http://www-genome.wi.mit.edu/cgibin/primer/primer3_www.cgi) and are available upon request. ANO5 exon 5 was amplified by PCR (Moltaq PCR kit, Molzym, Bremen, Germany) for all patients and for 192 healthy white British normal control samples from anonymous blood donors. The remaining 21 exons of ANO5 were PCR amplified in gDNA from UK9, UK10, G2, G3A and G3B. Amplified PCR material was sequenced using bidirectional fluorescent sequencing on an ABI 3730 XL 96 capillary sequencer, with BigDye Version 3.1 chemistry.
Polymorphic marker analysis
To determine whether the ANO5 c.191dupA alleles derived from a common founder, we analysed the frequency of two polymorphic loci; an intragenic single-nucleotide polymorphism (SNP) in exon 10 (rs7481951; NCBI SNP database http://www.ncbi.nlm.nih.gov/snp) and a microsatellite marker 0.135 Mb downstream of ANO5 (D11S1359; NCBI UniSTS database http://www.ncbi.nlm.nih.gov/genome/sts). The frequency of rs7481951 was determined in 11 patients homozygous for the ANO5 common mutation, by direct sequencing, and compared to the expected allele frequency for this SNP (NCBI SNP database; http://www.ncbi.nlm.nih.gov/snp). The frequency of a particular allele with a 192 bp DNA segment length containing CA repeats (‘the 192 allele’) for microsatellite marker D11S1359 was determined for 22 ANO5 alleles, and in 192 control chromosomes. To provide an estimate of the size of the core haplotype, polymorphism of microsatellite markers 5′ (D11S899, D11S1755 and D11S4114) and 3′ (D11S915 and D11S4163) of the ANO5 gene were analysed in those patients with the common mutation. Statistical analysis was by Fishers two-tailed exact test.
Results
The exon 5 c.191dupA mutation of ANO5 is a frequent cause of LGMD
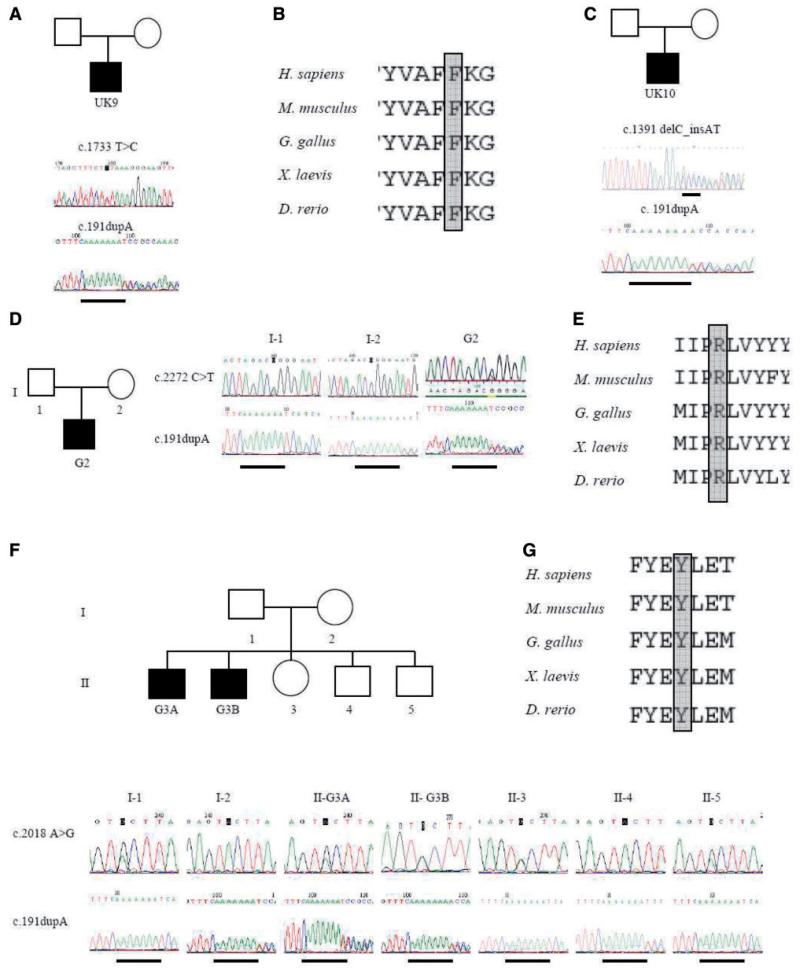
In 20 patients from 15 families, we identified the c.191dupA p.Asn64LysfsX15 mutation, giving a detection rate in our phenotypically suggestive cohort of ~32%, 25% (15/59) of the families studied. In 15 out the 20 patients, the mutation was identified as homozygous (UK patients UK1A, UK1B, UK2, UK3, UK4, UK5A, UK5B, UK6, UK7A, UK7B, UK8, UK11 and UK12 and German patients G1A and G1B). Where available, relatives were analysed for the common mutation. DNA was available from the unaffected siblings of UK1A and UK1B, G1A and G1B and G3A and G3B (which totalled five sisters and nine brothers). We found that only affected subjects were homozygous for the common mutation, whereas all heterozygous carriers of ANO5 c.191dupA were asymptomatic (both parents and siblings). Thus, the common mutation does not have a dominant effect in LGMD2L. Five patients (UK9, UK10, G2, G3A and G3B) were heterozygous for the common mutation but in these cases a second variant in the ANO5 gene was also detected on the other allele (Fig. 1). In addition to heterozygosity for the common mutation Patient UK9 (Fig. 1A), has a heterozygous nucleotide substitution in exon 16 of ANO5 (c.1733T > C p.Phe578Ser). The F578 residue is evolutionary conserved (Fig. 1B), and when substituted for serine results in a change in chemistry from non-polar to polar. This residue borders transmembrane domain 5 and the extracellular loop. In Patient UK10 a heterozygous variant in exon 14 was found in addition to the common mutation (Fig. 1C). This variant (c.1391delC_insAT p.Ala464AspfsX30) results in a shift in the reading frame and a premature termination codon in exon 15 of ANO5. In Patient G2, in addition to the common mutation in the heterozygous state, we identified a heterozygous missense variant in exon 20 of ANO5 (c.2272C > T p.Arg758Cys), with the parents being asymptomatic carriers of either the c.2272C > T variant (father) or the common mutation (mother) (Fig. 1D). The c.2272C > T mutation modifies an evolutionary conserved amino acid residue (Fig. 1E), located in the extracellular loop C-terminal to transmembrane domain 7, and when substituted alters the chemical properties of that position from polar and positively charged to non-polar neutral. In affected brothers G3A and G3B, in addition to the common mutation in the heterozygous state, a heterozygous missense variant in exon 18 of ANO5 (c.2018A > G p.Tyr673Cys) was identified. The parents and siblings of G3A and G3B are asymptomatic carriers of either the c.2018A > G variant or the common mutation (Fig. 1F). The c.2018A > G mutation modifies an evolutionary conserved amino acid residue (Fig. 1G), located in the DUF590 domain of unknown function, in the extracellular loop C-terminal to transmembrane domain 5.
Figure 1.
Identification of ANO5 mutations in UK and German families. Electrophoretograms of genomic DNA sequencing show heterozygous c.1733T > C p.Phe578Ser and c.191dupA mutations in patient UK9 (A), and protein alignment of residue Phe578 (B). Heterozygous c.1391delC_insAT and c.191dupA mutations in patient UK10 (C). Electrophoretograms of genomic DNA sequencing of patient G2 show heterozygous c.2272C > T (p.Arg758Cys) and c.191dupA mutations (D). His unaffected father carries the c.2272C > T mutation and unaffected mother carries the c.191dupA mutation. Protein alignment of residue Arg758 (E). Electrophoretograms of genomic DNA sequencing show heterozygous c.2018 A > G (p.Tyr673Cys) and c.191dupA mutations in patient G3A and G3B (F). Unaffected family members are carriers of either the c.2018A > G mutation (I-1, II-3, II-5) or for the c.191dupA mutation (I-2, II-4). Protein alignment of residue Tyr693 (G).
The common mutation in ANO5 is a founder allele
To investigate the hypothesis that the ANO5 common mutation is a founder allele, a SNP in exon 10 (NCBI SNP database; rs7481951 http://www.ncbi.nlm.nih.gov/snp) and a microsatellite marker 135 Kb downstream of ANO5 (D11S1359) were analysed (Fig 2A). All mutant alleles had the ‘A’ genotype for rs7481951, in contrast to the expected frequency of 13% in the normal European population. Similarly, 19 out of 22 mutant alleles (86%) carried the ‘192’ repeat allele for microsatellite marker D11S1359, whereas we found the ‘192’ allele in only 47 out of 192 normal northern European control chromosomes (24%). A Fisher’s exact test indicated strong linkage disequilibrium (P < 0.0001) between the common mutation and each of the two polymorphisms. Analysis of polymorphisms at microsatellite markers covering a region of 2.8 Mb upstream (D11S899, D11S1755 and D11S4114) and 2.8 Mb downstream (D11S915 and D11S4163) of ANO5 reveals the absence of an extended core haplotype (data not shown).
Figure 2.
Analysis of two polymorphisms in or downstream of the ANO5 gene reveal a founder effect for the common mutation. Exons 1–22 are indicated by boxes, with non-coding regions unfilled. The intragenic SNP in exon 10 lies 29 Kb downstream of the site of the common mutation c.191dupA, and the extragenic microsatellite marker D11S1359 is located 135 Kb 3′ of ANO5 (A). A Fisher’s exact test of the frequency of rs7481951 and D11S1359 in mutant alleles (from patients homozygous for c.191dupA) and the allele frequency expected (for rs7481951); NCBI SNP database (http://www.ncbi.nlm.nih.gov/snp) or measured (for D11S1359) shows both polymorphisms to be in strong linkage disequilibrium with c.191dupA (B).
Patients with the common ANO5 mutation have a homogenous phenotype
The 20 mutation positive patients were analysed in more detail (Table 1). The age of the patients at last examination ranged between 37 and 68 years. Eighteen patients were male and two were females. Prior to the detection of the ANO5 mutations, 15 patients had been classified as ‘unknown LGMD’, while five were labelled as non-dysferlin MM (UK7A, UK7B, UK9, G2 and G3A). At examination, the clinical phenotype was mainly characterized by an adult onset proximal lower limb weakness associated with highly raised CK values and variable degree of frequently asymmetrical muscle wasting mainly involving thigh and calf muscles. None of these patients presented with features of GDD, such as bone fragility, large jaws or frequent dental problems.
Table 1. Clinical phenotype of AN05 mutation positive patients.
| Patient | Age (years) and sex | Onset |
Creatine kinase (IU/I) | Pattern of muscle involvement |
Other features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Decade | Symptoms | Ambulant | UL prox | LL prox | LL distal | Walk on toes | Walk on heels | Muscle atrophy | Scapular winging | AS | ||||
| UK1A | 61, M | 40s | Walking difficulties | 3500 | Yes | +/− | + + | − | Able | Diff. | Medial gastrocnemius | No | + | Contractures (wrist, TA), sleep apnoea, diabetes |
| UK1B | 65, Ma | 40-50S | Walking difficulties | 4500 | Yes | − | + + | − | N.A. | NA | − | No | − | Diabetes, bladder cancer |
| UK2 | 50, M | 20s | Aches and pain | 4000–8000 | Yes | + | + + + | + | unable | Diff. | Quadriceps, hamstrings, gastrocnemius | No | + | Myoglobinuria |
| UK3 | 45, M | 20s | Walking difficulties | 4000–8000 | Yes | + + + | + + + | + /− | Diff. | Diff. | Biceps, brachioradialis, quadriceps, hamstrings | Yes | + | − |
| UK4 | 68, M | 20s | Walking difficulties | 3000 | Restricted | + | + + + | + + | Unable | Diff. | Deltoids, biceps, triceps, quadriceps | No | + | KH, foot drop, IHD |
| UK5A | 37, M | 20s | Walking difficulties | 5000 | Restricted | + /− | + + + | − | Able | Diff. | Medial gastrocnemius (AS) | Yes | + | KH, contractures (wrist, fingers) |
| UK5B | 43, M | 20s | Walking difficulties | 5300 | Yes | + | + + + | + | Able | Unable | Brachioradialis, hamstrings, medial gastrocnemius | No | + | KH |
| UK6 | 61, M | 40s | Upper limb weakness | 2400–3400 | Yes | + + + | + + + | + /− | Able | Able | Biceps, brachioradialis, pectoralis, quadriceps, hamstrings (AS) | Yes (AS) | + | KH, calf hypertrophy, contractures (wrist, fingers) |
| UK7A | 54, F | 20s | Difficulties standing on toes | 1800–10 000 | Yes | − | + + + | + | Unable | Unable | Quadriceps, medial gastrocnemius | Yes | + | KH |
| UK7B | 57, F | 40s | Difficulties standing on toes | 3900 | Yes | − | + | + | Unable | Able | Medial gastrocnemius | No | + | Contractures (TA) |
| UK8 | 56, M | 20s | Difficulties standing on toes | 2500 | Yes | − | + + + | + | Unable | Able | Biceps focally, glutei, quadriceps, hamstrings, medial gastrocnemius (AS) | No | + | KH |
| UK9 | 47, M | 30s | Calf wasting | 4500 | Yes | − | + | + + | Unable | Diff. | Quadriceps, calves (AS) | No | + | − |
| UK10 | 55, M | 30s | Stiffness, knee problems | 4100 | Yes | − | + + + | + /− | Able | Diff. | Biceps focally, quadriceps, hamstrings, medial gastrocnemius (AS) | Yes | + | KH |
| UK11 | 40, M | 30s | Walking difficulties | 3000–7000 | Yes | + /− | + + + | + | Unable | Able | Quadriceps and calves (AS) | No | + | − |
| UK12 | 58, M | 40s | Walking difficulties | 4400 | Yes | − | + + + | + /− | Unable | Unable | Biceps focally, thighs | No | + | − |
| G1A | 49, M | 30s | Walking difficulties | 2900–3500 | Severely restricted | + | + + + | + + | Unable | Unable | Severe wasting LL muscles | Yes (AS) | + | − |
| G1B | 48, M | 30s | ↓ sport performance | 800–4700 | Yes | + | + | + | Able | Diff. | Quadriceps | No | − | Calf hypertrophy, myoglobinuria |
| G2 | 35, M | Late teens | ↓ sport performance | 5000 | Yes | + | + + | + + + | Diff. | Diff. | Quadriceps, calves | No | + | − |
| G3A | 58, M | 40s | Elevated creatine kinase | 300–2000 | Yes | + | + | + | Able | Diff. | Medial gastrocnemius (AS) | No | + | − |
| G3B | 56, M | 30s | Difficulties standing on toes | 1700–3000 | Restricted | + | + + + | + + | Unable | Unable | Quadriceps, calves | Yes (AS) | + | Myoglobinuria |
Patient UK1B deceased at the age of 68 years of Bladder cancer. The patient was last seen in clinic at the age of 65 years. The indicated age is the age at last assessment.
Pt, patient number; CK, creatine kinase; UL, upper limbs; LL, lower limbs; AS, asymmetry; diff, able with difficulties; NA, data not available; TA, Achilles tendons; KH, knee hyperextension; IHD, ischaemic heart disease; M = male patient; F = female patient.
Onset
The median age at onset was 35 years, with a single individual (G2A) who showed reduced sporting performance in his late teens and six patients with onset <40 years (Table 1). Eight patients had engaged in extensive physical activity before onset of symptoms, for example patient UK2 ran a marathon at the age of 22 years, patient UK3 worked as a farmer, and patient UK10 ran a half marathon and regularly played rugby in his youth. The most common first symptoms were difficulties walking long distances or walking uphill or upstairs (nine patients), while five patients showed difficulties in toe-walking (Table 1). Two individuals noticed a reduced sporting performance as their first symptom while two others described myalgia and muscle stiffness.
Muscle function
A detailed assessment of skeletal muscle function was available for all patients. Median length of disease duration at assessment was 18 years (range 5–43). Proximal leg weakness was observed in all individuals (Table 1). A considerably milder distal lower limb involvement was observed on examination in 12 patients (60%). The five patients who had shown distal weakness at disease onset had significant proximal involvement at the time of examination (UK7A, UK7B, UK9, G2 and G3A, Table 1). A more pronounced involvement of the lower limbs in comparison to the upper limbs was observed in all subjects, with well-preserved muscle function in arms and hands in 18/20 patients at time of examination (90%). Hip muscles as well as knee flexors and extensors (quadriceps femoris) and plantar flexors were the weakest lower limb muscle groups tested, while biceps brachii, brachioradialis and triceps were the weakest muscles in the upper limbs. Weakness was generally slowly progressive with most of patients being ambulant into late adulthood. Four patients showed restricted ambulation and four of them used a wheelchair for longer distances after a disease progression of about 20 years (range 14–43 years) (Table 1). Muscle wasting, mainly of quadriceps femoris (in particular vastus medialis), hamstrings and calves, but also of biceps and brachioradialis, was observed in 19/20 patients (95%) (Fig. 3; Table 1). In the calves wasting mainly involved the medial gastrocnemius (8/20 patients; 40%), while the lateral compartment was spared or appeared hypertrophic compared to the medial gastrocnemius (Fig. 3B). Actual calf hypertrophy was observed in two patients (Table 1). Knee hyperextension, causing progressive functional problems, was observed in 7/20 patients (35%; Fig. 3I). Scapular winging was observed in 6/20 subjects (30%). Asymmetry of muscle weakness or atrophy was observed in 18/20 patients (90%; Table 1). Hand muscles were completely preserved and minor contractures were found in only 4/20 patients (20%; Table 1). Myoglobinuria occurred in the early stages of the disease in three patients (15%). A single patient suffered ischaemic heart disease, likely unrelated to the underlying muscle disease.
Figure 3.
Clinical assessment of patients with ANO5 mutation. (A and B) Frontal and posterior view of the lower limbs of patient G3A showing atrophy of thighs and medial gastrocnemius and relative hypertrophy of lateral gastrocnemius. (C and D) Frontal and lateral view of the lower limbs of patient G2 showing severe atrophy of quadriceps and calves. (E) Focal atrophy of biceps muscles of patient UK12. (F, G and H) Severe hamstrings and quadriceps atrophy in patient UK3 and UK11. (I) Knee hyperextension in patient UK7A.
Clinical investigations
Serum CK levels were increased in all individuals. Median CK levels were 4500 IU/l (range 300–10 000; normal value <150 IU/l). Electromyography was performed in 11/20 patients and showed myopathic changes in all patients.
Muscle MRI findings
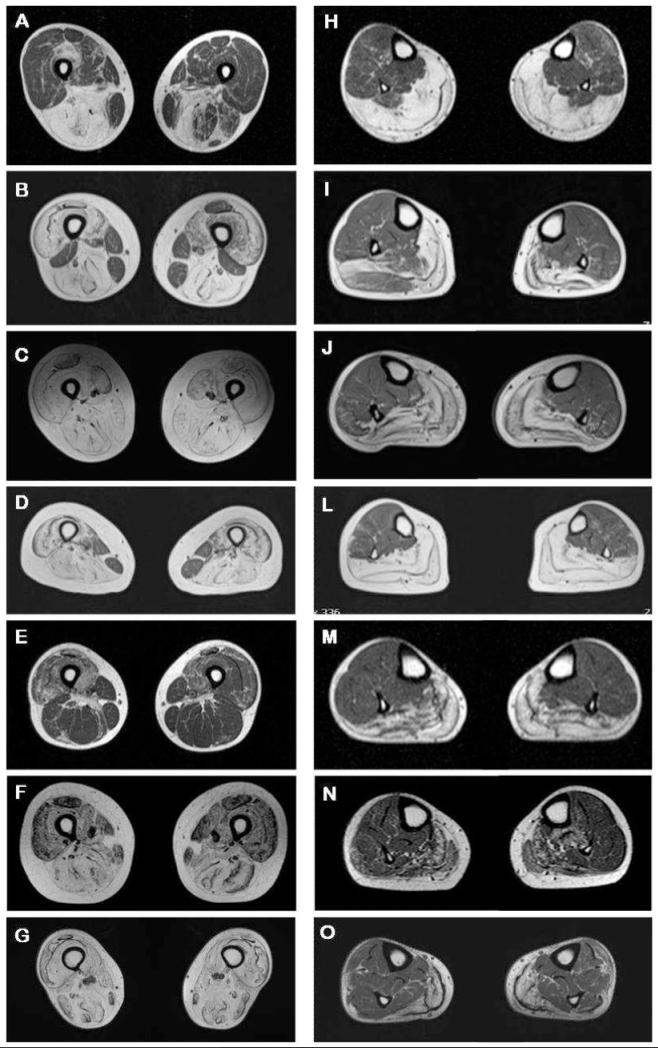
Muscle MRI of the pelvis and lower legs was performed in seven unrelated patients with variable clinical severity, clinical presentation and progression (UK1A, UK5A, UK5B, UK7A, UK11, G2 and G3A; Fig. 4, Table 1). Sections of the thigh muscles showed asymmetric variable fatty replacement of the posterior compartment with major involvement of adductor magnus, semi-membranosus and semitendinosus muscles (Fig 4A-D and 4F-G). Quadriceps muscles showed a more patchy involvement, especially in the later disease stages, such as in patients UK5A, UK5B, UK7A or G3A (Fig. 4B-D and 4G). Gracilis and sartorius muscles were least involved (Fig. 4A, B and D), except for patients UK5B or G3A (Fig. 4C and G), who showed a more severe generalized involvement of thigh muscles. Patient UK11 showed the mildest picture, with sparing of posterior compartment and relatively patchy quadriceps involvement (Fig. 4E). Moderate to severe atrophy of medial gastrocnemius and soleus muscles were evident in all individuals (Fig. 4H-O). Muscles of the anterior and lateral compartment of the lower legs, in particular tibialis anterior and posterior, peroneous longus and brevis as well as flexor digitorum longus and lateral gastrocnemius showed no or only minor involvement in all the patients examined (Fig. 4H-O). Visual comparison of the two MRIs performed on patient UK7A 7 years apart did not show any major difference or progression of atrophy (data not shown).
Figure 4.
Atrophy of thigh and calf muscles assessed for seven patients by MRI. Section of thighs and calves for each patient are at the same level (A and H for patient UK1A; B and I for UK5A; C and J for UK5B; D and L for UK7A; E and M for UK11; F and N for G2; G and O for G3A) (A) Asymmetric atrophy of hamstrings in patient UK1A. (B) Severe atrophy of quadriceps and hamstrings muscle in patient UK5A with asymmetric sparing of rectus femoris, biceps femoris, gracilis and sartorius muscles. (C) Severe atrophy of thigh muscles in patient UK5B. (D) Severe atrophy of thigh muscles in patient UK7A with sparing of gracilis and sartorius muscles. (E) Moderate asymmetric patchy atrophy of quadriceps and hamstrings in patient UK11. (F and G) Moderate and severe generalized atrophy of the anterior and posterior thigh muscles in patient G2 and G3A. (H-O) Moderate to severe atrophy of the posterior compartment of the lower legs in all examined patients with atrophy of soleus and medial gastrocnemius and relative sparing of muscles of the anterior and lateral compartments, in particular tibialis anterior and posterior, peroneous longus and brevis as well as flexor digitorum longus and lateral gastrocnemius.
Histological findings
Nineteen patients had a muscle biopsy. Muscle tissue was taken from quadriceps (six patients), gastrocnemius [four patients, biceps brachii (2), or deltoid muscle (1)], while for seven patients information on the site of the muscle biopsy was not available. Biopsies displayed myopathic or dystrophic changes, with variation in fibre size, central nuclei, fibre splitting, degeneration of muscle fibres and increase in interfascicular and intrafascicular connective tissue (Fig. 5). Regenerating fibres were detected ranging from 2% to 40% of fibres, as shown by neonatal myosin heavy chain staining (Fig. 5). Scattered fibres with inflammatory infiltrates were observed in 6/19 patients (31%), while a single patient (UK9) showed increased inflammatory changes (data not shown). Rimmed vacuoles were reported in the biopsies of patient G2 and G3B, while a single patient showed ring fibers (UK5B) (data not shown). Deficiency in proteins of the dystrophin–dystroglycan complex as well as deficiency of dysferlin and calpain was excluded by IHC and WBs in 13 patients with immunolabelling with a battery of 30 diagnostic antibodies consistently showing a normal pattern (Fig. 5 and data not shown).
Figure 5.
Histological, immunohistochemical and immunoblot findings in patients with ANO5 gene mutations. (A, B and C) H&E staining of muscle tissue from patients UK9, UK11 and UK5B showing myopathic or dystrophic changes. Notice the increase of internal nuclei (A), variation in fibre size, degeneration of muscle fibres (B) and increase in interfascicular and intrafascicular connective tissue (C). (D and E) neonatal myosin heavy chain labelling of muscle tissue from patient UK3 and UK11, respectively, indicating variable amounts of regenerating fibres. Brown fibres are undergoing regeneration. (F) WB analysis for dysferlin in patient UK9 showing a dysferlin band with normal size and amount. Equivalent protein loading was verified by Coomassie staining (blue band).
Genotype–phenotype correlations
This ANO5 patient cohort have a relatively homogenous phenotype, and no differences in overall phenotypes, mode of presentation or single clinic features was found when comparing patients homozygous for the common mutation and patients who were compound heterozygous for the common mutation and a second variant. No clear correlation between gender and phenotype was observed. However, females represented only 2/20 of the mutation positive patients, limiting this comparison but suggesting a strong bias towards affected males in the ANO5 population.
Prevalence
Eight patients with ANO5 mutation (UK1A, UK1B, UK3, UK4, UK5A, UK5B, UK6, UK8) belonged to the catchment area for the Institute of Human Genetics at Newcastle University (Northern Region of England, i.e. Northumberland, Durham, Cumbria and part of Yorkshire and Lancashire) and were part of the central muscle clinic database (Norwood et al., 2009). The estimated total population according to the last census of the Northern region of England is 2.99 million (Norwood et al., 2009). With eight confirmed cases positive for the common mutation (seven homozygous, one heterozygous) in our clinic population, the estimated minimum point prevalence for LGMD2L in the population of Northern England is 0.26/100 000 (Norwood et al., 2009).
Discussion
In this study, we report on the largest cohort to date of patients with ANO5 gene mutations. Our results indicate that mutations in the ANO5 gene, in particular, the common c.191dupA (p.Asn64LysfsX15) mutation, are responsible for a clinically recognizable adult onset LGMD, mainly involving the pelvic girdle and the proximal lower limbs, with high CK values. In this cohort, distal presentation was much less common. Mutations in the ANO5 gene were first described in five families apparently showing two distinct phenotypes, LGMD2L and MMD3 (Bolduc et al., 2010). The authors concluded that this phenotypic heterogeneity was reminiscent of what was observed with dysferlin gene mutations and the two major distinct clinical phenotypes, LGMD2B and MM. However, current opinion is that while patients with dysferlinopathy may present with symptomatology and muscle weakness predominantly in either the proximal or distal musculature, involvement of both the proximal and distal muscles becomes the norm as the disease progresses (Nguyen et al., 2007; Klinge et al., 2009; Paradas et al., 2010).The common ANO5 mutation can also lead to a variable clinical presentation at onset, but we have shown here that with disease progression the phenotypes largely overlap and merge into a more homogenous clinic entity.
The mutation c.191dupA is frequent in the British and German LGMD cohorts, occurring in all 20 of our mutation positive patients, and was found to be in strong linkage disequilibrium with an intragenic SNP in exon 10 of ANO5, and an extragenic microsatellite marker 135 Kb downstream of the gene. It is therefore likely that the high frequency of the common mutation is the result of a founder effect. While we have not elucidated the origin of the founder mutation, lack of variation at the closest microsatellite is consistent with this being a relatively young allele. The fact that it has been observed in three Dutch and French Canadian LGMD2L families (Bolduc et al., 2010), as well as the high occurrence in the UK (and German) cohort may suggest a founder of Northern European origin.
We have previously shown that the combined population prevalence figure for all inherited muscle disease categories in our clinic population was 37/100 000 (Norwood et al., 2009). Having screened our unknown local LGMD patients for the common ANO5 mutation, our prevalence data indicate that LGMD2L is the third most common form of LGMD in the Northern English population behind LGMD2A (0.6/100 000) and LGMD2I (0.43/100 000), but similar to the combined frequency of the sarcoglycanopathies (0.27/100 000), and twice as common as LGMD2B (0.13/100 000) (Norwood et al., 2009). Our data represent the minimum prevalence of LGMD2L and screening of the rest of the gene will be necessary in order to confirm the absolute prevalence for LGMD2L in this population group. The apparent frequency of this condition in this group compared to some of the other forms of LGMD confirm the need to incorporate ANO5 mutation screening (at least for the common mutation) at an early stage in the diagnostic algorithm for patients presenting with an LGMD phenotype.
We believe that there are clear supportive data that the variants found in compound heterozygosity with the common mutation are pathogenic. The c.2272C > T (p.Arg758Cys) mutation (patient G2) was previously described as homozygous in a Finnish family (Bolduc et al., 2010). In addition to this, we identified three new mutations; c.1733T > C (UK9), c.1391delC_insAT (UK10) and c.2018A > G (G3A and G3B), all in compound heterozygosity with the common mutation. c.1391delC_insAT, shifts the reading frame and introduces a premature stop codon, while the other two variants introduce missense amino acid changes. c.2018A >G segregates with disease in two affected brothers but not five unaffected family members, showing that carrying the common mutation or c.2018A > G heterozygously is insufficient to cause disease. Other evidence such as absence from the NCBI SNP database, amino acid conservation across species and altered amino acid chemical properties all suggest that the novel second variants described here are pathogenic. The mutations that we and others (Bolduc et al., 2010) have identified are spread throughout the gene, in exons 5, 8, 13, 14, 16, 18 and 20. There is no apparent trend in terms of the position of mutations within the protein in relation to specific motifs, transmembrane domains or cytoplasmic versus extracellular regions.
Studying a larger group of patients with ANO5 mutations allows us to establish the phenotype in more detail (Table 2). Although clear overlaps with LGMD2B/Miyoshi myopathy are evident, some features may help in distinguishing LGMD2L from other LGMDs. LGMD2L is an adult-onset disease and the average age at onset is about a decade later than in LGM2B (35 versus 20 years) (Klinge et al., 2009). At onset, muscle weakness mainly affects the pelvic girdle and lower limbs, with hip and thigh muscles the most severely affected, although it is important to note that the larger number of patients with proximal presentation in our study may also reflect a referral bias as our diagnostic service is primarily orientated towards LGMD. Involvement of the distal musculature is characterized by difficulty in standing on toes and calf wasting, and was present to some extent in 17/20 patients (85%) showing some degree of distal weakness or difficulty in standing on toes and/or heels (Table 1). Although difficulty in standing on toes may also be a sign of pelvic girdle weakness, muscle MRI confirmed distal involvement in the patients examined (Fig. 4). Muscle wasting mainly affects quadriceps, hamstrings and the medial gastrocnemius (Figs 3 and 4). Knee hyperextension appears to be a common feature of the disease often causes progressive functional disability that may be counteracted by appropriate splinting. In later stages of the disease, mild weakness and wasting of the upper limbs, in particular of biceps brachii and brachioradialis, is also observed (Fig. 3E). Asymmetry of muscle weakness and atrophy is commonly observed (18/20; 90%) and represents, together with thigh muscle atrophy and later age at onset a useful predictor to differentiate LGMD2L from LGMD2B. Similarly to dysferlinopathy, significant cardiac and respiratory involvement is not observed, suggesting that life expectancy might not be affected. Intra-familial variability in mode of presentation (families UK7 and G3) and disease progression (family UK5) was observed in 3/5 families. In particular, subject UK7B had been asymptomatic until her late 40s. Following the diagnosis in her sister she showed raised CK values and mild distal weakness. Intra-familial variability has also been reported in other autosomal recessive LGMDs, such as LGMD2A (Schessl et al., 2008) and sarcoglycanopathies (Angelini et al., 1998). Measurement of CK and molecular testing represent useful tools to screen for the identification of asymptomatic or mildly affected family members.
Table 2. The LGMD2L phenotype.
| Clinical characteristic | Number of patients |
|---|---|
| Increased creatine kinase value (>10×) | 20/20 |
| Proximal lower limb weakness | 20/20 |
| Adult onset (>20 years) | 19/20 |
| Muscle atrophy | 19/20 |
| Quadriceps/hamstrings atrophy | 15/20 |
| Calf (medial part) atrophy | 14 (8)/20 |
| Quadriceps/hamstrings and calf muscle atrophy | 10/20 |
| Upper limb muscle atrophy (mainly biceps) | 7/20 |
| Asymmetry of muscle weakness or atrophy | 18/20 |
| Distal lower limb weakness | 17/20 |
| Distal lower limb weakness (mild) | 13/20 |
| Distal lower limb weakness (moderate to severe) | 5/20 |
| Upper limb proximal weakness | 13/20 |
| Upper limb proximal weakness (mild) | 11/20 |
| Upper limb proximal weakness (moderate to severe) | 2/20 |
| Good sporting performance in presymptomatic period | 8/20 |
| Knee hyperextension | 7/20 |
| Scapular winging | 6/20 |
| Restriction/loss of ambulation | 4/20 |
| Contractures | 4/20 |
| Myoglobinuria | 3/20 |
Our results indicate a male predominance in LGMD2L, confirming previous findings (Bolduc et al., 2010), with only 5/32 reported ANO5 mutation positive patients (16%) so far being female. Gender predominance is not an unusual observation in LGMDs and a correlation between gender and onset or severity in LGMDs has also been reported (Zatz et al., 2003). In particular, male predominance and more rapid progression in males was observed in LGMD2A (Fanin et al., 2001), although these findings were not confirmed by later studies (Guglieri et al., 2008). Another report indicated that males with LGMD2G are more severely affected than females (Zatz et al., 2003). One possible explanation for the observed gender predominance is that females might be less severely affected and therefore less likely to be ascertained. We have excluded the presence of ANO5 mutations in all available unaffected female siblings, ruling out a possible misdiagnosis in mildly affected females. The two affected females reported here showed an onset and disease progression similar to males and there were no obvious differences in the phenotype. It is of interest to note however that patient UK7A developed symptoms after her first pregnancy, while her sister (UK7B), who did not have any children, started showing symptoms after menopause. A possible effect of pregnancy in triggering onset of symptoms has previously been observed for LGMD2B (Klinge et al., 2009). Though it is intriguing to speculate a hormonal component to disease onset and/or progression the very small number of females in this group makes it impossible to comment further.
Eight out of 20 patients reported a high level of sporting prowess prior to onset of symptoms. This finding has also been reported in dysferlinopathies but it is not a feature commonly associated with other forms of muscular dystrophy (Klinge et al., 2009). Highly raised CK values were detected in two individuals from our cohort before the onset of any clinical symptoms, similar to what is observed in dysferlinopathy. This is suggestive of an ongoing pathological process prior to the onset of clinical symptoms (Miyoshi et al., 1986; Okahashi et al., 2008).
Based on our molecular and clinical results, and in view of our data suggesting that LGMD2L is more common than dysferlinopathy at least in the UK population, we suggest that the diagnostic algorithm for LGMD needs to be amended. Mutations in ANO5 represent a relatively frequent cause of adult-onset muscular dystrophy with high CK. Diagnosis on the basis of muscle biopsy findings is currently not possible due to the lack of relevant antibodies. Mutation screening, particularly of the common mutation c.191dupA, should be an early step in the diagnostic testing of patients fitting this clinical description.
Acknowledgements
The authors thank Petra Mitzscherling for excellent technical assistance. They also would like to thank the patients who took part in this study.
Funding: Diagnostic facilities at the Newcastle Muscle Centre is supported by the National Commissioning Group (NCG) for rare neuromuscular disorders. The Institute of Human Genetics in Newcastle is part of the MRC centre for Neuromuscular Diseases. S.K., M.C.W., V.S., H.L. and A.H. are members of the German Muscular Dystrophy Network (MD-NET 01GM0887) funded by the German Ministry of Education and Research (BMBF, Bonn, Germany); www.md-net.org. Newcastle University and MD-NET are partner organization of TREAT-NMD (EC, 6th FP, proposal #036825; www.treat-nmd.eu).
Abbreviations
- CK
creatinine kinase
- ECG
electrocardiogram
- EMG
electromyography
- IHC
immunohistochemistry
- LGMDs
limb-girdle muscular dystrophy
- MMD3
Miyoshi muscular dystrophy
- MM
Miyoshi myopathy
- SNP
single-nucleotide polymorphism
- WB
western blot
References
- Almaca J, Tian Y, Aldehni F, Ousingsawat J, Kongsuphol P, Rock JR, et al. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284:28571–8. doi: 10.1074/jbc.M109.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelini C, Fanin M, Menegazzo E, Freda MP, Duggan DJ, Hoffman EP. Homozygous alpha-sarcoglycan mutation in two siblings: one asymptomatic and one steroid-responsive mild limb-girdle muscular dystrophy patient. Muscle Nerve. 1998;21:769–75. doi: 10.1002/(sici)1097-4598(199806)21:6<769::aid-mus9>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Bolduc V, Marlow G, Boycott KM, Saleki K, Inoue H, Kroon J, et al. Recessive mutations in the putative calcium-activated chloride channel Anoctamin 5 cause proximal LGMD2L and distal MMD3 muscular dystrophies. Am J Hum Genet. 2010;86:213–21. doi: 10.1016/j.ajhg.2009.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanin M, Pegoraro E, Matsuda-Asada C, Brown RH, Jr, Angelini C. Calpain-3 and dysferlin protein screening in patients with limb-girdle dystrophy and myopathy. Neurology. 2001;56:660–5. doi: 10.1212/wnl.56.5.660. [DOI] [PubMed] [Google Scholar]
- Guglieri M, Straub V, Bushby K, Lochmüller H. Limb-girdle muscular dystrophies. Curr Opin Neurol. 2008;21:576–84. doi: 10.1097/WCO.0b013e32830efdc2. [DOI] [PubMed] [Google Scholar]
- Illa I, Serrabo-Munuera C, Gallardo E, Lasa A, Rojas-Garcia R, Palmer J, et al. Distal anterior compartment myopathy: a dysferlin mutation causing a new muscular dystrophy phenotype. Ann Neurol. 2001;49:130–4. [PubMed] [Google Scholar]
- Jarry J, Rioux MF, Bolduc V, Robitaille Y, Khoury V, Thiffault I, et al. A novel autosomal recessive limb-girdle muscular dystrophy with quadriceps atrophy maps to 11p13-p12. Brain. 2007;130:368–80. doi: 10.1093/brain/awl270. [DOI] [PubMed] [Google Scholar]
- Klinge L, Aboumousa A, Eagle M, Hudson J, Sarkozy A, Vita G, et al. New aspects on patients affected by dysferlin deficient muscular dystrophy. J Neurol Neurosurg Psychiatry. 2010;81:946–53. doi: 10.1136/jnnp.2009.178038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge L, Dean AF, Kress W, Dixon P, Charlton R, Müller JS, et al. Late onset in dysferlinopathy widens the clinical spectrum. Neuromuscular Disorders. 2008;18:288–90. doi: 10.1016/j.nmd.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Miyoshi K, Kawai H, Iwasa M, Kusaka K, Nishino H. Autosomal recessive distal muscular dystrophy as a new type of progressive muscular dystrophy: seventeen cases in eight families including an autopsied case. Brain. 1986;109:31–54. doi: 10.1093/brain/109.1.31. [DOI] [PubMed] [Google Scholar]
- Nguyen K, Bassez G, Krahn M, Bernard R, Laforet P, Labelle V, et al. Phenotypic study in 40 patients with dysferlin gene mutations: high frequency of atypical phenotypes. Arch Neurol. 2007;64:1176–82. doi: 10.1001/archneur.64.8.1176. [DOI] [PubMed] [Google Scholar]
- Norwood FLM, Harling C, Chinnery PF, Eagle M, Bushby K, Straub V. Prevalence of genetic muscle disease in Northern England: in-depth analysis of a muscle clinic population. Brain. 2009;132:3175–86. doi: 10.1093/brain/awp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okahashi S, Ogawa G, Suzuki M, Ogata K, Nishino I, Kawai M. Asymptomatic sporadic dysferlinopathy presenting with elevation of serum creatine kinase. Typical distribution of muscle involvement shown by MRI but not by CT. Intern Med. 2008;47:305–7. doi: 10.2169/internalmedicine.47.0519. [DOI] [PubMed] [Google Scholar]
- Paradas C, González-Quereda L, De Luna N, Gallardo E, García-Consuegra I, Gómez H, et al. A new phenotype of dysferlinopathy with congenital onset. Neuromuscul Disord. 2009;19:21–5. doi: 10.1016/j.nmd.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Paradas C, Llauger J, Diaz-Manera J, Rojas-Garcia R, De Luna N, Iturriaga C, et al. Redefining dysferlinopathy phenotypes based on clinical findings and muscle imaging studies. Neurology. 2010;75:316–23. doi: 10.1212/WNL.0b013e3181ea1564. [DOI] [PubMed] [Google Scholar]
- Personius KE, Pandya S, King WM, Tawil R, McDermott MP. The FSHDYG. facioscapulohumeral dystrophy natural history study: standardization of testing procedures and reliability of measurements. Phys Ther. 1994;74:253–63. doi: 10.1093/ptj/74.3.253. [DOI] [PubMed] [Google Scholar]
- Pogue R, Anderson LVB, Pyle A, Sewry C, Pollitt C, Johnson MA, et al. Strategy for mutation analysis in the autosomal recessive limb-girdle muscular dystrophies. Neuromuscul Disord. 2001;11:80–7. doi: 10.1016/s0960-8966(00)00154-1. [DOI] [PubMed] [Google Scholar]
- Schessl J, Walter M, Schreiber G, Schara U, Müller C, Lochmüller H, et al. Phenotypic variability in siblings with calpainopathy (LGMD2A) Acta Myol. 2008;27:54–8. [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–29. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan AB, Shum EY, Hirsh S, Cygnar KD, Reisert J, Zhao H. ANO2 is the cilial calcium-activated chloride channel that may mediate olfactory amplification. Proc Natl Acad Sci. 2009;106:11776–81. doi: 10.1073/pnas.0903304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr H, Heisig JB, Benz PM, Schoberl S, Milenkovic VM, Strauss O, et al. TMEM16B, A novel protein with calcium-dependent chloride channel activity, associates with a presynaptic protein complex in photoreceptor terminals. J Neurosci. 2009;29:6809–18. doi: 10.1523/JNEUROSCI.5546-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi S, Kamata N, Vokes TJ, Maruoka Y, Nakakuki K, Enomoto S, et al. The novel gene encoding a putative transmembrane protein is mutated in gnathodiaphyseal dysplasia (GDD) Am J Hum Genet. 2004;74:1255–61. doi: 10.1086/421527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–5. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- Zatz M, de Paula F, Starling A, Vainzof M. The 10 autosomal recessive limb-girdle muscular dystrophies. Neuromuscul Disord. 2003;13:532–44. doi: 10.1016/s0960-8966(03)00100-7. [DOI] [PubMed] [Google Scholar]