Abstract
Inotrope use is one of the most controversial topics in the management of heart failure. While the heart failure community utilizes them and recognizes the state of inotrope dependency, retrospective analyses and registry data have overwhelmingly suggested high mortality, which is logically to be expected given the advanced disease states of those requiring their use. Currently, there is a relative paucity of randomized control trials due to the ethical dilemma of creating control groups by withholding inotropes from patients who require them. Nonetheless, results of such trials have been mixed. Many were also performed with agents no longer in use, on patients without an indication for inotropes, or at a time before automatic cardio-defibrillators were recommended for primary prevention. Thus, their results may not be generalizable to current clinical practice. In this review, we discuss current indications for inotrope use, specifically dobutamine and milrinone, depicting their mechanisms of action, delineating their patterns of use in clinical practice, defining the state of inotrope dependency, and ultimately examining the literature to ascertain whether evidence is sufficient to support the current view that these agents increase mortality in patients with heart failure. Our conclusion is that the evidence is insufficient to link inotropes and increased mortality in low output heart failure.
Keywords: inotropes, dobutamine, milrinone, heart failure
Introduction
It is widely recognized that decreased cardiac output is the trigger of a pathologic chain of events that results in the clinical syndrome of systolic heart failure (HF). Treatments that increase cardiac output, such as cardiac transplantation or left ventricular assist devices, are curative. A similar effect should be expected of inotropes because they, too, increase cardiac output.
At present, however, the use of inotropic agents in the management of HF is largely controversial. On one hand, almost everyone who manages patients with advanced HF utilizes them. The Acute Heart Failure Global Survey of Standard Treatment (ALARM-HF) global survey of 666 hospitals in nine countries showed that inotropes were used in 39% of all admissions for acute HF.1 In the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial, 72% of patients in the medical arm and 65% of patients in the ventricular assist device arm were on inotropes.2 Indeed, the HF community uniformly recognizes the state of “inotrope dependency”. On the other hand, current guidelines caution that these drugs may be potentially detrimental: “Despite improving hemodynamic compromise, positive inotropic agents have not demonstrated improved outcomes in patients with HF in either the hospital or outpatient setting”.3
The purpose of this paper is to present a thorough review of the evidence on inotrope use in HF, and to ascertain whether the strength of the evidence is sufficient to support the current view that long-term use of these agents may lead to increased rates of mortality among HF patients. We grouped the evidence, separating the sources demonstrating inotrope benefit from those indicating their detriment. Moreover, due to their availability in the US, this review will focus mainly on dobutamine and milrinone.
Current guidelines on inotropes
Guidelines of the American College of Cardiology Foundation/American Heart Association (ACCF/AHA) (2013),3 Heart Failure Society of America (2010),4 European Society of Cardiology (2012),5 and International Society for Heart and Lung Transplantation6–8 have recommendations on inotropes. While the guidelines on mechanical circulatory support (2013)7 and the guidelines for the care of heart transplant recipients (2010)8 address very specific indications of post-left ventricular assist device implantation right ventricular failure7 and acute cellular or antibody-mediated rejection and hemodynamic support in the early post-operative period,8 respectively, the rest make recommendations on the use of positive inotropic agents in HF. The recommendations of various societies are summarized in Table 1. In general, inotropes are indicated in the presence of acute or chronic hemodynamic compromise with end organ dysfunction due to low output, and are considered to be detrimental and contraindicated if this syndrome is not present.
Table 1.
Guideline recommended indications for inotropic agents in heart failure
| Guidelines | Strength | Level of evidence | |
|---|---|---|---|
| American College of Cardiology Foundation/American Heart Association 20133 | Until definitive therapy (eg, coronary revascularization, mechanical circulatory support, heart transplantation) or resolution of the acute precipitating problem, patients with cardiogenic shock should receive temporary intravenous inotropic support to maintain systemic perfusion and preserve end-organ performance. | I | C |
| Continuous intravenous inotropic support is reasonable as “bridge therapy” in patients with stage D refractory to guideline determined medical therapy and device therapy who are eligible for and awaiting mechanical circulatory support or cardiac transplantation. | IIA | B | |
| Short-term, continuous intravenous inotropic support may be reasonable in those hospitalized patients presenting with documented severe systolic dysfunction who present with low blood pressure and significantly depressed cardiac output to maintain systemic perfusion and preserve end-organ performance. | IIB | B | |
| Long-term, continuous intravenous inotropic support may be considered as palliative therapy for symptom control in select patients with stage D despite optimal guideline determined medical therapy and device therapy who are not eligible for either mechanical circulatory support or cardiac transplantation. | IIB | B | |
| Long-term use of either continuous or intermittent, intravenous parenteral positive inotropic agents, in the absence of specific indications or for reasons other than palliative care, is potentially harmful in the patient with HF. | III | B | |
| Use of parenteral inotropic agents in hospitalized patients without documented severe systolic dysfunction, low blood pressure, or impaired perfusion, and evidence of significantly depressed cardiac output, with or without congestion, is potentially harmful. | III | B | |
| European Society of Cardiology 20125 | An intravenous infusion of an inotrope (eg, dobutamine) should be considered in patients with hypotension (systolic blood pressure <85 mmHg) and/or hypoperfusion to increase cardiac output, increase blood pressure, and improve peripheral perfusion. | IIA | C |
| Inotropic agents are not recommended unless the patient is hypotensive (systolic blood pressure <85 mmHg), hypoperfused, or shocked because of safety concerns (atrial and ventricular arrhythmias, myocardial ischemia, and death). | III | C | |
| Heart Failure Society of America 20104 | Intravenous inotropes (milrinone or dobutamine) may be considered to relieve symptoms and improve end-organ function in patients with advanced HF characterized by LV dilation, reduced LVEF, and diminished peripheral perfusion or end-organ dysfunction (low output syndrome), particularly if these patients have marginal systolic blood pressure (<90 mmHg), have symptomatic hypotension despite adequate filling pressure, or are unresponsive to, or intolerant of, intravenous vasodilators. | May be considered | C |
| These agents may be considered in similar patients with evidence of fluid overload if they respond poorly to intravenous diuretics or manifest diminished or worsening renal function. | May be considered | C | |
| Intravenous inotropes (milrinone or dobutamine) are not recommended unless left heart filling pressures are known to be elevated or cardiac index is severely impaired based on direct measurement or clear clinical signs. | Not recommended | C | |
| International Society for Heart and Lung Transplantation Guidelines for Management of Heart Transplant Candidates 20066 | In patients with decompensated heart failure and hypoperfusion in spite of adequate filling pressures, inotropic or pressor therapy should be used. | I | C |
| Long-term use of inotropic therapy should only be used as a pharmacologic bridge to transplantation or for palliation. | I | C |
Abbreviations: HF, heart failure; LV, left ventricle; LVEF, left ventricular ejection fraction.
Specifically, the ACCF/AHA guidelines state that use of parenteral inotropic agents in hospitalized patients without documented severe systolic dysfunction, low blood pressure, or impaired perfusion, and evidence of significantly depressed cardiac output, with or without congestion, is potentially harmful.3
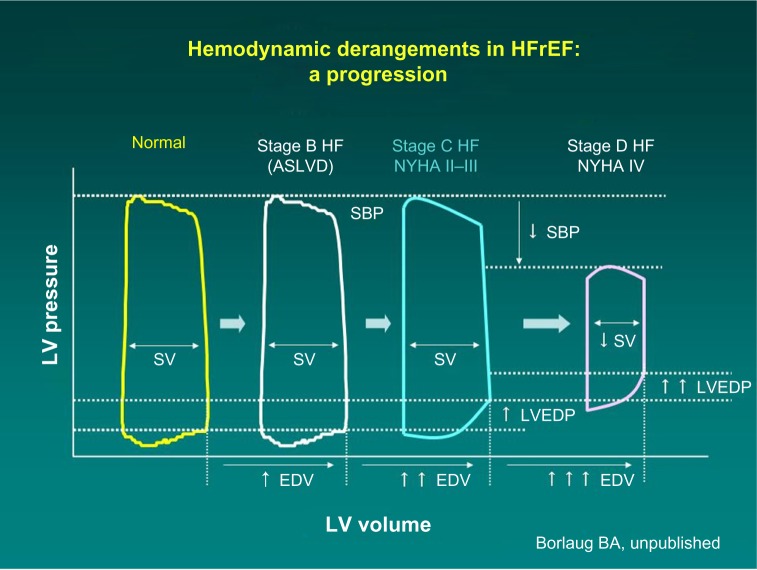
These recommendations are based on profound understanding of the pathophysiology of HF. As the disease progresses over time, the heart maintains normal cardiac output, but at the cost of rising left ventricular end diastolic pressure (Figure 1). The mainstay intervention at these stages is diuretic therapy, which decreases intracardiac filling pressures (congestion), along with medications favoring left ventricular reverse remodeling such as angiotensin-converting enzyme inhibitors. Eventually, compensatory mechanisms fail, and cardiac output decreases. Only at this advanced stage can inotropes be beneficial. Because low output is not present at the earlier stages, administration of inotropes cannot be favorable but can certainly cause harm because of side effects.
Figure 1.
Progression of hemodynamic derangements in heart failure (Barry Borlaug, with permission).
Abbreviations: ASLVD, asymptomatic systolic left ventricular dysfunction; EDV, end-diastolic volume; HF, heart failure; HFrEF, heart failure with reduced ejection fraction; LV, left ventricle; LVEDP, left ventricular end diastolic pressure; NYHA, New York Heart Association; SBP, systolic blood pressure; SV, stroke volume.
Inotropes: mechanism of action and hemodynamic effects
Milrinone and dobutamine are currently the only two inotropes approved for use in the US and both exert their actions by increasing the intracellular level of cyclic adenosine monophosphate (cAMP).9 Dobutamine achieves this effect indirectly through adrenergic agonism while milrinone, a phosphodiesterase inhibitor, directly blocks cAMP breakdown.10
Dobutamine is a sympathomimetic amine, which acts on beta-1, beta-2, and alpha-1 adrenergic receptors. The stimulation of these receptors produces a relative strong additive inotropic effect and a relatively weak chronotropic effect.11 Alpha-1 agonist activity in the vasculature causes vasoconstriction, which balances the beta-2 vasodilatory effect, permitting relatively unchanged blood pressure with administration.12 Dobutamine increases myocardial contractility, with accompanying reflex reduction in sympathetic tone. In HF patients, its use has actually been shown to cause a dose-dependent decrease in plasma norepinephrine.13 Overall, this leads to an increase in cardiac output by selective augmentation of stroke volume with a decrease in systemic vascular resistance. Because of its adrenergic properties, the use of dobutamine is problematic in patients who take beta blockers.
Milrinone is a bipyridine derivative of amrinone with 10–75 times more positive inotropic effect; additionally, unlike its parent drug, it has direct vasodilatory properties.14 Milrinone works by inhibiting phosphodiesterase 3 (PDE3), which in turn prevents the degradation of cAMP and ultimately leads to an increase in protein kinase A (PKA). PKA increases contractility of the left ventricle through cAMP dependent-PKA, which phosphorylates calcium channels, leading to a trans-sarcolemmal influx of calcium, increasing the rate that the sarcoplasmic reticulum uptakes calcium. PKA also causes the phosphorylation of myofilament proteins which facilitates the action of actin and myosin, and therefore increases cardiac contractility and cardiac output.15
Milrinone thus functions as an inodilator, both increasing cardiac contractility and reducing afterload with a consequent reduction in left ventricular filling pressures. When compared with adrenergic inotropic drugs such as dobutamine, milrinone has been shown to exert these hemodynamic effects with less myocardial oxygen consumption.14,16 Besides, milrinone can be used in patients on beta blockers, because its effects are not dependent on beta adrenoreceptors.
Milrinone not only acts as a systemic but also a pulmonary vasodilator. It was found to lower pulmonary vascular resistance in HF patients awaiting transplant by decreasing mean pulmonary arterial pressures, in addition to significantly lowering pulmonary capillary wedge pressure.17 Effects were more pronounced in severe pulmonary hypertension.18 Its actions on the pulmonary vasculature are comparable to sildenafil, as both medications increase the levels of cyclic nucleotides to exert an effect.19 Sildenafil causes mainly PDE5 inhibition, increasing cyclic guanosine monophosphate levels, while milrinone inhibits PDE3, causing an increase in cAMP as previously mentioned. Sildenafil lacks direct inotropic effects, due to relatively low concentrations of PDE5 in the myocardium. In a study of New York Heart Association (NYHA) class IV patients, Botha et al19 concluded that while both milrinone and sildenafil caused similar reductions in systemic and pulmonary vascular resistance, milrinone caused two times greater reduction in mean pulmonary artery pressure and significantly greater reduction of the pulmonary capillary wedge pressure, suggesting that milrinone may be the preferred agent in patients with pulmonary hypertension and HF. Milrinone also produced more cardio-specific effects due to the widespread distribution of PDE3 throughout the myocardium, resulting in lower filling pressures and higher heart rates in comparison.19
The magnitude of the hemodynamic effects of inotropes on cardiac index and cardiac output is remarkable. Insurance carriers look for a 20% increase in cardiac index or a similar decrease in pulmonary wedge pressure, in order to issue an approval for continuous home inotropes.20 However, greater response is common, with a two-fold increase in cardiac index commonly observed.21
Milrinone in currently approved doses typically increases cardiac index by 24%–42%, decreases pulmonary capillary wedge pressure by 24%–33%, and reduces systemic vascular resistance by 15%–31%, with dose-dependent effect. The drug is effective in most patients, and those with the worst hemodynamic profiles at baseline derive the most benefits.20
Most of the hemodynamic effects of dobutamine and milrinone are similar.22 Both dobutamine and milrinone:
increase cardiac output;
cause peripheral vasodilation;
and decrease pulmonary capillary wedge pressure.
There are some differences between dobutamine and milrinone.16,23–25
Dobutamine, in comparison with milrinone, causes:
greater increase in heart rate;
greater increase in myocardial oxygen consumption;
greater proarrhythmic effect, including ventricular tachycardia;26,27
and effects are attenuated in patients who receive beta blockers.
Milrinone, in comparison with dobutamine, causes:
more hypotension;
greater reduction in left and right heart filling pressures;
greater reduction in mean arterial pressure;
greater reduction in pulmonary arterial pressure;
longer duration of action after discontinuation of the intravenous infusion, especially in the presence of renal dysfunction;
and greater hemodynamic effects in general when the patient is on beta blockers.
The biggest difference between the two, especially in our expanding health care system, may be cost. Dobutamine is cheaper.28,29 For a course of in-hospital inotrope therapy, total acquisition cost of milrinone was significantly higher than that of dobutamine (US $16,270± $1,334 vs US $380± $533, P<0.00001).28 In terms of arrhythmogenicity, dobutamine causes atrial and ventricular arrhythmias more commonly than milrinone, although both agents have proarrhythmic potential and hence both require continuous rhythm monitoring, at least while in the hospital. Milrinone causes nonsustained ventricular tachycardia in 3.7% of patients and sustained ventricular tachycardia in 0.5%.20
Overall, hemodynamic properties of inotropes seem to be optimal for low output, or “cold” HF patients, especially if they are also “wet”,30 ie, have volume overload and increased intracardiac pressures. It is well-known that this type of HF patient has the worst prognosis.31 Besides, increase in cardiac output and decrease in congestion frequently results in improved urine output, a phenomenon widely known to HF doctors.24,32
It is quite counterintuitive that drugs with such remarkable hemodynamic effects can be detrimental in advanced HF.
Inotrope dependency
The term “inotrope dependent” is used liberally in the guidelines, without a formal definition. Patients are characterized as inotrope dependent if they cannot be weaned off inotropes at an experienced HF center.4 Inotrope dependence means that withdrawal of inotropes leads to symptomatic hypotension, recurrent congestive symptoms, or worsening renal function.33 It is recognized that symptoms and not purely the values of re-measured hemodynamic parameters have to be considered when deciding on inotrope dependence.33
Meanwhile, if we admit that there is a subset of patients who depend on inotropes, we have to logically conclude that inotropes prolong life. And indeed, the HFSA guidelines state that “these agents may help relieve symptoms due to poor perfusion and preserve end-organ function in patients with severe systolic dysfunction and dilated cardiomyopathy”.4 End organ function in HF is usually related to hepatic and renal function. If inotropes help preserve liver and kidney function, they ought to prolong life, or to “avoid imminent death”.34 The best definition of inotrope dependency we found in the paper by Hershberger et al.34 “Inotropic dependence was defined as the failure to wean from inotropes because of imminent (minutes to hours) worsening of the patient’s clinical status … such that death appeared imminent, and the patient was deemed highly unlikely to survive inotrope withdrawal to permit hospital discharge”. The authors state further that the attempted withdrawal of inotropic support in this cohort of patients can be acutely life-threatening.34
If we recognize that patients on inotropes cannot be weaned off of them, we have to admit that inotropes reduce mortality in this terminal end-stage HF population. Otherwise, the term “inotrope dependent” becomes oxymoranical.
Inotrope dependency is the condition which makes it unfeasible and ethically unacceptable to conduct any randomized controlled trials (RCTs) on inotropes versus placebo or inotrope versus no inotrope. The only comparison possible is one inotrope versus another, or inotropes versus a different means of inotropic support, like in the REMATCH trial.2 Indeed, Lynne Stevenson wrote in 200324 that randomized trials performed with and without inotropic infusions during HF hospitalizations have selected patients in whom intravenous therapy was not considered essential for management. Hershberger et al also wrote that a randomized clinical trial designed to remove dobutamine from patients deemed inotrope dependent would cause considerable discomfort from an ethical perspective.34 Ten years later, this statement still holds true. But if you enroll only patients in whom the intervention is not essential, you cannot establish the value of the very intervention that is tested.
Patterns of inotrope use
There are three distinct patterns of intravenous inotrope use: confined to hospital admission, intermittent home infusions (usually several times per week at the infusion center), and the infusions started in the hospital and continued at home continuously, weeks to months and even years in duration. Besides, some inotropes were used orally in the outpatient setting. Below, we briefly summarize non-randomized studies based on the setting of infusion. Randomized studies, where patients are randomized into inotrope versus placebo or inotrope versus no inotrope, regardless of the setting where infusion was performed, are summarized in Table 3. All studies, in the text and in the table, include patients with symptomatic HF and decreased left ventricular ejection fraction.
Table 3.
Randomized controlled trials of inotropes in heart failure
| Source, design | N | Follow-up | Inotrope | Cardiac index at baseline | Mortality | Other outcomes in the inotrope group vs placebo |
|---|---|---|---|---|---|---|
| Poor outcomes | ||||||
| Cohn et al, 199867 Vesnarinone Trial, randomized to vesnarinone in two different doses and placebo |
3,833 | 286 days | Vesnarinone, oral | NR | Mortality: Vesnarinone lower dose: 21% Vesnarinone higher dose: 22.9% Placebo: 18.9%, P<0.02 (vs placebo), the difference is presumably due to sudden (arrhythmic) death |
Improved quality of life |
| Cowley et al, 199466 The Enoximone Trial, a randomized, double-blind, placebo-controlled trial: enoximone vs placebo |
151 | One year | Enoximone, oral | NR | Number of deaths: Enoximone: 27 Placebo: 18, P<0.05 Sudden deaths: Enoximone: 11 Placebo: 5 Progressive HF death: Enoximone: 12 Placebo: 11 The trial was ended early because of an excess mortality in the patients treated with enoximone |
Improved quality of life |
| Uretsky et al, 199065 Enoximone trial Double-blind, randomized, placebo-controlled enoximone vs placebo |
102 | 4 months | Enoximone, oral | NR | Mortality: Enoximone: 5 patients Placebo: 0 patients, P<0.05 Two deaths were sudden, two were from progressive HF, and one was from acute myocardial infarction |
No differences in symptoms or exercise duration at the end of 4 months |
| Hampton et al, 199768 Randomized, placebo-controlled ibopamine vs placebo |
1,906 | About 1 year | Ibopamine, oral | NR | Mortality: Ibopamine: 232 (25%) Placebo: 193 (20%) RR: 1.26 (95% CI: 1.04–1.53), P=0.017. The trial was stopped early, because of an excess of deaths in the ibopamine group |
|
| Packer et al, 199169 Prospective Randomized Milrinone Survival Evaluation (PROMISE) trial Double-blind, randomized oral milrinone vs placebo |
1,088 | 6 months | Oral milrinone | NR | Mortality from all causes: Milrinone: 30% Placebo: 24% (A 28% increase in all cause mortality, P=0.038, and a 34% increase in cardiovascular mortality, P=0.016). The trial stopped prematurely because of survival compromise on milrinone |
Hospitalizations: Milrinone 44% Placebo 39%, P=0.041 |
| The Xamoterol in Severe Heart Failure Study, 199085 Randomized, double-blind, placebo-controlled Randomization: xamoterol vs placebo |
516 | 13 weeks | Xamoterol, oral (beta receptor agonist) | NR | Mortality: Xamoterol: 9.1% Placebo: 3.7%, P=0.02 |
|
| Metra et al, 200975 The Studies of Oral Enoximone Therapy in Advanced HF (ESSENTIAL), two identical, randomized, double-blind, placebo-controlled trials that differed only by geographic location |
1,854 | 17 months | Enoximone, oral | NR | All-cause mortality: no difference | The 6-minute walk distance increased with enoximone, compared with placebo, in ESSENTIAL-I (P=0.025, not reaching, however, the pre-specified criterion for statistical significance of P<0.020) |
| Elis et al, 199847 Randomized, double-blind, placebo-controlled dobutamine vs placebo over a 24-hour period every 2 to 3 weeks |
19 | 6 months | Dobutamine IV, intermittent | NR | The median survival: Dobutamine: 4.6 months Placebo: 8 months No difference |
No difference between the number of admissions for HF |
| Erlemeier et al, 199248 Dobutamine vs placebo |
20 | 4 weeks | Dobutamine, IV intermittent | NR | No mortality difference | Dobutamine: exercise duration increase, body weight decreased Placebo: no change |
| Oliva et al, 199949 DICE (Dobutaminanell’Insufficienza Cardiaca) trial: dobutamine vs standard treatment |
38 | 6 months | IV dobutamine, intermittent | 1.89±0.1 L/minute/m2 | Dobutamine: 5 deaths, 2 heart transplants Standard treatment: 3 deaths No difference |
Hospitalizations for all causes: no difference Dobutamine: 11 (7 for HF) Standard treatment: 17 (11 for HF) No difference in NYHA class and in 6-minute walking test |
| Massie et al, 198578 Double-blind, placebo-controlled amrinone vs placebo |
99 | 12 weeks | Amrinone, oral | NR | No mortality difference | Exercise tolerance: no difference |
| Narahara, 199179 The Western Enoximone Study Randomized, placebo-controlled enoximone vs placebo |
164 | 12 weeks | Enoximone, oral | NR | No mortality difference | Enoximone: greater increases in exercise time than placebo treatment at weeks 4 and 8 but not after 12 weeks |
| Van Veldhuisen et al, 199380 The Dutch Ibopamine Multicenter Trial Double-blind placebo-controlled, randomized ibopamine vs digoxin vs placebo |
161 | 6 months | Ibopamine, oral | NR | No mortality difference | |
| Good outcomes | ||||||
| Dubourg et al, 199081 A double-blind, randomized trial Enoximone vs placebo |
30 | 31 days | Enoximone, oral | 2.17±0.7 L/minute/m2 | Mortality: Enoximone: 1 Placebo: 3 |
Symptoms improvement on enoximone |
| Feldman et al, 200776 EMOTE trial (Enoximone in Intravenous Inotrope-Dependent Subjects Study) Enoximone vs placebo Enoximone was used to wean patients from IV inotropes |
201 | 6 months | Oral enoximone | NR | Alive and free of IV inotropes at 30 days: Enoximone: 62 (61.4%) Placebo: 51 (51%) At 60 days Enoximone: 46.5% Placebo: 30%, P=0.016 Time to death or re-initiation of IV inotropes: At 6 months: HR: 0.76 (95% CI: 0.55–1.04) At 60 days: HR: 0.62 (95% CI: 0.43–0.89), P=0.009 At 90 days: HR: 0.69 (95% CI: 0.49–0.97), P=0.031, favoring enoximone |
|
| Feldman et al, 199382 Vesnarinone Study Randomized, double-blind, placebo-controlled vesnarinone vs placebo |
477 | 6 months | Vesnarinone, oral | NR | Mortality plus worsening HF: Vesnarinone: 26 Placebo: 50, P=0.003 A 62% reduction (95% CI: 28%–80%) in the risk of dying from any cause among the patients receiving vesnarinone |
Vesnarinone: quality of life improved to a greater extent than in the placebo group over 12 weeks (P=0.008) |
| Nanas et al, 200471 Randomized, double-blind, placebo-controlled clinical trial Dobutamine vs placebo |
30 | 6 months | Dobutamine, IV intermittent, plus amiodarone | 2.3±0.7 L/minute/m2 | Survival: Dobutamine plus amiodarone vs placebo plus amiodarone HR: 0.403 (95% CI: 0.164–0.992; P=0.048) 1-year survival estimate: Dobutamine plus amiodarone: 69% Placebo plus amiodarone: 28%, P<0.05 2-year survival estimate: Dobutamine plus amiodarone: 44% Placebo plus amiodarone: 21%, P<0.05 |
|
| Likoff et al, 198483 Randomized, double-blind, placebo-controlled After being stabilized on amrinone, patients were randomized into continuation on amrinone or withdrawal of amrinone |
9 | Two 13-week stages | Amrinone, IV | 1.9±0.2 L/minute/m2 | Placebo: 7 patients had a significant deterioration of symptoms or exercise tolerance, or both. After 4 weeks of readministration of amrinone, clinical status improved | |
| Khalife et al, 198787 Double-blind, randomized, placebo-controlled Randomization: after the first phase when IV enoximone was given to all patients, they were randomized into oral enoximone or placebo |
17 | 12 weeks | Enoximone, IV and oral, in a 2-part study | 3.42±0.72 L/minute/m2 (after enoximone IV) | Enoximone: LVEF improved from 30.1%±6.8% to 33.9%±9.9% Placebo: unchanged |
|
Abbreviations: CI, confidence interval; HR, hazard ratio; IV, intravenous; NR, not reported; NYHA, New York Heart Association; RR, relative risk; LVEF, left ventricular ejector fraction; HF, heart failure; vs, versus.
Hospital infusions
Some studies report the experience with in-hospital inotrope infusions when the patients were admitted not because of hemodynamic compromise and low output syndrome, but electively. A 3-day dobutamine infusion in 29 patients resulted in hemodynamic and metabolic improvement, including elevation of sodium and improvement in renal function.35
Intravenous milrinone given to 14 patients resulted in improved hemodynamics and allowed higher doses of diuretics and other HF medications. Oral angiotensin-converting enzyme inhibitor and diuretic doses were increased by 318% and 89%, respectively. NYHA functional class improved from 3.8±0.4 to 2.6±0.6 following therapy, and there was a reduction in hospital admissions in ten patients who responded to therapy during the subsequent year compared with the year before treatment (4±17 versus [vs] 17±15).36
Intermittent infusions of either dobutamine (43 patients) or nitroprusside were given to a total of 113 patients for about 1 month. There was a higher rehospitalization rate (86% vs 57%, P<0.02) and higher mortality (58% vs 28%, P<0.006) in the dobutamine group. The decision of using dobutamine versus nitroprusside was made by individual physicians. Baseline systolic blood pressure was 90 mmHg in the dobutamine group and 95 mmHg in the nitroprusside group; there is no indication whether this difference was significant. Heart transplantation was done in 78% of those on dobutamine and only in 48% of those on nitroprusside.37
In 261 patients, in-hospital infusion of nesiritide in two different doses was compared with dobutamine. Six-month mortality was lower in the nesiritide groups.38
This last study was designed to compare the outcomes in patients with an infusion of nesiritide in a lower and higher dose versus any other vasoactive drug, at the discretion of the investigator, and patients were randomized into these three arms. Some patients in the arm with vasoactive drug were on dobutamine. The comparison between nesiritide and dobutamine was therefore a comparison between non-randomized groups, with very limited numbers of baseline characteristics and no invasive hemodynamic information. Moreover, mean baseline systolic blood pressure was 120 mmHg, and blood pressure below 90 mmHg was an exclusion criterion. Consequently, the study omitted all patients with low output HF syndrome, fundamentally excluding the only patients with an indication for dobutamine use. This essential design flaw makes the study inconclusive. The study of Capomolla et al37 was also inconclusive due to lack of randomization.
Comparison of dobutamine versus milrinone in hospitalized patients, awaiting heart transplantation, did not show a clear advantage of one or the other in terms of right heart hemodynamics, death, need for additional vasodilator/inotropic therapy, need for mechanical cardiac support before transplantation, or ventricular arrhythmias requiring increased antiarrhythmic therapy.28
Intermittent home infusions
Historically, intermittent infusions of inotropes were used as a treatment for end-stage HF with severe symptoms (NYHA III/IV). This practice is no longer supported and is a Class III recommendation as per ACC/AHA.3
No randomized trials are available, but there were several published series summarizing the outcomes.
Intravenous amrinone, given as intermittent infusions initially at the hospital, and then at home, to 41 patients, over the period of 51 months, resulted in improvement in NYHA class in 66% of patients, and a 50% reduction in number of days spent in the hospital and number of hospital admissions in the 6 months following the beginning of therapy, compared to the 6 months before the therapy.39
Intravenous dobutamine in four patients and milrinone in 32 patients, given as intermittent home infusions over the period of 294 days, resulted in a reduced number of hospital admissions, days spent in the hospital, and emergency room visits, compared with similar data from the year before entry in the program for each patient.40
Intravenous milrinone given as intermittent infusions at home for a short period of time (four cycles of 3 days per week) resulted in improved hemodynamics which was sustained throughout the treatment period and for 4 months after its discontinuation (mean pulmonary arterial pressure, pulmonary capillary wedge pressure, systemic vascular resistance, and pulmonary vascular resistance were significantly decreased and cardiac index was significantly increased).41
Intravenous intermittent dobutamine in 13 patients resulted in improved hemodynamics (a 25% increase in cardiac output) and, in seven patients, an improvement in functional class.42
Intravenous intermittent dobutamine in eleven patients for a period of time ranging 1.8–24 (mean: 7.8) months, resulted in significant increases in cardiac index and in NYHA functional class (3.8±0.4 to 2.8±0.7, P<0.01).43
Intermittent home infusions of milrinone in ten patients resulted in a four-fold decrease in hospitalizations during the study and symptomatic improvement.44
Intermittent dobutamine infusions in eleven patients for 3–24 months resulted in symptomatic improvement and a mean of 1.2 reduction in NYHA functional class.45
Intermittent dobutamine or milrinone infusions given to 73 patients resulted in subjective improvement.46
RCTs on intermittent home inotropes are included in Table 3. Elis et al47 did not demonstrate either a morbidity or mortality advantage of intermittent intravenous dobutamine. Erlemeier et al48 and Oliva et al49 also did not find any mortality difference, although the sample size was small in all three studies, with 19, 20, and 38 patients, respectively.
Multiple episodes of ventricular tachycardia have been reported on intermittent dobutamine infusion.26
The data on mortality are very variable. One study reported that only three out of 17 patients survived the 26-week study period of intermittent dobutamine, with six patients experiencing sudden death, and three other patients dying of progressive HF,42 while others reported no mortality at all.41,44 Because patients’ selection and infusion drugs, as well as the protocols, were not standardized, no conclusions on mortality are possible.
Continuous home infusions
Continuous inotrope infusion at home is more relevant to today’s practice than intermittent treatments. Such infusion may be used to improve symptoms and to better quality of life in hospice patients, in addition to acting as a bridge to cardiac transplant in candidates awaiting a donor. A decrease in the need for HF hospitalizations after initiation of continuous home inotrope infusions was suggested by the analysis of the Medicare data.50
Continuous home infusion of dobutamine or milrinone in 24 and seven patients, respectively, resulted in improvement in NYHA functional class from 4.0±0.0 to 2.7±0.9 (P<0.0001), decrease in the number of hospital admissions and length of stay from 20.9±12.7 to 5.5±5.4 days (P=0.0004), as well as a 16% reduction in cost of care in comparison to the control period preceding the therapy.51
Continuous home infusion of milrinone was used in 60 heart transplant candidates and resulted in hemodynamic and symptomatic improvement as well as cost reduction, with 88.3% of patients eventually undergoing heart transplant.52
Continuous home infusion of milrinone was given to 29 heart transplant candidates and resulted in hemodynamic and symptomatic improvement.53
Continuous home infusion of milrinone (eight patients) or dobutamine (twelve patients) given as a bridge to cardiac transplantation, resulted in improvement of functional status, serum creatinine, better hemodynamic parameters, and decreased numbers of hospitalizations during positive inotropic infusion therapy when compared with pre-treatment baseline.54
Continuous home infusion of dobutamine (four patients), dopamine (13 patients), or the combination of both (six patients) resulted in a reduction of the number of days spent in the hospital.55
Continuous (four patients) and intermittent (seven patients) home infusion of dobutamine in eleven patients resulted in symptomatic improvement.56
The number of reported deaths while on inotropes varied greatly among the studies, but since there were no control groups, and same patients’ historical data were used as control, no conclusion about mortality can be derived.
Mortality data and randomized studies
There is a relative paucity of RCTs on the mortality effect of inotropes in HF. Thus, to date, much of the data on the subject has been drawn from retrospective analysis. Overall, the data suggests that mortality of patients treated with intravenous inotropes is high. In the Evaluation Study of Congestive Heart Failure and Pulmonary Artery Catheterization Effectiveness (ESCAPE) trial, 6-month mortality in patients with HF receiving inotropes during hospitalization reached 19%,57 while the analysis of the Medicare data indicated that in patients treated with continuous home inotrope infusion, 6-month mortality exceeded 40%.50 Analysis of the Acute Decompensated Heart Failure National Registry (ADHERE), showed inotropic treatment with dobutamine and milrinone was associated with a 200% increase of in-hospital mortality in comparison to vasodilators.58 Moreover, the Flolan International Randomized Survival Trial (FIRST), determined that 6-month mortality among patients on dobutamine was 70%, with dobutamine being the strongest independent predictor of mortality in the study.59 Use of dobutamine or milrinone was consistent with very poor prognosis, even in comparison with other intravenous vasoactive drugs like vasodilators.58 The addition of more than one inotrope is associated with further mortality increase.60 High mortality rate alone, however, does not in itself prove that inotropes are detrimental. Indeed, mortality is expected to be high by virtue of the advanced disease states in those who require inotropes.
Meta-analyses and retrospective analyses examining the mortality effect of inotropes in HF have been largely mixed. A meta-analysis of multiple placebo-controlled trials by Thakray et al61 failed to demonstrate increased mortality on inotropes, while another meta-analysis on phosphodiestherase-3 inhibitors showed poorer outcomes on these agents.62 In another retrospective study, no mortality difference was found between dobutamine and milrinone at home in a single center experience,63 although milrinone was deemed more effective as a bridge to transplant, allowing more patients to be bridged by inotropes alone, without the need for mechanical circulatory support. Also, renal and hepatic function improved on milrinone.64
Some suggestions of increased mortality on inotropes come from post-hoc analyses of trials not designed to test the outcomes on inotropes where no randomization on inotrope versus no inotrope or placebo was conducted. For example, the FIRST trial was a RCT, designed to test the effects of continuous intravenous epoprostenol plus conventional therapy versus conventional therapy alone in patients with advanced HF. Some patients who entered the trial were also on intravenous dobutamine.59 The analysis of the outcomes depending on the use of dobutamine is therefore flawed because the patients who required inotropes were sicker (89% in NYHA IV) than those who did not (53%).
We grouped the randomized trials on inotropes into three categories: trials that demonstrate negative effects of inotropes on clinical outcomes, those that show neutral effects, and those that show beneficial effects of inotropes (Table 3).
Increased mortality was found on oral enoximone,65,66 oral vesnarinone,67 oral ibopamine,68 oral milrinone,68,69 and beta agonist xamoterol. Vesnarinone was associated with a dose-dependent increase in mortality, mostly due to arrhythmic death.67 None of these inotropes is currently in use, and hence none of these outcomes are pertinent to the effects of intravenous dobutamine or milrinone. Besides, inotropes are proarrhythmic, and sudden cardiac death is considered the main mechanism responsible for excess mortality on inotropes.67 Meanwhile, all the above studies were conducted before the time when implantation of automated cardioverter-defibrillators had become the routine. Today, many of the patients on inotropes are implanted with defibrillators by the time they are inotrope dependent and are largely protected from arrhythmic death.
Indirectly, this consideration is confirmed by the study of Drakos et al. Due to concern that arrhythmia might contribute to inotrope-induced mortality; they compared end-stage HF patients on intermittent inotropes versus conventional medical management, adding oral amiodarone to both groups (inotropes were represented by either dobutamine or levosimendan). The study was not randomized. The 6-month (51% vs 18%) and 1-year (36% vs 9%) survival rates were significantly higher (P=0.001 for both), and functional status was better, in patients on inotropes and amiodarone.70 Earlier, the same group of authors demonstrated similar results in a randomized, placebo-controlled study (Table 3).71 Interestingly, the survival benefit with this strategy was superior for ischemic compared to non-ischemic etiology of HF.72
The majority of randomized studies are neutral, demonstrating neither benefit nor detriment of inotropes. In the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations (OPTIME-CHF) trial of 951 patients admitted for acute decompensated HF, there were no significant differences of in-hospital mortality, 60-day mortality, or combined 60-day death when comparing milrinone versus placebo.73 Post-hoc subgroup analysis did reveal an increase in a composite of death or rehospitalization in patients with coronary artery disease treated with milrinone versus placebo (42% vs 36%), although no difference was found between the two groups in non-ischemic patients.74 The Studies of Oral Enoximone Therapy in Advanced HF (ESSENTIAL) trial examined the effect of low-dose enoximone on patients with advanced HF on optimal medical therapy, and also showed no mortality difference.75 In another study, oral enoximone used for weaning from intravenous inotropes, did not affect the mortality.76 Other authors47–49,77–80 also reported no difference in terms of mortality between inotropes and placebo.
Conversely, relatively few studies demonstrated beneficial effects of inotropes on mortality. Similarly to those trials showing increased mortality, most of these studied agents are not currently in use and are therefore not very pertinent: enoximone,76,81 vesnarinone,82 and amrinone.83 The only study on dobutamine in this group used it in combination with amiodarone to negate potential proarrhythmic effects. Mortality reduction on dobutamine plus amiodarone versus placebo plus amiodarone had a hazard ratio of 0.403 (95% confidence interval [CI]: 0.16–40.992; P=0.048).
Nevertheless, the main observation from reading reports of inotrope use, randomized or not randomized, is that very few authors report the data on central hemodynamics. We saw in multiple sets of guidelines cited in the beginning of this review that the only indication for inotropes in HF is low output syndrome. Meanwhile, very few papers provide hemodynamic data. It means that in most studies, cardiac index/cardiac output were not even measured, and patients were enrolled based on symptomatic HF and decreased left ventricular ejection fraction, which is not an equivalent for low output syndrome. Moreover, in the OPTIME-CHF trial, patients were excluded if their doctors thought that inotropes were indicated.73 It means that effects of inotropes were tested on patients who did not have indications for them, which is the best way to evaluate for side effects without therapeutic benefits.
In summary, most RCTs with inotropes share the following features:
They were performed with pharmacologic agents that are currently not in use. The reason for them being no longer used is the fact that they increase mortality. This does not mean, however, that the effects of the drugs, which proved to be detrimental, can be extrapolated to currently used agents.
They were performed in the years when automatic cardioverter-defibrillators were not recommended for primary prevention, and an excess of sudden death may not be pertinent to the current situation when patients with advanced cardiomyopathy are protected with implanted defibrillators.
They were performed on patients who did not have any evidence of low output syndrome and therefore did not have indications for inotropes.
The controversy in understanding the role of inotropes is very visible in modern literature. In the recent review, Francis et al88 acknowledge that use of inotropes “has been plagued by excessive mortality”. On the other hand, they state that “there are clinical settings where inotropic support … may be lifesaving”. These two statements are mutually exclusive. Either inotropes save lives, or they increase mortality. If patients cannot survive without inotropes, the inotropes are lifesaving. It is time to stop talking about “clear evidence that inotropic therapy increases mortality” and focus on definitions of the conditions where inotropes save lives.
Conclusion
In this review, we examined the quality of the current evidence, and found it insufficient to support the view that inotropes increase mortality in advanced heart failure patients with low output syndrome. Meta-analyses and randomized controlled trials results have been largely mixed, with inconclusive data. Moreover, randomized controlled trials have been scarce due to the ethical dilemma of withholding inotropes in patients who require them. Most randomized controlled trials shared certain common features: they were performed with inotropes that are not currently in use; they were performed before automated cardioverter-defibrillators were standard of care for primary prevention; and they were performed on patients without evidence of low output HF and without indications for inotropes. Thus, these studies may not be generalizable to our current clinical practice.
The use of inotropes should be limited to patients with systolic failure with evidence of hypoperfusion and inotrope dependence in whom weaning of inotropes may be life-threatening. Further studies should target these patient cohorts, using direct measurement of cardiac index/output as enrollment criteria, as they derive the most benefit from both acute and chronic inotrope therapy.
Table 2.
Properties of dobutamine and milrinone
| Inotrope | Dose | Onset and duration of action | Side effects | Comments |
|---|---|---|---|---|
| Dobutamine | 2.5–20 μg/kg/minute IV | Onset of action is 1–10 minutes, peak effect 10–20 minutes. The half-life is 2 minutes. | Ventricular ectopy, tachycardia, hypotension, angina, palpitations, fever, headache, nausea. | |
| Milrinone | 0.25–0.75 μg/kg/minute IV | Onset of action is 5–15 minutes. The half-life is 2.5 hours. | Ventricular and supraventricular arrhythmias, angina, hypotension, headache. | A 50 mcg/kg bolus is sometimes recommended,17 although it may increase the risk of side effects and does not add to beneficial hemodynamic effects.84 |
Abbreviation: IV, intravenous.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Follath F, Yilmaz MB, Delgado JF, et al. Clinical presentation, management and outcomes in the acute heart failure global survey of standard treatment (ALARM-HF) Intensive Care Med. 2011;37:619–626. doi: 10.1007/s00134-010-2113-0. [DOI] [PubMed] [Google Scholar]
- 2.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. New Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B, et al. American College of Cardiology Foundation. American Heart Association Task Force on Practice Guidelines 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College Of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1–e194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis And Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33:1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 6.Jessup M, Banner N, Brozena S, et al. Optimal pharmacologic and non-pharmacologic management of cardiac transplant candidates: approaches to be considered prior to transplant evaluation: International Society for Heart and Lung Transplantation guidelines for the care of cardiac transplant candidates – 2006. J Heart Lung Transplant. 2006;25:1003–1023. doi: 10.1016/j.healun.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Feldman D, Pamboukian SV, Teuteberg JJ, et al. The 2013 International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support: executive summary. J Heart Lung Transplant. 2013;32:157–187. doi: 10.1016/j.healun.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Costanzo MR, Dipchand A, Starling R, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914–956. doi: 10.1016/j.healun.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenblick EH, Frishman WH, LeJemtel TH. Dobutamine: A new synthetic cardioactive sympathetic amine. New Engl J Med. 1979;300:17–22. doi: 10.1056/NEJM197901043000105. [DOI] [PubMed] [Google Scholar]
- 10.Baim DS, McDowell AV, Cherniles J, et al. Evaluation of a new bipyridine inotropic agent – milrinone – in patients with severe congestive heart failure. New Engl J Med. 1983;309:748–756. doi: 10.1056/NEJM198309293091302. [DOI] [PubMed] [Google Scholar]
- 11.Vallet B, Dupuis B, Chopin C. Dobutamine: mechanisms of action and use in acute cardiovascular pathology. Ann Cardiol Angeiol (Paris) 1991;40:397–402. French. [PubMed] [Google Scholar]
- 12.Ruffolo RR., Jr The pharmacology of dobutamine. Am J Med Sci. 1987;294:244–248. doi: 10.1097/00000441-198710000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Colucci WS, Denniss AR, Leatherman GF, et al. Intracoronary infusion of dobutamine to patients with and without severe congestive heart failure. Dose-response relationships, correlation with circulating catecholamines, and effect of phosphodiesterase inhibition. J Clin Invest. 1988;81:1103–1110. doi: 10.1172/JCI113423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Young RA, Ward A. Milrinone. A preliminary review of its pharmacological properties and therapeutic use. Drugs. 1988;36:158–192. doi: 10.2165/00003495-198836020-00003. [DOI] [PubMed] [Google Scholar]
- 15.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase c and a sites on troponin i regulate myofilament ca2+ sensitivity and atpase activity in the mouse myocardium. J Physiol. 2003;552:845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colucci WS, Wright RF, Jaski BE, Fifer MA, Braunwald E. Milrinone and dobutamine in severe heart failure: differing hemodynamic effects and individual patient responsiveness. Circulation. 1986;73:III175–III183. [PubMed] [Google Scholar]
- 17.Givertz MM, Hare JM, Loh E, Gauthier DF, Colucci WS. Effect of bolus milrinone on hemodynamic variables and pulmonary vascular resistance in patients with severe left ventricular dysfunction: a rapid test for reversibility of pulmonary hypertension. J Am Coll Cardiol. 1996;28:1775–1780. doi: 10.1016/S0735-1097(96)00399-3. [DOI] [PubMed] [Google Scholar]
- 18.Pamboukian SV, Carere RG, Webb JG, et al. The use of milrinone in pre-transplant assessment of patients with congestive heart failure and pulmonary hypertension. J Heart Lung Transplant. 1999;18:367–371. doi: 10.1016/s1053-2498(98)00070-9. [DOI] [PubMed] [Google Scholar]
- 19.Botha P, Parry G, Dark JH, Macgowan GA. Acute hemodynamic effects of intravenous sildenafil citrate in congestive heart failure: comparison of phosphodiesterase type-3 and -5 inhibition. J Heart Lung Transplant. 2009;28:676–682. doi: 10.1016/j.healun.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Anderson JL. Hemodynamic and clinical benefits with intravenous milrinone in severe chronic heart failure: results of a multicenter study in the United States. Am Heart J. 1991;121:1956–1964. doi: 10.1016/0002-8703(91)90832-3. [DOI] [PubMed] [Google Scholar]
- 21.Klocke RK, Mager G, Kux A, Hopp HW, Hilger HH. Effects of a twenty-four-hour milrinone infusion in patients with severe heart failure and cardiogenic shock as a function of the hemodynamic initial condition. Am Heart J. 1991;121:1965–1973. doi: 10.1016/0002-8703(91)90833-4. [DOI] [PubMed] [Google Scholar]
- 22.Biddle TL, Benotti JR, Creager MA, et al. Comparison of intravenous milrinone and dobutamine for congestive heart failure secondary to either ischemic or dilated cardiomyopathy. Am J Cardiol. 1987;59:1345–1350. doi: 10.1016/0002-9149(87)90917-9. [DOI] [PubMed] [Google Scholar]
- 23.Monrad ES, Baim DS, Smith HS, Lanoue AS. Milrinone, dobutamine, and nitroprusside: comparative effects on hemodynamics and myocardial energetics in patients with severe congestive heart failure. Circulation. 1986;73:III168–III174. [PubMed] [Google Scholar]
- 24.Stevenson LW. Clinical use of inotropic therapy for heart failure: Looking backward or forward? Part I: inotropic infusions during hospitalization. Circulation. 2003;108:367–372. doi: 10.1161/01.CIR.0000078348.44634.BA. [DOI] [PubMed] [Google Scholar]
- 25.Lowes BD, Tsvetkova T, Eichhorn EJ, Gilbert EM, Bristow MR. Milrinone versus dobutamine in heart failure subjects treated chronically with carvedilol. Int J Cardiol. 2001;81:141–149. doi: 10.1016/s0167-5273(01)00520-4. [DOI] [PubMed] [Google Scholar]
- 26.David S, Zaks JM. Arrhythmias associated with intermittent outpatient dobutamine infusion. Angiology. 1986;37:86–91. doi: 10.1177/000331978603700203. [DOI] [PubMed] [Google Scholar]
- 27.Burger AJ, Horton DP, LeJemtel T, et al. Effect of nesiritide (b-type natriuretic peptide) and dobutamine on ventricular arrhythmias in the treatment of patients with acutely decompensated congestive heart failure: the precedent study. Am Heart J. 2002;144:1102–1108. doi: 10.1067/mhj.2002.125620. [DOI] [PubMed] [Google Scholar]
- 28.Aranda JM, Jr, Schofield RS, Pauly DF, et al. Comparison of dobutamine versus milrinone therapy in hospitalized patients awaiting cardiac transplantation: a prospective, randomized trial. Am Heart J. 2003;145:324–329. doi: 10.1067/mhj.2003.50. [DOI] [PubMed] [Google Scholar]
- 29.Toma M, Starling RC. Inotropic therapy for end-stage heart failure patients. Curr Treat Options Cardiovasc Med. 2010;12:409–419. doi: 10.1007/s11936-010-0090-9. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson LW, Massie BM, Francis GS. Optimizing therapy for complex or refractory heart failure: a management algorithm. Am Heart J. 1998;135:S293–S309. doi: 10.1016/s0002-8703(98)70257-1. [DOI] [PubMed] [Google Scholar]
- 31.Nohria A, Tsang SW, Fang JC, et al. Clinical assessment identifies hemodynamic profiles that predict outcomes in patients admitted with heart failure. J Am Coll Cardiol. 2003;41:1797–1804. doi: 10.1016/s0735-1097(03)00309-7. [DOI] [PubMed] [Google Scholar]
- 32.Leier CV, Webel J, Bush CA. The cardiovascular effects of the continuous infusion of dobutamine in patients with severe cardiac failure. Circulation. 1977;56:468–472. doi: 10.1161/01.cir.56.3.468. [DOI] [PubMed] [Google Scholar]
- 33.Stevenson LW. Clinical use of inotropic therapy for heart failure: looking backward or forward? Part II: chronic inotropic therapy. Circulation. 2003;108:492–497. doi: 10.1161/01.CIR.0000078349.43742.8A. [DOI] [PubMed] [Google Scholar]
- 34.Hershberger RE, Nauman D, Walker TL, Dutton D, Burgess D. Care processes and clinical outcomes of continuous outpatient support with inotropes (cosi) in patients with refractory endstage heart failure. J Card Fail. 2003;9:180–187. doi: 10.1054/jcaf.2003.24. [DOI] [PubMed] [Google Scholar]
- 35.Unverferth DV, Magorien RD, Altschuld R, Kolibash AJ, Lewis RP, Leier CV. The hemodynamic and metabolic advantages gained by a three-day infusion of dobutamine in patients with congestive cardiomyopathy. Am Heart J. 1983;106:29–34. doi: 10.1016/0002-8703(83)90434-9. [DOI] [PubMed] [Google Scholar]
- 36.Cusick DA, Pfeifer PB, Quigg RJ. Effects of intravenous milrinone followed by titration of high-dose oral vasodilator therapy on clinical outcome and rehospitalization rates in patients with severe heart failure. Am J Cardiol. 1998;82:1060–1065. doi: 10.1016/s0002-9149(98)00557-8. [DOI] [PubMed] [Google Scholar]
- 37.Capomolla S, Febo O, Opasich C, et al. Chronic infusion of dobutamine and nitroprusside in patients with end-stage heart failure awaiting heart transplantation: safety and clinical outcome. Eur J Heart Fail. 2001;3:601–610. doi: 10.1016/s1388-9842(01)00165-9. [DOI] [PubMed] [Google Scholar]
- 38.Silver MA, Horton DP, Ghali JK, Elkayam U. Effect of nesiritide versus dobutamine on short-term outcomes in the treatment of patients with acutely decompensated heart failure. J Am Coll Cardiol. 2002;39:798–803. doi: 10.1016/s0735-1097(01)01818-6. [DOI] [PubMed] [Google Scholar]
- 39.Levinoff Roth SN, Moe G. Intermittent intravenous amrinone infusion: a potentially cost effective mode of treatment of patients with refractory heart failure. Can J Cardiol. 1993;9:231–237. [PubMed] [Google Scholar]
- 40.Marius-Nunez AL, Heaney L, Fernandez RN, et al. Intermittent inotropic therapy in an outpatient setting: a cost-effective therapeutic modality in patients with refractory heart failure. Am Heart J. 1996;132:805–808. doi: 10.1016/s0002-8703(96)90315-4. [DOI] [PubMed] [Google Scholar]
- 41.Hatzizacharias A, Makris T, Krespi P, et al. Intermittent milrinone effect on long-term hemodynamic profile in patients with severe congestive heart failure. Am Heart J. 1999;138:241–246. doi: 10.1016/s0002-8703(99)70107-9. [DOI] [PubMed] [Google Scholar]
- 42.Krell MJ, Kline EM, Bates ER, et al. Intermittent, ambulatory dobutamine infusions in patients with severe congestive heart failure. Am Heart J. 1986;112:787–791. doi: 10.1016/0002-8703(86)90475-8. [DOI] [PubMed] [Google Scholar]
- 43.Applefeld MM, Newman KA, Sutton FJ, et al. Outpatient dobutamine and dopamine infusions in the management of chronic heart failure: clinical experience in 21 patients. Am Heart J. 1987;114:589–595. doi: 10.1016/0002-8703(87)90757-5. [DOI] [PubMed] [Google Scholar]
- 44.Cesario D, Clark J, Maisel A. Beneficial effects of intermittent home administration of the inotrope/vasodilator milrinone in patients with end-stage congestive heart failure: a preliminary study. Am Heart J. 1998;135:121–129. doi: 10.1016/s0002-8703(98)70352-7. [DOI] [PubMed] [Google Scholar]
- 45.Roffman DS, Applefeld MM, Grove WR, et al. Intermittent dobutamine hydrochloride infusions in outpatients with chronic congestive heart failure. Clin Pharm. 1985;4:195–199. [PubMed] [Google Scholar]
- 46.Lopez-Candales AL, Carron C, Schwartz J. Need for hospice and palliative care services in patients with end-stage heart failure treated with intermittent infusion of inotropes. Clin Cardiol. 2004;27:23–28. doi: 10.1002/clc.4960270107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elis A, Bental T, Kimchi O, Ravid M, Lishner M. Intermittent dobutamine treatment in patients with chronic refractory congestive heart failure: a randomized, double-blind, placebo-controlled study. Clin Pharmacol Ther. 1998;63:682–685. doi: 10.1016/S0009-9236(98)90092-3. [DOI] [PubMed] [Google Scholar]
- 48.Erlemeier HH, Kupper W, Bleifeld W. Intermittent infusion of dobutamine in the therapy of severe congestive heart failure – long-term effects and lack of tolerance. Cardiovasc Drugs Ther. 1992;6:391–398. doi: 10.1007/BF00054187. [DOI] [PubMed] [Google Scholar]
- 49.Oliva F, Latini R, Politi A, et al. Intermittent 6-month low-dose dobutamine infusion in severe heart failure: DICE multicenter trial. Am Heart J. 1999;138:247–253. doi: 10.1016/s0002-8703(99)70108-0. [DOI] [PubMed] [Google Scholar]
- 50.Hauptman PJ, Mikolajczak P, George A, et al. Chronic inotropic therapy in end-stage heart failure. Am Heart J. 2006;152:1096. e1–e8. doi: 10.1016/j.ahj.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harjai KJ, Mehra MR, Ventura HO, et al. Home inotropic therapy in advanced heart failure: cost analysis and clinical outcomes. Chest. 1997;112:1298–1303. doi: 10.1378/chest.112.5.1298. [DOI] [PubMed] [Google Scholar]
- 52.Brozena SC, Twomey C, Goldberg LR, et al. A prospective study of continuous intravenous milrinone therapy for status IB patients awaiting heart transplant at home. J Heart Lung Transplant. 2004;23:1082–1086. doi: 10.1016/j.healun.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 53.Canver CC, Chanda J. Milrinone for long-term pharmacologic support of the status 1 heart transplant candidates. Ann Thorac Surg. 2000;69:1823–1826. doi: 10.1016/s0003-4975(00)01313-8. [DOI] [PubMed] [Google Scholar]
- 54.Upadya S, Lee FA, Saldarriaga C, et al. Home continuous positive inotropic infusion as a bridge to cardiac transplantation in patients with end-stage heart failure. J Heart Lung Transplant. 2004;23:466–472. doi: 10.1016/S1053-2498(03)00203-1. [DOI] [PubMed] [Google Scholar]
- 55.Sindone AP, Keogh AM, Macdonald PS, McCosker CJ, Kaan AF. Continuous home ambulatory intravenous inotropic drug therapy in severe heart failure: safety and cost efficacy. Am Heart J. 1997;134:889–900. doi: 10.1016/s0002-8703(97)80012-9. [DOI] [PubMed] [Google Scholar]
- 56.Miller LW, Merkle EJ, Herrmann V. Outpatient dobutamine for end-stage congestive heart failure. Crit Care Med. 1990;18:S30–S33. [PubMed] [Google Scholar]
- 57.Elkayam U, Tasissa G, Binanay C, et al. Use and impact of inotropes and vasodilator therapy in hospitalized patients with severe heart failure. Am Heart J. 2007;153:98–104. doi: 10.1016/j.ahj.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 58.Abraham WT, Adams KF, Fonarow GC, et al. ADHERE Scientific Advisory Committee and Investigators. ADHERE Study Group In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: an analysis from the acute decompensated heart failure national registry (adhere) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 59.O’Connor CM, Gattis WA, Uretsky BF, et al. Continuous intravenous dobutamine is associated with an increased risk of death in patients with advanced heart failure: insights from the Flolan International Randomized Survival Trial (first) Am Heart J. 1999;138:78–86. doi: 10.1016/s0002-8703(99)70250-4. [DOI] [PubMed] [Google Scholar]
- 60.Rossinen J, Harjola VP, Siirila-Waris K, et al. The use of more than one inotrope in acute heart failure is associated with increased mortality: a multi-centre observational study. Acute Card Care. 2008;10:209–213. doi: 10.1080/17482940802262376. [DOI] [PubMed] [Google Scholar]
- 61.Thackray S, Easthaugh J, Freemantle N, Cleland JG. The effectiveness and relative effectiveness of intravenous inotropic drugs acting through the adrenergic pathway in patients with heart failure-a meta-regression analysis. Eur J Heart Fail. 2002;4:515–529. doi: 10.1016/s1388-9842(02)00041-7. [DOI] [PubMed] [Google Scholar]
- 62.Amsallem E, Kasparian C, Haddour G, Boissel JP, Nony P. Phosphodiesterase III inhibitors for heart failure. Cochrane Database Syst Rev. 2005;(1):CD002230. doi: 10.1002/14651858.CD002230.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gorodeski EZ, Chu EC, Reese JR, Shishehbor MH, Hsich E, Starling RC. Prognosis on chronic dobutamine or milrinone infusions for stage d heart failure. Circ Heart Fail. 2009;2:320–324. doi: 10.1161/CIRCHEARTFAILURE.108.839076. [DOI] [PubMed] [Google Scholar]
- 64.Mehra MR, Ventura HO, Kapoor C, Stapleton DD, Zimmerman D, Smart FW. Safety and clinical utility of long-term intravenous milrinone in advanced heart failure. Am J Cardiol. 1997;80:61–64. doi: 10.1016/s0002-9149(97)00284-1. [DOI] [PubMed] [Google Scholar]
- 65.Uretsky BF, Jessup M, Konstam MA, et al. Multicenter trial of oral enoximone in patients with moderate to moderately severe congestive heart failure. Lack of benefit compared with placebo. Enoximone multicenter trial group. Circulation. 1990;82:774–780. doi: 10.1161/01.cir.82.3.774. [DOI] [PubMed] [Google Scholar]
- 66.Cowley AJ, Skene AM. Treatment of severe heart failure: quantity or quality of life? A trial of enoximone. Enoximone investigators. Br Heart J. 1994;72:226–230. doi: 10.1136/hrt.72.3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cohn JN, Goldstein SO, Greenberg BH, et al. A dose-dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone trial investigators. New Engl J Med. 1998;339:1810–1816. doi: 10.1056/NEJM199812173392503. [DOI] [PubMed] [Google Scholar]
- 68.Hampton JR, van Veldhuisen DJ, Kleber FX, et al. Randomised study of effect of ibopamine on survival in patients with advanced severe heart failure. Second Prospective Randomised Study of Ibopamine on Mortality and Efficacy (PRIME II) Investigators. Lancet. 1997;349:971–977. doi: 10.1016/s0140-6736(96)10488-8. [DOI] [PubMed] [Google Scholar]
- 69.Packer M, Carver JR, Rodeheffer RJ, et al. Effect of oral milrinone on mortality in severe chronic heart failure. The PROMISE Study Research Group. New Engl J Med. 1991;325:1468–1475. doi: 10.1056/NEJM199111213252103. [DOI] [PubMed] [Google Scholar]
- 70.Drakos SG, Kanakakis JV, Nanas S, et al. Intermittent inotropic infusions combined with prophylactic oral amiodarone for patients with decompensated end-stage heart failure. J Cardiovasc Pharmacol. 2009;53:157–161. doi: 10.1097/FJC.0b013e31819846cd. [DOI] [PubMed] [Google Scholar]
- 71.Nanas JN, Tsagalou EP, Kanakakis J, et al. Long-term intermittent dobutamine infusion, combined with oral amiodarone for end-stage heart failure: a randomized double-blind study. Chest. 2004;125:1198–1204. doi: 10.1378/chest.125.4.1198. [DOI] [PubMed] [Google Scholar]
- 72.Tsagalou EP, Anastasiou-Nana MI, Terrovitis JV, et al. The long-term survival benefit conferred by intermittent dobutamine infusions and oral amiodarone is greater in patients with idiopathic dilated cardiomyopathy than with ischemic heart disease. Int J Cardiol. 2006;108:244–250. doi: 10.1016/j.ijcard.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 73.Cuffe MS, Califf RM, Adams KF, Jr, et al. Short-term intravenous milrinone for acute exacerbation of chronic heart failure: a randomized controlled trial. JAMA. 2002;287:1541–1547. doi: 10.1001/jama.287.12.1541. [DOI] [PubMed] [Google Scholar]
- 74.Felker GM, Benza RL, Chandler AB, et al. Heart failure etiology and response to milrinone in decompensated heart failure: results from the OPTIME-CHF study. J Am Coll Cardiol. 2003;41:997–1003. doi: 10.1016/s0735-1097(02)02968-6. [DOI] [PubMed] [Google Scholar]
- 75.Metra M, Eichhorn E, Abraham WT, et al. Effects of low-dose oral enoximone administration on mortality, morbidity, and exercise capacity in patients with advanced heart failure: the randomized, double-blind, placebo-controlled, parallel group ESSENTIAL trials. Eur Heart J. 2009;30:3015–3026. doi: 10.1093/eurheartj/ehp338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feldman AM, Oren RM, Abraham WT, et al. Low-dose oral enoximone enhances the ability to wean patients with ultra-advanced heart failure from intravenous inotropic support: results of the oral enoximone in intravenous inotrope-dependent subjects trial. Am Heart J. 2007;154:861–869. doi: 10.1016/j.ahj.2007.06.044. [DOI] [PubMed] [Google Scholar]
- 77.Colucci WS, Sonnenblick EH, Adams KF, et al. Efficacy of phosphodiesterase inhibition with milrinone in combination with converting enzyme inhibitors in patients with heart failure. The milrinone multicenter trials investigators. J Am Coll Cardiol. 1993;22:113A–118A. doi: 10.1016/0735-1097(93)90473-e. [DOI] [PubMed] [Google Scholar]
- 78.Massie B, Bourassa M, DiBianco R, et al. Long-term oral administration of amrinone for congestive heart failure: lack of efficacy in a multicenter controlled trial. Circulation. 1985;71:963–971. doi: 10.1161/01.cir.71.5.963. [DOI] [PubMed] [Google Scholar]
- 79.Narahara KA. Oral enoximone therapy in chronic heart failure: a placebo-controlled randomized trial. The Western Enoximone Study Group. Am Heart J. 1991;121:1471–1479. doi: 10.1016/0002-8703(91)90154-a. [DOI] [PubMed] [Google Scholar]
- 80.van Veldhuisen DJ, Man in ‘t Veld AJ, Dunselman PH, et al. Double-blind placebo-controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT) J Am Coll Cardiol. 1993;22:1564–1573. doi: 10.1016/0735-1097(93)90579-p. [DOI] [PubMed] [Google Scholar]
- 81.Dubourg O, Delorme G, Hardy A, Beauchet A, Tarral A, Bourdarias JP. Placebo-controlled trial of oral enoximone in end-stage congestive heart failure refractory to optimal treatment. Int J Cardiol. 1990;28(Suppl 1):S33–S42. doi: 10.1016/0167-5273(90)90149-y. discussion S43. [DOI] [PubMed] [Google Scholar]
- 82.Feldman AM, Bristow MR, Parmley WW, et al. Effects of vesnarinone on morbidity and mortality in patients with heart failure. Vesnarinone study group. New Engl J Med. 1993;329:149–155. doi: 10.1056/NEJM199307153290301. [DOI] [PubMed] [Google Scholar]
- 83.Likoff MJ, Weber KT, Andrews V, et al. Amrinone in the treatment of chronic cardiac failure. J Am Coll Cardiol. 1984;3:1282–1290. doi: 10.1016/s0735-1097(84)80189-8. [DOI] [PubMed] [Google Scholar]
- 84.Baruch L, Patacsil P, Hameed A, Pina I, Loh E. Pharmacodynamic effects of milrinone with and without a bolus loading infusion. Am Heart J. 2001;141:266–273. doi: 10.1067/mhj.2001.111404. [DOI] [PubMed] [Google Scholar]
- 85.Xamoterol in severe heart failure. The xamoterol in severe heart failure study group. Lancet. 1990;336:1–6. [PubMed] [Google Scholar]
- 86.DiBianco R, Shabetai R, Silverman BD, Leier CV, Benotti JR. Oral amrinone for the treatment of chronic congestive heart failure: results of a multicenter randomized double-blind and placebo-controlled withdrawal study. J Am Coll Cardiol. 1984;4:855–866. doi: 10.1016/s0735-1097(84)80044-3. [DOI] [PubMed] [Google Scholar]
- 87.Khalife K, Zannad F, Brunotte F, et al. Placebo-controlled study of oral enoximone in congestive heart failure with initial and final intravenous hemodynamic evaluation. Am J Cardiol. 1987;60:75C–79C. doi: 10.1016/0002-9149(87)90531-5. [DOI] [PubMed] [Google Scholar]
- 88.Francis GS, Bartos JA, Adatya S. Inotropes. J Am Coll Cardiol. 2014 doi: 10.1016/j.jacc.2014.01.016. [DOI] [PubMed] [Google Scholar]