Abstract
The composition and relative abundance of airborne pollen in urban areas of Australia and New Zealand are strongly influenced by geographical location, climate and land use. There is mounting evidence that the diversity and quality of airborne pollen is substantially modified by climate change and land-use yet there are insufficient data to project the future nature of these changes. Our study highlights the need for long-term aerobiological monitoring in Australian and New Zealand urban areas in a systematic, standardised, and sustained way, and provides a framework for targeting the most clinically significant taxa in terms of abundance, allergenic effects and public health burden.
Introduction
Pollen is the microscopic haploid stage of the plant life cycle. Pollen grains from different plant species have a remarkable diversity of shapes, sizes, and biochemical compositions. The identification of fossilised pollen in sedimentary sequences (palynology) has been pivotal in illuminating past environmental changes caused by, or associated with, climate change, human impacts and natural disturbances such as fires and tectonic activity. This technique is dependent on the assumption that deposited pollen provides a faithful representation of the vegetation patterns surrounding sedimentary traps. Palynologists test this assumption by collecting pollen from the ground surface within or between typical vegetation types or by sampling pollen in the atmosphere (e.g., D'Costa and Kershaw [1]; Wilmshurst and McGlone [2]; Fletcher and Thomas [3]; Tng et al. [4]). These investigations of ‘modern pollen rain’ have focused on wilderness or rural areas, to provide a basis for reconstructing historic biogeographic patterns. How pollen rain varies geographically within urban environments in Australia and New Zealand, where over 85% of the population live in Australia and New Zealand, remains poorly characterised (see Tng et al. [4] for an exception).
Parallel studies have produced substantial time series of airborne pollen data for urban locations to underpin investigations of seasonal allergic reaction such as rhinitis or asthma in humans. These large data sets have enabled the development of predictive models between meteorological variables and the concentration of pollen from specific allergenic pollen taxa such as grass and birch (e.g., Schäppi et al. [5]; Emberlin et al. [6]; Rodríguez-Rajo et al. [7]; Sofiev et al. [8]). In addition, in some urban areas, particularly in Europe, the aerobiological datasets now span several decades and are beginning to provide insights into biogeographic variation in landscape phenological patterns, and how these patterns respond to current climate change [9]–[12].
In contrast to the well-studied Northern Hemisphere, aerobiological studies of the Southern Hemisphere have been conducted in isolation and limited to monitoring specific cities for narrow time periods with few attempts made to discern generalised patterns across a country or region (see [13] for an exception). Indeed, many of the existing aerobiological studies located in Australia and New Zealand have been motivated by an interest in the public health burden of pollen sensitization and allergic asthma at major population centres. Aerobiology in the Southern Hemisphere is as complex as that in the Northern Hemisphere, given the variability of abiotic factors and environmental gradients that span the tropics to the temperate zones. The coexistence of very distinct indigenous vegetation, introduced Northern Hemisphere ornamentals, as well as exotic invasive species are additional variables that contribute to the aerobiology of each Southern Hemisphere regional center. Here we compile and analyse an historic atmospheric pollen dataset from 11 cities across Australia and New Zealand and examine the regional variations in pollen content and relative abundance. The geographical distribution of the 11 Australasian sites was broad enough to enable further examination of the role that biogeography plays in regional and urban variations in pollen composition. These data can potentially provide insights into how pollen production and dispersal derived from both native and exotic taxa may respond to changes in climate and urban and peri-urban land use. The influence of these variables and how that may affect allergic diseases is also discussed.
We emphasise that these results were achieved by collating data sets that were originally obtained for other purposes and were often of limited duration and collected using a variety of methods. The combined data set provides impetus for further work and suggests the potential of a more systematic and coordinated pollen monitoring network in Australasia for long-term studies into environmental change and the production of more accurate pollen forecasting systems to help allergy sufferers and their health care providers better manage their conditions. We conclude by considering some important issues that such an initiative could address.
Materials and Methods
Sampling Sites
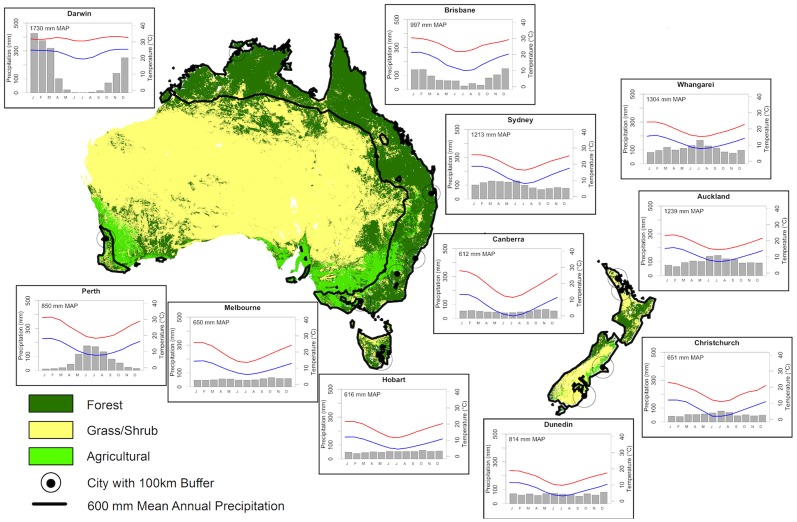
Aerobiological data have been collected across Australia and New Zealand using a range of methods and counting periods (see Table 1). This paper describes the re-analysis of approved university studies of aerobiological pollen in Australia and New Zealand. All studies have been published (Table 1) with the exception of pollen data from Perth and Canberra, where unpublished data were provided by the investigators at these sites (also authors in this paper FM and SGH). In cities where more than one station is operating the secondary station is not included in the numerical analysis as they overlap and are shorter records than the nearby stations in the same cities. Land cover attributes for Australia and New Zealand with climate summaries for each major urban centre associated with aerobiology studies are shown in Figure 1. Table 1 provides the location details of each aerobiological station including the collection periods of 14 aerobiology stations across 11 urban centres, which have been in operation for at least one season over the last 25 years. In aerobiology, pollen traps are situated well above ground level, both to avoid the complicating influence of local vegetation and anthropogenic disturbance as well as to better sample the regional pollen flux that are dominated by the major anemophilous (wind-dispersed) taxa [19]. This group of wind-pollinated species comprise many of the important allergenic sources [20]. The data presented here were obtained at pollen sampling sites ranging in elevation above ground level that, along with other differences in collection methods described below, are likely to affect the total volume of pollen sampled. Nevertheless the methods used at each location provide estimates of airborne pollen volume (in grains/cm3) that can be compared between regions and through time.
Table 1. Sites and methodologies employed in aerobiology recording stations across Australia and New Zealand.
| Location name | Suburb (site) | Country (State/Territory) | Latitude (S) | Longitude (E) | Elevation (m) | Monitor Height (m) | Start Date | End Date | Duration (days) | Time step | Time Start/End | Monitor type | Magnification | Counting method |
| Darwin | Casuarina (Charles Darwin University) | A (NT) | 12°22′ | 130°52′ | 13 | 14 | 18/03/2004 | 15/11/2005 | 607 | 24 hrs | 0:00 | Burkard | 400× | 4 transects |
| Darwin | Palmerston | A (NT) | 12°28′ | 130°58′ | 24 | 14 | 1/04/2004 | 31/03/2005 | 364 | 24 hrs | 0:00 | Burkard | 400× | 4 transects |
| Brisbane | Rocklea | A (QLD) | 27°29′ | 153°08′ | 13 | 2 | 7/06/1994 | 17/05/1999 | 1805 | 7 day | unknown | Burkard | 250× | entire slide |
| Perth | Murdoch (Murdoch University) | A (WA) | 32°04′ | 115°50′ | 30 | unknown | 1/09/2006 | 31/12/2006 | 121 | 24 hrs* | 0:00 | Burkard | ×400 | 3 transects |
| Sydney | Campbelltown (University of Western Sydney) | A (NSW) | 34°04′ | 150°47′ | 74 | 10 | 1/01/1993 | 31/12/1995 | 1094 | 24 hrs | unknown | Burkard | 400× | 3 transects |
| Canberra | Acton (Australian National University) | A (ACT) | 35°16′ | 149°7′ | 569 | 8 | 26/09/2007 | 13/12/2009 | 809 | 24 hrs | 0:00 | Burkard | 400× | 4 transects |
| Canberra | Holder | A (ACT) | 35°19′ | 149°2′ | 570 | 14 | 27/8/09, 29/9/10 | 31/1/10, 26/11/10 | 210 | 24 hrs | 10am (2009), 4pm (2010) | Burkard | ×40 (2009), ×20 (2010) | 1 transect |
| Melbourne | Parkville (Melbourne University) | A (VIC) | 37°48′ | 144°17′ | 14 | 1/09/2009 | 31/12/2011 | 852 | 24 hrs | 16:00 | Burkard | 20× with a field of view of 1 mm | 1 transect | |
| Hobart | Sandy Bay (University of Tasmania) | A (TAS) | 42°54′ | 147°19′ | 58 | 12 | 6/09/2007 | 31/12/2010 | 1212 | 24 hrs | 0:00 | Burkard | 400× | 4 transects |
| Kaikohe | Kaikohe | NZ | 35°24′ | 173°48′ | 198 | 2 | 15/11/1988 | 11/02/1989 | 88 | 24 hrs | 12:00 | Rotorod | 400× | n/a |
| Auckland | Grafton (Museum) | NZ | 36°51′ | 174°46′ | 61 | 8 | 28/10/1989 | 27/04/1990 | 181 | 24 hrs | 9:00 | Rotorod | 400× | n/a |
| Auckland | Onehunga | NZ | 36°51′ | 174°46′ | 15 | 3 | 27/10/1989 | 30/04/1990 | 185 | 24 hrs | 9:00 | Rotorod | 400× | n/a |
| Christchurch | Christchurch (Canterbury University) | NZ | 43°31′ | 172°35′ | 14 | 20 | 17/11/1988 | 13/02/1989 | 88 | 24 hrs | 12:00 | Rotorod | 400× | n/a |
| Dunedin | Dunedin | NZ | 45°51′ | 170°30′ | 0 | 5 | 1/10/1992 | 31/01/1993 | 122 | 24 hrs | 9:00 | Rotorod | 400× | n/a |
Data derived from unpublished and published data (Newnham et al. [13]; Bass and Morgan [14]; Ong et al. [15]; Green et al. [16]; Stevenson et al. [17]; Tng et al. [4]; Medek et al. [18]). Note that data from secondary stations in Darwin (Palmerston), Canberra (Holder) and Auckland (Grafton Museum) are not included in the numerical analysis in this paper. The raw pollen counts for each site used in this paper have been archived in excel files at the Australian Centre for Ecological Analysis and Synthesis data portal (http://aceas-data.science.uq.edu.au/portal/). Click on the following hyperlinked text to download data from each aerobiology recording station. Darwin, Brisbane, Perth, Sydney, Canberra, Melbourne, Hobart, Kaikohe, Auckland, Christchurch, Dunedin.
Assumptions: All air sampled at 10 l/minute (except NZ rotorod: 1.59×32 mm rods spun at 2400 rev/min for 6 min/hr), and all express concentration as grains/m3 of air.
*Not Saturdays and Sundays.
A; Australia, NZ; New Zealand, NT; Northern Territory, QLD; Queensland, WA; Western Australia, NSW; New South Wales, ACT; Australian Capital Territory, VIC; Victoria, TAS; Tasmania.
Figure 1. Land cover attribute for Australia and New Zealand with climatological summaries for each major urban centre associated with an aerobiology study.
Climate summaries include average monthly precipitation and minimum and maximum temperatures for each urban area (see Table 3 for data sources). Data shown for Whangarei represents the Kaikohe pollen count site.
Pollen Data Collection
The most common method of daily pollen counts in Australia is based on the deployment of a seven-day Hirst-type volumetric pollen and spore trap [21] located on structures (mostly rooftops) ranging from between 2–14 m above the ground. In New Zealand, an alternative device, the Intermittent Cycling Rotorod sampler [22] was deployed at all sites, but provides similar volumetric data. The Hirst-type sampler uses a range of adhesive surface compounds including vaseline and 10% paraffin wax in toluene on Melinex™ tape (Burkard Manufacturing Co. Ltd., Rickmansworth, Hertfordshire, UK), silicon-based adhesive (Lanzoni s.r.l., Bologna, Italy) or a 50% saturated Dow Corning high vacuum grease in solvent [23]. The seven-day tapes are then cut into 24-hr segments and mounted on glass slides with a stain such as fuchsine stained Gelvatol [24], Calberla's stain, or 2% Saffranin O in glycerol jelly. Alternatively daily pollen monitoring can be done using a glass microscope slide that is coated and stained as described. Analysis of each 24-hr period is conducted by counting between one and four transects at 400 magnification [25], though at one site the entire slide surface was counted (Brisbane, [26]). Pollen and spore counts were then converted to grains/m3 of air and expressed as a daily mean value [21]. Hirst-type pollen and spore traps are known to show an instrumental variation of about 25% [27]. In New Zealand the Intermittent Cycling Rotorod sampler is an impaction collector with a retracting collector rod sampling head [22]. Particles are collected on the leading, greased, edge of two 1.59×32 mm clear polystyrene collector rods spun intermittently by an electric motor at 2400 rev./min. The samplers were set-up to operate for 6 min every hour. Sampling rods were replaced every 24 hours, stained with Calberla's solution, and examined under a transmitted light microscope. Raw pollen counts for each of the pollen/spore types identified were converted to the volumetric index (grains/m3 of air) using a standard formula taking into account the sampling period and volume of air sampled [22]. The daily mean concentration of fungal spores, particularly Alternaria, was also recorded at some stations (Darwin, Brisbane, Sydney, Canberra, Melbourne and Hobart), though detailed analysis of these data is not presented here. The raw pollen counts for each site used in this paper have been archived in excel files at the Australian Centre for Ecological Analysis and Synthesis data portal (http://aceas-data.science.uq.edu.au/portal/). Links to specific station datasets are provided in Table 1.
Pollen Identification
Pollen identification was aided by the existing reference collections held by individual analysts or collated into digital or online reference collections (e.g., the Department of Archaeology and Natural History at the Australian National University, http://apsa.anu.edu.au, the CD published by Hjelmroos et al. [28]). In a number of these aerobiological surveys, it was not possible to place identifications at a species or genus taxonomic level. In these cases the taxon name reverted to the higher level (i.e., all genus-level identifications were placed into the relevant family) such as the Poaceae and Myrtaceae. The introduced tree taxa are referred to by their generic name (Alnus, Betula, Pinus, Quercus, Salix and Ulmus) as all are represented in Australia and New Zealand by more than one species in the genus and these are not distinguished in the available datasets.
Pollen Count Analysis
In order to reduce variation in pollen abundance records due to variations in equipment or handling protocols, the datasets were transformed into percentage values based on a total pollen sum (total pollen abundance of a single taxon recorded over the time of study divided by the total pollen abundance of all taxa recorded over the time of study) (Table 2). This data transformation mitigated other sources of variation in abundance between monitoring sites such as location, as coastal cities are exposed to a smaller proportion of land area and hence vegetation sources than inland cities (Figure 1).
Table 2. Aerobiologically significant pollen taxa contributing >80% to the total annual atmospheric pollen in urban areas across Australia and New Zealand.
| Taxa | Darwin | Brisbane | Perth | Sydney | Canberra | Melbourne | Hobart | Kiakohe | Auckland | Christchurch | Dunedin | Ave (rank) |
| Arecaceae | 26.0 | *26.0 | ||||||||||
| Casuarina | 3.1 | 6.5 | 6.2 | 1.8 | 0.4 | 4.3 | 3.7 (8) | |||||
| Urticaceae | 1.6 | 1.8 | 0.7 | 1.9 | 0.9 | 0.1 | 1.2 (16) | |||||
| Acacia | 3.1 | 0.2 | 0.3 | 0.3 | 0.3 | 0.5 | <0.1 | 0.7 (18) | ||||
| Myrtaceae | 31.0 | 3.1 | 6.0 | 11.0 | 5.7 | 5.6 | 8.2 | <0.1 | 5.2 | 3 | 7.9 (4) | |
| Chenopodiaceae | 0.3 | 0.1 | 0.8 | 0.2 | 0.3 | <0.1 | 11.0 | 6.8 | 2.4 (12) | |||
| Cyperaceae | 5.5 | 1.3 | 0.3 | 1.2 | 0.5 | 0.03 | 1.5 (13) | |||||
| Asteraceae | 0.1 | 0.7 | 2.5 | 1.0 | 0.8 | 0.4 | 0.5 | 0.3 | 2.1 | 2 | 1.0 (17) | |
| Cupressaceae | 5.2 | 9.0 | 27.5 | 23.0 | 22.5 | 58.0 | 13.3 | 0.4 | 1.0 | 1.7 | 28.5 | 17.3 (2) |
| Pinus | 0.1 | 4.5 | 31.0 | 3.0 | 17.0 | 1.2 | 3.3 | 0.3 | 0.5 | 2.4 | 11.4 | 6.8 (5) |
| Poaceae | 18.0 | 71.0 | 23.0 | 17.5 | 15.0 | 10.8 | 10.4 | 84.0 | 43.7 | 51.6 | 25.8 | 33.7 (1) |
| Plantago | 0.2 | 5.1 | 3.2 | 1.8 | 3.4 | 11.1 | 16.0 | 8.6 | 6.2 (7) | |||
| Ulmus | <0.1 | 3.8 | 0.4 | 1.6 | 1.5 (14) | |||||||
| Oleaceae | 20.0 | 1.8 | 5.2 | 5.2 | <0.1 | 7.2 | 6.6 (6) | |||||
| Salix | 0.2 | 1.7 | 5.0 | <0.1 | 0.2 | 1.4 (15) | ||||||
| Rumex | 4.3 | 0.1 | 2.3 | 3.6 | 3.5 | 6.5 | 3.4 (9) | |||||
| Quercus | 0.1 | 3.1 | 4.9 | 1.6 | <0.1 | 0.2 | 11.2 | 3.0 (10) | ||||
| Alnus | 3.9 | 1.8 | 2.0 | 2.6 (11) | ||||||||
| Betula | 1.7 | 4.5 | 16.7 | 0.6 | 0.35 | 27.0 | 8.5 (3) | |||||
| Coprosma | 0.4 | <0.1 | 0.1 | 1.7 | 0.6 (19) | |||||||
| Sub-total | 93.9 | 98.3 | 90.0 | 88.3 | 90.5 | 96.3 | 79.4 | 99.8 | 84.0 | 96.1 | 99.9 | 92.4 |
| Other | 6.1 | 1.7 | 10.0 | 11.7 | 9.5 | 3.7 | 20.6 | 0.2 | 16 | 3.9 | 0.1 | 7.6 |
Pollen taxa values are expressed as a percentage of the total pollen count for the record period available in each urban area (*excluded from ranking as only occurs in one urban area). The average and rank of percentage values across all urban areas is given in the right hand column. The category “Other” includes all pollen taxa counted in the total pollen sum and not identified to taxa here.
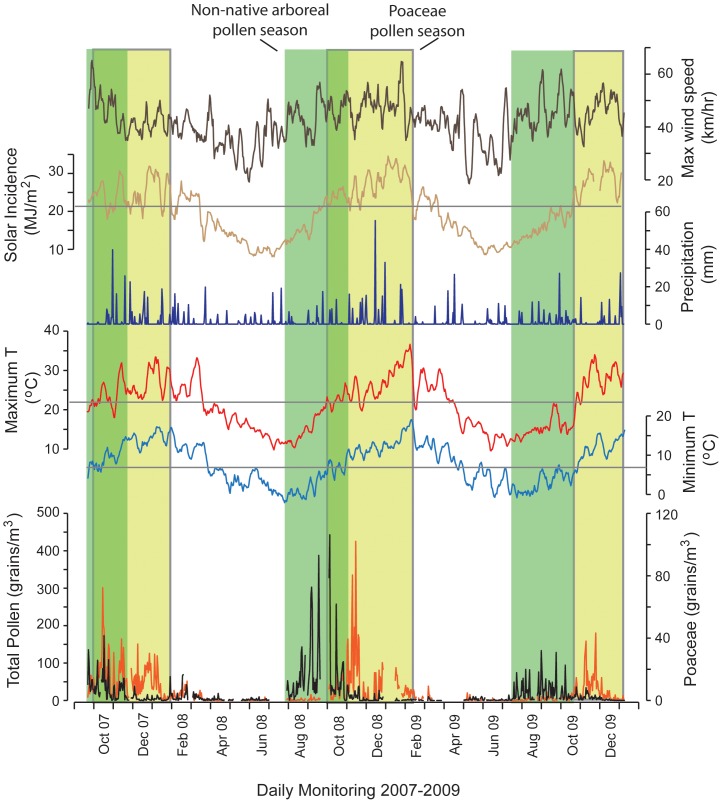
The pollen season has been defined in many ways by different authors [29]. To date, no standardized approach to identifying the pollen season has been defined in Australia or New Zealand [13], [15]–[17]. In general, the pollen season is said to have four parameters, the start, duration, peak and the end. In this study we adopted the Nilsson and Persson [30] approach, where the ‘start day’ is when the sum of daily pollen concentrations reaches 5% of the total yearly count and the ‘end day’ when the sum reaches 95% of the yearly count. The ‘year’ for all subtropical and temperate sites began on July 1 and ends on June 30. The exception was the tropical site (Darwin), where the ‘year’ begins on January 1 and ends on December 31. The season is thus the period when 90% of the total annual pollen count is collected on the trapping surface (see example given in Figure 2). Here we also point out that the years of sampling varied between certain sites, ranging from 1988 to 2012, with some sites sampled for a single season, a single year, or a small number of years. This factor must be considered when comparing phenological observations such as the timing of pollen season parameters between sites, as pollen season for the same species may vary substantially between years [31].
Figure 2. Climate summary and daily airborne pollen (Poaceae and Non-native arboreal taxa) for Canberra (26 Sept 2007–31 Dec 2009).
Pollen season is depicted by the shaded columns and defined by 90% of the airborne pollen falling in this time for each year (July 1 to June 30). Climate data from the Australian Bureau of Meteorology.
Similarities between the airborne pollen assemblages recorded within the 11 urban areas were calculated using non-metric multidimensional scaling ordination (nMDS, [32]), a technique that reduces the dimensionality of multivariate data, and graphically represented using the relative dissimilarities amongst (a) urban areas and (b) dominant taxa. The robust nature of the ordination is measured through the numerical stress value. A stress less than two corresponds to a good ordination and useful two-dimensional picture of sample similarity [32]. The nMDS outputs show vector positions for each variable within the environmental space. The length of the vectors correspond to the square-root of the r2 values, so weak predictors have a shorter length than strong predictors. High r2 values indicate a vector that is strongly associated with site/species variation in the ordination space. Low r2 values would indicate a vector that doesn't really explain much in the ordination space. Environmental vectors representing three meteorological variables (Mean annual maximum and minimum temperature and mean annual precipitation) and the proportion of four broad land cover types in a 100 km radius around each urban area were fitted to the species ordination using the envfit function of the vegan package in R [33], with random permutations of environmental variables performed in order to assess their significance. We make the assumption that the proportion of land cover types found in a 100 km radius around pollen sampling stations is representative of the total potential pollen dispersal area. However, it must be acknowledged that little is known of the pollen dispersal characteristics of Australasian pollen types and that further research is required to understand the influence that meteorological factors such as wind strength and distance from source vegetation might have on pollen concentrations in the atmosphere.
Results
Pollen Ranking Among Urban Areas
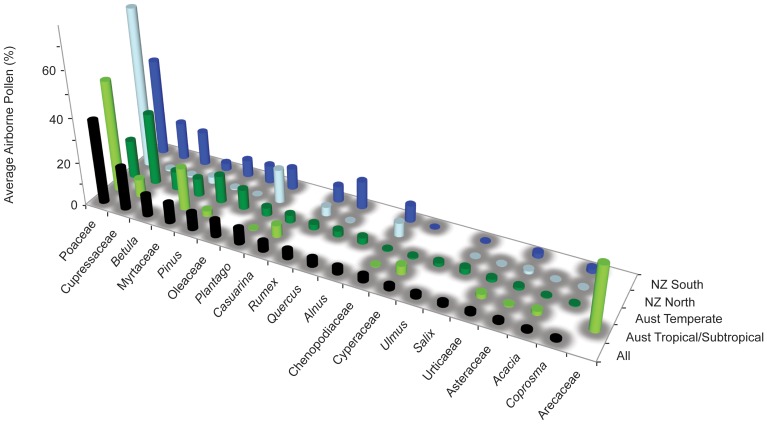
The airborne pollen data were ranked by percentage representation, with the top 20 pollen taxa making up >80% of the total pollen collected across 11 cities in Australia and New Zealand (Table 2 and Figure 3). The dataset incorporates taxa of high relative percentage representation that commonly occur in two or more urban areas (Arecaceae is an exception because of the high values recorded in Darwin). The most significant taxa across all sites are Poaceae and Cupressaceae, making up over 50% of the total airborne pollen in urban environments throughout the year. These are followed by Betula, the trees and shrubs in Myrtaceae, Pinus, Oleaceae, Casuarina, and the important herbaceous taxa such as Plantago and Rumex. Differences between the pollen taxa rankings (highest to lowest percentage representation) across the broad biogeographic regions of Australia (tropics to temperate: Australian Tropical/Subtropical = Darwin, Brisbane; Australian Temperate = Perth, Sydney, Canberra, Melbourne, Hobart) and New Zealand (North and South Island: NZ North = Kaikohe, Auckland; NZ South = Christchurch, Dunedin), reflect the strong climate controls on plant distributions, particularly those associated with Northern Hemisphere introductions such as Betula, Quercus, Alnus and Ulmus that contribute high levels of pollen into the atmosphere of the southern temperate cities.
Figure 3. Ranking for top 20 pollen taxa based on average percentage representation of airborne pollen areas across all urban areas (black bars).
These urban areas are then grouped into regional biogeographic zones (blank space = taxa not recorded). Australian Tropical/Subtropical = Darwin, Brisbane; Australian Temperate = Perth, Sydney, Canberra, Melbourne, Hobart; NZ North = Kaikohe, Auckland; NZ South = Christchurch, Dunedin.
Biogeographic Patterns
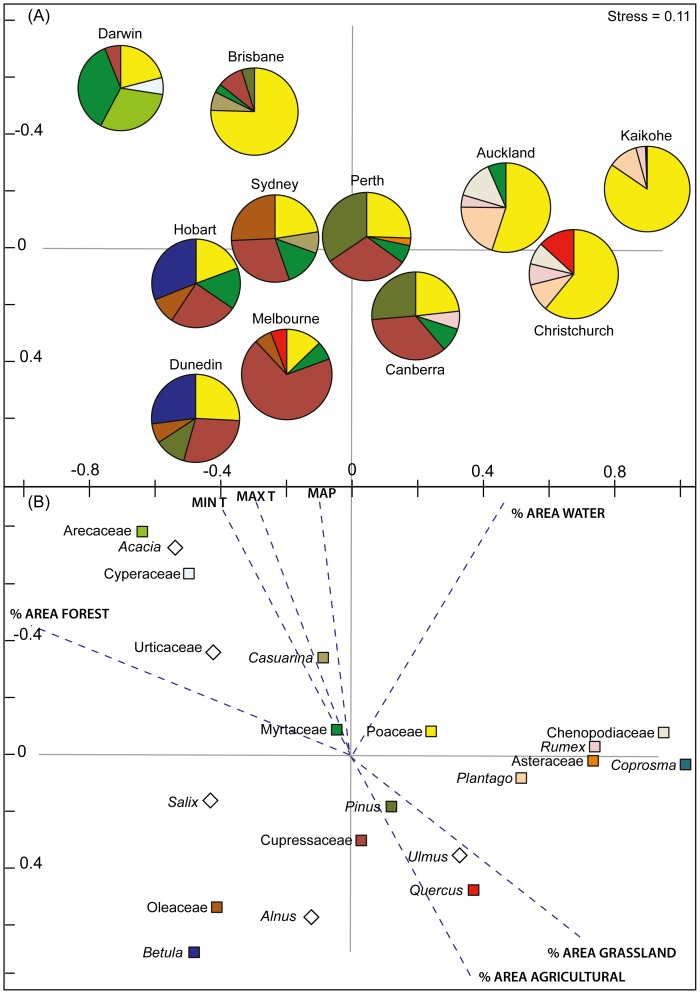
The two dimensional ordination had a low stress level (stress = 0.11) indicating it was a robust graphical representation of the similarity matrix among the cities and the dominant taxa. The ordination displays a gradation in vegetation formation (and land cover) across the set of urban areas (Figure 4A). The x-axis separates the tree dominant urban landscapes (negative values associated with pie charts dominated by tree taxa) from the grassland dominated urban landscapes (positive values associated with pie charts dominated by herbaceous taxa). The y-axis is associated with latitudinal position of Australian and New Zealand sites with positive values corresponding to lower latitude urban areas and negative values corresponding with higher latitude urban areas. The most significant environmental variable explaining the difference between airborne pollen in each urban area was minimum annual temperature (MinT, r2 = 0.77, P = 0.002), which is closely aligned to the latitudinal transect along which the urban areas lie (Table 3). The second environmental factor that showed a significant correlation with the airborne pollen was mean annual precipitation (MAP, r2 = 0.66, P = 0.01). Increased grass pollen dominance is clearly apparent in Figure 4, particularly in the savanna dominated tropics (Darwin), dry sclerophyll subtropical forests (Brisbane) and pastoral dominated temperate landscapes of New Zealand, where associated ruderal taxa such as Rumex, Plantago, Asteraceae and Chenopodiaceae are also prominent. The contribution of grass pollen was reduced in the southern temperate cities, where other woody taxa such as Cupressaceae (including both native and introduced species) and the introduced Northern Hemisphere trees (Betula, Alnus, Ulmus, and Quercus) become significant contributors.
Figure 4. Non-metric multidimensional scaling ordination (nMDS) of the major pollen taxa in Australia and New Zealand.
(A) Distribution of urban areas using a matrix of percentage representation of major pollen taxa (see Table 2 and using top eight pollen taxa at each site). The pie charts depict the relative contribution of the most abundant pollen taxa in each urban area. (B) Distribution of pollen taxa contributing to the differentiation of aerobiology of each urban area Note: coloured squares associated with each taxa depicted in (B) match the pie chart colours shown in (A), with diamonds showing taxa with a low percentage representation. The vectors (dotted lines) for the environmental variables show positions for each variable within the environmental space. Longer vectors (higher r2 values, see Table 3) indicate a stronger association of the environmental variable with site/species variation in the ordination space.
Table 3. Land cover attributes within a 100.
| Urban Area | %Area Water | %Area Agricultural | %Area Grass | %Area Forest | MaxT (°C) | MinT (°C) | MAP (mm) |
| Darwin | 45.5 | 0.1 | 0.2 | 54.3 | 33.3 | 19.3 | 1730 |
| Brisbane | 34.9 | 1.5 | 0.1 | 10.1 | 30.3 | 10.0 | 997 |
| Perth | 38.1 | 31.6 | 3.5 | 26.8 | 31.4 | 7.7 | 850 |
| Sydney | 51.1 | 4.2 | 0.3 | 44.3 | 25.9 | 8.0 | 1213 |
| Canberra | 2.2 | 30.2 | 5.1 | 62.5 | 28.0 | −0.1 | 612 |
| Melbourne | 19.0 | 27.6 | 14.3 | 39.1 | 25.9 | 6.0 | 650 |
| Hobart | 40.2 | 6.3 | 7.3 | 46.2 | 21.6 | 4.5 | 616 |
| Kaikohe | 65.1 | 0.6 | 22.2 | 12.2 | 24.3 | 7.8 | 1304 |
| Auckland | 62.9 | 2.2 | 24.8 | 10.1 | 23.7 | 7.1 | 1239 |
| Christchurch | 57.9 | 20.5 | 18 | 3.6 | 23.0 | 2.0 | 651 |
| Dunedin | 62.4 | 6.1 | 31.1 | 0.4 | 18.9 | 3.2 | 814 |
| nMDS Axis 1 | 0.48 | 0.36 | 0.72 | −0.89 | −0.29 | −0.40 | −0.11 |
| nMDS Axis 2 | −0.88 | 0.93 | 0.70 | −0.45 | −0.96 | −0.92 | −0.99 |
| r2 | 0.14 | 0.39 | 0.33 | 0.34 | 0.38 | 0.77 | 0.66 |
| Pr(>r) | 0.568 | 0.164 | 0.205 | 0.195 | 0.154 | 0.002 | 0.013 |
Pollen Seasons
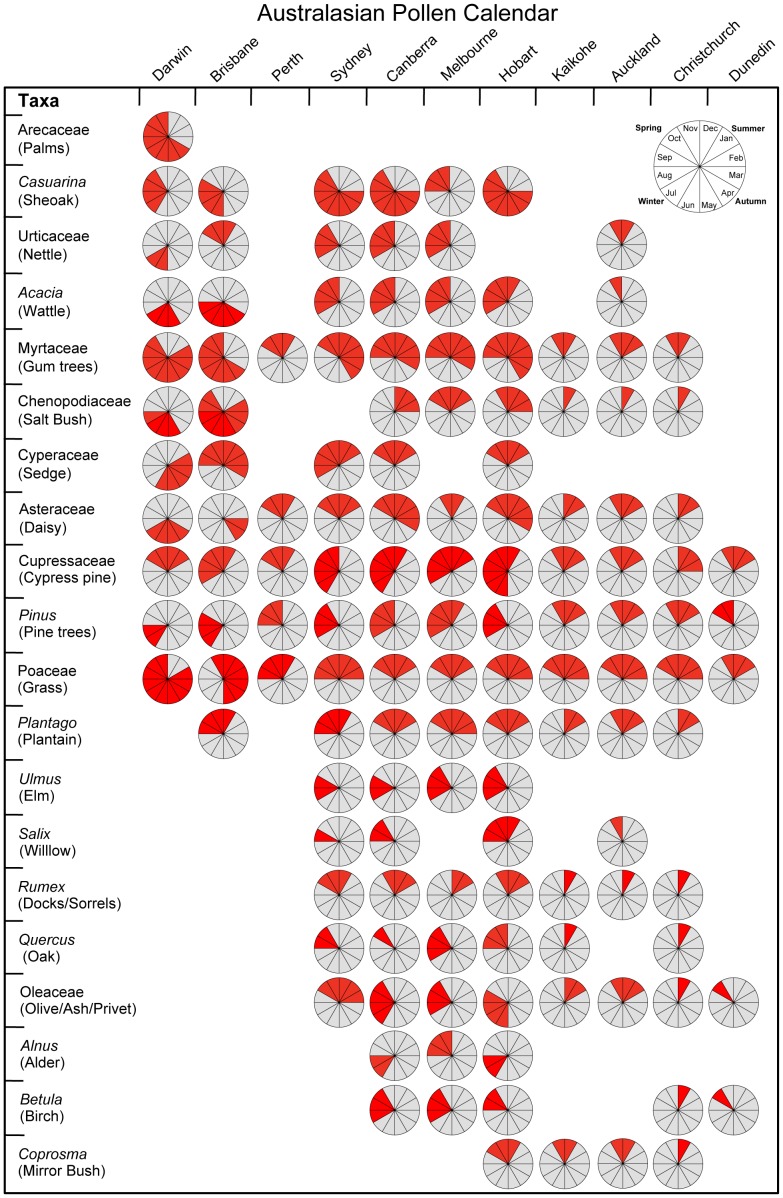
Figure 5 illustrates the Australasian pollen calendar for the top 20 taxa across 11 urban areas. The matrix is organised according to geographical distribution from tropical to temperate for both urban areas and pollen taxa. The clear shifts from long pollen seasons in the tropics to shorter periods in the temperate regions reflects the strong control of solar radiation incidence on pollen production during spring and summer months in the southern urban areas [36]. There is also a discernible shift in the initiation and length of pollen season towards higher latitude. For example, Poaceae has a long flowering season in the northern cities of Darwin and Brisbane (across the dry season and into the wet season as different grass species flower through the year), compared to the progressively shorter and later initiation of the pollen season in the southern cities.
Figure 5. Pollen calendar for aerobiologically significant pollen taxa (contributing 80%+ to the annual atmospheric pollen) in Australian and New Zealand urban areas.
The pie charts are divided into monthly segments with the red shade depicting the pollen season for each taxa to the nearest month in a given urban area. The pollen season for each taxon is determined using the period encompassing 90% of the annual pollen rain (see Figure 2).
The effect of temperature and solar incidence on the beginning and end of the pollen season in the temperate zone is illustrated with an example from Canberra in Figure 2, where the relationship between airborne pollen and key climate variables for Poaceae and total non-native tree species (Northern Hemisphere) is shown. While this is simply a visual comparison, previous studies from Melbourne [15], [37], Brisbane [26] and New Zealand [38] have also documented the significant influence of temperature and precipitation on the commencement and duration of the grass pollen season. The lack of consistent pollen season in some taxa such as Oleaceae may be a function of the multiple species from different genera being incorporated into this taxon (including Olea and Fraxinus), reflecting a significant limitation imposed by the inability to resolve pollen to the species level.
Discussion
The history of aerobiology research in Australia and New Zealand, like the rest of the world, can be characterised by its focus on local site (urban area) issues. Unlike other continents however, where considerable effort has been applied to standardise methodologies, teams working in Australia and New Zealand have tended to use a variety of collection and counting methodologies with little or no co-ordination between aerobiology stations in different urban areas. Despite the idiosyncrasies of the data, this study supports a basic principle that underpins palynology – that airborne pollen is a sensitive proxy of the current climate and flora of a region. Our results indicate that this principle applies as much to the introduced flora as to indigenous vegetation, despite concerted human effort to manipulate the former.
We found that Australian and New Zealand urban areas from a similar climate zones have similar pollen spectra but important differences occur due to surrounding land use and the establishment of non-native plants. Likewise, in similar climate zones, pollen season of each taxon is similar but not the same. For example, urban areas are often surrounded by agricultural landscapes that have a diversity of pollen predominantly represented by grass pollen and characterized by short seasons (Figure 5) compared to urban areas where there is more surrounding forest cover (e.g. Sydney and Hobart). Changes in land use on urban boundaries also have the potential to affect the types of airborne pollen inside the urban area [39]. The predominance of exotic and invasive tree and shrub species (and certainly non-native grasses, albeit these cannot be taxonomically resolved) highlights the profound changes that have occurred following European colonisation of Australia and New Zealand. For instance, in the urban areas of southern Australia and New Zealand, the widely planted birches are particularly dominant.
Temperature and rainfall are known to be primary controls on the daily distribution of airborne pollen in Australia and New Zealand, however, other climate variables that may influence the dissemination of these allergenic pollen types in the atmosphere are not well understood. Other variables such as the El Niño-Southern Oscillation (ENSO) have not been investigated, even though this large-scale ocean-atmosphere anomaly has been shown to modify climatic patterns, leading to droughts and floods, which have local and regional implications on the biosphere as well as vector borne diseases. A similar climate oscillation in western Europe, the North Atlantic Oscillation, has been linked with seasonal variation in grass pollen (e.g. [40]). ENSO and other short term climate oscillations such as the Southern Annular Mode and Indian Ocean Dipole may be important variables that could account for interseasonal differences observed in airborne pollen counts and seasonal starting dates in Australasia. The broad-scale patterns in the distribution, abundance and season of pollen amongst Australasian urban areas provide some clues as to potential changes in aerobiology due to climate change. Evidence from Europe and North America demonstrates that climate change has already increased the abundance and seasonal duration of allergenic pollens such as birch and ragweed and possibly increased concentrations of allergenic compounds [9], [12], [41]. Changes in the burden of allergenic disease related to changing climate have also been demonstrated [41], [42].
In marked contrast to developed countries of the northern hemisphere [43], the potential allergenic impacts of endemic southern hemisphere plants are poorly characterized despite many being identified as important allergens. Given the widespread population exposure to known allergenic plants we endorse the recommendation of the 2007 study of the economic impact of allergic diseases in Australia by Access Economics that ‘studies of the aerobiology and clinical significance of potential native Australian triggers of respiratory allergic disease should be made a priority’ [44]. Possible triggers include both native and non-native taxa. Native genera include common woody plants (Eucalyptus, Melaleuca, Callistemon and Acacia [45]–[51]) and grasses (Sorghum, Sarga and Andropogon [52]–[54]). Non-native plants include a number of taxa known to be allergenic. For example, birch is an important allergenic pollen in Scandinavia and a notable allergen throughout north-central Europe [6], [55] and it has been identified as an important allergenic pollen type in southern Australian urban areas [56]. Similarly, the non-native gamba grass (Andropogon gayensis) is thought to have lengthened the pollen season and increased the community burden of allergic rhinitis in Darwin [57].
Exotic allergenic plants have the potential to cause profound public health impacts if their ranges were to expand and their population to increase. For example, pollen produced by olive trees (Olea europaea) is the leading cause of seasonal allergic diseases in some regions of southern Europe [58], and this species is expanding its range across mediterranean climate zones in Australia [59] adding a new allergic pollen in areas where there are already allergic pollen in the atmosphere. If Ragweed species (Ambrosia spp.) were to expand their range and abundance they would add a new source of allergenic into autumn [60]–[62], a season currently with very few types of pollen (see Figure 5). Because allergic sensitisation to multiple plant allergens is common [63], our results show that the juxtaposition of non-native tree pollen such as birch in early-mid spring with Poaceae pollen in mid-late spring and summer could result in a lengthened period of risk for people allergic to pollens (cf. Figure 2).
Conclusions
Our study highlights the need to monitor changes to the aerobiology and provides a framework for targeting the most important taxa in terms of abundance and allergenic effects for each urban area. Establishing systematic regionally-based monitoring of airborne pollen will enable Australian and New Zealanders to better understand the high taxonomic diversity and seasonal variability of allergenic pollens. This will redress the paucity of research on the clinical and public health impacts and treatments for common endemic allergenic species such as Eucalyptus and Sorghum. Monitoring the increasing abundance of allergenic exotic species populations that can move from a dormant ‘sleeper’ populations to aggressive expansion phases, Ragweed (Ambrosia spp.) being a example, will also be a priority. Understanding the impacts that climate change will have on the phenological cycles and range of allergenic species into the future will be a critical step in the advancement of aerobiology studies in the Australasian region [9].
Acknowledgments
The authors wish to thank the staff of Australian Centre for Ecological Analysis and Synthesis, Terrestrial Ecosystem Research Network for assistance in organising the Workshop 1 of the Working Group “Understanding Australian aerobiology to monitor environmental change and human allergenic exposure”, North Stradbroke Island, Australia (11–15 March, 2013). Alison Specht, Kaitao Lai and Siddeswara Guru assisted with the construction and implementation of the online aerobiology data set. We thank Doctors Diana Bass and Geoffrey Morgan for provision of their published pollen count data from Sydney. Alison Jaggard has been assisted by the Environmental Trust (project reference number 2011/RD/0049), New South Wales Government. The authors also acknowledge the contributions a diverse group of people made to this study through counting pollen and maintaining the pollen records that we consolidated and analysed.
Funding Statement
Funding support for the Working Group came from the Australian Centre for Ecological Analysis and Synthesis (ACEAS). Terrestrial Ecosystem Research Network (TERN). Merck Sharp and Dohme provided additional independent untied co-sponsorship for the Working Group. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. D'Costa D, Kershaw AP (1997) An expanded recent pollen database from south-eastern Australia and its potential for refinement of palaeoclimatic estimates. Aust J Bot 45: 583–605. [Google Scholar]
- 2. Wilmshurst JM, McGlone MS (2005) Origin of pollen and spores in surface lake sediments: comparison of modern palynomorph assemblages in moss cushions, surface soils and surface lake sediments. Rev Palaeobot Palynol 136: 1–15. [Google Scholar]
- 3. Fletcher M-S, Thomas I (2007) Modern pollen–vegetation relationships in western Tasmania, Australia. Rev Palaeobot Palynol 146: 146–168. [Google Scholar]
- 4. Tng DYP, Hopf F, Haberle SG, Bowman DMJS (2010) Seasonal pollen distribution in the atmosphere of Hobart, Tasmania: preliminary observations and congruence with flowering phenology. Aust J Bot 58: 440–452. [Google Scholar]
- 5. Schäppi GF, Taylor PE, Kenrick J, Staff IA, Suphioglu C (1998) Predicting the grass pollen count from meteorological data with regard to estimating the severity of hayfever symptoms in Melbourne (Australia). Aerobiologia 14: 29–37. [Google Scholar]
- 6. Emberlin J, Detandt M, Gehrig R, Jaeger S, Nolard N, et al. (2002) Responses in the start of Betula (birch) pollen seasons to recent changes in spring temperatures across Europe. Int J Biometeorol 46: 159–170 [Erratum published (2003) 47: 113–115.]. [DOI] [PubMed] [Google Scholar]
- 7. Rodríguez-Rajo FJ, Frenguelli G, Jato MV (2003) Effect of air temperature on forecasting the start of the Betula pollen season at two contrasting sites in the south of Europe (1995–2001). Int J Biometeorol 47: 117–125. [DOI] [PubMed] [Google Scholar]
- 8. Sofiev M, Siljamo P, Ranta H, Linkosalo T, Jaeger S, et al. (2013) A numerical model of birch pollen emission and dispersion in the atmosphere. Description of the emission module. Int J Biometeorol 57: 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ziska L, Knowlton K, Rogers C, Dalan D, Tierney N, et al. (2011) Recent warming by latitude associated with increased length of ragweed pollen season in central North America. Proc Natl Acad Sci U S A 108: 4248–4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ziello C, Sparks TH, Estrella N, Belmonte J, Bergmann KC, et al. (2012) Changes to airborne pollen counts across Europe. PLoS ONE 7: e34076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ziska LH, Beggs PJ (2012) Anthropogenic climate change and allergen exposure: The role of plant biology. J Allergy Clin Immunol 129: 27–32. [DOI] [PubMed] [Google Scholar]
- 12. Newnham RM, Sparks TH, Skjøth CA, Head K, Adams-Groom B, et al. (2013) Pollen season and climate: Is the timing of birch pollen release in the UK approaching its limit? Int J Biometeorol 57: 391–400. [DOI] [PubMed] [Google Scholar]
- 13. Newnham RM, Fountain DW, Cornford CC, Forde MB (1995) A national survey of airborne pollen and grass flowering in New Zealand, with implications for respiratory disorder. Aerobiologia 11: 239–252. [Google Scholar]
- 14. Bass D, Morgan G (1997) A three year (1993–1995) calendar of pollen and Alternaria mould in the atmosphere of south western Sydney. Grana 36: 293–300. [Google Scholar]
- 15. Ong EK, Singh MB, Knox RB (1995) Seasonal distribution of pollen in the atmosphere of Melbourne: an airborne pollen calendar. Aerobiologia 11: 51–55. [Google Scholar]
- 16. Green BJ, Dettmann ME, Yli-Panula E, Rutherford S, Simpson R (2004) Aeropalynology of Australian native arboreal species in Brisbane, Australia. Aerobiologia 20: 43–52. [Google Scholar]
- 17. Stevenson J, Haberle SG, Johnston FH, Bowman DMJS (2007) Seasonal distribution of pollen in the atmosphere of Darwin, tropical Australia: Preliminary results. Grana 46: 34–42. [Google Scholar]
- 18. Medek DE, Kljakovic M, Fox I, Pretty DG, Prebble M (2012) Hay fever in a changing climate: linking an internet-based diary with environmental data. Ecohealth 9: 440–447. [DOI] [PubMed] [Google Scholar]
- 19. European Aerobiology Society (2011) Minimum requirements to manage aerobiological monitoring stations included in a national network involved in the EAN. International Aerobiology Newsletter 72: 1. [Google Scholar]
- 20.Knox RB (1979) Pollen and Allergy. Southampton, Edward Arnold, 60pp. [Google Scholar]
- 21. Hirst JM (1952) An automatic volumetric spore trap. Ann Appl Biol 39: 257–265. [Google Scholar]
- 22. Chapman JA (1982) The enhancement of the practice of clinical allergy with daily pollen and spore counts. Immunol Allergy Pract 4: 13–18. [Google Scholar]
- 23. Comtois P, Mandrioli P (1997) Pollen capture media: a comparative study. Aerobiologia 13: 149–154. [Google Scholar]
- 24.Lacey ME, West JS (2006) The air spora. A manual for catching and identifying airborne biological particles. Dordrecht: Springer. [Google Scholar]
- 25. Comtois P, Alcazar P, Néron D (1999) Pollen counts statistics and its relevance to precision. Aerobiologia 15: 19–28. [Google Scholar]
- 26. Green BJ, Dettmann ME, Rutherford S, Simpson RW (2002) Airborne pollen of Brisbane, Australia: a five-year record, 1994–1999. Grana 41: 242–250. [Google Scholar]
- 27. Buters JTM, Thibaudon M, Smith M, Kennedy R, Rantio-Lehtimäki A, et al. (2012) Release of Bet v 1 from birch pollen from 5 European countries. Results from the HIALINE study. Atmos Environ 55: 496–505. [Google Scholar]
- 28.Hjelmroos M, Beyon F, Culliver S, Jones AS, Tovey E (1999) Airborne allergens: Interactive identification of allergenic pollen and fungal spores. Compact Disc, Institute of Respiratory Medicine Limited, Sydney.
- 29. Jato V, Rodríguez-Rajo FJ, Alcázar P, De Nuntiis P, Galán C, et al. (2006) May the definition of pollen season influence aerobiological results? Aerobiologia 22: 13–25. [Google Scholar]
- 30. Nilsson S, Persson S (1981) Tree pollen spectra in the Stockholm region (Sweden), 1973–1980. Grana 20: 179–182. [Google Scholar]
- 31. Spieksma FThM, Emberlin JC, Hjelmroos M, Jäger S, Leuschner RM (1995) Atmospheric birch (Betula) pollen in Europe: Trends and fluctuations in annual quantities and the starting dates of the seasons. Grana 34: 51–57. [Google Scholar]
- 32. Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18: 117–143. [Google Scholar]
- 33.Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, et al. (2013) vegan: Community Ecology Package. R package version 2.0-8. http://CRAN.R-project.org/package=vegan
- 34. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A (2005) Very high resolution interpolated climate surfaces for global land areas. Int J Climatol 25: 1965–1978. [Google Scholar]
- 35. Giri C, Zhu Z, Reed B (2005) A comparative analysis of the Global Land Cover 2000 and MODIS land cover data sets. Remote Sens Environ 94: 123–132. [Google Scholar]
- 36. Hutchinson MF, McIntyre S, Hobbs RJ, Stein JL, Garnett S, et al. (2005) Integrating a global agro-climatic classification with bioregional boundaries in Australia. Global Ecol Biogeogr 14: 197–212. [Google Scholar]
- 37. Ong EK, Singh MB, Knox RB (1995) Grass pollen in the atmosphere of Melbourne: seasonal distribution over nine years. Grana 34: 58–63. [Google Scholar]
- 38. Newnham RM (1999) Monitoring biogeographical response to climate change: The potential role of aeropalynology. Aerobiologia 15: 87–94. [Google Scholar]
- 39. Emberlin J, Mullins J, Corden J, Jones S, Millington W, et al. (1999) Regional variations in grass pollen seasons in the UK, long-term trends and forecast models. Clin Exp Allergy 29: 347–356. [DOI] [PubMed] [Google Scholar]
- 40. Smith M, Emberlin J, Stach A, Rantio-Lehtimaki A, Caulton E, et al. (2009) Influence of the North Atlantic Oscillation on grass pollen counts in Europe. Aerobiologia 25 4: 321–332. [Google Scholar]
- 41. Beggs PJ (2004) Impacts of climate change on aeroallergens: past and future. Clin Exp Allergy 34: 1507–1513. [DOI] [PubMed] [Google Scholar]
- 42. Beggs PJ, Bambrick HJ (2005) Is the global rise of asthma an early impact of anthropogenic climate change? Environ Health Perspect 113: 915–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Patel NJ, Bush RK (2000) The role of environmental allergens in rhinitis. Immunol Allergy Clin North Am 20: 323–353. [Google Scholar]
- 44.Access Economics PL (2007) The economic impact of allergic disease in Australia: not to be sneezed at. Australasian Society of Clinical Immunology and Allergy (ASCIA). 111 p.
- 45. Howlett BJ, Hill DJ, Knox RB (1982) Cross-reactivity between Acacia (wattle) and rye grass pollen allergens: Detection of allergens in Acacia (wattle) pollen. Clin Allergy 12: 259–268. [DOI] [PubMed] [Google Scholar]
- 46. Sweeney M, Hosseiny S, Hunter S, Klotz SD, Gennaro RN, et al. (1994) Immunodetection and comparison of melaleuca, bottlebrush, and bahia pollens. Int Arch Allergy Immunol 105: 289–296. [DOI] [PubMed] [Google Scholar]
- 47. Liam C-K, Loo K-L, Wong CM-M, Lim K-H, Lee T-C (2002) Skin prick test reactivity to common aeroallergens in asthmatic patients with and without rhinitis. Respirology 7: 345–350. [DOI] [PubMed] [Google Scholar]
- 48. Altintaş DU, Karakoç GB, Yilmaz M, Pinar M, Kendirli SG, et al. (2004) Relationship between pollen counts and weather variables in East-Mediterranean Coast of Turkey: Does it affect allergic symptoms in pollen allergic children? Clin Dev Immunol 11: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sritipsukho P (2004) Aeroallergen sensitivity among Thai children with allergic respiratory diseases: a hospital-based study. Asian Pac J Allergy Immunol 22: 91–95. [PubMed] [Google Scholar]
- 50.Rutherford S (2001) Air pollution and asthma severity in South East Queensland: exposure and effects [PhD]. Griffith University.
- 51. Hanigan IC, Johnston FH (2007) Respiratory hospital admissions were associated with ambient airborne pollen in Darwin, Australia, 2004–2005. Clin Exp Allergy 37: 1556–1565. [DOI] [PubMed] [Google Scholar]
- 52. Davies JM, Li H, Green M, Towers M, Upham JW (2012) Subtropical grass pollen allergens are important for allergic respiratory diseases in subtropical regions. Clin Transl Allergy 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lazarides M, Hacker JB, Andrew MH (1991) Taxonomy, cytology and ecology of indigenous Australian sorghums (Sorghum Moench: Andropogoneae: Poaceae). Aust Syst Bot 4: 591–635. [Google Scholar]
- 54. Spangler RE (2003) Taxonomy of Sarga, Sorghum and Vacoparis (Poaceae: Andropogoneae). Aust Syst Bot 16: 279–299. [Google Scholar]
- 55. Burbach GJ, Heinzerling LM, Edenharter G, Bachert C, Bindslev-Jensen C, et al. (2009) GA2LEN skin test study II: Clinical relevance of inhalant allergen sensitizations in Europe. Allergy 64: 1507–1515. [DOI] [PubMed] [Google Scholar]
- 56. Baldo BA, Wrigley CW (1984) Allergy in Australia: Symptoms, diagnosis and treatment. Special Supplement. Med J Aust 141: S12–S18.6482773 [Google Scholar]
- 57. Johnston FH, Hanigan IC, Bowman DMJS (2009) Pollen loads and allergic rhinitis in Darwin, Australia: a potential health outcome of the grass-fire cycle. Ecohealth 6: 99–108. [DOI] [PubMed] [Google Scholar]
- 58. D'Amato G, Liccardi G (2002) The increasing trend of seasonal respiratory allergy in urban areas. Allergy 57: 35–36. [DOI] [PubMed] [Google Scholar]
- 59. Cuneo P, Leishman MR (2006) African olive (Olea europaea subsp. cuspidata) as an environmental weed in eastern Australia: a review. Cunninghamia 9: 545–77. [Google Scholar]
- 60. White JF, Bernstein DI (2003) Key pollen allergens in North America. Ann Allergy Asthma Immunol 91: 425–435; quiz 35–6, 92. [DOI] [PubMed] [Google Scholar]
- 61. Bass DJ, Delpech V, Beard J, Bass P, Walls RS (2000) Ragweed in Australia. Aerobiologia 16: 107–111. [Google Scholar]
- 62. Bass DJ, Delpech V, Beard J, Bass P, Walls RS (2000) Late summer and fall (March–May) pollen allergy and respiratory disease in Northern New South Wales, Australia. Ann Allergy Asthma Immunol 85: 374–381. [DOI] [PubMed] [Google Scholar]
- 63. Greiner AN, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378: 2112–2122. [DOI] [PubMed] [Google Scholar]