Abstract
Maintenance of cell survival is essential for proper embryonic development. In the mouse, Notchless homolog 1 (Drosophila) (Nle1) is instrumental for survival of cells of the inner cell mass upon implantation. Here, we analyze the function of Nle1 after implantation using the Meox2tm1(cre)Sor mouse that expresses the Cre recombinase specifically in the epiblast at E5.5. First, we find that NLE1 function is required in epiblast cells, as Nle1-deficient cells are rapidly eliminated. In this report, we also show that the Meox2Cre transgene is active in specific tissues during organogenesis. In particular, we detect high Cre expression in the vertebral column, ribs, limbs and tailbud. We took advantage of this dynamic expression profile to analyze the effects of inducing mosaic deletion of Nle1 in the embryo. We show that Nle1 deletion in this context, results in severe developmental anomalies leading to lethality at birth. Mutant embryos display multiple developmental defects in particular during axial skeletal formation. We also provide evidence that axial defects are due to an increase in apoptotic cell death in the somite at E9.5. These data demonstrate an essential role for Nle1 during organogenesis and in particular during axial development.
Introduction
The Notchless homolog 1 (Drosophila) (Nle1) gene codes for a member of the WD-repeat containing protein family involved in a wide range of cellular functions including cytoskeleton assembly, cell division, transcriptional regulation, RNA processing and signal transduction [1]–[4]. Initially, NLE1 was identified in Drosophila as a direct regulator of Notch activity, although the molecular mechanism underlying this regulatory process has not been characterized [2]. Interestingly enough, NLE1 was shown to have an ancient evolutionary origin, appearing before the emergence of pluricellularity and intercellular signaling pathways [5]. In yeast, the NLE1 ortholog Rsa4 is essential for ribosome biogenesis. It assembles in the nucleolus with the pre-60S ribosomal subunit and interacts through its well-conserved amino-terminal region with the AAA-ATPase Rea1. This interaction is required for the disassembly of non-ribosomal factors prior to export of the mature large subunit to the cytoplasm [6]. We recently showed that the key role of NLE1 in 60S biogenesis is conserved during evolution. Using conditional inactivation in adult mice, we demonstrated that NLE1 regulated ribosome biogenesis in hematopoietic stem cells and immature progenitors and was required for the maintenance of these populations [7]. Strikingly, NLE1 was dispensable for ribosome biogenesis, proliferation and differentiation of B lymphocytes, suggesting that alternative pathways for 60S subunit production might exist and be differentially active depending on cell type or degree of differentiation.
Limited data is available so far concerning the role of NLE1 during embryonic development. We previously reported that constitutive Nle1 inactivation leads to embryonic lethality around the time of implantation due to selective apoptosis of pluripotent cells of the blastocyst [1]. Early embryonic lethality has recently been reported for mice homozygous for non-conservative missense Nle1 mutations obtained by ENU mutagenesis [8]. To bypass the early embryonic lethality caused by Nle1 deficiency and address the role of NLE1 following implantation development, we conditionally inactivated the Nle1 gene using the Meox2tm1(cre)Sor strain of mice (called MORE hereafter) harboring the Meox2Cre allele which directs Cre recombinase expression in the epiblast at embryonic day (E) 5.5 [9]. Using this approach, we showed that Nle1 is required in epiblast cells after implantation. We also uncovered a second wave of transcriptional activity of the Meox2Cre allele resembling endogenous Meox2 gene expression profile. Analysis of Nle1 conditional mutant embryos after gastrulation points to an important role for NLE1 in formation of the axial skeleton.
Results
NLE1 is required in epiblast cells after implantation
To analyze the function of NLE1 in post-implantation embryos, we adopted a conditional gene targeting strategy. Conditional Nle1flox/flox mice were crossed to Nle1null/+;Meox2Cre/+ mice carrying a Nle1-null allele (Nle1LacZ or Nle1 Δ) and the Meox2Cre allele, which drives expression of Cre recombinase in the post-implantation epiblast from E5.5 [1], [9], [10]. We first monitored the activity of the Meox2Cre allele in our crosses using the Rosa26STOP-LacZ reporter mice [11]. In E7.5 Rosa26STOP-LacZ/+; Nle1flox /+;Meox2cre/+ control embryos, we observed that Cre was active in the epiblast and the large majority of cells showed recombination at the Rosa26STOP-LacZ allele (blue cells) as expected (Fig. 1A). Noticeably, the number of blue cells was lower in Rosa26STOP-LacZ/+; Nle1flox /Δ;Meox2cre/+mutant embryos, suggesting incomplete recombination of the Nle1flox allele has occurred in the epiblast of mutant embryos. We next monitored the efficiency of recombination at the Nle1 locus by PCR analysis of whole embryo or dissected embryonic organs at various developmental stages (Fig. 1B–C). The unrecombined Nle1flox allele was readily detected in E7.5 to E15.5 embryos, indicating that recombination of the Nle1flox allele in pluripotent epiblast cells was incomplete. Accordingly, Nle1LacZ/flox;Meox2Cre/+ mutant embryos were named Nle1mcKO embryos for Nle1 mosaic conditional Knock Out embryos since they were composed of a mixture of Nle1-deficient (Nle1LacZ/ Δ) and Nle1-proficient (Nle1LacZ/flox) cells.
Figure 1. Partial recombination of the Nle1flox allele by Meox2Cre and detection of Nle1-deficient cells.
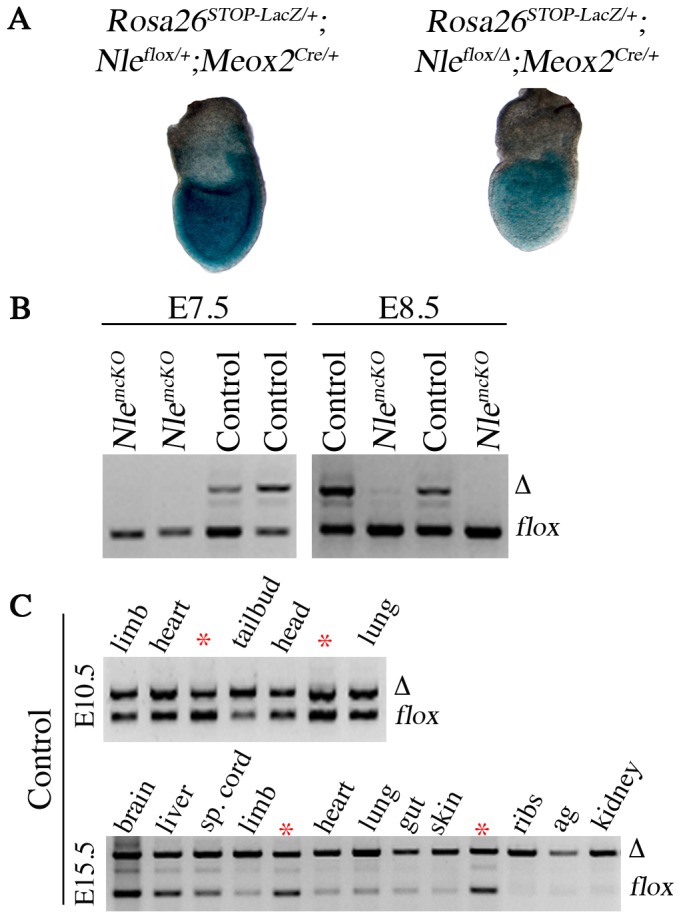
A. At gastrulation, Meox2Cre-mediated recombination was detected by staining for β-galactosidase activity using Rosa26STOP-LacZ reporter allele in Rosa26STOP-LacZ/+; Nle1flox/+; Meox2Cre/+ control embryo (left, n = 3) and in Rosa26STOP-LacZ/+; Nle1flox/Δ; Meox2Cre/+ mutant embryo (right, n = 3). B. PCR amplification of genomic DNA from Nle1flox/+; Meox2Cre/+ control embryos showing partial recombination of the Nle1flox allele at E7.5 (n = 4), E8.5 (n = 12). The recombined (Nle1 Δ) allele is detected as soon as E7.5. Note that PCR analysis underestimates the recombination efficiency at this stage. Indeed, PCR analysis was performed on whole embryo containing extraembryonic tissues (visceral endoderm, extraembryonic ectoderm) that do not express the Cre recombinase. Presence of Nle1-deficient cells was evaluated by PCR amplification of genomic DNA performed on Nle1mcKO mutant embryos at E7.5 (n = 9) and E8.5 (n = 6). Representative results of PCR are shown. C. PCR amplification of genomic DNA from Nle1flox/+; Meox2Cre/+ control embryos showing partial recombination of the Nle1flox allele in several tissues at E10.5 (n = 3) and E15.5 (n = 3). Efficiency of recombination was estimated through comparison with a reference DNA sample (*) derived from a Nle1flox/ Δ adult mouse. Nested PCR were performed for the E7.5 and E8.5 stages. sp. cord: spinal cord, ag: adrenal glands.
At E7.5 and E8.5, although the Nle1Δ allele could be detected in control embryos, it was hardly detectable in Nle1mcKO embryos (Fig. 1B). No morphological defects were observed in the mutant embryos at these stages suggesting that Nle1-deficient cells had been selected at the expense of Nle1-proficient cells during gastrulation. The higher proportion of epiblast cells with unrecombined Rosa26STOP-LacZ allele in mutant embryos compared to controls (Fig. 1A) suggests that selection of cells with low Cre activity had occurred during gastrulation. Importantly, when the Sox2-Cre driver line with higher Cre recombinase activity [12] was used to inactivate Nle1 in the epiblast cells, developmental arrest at E7.5-E8.5 was observed (Table S1). Collectively, these data indicate that Nle1 is required for survival and/or proliferation of epiblast cells of postimplantation embryos.
Meox2Cre expression is dynamic during embryonic development
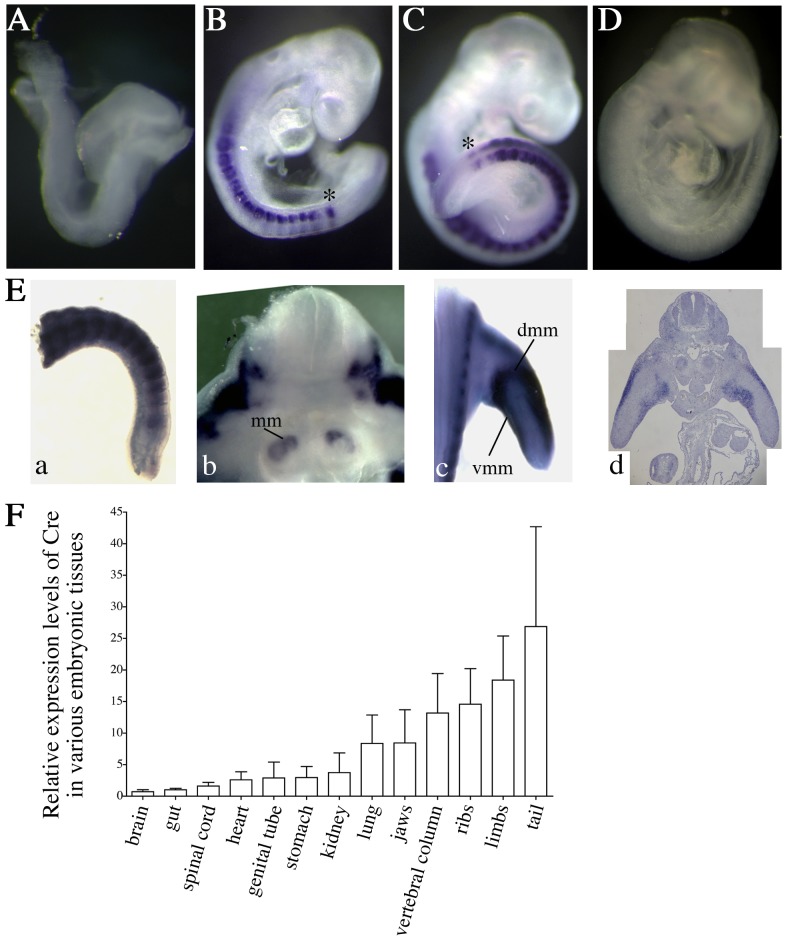
At later stages of development, we observed a dynamic profile of recombination at the Nle1flox allele. In E10.5 control embryos, all tissues displayed similar ratios of Nle1 Δ over Nle1flox allele (∼50% recombination efficiency) except for tailbud samples that contain the pre-somitic mesoderm (PSM) and the newly formed somites, which showed higher degrees of recombination (Fig.1C). Five days later, we observed near complete conversion of Nle1flox allele to Nle1 Δ allele was observed in most tissues except brain, spinal cord and liver (Fig. 1C). This indicated that Cre recombinase expression was not limited to the epiblast and that Cre activity was heterogeneous after gastrulation. The expression profile of the Meox2Cre allele has been determined using Cre activity reporter lines [12], [13]. Such a strategy allows us to determine the initial phase of Cre recombinase activity in a given lineage but is uninformative regarding the dynamics of Cre expression in the progenies of the primary recombined cells. Consequently, the Meox2Cre expression profile after gastrulation has not been determined so far. We therefore monitored Cre mRNA expression by in situ hybridization on E8.5 to E11.5 embryos recovered from crosses between wild-type females and Meox2Cre /+ males. At E8.5, no Meox2Cre expression could be observed (Fig. 2A). At E9.0 and E9.5, Meox2Cre transcriptional activity was detected in the somites and in the anterior PSM (Fig. 2B,C). At E11.5, the Meox2Cre allele was expressed in the newly formed somites, in limb muscles, metanephric mesenchyme and mesenchymal cells of palatal shelves (Fig. 2E and not shown). In addition, real-time RT-PCR on dissected tissues from E14.5 control embryos confirmed the heterogeneous Meox2Cre expression pattern showing highest levels in the ribs, limbs, vertebral column and tail (Fig. 2F). We conclude that the MORE mouse strain not only allows recombination of conditional alleles in epiblast cells but also during organogenesis.
Figure 2. Meox2Cre expression pattern during post-implantation development.
A–E. Whole-mount ISH performed on Meox2Cre/+ embryos at E8.5 (A), E9 (B), E9.5 (C) and E11.5 (E). (E8.5: n = 2, E9–E9.5: n = 9, E11.5: n = 2 Meox2Cre/+; Nle1+/+ embryos) No signal was observed in Meox2+/+ embryos at any developmental stages (result shown at E9, D). mm: metanephric mesenchyme, dmm and vmm: dorsal and ventral muscle masses, *: anterior part of pre-somitic mesoderm. F. Real-time RT-PCR from RNAs extracted from E14.5-organs (n = 3 Meox2Cre/+; Nle1+/+ embryos). The expression level of Cre in tissues was calculated with the 2-ΔΔC T method [42] after normalization with TBP and Tubuline β 5. We arbitrarily choose to compare Cre expression levels in various tissues relative to those expressed in the brain. Bars are means (SD).
Mosaic inactivation of Nle1 leads to abnormal organogenesis
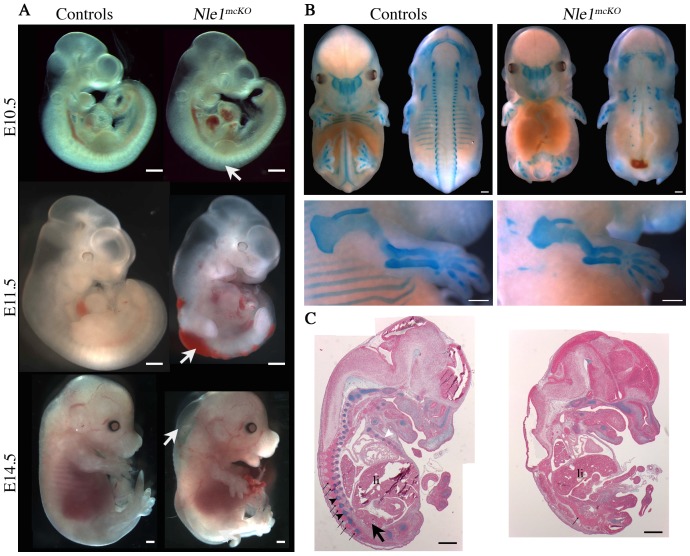
Nle1mcKO embryos were recovered at expected Mendelian ratios until birth but exhibited multiple and severe developmental anomalies (Table 1 and Fig. 3). Morphological defects were first observed at E10.5 when mutant embryos exhibited segmented but irregular somites (Fig. 3A). From E11.5, all Nle1mcKO embryos could be distinguished from their littermates by a shortened or absent tail, edema and hemorrhages along the entire length of the embryo (Fig. 3A). To study the formation of the axial skeleton, E14.5 embryos were stained with alcian blue. Analysis of mutant embryos revealed rare axial skeleton structures (absence of ribs, rare and disorganized vertebrae and vestigial tail), whereas cranial bones derived from cephalic mesoderm and neural crest and bones of the limbs derived from lateral mesoderm were present and displayed no gross morphological abnormalities except for a reduced size (Fig. 3B). To be precise about the developmental defects observed in mutant embryos, serial sections were stained with alcian blue. We found, when present, fused vertebrae in mutant embryos compared to controls (Fig. 3C, S1). In addition, Nle1mcKO embryos exhibited neural tube defects and small or absent kidneys (Fig 3C, S1). At E18.5, alcian blue (cartilage) and alizarin red (bone) staining of skeletal preparations showed that, in addition to the absence of axial skeleton, the elements of cranial and appendicular skeleton (limbs) were undersized and showed scarce mineralized bone tissue in Nle1mcKO embryos compared to controls (Fig S2). This phenotype was also evidenced at E17.5 using 3D µCT images acquisitions from E17.5 control and mutant embryos (Movies S1, S2). These results showed that Nle1 is essential during organogenesis and in particular for the formation of the axial skeleton.
Table 1. Number of mutant embryos and pups obtained from crosses between Nle1flox/flox and Nle1LacZ +/; Meox2Cre/+ mice at various stages.
| Stages | Total embryos | Mutant progenya |
| E6.5–E8.5 | 203 | 50 (25%) |
| E9.5–E11.5 | 364 | 104 (29%) |
| E13.5–E15.5 | 56 | 19 (34%) |
| E17.5–E18.5 | 48 | 13 (27%) |
| pups | 41 | 11 (27%)b |
Absolute number and frequency (%).
No mutant pups were found alive at birth.
Figure 3. Profound axial anomalies in Nle1mcKO mutant embryos.
A. Lateral views of E10.5, E11.5 and E14.5 control and Nle1mcKO embryos. Irregular somites (E10.5), edema and hemorrhages (E11.5 and E14.5) and vestigial tail (E14.5) are indicated (arrows). B. Cartilage staining of E14.5 control (left, ventral and dorsal views) and Nle1mcKO embryos (right, ventral and dorsal views). Note that ribs are missing and a few vertebrae remnants are detected along the neural tube. Magnified views of upper limbs (bottom) show that appendicular bones are present and exhibit reduced size in mutant embryos. C. Alcian blue staining of histological sections of E14.5 control (left) and Nle1mcKO (right) embryos. Vertebrae (black arrowhead), ribs (white arrowhead) and kidney (arrow) are indicated in the control embryo and are absent in the Nle1mcKO mutant embryo. Note that dorsal spinal ganglia (thin arrows) are regularly spaced along the vertebral column in control embryos and fused in the Nle1mcKO mutant embryo. li: liver. Scale bar: 500 µm.
Nle1mcKO mutant embryos have impaired somite formation
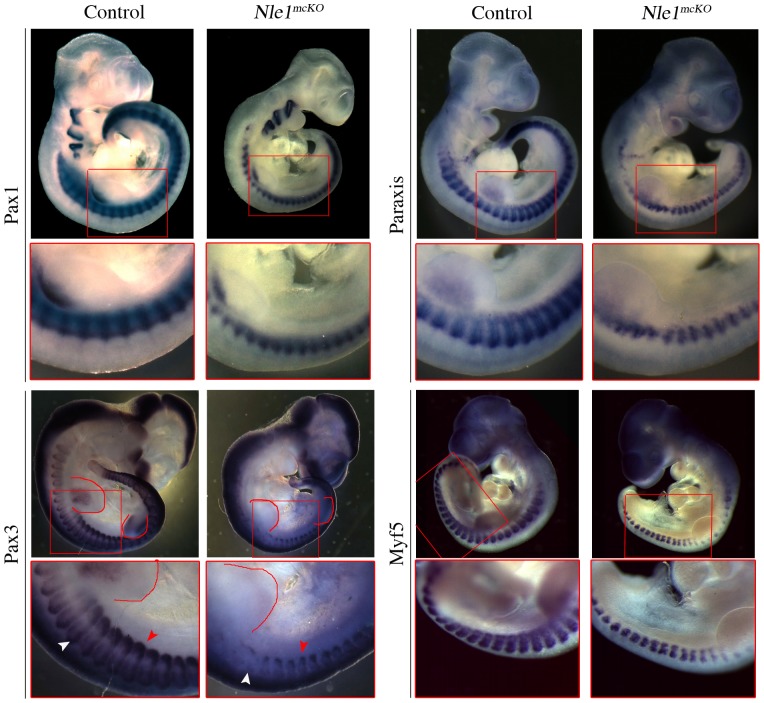
The observation that axial skeleton development is compromised in Nle1mcKO mutant embryos led us to analyze somitogenesis. After segmental border formation and epithelialization, cells located in the dorsal region of the somite give rise to dermomyotome and myotome, while cells in the ventro-medial region de-epithelialize to form the sclerotome responsible for the future axial skeleton. To characterize the nature of axial defects, we first performed histological analysis of somitic region of control and Nle1mcKO embryos between E9.5 (stage at which Meox2Cre is active again) and E11.5. At E9.5, while dermomyotome of Nle1mcKO somites was clearly visible on transverse sections, both the dermomyotome and the underlying mesenchyme appeared as loose tissues compared to controls (Fig. 4A). Sagittal sections of E11.5 Nle1mcKO embryos revealed fusion of the dorsal root ganglia and absence of sclerotome condensations that are indicative of abnormal somitogenesis (Fig. 4B). To further precise the origins of axial skeleton defects, we analyzed the expression pattern of somitic markers by whole mount in situ hybridization at E9.5 and E10.5. We observed that the expression pattern of markers of all somitic compartments (Paraxis for newly formed somites, Pax1 for sclerotome, Paraxis and Pax3 for the dermomyotome and Myf5 for the myotome) were similar in Nle1mcKO embryos compared to controls at E9.5 (Fig. S3). At E10.5, Pax1 was downregulated in the dorsal part of somites and especially in the most rostral somites in Nle1mcKO embryos (Fig. 5). This downregulation was specific for sclerotomal defects since normal Pax1 expression was observed elsewhere (pharyngeal arches and at the basis of the limb bud). We also examined the expression pattern of dermomyotome and myotome markers (Paraxis, Pax3 and Myf5) and showed that both compartments were perturbed in E10.5 Nle1mcKO embryos. Indeed, Paraxis exhibited a segmental pattern in the somites of control embryos, whereas expression in Nle1mcKO embryos was truncated dorsally with a more severe downregulation in the most rostral somites. In addition, no expression of Myf5 was detected in the dorsal part of the mutant somites. Similarly, Pax3 expression domain in the dermomyotome was severely reduced, especially in the dorsomedial lips, whereas a narrow signal was still observed in the dorsolateral lips. Absence of Pax3 expression was observed in the limb muscle primordial cells. In contrast, no major alterations of Pax3 expression profile was observed in the central nervous system. These data show that Nle1 function is critically required for the patterning of somitic derivatives.
Figure 4. Histological analysis of somitic regions of control and Nle1mcKO mutant embryos.
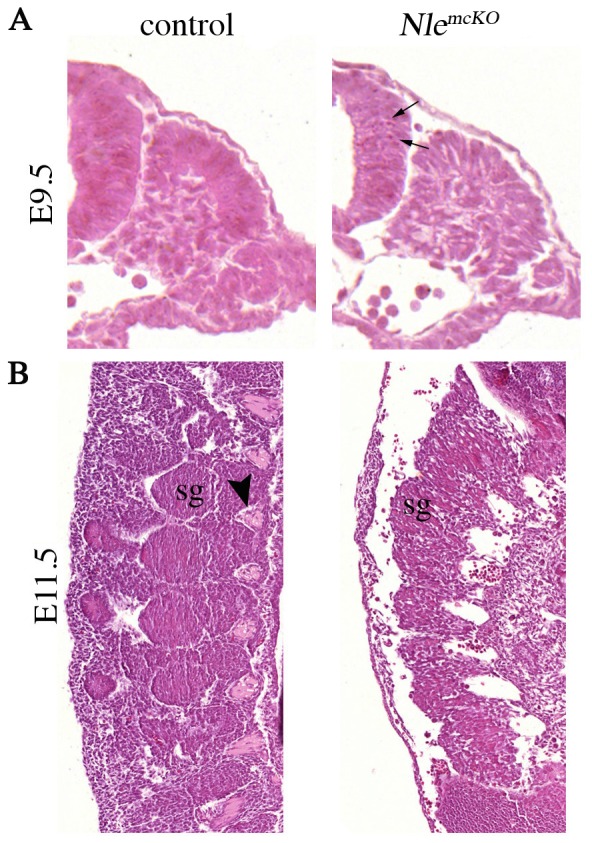
Embryos were sectioned and stained with hematoxylin and eosin at E9.5 (transverse section of the tail, A) and at E11.5 (sagittal section of interlimb region, B). Arrow indicated the picnotic nuclei visible in the neural tube of E9.5 Nle1mcKO embryos. At E11.5, absence of sclerotomal condensations (arrowhead in control) and fusion of dorsal spinal ganglia (sg) are shown.
Figure 5. Expression pattern of markers for somitic lineages in E10.5 control and Nle1mcKO embryos.
Whole-mount in situ hybridizations were performed with Pax1, Paraxis, Pax3 and Myf5 riboprobes (n = 3 control, n = 3 Nle1mcKO mutant embryos for Pax1, Paraxis and Pax3, n = 2 control and n = 2 control, n = 2 Nle1mcKO mutant embryos for Myf5. Pax3 is expressed in the dorsomedial (white arrowhead) and dorsolateral (red arrowhead) lips of the dermomyotome in E10.5 control embryos. The red line indicate the limb bud where Pax3-positive muscle progenitors are visible in the control embryo but not in the Nle1mcKO embryo. Red boxes mark an embryonic region shown enlarged below each embryo.
Nle1mcKO mutant embryos exhibit upregulated caspase3-dependent apoptosis in the somites and the neural tube
The observation that all somitic markers are rapidly downregulated in Nle1mcKO embryos led us to analyze if a loss of somitic cells had occurred due to cell death. Cell death was investigated by immunostaining using a specific antibody recognizing the active form of caspase 3 and by using LysoTracker® Red marker, previously demonstrated to be an accurate marker of cell death in embryos [14], [15]. Importantly, Cre-mediated apoptosis has been described in several Cre transgenic mouse lines [14], but so far not in the Meox2Cre line. Accordingly, we didn't observed an increase in cell death in Cre-expressing (Nle1LacZ/+; Meox2Cre/+) control embryos compared to non expressing (Nle1LacZ/+ or Nle1flox/+) ones (data not shown). In both types of control embryos, apoptosis was detected essentially in the head and in the ventral part of the mature somites (Fig. 6A, movie S3 and S5). In contrast, a dramatic increase in cell death was observed in the entire somites and in the neural tube from Nle1mcKO embryos at E9.5 and E10.5 (Fig. 6 and S4, movie S4 and S6). In the neural tube, upregulated apoptosis was detected caudally to the forelimb but not rostrally where caspase3-positive signals were equivalent to those observed in control embryos (Fig. S4). In the somites, upregulation of apoptosis was observed both in newly formed and mature somites. Apoptosis levels in other tissues were indistinguishable in mutant and control embryos. Of note, no increase in apoptosis was observed at E7.5, which is consistent with the “wild-type like” genotype of the Nle1mcKO embryos at these stages (not shown). Altogether, these results suggest that downregulation of Nle1 in post-implantation embryos leads to upregulation of apoptosis in the somites and the neural tube that probably contributes to abnormal somitogenesis and profound axial defects.
Figure 6. Increased cell death is observed in Nle1mcKO mutant embryos.
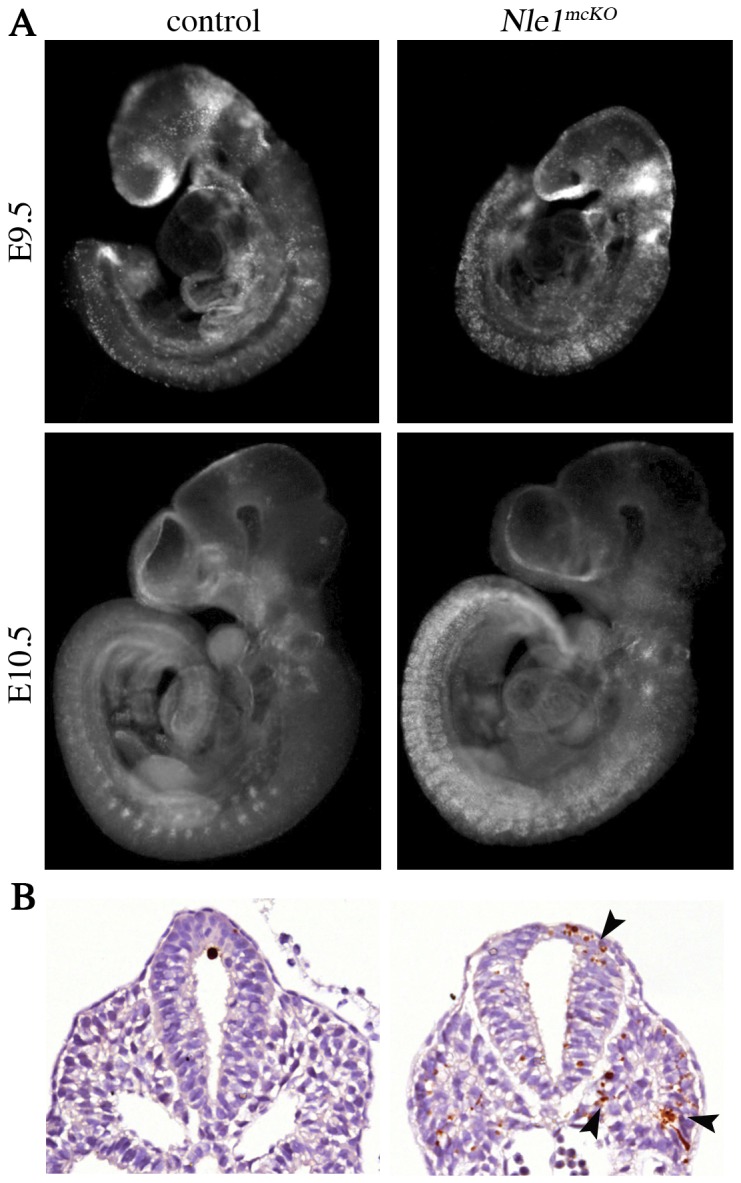
A. E9.5 and E10.5 control and mutant embryos were incubated in LysoTracker Red solution. Normal developmental apoptosis was observed in the head (E9.5 and E10.5) and in the ventral part of the somites (E10.5) of control embryos. In Nle1mcKO embryos, apoptosis in the head was similar as control embryos, whereas a marked increase in apoptosis was observed in the neural tube and the entire somites at E9.5 (n = 2 mutants and n = 6 controls including 2 Meox2Cre embryos) and E10.5 (n = 3 mutants and n = 8 controls including 2 Meox2Cre embryos). B. Immunostaining for the active form of caspase3 protein in E9.5 control and Nle1mcKO embryos. Representative results of immunostaining of transverse section of the caudal part of embryos are shown. In Nle1mcKO embryos, high number of apoptotic cells was observed (arrowheads) in the neural tube and the somites. (n = 3 mutants and n = 4 controls including a Meox2Cre embryo). No increase in apoptosis was observed due to the expression of the Meox2Cre allele.
Discussion
In the present study, the use of the Meox2Cre line shows that NLE1 is critically required during organogenesis and in particular for the axial skeleton formation. Based on our data, we propose that partial disruption of Nle1 leads to an increase in apoptotic cell death in the somites responsible for abnormal patterning of the somites leading to axial skeleton defects.
The MORE mouse strain is commonly used to generate null alleles from conditional floxed alleles or to determine if defects in extraembryonic tissues contribute to mutant phenotype by invalidating the gene of interest specifically in the epiblast [9]. The efficiency of recombination mediated by Meox2Cre depends on the floxed allele to be recombined. Hence, Meox2Cre activity allowed the full recombination of a floxed Retinoblastoma gene and the generation of Rb-deficient mice [16]. In contrast, Meox2Cre activity has been shown to mediate only partial recombination of the conditional alleles of Sonic Hedgehog, Bmpr1a, Nodal, Pcsk5, PPARγ and fgf8 genes during development [12], [13], [17]–[20]. Here, we show that Meox2Cre activity in the post-implantation epiblast was not sufficient to achieve full recombination of the Nle1flox allele as indicated by the fact that Nle1flox allele can be readily detected in E7.5 and E8.5 control embryos. At these stages, mutant embryos were almost exclusively composed of unrecombined cells, and we propose that Nle1-deficient epiblast cells are eliminated rapidly after their generation. Accordingly, when using a more active epiblast Cre driver line, i.e the Sox2-Cre line, embryonic lethality around E7.5 was observed. Collectively, our data demonstrate that the use of the Meox2Cre line does not allow the generation of embryos deficient for Nle1 prior to organogenesis despite Cre recombinase expression in the epiblast. While the MORE mouse has proven its interest in generating loss-of-function phenotypes in the embryo-proper, caution should be taken when using this strain to study gene function in early embryos. Indeed, in case of genes with essential functions for epiblast cells, low efficiency of recombination combined to rapid expansion of non-recombined epiblast cells may give rise to misleading interpretations.
The MORE line was obtained by inserting a nls-Cre recombinase cassette into the Meox2 gene by homologous recombination [9]. Cre activity was thus anticipated to mimic the expression profile of the endogenous Meox2 gene, which starts to be expressed in the epithelial somite and then becomes restricted to the sclerotome and limb musculature [21], [22]. Unexpectedly and for reasons that have remained unexplained so far, the Meox2Cre allele drives Cre expression in the epiblast at E5.5. The observation that the Nle1flox allele was recombined in a tissue-specific manner during the second half of gestation lead us to unravel a second wave of transcriptional activation of the Meox2Cre allele that starts between E8.5 and E9.5 and is restricted to the somites and the anterior part of the PSM. At later stage of development, Meox2Cre expression was detected in various organs, being low in endoderm-derived (liver, gut) and neurectoderm (brain, spinal cord) tissues and high in mesoderm-derived (ribs and vertebral column, heart) tissues. Although our analysis at late stages of development was performed by real-time RT-PCR and therefore lacks cellular resolution, Meox2Cre expression seems consistent with the widespread expression of the Meox2 gene in mesenchymal derivatives of E14.5 embryos [23]. Our data thus suggests that after a transient peak of expression in epiblast cell of early postimplantation embryos, Meox2Cre expression is likely to recapitulate endogenous Meox2 gene expression. It would be interesting to determine whether this secondary wave of Cre expression contributed to the axial skeleton-specific phenotype previously reported following forced Cyclooxygenase-2 expression using the MORE line [24].
One of the most striking abnormalities of Nle1mcKO embryos is the lack of axial skeleton. By the time the second wave of Meox2Cre expression, a dramatic increase in apoptosis was observed in the somites of E9.5 mutant embryos that likely contribute to somite mispatterning. Consistent with abnormal axial skeleton formation, histological and gene expression analyses showed that the sclerotome compartment was severely disorganized and that Pax1 was downregulated at E10.5. At E11.5, histological sections confirmed the dramatic effect of Nle1 invalidation on the sclerotomal compartment since no chondrogenic differentiation was observed. Other defects were observed in Nle1mcKO embryos including kidney dysgenesis, neural tube defects, edemas (probably due to cardiac defects [25], [26]) and hemorrhage. During embryonic development, Meox2 is expressed in the kidney (http://www.gudmap.org), heart and vasculature [27]–[29]. Nle1 inactivation may therefore be directly responsible for the defects observed in these tissues. In contrast, the causes for increased apoptosis in the neural tube of Nle1mcKO embryos at E9.5 and E10.5 are unclear. Indeed, no Cre expression was detected in the neural tube at these stages. An intriguing possibility would be that apoptosis of neural cells results from a cell non-autonomous process following Nle1 inactivation in the adjacent somitic compartment.
In Drosophila, NLE1 has been proposed to be a direct regulator of the Notch signaling pathway [2]. Since, the Notch pathway is known to tightly regulate somitogenesis in vertebrates [30], [31], abnormal Notch signaling could be a plausible explanation for the failure in somite patterning observed in the Nle1mcKO embryos. However, the somitic defects reported for embryos mutant for several members of the Notch pathway differ from those observed in Nle1mcKO embryos. Hence, inactivation of Notch1, Dll1 or RBPJκ genes lead to abnormal timing and coordination of somite segmentation, highlighted by disruptions in size and bilateral symmetry of the somites [32]–[34]. By contrast, Nle1mcKO somites were correctly segmented as judged from histological examinations and segmented expression of somitic markers. Moreover, absence of somites at the caudal most regions was also reported in embryos mutant for several members of the Notch pathway [32], [34]–[36], while this phenotype was not observed in Nle1mcKO embryos. While one should keep in mind that conditional inactivation of Nle1 by Meox2Cre is not temporally, spatially or quantitatively equivalent to the mentioned Notch mutants, the phenotypic discrepancies suggest that abnormal somite patterning in Nle1mcKO embryo is not caused by a dysregulation of the Notch pathway. In yeast, NLE1 ortholog, Rsa4, was shown to participate in pre-60S ribosomal subunit assembly [6], and conservation of the critical role of NLE1 in ribogenesis was recently demonstrated in mouse embryonic stem cells and immature hematopoietic cells in adult mice [10]. Defective ribosome biogenesis following Nle1 inactivation was shown to trigger the disappearance of hematopoietic stem cells and immature progenitors through p53-dependent mechanisms. It is therefore likely that the phenotypes observed in Nle1mcKO embryo were consecutive to defects in ribosome biogenesis. Interestingly, defects in the craniofacial, axial and limb skeleton were reported in patients and animals models with haploinsufficiency for ribosomal protein genes or mutations for ribosome biogenesis factors [37]–[40]. However, limited data are available concerning the mechanisms by which these mutations are affecting the developing skeleton. Strikingly, haploinsufficiency of ribosomal protein L38 (RPL38) in mice lead to abnormal axial skeletal patterning due to selective reduction in the translation of a subset of Hox mRNAs [41] indicating that ribosome composition may also impinges on developmental processes. Interestingly, outcompetition between wild-type and Rpl24+/− cells is observed in Rpl24+/− mutant mice [37], reminiscent of the selection of Nle1-deficient cells at the expense of Nle1-proficient cells that we observed in Nle1mcKO embryos before gastrulation. Further studies will be required to establish whether ribogenesis is affected in Nle1mcKO embryos and to determine the mechanisms responsible for the elimination or outcompetition of Nle1-deficient cells.
Materials and Methods
Ethics statement
Animals were housed in the Institut Pasteur animal facilities accredited by the French Ministry of Agriculture to perform experiments on live mice (accreditation B 75 15–06, issued on May 22, 2008) in appliance of the French and European regulations on care and protection of the Laboratory Animals (EC Directive 86/609, French Law 2001–486 issued on June 6, 2001). Protocols were approved by the veterinary staff of the Institut Pasteur animal facility and were performed in compliance with the NIH Animal Welfare (Insurance #A5476–01 issued on 02/07/2007).
Mice
To specifically inactivate Nle1 in the epiblast, Nle1 LacZ/+ mice [1] were crossed to Meox2Cre/+ [9] to produce Nle1LacZ/+;Meox2Cre/+ mice. These mice were then crossed with conditional Nle1flox/flox mice [10] to produce control and Nle1mcKO embryos. International strain nomenclature are Nle1tm1Cba for Nle1LacZ strain, Nle1tm1.1Cota for Nle1flox strain and Meox2tm1(cre)Sor for the Meox2Cre/+ strain. The colonies were maintained on a 129Sv/C57BL/6 mixed genetic background. Nle1mcKO mutant embryos were systematically compared to control littermates. Genotyping of embryos was then performed by PCR using the following primers LacZ-Forward: 5′- ACTATCCCGACCGCCTTACT -3′; LacZ-Reverse: 5′- GCTGGTTTCCATGAGTTGCT -3′; Cre-Forward: 5′- CACGACCAAGTGACAGCAAT -3′ and Cre-Reverse: 5′- TCCCCAGAAATGCCAGATTA -3′. Female mice were used between 6 and 14 weeks of age for most of the experiments. Pregnant mice were sacrificed by cervical dislocation for embryo recovery.
PCR genotyping of embryos, foetal tissues and mice
Biopsies from mice and portion of embryos (i.e., egg cylinders, yolk sacs) were used for genotyping by PCR. These tissues were lysed in 50 mM Tris-Hcl pH 8.5, 100 mM NaCl, 0,5% Tween and 0,1 mg/ml proteinase K at 56°C. Proteinase K was then inactivated at 95°C for 15 min before PCR amplification.
Real-time RT-PCR
Total RNA was isolated from organs of E14.5 Meox2Cre/+ embryos using Nucleospin RNA columns (Macherey Nagel). RT-PCR amplifications were performed using SuperScript III First-Strand Synthesis kit (Invitrogen) according to the manufacturer's instructions. Real time PCR was performed using SYBR Select Master Mix (Life Technologies) on an Mx3000P detection system (Agilent Technologies). The TBP and Tubuline β 5 genes were used as reference genes and expression differences were calculated as described [42]. Primers used are: Cre-Forward: 5′- CACGACCAAGTGACAGCAAT -3′ and Cre-Reverse: 5′- TCCCCAGAAATGCCAGATTA -3′, TBP-Forward: 5′-AGAACAATCCATACTAGCAGC-3′ and TBP-Reverse: 5′-GGGAACTTCACATCACAGCTC-3′, and Tubulineβ5-Forward: 5′-GATCGGTGCTAAGTTCTGGGA-3′ and Tubulinβ5-Reverse: 5′-AGGGACATACTTGCCACCTGT-3′.
Histological analysis
Embryos were fixed overnight in 4% paraformaldehyde and then dehydrated and embedded in paraffin. Next, 4 µm sections were stained with hematoxylin and eosin or alcian blue using standard procedures.
Skeletal preparations
For staining and visualization of whole skeletons, embryos were dissected and stained with alizarin red S and alcian blue 8GX (Sigma) as described [43]
3D- µCT image acquisitions
E17.5 embryos were collected, fixed in formol. Embryos were imaged using X-ray radiation micro-CT (SkyScan 1072). A microfocus X-ray tube was used as a source (50 kV, 173 µA). The specimen was mounted on a turntable that could be shifted automatically by 180° with a rotation step of 0.45° in the axial direction. The “SkyScan 1072” system provides an image pixel size of 18.88 µm. X-ray images were transformed by NRecon software (skyscan).
Whole-mount in situ hybridization
In situ hybridization was carried out as described [44]. Two to five embryos were used for each marker and stage. Antisense probes used during this study were Paraxis, Pax1, Pax3, Myf5 (provided by S. Tajbakhsh). Cre riboprobe was generated from PCR amplification of Cre cDNA by using specific primer for the Cre flanked by T7 and T3 promoters: Cre-T3Forward, 5′-GAGAATTAACCCTCACTAAAGGGGGACATGTTCAGGGATCGCCAGGCG and Cre-T7rev, 5′-GAGTAATACGACTCACTATAGGGTATTTACATTGGTCCAGCCACCAG.
Photographs of whole-mount stained embryos were taken with the SMZ 1500 stereomicroscope (Nikon) with the AxioCam HRc camera (Zeiss).
Cell death analysis
Cell death was detected by incubating whole embryos in 5 µM LysoTracker Red (Invitrogen L7528) in Hank's balanced salt solution for 30 min at 37°C in 5% CO2 in air. Embryos were then washed in phosphate buffered saline (PBS), dehydrated in methanol, and cleared in 1∶2 benzyl alcohol: benzyl benzoate. Photographs of stained embryos were taken with an inverted microscope Zeiss Axiovert 200M with a Zeiss apotome system controlled by the Zeiss axiovision 4.4 software. The CCD camera used was a Roper Scientific Coolsnap HQ. Immunohistochemistry using antibody against activated-caspase3 (Asp175; Cell Signalling) were used according to the manufacturer's instructions. Whole-mount immunochemistry was performed on E7.5 embryos using antibody against human activated caspase-3 (1∶200; Pharmingen) as described [45].
X-Gal staining
After fixation of embryos, β-galactosidase expression was visualized by staining with 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal for embryos; Life Technologies).
Supporting Information
Spinal cord and kidney anomalies in Nle1mcKO mutant embryos. A. Hematoxylin-eosin staining of histological section of E14.5 control (upper panel) and Nle1mcKO (lower panel) embryos. On sagittal sections, fused and rare cartilage primordium of spinal column is indicated (arrowheads). Edema (asterisks) and dilated central canal of the spinal cord (sp) are clearly visible. Kidneys (arrow) are indicated in the control embryos and in the Nle1mcKO mutant embryos (sagittal sections). li: liver, s: stomach, v: vertebra. Scale bars: 500 µm.
(TIF)
Alcian blue/alizarin red S double staining of the skeleton of E18.5 embryos. A. Whole skeleton staining of Nle1mcKO (left) and control (right) embryos. B. Magnification on the forelimb and humerus. Long bones of Nle1mcKO embryos are smaller and present a significant delay of mineralization.
(TIF)
Expression pattern of markers for somitic lineages in E9.5 control and Nle1mcKO embryos. Whole-mount in situ hybridizations were performed with Pax1, Paraxis, Pax3 and Myf5 riboprobes (n = 4 control, n = 3 Nle1mcKO mutant embryos for Pax1, n = 4 control, n = 5 Nle1mcKO mutant embryos Paraxis, n = 4 control, n = 4 Nle1mcKO mutant embryos for Pax3, n = 3 control, n = 6 Nle1mcKO mutant embryos for Myf5. No difference between Nle1mcKO and control embryos was observed for any marker tested.
(TIF)
Analysis of apoptosis in E9.5 control and Nle1mcKO embryos. Immunostaining for the active form of caspase3 protein at E9.5 in control (left) and Nle1mcKO (right) embryos are shown. Embryos were embedded in agarose before being embedded in paraffin. Serial transverse sections are shown. In control embryos, apoptotic cells (arrowhead) were observed in the epidermis, the neural tube and otic pit. In Nle1mcKO embryos, an abnormally high number of apoptotic cells was observed in the neural tube caudally to the forelimb and in the epithelial and mature somites. Black arrowheads indicate upregulated apoptosis and red arrowheads indicate normal developmental apoptosis (n = 3 mutants and n = 4 controls including a Meox2Cre embryo). ba: branchial arch, cr: caudal region of embryo, he: heart, ne: neuroepithelium, nt: neural tube, ot: otic vesicle, op: optic vesicle, so: somite.
(TIF)
Number of mutant embryos obtained from crosses between Nle1flox/flox and Nle1 Δ/+; Sox2Cre/+ mice at various embryonic stages.
(DOCX)
MicroCT three-dimensional images of E17.5 control embryo.
(MOV)
MicroCT three-dimensional images of E17.5 Nle1mcKO embryo showing absence of ribs and vertebrae.
(MOV)
Cell death analysis in E9.5 control embryo. Representative image of cell death status in one E9.5 control embryo. Normal developmental apoptosis is observed in the head.
(MOV)
Cell death analysis in E9.5 Nle1mcKO embryo. Representative image of cell death status in one E9.5 mutant embryo. Apoptosis in the head was similar as control embryos, whereas a severe increase in apoptosis is observed in the neural tube and the entire somites.
(MOV)
Cell death analysis in E10.5 control embryo. Representative image of cell death status in one E10.5 control embryo. Normal developmental apoptosis is observed in the head and in the ventral part of the somites.
(MOV)
Cell death analysis in E10.5 Nle1mcKO embryo. Representative image of cell death status in one E10.5 Nle1mcKO embryo. Apoptosis in the head was similar as control embryos, whereas a severe increase in apoptosis is observed in the neural tube and the entire somites.
(MOV)
Acknowledgments
We thank Murielle Almoussa and Clémire Cimper for technical help. We are grateful to S. Tajbakhsh for helpful discussions and for in situ probes. We thank David Hills, Aline Stedman and Anne Camus for reading the manuscript. We acknowledge Philippe Soriano and Michelle Tallquist for providing us Meox2Cre and Rosa26STOP-LacZ transgenic mice. We are grateful to A. Danckaert for her assistance with image analysis at the Institut Pasteur Imagopole. We are grateful to the electronic microscopy, microanalysis and microcharacterization platform (SC3M, U791-LIOAD) of the Nantes University. This work is dedicated to the memory of Dr Charles Babinet.
Funding Statement
This work was supported by the Centre National de la Recherche Scientifique, the Institut Pasteur and the Agence Nationale de la Recherche (contract n° JC05_41835). CS received grants from the Centre National de la Recherche Scientifique (Bourse de Doctorat pour les Ingénieurs). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cormier S, Le Bras S, Souilhol C, Vandormael-Pournin S, Durand B, et al. (2006) The murine ortholog of notchless, a direct regulator of the notch pathway in Drosophila melanogaster, is essential for survival of inner cell mass cells. Mol Cell Biol 26: 3541–3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Royet J, Bouwmeester T, Cohen SM (1998) Notchless encodes a novel WD40-repeat-containing protein that modulates Notch signaling activity. EMBO J 17: 7351–7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith TF, Gaitatzes C, Saxena K, Neer EJ (1999) The WD repeat: a common architecture for diverse functions. Trends Biochem Sci 24: 181–185. [DOI] [PubMed] [Google Scholar]
- 4. Xu C, Min J (2011) Structure and function of WD40 domain proteins. Protein Cell 2: 202–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gazave E, Lapébie P, Richards GS, Brunet F, Ereskovsky AV, et al. (2009) Origin and evolution of the Notch signalling pathway: an overview from eukaryotic genomes. BMC Evol Biol 9: 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bassler J, Kallas M, Pertschy B, Ulbrich C, Thoms M, et al. (2010) The AAA-ATPase Rea1 drives removal of biogenesis factors during multiple stages of 60S ribosome assembly. Mol Cell 38: 712–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Bouteiller M, Souilhol C, Beck-Cormier S, Stedman A, Burlen-Defranoux O, et al. (2013) Notchless-dependent ribosome synthesis is required for the maintenance of adult hematopoietic stem cells. J Exp Med 210: 2351–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lossie AC, Lo C-L, Baumgarner KM, Cramer MJ, Garner JP, et al. (2012) ENU mutagenesis reveals that Notchless homolog 1 (Drosophila) affects Cdkn1a and several members of the Wnt pathway during murine pre-implantation development. BMC Genet 13: 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tallquist MD, Soriano P (2000) Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis 26: 113–115. [DOI] [PubMed] [Google Scholar]
- 10.Le Bouteiller M, Souilhol C, Beck-Cormier S, Stedman A, Burlen-Defranoux O, et al.. (2013) Notchless-dependent ribosome synthesis is required for the maintenance of adult hematopoietic stem cells. The Journal of Experimental Medicine. [DOI] [PMC free article] [PubMed]
- 11. Soriano P (1999) Generalized lacZ expression with the ROSA26 Cre reporter strain. Nature Genetics 21: 70–71. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi S, Lewis P, Pevny L, McMahon AP (2002) Efficient gene modulation in mouse epiblast using a Sox2Cre transgenic mouse strain. Mech Dev 119 Suppl 1 S97–S101. [DOI] [PubMed] [Google Scholar]
- 13. Davis S, Miura S, Hill C, Mishina Y, Klingensmith J (2004) BMP receptor IA is required in the mammalian embryo for endodermal morphogenesis and ectodermal patterning. Dev Biol 270: 47–63. [DOI] [PubMed] [Google Scholar]
- 14. Naiche LA, Papaioannou VE (2007) Cre activity causes widespread apoptosis and lethal anemia during embryonic development. genesis 45: 768–775. [DOI] [PubMed] [Google Scholar]
- 15. Zucker RM, Hunter S, Rogers JM (1998) Confocal laser scanning microscopy of apoptosis in organogenesis-stage mouse embryos. Cytometry 33: 348–354. [DOI] [PubMed] [Google Scholar]
- 16. Wu L, de Bruin A, Saavedra HI, Starovic M, Trimboli A, et al. (2003) Extra-embryonic function of Rb is essential for embryonic development and viability. Nature 421: 942–947. [DOI] [PubMed] [Google Scholar]
- 17. Delgado I, Domínguez-Frutos E, Schimmang T, Ros MA (2008) The incomplete inactivation of Fgf8 in the limb ectoderm affects the morphogenesis of the anterior autopod through BMP-mediated cell death. Dev Dyn 237: 649–658. [DOI] [PubMed] [Google Scholar]
- 18. Duan SZ, Ivashchenko CY, Whitesall SE, D'Alecy LG, Duquaine DC, et al. (2007) Hypotension, lipodystrophy, and insulin resistance in generalized PPARgamma-deficient mice rescued from embryonic lethality. J Clin Invest 117: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Essalmani R, Zaid A, Marcinkiewicz J, Chamberland A, Pasquato A, et al. (2008) In vivo functions of the proprotein convertase PC5/6 during mouse development: Gdf11 is a likely substrate. Proc Natl Acad Sci USA 105: 5750–5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lu CC, Robertson EJ (2004) Multiple roles for Nodal in the epiblast of the mouse embryo in the establishment of anterior-posterior patterning. Dev Biol 273: 149–159. [DOI] [PubMed] [Google Scholar]
- 21. Mankoo BS, Collins NS, Ashby P, Grigorieva E, Pevny LH, et al. (1999) Mox2 is a component of the genetic hierarchy controlling limb muscle development. Nature 400: 69–73. [DOI] [PubMed] [Google Scholar]
- 22. Reijntjes S, Stricker S, Mankoo BS (2007) A comparative analysis of Meox1 and Meox2 in the developing somites and limbs of the chick embryo. Int J Dev Biol 51: 753–759. [DOI] [PubMed] [Google Scholar]
- 23. Diez-Roux G, Banfi S, Sultan M, Geffers L, Anand S, et al. (2011) A high-resolution anatomical atlas of the transcriptome in the mouse embryo. PLoS Biol 9: e1000582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shim M, Foley J, Anna C, Mishina Y, Eling T (2010) Embryonic expression of cyclooxygenase-2 causes malformations in axial skeleton. Journal of Biological Chemistry 285: 16206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Clark AL, Cleland JGF (2013) Causes and treatment of oedema in patients with heart failure. Nat Rev Cardiol 10: 156–170. [DOI] [PubMed] [Google Scholar]
- 26. Kim W, Essalmani R, Szumska D, Creemers JWM, Roebroek AJM, et al. (2012) Loss of endothelial furin leads to cardiac malformation and early postnatal death. Molecular and Cellular Biology 32: 3382–3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Douville JM, Cheung DYC, Herbert KL, Moffatt T, Wigle JT (2011) Mechanisms of MEOX1 and MEOX2 regulation of the cyclin dependent kinase inhibitors p21 and p16 in vascular endothelial cells. PLoS ONE 6: e29099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Skopicki HA, Lyons GE, Schatteman G, Smith RC, Andres V, et al. (1997) Embryonic expression of the Gax homeodomain protein in cardiac, smooth, and skeletal muscle. Circ Res 80: 452–462. [DOI] [PubMed] [Google Scholar]
- 29. Wu Z, Guo H, Chow N, Sallstrom J, Bell RD, et al. (2005) Role of the MEOX2 homeobox gene in neurovascular dysfunction in Alzheimer disease. Nat Med 11: 959–965. [DOI] [PubMed] [Google Scholar]
- 30. Pierfelice T, Alberi L, Gaiano N (2011) Notch in the vertebrate nervous system: an old dog with new tricks. Neuron 69: 840–855. [DOI] [PubMed] [Google Scholar]
- 31. Pourquié O (2011) Vertebrate segmentation: from cyclic gene networks to scoliosis. Cell 45: 650–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121: 1533. [DOI] [PubMed] [Google Scholar]
- 33. de la Pompa JL, Wakeham A, Correia KM, Samper E, Brown S, et al. (1997) Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124: 1139–1148. [DOI] [PubMed] [Google Scholar]
- 34. Hrabe˘ de Angelis M, McIntyre J, Gossler A (1997) Maintenance of somite borders in mice requires the Delta homologue DII1. Nature 386: 717–721. [DOI] [PubMed] [Google Scholar]
- 35. Dunwoodie SL, Clements M, Sparrow DB, Sa X, Conlon RA, et al. (2002) Axial skeletal defects caused by mutation in the spondylocostal dysplasia/pudgy gene Dll3 are associated with disruption of the segmentation clock within the presomitic mesoderm. Development 129: 1795–806. [DOI] [PubMed] [Google Scholar]
- 36. Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, et al. (1997) Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature 387: 288–292. [DOI] [PubMed] [Google Scholar]
- 37. Kirn-Safran CB, Oristian DS, Focht RJ, Parker SG, Vivian JL, et al. (2007) Global growth deficiencies in mice lacking the ribosomal protein HIP/RPL29. Dev Dyn 236: 447–460. [DOI] [PubMed] [Google Scholar]
- 38. Oliver ER, Saunders TL, Tarlé SA, Glaser T (2004) Ribosomal protein L24 defect in belly spot and tail (Bst), a mouse Minute. Development 131: 3907–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Trainor PA, Merrill AE (2013) Ribosome biogenesis in skeletal development and the pathogenesis of skeletal disorders. Biochim Biophys Acta 842: 769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Watkins-Chow DE, Cooke J, Pidsley R, Edwards A, Slotkin R, et al. (2013) Mutation of the diamond-blackfan anemia gene Rps7 in mouse results in morphological and neuroanatomical phenotypes. PLoS genetics 9: e1003094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kondrashov N, Pusic A, Stumpf CR, Shimizu K, Hsieh AC, et al. (2011) Ribosome-mediated specificity in hox mRNA translation and vertebrate tissue patterning. Cell 145: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 43. McLeod MJ (1980) Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. 'Teratology 22: 299–301. [DOI] [PubMed] [Google Scholar]
- 44. Tajbakhsh S, Rocancourt D, Cossu G, Buckingham M (1997) Redefining the genetic hierarchies controlling skeletal myogenesis: Pax-3 and Myf-5 act upstream of MyoD. Cell 89: 127–138. [DOI] [PubMed] [Google Scholar]
- 45. Jory A, Le Roux I, Gayraud-Morel B, Rocheteau P, Cohen-Tannoudji M, et al. (2009) Numb promotes an increase in skeletal muscle progenitor cells in the embryonic somite. Stem Cells 27: 2769–2780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Spinal cord and kidney anomalies in Nle1mcKO mutant embryos. A. Hematoxylin-eosin staining of histological section of E14.5 control (upper panel) and Nle1mcKO (lower panel) embryos. On sagittal sections, fused and rare cartilage primordium of spinal column is indicated (arrowheads). Edema (asterisks) and dilated central canal of the spinal cord (sp) are clearly visible. Kidneys (arrow) are indicated in the control embryos and in the Nle1mcKO mutant embryos (sagittal sections). li: liver, s: stomach, v: vertebra. Scale bars: 500 µm.
(TIF)
Alcian blue/alizarin red S double staining of the skeleton of E18.5 embryos. A. Whole skeleton staining of Nle1mcKO (left) and control (right) embryos. B. Magnification on the forelimb and humerus. Long bones of Nle1mcKO embryos are smaller and present a significant delay of mineralization.
(TIF)
Expression pattern of markers for somitic lineages in E9.5 control and Nle1mcKO embryos. Whole-mount in situ hybridizations were performed with Pax1, Paraxis, Pax3 and Myf5 riboprobes (n = 4 control, n = 3 Nle1mcKO mutant embryos for Pax1, n = 4 control, n = 5 Nle1mcKO mutant embryos Paraxis, n = 4 control, n = 4 Nle1mcKO mutant embryos for Pax3, n = 3 control, n = 6 Nle1mcKO mutant embryos for Myf5. No difference between Nle1mcKO and control embryos was observed for any marker tested.
(TIF)
Analysis of apoptosis in E9.5 control and Nle1mcKO embryos. Immunostaining for the active form of caspase3 protein at E9.5 in control (left) and Nle1mcKO (right) embryos are shown. Embryos were embedded in agarose before being embedded in paraffin. Serial transverse sections are shown. In control embryos, apoptotic cells (arrowhead) were observed in the epidermis, the neural tube and otic pit. In Nle1mcKO embryos, an abnormally high number of apoptotic cells was observed in the neural tube caudally to the forelimb and in the epithelial and mature somites. Black arrowheads indicate upregulated apoptosis and red arrowheads indicate normal developmental apoptosis (n = 3 mutants and n = 4 controls including a Meox2Cre embryo). ba: branchial arch, cr: caudal region of embryo, he: heart, ne: neuroepithelium, nt: neural tube, ot: otic vesicle, op: optic vesicle, so: somite.
(TIF)
Number of mutant embryos obtained from crosses between Nle1flox/flox and Nle1 Δ/+; Sox2Cre/+ mice at various embryonic stages.
(DOCX)
MicroCT three-dimensional images of E17.5 control embryo.
(MOV)
MicroCT three-dimensional images of E17.5 Nle1mcKO embryo showing absence of ribs and vertebrae.
(MOV)
Cell death analysis in E9.5 control embryo. Representative image of cell death status in one E9.5 control embryo. Normal developmental apoptosis is observed in the head.
(MOV)
Cell death analysis in E9.5 Nle1mcKO embryo. Representative image of cell death status in one E9.5 mutant embryo. Apoptosis in the head was similar as control embryos, whereas a severe increase in apoptosis is observed in the neural tube and the entire somites.
(MOV)
Cell death analysis in E10.5 control embryo. Representative image of cell death status in one E10.5 control embryo. Normal developmental apoptosis is observed in the head and in the ventral part of the somites.
(MOV)
Cell death analysis in E10.5 Nle1mcKO embryo. Representative image of cell death status in one E10.5 Nle1mcKO embryo. Apoptosis in the head was similar as control embryos, whereas a severe increase in apoptosis is observed in the neural tube and the entire somites.
(MOV)