Effective surveillance is crucial for early detection and successful mitigation of emerging diseases [1]. The current global approach to surveillance for wildlife diseases affecting biodiversity (“biodiversity diseases”) is still inadequate as demonstrated by the slow characterization and response to the two recent devastating epidemics, chytridiomycosis and white-nose syndrome [2]–[5]. Current surveillance for wildlife disease usually targets diseases that affect humans or livestock, not those impacting wildlife populations. Barriers to effective surveillance for biodiversity diseases include a relative lack of social and political will and the inherent complexity and cost of implementing surveillance for multiple and diverse free-ranging populations. Here we evaluate these challenges and the inadequacies of current surveillance techniques, and we suggest an integrated approach for effective surveillance.
Despite challenges in quantifying the role of disease in species declines [6], there are numerous clear examples of diseases (infectious, toxic, multifactorial, or of undetermined origin) that have caused severe population impacts; for example, avian malaria and poxvirus in Hawaii, diclofenac poisoning in Indian vultures, rinderpest in Africa, bighorn sheep pneumonia, chronic wasting disease, crayfish plague, avian trichomonosis, and Tasmanian devil facial tumor disease [7]–[15].
The emergence of the amphibian fungal skin disease chytridiomycosis is a pertinent example in which a lack of effective disease surveillance contributed to global biodiversity loss (Figure 1) [16]–[18]. Epidemiological investigation did not commence until 15 years after initial declines [19]. Despite recent listing of chytridiomycosis as a notifiable disease by the World Organization for Animal Health (OIE), the extended time before diagnosis very likely contributed to the decline and extinction of at least 200 species of frogs globally, helping to make amphibians the most endangered vertebrate class [3], [20].
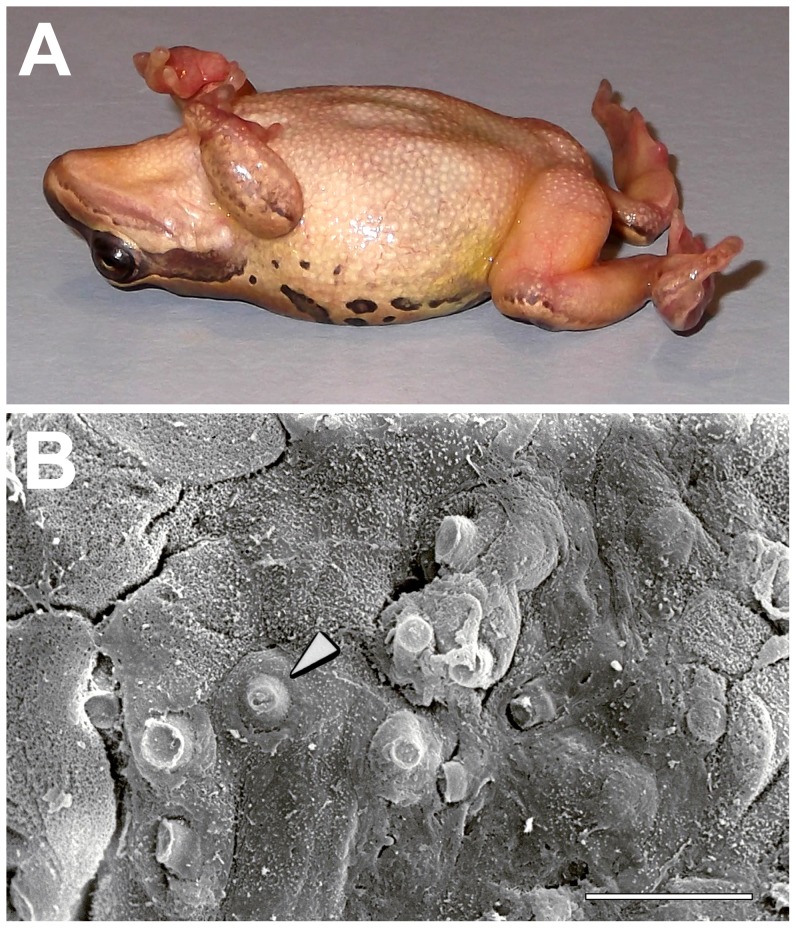
Figure 1. Chytridiomycosis: a catastrophic biodiversity disease causing amphibian declines.
Chytridiomycosis emerged in the 1970s but was not detected until the 1990s. (A) An alpine tree frog (Litoria verreauxii alpina) with severe chytridiomycosis, showing skin reddening and an inability to maintain normal upright posture; (B) skin surface of a stony creek frog (formerly Litoria lesueuri). Many cells are infected with sporangia, pushing discharge tubes (arrow) to the skin surface (scanning electron micrograph). Scale bar = 10 µm.
Here we define “biodiversity disease” as “a disease that has caused, or is predicted to cause, a decline in a wild species sufficient to worsen its conservation status.” This term can be applied to kingdoms other than Animalia, but those are outside the scope of the current paper. Our aim is to improve wildlife biodiversity disease surveillance, which could have important socioeconomic benefits, including reducing long-term disease management costs, protecting biodiversity and ecosystem services, and contributing to prespillover surveillance for public health and agricultural diseases [21]–[31]. Preventing disease-linked species extirpation will stabilize ecosystems, curtailing cascades of trophic coextinctions and global biodiversity loss [32]–[34]. Biodiversity and ecosystem stability are also increasingly linked with decreased risk of disease emergence [25], [35]–[39].
Current funding priorities for wildlife health surveillance tend to rely on overlap with human and livestock diseases [1]. Cost-benefit analyses applied to zoonotic and agricultural diseases in funding prioritization models, including, for example, the “willingness to pay” framework based on societal values and the concept of paying for “ecosystem services,” typically do not adequately address the consequences of biodiversity loss [4], [40], [41]. Appropriately quantifying the value of biodiversity would assist leveraging more appropriate resource allocation.
Responsibility for wildlife health is often spread across multiple agencies, levels of government, universities, and nongovernment agencies. This fragmentation of accountability may contribute to lower prioritization of biodiversity disease surveillance and control compared with human and livestock health threats, which are managed by specific departments.
To promote effective implementation of surveillance programs, a greater focus on emerging biodiversity diseases is needed in international policy and practice and more support must be given to existing regional wildlife health frameworks, recognizing their crucial role in identifying and managing biodiversity diseases. This recognition should encourage coordination at international, national, and local levels, as well as resourcing on-the-ground surveillance.
Several international bodies concerned with animal health are appropriately situated to take on this coordinating role, and collaborations between bodies such as OIE and the World Conservation Union (IUCN) may provide the necessary transdisciplinary expertise required [3]. The OIE has already taken steps in this direction by listing notifiable and non-notifiable infectious diseases, highlighting current issues through their Working Group on Wildlife Diseases, and developing their “Training Manual on Wildlife Diseases and Surveillance” [42]. International coordination can result in rapid disease assessments, prioritization of resources, and targeted response via regional frameworks for wildlife health (for example, the successfully coordinated, multiagency response to highly pathogenic avian influenza virus, H5N1 [4]).
A number of regional frameworks are already established, while others are new and emerging. With improved funding, regional frameworks for wildlife health will be better equipped to provide direction, facilities, and expertise for surveillance. These centers typically involve collaboration of veterinarians, ecologists, wildlife biologists, microbiologists, and molecular biologists. They require salaries for field staff, epidemiologists, and pathologists; funding for diagnostic testing; and data management systems to collect and analyze surveillance data. Agreement on methodologies, risk assessment pathways, and contingency plans for emerging infectious biodiversity diseases across these regional frameworks will support prompt responses to outbreaks [43].
Current biodiversity disease surveillance is often ad hoc and relies on passive surveillance (data collected from community submissions) or activities that overlap with human and livestock diseases. This approach is unable to elucidate the impact of disease on the population because only the diseased subpopulation is detected, and it is less likely to detect subtle clinical signs or alterations in species fitness, such as reduced fecundity, despite potentially large population impacts [44]–[52]. Some diseases may also be underrepresented due to the cryptic or noncharismatic nature of the hosts, the remote nature of the location, or apathy or acceptance of consequences once a diagnosis has been reached [47], [53]–[55].
Considering the potential deficiencies of current approaches to detect emerging biodiversity diseases, a new, transdisciplinary, systematic surveillance approach is needed. Essential elements of this approach are established in many countries, but are not specifically being utilized to detect biodiversity diseases. The following aspects could be incorporated into this approach:
-
Combine current strategies (integrate passive and active or general and targeted techniques with outbreak investigations that characterize emerging pathogens or multifactorial disease pathways to enable implementation of effective control) [56]. Surveillance techniques in use for human and domestic animal diseases that may be adapted include:
Disease-specific screening for incursions of important pathogens.
-
Use of sentinel species or individuals at sentinel locations (such as key wildlife trade sites) [27], [53], [57]. Species could be ranked for use as sentinels by evaluating:
Species value based on conservation status, taxonomy, ecosystem representation, and phylogenetic uniqueness.
Sentinel value based on ecological role (keystone species and predators/scavengers), ease of observation and representative sampling, current level of study, and probability as a disease-emergence host [58].
Target both known and unknown pathogens and hosts and regions predicted to be at high risk for disease emergence through predictive modeling. Retrospective and risk factor analyses show correlations between the incidence of disease emergence in general and socioeconomic and ecological factors (for example, highly biodiverse developing regions constitute infectious-disease–emergence hotspots which could be targeted [28], [59]–[61]). Deterministic models based on general pathogen characteristics and sensitivity analysis, combined with metagenomic studies, hold potential for predicting future disease emergence [62]–[65].
Ensure spatial and taxonomic representation to prevent the loss of biodiversity in important taxonomic clades or small regions with high levels of endemism [66].
Focus on multiple biological levels, such as ecosystems and species [67].
Integrate essential baseline ecological data collection for an understanding of the population impact of disease. Mark-recapture studies provide long-term data on population dynamics and are appropriate for wildlife population impact assessment, despite imperfect detection [68]. Integration of epidemiological transmission models with disease, population, and environmental data will better elucidate the roles of infectious disease, anthropogenic environmental disturbance, and other factors in driving changes in population structure, distribution, or size [69].
Incorporate self-evaluative mechanisms to ensure adaptability and prioritization strategies. Strategies should evolve as diagnostic and ecological monitoring techniques emerge, and as global circumstances change [1], [70], [71]. Frameworks for structured decision making and prioritization will ensure that surveillance approaches remain cost effective [72], [73].
In conclusion, we suggest that improved integration, capacity, and a systematic approach to disease surveillance in wildlife are imperative for future biodiversity conservation.
Acknowledgments
The authors thank A. Roberts, S. Young, R. Puschendorf, and D. Mendez for helpful comments on the manuscript.
Funding Statement
LFG and LB were supported by Australian Research Council grants LP110200240 and FT100100375. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vrbova L, Stephen C, Kasman N, Boehnke R, Doyle-Waters M, et al. (2010) Systematic review of surveillance systems for emerging zoonoses. Transbound Emerg Dis 57: 154–161. [DOI] [PubMed] [Google Scholar]
- 2. Foley J, Clifford D, Castle K, Cryan P, Ostfeld RS (2011) Investigating and managing the rapid emergence of white-nose syndrome, a novel, fatal, infectious disease of hibernating bats. Conserv Biol 25: 223–231. [DOI] [PubMed] [Google Scholar]
- 3. Skerratt LF, Berger L, Speare R, Cashins S, McDonald KR, et al. (2007) Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4: 125–134. [Google Scholar]
- 4. Carmichael C (2012) Coordinating an effective response to wildlife diseases. Wildl Soc Bull 36: 204–206. [Google Scholar]
- 5. Kuiken T, Ryser-Degiorgis M-P, Gavier-Widen D, Gortazar C (2011) Establishing a European network for wildlife health surveillance. Rev Sci Tech 30: 755–761. [DOI] [PubMed] [Google Scholar]
- 6. Smith KF, Sax DF, Lafferty KD (2006) Evidence for the role of infectious disease in species extinction and endangerment. Conserv Biol 20: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 7. Atkinson CT, Samuel MD (2010) Avian malaria Plasmodium relictum in native Hawaiian forest birds: epizootiology and demographic impacts on 'apapane Himatione sanguinea . J Avian Biol 41: 357–366. [Google Scholar]
- 8. Besser TE, Cassirer EF, Highland MA, Wolff P, Justice-Allen A, et al. (2013) Bighorn sheep pneumonia: Sorting out the cause of a polymicrobial disease. Prev Vet Med 108: 85–93. [DOI] [PubMed] [Google Scholar]
- 9. Williams ES (2005) Chronic wasting disease. Vet Pathol 42: 530–549. [DOI] [PubMed] [Google Scholar]
- 10. Vogel SW, Heyne H (1996) Rinderpest in South Africa - 100 years ago. J S Afr Vet Assoc (Tydskrif van die Suid-Afrikaanse Veterinere) 67: 164–170. [PubMed] [Google Scholar]
- 11. Chaudhry MJI, Ogada DL, Malik RN, Virani MZ, Giovanni MD (2012) First evidence that populations of the critically endangered Long-billed Vulture Gyps indicus in Pakistan have increased following the ban of the toxic veterinary drug diclofenac in south Asia. Bird Conservation International 22: 389–397. [Google Scholar]
- 12. Cassirer EF, Plowright RK, Manlove KR, Cross PC, Dobson AP, et al. (2013) Spatio-temporal dynamics of pneumonia in bighorn sheep. J Anim Ecol 82: 518–528. [DOI] [PubMed] [Google Scholar]
- 13. Kozubikova E, Petrusek A, Duris Z, Martin MP, Dieguez-Uribeondo J, et al. (2008) The old menace is back: Recent crayfish plague outbreaks in the Czech Republic. Aquaculture 274: 208–217. [Google Scholar]
- 14. Robinson RA, Lawson B, Toms MP, Peck KM, Kirkwood JK, et al. (2010) Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 5: e12215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hawkins CE, Baars C, Hesterman H, Hocking GJ, Jones ME, et al. (2006) Emerging disease and population decline of an island endemic, the Tasmanian devil Sarcophilus harrisii . Biol Conserv 131: 307–324. [Google Scholar]
- 16. Pech R, Byrom A, Anderson DR, Thomson C, Coleman M (2010) The effect of poisoned and notional vaccinated buffers on possum (Trichosurus vulpecula) movements: minimising the risk of bovine tuberculosis spread from forest to farmland. Wildlife Research 37: 283–292. [Google Scholar]
- 17. Robbins AH, Borden MD, Windmiller BS, Niezgoda M, Marcus LC, et al. (1998) Prevention of the spread of rabies to wildlife by oral vaccination of raccoons in Massachusetts. J Am Vet Med Assoc 213: 1407–1412. [PubMed] [Google Scholar]
- 18. Gagliardo R, Crump P, Griffith E, Mendelson J, Ross H, et al. (2008) The principles of rapid response for amphibian conservation, using the programmes in Panama as an example. International Zoo Yearbook 42: 125–135. [Google Scholar]
- 19. Skerratt L, Speare R, Berger L (2011) Mitigating the Impact of Diseases Affecting Biodiversity - Retrospective on the Outbreak Investigation for Chytridiomycosis. Ecohealth 7: S26–S26. [Google Scholar]
- 20. Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, et al. (2006) Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc Natl Acad Sci U S A 103: 3165–3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Boyles JG, Cryan PM, McCracken GF, Kunz TH (2011) Economic importance of bats in agriculture. Science 332: 41–42. [DOI] [PubMed] [Google Scholar]
- 22.Kemere P, Liddel MK, Evangelou P, Slate D, Omsmek S (2000) Economic analysis of a large scale oral vaccination program to control raccoon rabies. Lincoln: University of Nebraska - Lincoln. [Google Scholar]
- 23. Tambi EN, Maina OW, Mukhebi AW, Randolph TF (1999) Economic impact assessment of rinderpest control in Africa. Rev Sci Tech OIE 18: 458–477. [DOI] [PubMed] [Google Scholar]
- 24. Childs JE, Gordon ER (2009) Surveillance and control of zoonotic agents prior to disease detection in humans. Mt Sinai J Med 76: 421–428. [DOI] [PubMed] [Google Scholar]
- 25.Chivian E, Bernstein A, editors(2008) Sustaining Life: How Human Health Depends on Biodiversity. Oxford: Oxford University Press, USA. [Google Scholar]
- 26.Childs JE (2007) Pre-spillover prevention of emerging zoonotic diseases: What are the targets and what are the tools? In: Childs JE, Mackenzie JS, Richt JA, editors. Wildlife and Emerging Zoonotic Diseases: The Biology, Circumstances and Consequences of Cross-Species Transmission. Berlin: Springer-Verlag Berlin. pp. 389–443. [Google Scholar]
- 27. Aguirre AA (2009) Wild canids as sentinels of ecological health: a conservation medicine perspective. Parasit Vectors 2 Supplement 1 S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, et al. (2008) Global trends in emerging infectious diseases. Nature 451: 990–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Morens DM, Folkers GK, Fauci AS (2004) The challenge of emerging and re-emerging infectious diseases. Nature 430: 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yule JV, Fournier RJ, Hindmarsh PL (2013) Biodiversity, extinction, and humanity's future: The ecological and evolutionary consequences of human population and resource use. Humanities 2: 147–159. [Google Scholar]
- 31.Watson R, Albon S, Aspinall R, Austen M, Bardgett R, et al.. (2011) The UK National Ecosystem Assessment: Synthesis of the Key Findings. Cambridge: UNEP-WCMC. [Google Scholar]
- 32. Aguirre AA, Tabor GM (2008) Global factors driving emerging infectious diseases impact on wildlife populations. Ann N Y Acad Sci 1149: 1–3. [DOI] [PubMed] [Google Scholar]
- 33. Daszak P (2000) Emerging infectious diseases of wildlife - threats to biodiversity and human health. Science 287: 443. [DOI] [PubMed] [Google Scholar]
- 34. Maillard JC, Gonzalez JP (2006) Biodiversity and emerging diseases. Ann N Y Acad Sci 1081: 1–16. [DOI] [PubMed] [Google Scholar]
- 35. Keesing F, Belden LK, Daszak P, Dobson A, Harvell CD, et al. (2010) Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468: 647–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ostfeld RS (2009) Biodiversity loss and the rise of zoonotic pathogens. Clinical Microbiology and Infection 15: 40–43. [DOI] [PubMed] [Google Scholar]
- 37. Pongsiri MJ, Roman J, Ezenwa VO, Goldberg TL, Koren HS, et al. (2009) Biodiversity loss affects global disease ecology. Bioscience 59: 945–954. [Google Scholar]
- 38. Vaz VC, D'Andrea PS, Jansen AM (2007) Effects of habitat fragmentation on wild mammal infection by Trypanosoma cruzi . Parasitology 134: 1785–1793. [DOI] [PubMed] [Google Scholar]
- 39. Mills JN (2006) Biodiversity loss and emerging infectious disease: An example from the rodent-borne hemorrhagic fevers. Biodiversity 7: 9–17. [Google Scholar]
- 40. Spangenberg JH, Settele J (2010) Precisely incorrect? Monetising the value of ecosystem services. Ecological Complexity 7: 327–337. [Google Scholar]
- 41. Richardson L, Loomis J (2009) The total economic value of threatened, endangered and rare species: An updated meta-analysis. Ecol Econ 68: 1535–1548. [Google Scholar]
- 42.OIE (2010) Training manual on wildlife diseases and surveillance. Workshop for OIE national focal points for wildlife. Paris, France: World Organisation for Animal Health (OIE). [Google Scholar]
- 43.Jackson VS, Huntley S, Tomlinson A, Smith GC, Taylor MA, et al.. (2009) Chapter 9: Risk assessment and contingency planning for exotic disease introductions. In: Delahay RJ, Smith GC, Hutchings MR, editors. Management of disease in wild mammals. Tokyo: Springer. [Google Scholar]
- 44.Case TJ (2000) An Illustrated Guide to Theoretical Ecology. New York: Oxford University Press. [Google Scholar]
- 45. Knowles SCL, Wood MJ, Sheldon BC (2010) Context-dependent effects of parental effort on malaria infection in a wild bird population, and their role in reproductive trade-offs. Oecologia 164: 87–97. [DOI] [PubMed] [Google Scholar]
- 46. Reiner G, Fresen C, Bronnert S, Willems H (2009) Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) infection in wild boars. Vet Microbiol 136: 250–258. [DOI] [PubMed] [Google Scholar]
- 47. Stallknecht DE (2007) Impediments to wildlife disease surveillance, research, and diagnostics. Curr Top Microbiol Immunol 315: 445–461. [DOI] [PubMed] [Google Scholar]
- 48. Thulke HH, Eisinger D, Freuling C, Frohlich A, Globig A, et al. (2009) Situation-based surveillance: adapting investigations to actual epidemic situations. J Wildl Dis 45: 1089–1103. [DOI] [PubMed] [Google Scholar]
- 49.Wobeser GA (2007) Disease in Wild Animals: Investigation and Management. New York: Springer. [Google Scholar]
- 50. Duncan C, Backus L, Lynn T, Powers B, Salman M (2008) Passive, opportunistic wildlife disease surveillance in the Rocky Mountain Region, USA. Transbound Emerg Dis 55: 308–314. [DOI] [PubMed] [Google Scholar]
- 51. Ryser-Degiorgis M-P (2013) Wildlife health investigations: needs, challenges and recommendations. BMC Vet Res 9: 223–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Young S, Skerratt LF, Mendez D, Speare R, Berger L, et al. (2012) Using community surveillance data to differentiate between emerging and endemic amphibian diseases. Dis Aquat Organ 98: 1–10. [DOI] [PubMed] [Google Scholar]
- 53. Kuiken T, Leighton FA, Fouchier RAM, LeDuc JW, Peiris JSM, et al. (2005) Public health - pathogen surveillance in animals. Science 309: 1680–1681. [DOI] [PubMed] [Google Scholar]
- 54. Merianos A (2007) Surveillance and response to disease emergence. Curr Top Microbiol Immunol 315: 477–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Morner T, Obendorf DL, Artois M, Woodford MH (2002) Surveillance and monitoring of wildlife diseases. Rev Sci Tech 21: 67–76. [DOI] [PubMed] [Google Scholar]
- 56. Kane AJ, Morley PS (1999) How to investigate a disease outbreak. Proceedings of the Annual Convention of the AAEP 45: 137–141. [Google Scholar]
- 57. Aguirre AA, Tabor GM (2004) Introduction: Marine vertebrates as sentinels of marine ecosystem health. EcoHealth 1: 236–238. [Google Scholar]
- 58. Halliday JEB, Meredith AL, Knobel DL, Shaw DJ, Bronsvoort B, et al. (2007) A framework for evaluating animals as sentinels for infectious disease surveillance. J R Soc Interface 4: 973–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Taylor LH, Latham SM, Woolhouse EJ (2001) Risk factors for human disease emergence. Philos Trans R Soc Lond B Biol Sci 356: 983–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Real LA, Biek R (2007) Infectious disease modeling and the dynamics of transmission. Curr Top Microbiol Immunol 315: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Daszak P (2009) A Call for “Smart Surveillance”: A Lesson Learned from H1N1. Ecohealth 6: 1–2. [DOI] [PubMed] [Google Scholar]
- 62. Stephens CR, Heau JG, Gonzalez C, Ibarra-Cerdena CN, Sanchez-Cordero V, et al. (2009) Using biotic interaction networks for prediction in biodiversity and emerging diseases. PLoS ONE 4: e5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Parrish CR, Holmes EC, Morens DM, Park E-C, Burke DS, et al. (2008) Cross-species virus transmission and the emergence of new epidemic diseases. Microbiol Mol Biol Rev 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Relman DA (2013) Metagenomics, infectious disease diagnostics, and outbreak investigations: Sequence first, ask questions later? JAMA 309: 1531–1532. [DOI] [PubMed] [Google Scholar]
- 65. Pulliam JRC (2008) Viral host jumps: moving toward a predictive framework. EcoHealth 5: 80–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vieites DR, Wollenberg KC, Andreone F, Kohler J, Glaw F, et al. (2009) Vast underestimation of Madagascar's biodiversity evidenced by an integrative amphibian inventory. Proc Natl Acad Sci U S A 106: 8267–8272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Tompkins DM, Dunn AM, Smith MJ, Telfer S (2011) Wildlife diseases: from individuals to ecosystems. J Anim Ecol 80: 19–38. [DOI] [PubMed] [Google Scholar]
- 68. Cooch EG, Conn PB, Ellner SP, Dobson AP, Pollock KH (2012) Disease dynamics in wild populations: modeling and estimation: a review. J Ornithol 152 Supplement 2 S485–S509. [Google Scholar]
- 69. Skerratt LF, Garner TWJ, Hyatt AD (2010) Determining causality and controlling disease is based on collaborative research involving multidisciplinary approaches. Ecohealth 6: 331–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Thurmond MC (2003) Conceptual foundations for infectious disease surveillance. J Vet Diagn Invest 15: 501–514. [DOI] [PubMed] [Google Scholar]
- 71. Scholes RJ, Mace GM, Turner W, Geller GN, Jurgens N, et al. (2008) Toward a global biodiversity observing system. Science 321: 1044–1045. [DOI] [PubMed] [Google Scholar]
- 72. Carwardine J, O'Connor T, Legge S, Mackey B, Possingham HP, et al. (2012) Prioritizing threat management for biodiversity conservation. Conservation Letters 5: 196–204. [Google Scholar]
- 73. Joseph LN, Maloney RF, Possingham HP (2009) Optimal allocation of resources among threatened species: a project prioritization protocol. Conserv Biol 23: 328–338. [DOI] [PubMed] [Google Scholar]