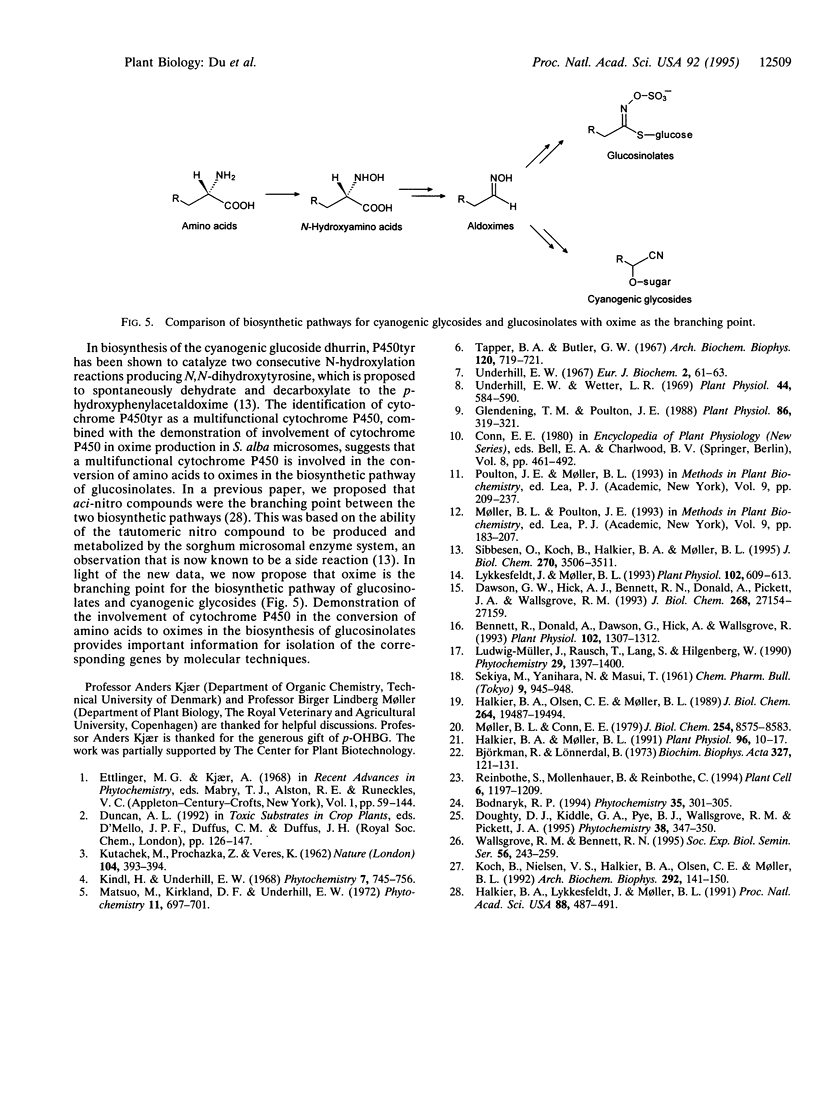
Abstract
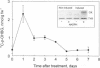
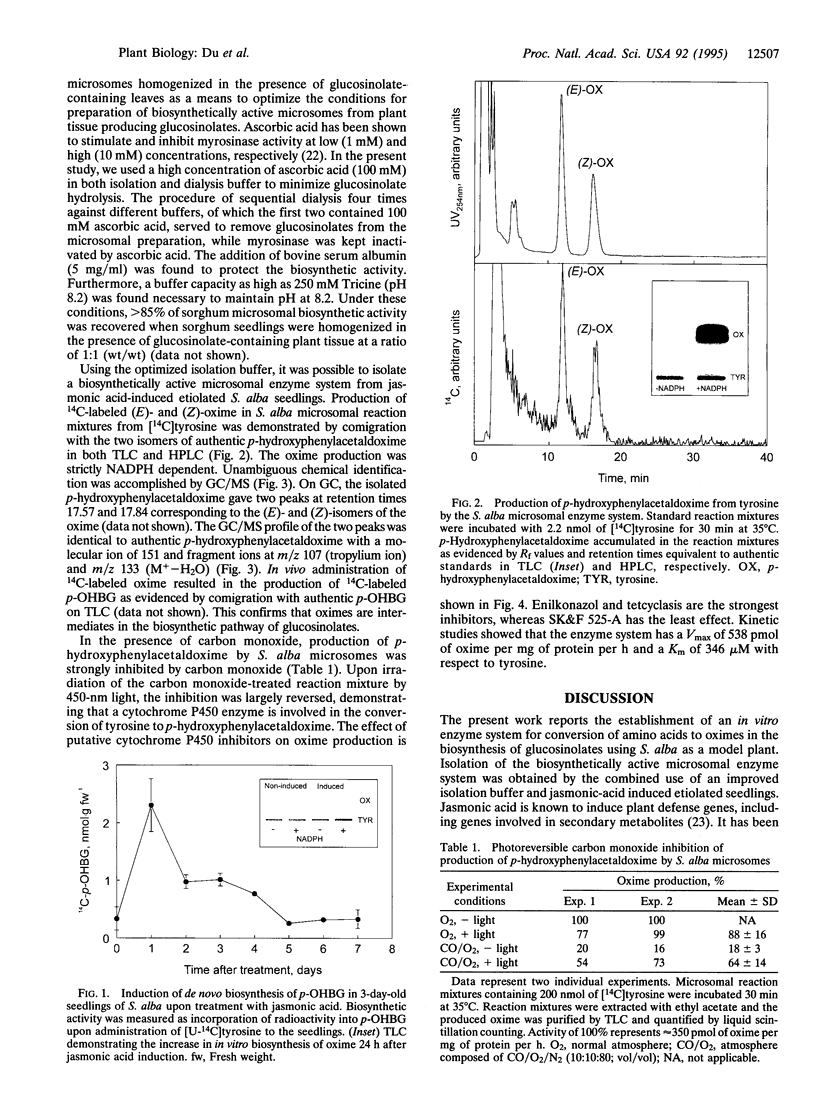
An in vitro enzyme system for the conversion of amino acid to oxime in the biosynthesis of glucosinolates has been established by the combined use of an improved isolation medium and jasmonic acid-induced etiolated seedlings of Sinapis alba L. An 8-fold induction of de novo biosynthesis of the L-tyrosine-derived p-hydroxybenzylglucosinolate was obtained in etiolated S. alba seedlings upon treatment with jasmonic acid. Formation of inhibitory glucosinolate degradation products upon tissue homogenization was prevented by inactivation of myrosinase by addition of 100 mM ascorbic acid to the isolation buffer. The biosynthetically active microsomal enzyme system converted L-tyrosine into p-hydroxyphenylacetaldoxime and the production of oxime was strictly dependent on NADPH. The Km and Vmax values of the enzyme system were 346 microM and 538 pmol per mg of protein per h, respectively. The nature of the enzyme catalyzing the conversion of amino acid to oxime in the biosynthesis of glucosinolates has been subject of much speculation. In the present paper, we demonstrate the involvement of cytochrome P450 by photoreversible inhibition by carbon monoxide. The inhibitory effect of numerous cytochrome P450 inhibitors confirms the involvement of cytochrome P450. This provides experimental documentation of similarity between the enzymes converting amino acids into the corresponding oximes in the biosynthesis of glucosinolates and cyanogenic glycosides.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett R., Donald A., Dawson G., Hick A., Wallsgrove R. Aldoxime-Forming Microsomal Enzyme Systems Involved in the Biosynthesis of Glucosinolates in Oilseed Rape (Brassica napus) Leaves. Plant Physiol. 1993 Aug;102(4):1307–1312. doi: 10.1104/pp.102.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkman R., Lönnerdal B. Studies on myrosinases. 3. Enzymatic properties of myrosinases from Sinapis alba and Brassica napus seeds. Biochim Biophys Acta. 1973 Nov 15;327(1):121–131. doi: 10.1016/0005-2744(73)90109-5. [DOI] [PubMed] [Google Scholar]
- Dawson G. W., Hick A. J., Bennett R. N., Donald A., Pickett J. A., Wallsgrove R. M. Synthesis of glucosinolate precursors and investigations into the biosynthesis of phenylalkyl- and methylthioalkylglucosinolates. J Biol Chem. 1993 Dec 25;268(36):27154–27159. [PubMed] [Google Scholar]
- Glendening T. M., Poulton J. E. Glucosinolate Biosynthesis: Sulfation of Desulfobenzylglucosinolate by Cell-Free Extracts of Cress (Lepidium sativum L.) Seedlings. Plant Physiol. 1988 Feb;86(2):319–321. doi: 10.1104/pp.86.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B. A., Lykkesfeldt J., Møller B. L. 2-nitro-3-(p-hydroxyphenyl)propionate and aci-1-nitro-2-(p-hydroxyphenyl)ethane, two intermediates in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):487–491. doi: 10.1073/pnas.88.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B. A., Møller B. L. Involvement of Cytochrome P-450 in the Biosynthesis of Dhurrin in Sorghum bicolor (L.) Moench. Plant Physiol. 1991 May;96(1):10–17. doi: 10.1104/pp.96.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkier B. A., Olsen C. E., Møller B. L. The biosynthesis of cyanogenic glucosides in higher plants. The (E)- and (Z)-isomers of p-hydroxyphenylacetaldehyde oxime as intermediates in the biosynthesis of dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem. 1989 Nov 25;264(33):19487–19494. [PubMed] [Google Scholar]
- KUTACEK M., PROCHAZKA Z., VERES K. Biogenesis of glucobrassicin, the in vitro precursor of ascorbigen. Nature. 1962 Apr 28;194:393–394. doi: 10.1038/194393b0. [DOI] [PubMed] [Google Scholar]
- Koch B., Nielsen V. S., Halkier B. A., Olsen C. E., Møller B. L. The biosynthesis of cyanogenic glucosides in seedlings of cassava (Manihot esculenta Crantz). Arch Biochem Biophys. 1992 Jan;292(1):141–150. doi: 10.1016/0003-9861(92)90062-2. [DOI] [PubMed] [Google Scholar]
- Lykkesfeldt J., Moller B. L. Synthesis of Benzylglucosinolate in Tropaeolum majus L. (Isothiocyanates as Potent Enzyme Inhibitors). Plant Physiol. 1993 Jun;102(2):609–613. doi: 10.1104/pp.102.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller B. L., Conn E. E. The biosynthesis of cyanogenic glucosides in higher plants. N-Hydroxytyrosine as an intermediate in the biosynthesis of dhurrin by Sorghum bicolor (Linn) Moench. J Biol Chem. 1979 Sep 10;254(17):8575–8583. [PubMed] [Google Scholar]
- Reinbothe S., Mollenhauer B., Reinbothe C. JIPs and RIPs: the regulation of plant gene expression by jasmonates in response to environmental cues and pathogens. Plant Cell. 1994 Sep;6(9):1197–1209. doi: 10.1105/tpc.6.9.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibbesen O., Koch B., Halkier B. A., Møller B. L. Cytochrome P-450TYR is a multifunctional heme-thiolate enzyme catalyzing the conversion of L-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. J Biol Chem. 1995 Feb 24;270(8):3506–3511. doi: 10.1074/jbc.270.8.3506. [DOI] [PubMed] [Google Scholar]
- Underhill E. W. Biosynthesis of mustard oil glucosides: conversion of phenylacetaldehyde oxime and 3-phenylpropionaldehyde oxime to glucotropaeolin and gluconasturtiin. Eur J Biochem. 1967 Jul;2(1):61–63. doi: 10.1111/j.1432-1033.1967.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Underhill L. E., Wetter L. R. Biosynthesis of Mustard Oil Glucosides: Sodium Phenylacetothiohydroximate and Desulfobenzylglucosinolate, Precursors of Benzylglucosinolate in Tropaeolum majus. Plant Physiol. 1969 Apr;44(4):584–590. doi: 10.1104/pp.44.4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]