Abstract
Background
Malignant pleural mesothelioma is a rare lethal malignancy caused by asbestos exposure. It is more frequently seen in certain regions in Turkey. In this retrospective study, we aimed to analyse demographic, clinical, and pathological data and treatment-related features in 54 patients.
Material/Methods
The study included 54 patients diagnosed with malignant mesothelioma that were followed and treated.
Results
Of the 54 patients, 34 (55.6%) were male. The median age in men and women were 60.3 (38.2–77.2) and 65.8 (37.7–77.5) years, respectively. In 35 (64.8%), exposure to asbestosis was present. Epithelial type was found in 27 (50.0%), followed by mixed type in 7 (13.0%) patients, and in 20 (37.0%) patients the subtype could not be determined. The disease was staged as IV in 37 (68.5%) patients. In 28 patients (51.9%), it was right-sided and in 1 (1.9%) it was bilateral. The most frequent metastatic sites (in decreasing order) were lungs, mediastinum, diaphragm, liver, and thoracal wall. Of the 54 patients, 36 (66.6%) received 1st-line chemotherapy and 20 (37%) 2nd-line chemotherapy. Eighteen patients (33.3%) received radiotherapy; 11 (20.3%) with palliative intention and 7 (12.9%) with curative intention. Median overall survival (OS) was 12.03 months (95% CI 7.2–16.8). OS was not affected by sex (p=0.32), smoking history (p=0.51), alcohol consumption (p=0.36), family history (p=0.67), pleural effusion presence (p=0.80), operation (p=0.14), clinical stage (p=0.072), symptom at presentation (p=0.66), having mixed type histology (p=0.079), asbestos exposure (p=0.06), and type of 1st-line chemotherapy (p=0.161). On the contrary, it may be positively affected by good ECOG PS (0–1) (p<0.01), age below 65 (p=0.03), left-sided disease (p=0.01), receiving chemotherapy (p<0.01), having unilateral pleural effusion (p=0.018), and type of 2nd-line chemotherapy (p=0.025).
Conclusions
OS of our patients was better than that found in the literature, seeming to be positively affected by early stages, better ECOG PS, age below 65 years, left side involvement, and having second-line chemotherapy with cisplatin-gemcitabine or 3M. Overall treatment success seems to be comparable to what is currently expected.
MeSH Keywords: Risk Factors, Overall Survival, Risk Factors, Mesothelioma, Survival Rate
Background
Malignant pleural mesothelioma (MPM) is an insidious cancer that emerges in pleura, peritoneal cavities, tunica vaginalis, or on pericardial surfaces. Of the total cases, 80% are pleural. In 70% of the cases, a relationship with exposure to asbestos is detected. Other possible etiologic factors are radiotherapy, viral oncogenes, and genetic factors [1–3]. Mesothelioma is mainly seen in adults. It generally occurs in the 5th and 6th decades of the life and 70–80% of the patients are male. Most of these patients have a history of long-term asbestos exposure during childhood [4]. The incidence of MPM in people without asbestos exposure is expected to be 1 in 1 million persons, whereas in people with exposure this ratio may be up to 10 in 100 persons [5]. Median overall survival ranges from 6 to 18 months. In patients with localized disease, survival can be increased by multimodality therapy [6]. Although extrapleural pneumonectomy is advocated by some groups, pleurectomy and decortication is most widely utilized [7]. In advanced stages, combination chemotherapy of cisplatin and pemetrexed is the current standard [8]. Radiotherapy can be applied as a part of multimodality therapy for rather early stages and as a palliative tool at later stages [9].
The incidence of mesothelioma is decreasing in developed countries due to reduced exposure in working and living areas, but in underdeveloped and developing countries it is increasing because of increased industrial and environmental exposure [5,10]. In Western countries mesothelioma occurs as a result of environmental exposure in people living near asbestos mines and through occupational exposure, but in countries such as Turkey, Cyprus, Greece, and Afghanistan it results from secondary contact with soil (white soil) mixed with asbestos for domestic use [11–15]. In Turkey, the problem is mainly environmental and is an important public health problem in central and southeastern Anatolia. A study conducted by the Turkish Ministry of Health, Directorate of Cancer Control Department, in 8 Turkish cities of during 2004–2006, reported that MPM was not among the 10 most common types of cancer in men and women, and its incidence in both sexes was below 1% [16].
As MPM is a rare disease, each study performed in large series of patients and published in the literature will advance the therapeutic approach to this disease. We think that sharing our experience about this disease, which is more commonly encountered in the neighboring cities compared to other regions of our country, both by revealing its clinico-pathological data and evaluating our therapeutic approaches and their outcomes, will make a substantial contribution to the medical literature.
Material and Methods
Prior to study initiation, approval was obtained from the Başkent University ethics committee (Date and number: 20/09/2010-KA10/133). In this retrospective study, we aimed to enroll patients treated at Adana Başkent Research and Training Hospital. The charts of 54 patients followed between January 2000 and June 2010 at Başkent University Medical Oncology Department were enrolled in this study. Key clinical parameters, disease, and therapies had been recorded in the electronic health records, including data on sex, age, smoking and drinking habits, exposure to asbestosis, clinic stages, ECOG PS, disease extension, and treatment protocols).
The patients were generally diagnosed using VATS and pleural biopsy (25 with pleural biopsy, 7 with thoracotomy, 20 with VATS, and 2 with pleural fluid cytology). All patients had histological diagnosis, including hematoxylin-eosin and immunohistochemistry for keratin, calretinin, EMA, mesothelin, CEA, and TTF-1. CT was used 25% of patients, MRI for 20% of patients, and PET CT for 55% of patients for initial staging. Operation decisions are taken in oncology tumor boards for patients suitable for operation and operation notes are also considered in our study. Only 5 of the patients with extrapulmonary pleurectomy had lymph node sampling; all other patients’ lymph node status was considered and compared with PET CT.
All patients were followed and screened with computerized tomography at 3-month intervals, in line with general follow-up protocols of the medical oncology department.
Statistical analysis
Statistical analysis was performed by using SPSS 15.0 software. For all data, frequency, mean, and standard deviation were calculated. Survival was analyzed by Kaplan-Meier method and factors that might have an effect on the survival were examined using the log-rank Test. Statistical data were considered to be significant at p<0.05.
Results
A total of 54 patients, of whom 30 (55.6%) were male, were enrolled in the study. Demographic and clinical characteristics features of patients are given in Table 1.
Table 1.
Demographic and clinical characteristics of the patients.
| Characteristic | N=54 | % | |
|---|---|---|---|
| Gender | Male | 30 | 55.6 |
| Female | 24 | 44.4 | |
| Age | Male | 60.3 | 38.2–77.2* |
| Female | 65.8 | 37.7–79.5* | |
| Classification by the age of 65 | <65 | 32 | 59.3 |
| ≥65 | 22 | 40.7 | |
| Asbestos exposure | Yes | 35 | 64.8 |
| No | 9 | 16.7 | |
| Unknown | 10 | 18.5 | |
| Smoking | Yes | 22 | 40.7 |
| No | 32 | 59.3 | |
| Ecog ps* at the time of diagnosis | 0 | 4 | 7.4 |
| 1 | 30 | 55.6 | |
| 2 | 17 | 31.5 | |
| 3 | 3 | 5.6 | |
| Histological type | Epithelial | 27 | 50.0 |
| Sarcomatoid | 0 | 0.0 | |
| Mixed | 7 | 13.0 | |
| Undefined | 20 | 37.0 | |
| Pathological stage | 2 | 2 | 3.7 |
| 3 | 15 | 27.8 | |
| 4 | 37 | 68.5 | |
| Clinical stage | 2 | 5 | 9.2 |
| 3 | 16 | 29.6 | |
| 4 | 33 | 61.2 | |
| Presenting symptom | Shortness of breath | 23 | 42.6 |
| Shortness of breath + chest pain | 23 | 42.6 | |
| Shortness of breath + effusion | 8 | 14.8 | |
| Side of involvement | Right | 28 | 51.9 |
| Left | 25 | 46.2 | |
| Bilateral | 1 | 1.9 | |
| Pleural effusion | Yes | 46 | 85.2 |
| No | 8 | 14.8 | |
| Pleural effusion | Unilateral | 45 | 83.3 |
| Bilateral | 1 | 1.9 |
ECOG-PS – Eastern Cooperative Oncology Group-performance status;
95% Confidence interval (CI).
At the time of diagnosis, 22 patients were found to have non-pleural metastasis. Of the patients, 22 had palliative or curative operations (16 patients had pleurectomy/decortication, 5 patients had extrapleural pneumonectomy, and 1 patient had pleurodesis). No mortality was seen in patients with surgical operation. Five of them had postoperative complication (e.g., empyema, bleeding). Mean hospitalization of patients who had surgery was 8.1 days.
Only 1 patient was treated with neoadjuvant therapy. In the first-line treatment, 36 patients received a total of 176 cycles of therapy (median 5.1) and the most common adverse effects were anemia (13.8%, grade I–II), neutropenia (13.8%, grade I–III), nausea and vomiting (10.2%, grade II), and thrombosis and asthenia (2%). In the second-line treatment, 20 patients received a total of 75 cycles of therapy (median 3.75) and common adverse effects were neutropenia (25%, grade I–IV), thrombocytopenia (10%, grade II–III), anemia (grade I–II), and neuropathy (5%). The therapies given to the patients are shown in Table 2.
Table 2.
Treatment modalities initiated to the patients.
| Treatment | Type | N, (%) |
|---|---|---|
| First-line CT* (N=36) | Cisplatin-pemetrexed | 24 (44.4) |
| Cisplatin-gemsitabine | 2 (3.7) | |
| 3M*** | 10 (18.5) | |
| Second-line CT (N=20) | Cisplatin-pemetrexed | 5 (9.2) |
| Cisplatin-gemsitabine | 12 (22.2) | |
| 3M*** | 3 (5.5) | |
| RT** (N=18) | Palliative | 11 (20.4) |
| Curative | 7 (13.0) |
CT – chemotherapy;
RT – radiotherapy;
3M – mitoxantrone, methotrexate, mitomycine-c.
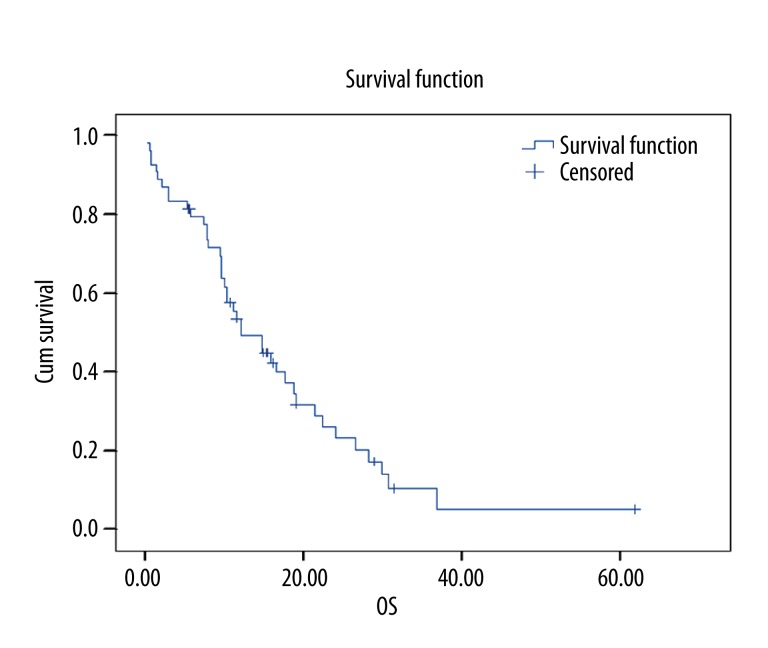
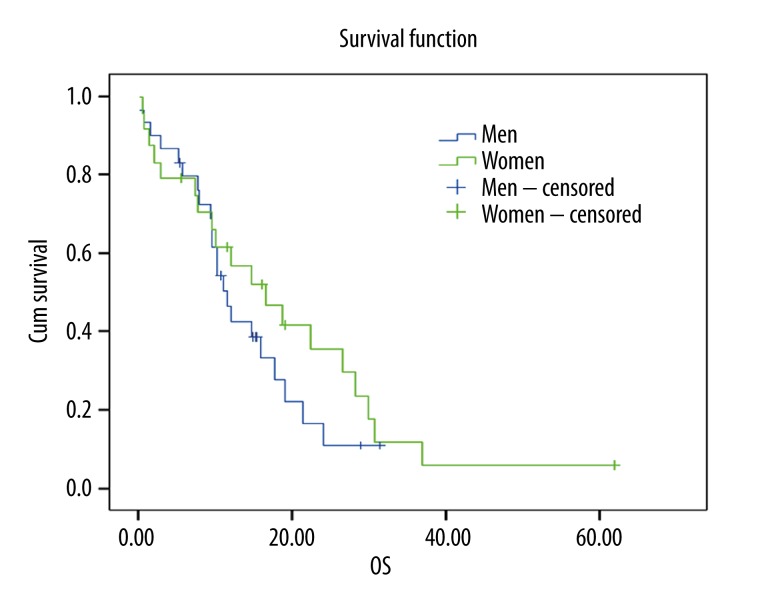
Overall survival data and their relation with other factors are shown in Table 3. Kaplan-Meier curve for overall survival is given in Figure 1. An insignificant correlation was observed between sex and overall survival (12.03, 95% CI: 7.24–16.83) (Figure 2).
Table 3.
Overall survival data and their relation with other clinical factors.
| Parameters | Median (95% CI, months) | P | |
|---|---|---|---|
| Survival | Overall | 12.03 (7.2–16.8) | |
| Gender | Male | 11.38 (9.0–13.7) | 0.32 |
| Female | 16.51 (7.4–25.5) | ||
| Age | <65 | 15.88 (13.3–18.4) | 0.03 |
| ≥65 | 9.27 (5.1–13.4) | ||
| Smoking | Yes | 17.69 (5.5–29.8) | 0.51 |
| No | 12.03 (7.6–16.4) | ||
| ECOG-PS* | O–1 | 15.88 (7.7–24.0) | <0.01 |
| >1 | 9.93 (4.3–15.5) | ||
| Clinical stage | 2 | 10.95 (0.0–31.7) | 0.07 |
| 3 | 18.58 (8.4–28.7) | ||
| 4 | 11.38 (8.0–14.7) | ||
| Histological type | Epithelial | 12.00 (4.9–19.0) | 0.07 |
| Mixed | 17.69 (5.0–30.3) | ||
| Site of involvement | Right | 10.95 (8.4–13.4) | 0.01 |
| Left | 18.58 (6.1–31.0) | ||
| Bilateral | 1.480 | ||
| Pleural effusion | Yes | 12.03 (5.1–18.9) | 0.80 |
| No | 11.38 (5.1–17.6) | ||
| Pleural effusion | Bilateral | 1.480 | 0.01 |
| Unilateral | 12.03 (5.2–18.8) | ||
| Operation | Yes | 16.51 (11.2–21.7) | 0.14 |
| No | 9.93 (5.9–13.9) | ||
| Chemotherapy | Yes | 17.69 (13.0–22.3) | <0.01 |
| No | 2.82 (0.0–7.8) | ||
| 1st line treatments | Cisplatin/pemetrexed | 18.94 (7.8–30.0) | 0.16 |
| 3M** | 16.51 (10.0–23.0) | ||
| 2nd line treatments | Cisplatin/pemetrexed | 12.00 (6.4–17.5) | 0.02 |
| Cisplatin/gemzar | 18.94 (15.3–22.5) | ||
| 3M | 44.35 (16.4–72.2) |
ECOG-PS – Eastern Cooperative Oncology Group-Performance status;
3M – mitoxantrone, methotrexate, mitomycine-c.
Figure 1.
Overall survival curve for all patients (n: 54).
Figure 2.
Overall survival curve according to gender (p=0.32).
A statistically significant difference was found between overall survival and age above or below 65, ECOG PS status 0–1 or >1, second-line treatment with cisplatin-gemcitabine or cisplatin-pemetrexed, 1-sided or 2-sided pleural effusion with, site of involvement left or right side, and taking chemotherapy or not.
Median progression-free survival was 7.33±0.87 (95% CI: 5.61–9.05) months. No statistically significant correlation was found between sex and receiving chemotherapy and progression-free survival (Table 4).
Table 4.
Progression-free survival after firstline chemotherapy.
| Characteristic | Median (95% CI; months) | P | |
|---|---|---|---|
| General | 7.66 (4.7–10.5) | ||
| Gender | Male | 7.33 (3.6–10.9) | 0.314 |
| Female | 7.76 (2.7–12.7) | ||
| Chemotherapy | Yes | 7.76 (3.6–11.8) | 0.572 |
| No | 6.57 (1.3–11.8) |
Discussion
MPM is a rare, insidious type of cancer. Progress in its treatment has been slow. This disease is also an important health problem in Turkey. Asbestos exposure is still a problem in developing countries such as Turkey. In this study, at one of the most comprehensive oncology centers of the region, we studied the patients who were treated at our hospital.
Of 54 patients enrolled to our study, 30 were male and 24 were female. We found that, in line with the published literature, the disease seems to be more common in men, probably because of higher levels of occupational and environmental exposure to asbestos, as in other countries [14,17,18]. In our study, the M/F ratio was 1.25. Among our patients, mean age was calculated to be slightly lower in men compared to women, which may be because men are exposed to asbestos most intensely and at an earlier age [13,19].
The disease initiation period after asbestos exposure is not exactly known, but the mean period was calculated as 32 years in 1 study [20]. Of the patients, 35 (64.8%) were definitively exposed to asbestos for at least a part of their lives and only 9 (18.5%) had no history of exposure to asbestos. Thirty-six patients were born in the regions of Turkey in which the exposure to asbestos is high and, although they immigrated to other regions after spending an average of 20–30 (mean 23.4 years) years in high-risk regions, they could not escape from malignant mesothelioma after the 6th decade of life. This is especially important for showing that in some places of our country, there is a high level of asbestos exposure (men who work in marble and stone quarries where there are high levels of asbestos exposure and women who paint their house annually with asbestos-containing lime) [21]. When we evaluated our patients for other additional factors, we found that 40% smoked and only 1.9% consumed alcohol. Previous administration of radiotherapy, which is an etiological risk factor for malignant mesothelioma, was not reported in any patient. The fact that nearly all of our female patients are housewives suggests that, in our country, in this group, painting the house with asbestos-containing lime paint rather than industrial or occupational exposure is the leading type risk [22]. Interestingly, only 1 of the patients had a familial history of malignant mesothelioma.
In MPM, age (below vs. above 65 years) is an important factor affecting prognosis [23]. Aggressive treatment is suggested for patients under age 65, even if there are additional risk factors [24]. In our patient group, overall survival was significantly higher in the patients below age 65 compared to those over 65 (15.8 and 9.2 months, p=0.037). Another factor that shows a good prognosis is the performance status of the patient at the time of diagnosis [23]. As expected in the patients with a performance status of 0–1 at the time of diagnosis, overall survival is better. In our study, 34 patients (62.9%) had a performance status of 0–1 at the time of diagnosis and a better overall survival was calculated compared to the patients with ECOG performance >1 (p<0.01). Life expectancy is longer in the patients with earlier clinical and pathological stage [23]. In our study, the patients with stage III disease had longer lifespan compared to those with stage II or stage IV diseases (18.5 months, 10.9 months, and 11.3 months, respectively). This difference may be explained by the fact that the patients have other risk factors (e.g., histology, age, ECOG PS). Reports in the general literature suggest aggressive therapies will not contribute to overall survival, patients with epithelial histology have a better prognosis, and aggressive therapy may not be suitable in patients with non-epithelial histology [24,25]. In our patient group, the patients with mixed histology had surprisingly prolonged survival compared to those with epithelial histology (17.6 months and 12.0 months, respectively, p=0.079). This result, which differs from results in the general literature, may be due to the lack of histopathologic subgroup description in 20 patients in our series, which might have changed the statistical results. Difficulties in the diagnosis of malignant mesothelioma should be kept in mind [26].
At the time of diagnosis, the most commonly reported complaints were shortness of breath and chest pain [27]. Although the presence of chest pain at the time of admission was reported to be a good prognostic factor [23], no significant correlation could be detected between the presenting symptom and overall survival (p=0.661). In more than one-third of patients, there is pleural effusion-related shortness of breath without chest pain [28]. In the patients with pleural fluid, cytological examination may demonstrate malignant cells in one-third of the cases [4]. In our case series, 85.2% of the patients were reported to have pleural effusion at the time of diagnosis and all but 1 had unilateral effusion. Although the majority of the patients have pleural effusion, in our clinic, only 2 patients had the diagnosis using pleural cytology. Histological subgroup classification of mesothelioma may not be performed using other closed biopsy methods such as pleural cytology [29]. However, biopsy using VATS and open thoracotomy is more likely to lead to a diagnosis [30]. In our study, 44.4% of the patients were diagnosed by VATS and open thoracotomy surgery. Inadequate biopsy material is a problem in our study and inability to perform subgroup analysis in the majority of the patients may be caused by this factor.
For the treatment of MPM, 3 important surgical procedures were defined: surgical pleurodesis, P/D, and EPP with VATS [25,31,32]. In the surgical procedures, mortality is less than 5% [31]. In our study, 22 patients underwent surgery (9 pleurectomy, 1 pleurodesis, 5 EPP, and 7 P/D) and no mortality occurred. Despite the substantial potential for morbidity, surgery may be beneficial in the palpation of the major symptoms such as resection pain and dyspnea [33]. Reports on the effect of P/D on overall survival are contradictory. Based on some data, VATS + P/D may be beneficial to ensure the survival benefit in the patients who are not candidates for EPP [34]. Despite the high morbidity (60%) and mortality (4–9%) observed with EPP, when administered along with chemotherapy and radiotherapy, this procedure may allow use of higher doses of radiotherapy, as well as prolonging survival in the presence of local recurrence and early disease [35]. However, EPP alone does not seem to prolong life expectancy [24]. In our study, there was not difference of overall survival between the patients who underwent surgery and those who did not undergo surgery. In Turkey most patients are diagnosed at an advanced stage, so there is scant opportunity to initiate trimodal therapy. A study considering trimodal therapy in stage 1–3 MPM patients revealed that median overall survival is approximately 16.8 months after neoadjuvant treatment with cisplatin-pemetrexed and following EPP and radiotherapy [36].
Although local control of the patients is ensured using surgery and radiotherapy, many patients develop systemic metastasis. Chemotherapy leads to prolonged life expectancy and improved quality of life and relieves the symptoms. The response rates obtained with chemotherapy ranged between 0% and 45% [37]. A phase 2 study reported by Nowak et al. revealed that median overall survival was 11.2 months with cisplatin-gemcitabine therapy [38]. In our study, overall survival time was found to be significantly longer in the patients treated with chemotherapy compared to those who did not receive it (17.6 months and 2.8 months, respectively, p<0.001). In the first-line treatment, we administered cisplatin-pemetrexed, cisplatin-gemcitabine and 3M (mitoxantrone, methotrexate, mitomycin-c) protocols and we did not find a difference in survival times among these 3 groups (p=0.161). In the second-line treatment, both the patients who received cisplatin-gemcitabine and those who received 3M protocols showed a significant difference of survival in second-line therapy compared with best supportive care (p=0.025). In the analyses of progression-free survival of the patients, the contribution of the chemotherapy did not show a significant difference between sexes.
Conclusions and Recommendations
MPM patients have a poor life expectancy, with a mean survival of approximately 1 year after diagnosis.
Because exposure to asbestos is the main etiological factor in 80% of the cases, primary prevention should be the first goal, especially in developing countries.
Selection of the potentially most appropriate surgical method in the patients who were diagnosed allows at least controlling the symptoms.
In the patients with early-stage disease, triple therapy (surgery, radiotherapy, and chemotherapy) options may be tried.
In the patients with advanced-stage disease, in light of many studies that showed significant survival differences by the best supportive therapy, we recommend giving at least first-line therapies to all patients with an appropriate performance status.
Footnotes
Conflict of interest
The authors of this manuscript have no conflicts of interest to disclose. The authors have full control of all the primary data and agree to allow the journal to review the data if requested.
Source of support: Departmental sources
References
- 1.Tward JD, Wendland MM, Shrieve DC, et al. The risk of secondary malignancies over 30 years after the treatment of non-hodgkin lymphoma. Cancer. 2006;107:108–15. doi: 10.1002/cncr.21971. [DOI] [PubMed] [Google Scholar]
- 2.De Luca A, Baldi A, Espesito V, et al. The retinoblastoma gene family pRb/p105, p107, pRb2/p130, and simian virus 40 large T-antigen in human mesotheliomas. Nat Med. 1997;3:913–16. doi: 10.1038/nm0897-913. [DOI] [PubMed] [Google Scholar]
- 3.Sekido Y. Genomic abnormalities and signal transduction dysregulation in malignant mesothelioma cells. Cancer Sci. 2010;101(1):1–6. doi: 10.1111/j.1349-7006.2009.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jett J, Aubry M. Clinical Respiratory Medicine. 2nd ed. Mosby; Malignant pleural Mesothelioma; pp. 735–41. [Google Scholar]
- 5.Antman KH. Natural history and epidemiology of malignant mesothelioma. Chest. 1993;103:373S–76. doi: 10.1378/chest.103.4_supplement.373s. [DOI] [PubMed] [Google Scholar]
- 6.Herndon JE, Gren MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–31. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 7.Treasure T, Lang-Lazdunski L, Waller D, et al. Extra-pleural pneumonectomy versus no extra-pleural pneumonectomy for patients with malignant pleural mesothelioma: clinical outcomes of the Mesothelioma and Radical Surgery (MARS) randomised feasibility study. Lancet Oncol. 2011;12(8):763–72. doi: 10.1016/S1470-2045(11)70149-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer. 2009;64:211–18. doi: 10.1016/j.lungcan.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Flores RM, Krug LM, Rosenzweig KE, et al. Induction chemotherapy, extrapleural pneumonectomy, and postoperative high-dose radiotherapy for locally advanced malignant pleural mesothelioma: a phase II trial. J Thorac Oncol. 2006;1:289–95. [PubMed] [Google Scholar]
- 10.Hodgson JT, Mcelvenny DM, Darton AJ, et al. The expected burden of mesothelioma mortality in Great Britain from 2002 to 2050. Br J Cancer. 2005;92:587–93. doi: 10.1038/sj.bjc.6602307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Selçuk ZT, Coplu I, Emri S, et al. Malignant pleural mesothelioma due to environmental mineral fiber exposure in Turkey. Analysis of 135 cases. Chest. 1992;102:790–96. doi: 10.1378/chest.102.3.790. [DOI] [PubMed] [Google Scholar]
- 12.Coplu I, Dumortıer P, Demir AU. An epidemiological study in an Anatolian village in Turkey environmentally exposed to tremolite asbestos. J Environ Pathol Toxicol Oncol. 1996;15:177–82. [PubMed] [Google Scholar]
- 13.Metintaş M, Ozdemir N, Hıllerdal G. Environmental asbestos exposure and malignant pleural mesohelioma. Respir Med. 1999;93:349–55. doi: 10.1016/s0954-6111(99)90318-9. [DOI] [PubMed] [Google Scholar]
- 14.Senyiğit A, Bayram H, Babayiğit C. Malignant pleural mesothelioma caused by environmental exposure to asbestos in the southeast of Turkey: CT findings in 117 patients. Respiration. 2000;67:615–22. doi: 10.1159/000056290. [DOI] [PubMed] [Google Scholar]
- 15.Emri S, Semir AU. Malign pleural mesothelioma in Turkey, 2000–2002. Lung Cancer. 2004;45S:S17–20. doi: 10.1016/j.lungcan.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 16.Eser S, Olcayto E, Karakılınç H, et al. Sağlık Bakanlığı Kanserle Savaş Dairesi Başkanlığı (Epidemiyoloji ve Koruma Şube Müdürlüğü) Nüfus tabanlı kanser kayıt merkezleri veri havuzu: sekiz il, 2004–2006 değerlendirilmesi. [tarihi 03.01.2011]. http://www.kanser.gov.tr/folders/file/8iL-2006-SON.pdf-erişim. [in Turkish]
- 17.Schwartz DA. New developments in asbestos induced pleural disease. Chest. 1991;99:191–98. doi: 10.1378/chest.99.1.191. [DOI] [PubMed] [Google Scholar]
- 18.Adams VI, Unnı KK, Muhm JR, et al. Diffuse malignant mesothelioma of pleura: Diagnosis and survival in 92 cases. Cancer. 1986;58:1540–51. doi: 10.1002/1097-0142(19861001)58:7<1540::aid-cncr2820580727>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 19.Gennaro V, Ugolini D, Viarengo P, et al. Incidence of pleural mesothelioma in Liguria Region, Italy (1996–2002) Eur J Cancer. 2005;41:2709–14. doi: 10.1016/j.ejca.2005.04.047. [DOI] [PubMed] [Google Scholar]
- 20.Lanphear BP, Buncher CR. Latent period for malignant mesothelioma of occupational origin. J Occup Med. 1992;34(7):718–21. [PubMed] [Google Scholar]
- 21.Yazıcıoglu S, Ilcayto R, Balcı K, et al. Pleural calcification, pleural mesotheliomas, and bronchial cancers caused by tremolite dust. Thorax. 1980;35:564–69. doi: 10.1136/thx.35.8.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senyigit A, Babayigit C, Gökirmak M, et al. Incidence of Malignant Pleural Mesothelioma due to Environmental Asbestos Fiber Exposure in the Southeast of Turkey. Respiration. 2000;67:610–14. doi: 10.1159/000056289. [DOI] [PubMed] [Google Scholar]
- 23.Curran D, Sahmoud T, Therasse P, et al. Prognostic factors in patients with pleural mesothelioma: the European Organization for Research and Treatment of Cancer experience. J Clin Oncol. 1998;16:145–52. doi: 10.1200/JCO.1998.16.1.145. [DOI] [PubMed] [Google Scholar]
- 24.Balduyck B, Trousse D, Nakas A, et al. Therapeutic surgery for nonepitheloid malignant pleural mesothelioma: is it really worthwhile? Ann Thorac Surg. 2010;89:907–11. doi: 10.1016/j.athoracsur.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 25.Sugarbaker DJ, Jaklitsch MT0, Liptay MJ. Mesothelioma and radical multimodality therapy: who benefits? Chest. 1995;107:345S–50. doi: 10.1378/chest.107.6_supplement.345s. [DOI] [PubMed] [Google Scholar]
- 26.Addis B, Roche H. Problems in mesothelioma diagnosis. Histopathology. 2009;54(1):55–68. doi: 10.1111/j.1365-2559.2008.03178.x. [DOI] [PubMed] [Google Scholar]
- 27.Stewart DJ, Edwards JG, Symthe WR, et al. Malignant pleural mesothelioma-an update. Int J Environ Health. 2004;10:26–39. doi: 10.1179/oeh.2004.10.1.26. [DOI] [PubMed] [Google Scholar]
- 28.Yates DH, Corrin B, Stidolph PN, Browne K. Malignant mesothelioma in South east England: clinicopathologic experience of 272 cases. Thorax. 1997;52:507–12. doi: 10.1136/thx.52.6.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bueno R, Reblando J, Glickman J, et al. Pleural biopsy: a reliable method for determining the diagnosis but not subtype in mesothelioma. An Thorac Surg. 2004;78:1774–76. doi: 10.1016/j.athoracsur.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 30.British Thoracic Society Standart of Care Committee. Statement on malignant mesothelioma in the United Kingdom. Thorax. 2001;56:250–65. doi: 10.1136/thorax.56.4.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rice DC, Erasmus JJ, Stevens CW, et al. Extended surgical staging for potentially resectable malignant pleural mesothelioma. Ann Thorac Surg. 2005;80:1988–92. doi: 10.1016/j.athoracsur.2005.06.014. discussion 1992–93. [DOI] [PubMed] [Google Scholar]
- 32.Van Ruth S, Baas P, Zoetmulder FA. Surgical treatment of malignant pleural mesothelioma: a review. Chest. 2003;123(2):551–61. doi: 10.1378/chest.123.2.551. [DOI] [PubMed] [Google Scholar]
- 33.Herndon JE, Gren MR, Chahinian AP, et al. Factors predictive of survival among 337 patients with mesothelioma treated between 1984 and 1994 by the Cancer and Leukemia Group B. Chest. 1998;113:723–31. doi: 10.1378/chest.113.3.723. [DOI] [PubMed] [Google Scholar]
- 34.Halstead JC, Lim E, Venkateswaran RM, et al. Improved survival with VATS pleurectomy-decortication in advanced malignant mesothelioma. Eur J SurgOncol. 2005;31:314–20. doi: 10.1016/j.ejso.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 35.Rusch VW, Rosenzweig K, Venkatraman E, et al. A phase II trial of surgical resection and adjuvant high-dose hemithoracic radiation for malignant mesothelioma. J Thorac Cardiovasc Surg. 2001;122:788–95. doi: 10.1067/mtc.2001.116560. [DOI] [PubMed] [Google Scholar]
- 36.Krug ML, Pass JH, Rusch WV, et al. Multicenter Phase II Trial of Neoadjuvant Pemetrexed Plus Cisplatin Followed by Extrapleural Pneumonectomy and Radiation for Malignant Pleural Mesothelioma. J Clin Oncol. 2009;27:3007–13. doi: 10.1200/JCO.2008.20.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Green J, Dundar Y, Dodd S, et al. Pemetrexed disodium in combination with cisplatin versus other cytotoxic agents or supportive care for the treatment of malignant pleural mesothelioma. Cochrane Database Syst Rev. 2007:CD005574. doi: 10.1002/14651858.CD005574.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak AK, Byrne MJ, Williamson R, et al. A multicenter phase II study of cisplatin and gemcitabine in malignant mesothelioma. Br J Cancer. 2002;87:491–96. doi: 10.1038/sj.bjc.6600505. [DOI] [PMC free article] [PubMed] [Google Scholar]