Abstract
Background
Down-regulation of microRNA-101 (miR-101) expression has been linked to bladder transitional cell carcinoma (BTCC) development. However, the relationship between the expression of miR-101 in BTCC and a patient’s prognosis has not yet been studied. Thus, we attempted to explore the correlation of miR-101 and clinicopathological factors of BTCC patients, and evaluate the impact of miR-101 on prognosis of BTCC.
Material/Methods
In 88 samples of BTCC (n=72) and normal tissues (n=16), the expressions of miR-101 were detected by quantitative reverse-transcriptase polymerase chain reaction (qRT-PCR). The relationship of miR-101 and clinicopathological factors in BTCC was analyzed statistically. Survival analysis was performed to assess the prognostic significance of miR-101.
Results
Down-regulation of miR-101 was found in BTCC tissues, compared with normal tissues (P<0.05). MiR-101 expression was significantly associated with tumor diameter, tumor stage, tumor grade, lymph node involvement, and lymph node metastasis (all P<0.05). Low-level expression of miR-101 was significantly correlated with shortened survival time (P<0.01). Multivariate Cox regression analysis revealed this significant prognostic impact was independent of other clinicopathologic factors (P<0.01).
Conclusions
Our results suggest that the expression of miR-101 is down-regulated in BTCC, which consequently favored tumor progression. MiR-101 may play an important role as a diagnostic and prognostic marker in BTCC.
MeSH Keywords: MicroRNAs, Carcinoma, Transitional Cell, Urinary Bladder Neoplasms, Prognosis
Background
MicroRNAs (miRNAs) are a class of endogenous small noncoding regulatory RNAs, approximately 22 nucleotides in length. They can bind with imperfect complementarity to the 3′ untranslated region (3′UTR) of the target mRNA via the RNA-induced silencing complex, regulating gene expression by repressing translation or decreasing the stability of mRNAs [1]. Many studies have shown that miRNAs has a role in cell differentiation, proliferation and apoptosis [2]. More importantly, miRNAs are also reported to be closely associated with various human cancers, as tumour suppressors or oncogenes [3–5]. BTCC is one of the most common cancers in the urinary system. Although significant advances have been achieved, the molecular pathogenesis of BTCC still remains to be elucidated. In recent years, the roles of miRNAs in BTCC have been explored [6–8]. These data highlight the importance of miRNAs in development of BTCC and provide more insights into the mechanisms regarding tumorigenesis.
Numerous studies have indicated that miR-101 is significantly down-regulated in various cancers, including prostate, breast, liver, and endometrial cancer, and displays a suppressive effect on cellular proliferation, migration, and invasion [9–12]. As to the BTCC, it has been demonstrated that miR-101 is down-regulated in BTCC, and miR-101 inhibits cell proliferation and colony formation in BTCC cell lines [1]. However, the relationship between the expression of miR-101 in BTCC and a patient’s prognosis has not yet been studied. Therefore, in the present study, we aimed to investigate whether miR-101 expression was associated with clinicopathologic parameters of BTCC, and we evaluated the predictive value of miR-101 on clinical outcomes of patients with BTCC.
Material and Methods
Patients and samples
Our study was approved by the Ethics Review Board of The First Affiliated Hospital of the University of South China and Xiangya Hospital. Informed consent was obtained from all patients. We studied bladder cancer specimens (cancer lesions and adjacent non-tumor tissues) from 72 patients who had undergone resection at the First Affiliated Hospital of University of South China and Xiangya Hospital between 2005 and 2012. No patient had received preoperative adjuvant therapy. We gathered all samples in the same manner following surgical removal, and they were snap-frozen immediately in liquid nitrogen and stored at −80°C until RNA extraction could be performed. All the procedures were performed by a single, experienced surgeon. The patients included 42 men and 30 women with ages ranging from 32 to 84 (median age: 57 years). The initial symptoms were hematuria (58%), urinary infection (15%), frequent micturition (13%), dysuresia (9%) or physical examination (5%). Before surgery, all the patients were diagnosed with ultrasound, cystoscopy, and computed tomography. Transurethral resection of bladder tumor (TURbt), partial cystectomy, and radical cystectomy were performed in 62%, 10% and 28% of patients, respectively. All the patients were diagnosed with BTCC by pathology. Average duration of hospitalization was 7.2±1.8 days. We obtained 16 normal bladder specimens from patients with benign prostatic hypertrophy through transurethral resection of the prostate (TURP). Information on other clinicopathological factors, including age, histological grade, tumor stage, tumor number, tumor diameter, lymph node status, and occurrence status were also collected. Telephone follow-up was conducted every 3 months after surgery to evaluate the survival status. The last follow-up date in this study was June 30, 2013.
qRT-PCR of miR-101 expression
Total RNA was extracted from frozen tissue using TRIzol Reagent (Applied Invitrogen, Carlsbad, CA, USA). The miR-101-specific primers and the internal control RNU6B gene were purchased from Ambion (Applied Biosystems, Foster City, CA). cDNA synthesis was performed by the High Capacity cDNA Synthesis Kit (Applied Biosystems) with miRNA-specific primers. QRT-PCR was performed on an Applied Biosystems 7500 Real-time system (ABI 7500HT instrument) with miRNA-specific primers by TaqMan Gene Expression Assay. It was programmed as: 95°C, 10 minutes; 95°C, 15 seconds; 60°C, 1 minute, which were repeated for 40 cycles. Fluorescent signals from each sample were collected at the endpoint of every cycle. Expression of miR-101 was normalized according to the internal RNU6B control, and plotted as relative value. The fold change between a sample and a normal control for miR-101 was calculated with the 2−ΔΔCT method. QRT-PCR was repeated in triplicate for each sample.
Statistical analysis
Statistical analysis was performed using SPSS version 13.0 software (Chicago, IL, USA). Data were analyzed using the chi-square test. Kaplan-Meier curves were constructed, and the log-rank test was performed for analysis of survival data. Multivariate analysis of the prognostic factors was performed with Cox regression model. The results are expressed as mean ±SD. The significance level was set at P < 0.05.
Results
MiR-101 expression in BTCC and its relationship with clinicopathological factors
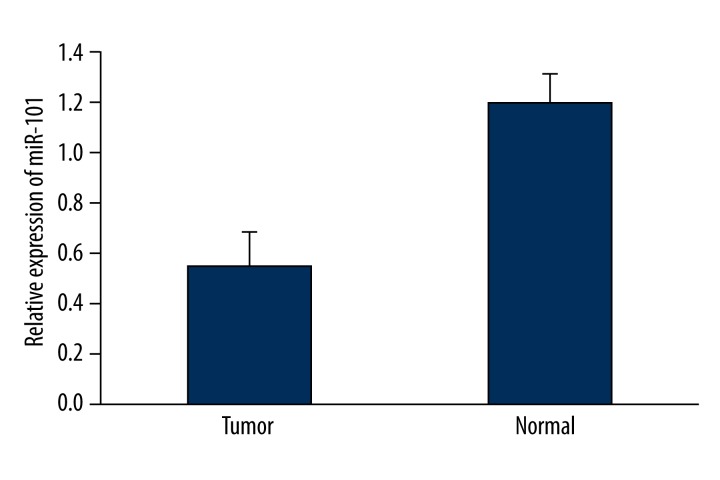
QRT-PCR was used to evaluate expression levels of miR-101 in 72 BTCC and 16 normal bladder specimens. Consistent with the previous study, BTCC tissues showed decreased levels of miR-101 compared to the normal tissues (0.54±0.14 vs. 1.20±0.11), with a median down-regulation of 0.45-fold (P<0.05) (Figure 1). Each BTCC patient was divided into high (>0.45-fold) or low (<0.45-fold) mir-101 expression group accordingly. Then the association of the expression of mir-101 with sex, age, tumor number, tumor diameter, tumor stage, tumor grade, lymph node involvement, lymph node metastasis, and tumor occurrence was analyzed. As shown in Table 1, miR-101 expression was significantly associated with tumor diameter, tumor stage, tumor grade, lymph node involvement, and lymph node metastasis (all P<0.05). There was no correlation between miR-101 expression and other clinicopathological factors (sex, age, tumor number, and recurrence).
Figure 1.
A comparison of miR-101 expression between normal bladder tissues and bladder TCC. miR-101 expressions are significantly decreased in BTCC compared to normal tissues (P<0.05). Data are presented as mean ±SD. P values were calculated by the chi-square test.
Table 1.
A comparison of miR-101 expression in BTCC and clinicopathological features.
| Case number | miR-101 expression | P-value | ||
|---|---|---|---|---|
| High (>0.45-fold) | Low (<0.45-fold) (>0.45-fold) | |||
| Sex | 0.561 | |||
| Male | 42 | 28 | 14 | |
| Female | 30 | 18 | 12 | |
| Age | 0.729 | |||
| <60 years | 48 | 30 | 18 | |
| ≥60 years | 24 | 16 | 8 | |
| Tumor number | 0.813 | |||
| Single | 43 | 27 | 16 | |
| Multiple | 29 | 19 | 10 | |
| Tumor diameter | 0.007 | |||
| <3 cm | 40 | 31 | 9 | |
| ≥3 cm | 32 | 15 | 17 | |
| Tumor stage | 0.001 | |||
| T1–T2 | 35 | 29 | 6 | |
| T3–T4 | 37 | 17 | 20 | |
| Tumor grade | 0.008 | |||
| I–II | 45 | 34 | 11 | |
| III | 27 | 12 | 15 | |
| Lymph node involvement | <0.001 | |||
| Positive | 26 | 8 | 18 | |
| Negative | 46 | 38 | 8 | |
| Lymph node metastasis | <0.001 | |||
| Positive | 19 | 3 | 16 | |
| Negative | 53 | 43 | 10 | |
| Tumor occurrence | 0.690 | |||
| Primary | 31 | 14 | 17 | |
| Recurrent | 41 | 12 | 29 | |
Significance of miR-101 expression in BTCC prognosis
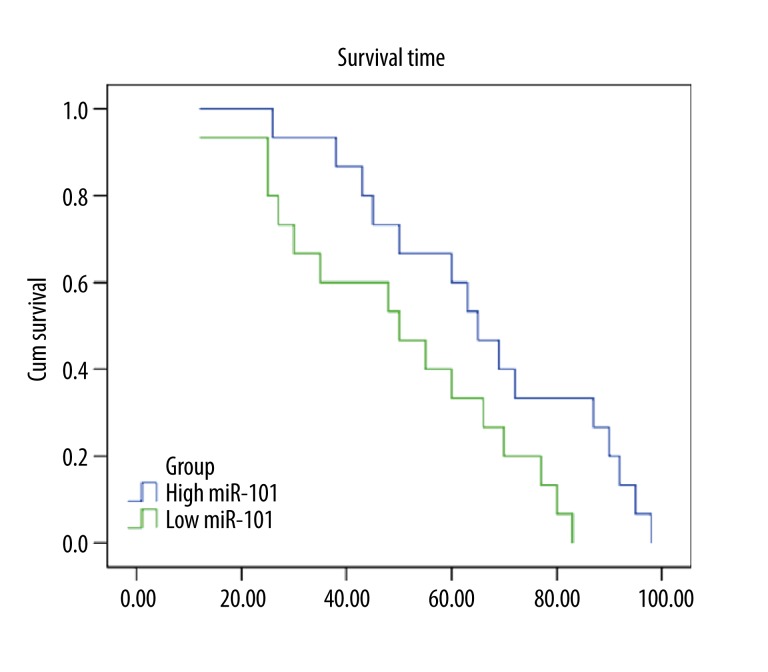
Survival analysis of 72 patients was performed with information available on clinical follow-up. Kaplan-Meier survival analysis revealed that the patients with low miR-101 expression had significantly poorer survival times compared with those with high miR101 expression (P=0.004). Figure 2 shows the survival curves of the patients with high or low miR-101. The median postoperative survival time of high and low miR-101 were 65±5.8 and 50±12.9 months, respectively. The multivariate Cox regression model revealed that low expression level miR-101 expression (P=0.028, HR 0.451; 95% CI 0.237–0.735) was also a significantly unfavorable prognostic factor, in addition to lymph node metastasis (P=0.042, HR 4.228; 95% CI 1.941–8.267) (Table 2).
Figure 2.
Kaplan-Meier analysis curve of postoperative survival times of BTCC patients according to the miR-101 relative expression. The expression levels of miR-101 in 72 BTCC patients were measured by qRT-PCR. High or low expression is based on the median fold change (0.45-fold higher than normal or 0.45-fold lower than normal). Patients with low miR-101 expression had significantly poorer survival times compared to those with high miR101 expression. P values were calculated by Kaplan-Meier analysis (P<0.01).
Table 2.
Multivariate analysis of the factors with survival time.
| Factors | β | SE | Wald | Exp(β) | 95% CI for Exp(β) | P value | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Lymph node metastasis | 1.437 | 0.601 | 3.884 | 4.228 | 1.941 | 8.267 | 0.042 |
| miR-101 | −1.133 | 0.462 | 5.013 | 0.451 | 0.237 | 0.735 | 0.028 |
Discussion
BTCC is one of the most common urogenital cancers. The challenges in controlling bladder cancer are the prevention of the recurrent disease and the inhibition of the disease progression during the treatment course. Clinicopathological parameters such as tumor grade and stage have been used clinically to evaluate pathologic events of bladder cancer, but their sensitivity is relatively low [13]. Therefore, identification of novel effective molecular markers is of great significance for the improvement of diagnostic and prognostic techniques, and for the development of more efficient therapeutic strategies for patients with bladder cancer.
miRNAs have an important role in the control of gene expression, because they can regulate genes involved in cellular proliferation, differentiation, or apoptosis. Bioinformatics predictions indicate that miRNAs regulate 30% of all protein coding genes [14]. miRNA deregulation has been observed in a large variety of tumors and association of miRNA expression with clinical data and outcome was described. Previous studies have demonstrated that several miRNAs were mis-regulated in bladder cancer tissues. It was reported that miR-16 inhibits bladder cancer proliferation by targeting Cyclin D1 [15]. miR-125b inhibits cell migration and invasion by targeting matrix metallopeptidase 13 in bladder cancer [16]. Moreover, miR-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer [17]. Clinical data shows that reduced expression of miR-100 confers unfavorable prognosis in patients with bladder cancer [13]. These observations suggest that miRNAs may be useful as therapeutic targets and prognostic biomarkers in BTCC.
Reduced expression of miR-101 has been observed in different types of cancers, including BTCC. In addition, reduced expression of miR-101 is associated with worse survival of these cancer patients [18], thus miR-101 is considered as a tumor suppressor. It was demonstrated that miR-101 suppresses motility of bladder cancer cells [19]. However, data concerning the relationship between miR-101 expression, clinicopathologic factors, and the prognosis of BTCC remains unclear.
For the use of miRNAs as prognostic biomarkers, it is important to evaluate the relationships between miRNAs and clinical data. In the present study, the potential relationship between the expression level of miR-101 and various clinicopathological factors and postoperative survival times were analyzed. Expression levels of miR-101 correlate with tumor diameter, tumor stage, tumor grade, lymph node involvement, and lymph node metastasis, but not other clinicopathological factors. Furthermore, we showed that BTCC patients with low miR-101 expression had a significantly shorter survival time than those with high miR-101 expression (P<0.01). Multivariate Cox hazard regression analysis revealed that the correlation was independent of other clinicopathological factors, indicating that low expression of miR-101 may be a useful indicator of an unfavorable prognosis (P=0.028, HR 0.451; 95% CI 0.237–0.735) independent of another factor, lymph node metastasis (P=0.042, HR 4.228; 95% CI 1.941–8.267). The potential prognostic value of miR-101 may be able to help physicians identify and select the patients who are most likely to benefit from therapy, in order to improve the treatment outcome of BTCC.
Data from multiple studies have already demonstrated that miR-101 acts as a reliable clinical tool in cancer diagnosis and prognosis. For example, it was reported that reduced expression of miR-101 is associated with a poorer prognosis in non-small-cell lung cancer [20] and the down-regulation of miR-101 in clinical HCC tissues correlates with tumor aggressiveness and poor prognosis [21]. However, in Schee’s [22] study, miR-101 was hardly expressed in 193 colorectal tumor samples and no significant associations were found between expression of miR-101 and metastasis-free or overall survival. These conflicting outcomes could be due to different samples sizes and end points set for analysis. Recent studies suggest that in addition to being a diagnostic and prognostic tool for patients with cancer, miR-101 might also be a potential drug target or a biomarker to predict the risk of radiotherapy or chemotherapy resistance, which could be used in a broad range of cancer therapies. It was reported that the ectopic miR-101 is able to radiosensitize most NSCLC cells [23]. Xu’s [24] study showed that miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. These studies suggest a novel potential approach of miR-101 to the improvement of BTCC treatment.
Until recently, many studies have explored the exact molecular mechanism of miR-101 in cancer development and progression. Abnormal down-regulation of miR-101 could frequently lead to the overexpression of enhancer of zeste homologue 2 (EZH2) in cancer [25]. EZH2 has been shown to be overexpressed in BTCC, and elevated EZH2 protein levels are associated with more aggressive bladder cancer. Efficient down-regulation of EZH2 resulted in significantly decreased cell proliferation in bladder cancer cells and retarded transition of G phase to S phase [26]. Additionally, miR-101 was shown to be a novel suppressor of bladder cancer T24 cell migration and invasion through its negative regulation of c-Met [19]. Wang’s [11] study demonstrates that down-regulation of miR-101 in different subtypes of human breast cancer tissues is linked to the increase of cellular proliferation and invasiveness via targeting Stmn1. Cyclooxygenase-2 (COX-2) is considered to be a mediator of inflammation, and overexpressed COX-2 strongly contributes to the growth and invasiveness of tumoral cells. MiR-101 directly silences COX-2 in vitro in colon cancer cell lines and regulates COX-2 expression through a translational repression mechanism [27]. Mcl-1 is an antiapoptotic member of the Bcl-2 family. It was shown that miR-101 significantly repressed the expression of luciferase carrying the 3-untranslated region of Mcl-1 and reduced the endogenous protein level of Mcl-1, whereas the miR-101 inhibitor obviously up-regulated Mcl-1 expression and inhibited cell apoptosis [20]. These results indicate that miR-101 may exert its proapoptotic function via targeting Mcl-1. Thus, miR-101 is likely to function as a tumor suppressor in multiple pathways, which are still largely unknown.
Conclusions
In conclusion, down-regulated miR-101 in BTCC was correlated with tumor diameter, tumor stage, tumor grade, lymph node involvement, and lymph node metastasis. Furthermore, the status of miR-101 expression might be also an independent prognostic factor for BTCC patients. The survival time of BTCC patients with high miR-101 expression are longer than those with low miR-101 expression. Although further testing on larger populations will be necessary, results indicate that miR-101 may be a promising candidate as a molecular diagnostic and prognostic biomarker for BTCC.
Abbreviations
- miR-101
microRNA-101
- miRNAs
microRNAs
- BTCC
bladder transitional cell carcinoma
- qRT-PCR
quantitative reverse-transcriptase polymerase chain reaction
- 3′UTR
3′ untranslated region
- EZH2
enhancer of zeste homologue 2
- COX-2
cyclooxygenase-2
Footnotes
Conflict of interest
The authors declare they have no conflicts of interest.
Source of support: This study was supported by the Natural Science Foundation of Hunan Province (No. 11JJ2040) and the National Natural Science Foundation of China (No. 81272838 and No. 81202005)
References
- 1.Friedman JM, Liang G, Liu CC, et al. The Putative Tumor Suppressor microRNA-101 Modulates the Cancer Epigenome by Repressing the Polycomb Group Protein EZH2. Cancer Res. 2009;69:2623–29. doi: 10.1158/0008-5472.CAN-08-3114. [DOI] [PubMed] [Google Scholar]
- 2.Wang HJ, Ruan HJ, He XJ, et al. MicroRNA-101 is down-regulated in gastric cancer and involved in cell migration and invasion. Eur J Cancer. 2010;46:2295–303. doi: 10.1016/j.ejca.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Li P, Mao WM, Zheng ZG, et al. Down-Regulation of PTEN Expression Modulated by Dysregulated miR-21 Contributes to the Progression of Esophageal Cancer. Dig Dis Sci. 2013;58(12):3483–93. doi: 10.1007/s10620-013-2854-z. [DOI] [PubMed] [Google Scholar]
- 4.Ma Q, Jiang Q, Pu Q, et al. MicroRNA-143 inhibits migration and invasion of human non-small-cell lung cancer and its relative mechanism. Int J Biol Sci. 2013;9:680–92. doi: 10.7150/ijbs.6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao G, Wang B, Liu Y, et al. miRNA-141, Downregulated in Pancreatic Cancer, Inhibits Cell Proliferation and Invasion by Directly Targeting MAP4K4. Mol Cancer Ther. 2013;12:2569–80. doi: 10.1158/1535-7163.MCT-13-0296. [DOI] [PubMed] [Google Scholar]
- 6.Xu X, Li S, Lin Y, et al. MicroRNA-124-3p inhibits cell migration and invasion in bladder cancer cells by targeting ROCK1. J Transl Med. 2013;11:276. doi: 10.1186/1479-5876-11-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xu X, Chen H, Lin Y, et al. MicroRNA-409-3p inhibits migration and invasion of bladder cancer cells via targeting c-Met. Mol Cells. 2013;36:62–68. doi: 10.1007/s10059-013-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo Y, Ying L, Tian Y, et al. miR-144 downregulation increases bladder cancer cell proliferation by targeting EZH2 and regulating Wnt signaling. FEBS J. 2013;280:4531–38. doi: 10.1111/febs.12417. [DOI] [PubMed] [Google Scholar]
- 9.Hao Y, Gu X, Zhao Y, et al. Enforced expression of miR-101 inhibits prostate cancer cell growth by modulating the COX-2 pathway in vivo. Cancer Prev Res (Phila) 2011;4:1073–83. doi: 10.1158/1940-6207.CAPR-10-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su H, Yang JR, Xu T, et al. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009;69:1135–42. doi: 10.1158/0008-5472.CAN-08-2886. [DOI] [PubMed] [Google Scholar]
- 11.Wang R, Wang HB, Hao CJ, et al. MiR-101 is involved in human breast carcinogenesis by targeting Stathmin1. PLoS One. 2012;7:e46173. doi: 10.1371/journal.pone.0046173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiroki E, Akahira JI, Suzuki F, et al. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010;101:241–49. doi: 10.1111/j.1349-7006.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang S, Xue S, Dai Y, et al. Reduced expression of microRNA-100 confers unfavorable prognosis in patients with bladder cancer. Diagn Pathol. 2012;7:159. doi: 10.1186/1746-1596-7-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–14. doi: 10.1038/nrg2290. [DOI] [PubMed] [Google Scholar]
- 15.Jiang QQ, Liu B, Yuan T, et al. MicroRNA-16 Inhibits Bladder Cancer Proliferation by Targeting Cyclin D1. Asian Pac J Cancer Prev. 2013;14:4127–30. doi: 10.7314/apjcp.2013.14.7.4127. [DOI] [PubMed] [Google Scholar]
- 16.Wu D, Ding J, Wang L, et al. microRNA-125b inhibits cell migration and invasion by targeting matrix metallopeptidase 13 in bladder cancer. Oncol Lett. 2013;5:829–34. doi: 10.3892/ol.2013.1123. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Majid S, Dar AA, Saini S, et al. MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in bladder cancer. PLoS One. 2013;8:e67686. doi: 10.1371/journal.pone.0067686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Guo X, Xiong L, et al. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362–70. doi: 10.1016/j.febslet.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 19.Hu Z, Lin Y, Chen H, et al. MicroRNA-101 suppresses motility of bladder cancer cells by targeting c-Met. Biochem Biophys Res Commun. 2013;435:82–87. doi: 10.1016/j.bbrc.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Luo L, Zhang T, Liu H, et al. MiR-101 and Mcl-1 in non-small-cell lung cancer: expression profile and clinical significance. Med Oncol. 2012;29:1681–86. doi: 10.1007/s12032-011-0085-8. [DOI] [PubMed] [Google Scholar]
- 21.Wang L, Zhang X, Jia LT, et al. c-Myc-mediated epigenetic silencing of microRNA-101 contributes to dysregulation of multiple pathways in hepatocellular carcinoma. Hepatology. 2013 doi: 10.1002/hep.26720. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Schee K, Boye K, Abrahamsen TW, et al. Clinical relevance of microRNA miR-21, miR-31, miR-92a, miR-101, miR-106a and miR-145 in colorectal cancer. BMC Cancer. 2012;12:505. doi: 10.1186/1471-2407-12-505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen S, Wang H, Ng WL, et al. Radiosensitizing effects of ectopic miR-101 on non-small-cell lung cancer cells depend on the endogenous miR-101 level. Int J Radiat Oncol Biol Phys. 2011;81:1524–29. doi: 10.1016/j.ijrobp.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, An Y, Wang Y, et al. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol Rep. 2013;29:2019–24. doi: 10.3892/or.2013.2338. [DOI] [PubMed] [Google Scholar]
- 25.Cho HM, Jeon HS, Lee SY, et al. microRNA-101 inhibits lung cancer invasion through the regulation of enhancer of zeste homolog 2. Exp Ther Med. 2011;2:963–67. doi: 10.3892/etm.2011.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang YB, Niu HT, Chang JW, et al. EZH2 silencing by RNA interference inhibits proliferation in bladder cancer cell lines. Eur J Cancer Care (Engl) 2011;20:106–12. doi: 10.1111/j.1365-2354.2009.01148.x. [DOI] [PubMed] [Google Scholar]
- 27.Strillacci A, Griffoni C, Sansone P, et al. MiR-101 downregulation is involved in cyclooxygenase-2 overexpression in human colon cancer cells. Exp Cell Res. 2009;315:1439–47. doi: 10.1016/j.yexcr.2008.12.010. [DOI] [PubMed] [Google Scholar]