To the Editor
Diuretics are a mainstay of treatment in both chronic and acute decompensated heart failure (HF). Studies during the 1990s and early 2000s show that roughly 90% of HF patients receive at least 1 class of diuretics, particularly a loop diuretic, for management of chronic (1,2) or acute (3) HF. There are at least 3 widely known loop diuretics—furosemide, bumetanide, and torsemide—all of which are available as generic formulations.
The available evidence suggests that newer loop diuretics and furosemide may not be identical. Although markedly limited by methodological problems and inadequate power, the few existing pharmacological and clinical studies propose that there might be superior and more consistent oral bioavailability, longer duration of action, improved tolerability, and better outcomes with newer loop diuretics, particularly torsemide, as compared with furosemide (4–7). Unlike bumetanide for which there is a dearth of clinical studies, only a few small studies have compared the effects of torsemide versus furosemide. The TORIC (Torasemide In Congestive Heart Failure) study, an open-label study of 1,337 patients with New York Heart Association class II to III HF, was the largest study comparing furosemide with newer loop diuretics. Although the study had several methodological limitations, TORIC showed that a greater proportion of patients receiving torsemide improved their functional class (45.8% vs. 37.2%, p < 0.00017) and that fewer patients receiving torsemide died (2.2% vs. 4.5%, p < 0.05) (5). Additionally, a meta-analysis of the existing studies (4 – 6,8–10) (Fig. 1), although demonstrating remarkable heterogeneity, suggests trends toward improved functional status and mortality with torsemide compared with furosemide. Previous research also suggested that torsemide could be cost-saving compared with furosemide (11). Although there are no existing clinical studies that have compared the efficacy of newer loop diuretics versus furosemide for episodes of acute HF, it might be possible that the newer agents are also beneficial in various stages of care of acutely decompensated HF. In light of the potential advantages of newer loop diuretics, we sought to characterize current patterns of use of these agents in U.S. hospitals.
Figure 1. Functional Status and Mortality With Torsemide Compared With Furosemide.
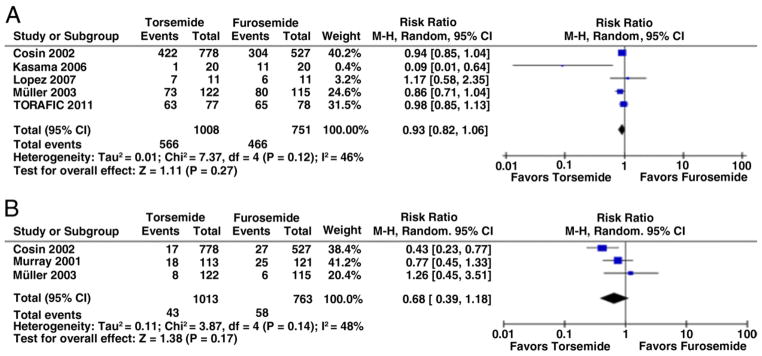
No improvement in New York Heart Association functional classification (A) and all-cause death (B) with torsemide versus furosemide. CI = confidence interval; M-H = Mantel-Haenszel.
Using the data from the Perspective database, a voluntary, fee-supported database of more than 500 U.S. hospitals developed by Premier, Inc., we studied HF hospitalizations during 2009 and 2010 to determine the proportion of adult (age >18 years) patients treated with major loop diuretic formulations. We identified HF hospitalizations by the International Classification of Diseases-Ninth Revision-Clinical Modification principal discharge codes: 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, or 428.xx.
Among the 274,515 patients with a principal discharge diagnosis of HF in the Perspective database, 251,472 (92%) patients received loop diuretic therapy during their hospital stay. Of those, 218,787 (87%) received furosemide as their only loop diuretic, 6,776 (3%) only received bumetanide, 972 (0.4%) only received torsemide, whereas 24,937 (10%) were treated with a combination of these agents.
Most patients with HF received a loop diuretic. However, torsemide, a new agent with potentially superior clinical effectiveness, was rarely used. Given the common usage of loop diuretics in HF and their potential nonequivalence in HF outcomes and safety endpoints, perhaps it is time for well-designed randomized controlled trials, powered for clinical endpoints such as mortality, readmission, and quality of life, to determine whether there are differences in the safety and effectiveness of these agents both for management of chronic HF and for episodes of acute decompensation.
Acknowledgments
This study was supported by grant #DF10-301 from the Patrick and Catherine Weldon Donaghue Medical Research Foundation in West Hartford, Connecticut, and by grant #UL1 RR024139-06S1 from the National Center for Advancing Translational Sciences in Bethesda, Maryland. The content is solely the responsibility of the authors and does not necessarily represent the official views of the sponsor. Dr. Bikdeli is a Post-doctoral Associate in Cardiovascular Medicine at Yale University School of Medicine; and is partially supported by grant #U01 HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr. Dharmarajan is supported by grant #T32 HL007854-16A1 from the Division of Cardiology at Columbia University; he is also supported as a Centers of Excellence Scholar in Geriatric Medicine at Yale by the John A. Hartford Foundation and the American Federation for Aging Research. Dr. Krumholz is supported by grant #U01 HL105270-03 (Center for Cardiovascular Outcomes Research at Yale University) from the National Heart, Lung, and Blood Institute. Dr. Krumholz also reports that he is the recipient of a research grant from Medtronic through Yale University and chairs a cardiac scientific advisory board for UnitedHealth.
Footnotes
All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
References
- 1.The Digitalis Investigators Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 2.The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. N Engl J Med. 1991;325:293–302. doi: 10.1056/NEJM199108013250501. [DOI] [PubMed] [Google Scholar]
- 3.Peacock WF, Costanzo MR, De Marco T, et al. for the ADHERE Scientific Advisory Committee and Investigators. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–9. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 4.Murray MD, Deer MM, Ferguson JA, et al. Open-label randomized trial of torsemide compared with furosemide therapy for patients with heart failure. Am J Med. 2001;111:513–20. doi: 10.1016/s0002-9343(01)00903-2. [DOI] [PubMed] [Google Scholar]
- 5.Cosin J, Diez J TORIC Investigators. Torasemide in chronic heart failure: results of the TORIC study. Eur J Heart Fail. 2002;4:507–13. doi: 10.1016/s1388-9842(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 6.Müller K, Gamba G, Jaquet F, Hess B. Torasemide vs. furosemide in primary care patients with chronic heart failure NYHA II to IV—efficacy and quality of life. Eur J Heart Fail. 2003;5:793–801. doi: 10.1016/s1388-9842(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 7.Masuyama T, Tsujino T, Origasa H, et al. Superiority of long-acting to short-acting loop diuretics in the treatment of congestive heart failure. Circ J. 2012;76:833–42. doi: 10.1253/circj.cj-11-1500. [DOI] [PubMed] [Google Scholar]
- 8.Kasama S, Toyama T, Hatori T, et al. Effects of torasemide on cardiac sympathetic nerve activity and left ventricular remodelling in patients with congestive heart failure. Heart. 2006;92:1434–40. doi: 10.1136/hrt.2005.079764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.López B, González A, Beaumont J, Querejeta R, Larman M, Díez J. Identification of a potential cardiac antifibrotic mechanism of torasemide in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:859–67. doi: 10.1016/j.jacc.2007.04.080. [DOI] [PubMed] [Google Scholar]
- 10.TORAFIC Investigators Group. Effects of prolonged-release torasemide versus furosemide on myocardial fibrosis in hypertensive patients with chronic heart failure: a randomized, blinded-end point, active-controlled study. Clin Ther. 2011;33:12074–13. e3. doi: 10.1016/j.clinthera.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Stroupe KT, Forthofer MM, Brater DC, Murray MD. Healthcare costs of patients with heart failure treated with torasemide or furosemide. Pharmacoeconomics. 2000;17:429–40. doi: 10.2165/00019053-200017050-00002. [DOI] [PubMed] [Google Scholar]


