Abstract
Diminished social motivation constitutes one of the core impairments of Autism Spectrum Disorder (ASD) and is thought to have a strong impact on the way individuals with autism respond to the presence of others. In this study, we hypothesized that experimental contexts involving direct interaction with an experimenter might elicit different reactions in ASD and thus act as a potential confound in the interpretation of group differences during social cognitive tests. Following classic work in social psychology on the ‘audience effect’ –wherein individuals act differently when they are being watched in a more or less conscious attempt to enhance their reputation in the eyes of others, we reasoned that social contexts are indeed likely to produce an increase in performance in typically developing (TD) individuals but that children with ASD would be less susceptible to such audience effects. More specifically, we were interested in testing the idea that susceptibility to the audience effect might explain part of the performance gap between children with and without autism in Theory of Mind (ToM) tasks, which are typically administered by a human experimenter. We tested this hypothesis by comparing performance on a ToM task administered in a social vs. a non-social setting. We found that ASDs and controls performed similarly when the task was administered using a non-social medium. However, control participants outperformed children with autism when an experimenter administered the task. Thus, TD controls demonstrated a relative improvement in performance when in the presence of an experimenter that children with ASD did not. The implications of this diminished ‘audience effect’ in ASD are discussed.
Keywords: Audience effect, Autism Spectrum Disorders, Attribution of intentions, Motivation, Reputation, Theory of Mind
Introduction
Autism Spectrum Disorders (ASD) are characterized by marked impairments in socialization and, in particular, by a “lack of spontaneous seeking to share enjoyment, interests, or achievements with other people” and a “lack of social or emotional reciprocity” (APA, 1994). More recently, these deficits in social motivation have been linked to diminished concern for reputation management (Chevallier, Kohls, Troiani, Brodkin, & Schultz, 2012; Chevallier, Molesworth, & Happé, 2012). Deficits in reputation management have been observed in various contexts including tasks where participants are given the opportunity to engage in flattery (Chevallier et al., 2012) or tasks where prosocial behavior is enhanced by the presence of an observer watching the participant’s action (Izuma, Matsumoto, Camerer, & Adolphs, 2011; Cage, Pellicano, Shah, & Bird, 2013). Taken together, these findings suggest that individuals with ASD react differently to the presence of observers. This has potentially far-reaching implications since many assessments commonly used in autism research (including IQ testing) are delivered by a human experimenter, and, as such, constitute contexts wherein diminished sensitivity to the presence of others in ASD might act as a potential confound. Yet, most efforts to control for task demands traditionally focus on the task itself and not on the context in which it is embedded.
In the present paper, we asked how this diminished sensitivity to the presence of others might affect performance on Theory of Mind (ToM) tasks. ToM tasks assess the ability to attribute intention, thoughts, or beliefs to other people and to predict their behavior based on these attributed mental states (Leslie, 1987). ToM tasks have been widely used to characterize social cognition in ASD and ToM deficits have been linked to a variety of social issues in this population (e.g., pragmatic and communication deficits, Frith & Happé, 1994; Happé, 1993, 1995; Norbury, 2005; Surian & Siegal, 2008). However, the idea that ToM is a core impairment behind the communication and socialization deficits in ASD is increasingly disputed (e.g., Chevallier et al., 2012; Scheeren, de Rosnay, Koot, & Begeer, 2012; Dufour et al., 2013). Here, we reasoned that since ToM tasks are typically administered by a human experimenter, it is conceivable that at least part of the performance gap reported in the literature can be explained by differences in sensitivity to the presence of an observer. When testing ToM, an experimenter typically sits across from the child and presents scenarios involving mental state attribution using vignettes or puppets. Given deep-seated differences in social sensitivity between ASD and TDCs, this experimental context may not be comparable across groups. Specifically, TDCs might perform better in this social context due to an intrinsic motivation to please the observer. The goal of the present study was to assess whether differential susceptibility to the audience effect might account for the apparent deficit in ToM reported in ASD.
The audience effect
Social psychologists have long demonstrated that people behave differently when they are being observed (Leary & Allen, 2010): The mere presence of another person (or subtle signs indicative of a social presence) has effects on a multitude of tasks, including reduced stroop interference (Huguet, Galvaing, Monteil, & Dumas, 1999), increased prosocial behaviors during economic games (Haley & Fessler, 2005) and real life settings (Bateson, Nettle, & Roberts, 2006; Ernest-Jones, Nettle, & Bateson, 2011), enhanced stimulus detection (Platania & Moran, 2001) and improved video game performance (Bowman, Weber, Tamborini, & Sherry, 2013). Evidence of such ‘audience effects’ can be found early in development: children listening to a funny story laugh more when another child is in the room than when they are by themselves (Chapman, 1973), and EEG studies have confirmed that children’s brains respond to the presence of an observer in the earliest stages of processing a stimulus (Kim, Iwaki, Uno, & Fujita, 2005). Such sensitivity to the presence of others is important for managing successful social relationships, and comes with obvious benefits. Individuals who are seen as working hard, doing well, or being generous, send a signal that distinguishes them as good partners; and this ultimately increases their chances of being included in future (potentially fruitful) interactions. In line with this idea, evolutionary psychologists have traced back humans’ sensitivity to the presence of others to the ultimate necessity to manage one’s reputation in a highly cooperative environment (Cronk & Leech, 2012).
Given this extensive literature, we reasoned that because children with ASD are less engaged in reputation management than TDCs (and thus less susceptible to audience effects), their performance should look comparatively worse when performing in a social context, but should be otherwise equal. In other words, TDCs might have an advantage over children with ASDs when an audience is present due to increased social motivation. In this study, children were given a ToM task under one of two testing conditions: social (administration by a human being) or non-social (administration by a computer). We predicted that the presence of a human experimenter would result in relatively better performance in the TDC group (i.e., their scores would be higher in the human condition than in the computer condition). In the ASD group, in contrast, we predicted that this social facilitation effect would be absent or diminished, leading to equal performance in the computer and human administration conditions and a condition-specific ToM deficit compared to TDCs.
Methods
Participants
Our sample was drawn from a larger pool of subjects who volunteered to come to our lab to participate in a study investigating behavioral, brain and genetic correlates of social deficits in ASD. In order to increase the probability that the community diagnosis was accurate, participants were screened using caregivers’ responses to the Social Communication Questionnaire (Rutter, Bailey, & Lord, 2003) and to our detailed medical history form. The scientific goal of this larger study was to recruit a highly heterogeneous sample of youth who met gold standard research diagnostic criteria for ASD in order to investigate the genetic and brain correlates of the heterogeneous ASD clinical presentation.
In the current study, however, participants were only included if they met the following stricter criteria: no intellectual disability, no uncorrected auditory or visual impairment, no known genetic conditions, no history of traumatic brain injury, no evidence of birth related injury, no other significant medical or neurological abnormality, and evidence of a good understanding of the task based on test notes and above chance performance in the control condition assessing physical causality between objects. All participants were native speakers of English. Members of the TDC group were seen by licensed clinical psychologists who ruled out the presence of DSM-IV-TR Axis I disorders based on clinical judgment, review of the child’s medical history form and parent interview. Current diagnosis of autism was confirmed by expert clinical judgment, based on parent-reported developmental history (Autism Diagnostic Interview-Revised: ADI-R; Rutter et al., 2003) and symptom presentation (Autism Diagnostic Observation Schedule: ADOS; Lord et al., 2000). Our final sample included a total of 154 children (122 males). Seventy-seven (46 in the Computer condition, 31 in the Human condition) were diagnosed with an ASD and matched individually to control participants based on chronological age (plus or minus one year), full scale IQ and verbal IQ (plus or minus 10 points), according to the DAS-II (see Table 1). The groups did not differ on gender ratio.
Table 1.
Participant characteristics
| ASD (n=77) | TDC (n=77) | |||
|---|---|---|---|---|
| Mean(SD) | Mean(SD) | Significance | ||
| Computer | Age | 10.0 (2.4) | 10.0 (2.3) | t(90) = −0.08, p = .94 |
| FSIQ | 109.3 (15.1) | 108.7 (14.7) | t (90) = 0.20, p = .84 | |
| VIQ | 108. 7 (14.6) | 111.0 (13.9) | t (90) = −0.80, p = .43 | |
| NVIQ | 109.1 (15.5) | 104.8 (13.9) | t (90) = 1.39, p = .17 | |
| Sex | M=41, F=5 | M=40, F=6 | χ2(1) = 0.10, p = 1.00 | |
| Human | Age | 9.7 (2.7) | 9.8 (2.7) | t(60) = −0.09, p = .93 |
| FSIQ | 107.5 (11.0) | 109.6 (10.1) | t(60) = −0.78, p = .44 | |
| VIQ | 108.2 (10.1) | 109.3 (10.0) | t(60) = −0.43, p = .67 | |
| NVIQ | 107.4 (13.0) | 105.7 (11.2) | t(60) = 0.57, p = .57 | |
| Sex | M=24, F=7 | M=17, F=14 | χ2(1) = 3.52, p = .11 | |
| Total | Age | 9.9 (2.5) | 9.9 (2.5) | t(152) = −0.12, p = .91 |
| FSIQ | 108.6 (13.5) | 109.1 (13.0) | t(152) = −0.23, p = .82 | |
| VIQ | 108.5 (12.9) | 110.3 (12.4) | t(152) = −0.91, p = .36 | |
| NVIQ | 108.4 (14.5) | 105.1 (12.8) | t(152) = 1.48, p = .14 | |
| Sex | M= 65, F=12 | M=57, F=20 | χ2(1) = 2.53, p = .16 | |
Stimuli
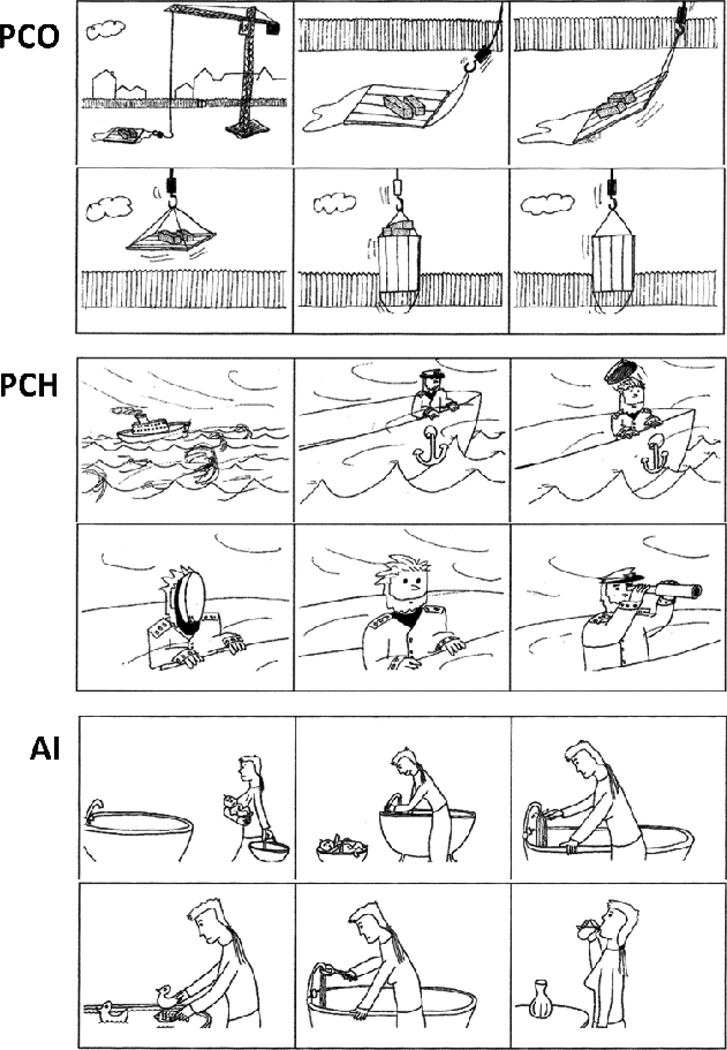
Forty-eight comic-strip-style storylines and their corresponding response vignettes were included (vignettes were designed by Brunet et al., 2003 and used to collect data for the present study with permission). These tapped three different aspects of understanding (16 stories of each type): Physical causality between objects (PCO), which allowed us to assess basic task demands; Physical causality with people as agents in the story (PCH), which allowed us to assess the impact of the presence of social characters within the stories; and attribution of intentions (AI), which probed ToM directly by requiring participants to attribute intentions to the story character and predict his or her actions accordingly (see Figure 1 for examples).
Figure 1.
Storyline and response options for PCO (physical causality – objects, correct answer on the right), PCH (physical causality – humans, correct answer in the middle) and AI (Attribution of intentions correct answer on the left).
Procedure
In the Human condition, a trained female experimenter provided instructions for the task and presented stories in a fixed-random order. In the Computer condition, the instructions and the stories were presented on a touchscreen computer using E-Prime (in the same fixed-random order as the Human condition). An experimenter was present in the room in the Computer condition, but out of direct line of sight, provided no feedback and was otherwise uninvolved in the task. A pre-recorded female voice provided all feedback for the task. Each story consisted of a series of three-panel comic-strip-style storylines, followed by another three pictures from which the child chose the most appropriate ending to complete the narrative. The child’s attention was drawn to the three pictures that composed the storyline (“Look at the story carefully”) either by the experimenter or by the recorded voice on the computer. After approximately five seconds, the matching answer strip (also three pictures) was shown by the experimenter or on the computer screen and the children heard: “Now pick one of these three options to best finish the story”. Prior to the test phase, children were given six practice items, two from each of the three conditions. Performance on the practice items was reinforced with comments like “good” or “that’s right” if children responded correctly, and the correct answer was briefly explained if they answered incorrectly. The explanation was provided by the experimenter in the Human condition and by the pre-recorded voice in the Computer version. In brief, the key differences between the Computer and the Human conditions were the way in which the information was transmitted to the participant and the possibility of establishing a rapport with between the participant and the experimenter.
Results
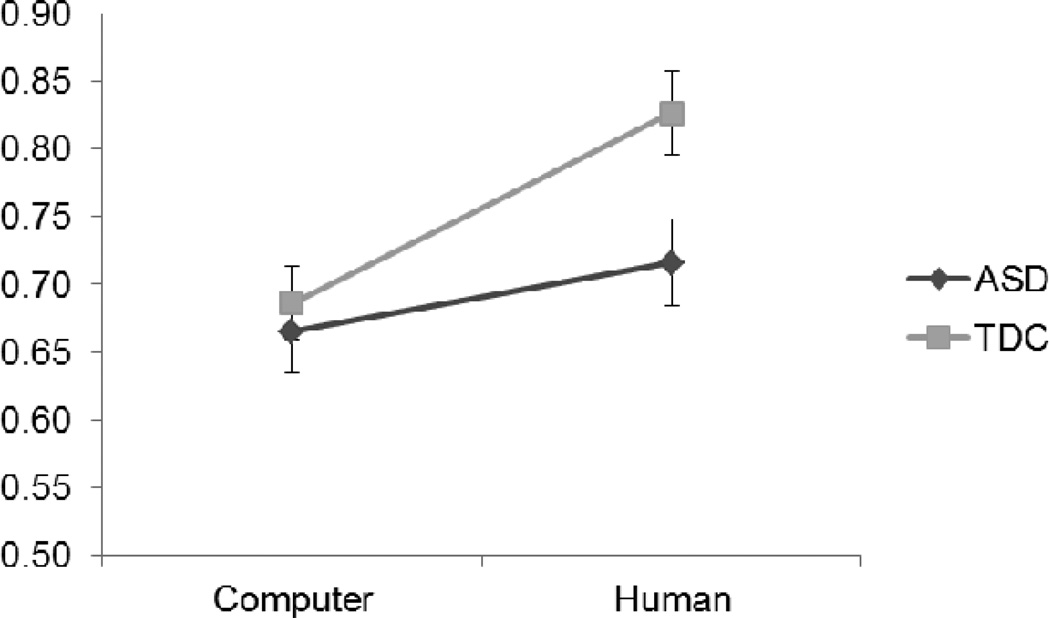
Preliminary analyses revealed no effect of gender on performance, so subsequent analyses were collapsed across this variable. A repeated-measures GLM with Diagnosis (ASD vs. TDC) and Condition (Computer vs. Human) as between-groups variable and question Type (PCO, PCH, AI) as a within-groups repeated measure was computed. A Mauchly’s test revealed that the data did not meet the sphericity assumption so we report Greenhouse-Geisser corrected values for the univariate analysis. The GLM revealed no main effect of Diagnosis, F(1, 150) = 1.64, p = .202, ηp2 = .012, a main effect of question Type, F(2, 149) = 196.71, p < .001, ηp2 = .57, and Condition, F(1, 150) = 6.38, p = .013, ηp2 = .041, a question Type × Diagnosis interaction, F(2, 149) = 5.21, p = .011, ηp2 = .034, a question Type × Condition interaction, F(2, 149) = 7.67, p = .002, ηp2 = .049 and a question Type × Condition × Diagnosis interaction, F(2, 149) = 3.21, p = .054, ηp2 = .021. The Type × Diagnosis interaction was due to ASD participants having lower scores in the AI condition than TDC participants and the Type × Condition interaction was due to the AI condition being harder overall than the PCH and PCO condition. Planned comparisons revealed that significant effects in the three way interaction were concentrated on the AI question type in the Human condition (see Figure 2), with TDCs performing better than in the Computer condition, t(75) = 3.40, p = .001, d=0.79 and better than ASDs, t(60) = −2.50, p = .015, d=−0.65 (see Table 2). By contrast ASD performance did not improve with human administration, t(75) = −1.12, p = .267, d=0.26. Performance in the PCH and in the PCO condition was equivalent for the two groups in the Human and in the Computer condition, PCH: t(152) = −1.75, p = .09, d=0.30, PCO: t(152) = −0.72, p = .474, d=0.12. Importantly, PCH and PCO trials gave rise to near-ceiling performance (above 90%), which may have precluded our ability to detect possible audience effects in these conditions.
Figure 2.
Rate of correct responses in the Computer vs. Human condition for the ASD group (dark line) compared to the TDC group (light line) in the Attribution of Intention condition.
Table 2.
Mean accuracy in the PCO, PCH and AI conditions for computer administration and human administration.
| ASD (n=77) | TDC (n=77) | |||
|---|---|---|---|---|
| Task | Mean(SD) | Mean(SD) | Significance | |
| Computer | PCO | 92.39 (10.78) | 92.12 (9.81) | t(90) = 0.13, p = .90 |
| PCH | 87.95 (12.89) | 89.54 (12.58) | t(90) = −0.06, p = .55 | |
| AIT | 66.49 (20.66) | 68.61 (18.19) | t(90) = −0.53, p = .60 | |
| Human | PCO | 93.15 (8.28) | 93.55 (6.75) | t(60) = −0.02, p = .83 |
| PCH | 92.14 (7.90) | 91.73 (8.13) | t(60) = 0.20, p = .84 | |
| AIT | 71.57 (17.81) | 82.66 (17.13) | t(60) = −2.50, p = .02* | |
Discussion
Research on the audience effect dates back to classic literature on social facilitation (Zajonc, 1965). Although the roots of this phenomenon are multi-determined, there is little doubt that a desire for acceptance plays an important role in biasing individuals’ performance when they are being watched (Leary & Allen, 2010). Consistent with this idea, we found that TDCs, who are, on average, more socially motivated than children with ASD, experienced social facilitation in a Theory of Mind task administered by a human being whereas children with ASD did not. When the audience was removed and the task was administered via computer, however, groups performed equivalently. Thus, there are two important findings of this study: 1) ToM deficits in ASD are only evident when the task is administered by a human but not when it is administered by a computer, 2) TDCs benefit from the presence of a human experimenter whereas ASD children do not. We now discuss these two points in turn.
Evidence for a ToM deficit in ASD
The idea that all or most social difficulties in autism are due to a ToM deficit is both highly prominent (Baron-Cohen, Leslie, & Frith, 1985; Baron-Cohen, 1995, 2000; Frith, 2013; White, Hill, Happé, & Frith, 2009; White, Coniston, Rogers, & Frith, 2011) and increasingly disputed (Chevallier, 2012; Chevallier et al., 2012; Dufour et al., 2013; Ponnet, Roeyers, Buysse, De Clercq, & Van Der Heyden, 2004; Scheeren et al., 2012). Here, we found that typically developing children performed better than children with ASD in the human condition but not in the computer condition of a ToM task. One interpretation of these findings is that the performance difference in the human condition does not reflect an actual difference in competence but rather reveals that sensitivity to task context is different for the two groups. This interpretation is in line with the fact that social interactions are –depending on theoretical accounts– confusing, cognitively taxing, or not motivating for individuals with ASD and with the fact that children with autism generally do well in most ToM tasks once they reach a certain verbal mental age (Bowler, 1992; Chevallier, Noveck, Happé, & Wilson, 2011; Fisher, Happé, & Dunn, 2005). Based on this existing body of research, we suggest that when individuals with autism perform less well than the controls on specific tasks, it is vital to consider the possibility that their difficulties are the result of differential sensitivity to task demands rather than to always assume that differences are due to diminished competence. To take a concrete example, in Chevallier, Molesworth and Happé’s 2012 study, children with ASD consistently provided lower ratings than TDCs for a set of pictures drawn by the experimenter. Taken in isolation, these results could be taken to indicate that children with autism are more negative overall or show diminished appreciation of the aesthetic value of drawings. However, the control condition, which requires rating pictures that were not drawn by the experimenter, reveals that ASD and TDC participants provide comparable ratings. The results therefore indicate that the most parsimonious interpretation is that the presence of the artist who drew the pictures in the first condition triggers higher scores in the TDC group but not in the ASD group (Chevallier et al., 2012).
It is important to note, however, that this interpretation relies on the premise that the computer condition is equally motivating for both groups. An alternative explanation for our findings is that typically developing children are in fact not optimally motivated during the computer condition while ASD children are. If TDC performance in the computer condition is not a valid baseline against which to compare the performance of children with ASD, the absence of a between-group difference during this condition would reflect a decrement in the performance of the TDC group, and it would follow that the TDC group shows their overall ToM skill more accurately in the Human condition. It is plausible that the ASD group would show performance equal to the TDC group with tailored incentives, e.g., money or a competitive context, as has been shown in some recent research (Peterson, Slaughter, Peterson, & Premack, 2013). In Peterson et al.’s study, for instance, children with ASD failed a standard ToM task, but performance improved drastically when the same children participated in a novel test involving competition to win a reward as the motive for tracking other players’ beliefs (Peterson, Slaughter, Peterson, & Premack, 2013). In the present study, it would have been interesting to include a similar third condition, but in the absence of such a condition, the idea that the results in the Human condition suggest real ToM deficits in the ASD group is a logical alternative. However, our current interpretation fits well with existing data on ToM skills in high functioning autism and with Peterson et al.’s recent work demonstrating that adequate incentives can boost ToM skills in ASD.
Finally, we would like to emphasize that the abstract ability to attribute intentions and mental states (e.g., the computer condition of our task) may not translate to real world settings. In fact, most instances of mental state attribution occur in interactive settings where an audience is present and the speedy integration of multiple social cues is required. Therefore, the fact that some individuals with ASD are genuinely capable of attributing thoughts, beliefs and intentions to others in a computerized task is no guarantee that they will make use of these skills in everyday situations (Chevallier, et al. 2011). In particular, one of the challenges of real life situations is that “the individual needs to go about defining a social task as such by paying attention to, and identifying, the relevant aspects of a social situation prior to having an opportunity to use their available social cognitive problem solving skills” (Klin, 2003, p. 347). Although diminished social motivation probably has a limited impact on the emergence of the basic building blocks of social cognition (e.g., recognition of basic emotions or ability to attribute mental states), the development of expert social skills and the fluidity with which an individual is able to use them will likely be hindered by diminished motivation. As Schultz (2005) explained, diminished motivation to attend to social cues early in life leads to fewer exposures to social information, which ultimately precludes the development of social expertise.
Evidence for a diminished audience effect in ASD
The existing literature on audience effects often links improved performance in the presence of an observer to the fact that the individual has noticed (often unconsciously) an opportunity for reputation enhancement. However, other mechanisms may explain this phenomenon. For example, joint attention skills or familiarity and comfort may lead people to be more attentive when they are involved in a dyadic task. Therefore, in order to lend credence to our interpretation that diminished sensitivity to the presence of an observer is linked to diminished concern for reputation, it would have been useful to include direct measures of concern for reputation and self-image, e.g., by using instruments such as the Fear of Negative Evaluation Scale (Rodebaugh et al., 2004), or by collecting and coding video tapes of participants’ behavior during the ToM task. Another way of testing this hypothesis would be to compare different clinical subgroups within ASD where social motivation is thought to be more or less impaired. Indeed, although social motivation is diminished overall in ASD, there appears to be subgroups of individuals with autism (e.g., those falling under the “active-but-odd” subtype; Wing & Gould, 1979) who appear to be motivated to interact with others. If our hypothesis is right, we should find that individuals with such a clinical profile will be affected by the audience effect and will therefore perform better in the human than in the computer condition. In future research, filming participants and experimenters as they interact during the ToM experiment would also enable us to rule out the possibility that the experimenter engages differently, or more fully, with one group than the other.
Despite these limitations, we believe that our explanation for the diminished audience effect in ASD (i.e., blunted sensitivity to reputational opportunities) is reasonable. Indeed, this interpretation fits well with existing data highlighting diminished concern for reputation in autism (Cage et al., 2013; Chevallier et al., 2012; Izuma et al., 2011) and with a growing body of literature demonstrating diminished social motivation in ASD (Chevallier et al., 2012; G. Dawson et al., 2002; Dichter, Richey, Rittenberg, Sabatino, & Bodfish, 2012; Klin, Jones, Schultz, Volkmar, & Cohen, 2002; Mundy & Neal, 2001). Although these preliminary findings require further investigation and replication, they nonetheless raise the interesting (and novel) possibility that diminished concern for reputation can widen performance gaps between TDCs and ASDs. In our task, for instance, what might have appeared to be a deficit in autism may also be described as a social advantage that control participants gain through social interaction. It is important to note, however, that differences were limited to the attribution of intention condition and that the understanding of physical causality was not facilitated by human administration. We believe that this is best explained by the fact that understanding of physical causality (involving both objects, PCO, and humans, PCH) functioned as a control in our task and appropriately high levels of performance were thus found in both participant groups. It is likely that this near-ceiling effect, with performance hovering around 90% in both physical causality conditions as opposed to 70% in the ToM condition, prevented us from identifying a general facilitatory effect of human test administration.
Understanding whether or not the effect described in the present paper is specific to ToM tasks or constitutes a more general phenomenon has important implications for the way we administer a range of cognitive assessments in ASD. To give just one example, experimenters administering intelligence tests aim to assess children’s ‘true’ competence and they scaffold performance using the appropriate level of support when they feel that the child’s responses do not reflect her potential (Duckworth, Quinn, Lynam, Loeber, & Stouthamer-Loeber, 2011). This is achieved, in part, through the use of non-evaluative social encouragement (e.g., “Tell me more about that” or “Thanks for working so hard!”) which testers are encouraged to use as a lever to help the child demonstrate maximal intellectual performance. This is innocuous insofar as social responsiveness is orthogonal to the dependent variable at stake (intelligence) and in cases where it can be safely assumed that individual variations in reaction to social scaffolding are reasonably small and randomly distributed in the population. However, when comparing populations where social responsiveness varies systematically—such as ASD vs. TDCs—this can act as an important confound because natural social incentives will fail to be equally motivating for both participant groups.
In IQ tests, for instance, it is possible that the commonly reported difference between ASD and TDCs (Baio et al., 2012) can be partially explained by differences in motivation rather than ability (see also, M. Dawson, Soulières, Gernsbacher, & Mottron, 2007). Additionally, one might argue that the various IQ subtests (i.e., block design vs. comprehension) are likely differentially affected by such motivational differences and this might amplify measurement error when assessing intelligence in autism. Interestingly, Wechsler himself insisted on the idea that “factors other than intellectual enter into our concept of general intelligence, and that … what is needed is that these factors be rigorously appraised” (Wechsler, 1950, p. 83).
Beyond IQ testing, taking motivational (or, going back to Wechsler’s terminology, “conative”) factors into account is crucial in many experimental contexts and scholars have long emphasized that individual differences and situational manipulations affect the availability and allocation of cognitive resources (see, e.g., Revelle, 1993, Duckworth et al., 2011). Yet, as Revelle (1993) pointed out, “A common assumption when studying human performance is that subjects are alert and optimally motivated…And although individual differences in cognitive ability are assumed to exist, differences in motivation are ignored” (pp. 352 – 353). The present study suggests that taking social motivation into account is indeed crucial when assessing cognitive skills in ASD.
Acknowledgments
This study was funded by grants from the Pennsylvania Department of Health (SAP # 4100047863) and the National Institute of Mental Health (RC1MH08879) awarded to the last author. The authors wish to warmly thank all the children and families who took part in this study. The authors would also like to thank the reviewers and editor who helpfully commented on previous drafts of this paper.
Footnotes
C. Chevallier, J. Parish-Morris, and R.T. Schultz developed the study concept. Testing and data collection were performed by L. Le, N. Tonge and J. Miller. C. Chevallier, J. Parish-Morris performed the data analysis and interpretation under the supervision of J. Miller and R.T. Schultz. C. Chevallier, J. Parish-Morris and N. Tonge drafted the paper. R.T. Schultz and J. Miller provided critical revisions. All authors approved the final version of the paper for submission.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. Vol. 4th. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Baio J ADDM Network Surveillance Year 2008 investigators. Prevalence of Autism Spectrum Disorders — Autism and Developmental Disabilities Monitoring Network, 14 Sites, United States, 2008 (MMWR No. 61(SS03)) 2012:1–19. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/ss6103a1.htm. [PubMed]
- Baron-Cohen S. Mindblindness: An Essay on Autism and Theory of Mind. Cambridge: MIT Press; 1995. [Google Scholar]
- Baron-Cohen S. Theory of mind and autism: a 15-year review. In: Baron-Cohen S, Tager-Flusberg H, Cohen DJ, editors. Understanding other minds: Perspectives from developmental cognitive neuroscience. Oxford: Oxford University Press; 2000. pp. 3–21. [Google Scholar]
- Baron-Cohen S, Leslie A, Frith U. Does the autistic child have a “theory of mind”. Cognition. 1985;21(1):13–125. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bateson M, Nettle D, Roberts G. Cues of being watched enhance cooperation in a real-world setting. Biology letters. 2006;2(3):412–414. doi: 10.1098/rsbl.2006.0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler DM. “Theory of Mind” in Asperger’s Syndrome. Journal of Child Psychology and Psychiatry. 1992;33(5):877–893. doi: 10.1111/j.1469-7610.1992.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Bowman ND, Weber R, Tamborini R, Sherry J. Facilitating Game Play: How Others Affect Performance at and Enjoyment of Video Games. Media Psychology. 2013;16(1):39–64. [Google Scholar]
- Cage E, Pellicano E, Shah P, Bird G. Reputation management: evidence for ability but reduced propensity in Autism. Autism Research. 2013;6(5):433–442. doi: 10.1002/aur.1313. [DOI] [PubMed] [Google Scholar]
- Chapman AJ. Social facilitation of laughter in children. Journal of Experimental Social Psychology. 1973;9(6):528–541. [Google Scholar]
- Chevallier C, Molesworth C, Happé F. Diminished social motivation negatively impacts reputation management: Autism Spectrum Disorders as a case in point. PLoS ONE. 2012 doi: 10.1371/journal.pone.0031107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier C. Theory of mind and autism: Revisiting Baron-Cohen et al.’s Sally-Anne study. In: Slater A, Quinn P, editors. Developmental Psychology: Revisiting the Classic Studies. London: Sage; 2012. [Google Scholar]
- Chevallier Coralie, Kohls G, Troiani V, Brodkin ES, Schultz RT. The social motivation theory of autism. Trends in cognitive sciences. 2012 doi: 10.1016/j.tics.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevallier Coralie, Noveck I, Happé F, Wilson D. What’s in a voice? Prosody as a test case for the Theory of Mind account of autism. Neuropsychologia. 2011;49(3):507–517. doi: 10.1016/j.neuropsychologia.2010.11.042. [DOI] [PubMed] [Google Scholar]
- Cronk L, Leech BL. Meeting at Grand Central: Understanding the Social and Evolutionary Roots of Cooperation. Princeton University Press; 2012. [Google Scholar]
- Dawson G, Carver L, Meltzoff A, Panagiotides H, McPartland J, Webb S. Neural correlates of face recognition in young children with autism spectrum disorder. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson M, Soulières I, Gernsbacher MA, Mottron L. The Level and Nature of Autistic Intelligence. Psychological Science. 2007;18(8):657–662. doi: 10.1111/j.1467-9280.2007.01954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW. Reward Circuitry Function in Autism During Face Anticipation and Outcomes. Journal of Autism and Developmental Disorders. 2012;42(2):147–160. doi: 10.1007/s10803-011-1221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufour N, Redcay E, Young L, Mavros PL, Moran JM, Triantafyllou C, Saxe R. Similar Brain Activation during False Belief Tasks in a Large Sample of Adults with and without Autism. PloS one. 2013;8(9):e75468. doi: 10.1371/journal.pone.0075468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth AL, Quinn PD, Lynam DR, Loeber R, Stouthamer-Loeber M. Role of test motivation in intelligence testing. Proceedings of the National Academy of Sciences. 2011;108(19):7716–7720. doi: 10.1073/pnas.1018601108. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ernest-Jones M, Nettle D, Bateson M. Effects of eye images on everyday cooperative behavior: a field experiment. Evolution and Human Behavior. 2011;32(3):172–178. [Google Scholar]
- Fisher N, Happé F, Dunn J. The relationship between vocabulary, grammar, and false belief task performance in children with autistic spectrum disorders and children with moderate learning difficulties. Journal of Child Psychology and Psychiatry. 2005;46(4):409–419. doi: 10.1111/j.1469-7610.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- Frith U. Are there innate mechanisms that make us social beings? Scripta Varia. 2013;121 [Google Scholar]
- Frith U, Happé F. Language and Communication in Autistic Disorders. Philosophical Transactions: Biological Sciences. 1994;346(1315):97–104. doi: 10.1098/rstb.1994.0133. [DOI] [PubMed] [Google Scholar]
- Haley K, Fessler D. Nobody’ s watching? Subtle cues affect generosity in an anonymous economic game. Evolution and Human Behavior. 2005;26(3):245–256. [Google Scholar]
- Happé F. Communicative competence and theory of mind in autism: a test of relevance theory. Cognition. 1993;48(2):101–119. doi: 10.1016/0010-0277(93)90026-r. [DOI] [PubMed] [Google Scholar]
- Happé F. Understanding Minds and Metaphors: Insights from the Study of Figurative Language in Autism. Metaphor and Symbol. 1995;10(4):275–295. [Google Scholar]
- Huguet P, Galvaing MP, Monteil JM, Dumas F. Social presence effects in the Stroop task: Further evidence for an attentional view of social facilitation. Journal of Personality and Social Psychology. 1999;77(5):1011–1025. doi: 10.1037//0022-3514.77.5.1011. [DOI] [PubMed] [Google Scholar]
- Izuma K, Matsumoto K, Camerer CF, Adolphs R. Insensitivity to social reputation in autism. Proceedings of the National Academy of Sciences. 2011;108(42):17302–17307. doi: 10.1073/pnas.1107038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Iwaki N, Uno H, Fujita T. Error-Related Negativity in Children: Effect of an Observer. Developmental Neuropsychology. 2005;28(3):871–883. doi: 10.1207/s15326942dn2803_7. [DOI] [PubMed] [Google Scholar]
- Klin A. The enactive mind, or from actions to cognition: Lessons from autism. Philosophical Transactions: Biological Sciences, 2003;358(1430):345–360. doi: 10.1098/rstb.2002.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klin A, Jones W, Schultz R, Volkmar F, Cohen D. Visual Fixation Patterns During Viewing of Naturalistic Social Situations as Predictors of Social Competence in Individuals With Autism. Archives of General Psychiatry. 2002;59(9):809–816. doi: 10.1001/archpsyc.59.9.809. [DOI] [PubMed] [Google Scholar]
- Leary MR, Allen AB. Social Motivation. New York, NY: Psychology Press; 2010. Belonging Motivation Establishing, Maintaining, and Repairing Relational Value; pp. 37–55. (David Dunning) [Google Scholar]
- Leslie A. Pretense and representation: The origins of “theory of mind”. Psychological Review. 1987;94(4):412–426. [Google Scholar]
- Mundy P, Neal A. Neural plasticity, joint attention, and a transactional social-orienting model of autism. International review of research in mental retardation. 2001;23:139–168. [Google Scholar]
- Norbury C. The relationship between theory of mind and metaphor: Evidence from children with language impairment and autistic spectrum disorder. British Journal of Developmental Psychology. 2005;23:383–399. [Google Scholar]
- Peterson CC, Slaughter V, Peterson J, Premack D. Children with autism can track others' beliefs in a competitive game. Developmental science. 2013 doi: 10.1111/desc.12040. [DOI] [PubMed] [Google Scholar]
- Platania J, Moran GP. Social facilitation as a function of the mere presence of others. The Journal of social psychology. 2001;141(2):190–197. doi: 10.1080/00224540109600546. [DOI] [PubMed] [Google Scholar]
- Ponnet KS, Roeyers H, Buysse A, De Clercq A, Van Der Heyden E. Advanced Mind-Reading in Adults with Asperger Syndrome. Autism. 2004;8(3):249. doi: 10.1177/1362361304045214. [DOI] [PubMed] [Google Scholar]
- Revelle W. Individual differences in personality and motivation: “noncognitive” determinants of cognitive performance. In: Baddeley AD, Weiskrantz AD, editors. Attention: Selection, Awareness, and Control. A Tribute to Donald Broadbent. Oxford: Oxford University Press; 1993. pp. 346–373. [Google Scholar]
- Rodebaugh TL, Woods CM, Thissen DM, Heimberg RG, Chambless DL, Rapee RM. More information from fewer questions: the factor structure and item properties of the original and brief fear of negative evaluation scale. Psychological assessment. 2004;16(2):169. doi: 10.1037/1040-3590.16.2.169. [DOI] [PubMed] [Google Scholar]
- Rutter M, Bailey A, Lord C. The Social Communication Questionnaire. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Scheeren AM, de Rosnay M, Koot HM, Begeer S. Rethinking theory of mind in high-functioning autism spectrum disorder. Journal of Child Psychology and Psychiatry. 2012 doi: 10.1111/jcpp.12007. [DOI] [PubMed] [Google Scholar]
- Surian L, Siegal M. Language and communication in autism and Asperger syndrome. In: Stemmer B, Whitaker HA, editors. Handbook of neuroscience of language. Amsterdam: Elsevier; 2008. [Google Scholar]
- Wechsler D. Cognitive, conative, and non-intellective intelligence. American Psychologist. 1950;5(3):78. [Google Scholar]
- White S, Hill E, Happé F, Frith U. Revisiting the strange stories: Revealing mentalizing impairments in autism. Child Development. 2009;80(4):1097–1117. doi: 10.1111/j.1467-8624.2009.01319.x. [DOI] [PubMed] [Google Scholar]
- White SJ, Coniston D, Rogers R, Frith U. Developing the Frith-Happé animations: A quick and objective test of Theory of Mind for adults with autism. Autism Research. 2011;4(2):149–154. doi: 10.1002/aur.174. [DOI] [PubMed] [Google Scholar]
- Wing L, Gould J. Severe impairments of social interaction and associated abnormalities in children: Epidemiology and classification. Journal of Autism and Developmental Disorders. 1979;9(1):11–29. doi: 10.1007/BF01531288. [DOI] [PubMed] [Google Scholar]
- Zajonc RB. Social facilitation. University of Michigan: Research Center for Group Dynamics, Institute for Social Research; 1965. [Google Scholar]