Abstract
To reduce maintenance costs, municipalities and schools are starting to replace natural grass fields with a new generation synthetic turf. Unlike Astro-Turf, which was first introduced in the 1960’s, synthetic field turf provides more cushioning to athletes. Part of this cushioning comes from materials like crumb rubber infill, which is manufactured from recycled tires and may contain a variety of chemicals. The goal of this study was to evaluate potential exposures from playing on artificial turf fields and associated risks to trace metals, semivolatile organic compounds (SVOCs), and polycyclic aromatic hydrocarbons (PAHs) by examining typical artificial turf fibers (n=8), different types of infill (n=8), and samples from actual fields (n=7). Three artificial biofluids were prepared which included: lung, sweat, and digestive fluids. Artificial biofluids were hypothesized to yield a more representative estimation of dose than the levels obtained from total extraction methods. PAHs were routinely below the limit of detection across all three biofluids precluding completion of a meaningful risk assessment. No SVOCs were identified at quantifiable levels in any extracts based on a match of their mass spectrum to compounds that are regulated in soil. The metals were measurable but at concentrations for which human health risk was estimated to be low. The study demonstrated that for the products and fields we tested, exposure to infill and artificial turf was generally considered de minimus, with the possible exception of lead for some fields and materials.
1. Introduction
In an effort to reduce costs and increase field use, municipalities across the US and Europe have been replacing natural grass with artificial turf at parks and athletic fields. (1) However, a number of public health concerns have been raised regarding artificial turf fields (2–5) including potential exposure to toxic substances contained in the turf fibers and crumb rubber infill. (6–10) Crumb rubber, which is manufactured from recycled tires, is used to provide cushioning and extend the life of the field. However, it can contain polycyclic aromatic hydrocarbons (PAH), volatile organic chemicals (VOCs), semi volatile organic chemicals (SVOCs), and heavy metals. The concentration of each contaminant can vary depending on the basic composition and age of the parent material used in the infill and turf.(11–14)
Previous studies completed in the US and Europe have provided insight into the composition and safety of primarily older turf material. Levels of PAHs and other SVOCs in the raw in-fill material have been reported to be above health based standards (9, 15) which is not surprising given the in-fill is often composed of used shredded tires which contain many hazardous organic compounds. In one study, measurements of airborne measurements of PAHs, PCBs, PCDDs, and PCDFs at actively used artificial-turf playing fields suggested in excess of one in a million lifetime cancer risk based on BaP air concentrations.(16) Several other studies have evaluated personal and stationary VOC, SVOC, and PM10 air samples near synthetic turf fields without seeing an elevated risk. In a study that collected air samples across five synthetic turf fields (4 outdoor and 1 indoor) in Connecticut no elevated health risk was found for either cancerous or non-cancerous endpoints. (13) A Norwegian study evaluating the risk from exposure to a wide range of contaminants concluded artificial turf fields do not cause any elevated health risk to children or adult athletes. (17) Air and wipe samples were collected for PM2.5 and PM10, VOCs, and SVOCs at artificial fields in New York State. Again, no elevated health risks were identified in air samples collected from on-field or downwind locations. (18) In addition to organic compounds, metals have been identified as a potential contaminant of concern, particular lead which can cause severe permanent neurological health effects in children.(9, 19–21) Lead based coloring agents used in fibers of some artificial turf can be released into the environment as the field ages. Measured concentrations have exceeded the statutory lead limit for consumer products intended for children and EPA lead-dust standard for floors.(19)
A few studies have considered the bio-accessibility of measured contaminants in selected samples of artificial turf fibers or crumb rubber in fill. Bio-accessibility takes into consideration the maximum concentration of an analyte that is soluble in laboratory prepared synthetic bio-fluids. Bio-fluids simulate the pH and composition of sweat, digestive juices, and lung fluid and are more representative of an individual’s overall potential dose than total extraction alone. A study using artificial digestive fluid to extract metals and VOCs from crumb rubber found no increased risk of acute or chronic health effects to children. (10) Zhang et al. (2008) observed high total levels of PAHs and some trace metal in the total extracts. However, it was found that the PAHs had a low bio-accessibility and only between one quarter and one half of the lead and chromium in the turf were bio-accessible.(9)
No published study has considered the bio-accessibility of SVOCs or metals via all three potential exposure routes: dermal, ingestion and inhalation. The goal of this study was to determine whether the bio-accessible fraction of metals and SVOCs found in artificial turf fields exceeded non-cancerous risk-based guidance values for children and adult athletes.
2. Methods
2.1 Turf fibers, crumb rubber infill, and field samples
New crumb infill (n=9) and new turf fiber products (n=8) were obtained from an architectural firm that specialized in artificial turf installation. Actual field samples were collected from seven New Jersey area communities that use crumb rubber infill products for athletic fields. This was accomplished by sweeping the infill material into a clean plastic shovel using new brushes and storing the crumb rubber collected in new Ziploc bags at room temperature until subsampled for analysis. For the selected fields, information was obtained on the manufacturer, age of the field, and general use of the field. All samples underwent the same bio-fluid extraction for SVOCs and metals. The “total” extractable fraction was measured by direct solid phase micro-extraction (SPME) for SVOCs and nitric acid extraction digestion for metals and used as the basis for the bio-accessibility comparisons.
2.2 Bio-fluid extractions
Using prepared synthetic solutions (Table I), bio-accessibility analyses for metals and SVOCs were performed for the following three routes of exposure: digestive system, respiratory system, and dermal. The artificial fluids used in these extracts have been documented to be functional analogs of actual biological fluids in previous studies. (22–26) Briefly, for the digestive system 200 mg of infill or a 4cm × 4cm piece of turf was extracted with 8 ml of the artificial saliva and 100 ml of gastric fluid (pH = 1.4 ± 0.2) for 2 hours at 37°C while being shaken at 30 RPM. Half the extract was then shaken again for 2 h after adding 100 ml of intestinal fluid, adjusted to a pH of 6.5 ± 0.1 and then the two digestive extracts were recombined and shaken for 2 more hours to simulate the digestive process. For the dermal pathway, approximately 200 mg of infill or a 4cm × 4cm piece of turf was extracted with 20 ml of the sweat solution by shaking at 30 RPM in a water bath at 37°C for 1 hr. For the respiratory system, approximately 100 mg of infill or a 4cm × 4cm piece of turf was extracted with 10 ml of the synthetic lung solution by shaking at 30 RPM at 37°C for 24 hours. The metals were extracted using a nitric acid microwave digestion procedure and analyzed by Inductively Coupled Plasma/Mass Spectroscopy (ICP/MS).(26) Mixed standards were prepared in nitric acid daily for the target metals selected based on their prevalence in the turf materials determined by qualitative scans and potential adverse health effect. Quality assurance checks were run routinely.
Table I.
Composition of the Synthetic Biofluids
| Synthetic Biofluid | Composition |
|---|---|
| Ingestion – Digestive Fluid | Saliva: 4mM Calcium Chloride Dehydrate, 0.4%w/v Mucin, 5mM Potassium Chloride,7mM Sodium Chloride,4mM Sodium Phosphate Dibasic, and 17mM Urea |
| Gastric: 30mM Sodium Chloride, 84mM Hydrochloric Acid, and 0.32%(w/v) Pepsin | |
| Intestinal: 200mM Sodium Bicarbonate | |
| Dermal – Sweat | 340mM Sodium Chloride, 330mM Ammonium Chloride, 83mM Urea, 170mM Lactic Acid, and 42mM Glacial Acetic Acid |
| Inhalation - Lung | 10mM Magnesium Chloride, 150mM Sodium Chloride, 4mM Potassium Chloride, 1mM Disodium Phosphate,5mM Sodium Sulfate, 25mM Calcium Chloride,7mM Sodium Acetate, 24mM Sodium Bicarbonate, 3mM Sodium Citrate and 0.20%9(w/v) Dipalmitoyl Lecitin |
The SVOCs were analyzed using a direct solid phase micro extraction (DI-SPME) to concentrate the semi-volatile organic analytes from 10 mL of the biofluid extract followed by GC/MS. The GC/MS ion trap was operated in the EI positive mode and scanned between 40 and 600 amu to obtain maximum information about each peak. Quantification of the PAHs was done by comparison to commercially purchased standards. For the SVOC characterization, the mass spectrum acquired from each analyte was matched to a mass spectrum from the NIST/EPA/NIH 2008 mass spectral library database of more than 64,000 compounds, with a >70% fit required for a positive identification. Approximately half the peaks were found to match an entry in the NIST library. The peaks with the top 10% area abundances were compared to the compounds listed in a series of toxicological databases (US EPA PALs, ATSDR Toxicology Profiles, ATSDR MRL 2010, CERCLA Priority Hazardous List 2007, EPA Final AEGLs, EPA Drinking Water Advisory, and Environment, Health and Safety Online Carcinogens) to identify potential compounds of concern. Of the several hundred compounds identified in the top 10% area abundances the only compound identified that was listed in any of the toxicological databases was toluene. Listings of the compounds in the various biofluids are given in the Supplementary Material. It includes a large number and variety of organic materials identified at very low concentrations but for which no hazard data are currently in toxicological databases used for regulatory purposes.
The nitric acid extraction was conducted by digesting the acid soluble fraction of approximately 0.2g of sample in a microwave pressure vessel containing 2mL of concentrated nitric acid under high temperature and pressure for 30 minutes. A total SVOC analysis was done by directly analyzing several grams of turf or infill by SPME coupled to GC/MS analysis.
2.3 Blanks for QA/QC
A single blank was run with each set of sample extracts. Since the salts used to prepare the artificial biofluids contained trace levels of metals, several of the blanks had detectable metal concentrations. To evaluate whether a blank correction was needed for any metal above the limit of detection the following criteria was used: if the sample value was less than twice the blank value then the sample concentration was replaced by twice the blank value, otherwise the blank value was subtracted from the sample extract concentration (see the Supplementary Material for the blank levels in the different extracts).
Blank samples of each biofluid were also analyzed for SVOCs using the same protocol as those used to extract turf samples. Compounds found to be present in the blank were not included in the list of compounds evaluated for toxicological properties. Standards spiked into blank samples were measured along with other figures of merit when the method was developed. The coefficient of variance (CV) was compound dependent as expected but ranged from 2.3 to 12%. The linearity for the compounds that were quantified were almost all 1 – 50 ppb and the method detection limits ranged from 0.3 to 1 ppb, all well below the quantitation of analyte when reported. Benzo(a) pyrene had a much higher detection limit (40 ppb) as it was not retained well on the fiber. While the expectation of homogeneity of a manufactured product may have been greater than observed, the measurement of a lower level analyte under very inhomogeneous extraction conditions was not unexpected. The CV for the method demonstrated that it was within expected analytical reproducibility.
2.4 Risk Assessment
Based upon the analytical results below, a risk assessment for potential metal exposure was conducted in accordance with standard EPA risk assessment guidance for non-cancerous health endpoints.(27, 28) No risk assessment was conducted for the SVOCs, since no regulated SVOCs were detected in the bioextracts and in most circumstances the values were not quantifiable. Routes of exposure that were considered in this assessment included dermal, ingestion, and inhalation pathways. Values were calculated for field samples only, since they were considered the most representative estimate of exposure. Male and female exposure values were averaged. Exposure was assumed to occur 3 hours per day, 130 days a year, and across five age groups (age ≥ 6 to < 11 years; age ≥ 11 to < 16 years; age ≥ 16 to < 19 years; age ≥ 19 years to < 39 years). For the lead risk assessment we considered two age groups: ≥ 2 to < 6 years and age ≥ 6 to < 7 years. In order to be as conservative as possible, the largest measured concentrations of each analyte were used for the screening values. If all samples were below the limit of detection (LOD) the largest LOD value for that compound was used. EPA recommended age specific body weight were applied to each group to determine dermal, ingestion, and inhalation doses (age ≥ 6 to < 11 years, 31.8 kg; age ≥ 11 to < 16 years, 56.8 kg; age ≥ 16 to < 19 years 71.6 kg; age ≥ 19 years to < 39 years, 80 kg).(28)
Skin contact with the turf from sliding or running on the field was considered as the primary route of dermal exposure. Since dermal exposure can differ by the sport and the amount of equipment and clothing worn by athletes, soccer was considered as a worst case scenario for dermal exposure. Skin adherence factors used in this study were 0.04 mg/cm2 (children playing) and 0.012 mg/cm2 (adult soccer players).(27)The following surface area to body weight averages were used: children age ≥ 6 to < 11 years 352 cm2/kg; children age ≥ 11 to < 16 years 288 cm2/kg; children age ≥ 16 to < 19 years 257 cm2/kg; and adults age ≥ 19 years 246 cm2/kg. (28) Chemical specific skin absorption fractions used were: 0.01 for beryllium, chromium, copper, lead, magnesium, titanium, selenium, silver, and vanadium; 0.03 for arsenic; and 0.001 for cadmium.(27) Dermal reference doses were calculated by the product of the ingestion reference dose and the gastrointestinal absorption factor (arsenic 100%; cadmium 2.5%; chromium 2.5%; copper 67.5%; lead 35%; vanadium 2.6%).(27, 29, 30)
Ingestion could be an important route of exposure to children since they have a greater propensity for hand-to-mouth contact and pica behavior compared to adults; although incidental ingestion by adults during sport related activities cannot be ignored. Ingestion rates used in this risk assessment were 50 mg/day for children and 20 mg/day for adults.(28)
Based upon the study objectives and available resources, air samples were not collected in the laboratory or the field. Therefore, we relied on air measurements collected at artificial turf fields from previous studies to estimate the bio-accessible concentrations of metals for the risk assessment. Two studies were identified that published PM10 (particles with an aerodynamic size <10 μm) concentrations.(31, 14) One field in North Carolina was sampled for PM10 at four different locations on two separate days. Concentrations ranged from 14.2 μg/m3 to 33.4 μg/m3 depending on the field location and day.(31) Five fields in Connecticut were sampled and concentrations ranged from 4.5 μg/m3 to 16.5 μg/m3.(14) To be conservative, we used the largest PM10 concentration for the risk assessment (33.4 μg/m3). This value was then multiplied by the maximum concentration that was found in the lung bio-fluid extract for each metal which in most cases was the LOD. The 95th percentile male and female combined inhalation rate was used for each age strata: children age ≥ 6 to < 11 years 17.0 m3/day; children age ≥ 11 to < 16 years 19.3 m3/day; children age ≥ 16 to < 19 years 20.8 m3/day; and adults age ≥ 19 years 22.4 m3/day.(28) Inhalation reference doses were calculated from inhalation reference concentrations using standard EPA equations. (32)
The EPA does not publish a reference dose for lead since toxicological effects can occur at low concentrations in children. To protect against permanent neurological effects the Centers for Disease Control and Prevention (CDC) recommends that children’s (age 1 to 5 years old) blood lead levels (BLL) not exceed 5 μg/dL.(33) To predict BLL we used the EPA’s Integrated Exposure Uptake Biokinetic Model for Lead in Children (IEUBK) Version 1.1. The model only predicts BLL in children younger than 7 years old. We evaluated two exposure scenarios in this risk assessment: children potentially using artificial turf fields for soccer and other sports (6–7 years old); and younger children that who may also use the field for non-sporting activities (2–6 years old). To calculate BLLs, we used the maximum measured bioavailable lead concentration of 260 mg/kg, a PM10 concentration of 33.4 μg/m3, exposure duration of 3 hours, and a soil ingestion rate of 50 mg/day for both age groups. No other sources of lead exposures were considered and default age specific ventilation rates were used.
3. Analytical Results
3.1 SVOCs
There were some differences in the LOD based on the mass of material extracted and the volume used of each artificial fluid. A larger volume was used for the digestive fluid since that is more representative of actual digestive tract conditions. In general, the detection limits for the various SVOCs and PAHs measured were between <0.1 – <5 mg/kg, <0.01 – <0.5mg/kg and <0.1–<5mg/kg for the sweat, lung and saliva/gastric/intestinal fluid, respectfully. The full listings of the SVOC compounds identified in each of the extracts of the turf samples based on our search for library matches of the mass spectra are given in supplemental materials.
For comparison with previous studies, sixteen PAHs were specifically targeted (Table II). With the exception of acenaphthylene and naphthalene, which were just above their detection limits in a small number of samples, all PAHs quantified in this study were below the LOD. Additionally, several SVOCs which had previously been observed in other turf studies and reported to be hazardous but not regulated in soil, were specifically searched for (benzothiazole, butylated hydroxyanisole, n-hexadecane, and 4-(tert-octyl) phenol). (34, 12) Benzothiazole and 4-tert-octyl phenol, were found in the total extraction at 1.0 mg/kg and 0.2 mg/kg, respectively. The compound 4-tert-ocyl phenol was present in the lung (0.2 mg/kg) and sweat (1.0 mg/kg) biofluids. Although, benzothiazole was not detected in any the biofluids, 2,2 benzothiazole, a dimer of benzothiazole, was identified at the 10 mg/kg in the digestive fluid. In addition, a similar compound Phenol, 2,5-bis(1,1-dimethylethyl)- which is used as a UV stabilizer and has environmental but not reported human toxicity was also present in the total extract at 10mg/kg.
Table II.
Maximum Concentration (mg/kg) of PAHs specifically quantified in the study
| Compound | Sweat | Lung | Digestive | Total Extract |
|---|---|---|---|---|
| Acenaphthene | <0.11 | <0.05 | <0.56 | <0.03 |
| Acenaphthylene | <0.17 | <0.09 | <0.68 | 2.48 |
| Anthracene | <0.08 | <0.04 | <0.42 | <0.02 |
| Azobenzene | <0.49 | <0.24 | <2.5 | <0.12 |
| Benzo[a] anthracene | <0.31 | <0.16 | <1.7 | <0.08 |
| Benzo[a]pyrene | <1.4 | <0.74 | <7.6 | <0.37 |
| Benzo[b] fluoranthene | <1.2 | <0.56 | <6.4 | <0.31 |
| Benzo[k] fluoranthene | <1.9 | <0.69 | <7.2 | <0.34 |
| Carbazole | <0.35 | <0.18 | <1.9 | <0.09 |
| Chrysene | <1.1 | <0.54 | <5.5 | <0.27 |
| Dibenzo[a,h] anthracene | <2.0 | <0.98 | <10 | <0.49 |
| Fluoranthene | <0.11 | <0.06 | <0.62 | <0.03 |
| Fluorene | <.07 | <0.03 | <0.35 | <0.02 |
| Naphthalene | <0.03 | <0.02 | <0.12 | 0.27 |
| Phenanthrene | <0.10 | <0.05 | <0.52 | <0.02 |
| Pyrene | <0.10 | <0.05 | <0.52 | <0.02 |
The total extraction protocol released more organic compounds than were extracted by any individual biofluids. However, the amount of organic material extracted varied greatly across the different compounds. Of the three artificial biofluids, the artificial sweat fluid extracted more compounds, while the least number of organic compounds were present in the digestive fluid. This may be a result of the digestive fluid’s acidic nature, though it did contain the organic compounds mucin, urea, and pepsin. The transfer between the different pHs, which was meant to replicate the digestive process, could also precipitate some larger organic compounds or cause them to adsorb to surfaces.
3.2 Metals
The range of metal concentrations (mg/kg) and the number of samples below the LOD are shown in Table III. The majority of the target metals in most samples were below the LOD (63%) with field samples having a larger percentage of censored observations (71%) compared to the new infill (60%), and new fiber samples (57%). Arsenic, beryllium, cadmium, selenium, and silver were below the limit of detection in all or nearly all the samples for the artificial bio-fluids and the nitric acid digestion. Lead had the smallest percentage of censored measurements (23%), followed by titanium (32%), and vanadium (38%).
Table III.
Number and range (mg/kg) of metal concentrations by extract
| Sweat Bio-fluid Extract
| ||||||
|---|---|---|---|---|---|---|
| Metal | New Infill | New Fiber | Field Sample | |||
| n (< LOD) | Range (mg/kg) | n (< LOD) | Range (mg/kg) | n (< LOD) | Range (mg/kg) | |
| Arsenic | 9 (9) | <0.50 | 7 (7) | <0.10 | 7 (1) | 1.4–1.7 |
| Beryllium | 9 (9) | <0.20 | 7 (7) | <0.20 | 7 (7) | <0.20 |
| Cadmium | 9 (8) | <0.090–0.11 | 7 (7) | <0.030 | 7 (7) | <0.20 |
| Chromium | 9 (0) | 0.70–1.2 | 7 (0) | 0.10–1.3 | 7 (1) | 2.1–2.7 |
| Copper | 9 (5) | <0.080–0.54 | 7 (0) | 0.030–1.6 | 7 (1) | 1.8–2.2 |
| Lead | 9 (0) | 0.090–1.6 | 7 (0) | 0.030–12 | 7 (1) | <0.20–1.5 |
| Magnesium | 9 (2) | <7.0–980 | 7 (0) | 3.3–18 | 7 (7) | <10 |
| Selenium | 9 (9) | <1.9 | 7 (7) | <0.60 | 7 (7) | <0.70 |
| Silver | 9 (9) | <0.10 | 7 (7) | <0.060 | 7 (7) | <0.70 |
| Titanium | 9 (0) | 0.60–1.3 | 7 (0) | 0.10–1.1 | 7 (0) | 3.2–4.0 |
| Vanadium | 9 (0) | 6.0–21 | 7 (0) | 0.50–1.6 | 7 (0) | 15–18 |
|
| ||||||
| Digestive Bio-fluid Extract
| ||||||
| Arsenic | 6 (3) | <0.10–0.48 | 7 (7) | <0.040 | 7 (7) | <3.0 |
| Beryllium | 6 (6) | <0.40 | 7 (7) | <0.40 | 7 (7) | <0.40 |
| Cadmium | 6 (6) | <4.0 | 7 (7) | <0.30 | 7 (0) | 2.5–11 |
| Chromium | 6 (6) | <7.0 | 7 (6) | <0.60–0.74 | 7 (7) | <6.0 |
| Copper | 6 (2) | <20–32 | 7 (6) | <1.0–1.6 | 7 (7) | <20 |
| Lead | 6 (0) | 5.3–66 | 7 (3) | <0.30–4.7 | 7 (0) | 2.5–260 |
| Magnesium | 6 (5) | <1000–4600 | 7 (7) | <90 | 7 (7) | <900 |
| Selenium | 6 (5) | <0.90–1.5 | 7 (7) | <0.10 | 7 (7) | <2.0 |
| Silver | 6 (3) | <0.20–0.23 | 7 (7) | <0.20 | 7 (6) | <0.40–0.90 |
| Titanium | 6 (6) | <10 | 7 (7) | <0.10 | 7 (7) | <10 |
| Vanadium | 6 (6) | <1.0 | 7 (3) | <0.10–0.12 | 7 (7) | <1.0 |
|
| ||||||
| Lung Bio-fluid Extract
| ||||||
| Arsenic | 9 (9) | <0.50 | 4 (4) | <0.20 | 7 (7) | <0.050 |
| Beryllium | 9 (9) | <0.50 | 4 (4) | <0.20 | 7 (7) | <0.030 |
| Cadmium | 9 (9) | <0.20 | 4 (4) | <0.090 | 7 (7) | <0.090 |
| Chromium | 9 (7) | <0.20–0.66 | 4 (1) | <0.090–0.12 | 7 (7) | <0.050 |
| Copper | 9 (7) | <0.40–0.58 | 4 (3) | <0.2–2.0 | 7 (7) | <0.20 |
| Lead | 9 (7) | <0.20–0.26 | 4 (2) | <0.02–0.61 | 7 (5) | <0.020–0.023 |
| Magnesium | 9 (0) | 650–970 | 4 (0) | 77–300 | 7 (7) | <100 |
| Selenium | 9 (9) | <2.0 | 4 (4) | <0.90 | 7 (7) | <0.10 |
| Silver | 9 (9) | <0.50 | 4 (4) | <0.20 | 7 (7) | <0.10 |
| Titanium | 9 (0) | 1.5–6.7 | 4 (0) | 0.20–0.96 | 7 (7) | <0.40 |
| Vanadium | 9 (0) | 0.65– 3.0 | 4 (0) | 0.39–1.5 | 7 (7) | <0.70 |
|
| ||||||
| Nitric Acid Digestion
| ||||||
| Arsenic | 9 (8) | <0.70–0.80 | 8 (5) | <0.040–4.0 | 7 (7) | <0.70 |
| Beryllium | 9 (9) | <0.70 | 8 (6) | <0.040–0.51 | 7 (7) | <0.70 |
| Cadmium | 9 (7) | <0.70–1.1 | 8 (8) | <0.50 | 7 (7) | <0.70 |
| Chromium | 9 (2) | <0.70–16 | 8 (0) | 0.34–820 | 7 (5) | <0.70–0.92 |
| Copper | 9 (1) | <0.70–36 | 8 (0) | 0.69–110 | 7 (0) | 8.8–59 |
| Lead | 9 (3) | <0.010–17 | 8 (0) | 0.53–4400 | 7 (0) | 4.1–140 |
| Magnesium | 9 (2) | <7.0–7800 | 8 (2) | <30–12000 | 7 (3) | <70–160 |
| Selenium | 9 (9) | <1.0 | 8 (6) | <0.10–2.9 | 7 (6) | <0.60–1.3 |
| Silver | 9 (9) | <10 | 8 (8) | <8.0 | 7 (7) | <10 |
| Titanium | 9 (1) | <0.70–18 | 8 (0) | 0.81–820 | 7 (0) | 1.9–9.6 |
| Vanadium | 9 (3) | <0.10–2.1 | 8 (8) | <40 | 7 (1) | <0.80–0.74 |
Lead was detected in almost all field samples for digestive, sweat, and total extraction fluids with digestive fluid extract of one field sample as high as 260 mg/kg. Metal concentrations were not markedly different across the three different sample types (new infill, new fiber, field sample). However, one of the new turf fiber sample contained relatively large concentrations of chromium (820 mg/kg) and lead (4400 mg/kg) compared to the other samples tested. In general, chromium was not detected in the field samples with the majority of measurements below the LOD (71%) across all three bio-fluids and total extracts.
Concentrations found in the different bio-fluids extracts varied and were dependent on the individual metal and sample type. It was found that the nitric acid (50%) extraction yielded lower amounts of lead and cadmium than the digestive bio-fluids extract for several samples. To investigate whether these differences might be due to heterogeneity in the infill and turf material, one field sample, which had a two-fold discrepancy in lead loading between the two methods, was analyzed in triplicate. Two different extraction solutions were used- a 50% nitric acid solution and a more rigorous, destructive nitric acid method in which the infill material was completely digested. The results are given in Table IV and indicate that the variability of lead contained in the infill material is large and can span more than two orders of magnitude.
Table IV.
Repeated measurements of field sample (mg/kg)
| Metal | Nitric Acid (50%) Extract
|
Destructive Nitric Acid
|
||||
|---|---|---|---|---|---|---|
| A | B | C | A | B | C | |
|
|
|
|||||
| Arsenic | 0.800* | 0.800* | 0.800* | 1.40 | 1.86 | 1.57 |
| Beryllium | 0.200* | 0.200* | 0.200* | 0.100* | 0.100* | 0.100* |
| Cadmium | 0.100* | 0.144 | 0.22 | 0.446 | 0.455 | 0.452 |
| Chromium | 0.503 | 1.15 | 0.832 | 1.84 | 1.85 | 1.16 |
| Copper | 5.87 | 7.58 | 8.73 | 9.48 | 15.8 | 5.66 |
| Lead | 0.362 | 19.1 | 2.29 | 6.68 | 16.2 | 7.52 |
| Magnesium | 50.0 | 56.6 | 60.1 | 17.9 | 17.5 | 13.8 |
| Selenium | 0.800* | 0.800* | 0.800* | 5.72 | 8.34 | 6.1 |
| Silver | 0.300* | 0.300* | 0.300* | 0.200* | 0.200* | 0.200* |
| Titanium | 4.12 | 6.07 | 3.09 | 16.7 | 15.1 | 19.2 |
| Vanadium | 0.338 | 0.387 | 0.300* | 1.62 | 1.71 | 1.44 |
Below the LOD
3.3 Risk Analyses
Based upon the above analytical results, risks were calculated for metals that were detected in at least 90% of the samples tested and had a reference dose. The estimated dermal (Table V), ingestion (Table VI), and inhalation (Table VII) dose for each age strata was calculated and compared to the reference dose. The quotient of estimated dose divided by the reference dose was calculated and is shown as the hazard quotient (HQ) (Table VIII). A HQ greater than 1 indicates the potential for an adverse non-cancerous health outcome. In general, for each exposure route, the estimated dose was orders of magnitude smaller than the reference dose. Across all routes of exposure the greatest risk was for children in the lowest age strata. Risk decreased as age increased, with adults having a very small risk compared to youngest age group evaluated.
Table V.
Dermal reference dose (RfD), dermal average daily dose, and hazard quotient (HQ) by metal and age group
| Metal | RfD* mg/kg-day | Age ≥ 6 to < 11 years
|
Age ≥ 11 to < 16 years
|
Age ≥ 16 to < 19 years
|
Age ≥ 19 years
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | ||
| Arsenic | 3.00E-04a | 2.56E-07 | 8.53E-04 | 2.09E-07 | 6.97E-04 | 1.86E-07 | 6.20E-04 | 5.37E-08 | 1.79E-04 |
| Cadmium | 5.00E-05a | 1.00E-09 | 2.00E-05 | 8.21E-10 | 1.64E-05 | 7.30E-10 | 1.46E-05 | 2.10E-10 | 4.21E-06 |
| Chromium | 7.80E-05a | 1.35E-07 | 1.73E-03 | 1.11E-07 | 1.42E-03 | 9.86E-08 | 1.26E-03 | 2.84E-08 | 3.64E-04 |
| Copper | 2.70E-02b | 1.10E-07 | 4.07E-06 | 9.03E-08 | 3.34E-06 | 8.03E-08 | 2.97E-06 | 2.32E-08 | 8.58E-07 |
| Vanadium | 2.60E-04c | 9.02E-07 | 3.47E-03 | 7.39E-07 | 2.84E-03 | 6.57E-07 | 2.53E-03 | 1.89E-07 | 7.29E-04 |
Dermal reference doses were derived from the product of the oral reference dose and the gastrointestinal absorption fraction:
EPA Integrated Risk Information System chronic oral reference dose
EPA Health Effects Assessment Summary Tables chronic oral reference dose
Agency for Toxic Substances and Disease Registry Minimal Risk Levels intermediate oral dose.
Table VI.
Ingestion reference dose (RfD), ingestion average daily dose, and hazard quotient (HQ) by metal and age group
| Metal | RfD* mg/kg-day | Age ≥ 6 to < 11 years
|
Age ≥ 11 to < 16 years
|
Age ≥ 16 to < 19 years
|
Age ≥ 19 years
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | ||
| Arsenic | 3.00E-04a | 2.10E-07 | 7.00E-04 | 1.18E-07 | 3.93E-04 | 9.33E-08 | 3.11E-04 | 3.34E-08 | 1.11E-04 |
| Cadmium | 1.00E-03a | 7.70E-07 | 7.70E-04 | 4.31E-07 | 4.31E-04 | 3.42E-07 | 3.42E-04 | 1.22E-07 | 1.22E-04 |
| Chromium | 3.00E-03a | 4.20E-07 | 1.40E-04 | 2.35E-07 | 7.83E-05 | 1.87E-07 | 6.23E-05 | 6.68E-08 | 2.23E-05 |
| Copper | 4.00E-02b | 1.40E-06 | 3.50E-05 | 7.84E-07 | 1.96E-05 | 6.22E-07 | 1.56E-05 | 2.23E-07 | 5.58E-06 |
| Vanadium | 1.00E-02c | 1.40E-07 | 1.40E-05 | 7.84E-08 | 7.84E-06 | 6.22E-08 | 6.22E-06 | 2.23E-08 | 2.23E-06 |
Oral reference doses are from the following sources:
EPA Integrated Risk Information System chronic oral reference dose
EPA Health Effects Assessment Summary Tables chronic oral reference dose
Agency for Toxic Substances and Disease Registry Minimal Risk Levels intermediate oral dose.
Table VII.
Inhalation reference dose (RfD), inhalation average daily dose, and hazard quotient (HQ) by metal and age group
| Metal | RfD* mg/kg-day | Age ≥ 6 to < 11 years
|
Age ≥ 11 to < 16 years
|
Age ≥ 16 to < 19 years
|
Age ≥ 19 years
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Dose mg/kg-day | HQ | Dose mg/kg-day | HQ | Dose mg/kg- day | HQ | Dose mg/kg- day | HQ | ||
| Arsenic | 4.29E-06a | 3.98E-11 | 9.29E-06 | 2.53E-11 | 5.90E-06 | 1.20E-11 | 2.81E-06 | 2.08E-11 | 4.86E-06 |
| Cadmium | 2.86E-06b | 7.17E-11 | 2.51E-05 | 4.55E-11 | 1.59E-05 | 2.17E-11 | 7.58E-06 | 5.16E-13 | 1.81E-07 |
| Chromium | 2.86E-05C | 3.98E-11 | 1.39E-06 | 2.53E-11 | 8.85E-07 | 1.20E-11 | 4.21E-07 | 2.87E-13 | 1.00E-08 |
| Copper | 2.86E-02d | 1.59E-10 | 5.57E-09 | 1.01E-10 | 3.54E-09 | 4.81E-11 | 1.68E-09 | 1.15E-12 | 4.02E-11 |
| Vanadium | 2.86E-05b | 5.57E-10 | 1.95E-05 | 3.54E-10 | 1.24E-05 | 1.68E-10 | 5.90E-06 | 4.02E-12 | 1.41E-07 |
Inhalation reference doses were derived from the following reference concentrations:
California EPA chronic inhalation reference concentration
ATSDR chronic inhalation reference concentration
EPAReference Concentration for Chronic Inhalation Exposure
California EPA acute inhalation reference concentration
Table VIII.
Hazard index (HI) by metal and age group
| Metal | Age ≥ 6 to < 11 years
|
Age ≥ 11 to < 16 years
|
Age ≥ 16 to < 19 years
|
Adult
|
|---|---|---|---|---|
| HI | HI | HI | HI | |
| Arsenic | 1.56E-03 | 1.10E-03 | 9.34E-04 | 2.95E-04 |
| Cadmium | 8.15E-04 | 4.63E-04 | 3.64E-04 | 1.26E-04 |
| Chromium | 1.87E-03 | 1.50E-03 | 1.32E-03 | 3.87E-04 |
| Copper | 3.91E-05 | 2.29E-05 | 1.86E-05 | 6.44E-06 |
| Vanadium | 3.50E-03 | 2.86E-03 | 2.54E-03 | 7.31E-04 |
Of all the exposure routes, dermal was generally found to be primary route of metal exposure. However, all dermal HQs were three orders of magnitudes less than 1. The inhalation route of exposure was found to be inconsequential for all metals due to poor solubility in lung fluid. The HQs for dermal, ingestion, and inhalation exposures were summed and presented in Table VI as the hazard index (HI). Like the HQ, a HI above 1 represents a potential for adverse health outcomes. No metals quantified in this study had a HI that approached 1 for any age group.
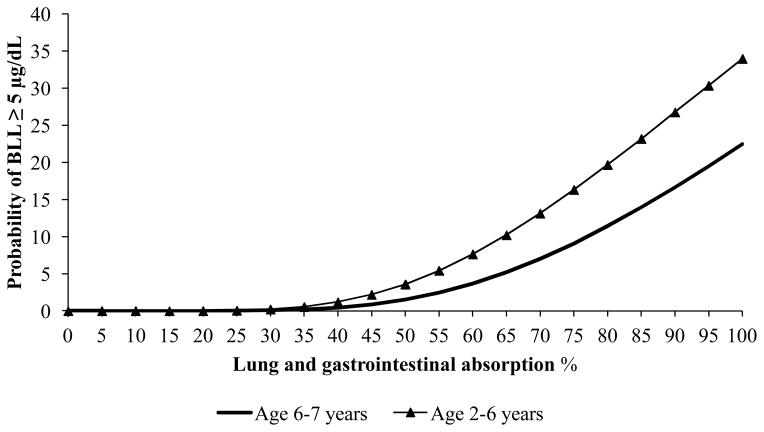
Figure 1 show the probability of a child having a BLL ≥ 5 μg/dL at different lung and gastrointestinal absorption rates using the maximum lead values measured in any biofluid. At a 100% absorption rate the probability of a child having a BLL ≥ 5 μg/dL was 22.5% (6–7 years old) and 34% (2–6 years old). At the EPA recommended gastrointestinal absorption rate (30%) and lung absorption rate (32%) there was less than a 0.5% chance of child’s BLL exceeding 5 μg/dL.
Figure 1.
Lung and gastroinstestinal absorption % vs. probablity of blood lead level (BLL) ≥ 5μg/dL for each age group
4. Discussion
The major driving force for this study was concern over metal and SVOC exposures to adult athletes and children using artificial turf fields. The extraction procedures we used indicated that little of the PAHs and SVOCs present in the new infill material or field samples we collected were bio-accessible across the three biofluids used in this study. Almost every sample we tested was found to be below the LOD for PAHs and none of the levels found in the biofluids or the SPME total analysis exceeded the New Jersey Department of Environmental Protection’s (NJDEP) soil cleanup criteria. (35) Therefore, the risk from long term exposure would be considered insignificant for PAHs.
Two SVOCs, 4-tert-ocyl phenol and butylated hydroxytoluene (BHT), were readily found in the lung and sweat extracts. BHT has few reported adverse toxicological effect and is used as a common component of cosmetics and as a food additive. (36) BHT and 4-tert-ocyl phenol are high volume production compounds and are used in the formation of rubber. They are currently not regulated or present in the toxicological databases searched. (37, 38) Therefore, exposure to those compounds is not expected to be a hazardous above other common environmental exposures.
Concentrations of the targeted SVOCs, including PAHs, measured in the field samples in this study were lower than levels that have been previously reported for actual fields or artificial turf products. (39, 9) Zhang et al. (2008) quantified a similar group of PAHs at eight turf fields across New York State. They reported total PAH levels as high as 38.15 mg/kg in the total extract, while the highest total PAH concentration in this study was 2.48 mg/kg.(9) A second study investigating total PAH content in unused crumb rubber material found concentrations approximately an order of magnitude larger than our study. (39) These differences in reported PAH concentrations may be due to the age of the fields sampled, or differences in the new products that were used in the current study. In the New York State study, the authors found a decrease in PAH concentrations as the field age increased, with highest concentrations found within a few months of the turf being installed. (9) All fields sampled in this study were installed more than two years prior to sampling.
Metals were detected in a large number of biofluids and total extracts. Nevertheless, for almost all the metals we evaluated the risk from exposure was small and below EPA criterion. Excluding lead, all Hazard Indices were three order of magnitude smaller than 1 across all age strata. Assuming exposure was an additive function, the hazard associated with metal exposure would still be inconsequential. Although cancerous health endpoints were not the focus of this study, cancer risk was calculated for metals that had cancer slope factors (arsenic, cadmium, chromium, and lead). The risk of cancer from exposure to all carcinogenic metals compounds was less than 1 chance in 1,000,000 and therefore risk was considered negligible.
One field sample did contain a high lead level (260 mg/kg) which was on the same order of magnitude as the NJ DEP cleanup value (400 mg/kg). Based on National Health and Nutrition Examination Survey (NHANES) only 2.5% of children (age 1–5 years old) in the US have BLLs exceeding 5 μg/dL.(33) Using the maximum lead values obtained from the field measurements, there was 2.5% probability that child’s BLL would exceed 5 μg/dL at a lung and gastrointestinal absorption rate of 55% in children 6–7 years old and 46% in children 2–6 years old. Although these values are larger than the EPA recommended absorption rate (32% and 30% lung and gastrointestinal respectively) our study used artificial bio-fluids which more closely estimates the metal’s solubility in the body compared to total extraction methods. Excluding the single high lead measurement and using the mean lead levels of the six other fields, there was a near 0% probability of a child’s BLL exceeding 5 μg/dL. However, in both exposure scenarios other sources of lead were not considered. Since it is possible that children may be exposed to potentially high concentrations of lead while using artificial turf fields we recommend at a minimum all infill and fibers should be certified for low or no lead content prior to purchase and installation.
We observed a large range in lead measurements within and between fields we sampled. When the high lead field was re-sampled, all measurements were found to be an order of magnitude smaller than the initial measurement. This is likely due to large amounts of variability in the composition of the crumb rubber and not due to the analytical methods. Previous studies have also found a large degree of variability in lead measurements from artificial turf fields. (31, 9) In North Carolina, the US Environmental Protection Agency sampled three fields for lead multiple times. Total extractable lead concentrations in the crumb rubber infill ranged from 11 to 61 mg/kg between samples, with up to a fourfold difference within samples. (31) In New York State, concentrations of lead were less than 6 mg/kg in four field samples, however one field had lead concentrations in excess of 50 mg/kg. (9) Based on results from this study and others, the heterogeneity of the turf and crumb rubber infill can be large and multiple samples from the same field may be needed to accurately characterize lead exposure.
One new fiber sample did contain large concentration of lead (4400 mg/kg) and chromium (820 mg/kg) which exceeded the NJ DEP soil cleanup levels (400 mg/kg and 20 mg/kg respectively). This finding is consistent with another study that sampled turf fibers for lead and found concentrations as large as 700 mg/kg. (31) It is likely that lead-chromate was used as the colorization agent in the turf material, though the Pb/Cr ratio was 5.38, which exceeds the theoretical ratio of lead chromate of 3.98; which may be suggestive of additional lead compounds present in the pigment. The presence of lead and chromium in a new turf material reinforces the need to independently check new fiber materials for the presence of lead-chromate paint before purchase and installation. Further, use of that type of coloring agent should be banned from use in turf/infill. As the turf material degrades from weathering the lead could be released, potentially exposing young children.
This study adds to the growing volume of literature regarding potential exposure to metals and SVOCs from synthetic turf fields. We sampled seven fields, with varying age throughout NJ, as well as unused crumb rubber and fiber samples. Our study is unique in that it considered the bio-accessibility of PAHs, SVOCs, and metal for dermal, ingestion, and inhalation exposure using artificial body fluids for various samples. Results from our study are consistent and expands upon previous studies that have found small concentrations of SVOCs and metals in the infill and fiber material. In addition to quantitative measurements we qualified the risk to metals from chronic exposure across different age strata.
Limitations to this study include possible selection bias and the small number of fields analyzed for comparisons. Since weathering and temperature has been shown to influence concentrations, field measurements may only be generalizable to areas and ages of the fields similar to our study. Additionally, a large amount of variability was found between field samples and different locales may use older or another version of crumb rubber in fill that contains different concentrations of compounds that may still be concern.
5. Conclusions
A comprehensive study was conducted to examine what could be extracted in biofluids from infill and turf products that have been placed on fields and the associated risk from metal and SVOC exposure to children and adult athletes. The extraction procedures included total extraction for metals and SVOCs as well as synthetic sweat, lung, and digestive fluids. Concentrations of PAHs were generally below the limit of detection for all targeted compounds. The SVOCs identified based on library matches of their mass spectra were not present in toxicological databases evaluated and many are ubiquitous part of consumer products. Similarly, the metal concentrations measured in field samples indicate that the risk would be de minimus among all populations expected to use artificial turf fields. However, since there were detectable levels of lead, it is prudent to reemphasize the need to avoid lead-based pigments in these materials as coloring agents and ensure that lead is not in the turf prior to purchase and installation. In the future, the types of bio-accessibily studies conducted as part of these experiments should be completed for all new turf/infill products.
Supplementary Material
Acknowledgments
We would like to thank Shahnaz Alimokhtari and Mordecai Weisel for sample preparation, Dr. Hilly Yang for the organic analyses by SPME/GC/MS and Dr. Elizabeth McCandlish for the metal analysis by ICP/MS. We would also like to thank John S. Butz, RLA of Abel Bainnson Butz, LLP for his expertise on installation of artificial turf fields.
Funding: The study was supported by contract #SHW10-004 from the NJ Department of Environmental Protection, Recycling Program and Planning. Drs. Weisel, Lioy and Buckley are also part of the NIEHS CEED, ES-P30ES05022.
References
- 1.McNitt A. Synthetic turf in the USA - trends and issues. International Turfgrass Society. 2005;10:27–33. [Google Scholar]
- 2.Yaghoobian N, Kleissl J. Modeling the thermal effects of artificial turf on the urban environment. Journal of Applied Meteorology and Climatology. 2010;49:332–345. [Google Scholar]
- 3.Akkaya S, Serinken M, Akkaya N, Türkçüer İ, Uyanik E. Football injuries on synthetic turf fields. Joint Diseases & Related Surgery. 2011;22 (3):155–9. [PubMed] [Google Scholar]
- 4.Stiles VH, James IT, Dixon SJ, Guisasola IN. Natural turf surfaces: The case for continued research. Sports Medicine. 2009;39 (1):65–84. doi: 10.2165/00007256-200939010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Claudio L. Synthetic turf: Health debate takes root. Environmental Health Perspectives. 2008;116 (3):A116. doi: 10.1289/ehp.116-a116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gomes J, Mota H, Bordado J, Cadete M, Sarmento G, Ribeiro A, Baiao M, Fernandes J, Pampulim V, Custódio M, Veloso I. Toxicological assessment of coated versus uncoated rubber granulates obtained from used tires for use in sport facilities. Journal of the Air & Waste Management Association. 2010;60 (6):741–6. doi: 10.3155/1047-3289.60.6.741. [DOI] [PubMed] [Google Scholar]
- 7.Anderson ME, Kirkland KH, Guidotti TL, Rose C. A case study of tire crumb use on playgrounds: Risk analysis and communication when major clinical knowledge gaps exist. Environmental Health Perspectives. 2006;114 (1):1–3. doi: 10.1289/ehp.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lisi RD, Park JK, Stier JC. Mitigating nutrient leaching with a sub-surface drainage layer of granulated tires. Waste Management. 2004;24 (8):831–9. doi: 10.1016/j.wasman.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Zhang JJ, Han IK, Zhang L, Crain W. Hazardous chemicals in synthetic turf materials and their bioaccessibility in digestive fluids. Journal of Exposure Science and Environmental Epidemiology. 2008;18 (6):600–7. doi: 10.1038/jes.2008.55. [DOI] [PubMed] [Google Scholar]
- 10.Office of Environmental Health Hazard Assessment. Health effects of recycled waste tires in playground and track products. California Environmental Protection Agency; 2007. [Google Scholar]
- 11.Li X, Berger W, Musante C, Mattina MI. Characterization of substances released from crumb rubber material used on artificial turf fields. Chemosphere. 2010;80 (3):279–85. doi: 10.1016/j.chemosphere.2010.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg G, Toal B, Kurland T. Benzothiazole toxicity assessment in support of synthetic turf field human health risk assessment. Journal of Toxicology & Environmental Health Part A. 2011;74 (17):1175–83. doi: 10.1080/15287394.2011.586943. [DOI] [PubMed] [Google Scholar]
- 13.Ginsberg G, Toal B, Simcox N, Bracker A, Golembiewski B, Kurland T, Hedman C. Human health risk assessment of synthetic turf fields based upon investigation of five fields in connecticut. Journal of Toxicology & Environmental Health Part A. 2011;74 (17):1150–74. doi: 10.1080/15287394.2011.586942. [DOI] [PubMed] [Google Scholar]
- 14.Simcox NJ, Bracker A, Ginsberg G, Toal B, Golembiewski B, Kurland T, Hedmane C. Synthetic turf field investigation in connecticut. Journal of Toxicology & Environmental Health Part A. 2011;74 (17):1133–49. doi: 10.1080/15287394.2011.586941. [DOI] [PubMed] [Google Scholar]
- 15.Llompart M, Sanchez-Prado L, Pablo Lamas J, et al. Hazardous organic chemicals in rubber recycled tire playgrounds and pavers. Chemosphere. 2012;90 (2):423–431. doi: 10.1016/j.chemosphere.2012.07.053. [DOI] [PubMed] [Google Scholar]
- 16.Menichini E, Abate V, Attias L, De Luca S, di Domenico A, Fochi I, Forte G, Iacovella N, Iamiceli AL, Izzo P, Merli F, Bocca B. Artificial-turf playing fields: Contents of metals, pahs, pcbs, pcdds and pcdfs, inhalation exposure to pahs and related preliminary risk assessment. Science of the Total Environment. 2011;409 (23):4950–7. doi: 10.1016/j.scitotenv.2011.07.042. [DOI] [PubMed] [Google Scholar]
- 17.Bjorge C. Artificial Turf Pitches--An Assessment of the Health Risks for Football Players, Prepared by Norwegian Institute of Public Health and the Radium Hospital. Oslo: 2006. Norwegian Public Health Report. [Google Scholar]
- 18.Lim L, Walker R. An assessment of chemical leaching, releases to air and temperature at crumb-rubber infilled synthetic turf fields. New York State Department of Health; May, 2009. [Google Scholar]
- 19.Van Ulirsch G, Gleason K, Gerstenberger S, Pulliam G, Ahmed T, Fagliano J. Evaluating and regulating lead in synthetic turf. Environmental Health Perspectives. 2010;118 (10):1345. doi: 10.1289/ehp.1002239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lanphear BP, Hornung R, Khoury, Yolton K, Baghurst P, Bellinger BC, Canfield RL, Dietrich KM, Bornschein R, Greene T, Rothenberg SJ, Needleman HL, Schnaas L, Wasserman G, Graziano J, Roberts R. Low-level environmental lead exposure and children’s intellectual function: An international pooled analysis. Environmental Health Perspectives. 2005;113 (7):894. doi: 10.1289/ehp.7688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Betts KS. CDC updates guidelines for children’s lead exposure. Environmental Health Perspectives. 2012;120 (7):a268. doi: 10.1289/ehp.120-a268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamel SC, Ellickson KM, Lioy PJ. The estimation of the bioaccessibility of heavy metals in soils using artificial biofluids by two novel methods: Mass-balance and soil recapture. Science of the Total Environment. 1999;243–244:273–83. doi: 10.1016/s0048-9697(99)00402-7. [DOI] [PubMed] [Google Scholar]
- 23.Ellickson KM, Meeker RJ, Gallo MA, Buckley BT, Lioy PJ. Oral bioavailability of lead and arsenic from a nist standard reference soil material. Archives of Environmental Contamination and Toxicology. 2001;40 (1):128–35. doi: 10.1007/s002440010155. [DOI] [PubMed] [Google Scholar]
- 24.Buckley B, Gilmartin C, Skorupsky S, Ellickson K, Hamel S, Lioy PJ, Meeker RJ, Gallo M. Estimates of bioavailability of metals in soils with synthetic biofluids: Is this a replacement for animal studies?. Presented at Bioavailability, Quantifying the Real Toxicity of Common Soil Contaminants; 1997. [Google Scholar]
- 25.Buckley B, Hamel S, Fang W, Gilmartin C, Lioy PJ. Estimation of the bio-available fraction of metal contaminant in soils using synthetic bio-fluid extraction. Presented at the Society for Risk Analysis and International Society of Exposure Analysis; 1996. [Google Scholar]
- 26.Buckley B, Skorupsky S, Johnson W, Fang W, Rashid I. Measurement of chromium speciation changes in in-vitro bio-availability assays by icp/ms. Presented at Analytical Chemistry and Applied Spectroscopy; 1998. [Google Scholar]
- 27.United States Environmental Protection Agency. Risk assessment guidance for superfund volume i: Human health evaluation manual (part e, supplemental guidance for dermal risk assessment) 2004. [Google Scholar]
- 28.United States Environmental Protection Agency. Exposure factors handbook. 2011. [Google Scholar]
- 29.Barceloux DG, Barceloux D. Copper. Clinical Toxicology. 1999;37 (2):217–30. doi: 10.1081/clt-100102421. [DOI] [PubMed] [Google Scholar]
- 30.Blake K, Mann M. Effect of calcium and phosphorus on the gastrointestinal absorption of pb in man. Environmental Research. 1983;30 (1):188–94. doi: 10.1016/0013-9351(83)90179-2. [DOI] [PubMed] [Google Scholar]
- 31.United States Environmental Protection Agency. A scoping-level field monitoring study of synthetic turf fields and playgrounds. Nov, 2009. [Google Scholar]
- 32.United States Environmental Protection Agency. [Accessed January 1, 2013];Superfund chemical data matrix. Available at: http://www.epa.gov/superfund/sites/npl/hrsres/tools/scdm.htm. Last updated August 01, 2012.
- 33.Advisory Committee on Childhood Lead Poisoning Prevention. Low level lead exposure harms children: A renewed call for primary prevention. Centers for Disease Control and Prevention; 2012. [Google Scholar]
- 34.Ansoborlo E, Henge-Napoli MH, Chazel V, Gibert R, Guilmette RA. Review and critical analysis of available in vitro dissolution tests. Health Physics. 1999;77 (6):638–45. doi: 10.1097/00004032-199912000-00007. [DOI] [PubMed] [Google Scholar]
- 35.New Jersey Department of Environmental Protection. [Accessed January 1, 2013];Residential soil cleanup criteria. Available at: http://www.nj.gov/dep/srp/guidance/scc/. Last Updated: June 4, 2008.
- 36.Lanigan RS, Yamarik TA. Final report on the safety assessment of sorbitan caprylate, sorbitan cocoate, sorbitan diisostearate, sorbitan dioleate, sorbitan distearate, sorbitan isostearate, sorbitan olivate, sorbitan sesquiisostearate, sorbitan sesquistearate, and sorbitan triisostearate. International Journal of Toxicology. 2002;21(Supplemental 1):93–112. doi: 10.1080/10915810290096414. [DOI] [PubMed] [Google Scholar]
- 37.Department of Environment, Food and Rural Affairs (DEFRA) 4-tert-octylphenol risk reduction strategy and analysis of advantages and drawbacks. United Kingdom: 2008. [Google Scholar]
- 38.TOXNET. [Accessed June 22, 2011];Benzothiazole. 2010 Available at: http://toxnet.nlm.nih.gov/cgi-bin/sis/search.
- 39.Gomes J, Mota H, Bordado J, Cadete M, Sarmento G, Ribeiro A, Baiao M, Fernandes J, Pampulim V, Custódio M. Toxicological assessment of coated versus uncoated rubber granulates obtained from used tires for use in sport facilities. Journal of the Air & Waste Management Association. 2010;60 (6):741–6. doi: 10.3155/1047-3289.60.6.741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.