Abstract
Background
Antiretrovirals (ARV) that achieve adequate concentrations in anatomical sites of transmission are of interest for HIV prevention. A Phase 1, open label, pharmacokinetic (PK) study was performed to describe first dose and steady-state PK of the integrase inhibitor dolutegravir (DTG) in blood plasma (BP), cervicovaginal fluid (CVF), cervical tissue (CT), and vaginal tissue (VT) in HIV-1 negative women.
Methods
Eight healthy females given DTG 50mg daily for 5–7d had eleven paired BP and CVF samples collected over 24h following the first dose (PK1) and multiple dosing (PK2). Each woman underwent CT and VT biopsies at 1/4 time points at PK1 and PK2 to generate composite PK profiles. DTG concentrations were analyzed by validated LC-MS/MS methods. Noncompartmental PK analysis was performed and Spearman Rank Correlations determined between matrices.
Results
BP AUCs were similar to previous reports and concentrations remained greater than the protein-adjusted IC90 for wild-type HIV (64 ng/mL). CVF exposures were ~6% of BP with low interindividual variability. CT and VT exposures were 7% of BP at PK1, and 9–10% of BP at PK2 with 94% of samples >PA-IC90. CT and VT concentrations were correlated to each other (rho=0.70, p=0.003), and to CVF at steady state (rho=0.52, p=0.04). Accumulation of DTG from PK1 to PK2 occurred in BP, CT, and VT, but only marginally in CVF.
Conclusions
DTG BP PK were consistent with previously published values. CVF, CT and VT exposures were highly correlated. At PK2, DTG accumulated to a greater extent in tissue than in BP or CVF, suggesting increased tissue affinity.
Keywords: dolutegravir, pharmacokinetics, cervicovaginal fluid, cervical tissue, vaginal tissue, female genital tract
Introduction
GSK1349572 (dolutegravir, DTG) is an integrase strand transfer inhibitor (INSTI) currently in clinical development. It has a 14-hour plasma half-life, which allows for once daily dosing without the need for pharmacokinetic (PK) boosting. In SPRING-1, a Phase 2b dose-ranging study in HIV-infected patients, 88–92% of patients dosed once daily with 10, 25, and 50 mg of DTG had undetectable HIV RNA at 48 weeks, compared with 82% of those taking the non-nucleoside reverse transcriptase inhibitor (NNRTI) efavirenz (EFV) based on the intention-to-treat (ITT) exposed population [1]. These subjects also received either abacavir (ABC)/lamivudine (3TC) or tenofovir (TDF)/emtricitabine (FTC). Two Phase 3 studies have evaluated the use of DTG in treatment naïve patients. In SPRING-2, a double-blind, active control, non-inferiority study, DTG 50mg daily was compared to the first integrase inhibitor to market, raltegravir (RAL) 400mg twice daily, each given with either ABC/3TC or TDF/FTC [2]. Using an FDA snapshot analysis of the ITT exposed population, at 48 weeks, 88% of the patients receiving DTG and 85% of those receiving RAL had HIV RNA suppressed to <50 copies/mL. In SINGLE, a double blind, double dummy, non-inferiority study, DTG given with ABC/3TC was compared to EFV given as the fixed-dose combination with TDF/FTC [3]. Also using an FDA snapshot analysis of the ITT exposed population, at 48 weeks, 88% of DTG recipients and 81% of EFV recipients had HIV RNA suppressed to <50 copies/mL primarily due to the higher discontinuation rates in the EFV arm. Additionally, DTG has been shown to maintain efficacy in raltegravir experienced patients when given twice daily in an open-label cohort study [4]. Based on the promising results of these studies, DTG is a welcome addition to currently available antiretrovirals.
The purpose of this study is to describe first dose and steady state pharmacokinetics of DTG in cervicovaginal fluid (CVF), vaginal tissue (VT) and cervical tissue (CT) compared to blood plasma (BP) in HIV-1 negative women. Although current antiretroviral therapy regimens decrease blood plasma HIV RNA to <50 copies/mL in a majority of patients, the risk of HIV-1 transmission remains, as viral shedding in the female genital tract may be incompletely suppressed [5,6]. To eradicate replication-competent virus at the site of transmission, it is hypothesized that adequate local concentrations of antiretroviral drugs must be achieved [7]. Understanding pharmacokinetic behavior of DTG in multiple female biological compartments will inform its role in preventing viral replication in the genital tract in HIV-infected women (particularly during acute HIV infection and in late-presenting pregnancy), and its potential in protecting mucosal surfaces against HIV infection for applications such as treatment as prevention, and post-exposure prophylaxis. This is the first study of female genital tract antiretroviral pharmacokinetics to be completed prior to market approval.
Methods
Study Design and Population
The study was approved by the UNC Biomedical Institutional Review Board and performed under FDA IND# 112,854. The trial was registered on clinicaltrials.gov under the identifier NCT01404806. All study activities were carried out in accordance with the ethical standards of the International Conference on Harmonisation E6 Good Clinical Practice guidances. Informed consent was obtained on all participants prior to any study activities.
The women underwent screening within 45 days prior to enrollment. Screening procedures consisted of a complete medical history and physical examination; 12-lead electrocardiogram (ECG) with cardiology interpretation, comprehensive laboratory studies (complete blood count with differential, liver function tests, serum chemistries, urinalysis, and urine toxicology). Subjects were screened for active hepatitis B and hepatitis C, HIV, and other sexually transmitted infections such as syphilis, gonorrhea, trichomonas and chlamydia. Women underwent screening prior to study enrollment and DTG dosing. Subjects were eligible to participate if they were females with regular menstrual cycles between 18–35 years of age, inclusive at the date of screening, and had a Body Mass Index (BMI) 18–30kg/m2 with total body weight greater than 50kg. Subjects were required to have fully intact genital and gastrointestinal tracts, with documentation of a normal Pap smear within one year. Subjects were excluded for any clinically significant abnormal lab value, physical examination finding or clinical condition that would interfere with study activities. Subjects were required to stop all prescription and nonprescription medications 7 days before and herbal supplements 14 days before study enrollment, and medications could not be restarted until study completion. Subjects were limited to consumption of less than 14 alcoholic drinks per week [1 drink = 5 ounces (150mL) of wine or 12 ounces (360mL) of beer or 1.5 ounces (45mL) of spirits], and acetaminophen at doses of <1gm/day.
All subjects had to use at least one acceptable form of contraception, including abstinence, bilateral tubal ligation, condoms with spermicide or foam, stable male partner with vasectomy or female only partners, or oral hormonal contraception started at least 3 months prior to enrollment. Subjects agreed to remain abstinent from all sexual activity and insertion of all vaginal products starting 72 hours prior to enrollment until completion of all study activities. While taking DTG, the women were assessed for adverse events and medication adherence daily in person or via telephone and using a standardized head-to-toe assessment form. Vital signs, serum chemistries, CBC, and urine pregnancy test were performed at each visit. All adverse events were graded by the study Physician Assistant and study Physician using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events.
Sample Collection and Processing
Subjects were admitted to the North Carolina Translational and Clinical Sciences (NC TraCS) Institute Clinical and Translational Research Center (CTRC) on day 1 (first dose) and either day 5, 6, or 7 of dosing with DTG 50mg daily. Steady-state conditions were expected to be achieved within 5 days of dosing. Enrollment was scheduled 5–10 days after the end of subject's menses. A witnessed DTG dose was given after an 8 hour fast and standard research meals were provided throughout each 24 hour inpatient sampling period. BP was collected in 3ml K2EDTA tubes (BD Diagnostics, Franklin Lakes, NJ) pre-dose, then at 0.5, 1, 2, 3, 4, 6, 8, 12, 18 and 24 hours following each dose. At those same time points, CVF was self-collected by the women using a volumetric aspiration device. Additionally, BP and CVF samples were collected at 48 and 72 hours following the final dose. Immediately following collection, the CVF was transferred from the vaginal aspirator to a 2mL cryovial and frozen at −80°C. Within 1 hour after blood collection, K2EDTA tubes stored on ice were processed by centrifugation at 800×g at 4°C for 10 minutes. The resultant BP was then transferred to a 2ml cryovial and frozen at −80°C.
Vaginal and cervical tissues were collected by biopsy at one time point during each PK visit. Two women were assigned to each of four time points (either 3, 6, 12, or 24 hours post-dose). Briefly, subjects were placed in the dorsal lithotomy position. After speculum insertion, the vaginal fornices and the entire cervix were anesthetized with topical 20% benzocaine spray (HurriCaine™, Beutlich Pharmaceuticals, Waukegan, IL) and Lidocaine Hydrochloride Oral Topical Solution 2% (Boehringer Ingelheim, Roxane Laboratories, Inc., Columbus, OH). Vaginal tissue biopsies were obtained at either the left or right vaginal fornix. Cervical biopsies were obtained at either the 3 or 9 o'clock position. Biopsies at the second time point were obtained at the opposite location. Approximately 4mm × 2mm × 2mm specimen with a median (range) weight of 0.014 (0.007–0.066) grams, was obtained at both the vaginal and cervical sites using Baby Tischler forceps (Cooper Surgical, Germany). Samples were placed in separate labeled screw-capped polypropylene tubes, immediately snap-frozen in liquid nitrogen, and stored at −80°C until analysis. In order to be considered evaluable, subjects had to provide tissue and 80% of BP and CVF samples, including the pre-dose sample and 24-hour sample at each PK visit.
Laboratory Analysis
Quantification of DTG plasma concentrations was performed by protein precipitation and LC-MS/MS analysis. Calibration curves were obtained using a 1/concentration2 weighted linear regression of analyte:internal standard peak area ratio versus nominal concentration. Compilation of concentration results and descriptive statistical analyses were performed using Sciex Analyst version 1.6.1. Thirty microliters of each stored plasma sample was mixed with 600μL of acetonitrile containing the isotopically-labeled internal standard, dolutegravir-d715N (DTG-IS). Following vortex and centrifugation, a portion of the supernatant was diluted with 50:50 methanol:water prior to LC-MS/MS analysis. DTG was eluted from a Varian (Agilent) Pursuit Diphenyl (2.1 × 50mm, 3μm particle size) analytical column. An API- 5000 triple quadrupole mass spectrometer (AB Sciex, Foster City, CA) was used to detect the analyte. For DTG, the precursor ion was 420m/z and the product ion was 277m/z. For DTG-IS, the precursor ion was 428m/z and the product ion was 283m/z. Data were collected using AB Sciex Analyst Chromatography Software. The dynamic range of the assay was 20–20,000 ng/mL. All calibrators and quality control (QC) samples were within 15% of the nominal value for both within-day and between-day runs. The high, medium, and low QC values used for plasma were 60.0, 600, and 16,000 ng/mL. Within-day and between-day precision calculations were < 15%. The recovery range for DTG in plasma was 98.0–103%, and the recovery of DTG-IS was 104%.
The extraction of DTG from CVF samples required a 5-fold dilution with 0.9% sodium chloride solution prior to analysis to lower the viscosity of the CVF. This dilution allowed for proper vortexing of the sample and facilitated quantitative transfer of the sample to the extraction tube. Calibration standards and QC samples were prepared in 5-fold diluted human CVF. The lower viscosity of the diluted CVF also allowed for accurate preparation of standards and QCs. Since samples were diluted following collection, a 5-x dilution factor was applied to all samples. Thirty microliters of each diluted CVF sample was mixed with 270 μL of acetonitrile containing DTG-IS. Following vortex and centrifugation, a portion of the supernatant was diluted with 50:50 methanol:water. DTG was detected using identical conditions to those described for plasma. The dynamic range of the assay was 1–1,000 ng/mL. The recovery range for DTG in CVF was 99.4–105%, and the recovery of DTG-IS was 101%. All calibrators and quality controls samples were within 15% of the nominal value. The low, medium, and high QC values used for CVF were 3.00, 30.0, and 800 ng/mL. Within-day and between-day precision was < 15%.
In order to extract DTG from VT and CT samples, the tissue samples were initially homogenized in 1mL of 80:20 water:acetonitrile. Fifty microliters of the resulting homogenate was extracted by protein precipitation with acetonitrile containing DTG-IS. Following vortex and centrifugation, a portion of the supernatant was diluted with water. DTG was detected on an LC-MS/MS system using identical conditions as described above. During method validation, calibration standards were prepared in human vaginal tissue homogenate. QC samples were prepared in human vaginal and cervical tissue homogenates. Method validation results indicated that calibration standards prepared in vaginal tissue homogenate could be used successfully to quantitate DTG in both tissues. For sample analysis, calibration standards and QC samples were prepared in human vaginal tissue homogenate. The dynamic range of the assay was 0.2–200 ng/mL homogenate. The recovery range of DTG in vaginal homogenate was 50.7–63.4%, and the recovery of DTG-IS was 72.0%. The recovery range of DTG in cervical homogenate was 48.3–65.9% and the recovery of DTG-IS was 71.4%. All calibrators and quality controls samples were within 15% of the nominal value with precision values < 15%. The low, medium, and high QC values used for tissues were 0.600, 6.00, and 160 ng/mL.
Pharmacokinetic and Statistical Analysis
No formal sample size calculation was performed for this study. The sample size was chosen to generate PK data adequate for understanding penetration of DTG into the female genital tract considering the number sufficient to describe interpatient variability and prior similarly performed PK studies. Noncompartmental analysis was performed using Phoenix Win Nonlin v6.3 (Certara L.P.; St. Louis, MO). Cmax and Tmax were determined by visualization of the concentration-time curves and the area-under-the-concentration-time curve over 24 hours (AUC0–24hr) was determined using the trapezoidal rule (linear up/log down interpolation). BP and CVF half-life (t1/2) was determined by concentrations collected 24–72h after the final dose. Composite AUCs were determined following the first dose (PK1) and at steady state (PK2) for CT and VT using the geometric mean of the two concentrations from each woman at each of the four biopsy time points. Summary statistics and Spearman Rank Correlations between BP and CVF, CVF and GT, and CT and VT, were calculated using SAS 9.3 (SAS Institute Inc; Cary, NC). PK parameters are separated by day and by matrix and PK graphs were made using SigmaPlot 12.0 (Systat Software, Inc; Chicago, IL). To calculate tissue penetration ratios, tissue VT and CT density was assumed to be 1.05 g/mL. Concentrations below the limit of detection (BLD) were imputed as 0 and concentrations below the limit of quantification (BLQ) were imputed as half the lower limit of quantification (LLOQ) for the particular matrix (the lowest point on the standard curve). Accumulation ratios were calculated between PK1 to PK2. Data are presented as median (25th–75th percentile) unless otherwise noted.
Results
Demographics
Eleven women were enrolled to reach the target total of eight women completing pharmacokinetic analysis for both PK1 and PK2. Three women dropped out during PK1 due to an inability to collect at least 80% of the required CVF samples. These three women are included in the adverse event data only. The median age of the eight evaluable women was 21 (range 18–27) years. The median BMI was 22.5 (range 21.0–29.2) kg/m2. All were Caucasian. The median PK profiles for BP and CVF, as well as the composite PK profiles for CT and VT are shown for PK1 (Figure 1) and for PK2 (Figure 2). The pharmacokinetic parameters for all matrices at both PK1 and PK2 are summarized (Table 1).
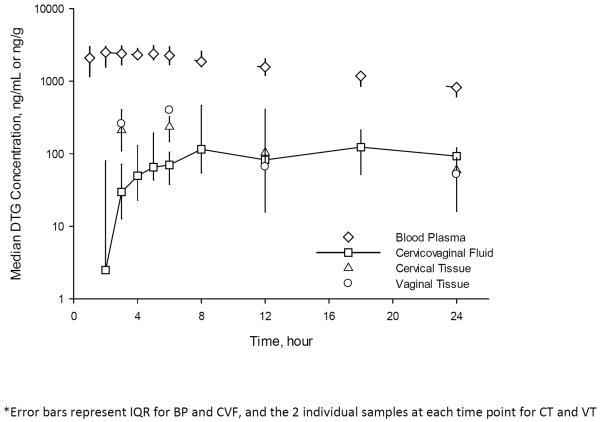
Figure 1. Median (IQR) Concentrations of dolutegravir (DTG) in blood plasma (BP), cervicovaginal fluid (CVF), cervical tissue (CT), and vaginal tissue (VT) following a single dose of DTG.
Following the first dose of DTG, the median (IQR) DTG concentrations at each time point are plotted for BP and CVF and the median (range) DTG concentrations of the two individual subject CT and VT samples at each time point are plotted as a composite pharmacokinetic curve. Error bars represent IQR for BP and CVF, and the 2 individual samples at each time point for CT and VT. The AUC ratio of CVF to BP is 0.07 (0.05–0.18) following the first dose and the AUC ratio of tissue to CVF is 0.09.
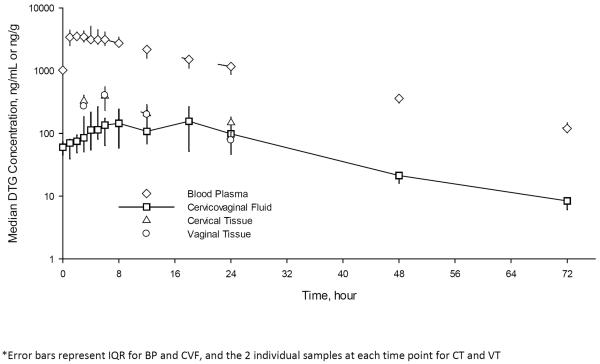
Figure 2. Pharmacokinetics of dolutegravir (DTG) in blood plasma (BP), cervicovaginal fluid (CVF), cervical tissue (CT), and vaginal tissue (VT) at steady state.
At steady state, the median (IQR) DTG concentrations at each time point are plotted for BP and CVF and the median (range) DTG concentrations of the two individual subject CT and VT samples at each time point are plotted as a composite pharmacokinetic curve. Error bars represent IQR for BP and CVF, and the 2 individual samples at each time point for CT and VT. The AUC ratio of CVF to BP is 0.06 (0.04–0.11) at steady state and the tissue concentrations are 46–63% higher than in CVF.
Table 1.
Pharmacokinetic parameters of dolutegravir in blood plasma, cervicovaginal fluid, cervical tissue, and vaginal tissue
| Blood Plasma | PK1 (first dose) Median (IQR) | PK2 (steady state) Median (IQR) | Accumulation ratio Median (IQR) |
| AUC0-24h(ng*h/mL) | 38,300 (31,600–45,400) | 55,400 (43,300–62,200) | 1.42 (1.28–1.50) |
| Clast (ng/mL) | 821 (610–956) | 120 (102–149) | - |
| Cmax (ng/mL) | 3,000 (2,380–3,470) | 3,770 (3,430–5,250) | - |
| Tmax (h) | 2.0 (1.0–4.8) | 2.5 (1.3–4.0) | - |
| t½ (h) | Not calculated* | 14.8 (13.6–16.1) | - |
| Cervicovaginal Fluid | PK1 (first dose) Median (IQR) | PK2 (steady state) (Median (IQR) | Accumulation ratio Median (IQR) |
| AUC0-24h (ng*h/mL) | 2,600 (1,280–8,100) | 3,160 (2,820–5,220) | 0.82 (0.71–1.12) |
| Clast (ng/mL) | 93.1 (41.2–122) | 8.7 (6.6–10.2) | - |
| Cmax (ng/mL) | 149 (84.4–435) | 230 (93.4–347) | - |
| Tmax (h) | 9.9 (5.7–17.9) | 7.9 (3.7–11.9) | - |
| t½ (h) | Not calculated* | 13.5 (11.1–14.9) | - |
| Cervical Tissue | PK1 (first dose) Median (IQR) | PK2 (steady state) Median (IQR) | Accumulation ratio (AUC0-24 PK2/AUC0-24 PK1) |
| AUC10–24h (ng*h/g) | 2,750 | 5,300 | 1.93 |
| Clast (ng/g) | 59.6 (41.6–77.5) | 149 (114–183) | - |
| Cmax (ng/ g) | 236 (148–324) | 395 (232–557) | - |
| Tmax (h) | 6 | 6 | - |
| t½ (h) | Not calculated* | Not calculated* | - |
| Vaginal Tissue | PK1 (first dose) Median (IQR) | PK2 (steady state) Median (IQR) | Accumulation ratio (AUC0-24 PK2/AUC0-24 PK1) |
| AUC0-24h (ng*h/g) | 2,730 | 4,740 | 1.74 |
| Clast (ng/ g) | 52.1 (16.0–88.1) | 77.8 (51.1–105) | - |
| Cmax (ng/ g) | 399 (392–407) | 405 (337–472) | - |
| Tmax (h) | 6 | 6 | - |
| t½ (h) | Not calculated* | Not calculated* | - |
Clast is C24h for PK1 and C72h for PK2
Tissue AUCs are composite geometric means of all subjects
t½ was not calculated for blood plasma or cervicovaginal fluid after the first dose because there was not a clear elimination phase in all subjects by 24 hours and was not calculated for cervical or vaginal tissue at either time point because each individual subject only had one sampling time and concentrations remained high at 24 hours.
Plasma Concentrations
Cmax and Tmax ranged from 1,350–4,380 ng/mL at 1–6 h after the first dose and 2,530–5,590 ng/mL at 1–4 h after multiple dosing. The median (IQR) AUC0-24h for PK1 was 38,300 (31,600–45,400) ng*h/mL and for PK2 was 55,400 (43,300–62,200) ng*h/mL. The half-life (t½) calculated using concentrations from 24–72h post-dose, was 14.8 (13.6–16.1) h. The median (IQR) accumulation ratio of DTG in blood plasma over 7 days of dosing was 1.42 (1.28–1.50).
Cervicovaginal Fluid Concentrations
After a single dose, DTG was detected in cervicovaginal fluid (CVF) at 1 hour post dose in 3/8 women, and at 3 hour post dose in 8/8 women. The median (IQR) AUC0-24h for PK1 was 2,600 (1,280–8,100) ng*h/mL and for PK2 was 3,160 (2,820–5,220) ng*h/mL. Over 24h following the first dose, an elimination phase was not evident in 4/8 women, and a reliable t1/2 could not be calculated. After 5–7 days of dosing, the median (IQR) t1/2 was 13.5 (11.1–14.9) h. The median (IQR) accumulation ratio of DTG in cervicovaginal fluid was 0.82 (0.71–1.12) indicating no significant accumulation in the CVF with multiple dosing. After a single dose, the AUC0-24h penetration ratios of CVF:BP were 0.07 (0.05–0.18); after multiple dosing, the ratios were 0.06 (0.04–0.11). These data demonstrates that CVF exposure is approximately 6–7% of blood plasma exposure.
Genital Tract Tissue Concentrations
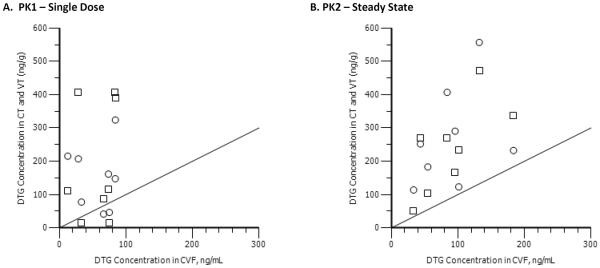
DTG concentrations were similar between CT and VT, after both single and multiple dosing (Spearmans rho=0.70; p=0.003) (Figure 3). For CT, the composite AUC0-24h for PK1 and PK2 was 2,750 ng*h/mL and 5,300 ng*h/mL, respectively. The accumulation ratio was 1.93. For vaginal tissue, the composite AUC0-24h for PK1 and PK2 was 2,730 ng*h/mL and 4,740 ng*h/mL, respectively. The accumulation ratio was 1.74. These data suggest greater drug accumulation of DTG in female genital tract tissues than in blood plasma. Penetration ratios for VT and CT, in relation to blood plasma, were both 0.07 after a single dose, and were 0.09–0.10 after multiple dosing. After a single dose of DTG, VT and CT exposure was nearly double CVF concentrations at the same time points (CT:CVF ratio = 1.97; VT:CVF ratio = 1.95). After multiple dosing, tissue concentrations were 2–3 times higher than CVF (CT:CVF ratio = 2.61; VT:CVF ratio = 2.34). The correlation between CVF and GT was 0.15 (p=0.55) at PK1, but correlation between CVF and GT was 0.52 (p=0.04) at PK2 (Figure 4).
Figure 3. The correlation between cervical tissue (CT) and vaginal tissue (VT) dolutegravir (DTG) concentrations.
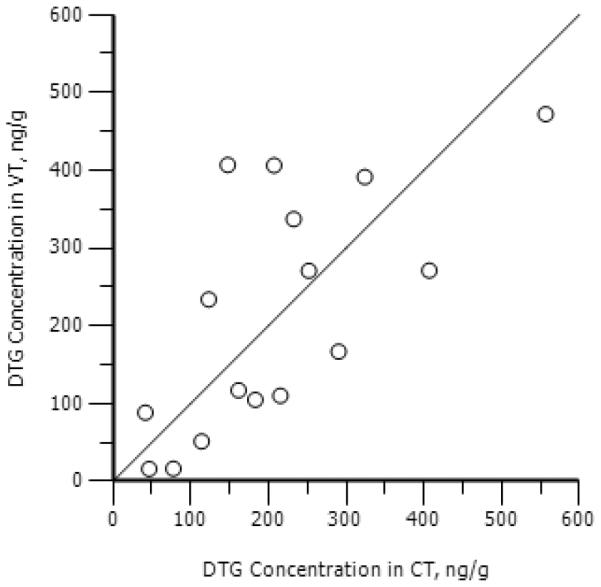
Individual concentration/time points are plotted for DTG in CT and VT. A significant (p=0.003) correlation is noted between CT and VT concentrations (rho=0.70). A line of unity is presented as reference.
Figure 4. The correlation between cervicovaginal fluid (CVF) and genital tissue dolutegravir (DTG) concentrations.
Individual concentration/time points are plotted for DTG in genital tract tissues and in CVF. a) Presents cervical tissue (circles) and vaginal tissues (squares) correlated with CVF following the first dose of DTG. b) Presents vaginal tissue (circles) and vaginal tissues (squares) correlated with CVF after multiple dosing. The correlation between CVF and genital tissues (GT) at PK1 is 0.16 (p=0.55), and between CVF and GT at PK2 is 0.52 (p=0.04). A line of unity is presented as reference.
Adverse Events
Adverse event data include all eleven enrolled women who received at least one dose of DTG. There were no serious adverse events and no women discontinued DTG as the result of an adverse effect. Non-serious adverse events that were grade 1 or 2 and determined to be possibly related to study drug involved 9 events in 8 women, and included headache (2 subjects), drowsiness (1 subject), dizziness/nausea (2 subjects), GI upset with gas (1 subject), lightheadedness upon standing (1 subject), and a grade 2 AST elevation at follow-up (1 subject). One subject experienced scalp tingling and it was unknown whether this was related to DTG. Adverse events that were determined to be unrelated to DTG administration included one episode of fainting in one subject with IV insertion prior to the first dose of DTG, irritation at the IV site (1 subject), and early menses (1 subject).
Discussion
The recently published trial, HPTN052, provided proof that effective antiretroviral treatment of HIV infected individuals decreases the risk of HIV transmission to negative partners by 96% [8]. Evidence from 3 large clinical trials also demonstrates that tenofovir and emtricitabine taken orally by HIV negative individuals can protect against infection through heterosexual or homosexual transmission [9,10,11]. Evidence also exists, however, that not all antiretrovirals penetrate the female genital tract equally well [12] and that viral shedding in the female genital tract can persist in patients who are have undetectable HIV RNA in blood plasma while on antiretroviral therapy [6,7]. Of importance to both treatment as prevention and PrEP strategies, are antiretroviral agents with long half-lives that allow for once daily dosing, with favorable adverse effect profiles and with the ability to penetrate the mucosal compartments involved in HIV transmission (eg the female genital tract).
In this study, BP exposures of DTG after single and multiple dosing were found to be consistent with previously published data [13]. Concentrations above the established protein binding-adjusted IC90 of 64 ng/mL were achieved within 1 h of the first dose and remained well above 64 ng/mL for the entire dosing interval within all subjects [14]. In 7/8 subjects, BP concentrations remained above the IC90 at 72h after the final dose. These data demonstrate prolonged exposure post-dose, and some degree of adherence “forgiveness”. No serious adverse events were seen in this study. The adverse effects that were reported were mild, and did not lead to discontinuation of DTG.
CVF concentrations were detectable 3 hrs after dosing in all subjects, and remained detectable through 72h after the last dose. Overall DTG exposure was approximately 6% of BP, with a CV% of 72% at steady state. In BP, DTG is >99% protein bound. Lopinavir, atazanavir, amprenavir, ritonavir and efavirenz, which are also highly protein bound (>85%), also have low penetration into CVF compared to blood plasma [12,15]. Raltegravir, which has 83% protein binding and exhibits more variable blood plasma pharmacokinetics (CV%=212) [16], has CVF exposures that are 100–400% of BP [17,18,19]. Elvitegravir, the other INSTI currently on the market, has yet to establish female genital tract exposure. However, it is also highly protein bound in BP (98%), and requires a pharmacokinetic booster in order to achieve a half-life adequate for once daily dosing [20].
Protein binding in the CVF was not specifically measured in this study. If we assume equivalent protein binding in CVF as in BP for DTG (>99%), concentrations above the established protein binding-adjusted IC90 of 64 ng/mL were achieved within 6 h of the first dose for 50% of the subjects and remained above 64 ng/mL for the entire dosing interval in those same subjects. However, the major drug binding proteins alpha 1-acid glycoprotein (AAG) and albumin, have concentrations in CVF that are <1% of what is measured in BP [21]. Additionally, we have measured maraviroc CVF protein binding, and have found it to be 10% of what is seen in blood plasma (7.6% versus ~76%) [22,23]. Since the protein-unbound DTG IC50 is 0.21 ng/mL, if at least 90% of DTG in CVF is unbound, it is likely that CVF exposures are above this value in all women by 4 h after a single dose, and maintained in all women after multiple dosing out to at least 72h post-dose.
DTG concentrations in CVF correlated with CT and VT at steady state, suggesting CVF may be a useful surrogate for tissue exposures. However, tissue sampling occurred at only a small number of time points (1 per subject), and should be investigated further to establish the relationship. DTG concentrations were similar in VT and CT, suggesting equal distribution of drug throughout the lower female genital tract. Penetration ratios for VT and CT, in relation to blood plasma, were 0.07 after a single dose and 0.09–0.10 after multiple dosing. Female genital tract tissue:plasma AUC ratios for other antiretrovirals range from 0.6 (tenofovir) to 42 (emtricitabine) [7]. DTG concentrations in these tissues were > IC90 in all eight women at all four steady state time points.
Additionally, a Phase II dose ranging study of dolutegravir identified an Emax model that best explained the relationship between DTG trough concentrations and efficacy. The Emax IC50 was 36 ng/ml. Trough concentrations were above this value in plasma in all subjects at 24 and 72 hours post dose. In CVF, trough concentrations above 36 ng/mL were achieved in 7/8 subjects at 24 hours, but in no subjects by 48 hours. All CT and VT concentrations were greater than 36 ng/mL after multiple dosing [13].
While it is important to investigate the pharmacokinetics in the female genital tract of healthy women, it would also be of interest to determine the pharmacokinetics and viral load suppression achieved in HIV infected women to determine the potential role ofdolutegravir in treatment as prevention. Given the similar plasma concentrations of dolutegravir in both healthy subjects and the HIV infected population, a significant difference in genital tract penetration of dolutegravir would not be expected [13,14].
Conclusions
DTG plasma PK parameters after single and repeat doses were similar to previously reported data. DTG exposure in CVF was 6% of plasma exposure under steady-state conditions. Delayed Tmax was observed in CVF (Tmax=6hr) compared to plasma (Tmax=2hr). There was only marginal accumulation after repeat dosing. DTG exposure in CT and VT were similar and ~ 10% of plasma exposure under steady-state conditions. Higher accumulation of DTG after multiple dosing was noted in both CT and VT compared to BP. DTG concentrations were above the protein binding-adjusted IC90 in 100% of CT and 88% of VT samples. DTG was well tolerated with no significant safety issues observed.
Acknowledgements
The authors would like to acknowledge Trenton Stevens and Maya Wai for contributions to study visit conduct. We would also like to thank the study participants and the UNC Center for AIDS Research. The project was supported by Shionogi-Viiv Healthcare, the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Award Number UL1TR000083. Sources of Funding: Shionogi-Viiv; UNC Center for AIDS Research (5P30AI050410-13; CS, JBD, ADMK), NC TraCS CTSA Grant (UL1TR000083), K23AI077355 (KBP), K23AI093156 (JBD), U01AI095031 (ADMK)
WinNonLin was provided to faculty and trainees in the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, by Certara as a member of the Pharsight Academic Center of Excellence Program.
Footnotes
Author Contributions:
JL Adams- subject recruitment and study visit conduct, pharmacokinetic and statistical data analysis, and primary author of manuscript
KB Patterson- study visit conduct, clinical study safety officer, critical review of manuscript
HMA Prince- subject recruitment and study visit conduct, critical review of manuscript
C Sykes- analytical data analysis, critical review of manuscript
BN Greener – study visit conduct, pharmacokinetic and statistical data analysis, critical review of manuscript
JB Dumond- pharmacokinetic and statistical data analysis, critical review of manuscript
ADM Kashuba- study design, analytical and pharmacokinetic data analysis, funding support, critical review of manuscript
Disclosures Conflicts of Interest: Financial support for the study was provided by Shionogi-Viiv Healthcare. Angela Kashuba's spouse is employed by GlaxoSmithKline. The remaining authors have no additional conflicts to declare.
The data was presented at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, USA, Mar 2–5, 2013.
References
- 1.van Lunzen J, Maggiolo F, Arribas JR, et al. Once-daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 2.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013 Jan 7; doi: 10.1016/S0140-6736(12)61853-4. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results--SINGLE (ING114467). 52nd Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 9–12, 2012; Abstract H-556b. [Google Scholar]
- 4.Eron JJ, Clotet B, Durant J, et al. Safety and Efficacy of Dolutegravir in Treatment-Experienced Subjects With Raltegravir-Resistant HIV Type 1 Infection: 24-Week Results of the VIKING Study. J Infect Dis. 2013 Mar;207(5):740–8. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–2098. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graham S, Holte M, Sarah E, et al. Initiation of antiretroviral therapy leads to a rapid decline in cervical and vaginal HIV-1 shedding. AIDS. 2007;21(4):501–507. doi: 10.1097/QAD.0b013e32801424bd. [DOI] [PubMed] [Google Scholar]
- 7.Cohen MS, Muessig KE, Smith MK, Powers KA, Kashuba AD. Antiviral agents and HIV prevention: controversies, conflicts, and consensus. AIDS. 2012 Aug 24;26(13):1585–98. doi: 10.1097/QAD.0b013e3283543e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011 Aug 11;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Damme L, Corneli A, Ahmed K, et al. Preexposure Prophylaxis for HIV Infection among African Women. N Engl J Med. 2012 Aug 2;367(5):411–22. doi: 10.1056/NEJMoa1202614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM, Donnell D, Ndase P, et al. Antiretriviral Prophylaxis for HIV Prevention in Heterosexual Men and Women. N Engl J Med. 2012 Aug 2;367(5):399–410. doi: 10.1056/NEJMoa1108524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral Preexposure Prophylaxis for Heterosexual HIV Transmission in Botswana. N Engl J Med. 2012 Aug 2;367(5):423–34. doi: 10.1056/NEJMoa1110711. [DOI] [PubMed] [Google Scholar]
- 12.Dumond JB, Yeh RF, Patterson KB, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS. 2007;21(14):1899–1907. doi: 10.1097/QAD.0b013e328270385a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25(14):1737–1745. doi: 10.1097/QAD.0b013e32834a1dd9. [DOI] [PubMed] [Google Scholar]
- 14.Min S, Song I, Borland J, et al. Pharmacokinetics and Safety of S/GSK1349572, a Next-Generation HIV Integrase Inhibitor, in Healthy Volunteers. Antimicrob Agents Chemother. 2010;54(1):254–8. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Min SS, Corbett AH, Rezk N, et al. Protease inhibitor and non-nucleoside reverse transcriptase inhibitor concentrations in the genital tract of HIV-1-infected women. JAIDS. 2004;37(5):1577–1580. doi: 10.1097/00126334-200412150-00008. [DOI] [PubMed] [Google Scholar]
- 16.Isentress (Raltegravir) Package Insert. Merck & Co., Inc.; Whitehouse Station, NJ, USA: 2011. [Google Scholar]
- 17.Patterson KB, Prince HA, White N, et al. Pharmacokinetics (PK) of raltegravir (RAL) in the blood plasma (BP) and genital tract (GT) in HIV+ and HIV− women. XVIII International AIDS Conference; Vienna, Austria. 18 – 23 July 2010; Late breaker poster LBPE18. [Google Scholar]
- 18.Jones AE, Talameh J, Patterson K, et al. First-dose and steady-state pharmacokinetics of raltegravir in the genital tract of HIV-negative women. 10th International Workshop on Clinical Pharmacology of HIV Therapy; Amsterdam, Netherlands. April 15–17, 2009; Abstract 0–06. [Google Scholar]
- 19.Clavel C, Peytavin G, Tubiana R, et al. Raltegravir concentrations in the genital tract of HIV-infected women treated with a raltegravir-containing regimen (DIVA 01 Study) Antimicrob Agents Chemother. 2011;55(6):3018–3021. doi: 10.1128/AAC.01460-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramanathan S, Mathia A, German P, Kearney BP. Clinical Pharmacokinetic and Pharmacodynamic Profile of the HIV Integrase Inhibitor Elvitegravir. Clin Pharmacokinet. 2011;50(4):229–244. doi: 10.2165/11584570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Salas Herrera IG, Pearson RM, Turner P. Quantitation of albumin and alpha-1-acid glycoprotein in human cervical mucus. Hum Exp Toxicol. 1991;10(2):137–139. doi: 10.1177/096032719101000209. [DOI] [PubMed] [Google Scholar]
- 22.Dumond JB, Patterson KB, Pecha AL, et al. Maraviroc concentrates in the cervicovaginal fluid and vaginal tissue of HIV-negative women. J Acquir Immune Defic Syndr. 2009;51(5):546–553. doi: 10.1097/QAI.0b013e3181ae69c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selzentry (maraviroc) Package Insert. Pfizer, Freiburg; Germany: 2012. [Google Scholar]