Abstract
Objective
The aim of this study was to determine the expression of S100 positive dendritic cells (DCs) and the relationship with clinicopathologic factors in endometrial carcinoma.
Methods
Samples were collected from 89 patients with endometrial endometrioid adenocarcinoma treated in Pusan National University Hospital from 2004 to 2011. Normal endometrial tissues were obtained from 30 hysterectomized women with benign adnexal masses and served as controls. Paraffin-embedded sections were immunohistochemically stained for S100 was performed, and the number of positive DCs was counted. The relationship of these cells to the stage, histological grade, myometrial invasion, and lymph node metastasis was analyzed.
Results
The proportion of S100-positive DCs in the endometrial endometrioid adenocarcinoma was 31.5% (28/89), which was significantly higher (P<0.05) than in the control group. The proportion of S100-positive DC expression was negatively correlated with the histologic grade, but was not associated with the stage, myometrial invasion, or lymph node metastasis.
Conclusion
High DC density was inversely correlated with histologic grade in endometrial carcinoma. Tumor-infiltrating S100+ DCs may be used as pathologic marker in endometrial carcinoma.
Keywords: Dendritic cells, Endometrial neoplasms, S100
Introduction
Endometrial carcinoma is the most common malignancy of the female genital tract in the United States. In Korea, the incidence of endometrial cancer has been increasing in recent years; the age standardized incidence rate per 100,000 during 2010 was 5.0 [1]. The recent increasing prevalence of risk factors such as obesity and diabetes will result in further increases in the incidence of endometrial carcinoma.
Patients with endometrial cancer generally have a good prognosis due to early presentation with postmenopausal bleeding. In addition, early stage cancer does not spread beyond the uterus. However, recurrent or metastatic endometrial cancers still have a poor prognosis. The improvement of clinical outcomes will require a much better understanding of the processes that inhibit and stimulate cancer progression [2].
Several investigations have assessed different biological variables in tissue and serum from endometrial carcinoma patients to detect biomarkers predicting the clinical outcome. These biomarkers could be used for the stratification of patients for better tailored treatment [3].
Recently, an influencing factor in the clinical outcome in human cancers has been found to be linked with the fight between host immunity and the tumor. One component in the tumor microenvironment that plays central role in antitumor immunity is the dendritic cells (DCs). DCs are recognized as the strongest antigen-presenting cells and are potent in the ability to activate initial T-lymphocytes to initiate immune responses [4]. The presence of a large number of DCs in tumor tissues may therefore suggest a favorable prognosis.
A positive association between tumor-infiltrating DCs and clinical prognosis has been reported in a variety of human solid tumors [5]. The S100+ DCs, in particular, represent one of the important factors reflecting the immune system's ability to inhibit tumor growth. Available evidence indicates that high numbers of infiltrating immune cells in the tumor microenvironment correlate with an improved prognosis for cancer patients [6].
In the present study, we analyzed the tumor infiltration of S100+ DCs to determine whether the presence of DCs was associated with known prognostic factors in endometrial carcinoma. Our data demonstrated that a high rate of infiltration of S100+ DCs was negatively correlated with histologic grade.
Materials and methods
1. Patients and tissue samples
A total of 89 patients with endometrial endometrioid carcinoma who underwent surgery and were diagnosed from 2004 to 2011 were selected from the archives of the Pusan National University Hospital in this study. All patients underwent a total abdominal hysterectomy and a bilateral salpingo-oophorectomy. More extensive treatment with pelvic and/or para-aortic lymph node dissection was performed in case of more advanced disease (stage II and higher) or unfavorable features (grade 2 and higher). H&E stained sections were reviewed and reclassified by World Health Organization guidelines [7]. The following parameters had been evaluated in the tissue materials: histologic type, grade of differentiation, stage, depth of myometrial invasion. This retrospective study was approved by the Ethical Review Committee of Pusan National University Hospital.
2. Immunohistochemistry
The tissue specimens were fixed in 10% formalin and embedded in paraffin. Sections, 4 µm in thickness, were deparaffinized in xylene and rehydrated through a series of graded ethanol. Endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol for 10 minutes. Antigen retrieval was performed by microwaving the slides in citrate buffer (pH 6.0). The sections were then incubated at 4℃ overnight with anti-S100 antibody (rabbit polyclonal, Z0311, 1:400; Dako-Cytomation, Glostrup, Denmark). Immunoreactivity was visualized using 3,3'-diaminobenzidine (Dako-Cytomation). Slides were counterstained with Meyer's hematoxylin. Human Schwannoma tissue was used as a positive control and phosphate-buffered saline without the primary antibody served as a negative control.
3. Evaluation of staining
Each slide was evaluated independently by two pathologists who were blinded to clinical and outcome data. Condensed staining areas in the tumor tissue were selected for observation. Areas with maximally positive cells were observed under a microscope at high magnification (×400) and the numbers of S100-positive DCs were counted. Five fields were selected for every section, and the cell numbers were averaged. The patients were divided into 2 groups based on the median S100+ cell counts. DC numbers 10 or greater per high power fields (HPF) were considered a positive group, whereas the one less than 10/HPF was considered a negative group [8].
4. Statistical analysis
For statistical analysis, SPSS ver. 15.0 (SPSS Inc., Chicago, IL, USA) was used. The χ2 test and Fisher's exact test were used to evaluate the correlation between the expression of S100 and the clinicopathologic parameters. P-values of <0.05 were considered statistically significant.
Results
1. Patients' characteristics
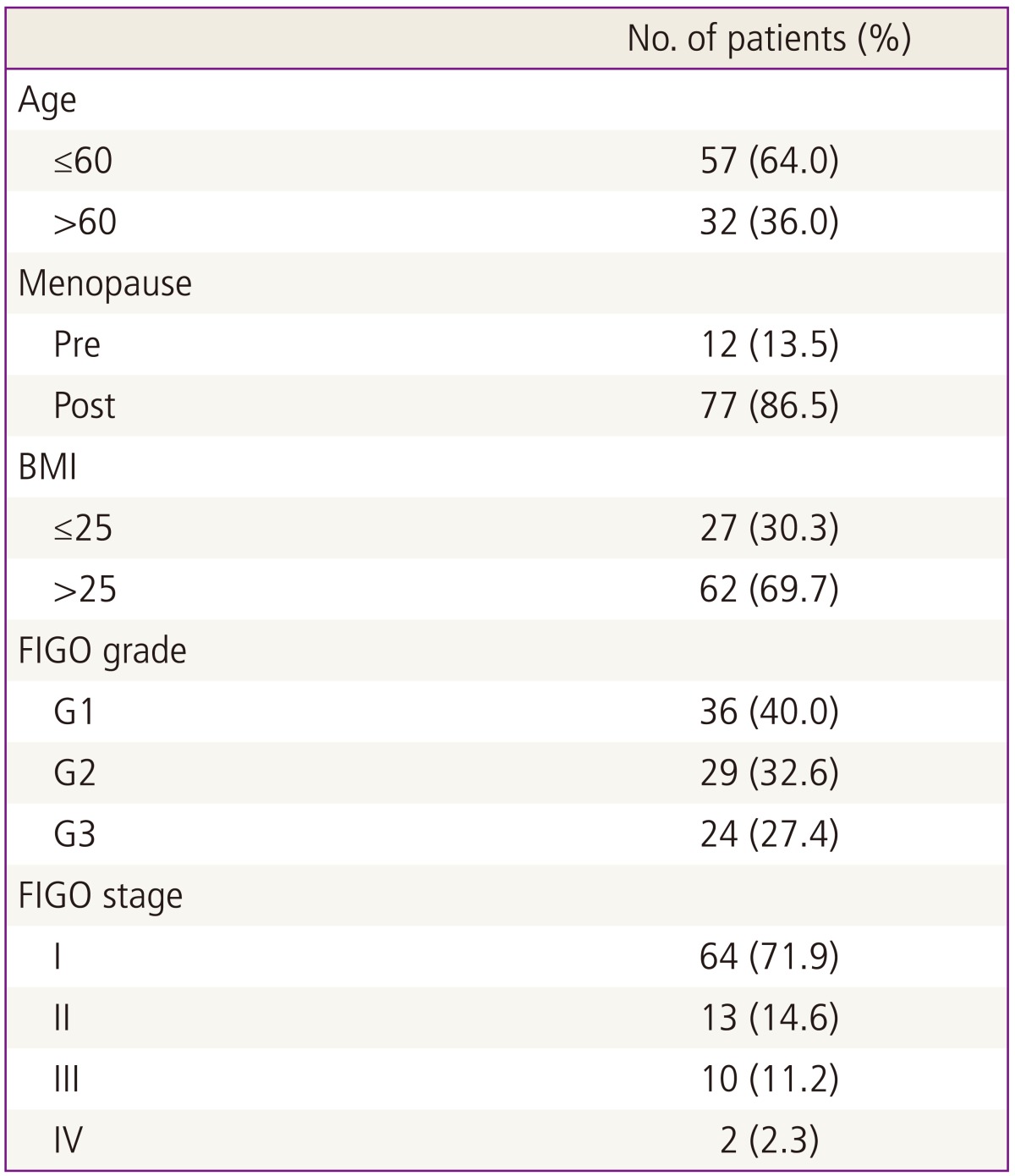
The clinicopathological characteristics of the 89 patients are presented in Table 1. The mean age of the patients was 61 years (range, 40-78 years). Patients with endometrial cancer included 12 with premenopausal status and 77 with postmenopausal status. Clinical follow-up was available for all cases. The median follow-up period was 64 months (range, 8-84 months). Overall, 86.5% of the patients had early-stage disease (I and II) and 13.5% had advanced-stage disease. In addition, 40.4% had well-differentiated cancer (G1), 32.65% had moderately differentiated cancer (G2), and 26.9% had poorly differentiated cancer (G3). None of the patients had undergone radiation or chemotherapy before surgery. A total of 30.3% underwent total hysterectomy and a bilateral salpingo-oophorectomy (BSO) alone, while 69.7% underwent total hysterectomy and BSO and pelvic and/or para-aortic lymph node dissection. No patients had remaining macroscopic tumors at the time of surgery.
Table 1.
Clinicopathological characteristics of the patients with endometrial carcinoma
BMI, body mass index; FIGO, International Federation of Gynaecology and Obstetrics.
2. S100+ dendritic cell infiltration into endometrial carcinoma tissues
S100 antigens were stained in endometrial carcinoma tissues. Expression of S100 protein was noted in the nucleus and/or in the cytoplasm of DCs, appearing as brown granules. Based on the analysis of the DCs, samples were divided into groups; one with less than 10 DCs per mm2 and the other with 10 or more DCs per mm2.
Positive DCs were sparse in normal endometrial tissue. S100+ DCs were observed more frequently in the stroma surrounding tumors than within the tumors. The proportion of S100+ DCs in the endometrial adenocarcinoma was 31.5% (28/89), which was significantly higher (P<0.001) than in the normal endometrial tissues.
3. S100+ dendritic cells infiltration and clinicopathological factors
Fig. 1 shows a representative case of endometrioid endometrial carcinoma that illustrates the distribution pattern of S100+ DCs. The relationships between the proportion of S100+ DCs and clinicopathological variables are assessed and summarized in Table 2. Tumor grade (P<0.001) was significantly associated with the rate of S100+ DCs. Stage (P=0.094), myometrial invasion (P=0.879), and lymph node metastasis (P=0.427) were not significantly associated with the proportion of S100+ DCs. Lymph node positive group are biopsy proven positive cases while lymph node negative group include biopsy proven negative cases and cases with no lymphadenectomy and no significant lymphadenopathy on magnetic resonance imaging.
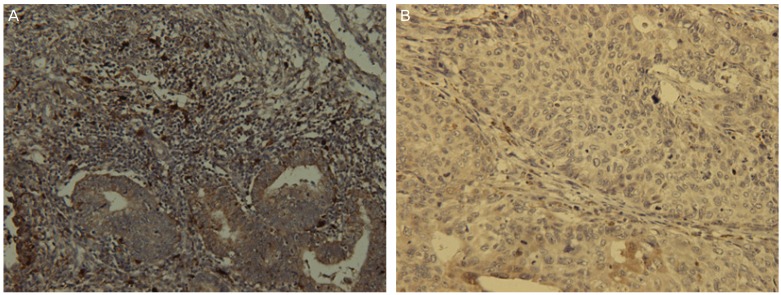
Fig. 1.
Immunohistochemical staining of dendritic cell infiltration in endometrial carcinoma. Representative patient tissues were highly stained for S100 (A). (B) was patient tissues with low S100 cell count (×400).
Table 2.
Association of tumor-infiltrating dendritic cells with clinicopathological parameters
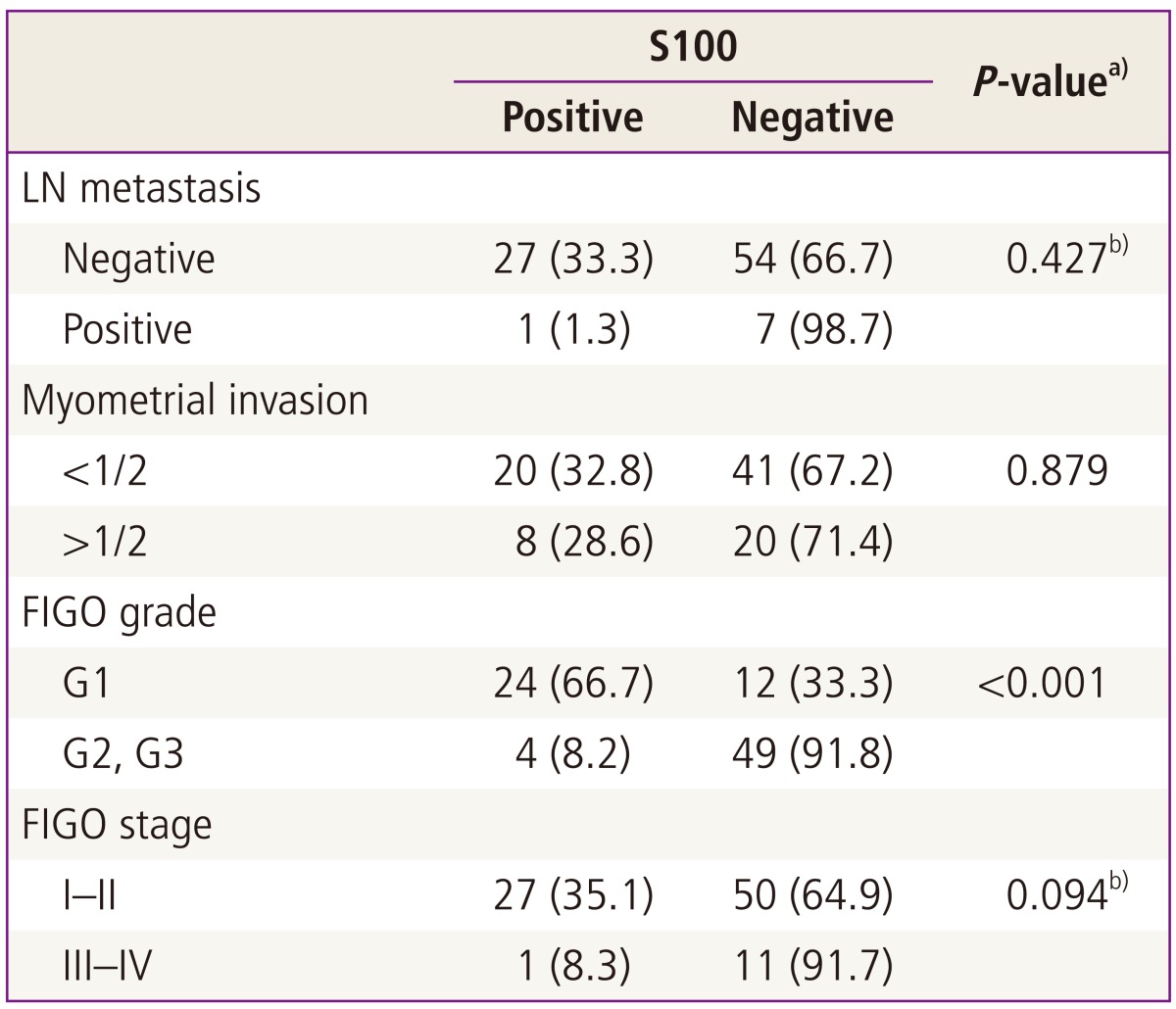
Values are presented as number (%).
FIGO, International Federation of Gynecology and Obstetrics.
a)P-values were calculated using the chi-square test. Bold signifies P<0.05; b)Fisher's exact test.
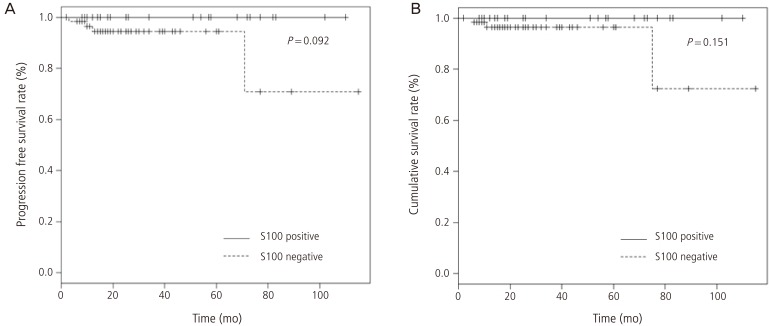
4. Correlation with survival rate
The correlation between the number of S100+ cells and survival rate is shown in Fig. 2. In the Kaplan-Meier plot for progression free survival and overall survival, a slight tendency towards a higher progression free and overall survival rates for endometrial carcinoma patients with S100 positive DCs, although these were not statistically significant (P=0.092, P=0.151, respectively).
Fig. 2.
Kaplan-Meier plot of survival rate. Significant progression free survival (A) and overall survival (B) rate for patients with S100 positive endometrial carcinoma are not demonstrated.
Discussion
This study examined the prognostic significance of the tumor infiltrating DCs in endometrial carcinoma patients. The present study showed that the proportion of S100-positive DCs was negatively correlated with the histologic grade.
DCs are recognized as the most potent antigen-presenting cells and are useful markers for immune responses because they are capable of inducing cytotoxic T lymphocytes from native T cells. T and B cell' activation by DCs occurs by presentation of antigens on major histocompatibility complex class I and II molecules. Thus, DCs play a central role in the regulation and maintenance of the cellular immune response against cancer. Their presence within the tumors is close related to favorable prognosis in patients [9]. Immunohistochemical studies have demonstrated that an increased number of S100 positive DCs correlates with a better prognosis in various types of tumors [10].
The tumor cells secret the pro-inflammatory cytokines, which promotes the infiltration of leukocytes of the innate and adaptive immune system, including macrophages, neutrophils, NK cells, DCs, mast cells, T and B lymphocytes, into the tumor microenvironment. The tumor microenvironment in the stroma is involved in the neoangiogenesis and in the proliferation and invasion of carcinoma cells. In endometrial carcinoma, higher numbers of stromal macrophages, neutrophils, B and T lymphocytes and DCs were infiltrated when compared with normal endometrial tissue [11].
Kara et al. [12] analyzed the number of S100 DCs in sentinel lymph nodes (SLNs) in early-stage endometrial cancer patients and demonstrated that the mean numbers of the S100+ DCs in the tumor-free SLNs were significantly higher than in the tumor-free non-SLNs. A similar result was found in the comparison of metastatic SLNs and metastatic non-SLNs. The underlying factor for the metastatic involvement of SLNs may be immunosuppression. The number of mature DCs in metastatic SLNs was significantly decreased when compared with tumor-free SLNs.
Similar results have also been were reported for other tumor types. Oka et al. [13] investigated the prognostic significance of S100-positive cells in patients with adenocarcinoma of the cervix and showed that patients with S100-positive cells had significantly better outcomes than women who were negative for S100. Nagorsen et al. [14] found a better survival for both high stromal and epithelial DC infiltration in colorectal carcinoma.
We showed that the proportion of S100+ DCs was significantly associated with the tumor grade (P<0.001), in agreement with previous studies. Honig et al. [8] reported that a higher DC infiltration was correlated with better tumor differentiation. Coppola et al. [9] demonstrated that high-grade tumors in endometrial adenocarcinoma were essentially DC-depleted. On the contrary, a higher degree of DC infiltration was associated with better prognosis, suggesting that DC infiltration might be a favorable prognostic factor in endometrial cancer. Elagoz et al. [15] investigated the distribution of DCs in endometrial carcinoma and correlated the results with FIGO (International Federation of Gynecology and Obstetrics) grades, and found a statistically significant difference between S100-positive DCs and grades, with higher numbers of positive DC cells found in grade 1 than in grade 2 and 3 tumors. The authors suggested that DC infiltration may be considered to be a reliable marker that correlated with prognosis in endometrial cancer.
Our present study results differ from some reported for uterine cervix, larynx, and colorectal carcinomas. For example, no significant association was found previously between the degree of S100-positive DC infiltration and tumor differentiation in cervical cancers [16]. The S100 results for laryngeal squamous cell carcinoma showed no association with grade, tumor stage, or survival [17]. The S100 positivity for colorectal cancers was not a significant prognostic factor for overall survival [18].
In some reports, S100 overexpression was associated with poor prognosis. The evidence showed that DCs might lose their ability to activate T cells following exposure to cancer cells. A high number of DCs in the tumor tissues might imply their accumulation due to inhibited migration, which could in turn be associated with poor prognosis [19]. In addition, S100 overexpression is linked with high tumor grade, advanced stage, metastasis and worse survival [5].
Recent data has suggested that S100 may play both positive and negative roles. Further research is therefore required in order to distinguish the complex expression patterns of S100 in tumor and stromal cells and to better understand their pro-tumorigenic and anti-tumorigenic actions [5].
Patients with unfavorable prognostic factors currently undergo a more radical surgery plus systemic lymphadenectomy and/or adjuvant radiotherapy. Further optimization and therapeutic strategy are required for patients with unfavorable prognostic factors in order to improve their clinical outcomes. In addition to the traditional prognostic variables, immunohistochemical staining for tumor infiltrating immune cells could be used a predictor of prognosis. Therefore, further exploration through immunohistochemical evaluation of tumor infiltrating immune cells should be conducted with the aim of developing novel therapeutic strategies for endometrial carcinoma [20].
Our study revealed that the proportion of S100-positive DC expression was negatively correlated with the histologic grade, but was not associated with the stage, myometrial invasion, or lymph node metastasis. However, Lijun et al. [21] reported that the proportion of invasion of the S100-positive DCs was negatively correlated with the clinical stage and lymph node metastasis but was not correlated with the grade and myometrial invasion. Discrepancy may be from some limiting factors in the studies. Our study has some limitations. First, demographic data include the limited number of patients, and unintended skewed patient distributions, especially in terms of stage and lymph node metastasis. Second, although S100 protein has been studied as functional DCs in many conditions, its functional implication is confusing. S100 protein is a nonspecific marker of DCs. CD83 antigen is a specific marker of mature human DCs and is likely to be a more reliable DC marker that reflects host antitumor immunity [22].
In conclusion, our results indicate that the proportion of stromal infiltration of S100+ DCs was correlated with histologic grade as a conventional prognostic marker in endometrial carcinoma. However, to define DCs as a pathologic prognostic marker, additional larger studies are required.
Acknowledgments
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (A100557).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Seo HG, Lee JS. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45:1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Horst PH, Wang Y, Vandenput I, Kuhne LC, Ewing PC, van Ijcken WF, et al. Progesterone inhibits epithelial-to-mesenchymal transition in endometrial cancer. PLoS One. 2012;7:e30840. doi: 10.1371/journal.pone.0030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadducci A, Cosio S, Genazzani AR. Tissue and serum biomarkers as prognostic variables in endometrioid-type endometrial cancer. Crit Rev Oncol Hematol. 2011;80:181–192. doi: 10.1016/j.critrevonc.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Movassagh M, Spatz A, Davoust J, Lebecque S, Romero P, Pittet M, et al. Selective accumulation of mature DC-Lamp+ dendritic cells in tumor sites is associated with efficient T-cell-mediated antitumor response and control of metastatic dissemination in melanoma. Cancer Res. 2004;64:2192–2198. doi: 10.1158/0008-5472.can-03-2969. [DOI] [PubMed] [Google Scholar]
- 5.Maletzki C, Bodammer P, Breitruck A, Kerkhoff C. S100 proteins as diagnostic and prognostic markers in colorectal and hepatocellular carcinoma. Hepat Mon. 2012;12(10 HCC):e7240. doi: 10.5812/hepatmon.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun. 2011;3:355–364. doi: 10.1159/000325465. [DOI] [PubMed] [Google Scholar]
- 7.Tavassoli FA, Devilee P International Agency for Research on Cancer; World Health Organization. Pathology and genetics of tumours of the breast and female genital organs. Lyon: IAPS Press; 2003. [Google Scholar]
- 8.Honig A, Schaller N, Dietl J, Backe J, Kammerer U. S100 as an immunohistochemically-detected marker with prognostic significance in endometrial carcinoma. Anticancer Res. 2005;25:1747–1753. [PubMed] [Google Scholar]
- 9.Coppola D, Fu L, Nicosia SV, Kounelis S, Jones M. Prognostic significance of p53, bcl-2, vimentin, and S100 protein-positive Langerhans cells in endometrial carcinoma. Hum Pathol. 1998;29:455–462. doi: 10.1016/s0046-8177(98)90060-0. [DOI] [PubMed] [Google Scholar]
- 10.Coventry B, Heinzel S. CD1a in human cancers: a new role for an old molecule. Trends Immunol. 2004;25:242–248. doi: 10.1016/j.it.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Wallace AE, Gibson DA, Saunders PT, Jabbour HN. Inflammatory events in endometrial adenocarcinoma. J Endocrinol. 2010;206:141–157. doi: 10.1677/JOE-10-0072. [DOI] [PubMed] [Google Scholar]
- 12.Kara PP, Ayhan A, Caner B, Gultekin M, Ugur O, Bozkurt MF, et al. Analysis of dendritic cells in sentinel lymph nodes of patients with endometrial and patients with cervical cancers. Int J Gynecol Cancer. 2009;19:1239–1243. doi: 10.1111/IGC.0b013e3181b3e616. [DOI] [PubMed] [Google Scholar]
- 13.Oka K, Nakano T, Arai T. Adenocarcinoma of the cervix treated with radiation alone: prognostic significance of S-100 protein and vimentin immunostaining. Obstet Gynecol. 1992;79:347–350. doi: 10.1097/00006250-199203000-00004. [DOI] [PubMed] [Google Scholar]
- 14.Nagorsen D, Voigt S, Berg E, Stein H, Thiel E, Loddenkemper C. Tumor-infiltrating macrophages and dendritic cells in human colorectal cancer: relation to local regulatory T cells, systemic T-cell response against tumor-associated antigens and survival. J Transl Med. 2007;5:62. doi: 10.1186/1479-5876-5-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elagoz S, Arici DS, Aker H. Relationship between FIGO grade and AgNOR, S100-positive langerhans cells in endometrial adenocarcinoma. Pathol Int. 2000;50:616–619. doi: 10.1046/j.1440-1827.2000.01099.x. [DOI] [PubMed] [Google Scholar]
- 16.Bethwaite PB, Holloway LJ, Thornton A, Delahunt B. Infiltration by immunocompetent cells in early stage invasive carcinoma of the uterine cervix: a prognostic study. Pathology. 1996;28:321–327. doi: 10.1080/00313029600169274. [DOI] [PubMed] [Google Scholar]
- 17.Karakok M, Bayazit YA, Ucak R, Ozer E, Kanlikama M, Mumbuc S, et al. Langerhans cell related inflammatory reaction in laryngeal squamous cell carcinoma. Auris Nasus Larynx. 2003;30:81–84. doi: 10.1016/s0385-8146(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 18.Liska V, Vycital O, Daum O, Novak P, Treska V, Bruha J, et al. Infiltration of colorectal carcinoma by S100+ dendritic cells and CD57+ lymphocytes as independent prognostic factors after radical surgical treatment. Anticancer Res. 2012;32:2129–2132. [PubMed] [Google Scholar]
- 19.Nestle FO, Burg G, Fah J, Wrone-Smith T, Nickoloff BJ. Human sunlight-induced basal-cell-carcinoma-associated dendritic cells are deficient in T cell co-stimulatory molecules and are impaired as antigen-presenting cells. Am J Pathol. 1997;150:641–651. [PMC free article] [PubMed] [Google Scholar]
- 20.De Jong RA, Leffers N, Boezen HM, ten Hoor KA, van der Zee AG, Hollema H, et al. Presence of tumor-infiltrating lymphocytes is an independent prognostic factor in type I and II endometrial cancer. Gynecol Oncol. 2009;114:105–110. doi: 10.1016/j.ygyno.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 21.Lijun Z, Xin Z, Danhua S, Xiaoping L, Jianliu W, Huilan W, et al. Tumor-infiltrating dendritic cells may be used as clinicopathologic prognostic factors in endometrial carcinoma. Int J Gynecol Cancer. 2012;22:836–841. doi: 10.1097/IGC.0b013e31825401c6. [DOI] [PubMed] [Google Scholar]
- 22.Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–3835. [PubMed] [Google Scholar]