Abstract
This article reviews the extant twin studies employing magnetic resonance imaging data (MRI), with an emphasis on studies of population-based samples. There have been approximately 75 twin reports using MRI, with somewhat under half focusing on typical brain structure. Of these, most are samples of adults. For large brain regions such as lobar volumes, the heritabilities of large brain volumes are consistently high, with genetic factors accounting for at least half of the phenotypic variance. The role of genetics in generating individual differences in the volumes of small brain regions is less clear, mostly due to a dearth of information, but rarely because of disagreement between studies. Multivariate analyses show strong genetic relationships between brain regions. Cortical regions involved in language, executive function, and emotional regulation appear to be more heritable than other areas. Studies of brain shape also show significant, albeit lower, genetic effects on population variance. Finally, there is evidence of significant genetically mediated relationships between intelligence and brain structure. At present, the majority of twin imaging studies are limited by sample sizes small by the standards of behavioral genetics; nevertheless the literature at present represents a pioneering effort in the pursuit of answers to many challenging neurobiological questions.
Over the last half century, studies of twin and familial relationships have generated a wealth of information on the causes of individual differences in behavior and psychopathology. Numerous family studies, particularly those using twin designs, have provided evidence that a multitude of human behavioral traits are strongly influenced by our genetic makeup (Boomsma et al., 2002; Sullivan & Kendler, 1999). More recently, quantitative genetic studies of neurobiological phenotypes (so called ‘endophenotypes’), including measures of brain structure and function using magnetic resonance imaging (MRI), have received increased interest, primarily as potential bridges between genes and selected behavioral measures. Even though the extant endophenotype literature already has made substantial contributions to our understanding of neurogenetics, all evidence indicates that we are on the verge of a dramatic revolution in our science. These changes will likely result in a closer relationship with conventional neuroscience, and possibly even the emergence of a novel subdiscipline in behavioral genetics.
Understanding the biological substrates of the mind ranks among the most ambitious scientific tasks ever attempted, and all available neurobiological tools must remain on the table, as each provides a unique perspective on a very complex problem. MRI, however, does have several advantages (as well as disadvantages) relative to other modalities. MRI is quite a versatile technology that enables (a) the examination of soft tissues in vivo with extremely high resolution (approximately 1 mm3) relative to other imaging techniques (Bushberg et al., 1994), (b) the assessment of function by measuring brain hemodynamics (fMRI), (c) visualization of white matter tracts via diffusion tensor imaging (DTI), and (d) assessment of neurotransmitter and metabolite levels in the living brain via magnetic resonance spectroscopy (MRS; Casey & de Haan, 2002). This versatility, in combination with its lack of ionizing radiation, makes MRI particularly useful for large neuroanatomic studies on typical populations. Disadvantages include expense compared to EEG, lower temporal resolution for functional studies compared to EEG or magnetoencephalography (MEG), substantially decreased anatomical resolution compared to pathological specimens, and a more limited ability to visualize the distribution of molecules of interest as compared to positron emission tomography (PET).
To date, nearly all reports on twins using MRI have been anatomic. An anatomic image effectively is a downsampled, digital version of the original human brain, in which each voxel (a three dimensional, or volumetric, pixel) in the image represents a true physical volume. Thus, anatomic MRI allows for several options in image processing which enable both volumetric (measurement of volumes) and morphometric (measurement of shape) analyses at multiple levels of resolution. The present manuscript represents a review of the existing twin reports using MRI, with an emphasis on normal brain structure. Relevant papers were identified via PubMed and ISI Web of Science database searches using the intersection of the terms twin, brain, and MRI (or magnetic resonance imaging). Reports were excluded from consideration if they represented case reports, or if they used twins as a convenience sample in order to address nongenetic questions.
Heritability of Volumetric Phenotypes
There have been approximately 35 reports on typical neurodevelopment in twins using MRI. About half of these reports have used structural equation modeling (SEM), with others basing their estimates on Falconer estimation. These reports employed a diversity of volumetric and morphometric techniques (Table 1), but most included a volumetric component. The analysis of data from studies of psychopathology provides some additional information, albeit limited, on the normal heritability of MRI-derived phenotypes (Table 2); many of these papers are focused on a few specific regions of interest (ROIs) that are pertinent to a particular neurobiological question.
Table 1.
Comparison of Qualitative Study Design Characteristics for All Existing Twin Reports Using MRI in Typically Developing Populations
| Study | Population | Substructures measured? |
Morphological measures? |
Voxel-level measures? |
Brain and behavior? |
Multivariate analyses? |
SEM-based statistics? |
Longitudinal design? |
|---|---|---|---|---|---|---|---|---|
| Reveley, 1984 | Adult | Y2 | N | N | N | N | Y | N |
| Oppenheim, 1989 | Adult | Y2 | Y2 | N | N | N | N | N |
| Steinmetz, 1994 | Adult | N | Y | N | N | N | N | N |
| Steinmetz, 1995 | Adult | Y | N | N | Y7 | N | N | N |
| Bartley, 1997 | Adult | Y | Y | N | N | N | Y | N |
| Biondi, 1998 | Adult | Y | Y | N | N | N | N | N |
| Bonan, 1998 | Adult | Y | Y | N | N | N | N | N |
| Carmelli 1998 | Adult | N | N | N | N | N | Y | N |
| Haidekker, 1998 | Adult | N | Y | N | N | N | N | N |
| Tramo, 1998 | Adult | Y | N | N | Y | N | N | N |
| Carmelli, 1999 | Adult | N | N | N | Y | N | N | N |
| Lohman, 1999 | Adult | N | Y | N | N | N6 | N | N |
| Le Goualher, 2000 | Adult | N | Y | N | N | N | N | N |
| Pennington, 2000 | Pediatric1 | Y | N | N | Y | Y | N | N |
| Pfefferbaum, 2000 | Adult | Y2 | N | N | N | Y3 | Y | N |
| Posthuma 2000 | Adult | N | N | N | N | Y | Y | N |
| Barré, 2001 | Adult | N | N | N | N | Y | Y | N |
| Pfefferbaum, 2001 | Adult | Y2 | N | N | N | N | Y | N |
| Sullivan, 2001 | Adult | Y | N | N | N | N | Y | N |
| Thompson, 2001 | Adult | N | N | Y | Y | N | N | N |
| Carmelli 2002a | Adult | Y | N | N | Y | N | Y | N |
| Carmelli 2002b | Adult | N5 | N | N | Y | N | Y | N |
| Eckert, 2002 | Pediatric | Y | N | N | N | N | N | N |
| Geschwind, 2002 | Adult | Y | N | N | Y7 | N | Y | N |
| Hulshoff Pol, 2002 | Adult | Y2 | N | N | N | N | Y | N |
| Posthuma 2002 | Adult | N | N | N | Y | Y | Y | N |
| Reed, 2002 | Adult | Y2 | N | N | N | N | N | N |
| White, 2002 | Adult | Y | N | N | N | N | N | N |
| Wright, 2002 | Adult | Y | N | N | N | Y3* | Y | N |
| Scamvougeras, 2003 | Adult | Y2 | N | N | N | N | N | N |
| Styner, 2003 | Adult | Y | Y | N | N | N | N | N |
| Mohr, 2004 | Adult | N | Y | N | N | N | N | N |
| Pfefferbaum, 2004 | Adult | Y2 | Y2 | N | N | Y | Y | Y4 |
| Wallace et al., 2006 | Pediatric | Y | N | N | N | N | Y | N |
| Hulshoff Pol, 2006 | Adult | Y | N | Y | Y | N | Y | N |
| Schmitt et al., 2007 | Pediatric | Y | N | N | N | Y | Y | N |
Note: ‘Substructures measured’ is an indication of whether parcellation data for ROIs are reported for structures other than total brain, intracranial volume, or hemispheric volumes. A ‘Y’ for brain and behavior is indicative that the study not only measured psychometric and imaging variables, but also attempted to describe brain-behavioral relationships. In contrast, the ‘Multivariate Analyses’ column identifies studies that model relationships between neuroanatomic variables.
Greater than 70% of the twin sample was reading disabled
Only midsagittal structures (lateral ventricles and/or corpus callosum) were measured
Bivariate, or bivariate with post-hoc principal component analysis (3*)
Two timepoints
White matter hyperintensities were the only neuroanatomic variable reported
PCA used, but to assess global, rather than structure-specific, eigenvalues
Handedness only
Table 2.
Comparison of Qualitative Study Design Characteristics for Reports on Neuropathology Using MRI in Twins
| Study | Condition of interest | Population | Structure of interest | Volumetric parcellation? | Morphological measures? | Voxel-level measures? | ||
|---|---|---|---|---|---|---|---|---|
| Casanova, 1990a | Schizophrenia | Adult | Corpus Callosum | N | N | N | ||
| Casanova, 1990b | Schizophrenia | Adult | Corpus Callosum | N | Y | N | ||
| Suddath, 1990 | Schizophrenia | Adult | Ventricles, hippocampus | Y | N | N | ||
| Casanova, 1991 | Schizophrenia | Adult | Corpus Callosum | N | Y | N | ||
| Weinberger 1991 | Schizophrenia | Adult | Cerebral lateralization | Y | N | N | ||
| Weinberger, 1992a | Schizophrenia | Adult | Hippocampus | Y | N | N | ||
| Prefrontal cortex | ||||||||
| Weinberger, 1992b | Schizophrenia | Adult | Limbic structures | Y | N | N | ||
| Bartley, 1993 | Schizophrenia | Adult | Sylvian fissure | Y | N | N | ||
| Kinnunen, 1993 | Lupus | Adult | Clinical readings | N | N | N | ||
| Goldberg, 1994 | Schizophrenia | Adult | Prefrontal Cortex, Hippocampus | Y | N | N | ||
| Thorpe, 1994 | Multiple sclerosis | Adult | Clinical readings | N | N | N | ||
| Hyde, 1995 | Tourette syndrome | Pediatric | Caudate | Y | N | N | ||
| Noga, 1996 | Schizophrenia | Adult | Hemispheric volumes and other measures of hemisphere size | Y | Y | N | ||
| Jackson, 1998 | Epilepsy | Adult | Hippocampus | Y | N | N | ||
| McNeil, 2000 | Schizophrenia | Adult | Hippocampus | Y | N | N | ||
| Barré, 2001 | Schizophrenia | Adult | Multiple substructures | Y | N | N | ||
| Briellmann, 2001 | Epilepsy | Adult | Clinical Findings, Hippocampus | Y | N | N | ||
| Noga, 2001 | Bipolar I | Adult | Mesial Temporal, Basal Ganglia | Y | N | N | ||
| Bridle, 2002 | Schizophrenia | Adult | Subcortical | Y | N | N | ||
| Cannon,2002 | Schizophrenia | Adult | Voxel-level | N | N | Y | ||
| Kiseppä, 2002 | Bipolar I | Adult | Cerebral lobar gray/white volumes ventricular volumes | Y | N | N | ||
| Narr, 2002a | Schizophrenia | Adult | Corpus Callosum | Y | N | N | ||
| Narr, 2002b | Schizophrenia | Adult | Hippocampus | Y | N | Y | ||
| Gilbertson, 2002 | PTSD | Adult | Hippocampus | |||||
| Castellanos, 2003 | ADHD | Pediatric | Caudate | Y | N | N | ||
| Järvenpää, 2004 | Cognitive Dysfunction | Adult | Hippocampus | Y | N | N | ||
| Kates, 2004 | Autism | Pediatric | Cerebral lobar and cerebellar gray/white volumes, ventricles | Y | N | N | ||
| May, 2004 | PTSD | Adult | Septum Pellucidum | N | N | N | ||
| Van Erp, 2004 | Schizophrenia | Adult | Hippocampus | Y | N | N | ||
| Van Haren, 2004 | Schizophrenia | Adult | Hippocampus | Y | N | N | ||
| Hulshoff Pol, 2004 | Schizophrenia | Adult | Whole brain volume, gray/white brain volumes | Y | N | N | ||
| Rijsdijk, 2005 | Schizophrenia | Adult | Whole brain, Hippocampus, Ventricles | Y | N | N | ||
| Spaniel,2005 | Schizophrenia | Adult | Global T1/T2 Relaxation | N | N | N | ||
| Styner, 2005 | Schizophrenia | Adult | Corpus Callosum | Y | Y | N | ||
| Terriberri, 2005 | Schizophrenia | Adult | Corpus Callosum | N | Y | N | ||
| Hulshoff Pol, 2006 | Schizophrenia | Adult | Voxel-based morphometry | N | N | Y | ||
| Levitt, 2006 | PTSD | Adult | Cerebellar Vermis | Y | N | N | ||
| Ettinger, 2007 | Schizophrenia | Adult | Thalamus | Y | N | N | ||
| de Geus, 2007 | Anxiety and depression | Adult | Voxel-based Morphometry | N | N | Y | ||
| van ’t Ent, 2007 | ADHD problems | Pediatric | Voxel-based Morphometry | N | N | Y | ||
Note: This summary table excludes case reports and studies using twin samples to address nongenetic questions. Reports that include information on the genetics of typical neuroanatomy, usually because they provide statistics on control samples, are shown in bold.
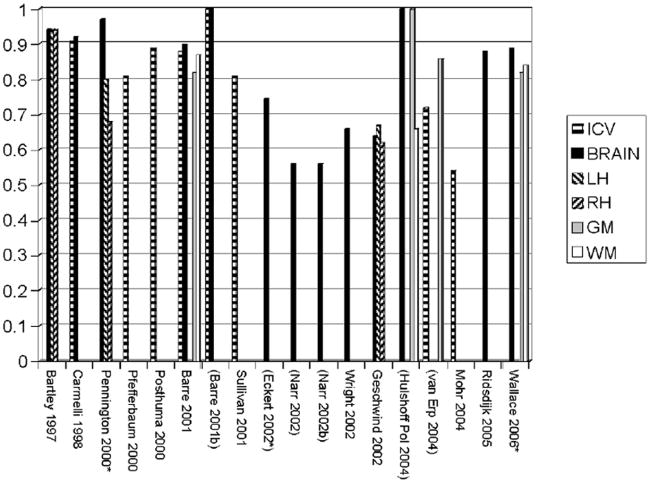
Considered together, twin MRI reports have demonstrated a strong, highly statistically significant role of genes in the generation of the variability in human brain volumes, particularly for larger structures (Baaré et al., 2001a; Pennington et al., 2000; Pfefferbaum et al., 2000). For example, in their large sample, methodologically-rigorous analyses, found that genetic factors were responsible for .90, .82, and .88 of the total variance in total brain, gray, and white matter volumes, respectively, in 112 adult twin pairs (Baaré et al., 2001a). Figure 1 provides a between-study comparison of heritability estimates for these large structures.
Figure 1. Summary of heritability estimates for volumes of large neuroanatomic structures.
Note: Reports are listed in parentheses if heritability is calculated via Falconer estimation, either by the authors of the study or post hoc based on correlations; the remaining studies use SEM.
* = Pediatric studies
ICV = intracranial volume
Brain = total brain or total cerebrum
LH = left hemisphere
RH = right hemisphere
GM = total gray matter
WM = total white matter
Heritability estimates based on variance components analysis for neuroanatomic substructures are substantially less frequent than for global volumetric measures. It is common that only one or two estimates of heritability have been reported for a given region of interest. For example, there are only three published twin studies that report findings for cerebral lobar tissue (Carmelli et al. 2002b; Geschwind et al., 2002; Wallace et al., 2006); all three suggest that genes play the predominant role in generating population variance in these structures, with some evidence that the role of genes in the occipital lobe is weaker than in other regions. Similarly, there are only three reports that parse cerebellar variance into genetic and non-genetic components (Posthuma et al., 2000; Wright et al., 2002; Wallace et al., 2006); heritability estimates from these studies are .89, .67, and .49.
Though large volumetric measures consistently show high heritabilities, the role of genetic factors on individual differences of smaller structures is more variable. Of these substructures, the lateral ventricles, corpus callosum, and the hippocampus are by far the most well studied. There is extremely strong evidence that the variance in the area of the corpus callosum is dominated by genetic factors (Oppenheim et al., 1989; Pfefferbaum et al., 2000; Scamvougeras et al., 2003; Sullivan et al., 2001). In contrast, heritability estimates for both the hippocampus (Baaré et al., 2001b; Narr et al., 2002; Rijsdijk et al., 2005; Sullivan et al., 2001; Van Erp et al., 2004; Van Haren et al., 2004; Wright et al., 2002) and lateral ventricles (Baaré et al., 2001a; Baaré et al., 2001b; Pfefferbaum et al., 2000; Reveley et al., 1984; Reveley et al., 1982; Rijsdijk et al., 2005; Wright et al., 2002) are generally lower, but with a great deal of variability from study to study.
Other neuroanatomic structures have rarely been measured in full twin designs, if at all. A few studies on monozygotic (MZ) pairs have shown high intertwin correlations for subcortical structures. For example, White et al. (2002) report correlations of .84 for caudate, .75 for putamen, and .75 for thalamic volumes in12 MZ pairs, while Ettinger et al. (2007) estimate the intraclass correlation for thalamus at .69 in 27 normal MZ pairs as part of a study on schizophrenia. For most ROIs, however, the information regarding variance components comes solely from a study by Wright et al. on 10 MZ and 10 dizygotic (DZ) twin pairs, which included measures from individual cerebral gyri (roughly based on Brodmann’s areas), as well as subcortical gray, thalamus, cerebellum, and brain stem: 92 parcellated regions (all gray matter) in total (Wright et al., 2002). While numerous regions had statistically significant familial influences, only the precentral gyrus had statistically significant effects due to genes specifically. Regions with heritability estimates greater than .50 included the superior parietal lobe, posterior cingulate gyrus, corpus striatum, putamen, and cerebellum. Though this study suffers from low power and multiple testing issues, it is currently the de facto source for estimates of the magnitude of genetic influences in typical populations for nearly all small substructures in the brain.
With the possible exception of the lateral ventricles, there is little evidence that the shared environment plays a role in generating neuroanatomic (either morphological or volumetric) variability, although this may be obscured by nonadditive effects of genes.
Voxel-Based Image Analyses
Rather than defining brain regions, an alternative approach is to analyze structure at the voxel level; analyses of these fundamental elements represent the highest level of magnification possible for a particular brain scan. A voxel-level approach has the advantage of vastly increased spatial resolution, as well as a lack of a priori constraints on the data. Disadvantages of voxel-level analyses include the more exploratory nature of the method (the inevitable flip side of effectively ignoring functional neuroanatomy), the necessity of many thousands of analyses and subsequent multiple testing issues that arise, and the potential for higher measurement error in these smaller measurements. Nevertheless, the technique offers great promise in identifying subtle effects of genetic factors on brain structure that may be obscured by more regional measures.
There have been only two published twin studies that report results in typical populations at high resolution, though voxel-level analyses are somewhat more common in neuropathological samples. In an influential paper, Thompson et al. examined gray matter density in 10 MZ and 10 DZ adult twin pairs and found that genetic factors strongly influenced language and executive processing centers. (Thompson et al., 2002). Probability maps suggested particularly strong genetic effects in middle frontal regions, and an asymmetry in Wernicke’s region (the center for receptive speech), with the left side highly significant but not the right. More recently,Holshoff Pol et al. (2006) examined both gray and white matter density in a substantially larger sample of 258 individuals. Their analysis found several highly significant gray matter foci, including in the superior and middle frontal lobe, Heschl’s gyrus, cingulate cortex, and portions of the occipital lobe. Additionally, these analyses discovered significant heritability in white matter tracts, including the superior occipitofrontal fascicle, corpus callosum, and corticospinal tracts.
Morphometric Measures
The role of the genome on differences in brain shape appears to be significant, but more modest than its influence on volume. Though less well studied, twin findings on shape differences have been fairly consistent (Bartley et al., 1997; Biondi et al., 1998; Bonan et al., 1998; Eckert et al., 2002; Haidekker et al., 1998; Lohmann et al.,1999; Mohr et al., 2004; Steinmetz et al., 1994; White et al., 2002). In general, brain morphology appears to be significantly heritable, but to a lesser extent than volume. For example, raters are able to successfully match surface renderings of MZ pairs, even when there are striking qualitative differences; this finding suggests some familial influences, but also a role of the unique environment in the development of gyral patterns (Biondi et al., 1998). More quantitative measures have produced similar findings. A study by Bartley et al. (1997) found low heritability for gyral patterning, despite high heritability estimates for volumes in 10 MZ and 9 DZ pairs. Similarly, a study by White et al. (2002) on 24 MZ twin pairs reported that within-pair correlations on volumetric measures were substantially higher than surface measures of cerebral morphology. Lohmann also found an effect of genes on sulcal patterns in 19 pairs of MZ twins, with stronger pairwise correlations for deeper (and ontogenetically older) sulci (Lohmann et al., 1999). Other groups have replicated these findings using different metrics of cortical shape and gyral complexity (Haidekker et al., 1998; Mohr et al., 2004). Similar conclusions are found when examining the morphology of the central sulcus (Bonan et al., 1998; Le Goualher et al., 2000), the planum temporale (Eckert et al., 2002), or the corpus callosum (Styner et al., 2005; Terriberry et al., 2005) specifically, rather than global sulcal patterns.
Multivariate Analyses
Despite the critical importance of understanding the etiology of interregional neuroanatomic associations, there are only four reports that investigate questions of this nature. Baaré et al. (2001a) examined relationships between height, intracranial volume (ICV), total gray matter, total white matter, and lateral ventricular volumes in a sample of 54 MZ and 58 DZ adult twin pairs and 34 sibs of DZ pairs, via variance component analyses. Between gray and white matter, they found a genetic correlation of .68, a unique environmental correlation of .04, and no statistically significant evidence of genetic correlations between the lateral ventricles and other regions of interest. A principal components analysis by Pennington et al. (2000) on a sample of 34 MZ and 32 DZ late teen or young adult twin pairs (most pairs had one or more twins with reading disability) parcellated the brain into 7 cortical gray compartments and 6 noncortical structures (white matter, basal ganglia, brain stem, hippocampus, cerebellum, and the central gray nuclei, including the thalamus). While cerebral structures loaded primarily on the first factor, all other structures loaded on the second (except central gray, which loaded equally on both). Both factors were significantly more correlated in MZ than in DZ pairs, suggesting a strong genetic component to each. The third extant multivariate volumetric study by Wright et al. (2002) parcellated the brain into regions with high spatial resolution. This study identified two putative supra-regional principal components under genetic control. Specifically, a frontoparietal limbic/paralimbic factor and a factor related to audition (lateral temporal cortex, insula, occipitofrontal, and other frontal regions) were found; factor loadings, however, were quite low (< |0.25|). These findings would suggest that genes are involved in generating functional relationships between distant brain regions.
Recently, our group completed a multivariate analysis of six ontogenetically diverse volumetric ROIs (cerebrum, cerebellum, lateral ventricles, thalamus, corpus callosum, and basal ganglia) using a large pediatric sample and traditional behavioral genetic techniques (Schmitt et al., 2007). In general, the genetic correlations were quite high (Table 3). Further, the relationships between these structures were almost entirely determined by a single genetic factor shared between brain tissues. The lateral ventricles and the corpus callosum were notable exceptions; while the genetic influences on the ventricles were low, genes seem to play a strong role in generating variability in human corpus callosum area, but these effects are largely independent of other tissues. We also found genetic and environmental correlations for cerebral gray and white matter (.84 and −.04, respectively) similar to those Baaré et al. (2001a) found for total gray and white matter in their adult sample.
Table 3.
Genetic and Environmental Correlations for Six ROIs in a Pediatric Twin Sample
| Cerebrum | LV | CC | Thalamus | SC | Cerebellum | |
|---|---|---|---|---|---|---|
| Cerebrum | 1 | .26 (.06 .43) | .37 (.17 .54) | .35 (.16 .51) | .23 (.03 .42) | .58 (.43 .70) |
| LV | .18 (−.33 .69) | 1 | −.05 (−.25 .15) | −.22 (−.40 −.03) | -.23 (−.41 −.03) | .29 (.10 .46) |
| CC | .30 (.05 .52) | .22 (−.54 .74) | 1 | .49 (32 .63) | .39 (.19 .55) | .10 (−.11 .30) |
| Thalamus | .97 (.83 1.0) | .00 (−.49 .65) | .42 (.11 .66) | 1 | .65 (.52 .75) | .07 (−.13 .27) |
| SC | .82 (.71 .92) | −.37 (−.80 .24) | .35 (.07 .64) | .91 (.81 .98) | 1 | .13 (−.07 .33) |
| Cerebellum | .82 (.59 1.0) | .20 (−.59 .71) | .12 (−.38 .57) | .79 (.44 1.0) | .63 (.29 .93) | 1 |
Note: Genetic correlations are provided below the diagonal, with environmental correlations above (From Schmitt et al. 2007).
LV = lateral ventricles
CC = corpus callosum
SC = subcortical nuclei including caudate nucleus, putamen, and globus pallidus
95% confidence intervals are given in parentheses.
Longitudinal Studies
There is only one paper that reports longitudinal data on neuroanatomic structures and changes with age. It is based on two volumetric measurements with an interval of 4 years between them, using subjects recruited from the National Heart, Lung and Blood Institute (NHLBI) study on World War II veterans (Pfefferbaum et al., 2004). The subsequent analyses on 71 twin pairs suggest genetic stability in both the corpus callosum and lateral ventricular volumes over this time interval. This study also found evidence for environmental factors increasing the variability in both measures with time, suggesting ongoing changes in brain structure even in the 8th decade of life.
Relationships Between Brain and Behavior
Though numerous twin studies have investigated the relationships between atypical behavior and neuroanatomic endophenotypes (Table 2), there are relatively few that have attempted to understand how the genetic and environmental effects on typical cognitive and behavioral measures are mediated through brain morphology. Most of the existing work has been on cognition. The detection of brain–cognition correlations has been particularly elusive in typical twin samples. An initial study on full scale IQ and several brain volumes in a small twin sample of MZ twins failed to find a significant correlation with any structure (range −.04 to .20; Tramo et al., 1998).
Evidence suggests, however, that a small correlation does exist. Using voxel-based morphometry, Thompson found strong evidence that intelligence (defined as a combination of selected subtests of the WAIS-R) was significantly correlated with frontal gray matter in his sample of 40 twins, but did not attempt to parcellate the correlation into genetic and nongenetic components (Thompson et al., 2001). Spurred by this discovery, Posthuma et al. (2002) compared WAIS-IIIR IQ scores to total gray and white matter volumes in an extended twin design with 24 MZ pairs, 31 DZ pairs, and 25 siblings. They found a small, but significant correlation between IQ and neuroanatomic structures, with all of the covariance between IQ and brain anatomy associated with genetic factors. A follow-up study examining WAIS-III subtests produced similar results, with small brain-behavioral correlations dominated by genetic effects (Posthuma et al., 2003). Using higher resolution imaging methods, Hulshoff Pol et al. (2006) identified significant genetic correlations between the right medial frontal gyrus and PIQ and VIQ, and between PIQ and the right parahippocampal gyrus. It is noteworthy, however, that since both intelligence and large brain volumes are strongly genetically influenced, the proportion of genetic effects shared between these phenotypes is small, even though genetic factors appear to predominate in explaining their (small) observed covariance.
The only brain and behavior study on a typical pediatric twin population was by Pennington (Pennington et al., 2000), which found WISC-R full scale IQ measures to be correlated with total cerebral volume (.42 in their reading disabled sample, .31 in a control group). The genetic correlation between these measures in the combined sample was .48.
The remaining studies on cognition are from the NHLBI, an all male geriatric population (Carmelli et al., 2002b; Carmelli et al., 2002a; Carmelli et al., 1999). Of these, one presents a systematic analysis of two cognitive factors (verbal memory and executive function; Carmelli et al., 2002b). These two factors were based on principal components analyses on data from several tests (Trails A and B, Stroop, California Verbal Learning Test, the Iowa Screening Battery for Mental Decline, and the WAIS Digit symbol substitution subtest), and were highly heritable (.62 and .64, respectively). Executive function was found to be positively correlated with frontal and temporal regions, and negatively correlated with lateral ventricular volume (magnitude of correlations approximately .20). A similar, slightly weaker pattern was found with brain–verbal memory correlations. However, out of all measures, only lateral ventricular volumes and executive function shared common genetic origins to a significant level (genetic correlation = −.25). Other studies from the NHLBI focus on the relationships between cognitive and physical performance, and white matter hyperintensities in the elderly (Carmelli et al., 2002a; Carmelli et al., 1999).
Functional Imaging
Functional MRI experiments on twin samples are extraordinarily rare. To our knowledge there are only three reports published, with two examining aspects of schizophrenia in very small samples (Karlsgodt et al., 2007; Spaniel et al., 2006). The remaining study examined the neural substrates of sadness in 8-year-olds; 47 MZ and 57 DZ twin pairs were included in the study (Côté et al., 2007). Despite the large sample size (by the standards of fMRI research), this study found no genetic influences on the relationships between sadness and brain activation in two areas of the brain (medial prefrontal cortex and ventrolateral prefrontal cortex) previously correlated with the subjective experience of sadness; rather, these relationships were dominated by unique environmental effects.
Other Reviews
There is only one other comprehensive review of the twin MRI literature (Peper et al. 2007). Although it is unusual for reviews to cite reviews, this paper is noteworthy for several reasons. First, it represents an independent evaluation of the literature. Second, it provides more detail on several aspects of volumetric neuroimaging. Third, like nearly all other studies reported here, it is not published in a genetics journal and therefore may have escaped the notice of many interested readers.
Limitations of Current Research
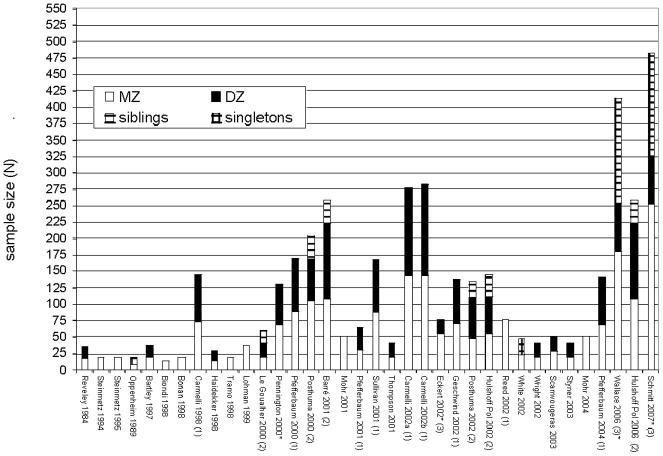
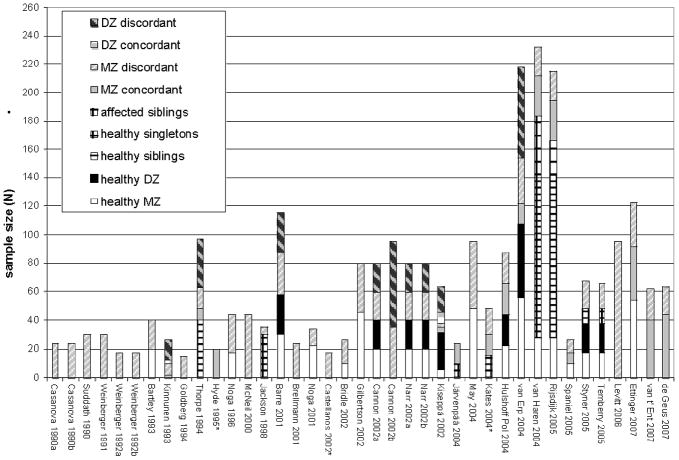
Despite the promise of these methods, numerous limitations of the field are readily apparent. First is a relative dearth of work and lack of replication in this novel field for all but the largest brain structures, exacerbated by the limited sample sizes of the great majority of existing studies (Figure 2). The information that can be gleaned from control groups from pathological studies suffers this problem to an even greater extent (Figure 3). Small samples lead to low confidence in parameter estimates, which is certainly responsible for much of the observed discrepancies between studies. Further, many studies restrict their samples exclusively to MZ twins, which prevents them from distinguishing genetic effects from those of the shared environment (Biondi et al., 1998; Mohr et al., 2001; Mohr et al., 2004; Reed et al., 2002; Steinmetz et al., 1995; Tramo et al., 1998). Of the papers on typical development that include MZ and DZ pairs in order to disentangle genetic and shared environmental effects most are based either on the NIMH pediatric imaging study, an adult sample acquired by the Netherlands Twin Registry, or on the male geriatric sample from NHLBI. Fortunately, these studies also are among the largest extant; nevertheless, there is a critical need for replication at all stages of the life cycle. Thus far, many of the structures demonstrate high familiarity, with MZ correlations of .9 or more. As (all other things being equal) large correlations have smaller standard errors than for smaller ones, the MZ correlations are relatively precisely known. However, the smaller DZ correlations — which often have been obtained from smaller samples — are particularly in need of additional data to increase their precision, and that of variance components estimates obtained from them.
Figure 2. Sample characteristics of the extant MRI twin studies on typical populations.
Note: To facilitate comparisons with singleton and sibling subsamples, individual twins are counted rather than twin pairs. Several reports share overlapping samples, either derived from the National Heart, Lung, and Blood Institute sample (1), the Netherlands Twin Registry (2), or NIMH’s longitudinal pediatric imaging study (3).
* = reports based on data from pediatric populations.
Figure 3. Sample characteristics of the extant MRI twin reports on neuropathology.
Note: Control twins are shown as solid colors (MZ white, DZ black). Qualitative characteristics of these studies are provided in Table 2.
The variety of image processing methodologies, anatomical parcellation strategies, and statistical analyses used are also likely contributors to non-replication. In general, studies that employ state of the art image processing techniques rarely use advanced statistical genetic methodologies, and vice versa. Of the studies that demonstrate advanced methodological approaches in both domains, many (e.g., Wright et al.) are limited by extraordinarily small samples. Some of the newer studies (e.g., Holshoff Pol et al., 2006) set new standards for the rigorous combination of neuroimaging, quantitative genetics, and statistical power that will be required in order to provide definitive answers to most important neurogenetic questions.
The Role of MRI in Twin Research and Prospects for the Future
Even though the field is in its infancy, the synthesis of imaging and twin research already has produced some intriguing findings. In addition to confirming our expectations that volumes of large brain structures are highly heritable, studies at higher resolution suggest that regions of the brain involved in language, executive function, multimodal association, and emotion may have particularly strong genetic determinants. There is limited, but tantalizing evidence of genetically mediated relationships between cognition and brain structure, and prominent genetic correlations between diverse neuroanatomical regions.
A recent special edition of Human Brain Mapping on genetics and imaging (Volume 28, Issue 8) reflects the increasing interest in fusing these sciences within the neuroimaging community. Combining genetics and imaging is hardly novel; since the emergence of MRI, for example, the functional anatomy of neurogenetic disease has been of great interest (Reiss et al., 2000). What seems different now is the sheer increase in scale for investigation of the genetics of typical neurodevelopment, with several large twin studies just beginning to report their initial findings. Several more large projects (e.g., the Vietnam Era Twin Study of Aging, and the Brisbane Twin Imaging Study) are well on their way towards gathering large samples by the standards of conventional brain imaging. With these samples, much more subtle questions can be asked, such as how genetic effects change over neurodevelopment and aging, how different neuroanatomic structures share common genetic determinants, how genetic effects on behavior are mediated through cortical networks, and whether individual differences in functional neuroanatomy are genetically mediated. Further, as many of these studies are longitudinal and population-based, the ability to measure brain structure in subjects before and after the development of common psychiatric conditions (e.g., ADHD, dementia) is a likely possibility.
The next decade promises to be an exciting time for individuals interested in the genetics of neurobiological phenotypes. It will also represent a critical moment in the evolution of behavioral genetics, a moment in which the field must decide how best to integrate these new techniques into its rigorous tradition of quantitative analysis. Simply ignoring neurobiological measures could have serious detrimental effects, as rapid advances in neurogenetics and subsequent changes in the scientific landscape are inevitable. On the other hand, scientists unfamiliar with neuroimaging should avoid being too mesmerized by the technological aspects of the methods. Rather, we must critically evaluate how best to bring these powerful tools together in a meaningful and productive manner.
Acknowledgments
This work was supported by NIH grants MH-65322, MH-20030, DA-18673, AG-18386, AG-18384, AG-22381, and AG-22982.
References
- Baaré WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, De Geus EJ, Schnack HG, Van Haren NE, Van Oel CJ, Kahn RS. Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex. 2001a;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Baaré WF, van Oel CJ, Hulshoff Pol HE, Schnack HG, Durston S, Sitskoorn MM, Kahn RS. Volumes of brain structures in twins discordant for schizophrenia. Archives of General Psychiatry. 2001b;58:33–40. doi: 10.1001/archpsyc.58.1.33. [DOI] [PubMed] [Google Scholar]
- Bartley AJ, Jones DW, Weinberger DR. Genetic variability of human brain size and cortical gyral patterns. Brain. 1997;120:257–269. doi: 10.1093/brain/120.2.257. [DOI] [PubMed] [Google Scholar]
- Biondi A, Nogueira H, Dormont D, Duyme M, Hasboun D, Zouaoui A, Chantome M, Marsault C. Are the brains of monozygotic twins similar? A three-dimensional MR study. AJNR American Journal of Neuroradiology. 1998;19:1361–1367. [PMC free article] [PubMed] [Google Scholar]
- Bonan I, Argenti AM, Duyme M, Hasboun D, Dorion A, Marsault C, Souaoui A. Magnetic resonance imaging of cerebral central sulci: A study of monozygotic twins. Acta Geneticae Medicae et Gemellologiae. 1998;47:89–100. doi: 10.1017/s000156600000026x. [DOI] [PubMed] [Google Scholar]
- Boomsma D, Busjahn A, Peltonen L. Classical twin studies and beyond. Nature Reviews Genetics. 2002;3:872–882. doi: 10.1038/nrg932. [DOI] [PubMed] [Google Scholar]
- Bushberg JT, Seibert JA, Leidholdt EM, Boone JM. The essential physics of medical imaging. Baltimore, MD: Williams and Wilkins; 1994. [Google Scholar]
- Carmelli D, Reed T, DeCarli C. A bivariate genetic analysis of cerebral white matter hyperintensities and cognitive performance in elderly male twins. Neurobiology of Aging. 2002a;23:413–420. doi: 10.1016/s0197-4580(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, DeCarli C, Reed T. Quantitative genetic modeling of regional brain volumes and cognitive performance in older male twins. Biological Psychology. 2002b;61:139–155. doi: 10.1016/s0301-0511(02)00056-x. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Wolf PA, Miller BL, DeCarli C. Midlife cardiovascular risk factors and brain morphology in identical older male twins. Neurology. 1999;52:1119–1124. doi: 10.1212/wnl.52.6.1119. [DOI] [PubMed] [Google Scholar]
- Casey BJ, de Haan M. Introduction: New methods in developmental science. Developmental Science. 2002;5:265–267. [Google Scholar]
- Côté C, Beauregard M, Girard A, Mensour B, Mancini-Marie A, Perusse D. Individual variation in neural correlates of sadness in children: A twin fMRI study. Human Brain Mapping. 2007;6:482–487. doi: 10.1002/hbm.20400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert MA, Leonard CM, Molloy EA, Blumenthal J, Zijdenbos A, Giedd JN. The epigenesis of planum temporale asymmetry in twins. Cerebral Cortex. 2002;12:749–755. doi: 10.1093/cercor/12.7.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger U, Picchioni M, Landau S, Matsumoto K, Van Haren NE, Marshall N, Hall MH, Schulze K, Toulopoulou T, Davies N, Ribchester T, McGuire PK, Murray RM. Magnetic resonance imaging of the thalamus and adhesio interthalamica in twins with schizophrenia. Archives of General Psychiatry. 2007;64:401–409. doi: 10.1001/archpsyc.64.4.401. [DOI] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidekker MA, Evertsz CJ, Fitzek C, Boor S, Andresen R, Falkai P, Stoeter P, Peitgen HO. Projecting the sulcal pattern of human brains onto a 2D plane — A new approach using potential theory and MRI. Psychiatry Research. 1998;83:75–84. doi: 10.1016/s0925-4927(98)00029-8. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RCW, Baaré WF, van Oel C, Van Haren NE, Collins DL, Evans AC, Amunts L, Burgel U, Zilles K, De Geus E, Boomsma DI, Kahn RS. Genetic contributions to human brain morphology and intelligence. Journal of Neuroscience. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsgodt KH, Glahn DC, van Erp TGM, Therman S, Huttunen M, Manninen M, Kaprio J, Cohen MS, Lonnqvist J, Cannon TD. The relationship between performance and fMRI signal during working memory in patients with schizophrenia, unaffected co-twins, and control subjects. Schizophrenia Research. 2007;89:191–197. doi: 10.1016/j.schres.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Le Goualher G, Argenti AM, Duyme M, Baaré WF, Hulshoff Pol HE, Boomsma DI, Zouaoui A, Barillot C, Evans AC. Statistical sulcal shape comparisons: Application to the detection of genetic encoding of the central sulcus shape. Neuroimage. 2000;11:564–574. doi: 10.1006/nimg.2000.0559. [DOI] [PubMed] [Google Scholar]
- Lohmann G, von Cramon DY, Steinmetz H. Sulcal variability of twins. Cerebral Cortex. 1999;9:754–763. doi: 10.1093/cercor/9.7.754. [DOI] [PubMed] [Google Scholar]
- Mohr A, Knauth M, Weisbrod M, Stippich C, Sartor K. Similarity of the brains of twins. Rofo. 2001;173:515–521. doi: 10.1055/s-2001-14994. [DOI] [PubMed] [Google Scholar]
- Mohr A, Weisbrod M, Schellinger P, Knauth M. The similarity of brain morphology in healthy monozygotic twins. Brain Research Cognitive Brain Research. 2004;20:106–110. doi: 10.1016/j.cogbrainres.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Narr KL, Van Erp TG, Cannon TD, Woods RP, Thompson PM, Jang S, Blanton R, Poutanen VP, Huttunen M, Lonnqvist J, Standerskjold-Nordenstam CG, Kaprio J, Mazziotta JC, Toga AW. A twin study of genetic contributions to hippocampal morphology in schizophrenia. Neurobiology of Disease. 2002;11:83–95. doi: 10.1006/nbdi.2002.0548. [DOI] [PubMed] [Google Scholar]
- Oppenheim JS, Skerry JE, Tramo MJ, Gazzaniga MS. Magnetic resonance imaging morphology of the corpus callosum in monozygotic twins. Annals of Neurology. 1989;26:100–104. doi: 10.1002/ana.410260117. [DOI] [PubMed] [Google Scholar]
- Pennington BF, Filipek PA, Lefly D, Chhabildas N, Kennedy DN, Simon JH, Filley CM, Galaburda A, DeFries JC. A twin MRI study of size variations in human brain. Journal of Cognitive Neuroscience. 2000;12:223–232. doi: 10.1162/089892900561850. [DOI] [PubMed] [Google Scholar]
- Peper JS, Brouwer RM, Boomsma DI, Kahn RS, Pol HEH. Genetic influence on human brain structure: A review of brain imaging studies in twins. Human Brain Mapping. 2007;28:464–473. doi: 10.1002/hbm.20398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Carmelli D. Morphological changes in aging brain structures are differentially affected by time-linked environmental influences despite strong genetic stability. Neurobiology of Aging. 2004;25:175–183. doi: 10.1016/s0197-4580(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Swan GE, Carmelli D. Brain structure in men remains highly heritable in the seventh and eighth decades of life. Neurobiology of Aging. 2000;21:63–74. doi: 10.1016/s0197-4580(00)00086-5. [DOI] [PubMed] [Google Scholar]
- Posthuma D, Baaré WF, Hulshoff Pol HE, Kahn RS, Boomsma DI, De Geus EJ. Genetic correlations between brain volumes and the WAIS-III dimensions of verbal comprehension, working memory, perceptual organization, and processing speed. Twin Research. 2003;6:131–139. doi: 10.1375/136905203321536254. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baaré WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Neale MC, Hulshoff Pol HE, Baaré WEC, Kahn RS, Boomsma D. Multivariate genetic analysis of brain structure in an extended twin design. Behavior Genetics. 2000;30:311–319. doi: 10.1023/a:1026501501434. [DOI] [PubMed] [Google Scholar]
- Reed T, Pfefferbaum A, Sullivan EV, Carmelli D. Influences of chorion type on measurements of the corpus callosum in adult monozygotic male twins? American Journal of Human Biology. 2002;14:338–346. doi: 10.1002/ajhb.10027. [DOI] [PubMed] [Google Scholar]
- Reiss AL, Eliez S, Schmitt JE, Patwardhan A, Haberecht M. Brain imaging in neurogenetic conditions: Realizing the potential of behavioral neurogenetics research. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6:186–197. doi: 10.1002/1098-2779(2000)6:3<186::AID-MRDD6>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Reveley AM, Reveley MA, Chitkara B, Clifford C. The genetic-basis of cerebral ventricular volume. Psychiatry Research. 1984;13:261–266. doi: 10.1016/0165-1781(84)90041-6. [DOI] [PubMed] [Google Scholar]
- Reveley AM, Reveley MA, Clifford C, Murray RM. Cerebral ventricular size in twins discordant for schizophrenia. Lancet. 1982;1:540–541. doi: 10.1016/s0140-6736(82)92047-5. [DOI] [PubMed] [Google Scholar]
- Rijsdijk FV, Van Haren NE, Picchioni MM, McDonald C, Toulopoulou T, Hulshoff Pol HE, Kahn RS, Murray R, Sham PC. Brain MRI abnormalities in schizophrenia: Same genes or same environment? Psychological Medicine. 2005;35:1–11. doi: 10.1017/S0033291705005167. [DOI] [PubMed] [Google Scholar]
- Scamvougeras A, Kigar DL, Jones D, Weinberger DR, Witelson SF. Size of the human corpus callosum is genetically determined: An MRI study in mono and dizygotic twins. Neuroscience Letters. 2003;338:91–94. doi: 10.1016/s0304-3940(02)01333-2. [DOI] [PubMed] [Google Scholar]
- Schmitt JE, Wallace GW, Rosenthal MA, Molloy EA, Ordaz S, Lenroot R, Chasen LS, Blumenthal JD, Kendler KS, Neale MC, Giedd JN. A multivariate analysis of neuroanatomic relationships in a genetically informative pediatric sample. Neuroimage. 2007;35:70–82. doi: 10.1016/j.neuroimage.2006.04.232. [DOI] [PubMed] [Google Scholar]
- Spaniel F, Tintera J, Hajek T, Horacek J, Dezortova M, Hajek M, Dockery C, Kozeny J, Hoschl C. Language lateralization in monozygotic twin pairs concordant and discordant for schizophrenia. A fMRI pilot study. Schizophrenia Research. 2006;81:152. doi: 10.1016/j.eurpsy.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Herzog A, Huang Y, Hacklander T. Discordant brain-surface anatomy in monozygotic twins. New England Journal of Medicine. 1994;331:951–952. doi: 10.1056/nejm199410063311419. [DOI] [PubMed] [Google Scholar]
- Steinmetz H, Herzog A, Schlaug G, Huang Y, Jancke L. Brain (A) symmetry in monozygotic twins. Cerebral Cortex. 1995;5:296–300. doi: 10.1093/cercor/5.4.296. [DOI] [PubMed] [Google Scholar]
- Styner M, Lieberman JA, McClure RK, Weinberger DR, Jones DW, Gerig G. Morphometric analysis of lateral ventricles in schizophrenia and healthy controls regarding genetic and disease-specific factors. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:4872–4877. doi: 10.1073/pnas.0501117102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A, Swan GE, Carmelli D. Heritability of hippocampal size in elderly twin men: Equivalent influence from genes and environment. Hippocampus. 2001;11:754–762. doi: 10.1002/hipo.1091. [DOI] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine and Tobacco Research. 1999;1:S51–S57. doi: 10.1080/14622299050011811. [DOI] [PubMed] [Google Scholar]
- Terriberry TB, Joshi SC, Gerig G. Hypothesis testing with nonlinear shape models. Information Processing in Medical Imaging, Proceedings. 2005;3565:15–26. doi: 10.1007/11505730_2. [DOI] [PubMed] [Google Scholar]
- Thompson P, Cannon TD, Toga AW. Mapping genetic influences on human brain structure. Annals of Medicine. 2002;34:523–536. doi: 10.1080/078538902321117733. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, Van Erp T, Poutanen VP, Huttunen M, Lonnqvist J, Standertskjold-Nordenstam CG, Kaprio J, Khaledy M, Dail R, Zoumalen CI, Toga AW. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Loftus WC, Stukel TA, Green RL, Weaver JB, Gazzaniga MS. Brain size, head size, and intelligence quotient in monozygotic twins. Neurology. 1998;50:1246–1252. doi: 10.1212/wnl.50.5.1246. [DOI] [PubMed] [Google Scholar]
- Van Erp TG, Saleh PA, Huttunen M, Lonnqvist J, Kaprio J, Salonen O, Valanne L, Poutanen VP, Standerskjold-Nordnestam CG, Cannon TD. Hippocampal volumes in schizophrenic twins. Archives of General Psychiatry. 2004;61:346–353. doi: 10.1001/archpsyc.61.4.346. [DOI] [PubMed] [Google Scholar]
- Van Haren NE, Picchioni MM, McDonald C, Marshall N, Davis N, Ribchester T, Hulshoff Pol HE, Sharma T, Sham P, Kahn RS, Murray R. A controlled study of brain structure in monozygotic twins concordant and discordant for schizophrenia. Biological Psychiatry. 2004;56:454–461. doi: 10.1016/j.biopsych.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Wallace GW, Schmitt JE, Lenroot R, Viding E, Ordaz S, Rosenthal MA, et al. A pediatric twin study of brain morphometry. Journal of Child Psychology and Psychiatry, and Allied Disciplines. 2006;47:987–993. doi: 10.1111/j.1469-7610.2006.01676.x. [DOI] [PubMed] [Google Scholar]
- White T, Andreasen NC, Nopoulos P. Brain volumes and surface morphology in monozygotic twins. Cerebral Cortex. 2002;12:486–493. doi: 10.1093/cercor/12.5.486. [DOI] [PubMed] [Google Scholar]
- Wright IC, Sham P, Murray RM, Weinberger DR, Bullmore ET. Genetic contributions to regional variability in human brain structure: Methods and preliminary results. Neuroimage. 2002;17:256–271. doi: 10.1006/nimg.2002.1163. [DOI] [PubMed] [Google Scholar]