Abstract
Bisphenol A (BPA) was administered by gavage (2.5–300,000 μg/kg body weight (bw)/day) to pregnant Sprague Dawley dams, newborn pups, and continuing into adulthood. Aglycone (i.e., unconjugated and active) and conjugated (i.e., inactive) BPA were evaluated by liquid chromatography electrospray tandem mass spectrometry (LC-ES/MS/MS) in serum to better interpret toxicological endpoints measured in the study. Ethinyl estradiol (EE2, 0.5 and 5 μg/kg bw/day) and the endogenous hormones, 17β-estradiol (E2) and testosterone, were similarly evaluated. Mean BPA aglycone levels in vehicle and naïve control rat serum (0.02–0.5 ng/ml) indicated sample processing artifact, consistent with literature reports of a propensity for postexposure blood contamination by BPA. Direct measurements of BPA-glucuronide in vehicle and naïve control serum (2–10nM) indicated unintentional exposure and metabolism at levels similar to those produced by 2.5 μg/kg bw/day BPA (7–10nM), despite careful attention to potential BPA inputs (diet, drinking water, vehicle, cages, bedding, and dust) and rigorous dosing solution certification and delivery. The source of this exposure could not be identified, but interpretation of the toxicological effects, observed only at the highest BPA doses, was not compromised. Internal exposures to BPA and EE2 aglycones were highest in young rats. When maximal serum concentrations from the two highest BPA doses and both EE2 doses were compared with concurrent levels of endogenous E2, the ERα binding equivalents were similar to or above those of endogenous E2 in male and female rats of all ages tested. Such evaluations of estrogenic internal dosimetry and comprehensive evaluation of contamination impact should aid in extrapolating risks from human BPA exposures.
Keywords: bisphenol A, ethinyl estradiol, pharmacokinetics, estrogen receptor, mass spectrometry
Bisphenol A (BPA) is an industrial chemical used extensively in the manufacture of polycarbonate plastic products and epoxy resins used for food can liners (National Toxicology Program, 2008). The U.S. Food and Drug Administration regulates BPA-containing products including food-contact materials and medical devices. Regulatory risk assessments of endocrine-active chemicals like BPA often incorporate data from animal toxicology studies that include explicit evaluation of hazards from perinatal exposure because aberrant developmental programming can induce irreversible adverse changes in phenotype.
The potential for toxicity of BPA exposure was evaluated in a subchronic study that included fetal, neonatal, and adult life stages (see Delclos et al., 2014). Pregnant Sprague Dawley rats were exposed by daily gavage to doses of BPA ranging from 2.5 to 300,000 μg/kg body weight (bw)/day starting on gestation day (GD) 6 until parturition. Direct daily gavage dosing of pups continued with starting on postnatal day (PND) 1 through termination of the study. For a point of reference, the lowest BPA dose tested in this study is approximately 70-fold above median American aggregate daily exposure (0.037 μg/kg bw/day; Lakind et al., 2012).
An important element of this study, which was jointly sponsored by the National Toxicology Program and the U.S. Food and Drug Administration (Birnbaum et al., 2013), was an evaluation of BPA internal dosimetry because its pharmacokinetics are well known to change during fetal and neonatal development in rodents (Doerge et al., 2010a), a phenomenon not observed in nonhuman primates (Doerge et al., 2010b). The critical role of phase II metabolism in detoxification made it important to measure both the aglycone (i.e., unconjugated and active) and conjugated (i.e., inactive glucuronide and sulfate) forms of BPA. Although many molecular and cell-based studies suggest the potential for interaction of BPA with many receptor systems and cellular targets (e.g., thyroid hormone, aryl hydrocarbon, androgen, and aromatase) as possible toxic modes of action, a prominent hypothesis for adverse potential of BPA centers on estrogenic mechanisms, via classical nuclear and nonclassical membrane-bound receptors (Richter et al., 2007). Therefore, it was also important to evaluate the internal dosimetry from concurrent treatment with a reference estrogen, EE2, as well as endogenous levels of the major steroid hormones, 17β-estradiol (E2) and testosterone, both of which are known to vary throughout life stages. This paper describes the analytical strategies used to determine these internal exposures and uses this information to compare relative estrogen receptor (ER) occupancy as a contributor to dose-dependent estrogenic effects reported in the companion paper (Delclos et al., 2014).
A goal of these two studies was to evaluate whether life-stage-dependent internal dosimetry of estrogenic compounds could be used to evaluate critically the hypothesis that aberrant estrogenic signaling during critical developmental windows could manifest as phenotypical changes in the adult animal. This goal is important for human risk assessment because consistency between internal dosimetry, receptor-mediated signaling, and demonstrably adverse effects in animal models would facilitate the interpretation of estrogenic mechanisms in the endocrine disruption debate.
EXPERIMENTAL
Materials
All high pressure liquid chromatography (HPLC) solvents including water were Optima LC/MS grade purchased from Fisher Scientific (Pittsburgh, PA). Native BPA, EE2, β-glucuronidase/sulfatase (Helix pomatia, H1, 16 units/mg), testosterone, and all other chemical reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO). The isotopically labeled internal standards 13C12-BPA, 13C6-E2, and d4-EE2 were obtained from Cambridge Isotope Labs (Andover, MA). The d6-BPA (99.5 atom%) was obtained from CDN Isotopes (Pointe-Claire, Quebec). The native and d3-testosterone (99.5 atom%) were purchased from Sigma-Aldrich Co. The unlabeled BPA-glucuronide (BPA-G) and 13C12-BPA-G were kind gifts from the National Toxicology Program. Sprague Dawley rat serum (unfiltered) was purchased from Bioreclamation LLC (Westbury, NY; lot no. 187248) and analyzed using LC/MS/MS. Only uncontaminated lots of serum [i.e., BPA levels below limit of blank (LOB)] were used subsequently as negative control samples and for spiking with known amounts of authentic standards. Certified reference standards of frozen male and female human serum were obtained from the National Institute of Standards and Technology (NIST, Gaithersburg, MD).
Animal Handling Procedures
Procedures involving care and handling of rats were reviewed and approved by the NCTR Laboratory Animal Care and Use Committee. Sprague Dawley rats, obtained from the NCTR colony, were maintained on a soy-free basal diet (irradiated Purina 5K96 pellets; Test Diets, Purina Mills, Richmond, IN).
For the d6-BPA pharmacokinetic study, Sprague Dawley pups were delivered and culled on PND 1 to 3–5 males and 3–5 females per litter to provide one pup per sex from each litter for blood collection at each of the postdose time points (body weights 10.5 ± 0.79 g). Pups were treated with a single gavage dose of d6-BPA in 0.3% CMC at 100 μg/kg bw on PND 4 and blood was collected by cardiac puncture at various times after dosing (untreated controls, 0.25, 0.5, 1, 2, 4, 8, and 24 h). At each postdose time point, groups of four pups (two males and two females) were sacrificed by CO2 asphyxiation after the dose administration. The concentration of the dosing solution was verified immediately prior to dosing by LC/MS/MS analysis to be within 10% of target concentration (data not shown).
In the 90-day subchronic study, pregnant Sprague Dawley rats were exposed by daily gavage starting on gestation day (GD) 6 until the initiation of labor. Direct daily gavage dosing of pups continued with starting on postnatal day (PND) 1 through termination of the study. The 13 treatment groups in this study included a vehicle control of 0.3% carboxymethyl-cellulose in water (CMC), an untreated group (naïve control), two reference estrogen groups (ethinyl estradiol, EE2, at 0.5 and 5.0 μg/kg bw/day), and nine BPA doses (2.5, 8, 25, 80, 260, 840, 2700, 100,000, and 300,000 μg/kg bw/day). The PND 80 females were staged to be in estrus at the time of blood collection, which occurred within a time period of 7:30–11:00 a.m. The pup body weights for the respective studies were: PND 4 dosimetry, 10.8 ± 3.1 g; PND 21 dosimetry, 53.5 ± 5.8 g; PND 80 dosimetry, 361.0 ± 102.2 g. Total serum volumes collected varied but were typically 100–200 μl at PND 4 and >200 μl for PND 21 and PND 80.
The PND 4 and PND 21 blood samples were collected by cardiac puncture using a 1 ml tuberculin syringe with a 25 G × 5/8″ needle (BD, Franklin Lakes, NJ, no. 309626), and the PND 80 blood samples were collected from the lateral tail vein using a SurSaver 1 ml syringe with 23 G × 1/2″ fixed needle (Terumo Medical Corporation, Elkton, MD, no. SS01D2313). PND 4 blood samples were transferred into Fisherbrand disposable culture tubes, lime glass, 6 × 50 mm (Fisher Scientific, no. 14-958-A), whereas PND 21 and PND 80 blood samples were collected into Fisherbrand disposable culture tubes, borosilicate glass, 12 × 75 mm (Fisher Scientific, no. 14-961-26). In all cases, serum was transferred from the glass tube to an Eppendorf Safe-lock polypropylene tube (Eppendorf) using a Fisherbrand Pasteur pipette, 53/4 disposable, borosilicate glass (Fisher Scientific, no. 13-678-20B). The syringes, needles, polypropylene Eppendorf tubes, and all glass tubes used were evaluated for contamination by rinsing with either methanol or water (1 ml), to which was added a known amount of 13C12-BPA internal standard (1 ng) before analyzing for BPA using LC/MS/MS as described below. The syringes, needles, glass, and polypropylene tubes selected for use in blood collection and processing were found to contain levels of BPA at or below the daily LOB (see below). It should be noted that these procedures did identify consistent contamination in rinsates from tubes that were subsequently rejected: 0.1–14 ng/ml in 2.0 ml screwcap cryo-vials (United Laboratory Plastics, St. Louis, MO, no. UP 2031); and 0.2–1.2 ng/ml in CryoElite vials, Wheaton, Millville, NJ, part no. 985868; 0.1–13 ng/ml in Microtainer tubes (BD, Franklin Lakes, NJ, no. 365957); 0.2–1.2 ng/ml in Sample cup blue Roche-USA, part no. 06789).
The volume of blood collected from PND 4 pups was often limited and the numbers of pups with adequate sample volumes for analysis of both aglycone and total BPA varied but were between 4 and 10 per sex, with roughly balanced numbers of males and females in each dose group. Based on the initial PK study where Cmax was determined to be 0.25 h for aglycone d6-BPA and 1 h for total d6-BPA, blood was collected in the time window between 0.25 and 0.75 h postdosing as required to accommodate the large numbers of pups being treated. The sampling times were recorded, and the mean was 0.47 ± 0.15 h on PND 4, 0.53 ± 0.17 h on PND 21, and 0.43 ± 0.03 h on PND 80. Blood samples were allowed to clot at room temperature, serum was obtained by centrifugation, and samples were stored at –80°C until analyzed.
Input media that could potentially contribute background native BPA exposure to the rats were evaluated. Analysis of BPA in the diet, CMC vehicle, drinking water, bedding, and cage materials is detailed in the companion paper (Delclos et al., 2014). A post hoc evaluation of other possible sources of exposure within the animal rooms was conducted to see whether aerosol/dust distribution could contribute. In a subsequent ongoing BPA dosing study, replicate cages fitted with sealed isolator tops were sampled using cotton swabs by rubbing over the entire surfaces, either interior or exterior, from control, 250 μg BPA/kg bw/day and 25,000 μg BPA/kg bw/day dose group cages. The replicate cages were selected from shelves located on the top of the cage rack, the middle, and the bottom (n = 3 per dose group). The used cotton swab was inserted directly into a silanized glass vial and eluted using methanol (1 ml total volume), to which was added a known amount of 13C12-BPA internal standard (1 ng) before analysis using LC/MS/MS as described below. The respective control/medium/high dose group cage mean total swab levels (n = 3) samples on the inside were 0.1, 0.1, and 0.6 ng BPA and on the outside 0.2, 0.4, and 2.3 ng total. The methanol blank swab contained 0.2 ng total and the blank mean + 2 SD was subtracted from each of the cage values. Levels of BPA glucuronide (BPA-G) were similarly evaluated and only the inside swab from the 25,000 μg/kg bw/day dose group cages contained above 0.1 ng (0.06 ng BPA-equivalent). The methanol blank swab contained 0.1 ng BPA-G that was subtracted from each of the cage values.
Pharmacokinetic Analysis
Plots of serum concentrations of total and aglycone d6-BPA versus time following gavage administration to PND 4 rats were analyzed using model-independent pharmacokinetic analysis (PK Solutions 2.0 software, Summit Research Services, Montrose, CO). Log-linear plots of group mean time point values were fit to three kinetic phases corresponding to elimination, distribution, and absorption. The first-order rate constants were determined from the slope of the respective phases after subtracting the contribution from the terminal elimination phase of the respective curve (i.e., feathering). Internal exposures to BPA were determined either as AUC0–∞, determined by using the trapezoidal rule, or as Cmax, which was determined by visual inspection and occurred at Tmax.
Statistical Analysis
Pharmacokinetic parameters for d6-BPA from PND 4 rats were determined from plots of mean values at each time point (n = 4, two males and two females). Serum concentration measurements for each native BPA dose group were comprised of roughly equal numbers of males and females and because no significant pharmacokinetic sex differences were observed previously (Doerge et al., 2010a), the sexes were combined. Data are expressed as means ± standard deviation (SD).
LC/MS/MS Quantification Methodologies
Native (unlabeled) BPA aglycone
The 13C12-BPA internal standard was validated as previously described (Twaddle et al., 2010). LC and tandem MS parameters for online column switching analysis of native BPA are those reported previously (Teeguarden et al., 2011). Calibration curves were linear over the ranges of 0 to 300/3000/300,000 ng/ml (1.3μM/13μM/1.3mM), based on the serum volume used, with a slope of 1.02. Pools of control rat serum and incurred rat serum were prepared for use, in duplicate, as daily quality control checks. In addition, four enzyme blanks (H. pomatia glucuronidase/sulfatase mixture) or four aglycone procedural blanks were also prepared with each sample set to establish background BPA levels from sample preparation (i.e., LOB). The method was validated over two days using negative control serum (i.e., BPA < LOB), spiked negative control serum, and incurred serum. The use of the incurred serum that contained both aglycone and conjugated BPA was used to validate completeness of enzyme hydrolysis for analysis of total BPA. Overnight incubation (>16 h) at 37ºC was used throughout to ensure total enzymatic hydrolysis even though hydrolysis was typically complete after times <4 h. Control rat serum was spiked at 1.0 and 1.8 ng/ml (2.3 and 7.9nM) for analysis of aglycone and total BPA, respectively, in 100 μl serum samples. For 10 μl serum samples, control serum was spiked at 10 ng/ml, and for total BPA analysis an incurred serum sample containing 30 ng/ml was used.
Method validation and quality control
The validation of the online column switching LC/MS/MS method was reported previously (Teeguarden et al., 2011). Measurable responses for BPA were observed in all procedural blanks because trace level contamination by native BPA is difficult to avoid (Ye et al., 2013; Teeguarden et al., 2011; Twaddle et al., 2010). Accordingly, four replicate procedural blanks were analyzed with each sample set to determine a daily LOB. These samples replaced serum with water and went through the entire sample preparation process. The LOB was defined as the mean value + 2 SD of the replicates and the daily LOB was subtracted from each serum sample concentration (with enzymatic hydrolysis, 0.5–1.8nM; without enzyme, 0.3–1.1nM). In addition, daily limits of detection (LOD) were estimated from the amount of BPA producing a signal/noise ratio >3 above the LOB (with enzymatic hydrolysis, 0.2–1.1nM; without enzyme, 0.1–0.4nM). If the sample quantification value after subtraction of the LOB was not higher than the daily calculated LOD, it was reported as <LOD. Validation was performed on both 10 and 100 μl aliquots of serum. Intra- and interday precision ranged from 0.6 to 5.3% RSD. Intra- and interday accuracy ranged from 98 to 105%.
d6-BPA
Analysis of d6-BPA was performed by using a validated high throughput LC-ES/MS/MS method as previously described (Twaddle et al., 2010). Briefly, labeled internal standard (13C12-BPA) was added to each thawed serum sample (10–100 μl), then samples were purified using supported liquid extraction in 96-well plates and analyzed using LC-ES/MS/MS in the multiple reaction monitoring mode of specific negative ion transitions for d6- and 13C12-BPA. Total serum concentrations of d6-BPA (i.e., aglycone + conjugates) were quantified following incubation with a H. pomatia glucuronidase/sulfatase mixture. Special precautions, similar to those previously published (Twaddle et al., 2010), were used to minimize adventitious hydrolysis of conjugates during sample preparation and no evidence for hydrolysis was observed. Method validation included replicate analysis of spiked serum at levels from 0.4 to 40nM with accuracy and precision ranging from 89 to 105% and 2.8 to 18%, respectively, as previously described (Twaddle et al., 2010). The method detection limit (LOD) for d6-BPA, defined as a signal/noise ratio of greater than or equal to 3, was approximately 0.2nM (0.05 ng/ml) in 100 μl serum. When calculating group mean values, samples <LOD were replaced with a value of 1/2LOD (0.1nM).
BPA-glucuronide
The standard method of quantifying conjugated BPA as the aglycone after an enzymatic hydrolysis step (i.e., indirect) was confirmed by developing and validating an LC-ES/MS/MS method for direct quantification of the major BPA metabolite in rats, BPA-G, using an isotopically labeled internal standard. The purity of isotopically labeled 13C12-BPA-G internal standard and unlabeled BPA-G was characterized by liquid chromatography with ultraviolet detection (LC-UV) (280 nm) and full-scan LC-ES/MS/MS. The transitions and voltages used to analyze labeled and unlabeled BPA-G are listed in Supplementary table 1. The labeled BPA-G was found to contain 0.02% unlabeled BPA-G and 0.01% 13C12-BPA. The time course (1–16 h) and extent of enzymatic hydrolysis of the unlabeled BPA-G (5–5.7 ng/ml, 12–14nM) were evaluated in spiked negative control serum and water using the procedures described previously (Twaddle et al., 2010). By 1 h, the reaction was ∼90% complete, and by 16 h (the overnight incubation time used throughout), 94 ± 1.2 to 102 ± 1.9% (mean ± SD, n = 3 at each time point) of the expected BPA was observed on two separate days of analysis. The acetonitrile extraction and online LC/MS/MS procedures used were modifications of those described above for native BPA (not shown) and the method was validated using negative control rat serum for spiking and blank determinations. BPA-G was <LOD in the rat serum sample tested, and serum spiked at 1 ng/ml (2.5nM) showed 101 ± 2.7% recovery (n = 20 over two separate days). The LOD for 100 μl serum was approximately 0.4-0.6 nM.
E2 and EE2
Preparation of standards and quality control samples
The purities of labeled E2 and EE2 were evaluated by LC-UV (220 nm detection) and full scan LC/MS (negative ion ES detection). Labeled EE2 and E2 contained undetectable levels of the unlabeled compound (<0.04%). Concentrations of labeled and unlabeled E2 and EE2 solutions were matched by using LC-UV (220 nm detection). Calibration curves were generated by derivatizing varying concentrations of unlabeled E2 and EE2, while keeping the internal standard concentrations constant. The curves were linear over the ranges of 0–1000 pg/ml with slopes of 0.97 and 1.15 for E2 and EE2, respectively. Pools of negative control rat serum and negative control rat serum spiked at 10 pg/ml were prepared for use as daily quality control checks.
Sample preparation
Published procedures using liquid-liquid extraction and dansyl chloride derivatization were adapted for the analysis of E2 and related phenolic compounds (Kushnir et al., 2008 ; Xiong et al., 2010; Xu et al., 2007). Briefly, 100 μl of serum plus 10 μl internal standards were extracted with an equal volume of methyl-t-butyl ether (MTBE), the organic layer was separated using a glass pipet, transferred to a 96-well plate, and then evaporated to dryness. The residue was derivatized with dansyl chloride (1 mg/ml in acetone) and sodium bicarbonate (0.1M in water) by heating at 60°C for 10 min. A 20 μl aliquot was injected into the UPLC column. For analysis of total E2 and EE2, serum was first enzymatically deconjugated at 37ºC overnight with β-glucuronidase/sulfatase (3.2 units). Then 200 μl of water was added to each sample and the samples were extracted using supported liquid extraction plate with MTBE as the elution solvent (Biotage, Charlotte, NC). The MTBE was evaporated and the samples were derivatized as described above.
Liquid chromatography was conducted with an Acquity UPLC system (Waters, Inc., Milford, MA), a 1260 Infinity HPLC pump (Agilent, Santa Clara, CA), and an automated six port switching valve (Rheodyne, Cotati, CA). The switching valve was used to divert the HPLC column effluent either to waste or the mass spectrometer. The Agilent pump was used to provide continuous flow to the mass spectrometer when the column effluent was diverted to waste. The analytes were separated using a Luna Phenyl-Hexyl column (2.0 × 150 mm, 3 μm particle size, Phenomenex, Torrance, CA). The mobile phase consisted of 0.01% formic acid in both water and acetonitrile. The gradient program consisted of 60% acetonitrile for 2 min and then a linear gradient up to 95% acetonitrile from 2 to 12 min. The column was held at 95% acetonitrile for an additional 3 min before resetting to starting conditions. The total run time was 19 min and the flow rate was 0.3 ml/min with a column temperature of 30°C. The analytical column was inline with the mass spectrometer from 7 to 9.7 min and diverted to waste for all remaining time. The Agilent pump ran isocratically with 0.01% formic acid in 20% water and 80% acetonitrile at 0.3 ml/min. An Acquity CSH Phenyl-Hexyl column (2.1 × 100 mm, 1.7 μm particle size, Waters, Inc.) was used for separation of enzymatically deconjugated samples. The gradient program consisted of 55% acetonitrile for 2 min followed by a linear gradient up to 75% acetonitrile from 2 to 7 min and then stepped up to 95% acetonitrile. The column was held at a temperature of 50°C and eluted with 95% acetonitrile for an additional 3 min before resetting to starting conditions. The total run time was 13.5 min. The flow rate was 0.5 ml/min for 2 min and then a linear flow gradient from 0.5 to 0.55 was used over the 2 to 7 min time period. The analytical column was inline with the mass spectrometer from 5.3 to 7 min and diverted to waste for all remaining time. The Agilent pump ran isocratically with 0.01% formic acid in 20% water and 80% acetonitrile at 0.5 ml/min.
A Xevo TQ-S triple quadrupole mass spectrometer (Waters, Inc.) equipped with an electrospray interface was used in selected reaction monitoring mode for analysis of positive ions. The transitions monitored for E2 and EE2 and the corresponding voltages and energies are shown in Supplementary table 1. Other MS parameters included source and desolvation temperatures of 150°C and 500°C, respectively, argon as collision gas (0.15 ml/min) and nitrogen as the desolvation (1000 l/h) and cone gas (150 l/h).
Method Validation and Quality Control
Various extraction, cleanup, and separation parameters were optimized for quantification of E2 and EE2 in serum. The methods were validated for 100 μl aliquots of negative control rat serum over two days using replicates of commercial charcoal-stripped male rat serum, spiked at two levels (10 and 100 pg/ml), pooled control serum, and spiked pooled control serum (10 pg/ml). Intra- and interday precision was expressed as relative standard deviation (RSD), which ranged from 2.1 to 13% for E2 and from 0.6 to 5.5% for EE2. Intra- and interday accuracy ranged from 83 to 94% for E2 and from 89 to 106% for EE2. The LOD for both E2 and EE2 was 1.5 pg/ml (5pM). When calculating group mean values, samples <LOD were replaced with a value of 1/2LOD (0.8 pg/ml). Levels of EE2 were determined in control rats (combined naïve and vehicle-treated) and EE2 dosed rats with group sizes of five males and five to seven females at each age. Levels of E2 were determined from control rats (combined naïve and vehicle-treated) with group sizes of 10 males and 10–14 females at each age.
Testosterone
Testosterone was analyzed by isotope dilution LC/MS/MS using procedures based on those published previously (Hsing et al., 2007). Labeled testosterone contained undetectable levels of the unlabeled compound (<0.04%). Concentrations of labeled and unlabeled testosterone solutions were matched by using LC-UV (260 nm detection). Calibration curves were generated by varying the concentrations of unlabeled testosterone, although keeping the internal standard concentration constant. The transitions and voltages used to analyze labeled and unlabeled testosterone are listed in Supplementary table 1. Calibration curves were linear over the ranges of 0 to 1000 pg/ml with a slope of 1.0. Pools of control female rat serum, incurred serum containing 0.77 ng/ml, and control rat serum spiked at 5 ng/ml were prepared for use as daily quality control checks. Method validation included analysis of incurred and spiked female serum samples on two separate days and the analysis of standard reference materials (NIST frozen male and female human serum containing 6.43 and 0.28 ng/ml, respectively). Accuracy was 90% for the low range and 95% for the high range NIST standards and intra- and interday precision were 2.7–3.8% RSD on three separate days. The LOD for analysis of 100 μl serum was approximately 0.05 ng/ml (0.17nM). When calculating mean values, samples containing <LOD were replaced with a value of 1/2LOD (0.025 ng/ml).
RESULTS
Pharmacokinetics of Oral d6-BPA in PND 4 Rats
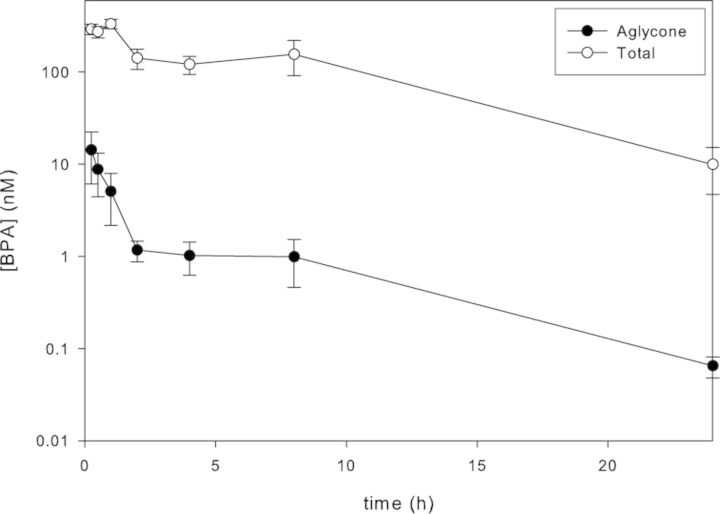
The serum concentration-time profiles for total and aglycone d6-BPA following gavage administration of 100 μg/kg bw to PND 4 pups are shown in Figure 1. Aglycone d6-BPA concentration was maximal at the initial sampling point (Cmax, 14nM at Tmax,15 min), whereas the Tmax for total d6-BPA was reached at 1 h. At Tmax (15 min), aglycone d6-BPA represented 4.9% of the total. The AUC0–∞ for aglycone d6-BPA was 26.3 nmol × h × l−1, which represented 0.97% of the total AUC (2698 nmol × h × l−1). The half-time for absorption, distribution, and terminal elimination of aglycone d6-BPA were 0.15, 2.6, and 4.1 h, respectively.
FIG. 1.
Serum concentration-time profiles for total and aglycone d6-BPA in PND 4 Sprague Dawley rats (mean ± SD, n = 4 pups at each time point) treated by gavage with a single dose of 100 μg d6-BPA/kg bw in 0.3% carboxymethylcellulose in water.
Analysis of BPA in Serum from Vehicle and Naïve Control Rats
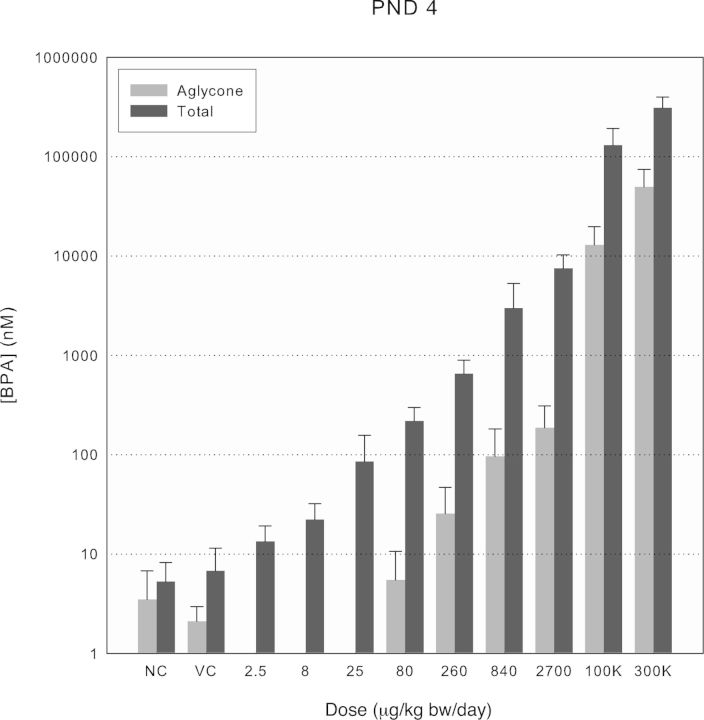
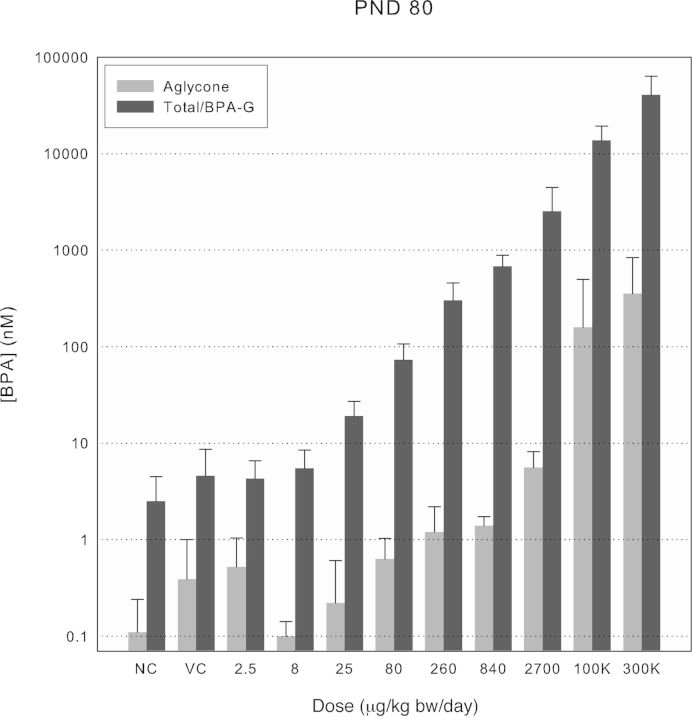
Analysis of serum samples from naïve and vehicle control rats of all ages consistently contained measurable levels of native aglycone BPA above the respective daily LOB with mean values in the range of 0.1–2nM (0.02–0.5 ng/ml; Figs. 2–4). The mean percentages of total BPA present as aglycone in the control serum samples (17–58%) exceeded those measured under controlled conditions in the oral PK study with d6-BPA (i.e., maximally 4.2% at Cmax in PND 4 pups; Fig. 1) and are best interpreted as resulting from contamination by native BPA during the sample collection and storage. The background levels were consistently higher in PND 4 and PND 21 pups than in the PND 80 adults, presumably because of the different blood withdrawal procedures and the volumes of blood collected (cardiac puncture in young rats and tail vein withdrawal in adults). The evidence for sample contamination by native BPA despite evaluation of all blood handling materials prior to sample collection underscores the difficulty of preventing contamination of serum by native BPA because of numerous sources in laboratories (e.g., dust, plastic blood collection, and laboratory products; Twaddle et al., 2010; Ye et al., 2013). As a result, measurements of aglycone BPA were often foregone in favor of using the limited sample volumes available for analysis of total BPA in rats receiving the 2.5, 8, and 25 μg/kg bw/day doses. This strategy was deemed appropriate because extrapolation from d6-BPA pharmacokinetic studies (Fig. 1 and Doerge et al., 2010a) showed the peak aglycone levels in serum (0.14nM per μg/kg bw dose unit in PND 4 rats) would be below those measured in the controls (Supplementary fig. 1). The levels of total BPA in naïve and vehicle control rats were consistently observed in the 1–10nM range (Figs. 2–4). Because the postexposure contamination of the blood samples by aglycone BPA made interpretation of total BPA levels difficult, it was deemed important to verify conjugated BPA levels directly as BPA-G. A high degree of concordance between the two methods was observed through paired sample comparisons of conjugated BPA (total − aglycone) versus BPA-G (e.g., in PND 21 controls, 105 ± 10%, n = 16; PND 80 controls, 100 ± 25%, n = 13). Small sample volumes precluded a similar comparative analysis for PND 4 serum. This concordance is also consistent with previous analyses using purified β-glucuronidase and sulfatase enzymes separately, which showed that BPA-G was the predominant BPA conjugate in rats (Doerge et al., 2010a).
FIG. 2.
Aglycone and total BPA serum concentrations at Cmax across treatment groups for PND 4 rats. Values shown by treatment group are means of either total or aglycone BPA ± SD for dosed males and females, combined (n ≥ 8). Aglycone BPA was not quantified in 2.5–25 μg/kg bw dose groups.
FIG. 4.
Aglycone and total BPA/BPA-glucuronide serum concentrations at Cmax across treatment groups for PND 80 rats. Values shown by treatment group are means of either total or aglycone BPA ± SD for dosed males and females, combined (n ≥ 8), and BPA-glucuronide combined means ± SD for naïve and vehicle control groups (NC, n = 17 and VC, n = 15, respectively).
Total BPA exposures in naïve and vehicle control rats produced blood concentrations of BPA-G similar to those observed from dosing with the two lowest doses (2.5 and 8 μg/kg bw/day, Figs. 2–4). Although the low level of BPA contamination measured in the basal diet (<5 ppb) would contribute to total native BPA in serum, this was deemed unlikely to contribute significantly. Daily consumption of as much as 5 μg BPA/kg diet at a rate of approximately 15% of body weight, even as a bolus, would result in <1 μg/kg bw contribution associated with a predicted 5pM increment at peak serum concentration based on pharmacokinetics in previous studies in PND 21 and adult rats (0.4–0.7nM for 100 μg/kg bw given by bolus gavage; Doerge et al., 2010a). Potential exposure to BPA from the vehicle, cage materials, bedding, and drinking water was also carefully evaluated and found to be minimal (Delclos et al., 2014), and the additional source(s) of exposure has not been identified.
Analysis of Serum from BPA-Treated Rats
Increases in serum levels for both aglycone and total BPA above levels measured in controls were observed for all ages of rats tested above approximately 80 μg/kg bw (Figs. 2–4). Although differences in levels of aglycone BPA observed in PND 21 and PND 80 rats were minimal, levels in PND 4 pups were consistently higher in accordance with the immaturity of phase II metabolism of BPA previously found in rodents, but not nonhuman primates (Doerge et al., 2010a; Yang et al., 2013). The fraction of total BPA present as aglycone was evaluated for doses above 80 μg/kg bw, where the impact of contamination was minimal, to evaluate further the possible impact of dose on phase II metabolism. The aglycone fraction was higher in PND 4 pups and appeared to diverge from the ∼4% range to >10% at the two highest doses (Supplementary fig. 1). No deviation from the 1–2% aglycone fraction was seen in PND 21 and PND 80 rats except for the highest dose of BPA in PND 21 rats.
The peak levels of aglycone and total d6-BPA determined under similar dosing conditions in the pharmacokinetic study for PND 4 pups (Fig. 1) and our previous evaluation of Cmax for d6-BPA gavage on PND 21 and PND 90 (Doerge et al., 2010a) made it possible to compare measured and simulated serum levels for all doses for newborn, weanling, and adult rats (Supplementary figs. 1–3).
Analysis of Serum from EE2-Treated Rats
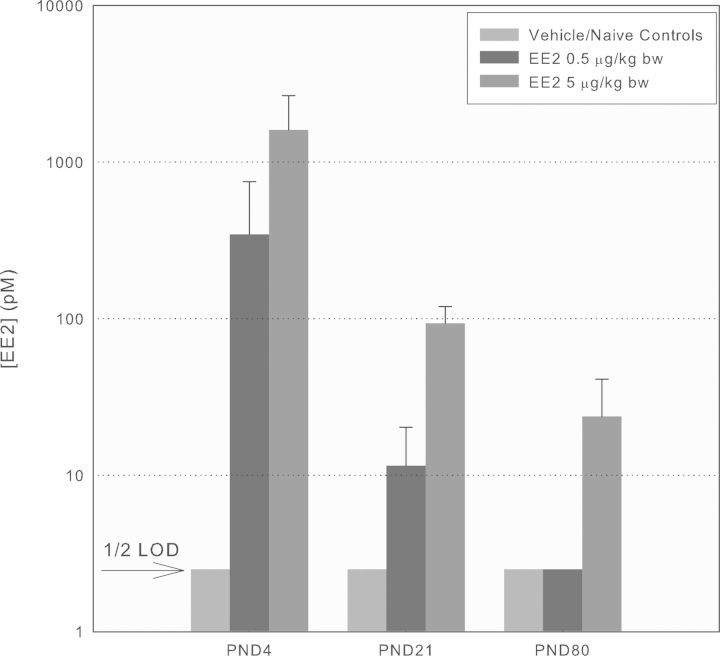
Serum levels of EE2 in male and female PND 4, PND 21, and PND 80 rats were determined in controls, 0.5 and 5 μg EE2/kg bw-treated rats using identical sample collection to that described for the BPA-treated rats (n = 5–7 per sex per group). Because there were no significant differences between levels in male and females (t-test), the values shown in Figure 5 are combined by treatment group. Serum levels of EE2 in vehicle and naïve control rats of all ages were consistently below the LOD (5pM or 1.5 pg/ml, Fig. 5). As seen with BPA, EE2 levels were far higher in PND 4 and decreased successively on PND 21 and PND 80. The effect of age on the degree of conjugation was evaluated in a subset of samples by comparing EE2 levels before and after total enzymatic deconjugation. On PND 4, 100% of EE2 was present unconjugated, on PND 21, 50% was unconjugated, and on PND 80, 10% was unconjugated (not shown).
FIG. 5.
EE2 Cmax values following gavage treatment of Sprague Dawley rats at different ages. EE2 concentrations (unconjugated) were quantified using LC/MS/MS from serum collected from male and female rats approximately 30 min after gavage treatment (mean ± SD, n = 5–7/sex/group/age) of the designated ages (note: log ordinate scale; LOD 5pM, 1.5 pg/ml).
Endogenous Steroid Hormone Levels
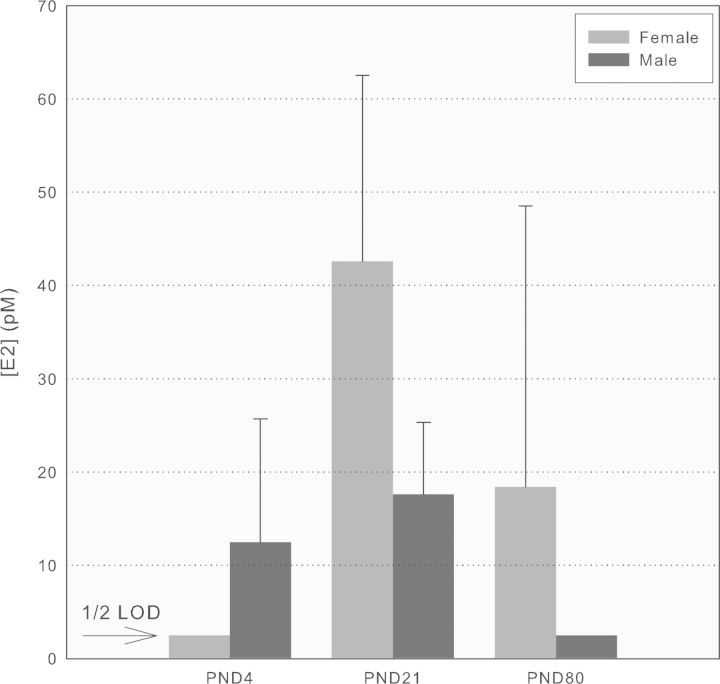
The derivatization method for E2 produced a level of analytical sensitivity that was useful for evaluating life-stage-dependent changes in serum levels in Sprague Dawley rats using the naïve and vehicle control groups. Levels of E2 in males and females were greater than LOD (1.5 pg/ml, 5pM) in all but newborn females and adult males (Fig. 6). E2 was measured in male pups at PND 4 and PND 21, but was undetectable in adult male rats at PND 80 and PND 180 (not shown). In females, E2 was <LOD on PND 4, and similar at PND 21 and PND 80. Enzymatic deconjugation studies showed that E2 was present exclusively in the unconjugated form.
FIG. 6.
E2 levels in male and female Sprague Dawley rats at different ages. Serum concentrations of E2 (unconjugated) were quantified using LC/MS/MS (LOD 5pM, 1.5 pg/ml) from naïve and vehicle control rats (mean ± SD, n = 5–10/sex/age).
Testosterone levels were measured in rats at different ages (Supplementary fig. 4). Because insufficient serum volumes were available from PND 4 pups, serum was instead collected from pups culled at PND 1. Sufficient serum volumes were available from control groups of PND 21 and PND 80 rats in the BPA study. In addition, serum was collected from older untreated Sprague Dawley rats (PND 100 and PND 180) that were obtained as retired breeders from the NCTR colony. Testosterone levels were detectable in male rats as young as PND 1 and increased until PND 100, with lower levels observed in PND 180 rats. Testosterone levels in female rats were <LOD (0.05 ng/ml, 0.17nM) on PND 1 and increased to measurable levels on PND 21 and PND 80. Testosterone levels in female rats did not exceed those observed in PND 1 males at any age.
The effect of BPA dosing on PND 80 serum hormone levels is described in the companion paper (Delclos et al., 2014). Briefly, mean serum estradiol as measured by radioimmunoassay in females was significantly increased by approximately 67% and 113% in the 100,000 and 300,000 μg BPA/kg bw/day groups, respectively. In males, there were no treatment-related differences for serum testosterone levels as measured by radioimmunoassay.
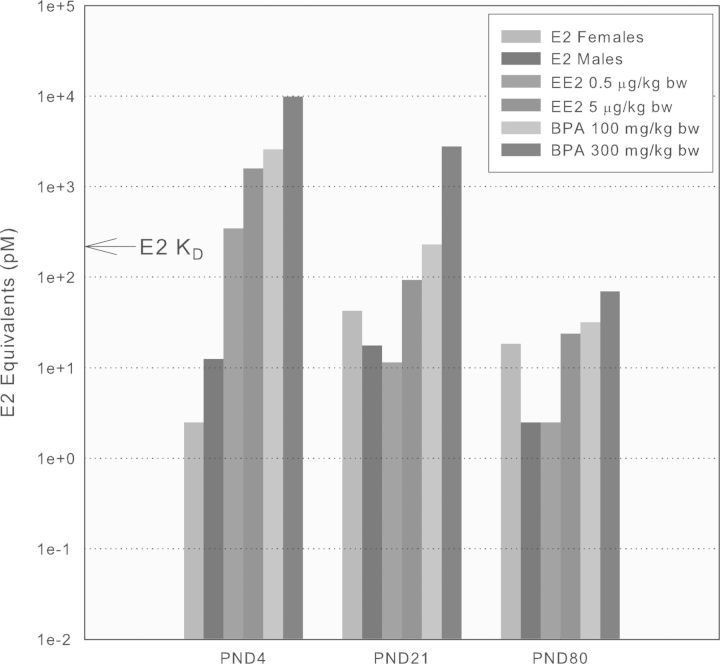
In order to critically test an estrogenic mode of action for BPA, internal dosimetry of aglycone BPA was compared where toxicological effects were observed in estrogen-responsive tissues and overlapped with those produced by the reference estrogen, EE2. As shown in the companion paper (Delclos et al., 2014), only the two highest BPA doses met these criteria. E2-equivalent concentrations for binding to ERα were calculated by adjusting combined male and female group mean Cmax values determined for the 100,000 and 300,000 μg/kg bw/day BPA dose groups (Figs. 2–4), group mean Cmax values for both doses of EE2 (Fig. 5), and endogenous E2 levels in either male or female rats (Fig. 6) by using the respective relative ERα ligand binding affinity (1 for E2, 1.2 for EE2, and 0.0002 for BPA) where the KD for E2 with ERα is 200pM (Gutendorf and Westendorf, 2001). Figure 7 shows the estrogenic internal exposures for each treatment group and life stage.
FIG. 7.
Comparison of estrogen receptor α (ERα) binding-equivalent serum concentrations of endogenous E2 with EE2- and BPA-treated rats of different ages (see the Results section for description of the process used). The combined male + female Cmax values for BPA in the 100,000 and 300,000 μg/kg bw treatment groups are shown along with combined Cmax values for both doses of EE2 and the endogenous E2 values obtained in either male or female pups at various life stages. The KD for E2 with ERα of 200pM is shown for reference.
DISCUSSION
Internal Dosimetry as a Critical Design Element in the Interpretation of Toxicology Studies
A limitation in the design of most BPA toxicity studies, including previous guideline-compliant studies conducted in Sprague Dawley rats (Stump et al., 2010; Tyl et al., 2002), is the lack of evaluation of internal dosimetry, particularly in the “low dose region” (Teeguarden and Hanson-Drury, 2013). The comprehensive evaluation of tissue histopathology and reproductive and developmental endpoints in this subchronic BPA exposure study was matched by comprehensive evaluation of the levels of aglycone (i.e., unconjugated and receptor-active) and conjugated (i.e., inactive) BPA from daily oral administration throughout perinatal and adult life stages. The ages of rats evaluated were determined by sacrifice times chosen to include evaluation of development during early neonatal (PND 4), weaning (PND 21), and adult (PND 80) periods. These measurements in this rat model complement previous evaluations of pharmacokinetics of d6-BPA during fetal (GD 12–20), neonatal (PNDs 3–21), and adult (PND 90) life stages in the same rat strain (Doerge et al., 2010a, 2011a), as well as in CD-1 mice (Doerge et al., 2011b), nonhuman primates (Doerge et al., 2010b; Patterson et al., 2013), and PBPK modeling of the rat, monkey, and human data sets (Fisher et al., 2011; Yang et al., 2013). The pervasive postexposure contamination of serum by native aglycone BPA observed in vehicle and naïve control rats required specific evaluation of methodological blanks using commercial rat serum determined to contain undetectable levels of BPA. In addition, the wide range of BPA doses required complementary analytical strategies in order to quantify the resulting serum levels of aglycone and total BPA. Finally, the finding of the glucuronide metabolite of BPA indicated low level unintended exposure in vehicle and naïve control rats, and necessitated an independent method for directly quantifying native BPA-G. The use of two doses of the reference estrogen, EE2, required implementation of additional methodology, which conveniently permitted concurrent evaluation of the principal endogenous estrogen, E2. Testosterone levels were also determined at different life stages. A goal was to compare directly the dose-response for BPA and the reference estrogen, EE2, in the context of normal endogenous E2 levels in males and females at different ages. The overall goal of these measurements was to use internal dosimetry for BPA to interpret the dose-response for any histopathological or clinical chemistry endpoints affected (see Delclos et al., 2014) and to test the hypothesis of estrogen receptor-mediated signaling disruption during critical developmental windows in rats.
Life-Stage Dependence of BPA Pharmacokinetics
The pharmacokinetics of d6-BPA in PND 4 rat pups were evaluated initially because different vehicle (0.3% CMC vs. 10% ethanol in water) and age (PND 4 vs. PND 3) were used than in the previous study of newborn rats, even though the same rat strain and dose were used (100 μg/kg bw; Doerge et al., 2010a). The pharmacokinetic parameters for aglycone d6-BPA in PND 4 pups (Cmax, AUC, and elimination half-time) were intermediate between those reported previously for PND 3 and PND 10, consistent with the age-dependent development of metabolic and excretory function in neonatal rats that combine to decrease internal exposures as maturation proceeds (Doerge et al., 2010a). Because pharmacokinetics of BPA aglycone appeared to be linear with dose up to at least 840 μg/kg bw (Figs. 2–4), dose extrapolation of Cmax from this controlled exposure was used to simulate levels in PND 4 rat serum from all doses of BPA tested (Supplementary fig. 1). Similarly, Cmax values from Doerge et al. (2010a) were used to simulate BPA levels in PND 21 and PND 90 rats using the range of doses tested here in comparison with the measured values (Figs. 3 and 4 vs. Supplementary figs. 2 and 3).
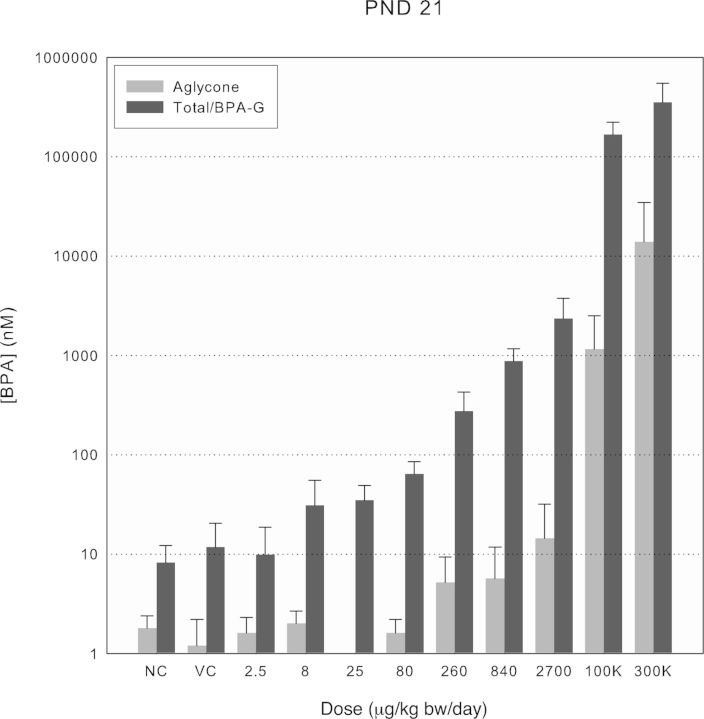
FIG. 3.
Aglycone and total BPA/BPA-glucuronide serum concentrations at Cmax across treatment groups for PND 21 rats. Values shown by treatment group are means of either total or aglycone BPA ± SD for dosed males and females, combined (n ≥ 8), and BPA-glucuronide combined means ± SD for naïve and vehicle control groups (NC, n = 8 and VC, n = 8, respectively). Aglycone BPA was not quantified in the 25 μg/kg bw dose group.
The large numbers of rat pups being dosed daily during the main study made adherence to a single time point for collection of blood samples impossible, so a time window of 0.25–0.75 h was selected as a practical compromise to evaluate Cmax for both aglycone and total BPA at the different ages. The mean postdose times at the three ages were in the range of 0.43–0.53 h (see the Experimental section for details). The serum concentrations of EE2 and E2 were also evaluated using the same postdosing time window and were not evaluated specifically for serum concentration-time profiles. The pharmacokinetics of orally administered EE2 in adult female Sprague Dawley rats have been previously reported (Twaddle et al., 2003).
Figures 2–4 show the aglycone and total BPA (i.e., aglycone + conjugated forms) or BPA-G in the vehicle and naïve controls levels at Cmax using a log scale. Aglycone levels were as much as 1000-fold lower than the corresponding total levels, depending on age and dose. In PND 4, and to a more limited extent in PND 21 rats, plots of the aglycone fraction of total BPA showed an apparent discontinuity in the higher dose region (Supplementary fig. 5), which suggested that saturation of phase II metabolism in the gastrointestinal tract or liver could be occurring at the two highest doses of BPA. No such discontinuity was observed in PND 80 rats.
Methodological Limitations in the Conduct and Interpretation of BPA Dosimetry
The impact of serum contamination by BPA was evident in measurements of aglycone BPA in vehicle and naïve controls (Figs. 2–4). Measured and simulated levels of aglycone BPA levels in dosed-rat serum did not exceed the levels observed in controls until the 80 μg/kg dose (Figs. 2–4 and Supplementary figs. 1–3). The aglycone fractions were higher than those observed after gavage with doses uncompromised by contamination or saturation of metabolism (<4%, Supplementary fig. 5). These findings were consistent with a significant body of evidence supporting the propensity for contamination of blood by aglycone BPA during sample collection and processing (Markham et al., 2010; Twaddle et al., 2010; Ye et al. (2013). This problem was encountered in our measurements of aglycone BPA despite careful prescreening of sample collection apparatus before initiation of the experimentation (see the Experimental section). Because procedural blanks were determined daily and subtracted from each determination, we conclude that the contamination occurred during sample collection and/or storage (i.e., postexposure). It is for this reason that internal dosimetry was independently evaluated by using a “low dose” of isotopically labeled BPA in Figure 1 and previous pharmacokinetic studies, for which environmental contamination is not problematic (Doerge et al., 2010a,b).
The serum concentrations of conjugated BPA, determined in dosed rats after complete enzymatic hydrolysis of conjugated metabolites and subtraction of aglycone, were similar to those predicted from single dose pharmacokinetic studies (Fig. 1 for PND 4, and Doerge et al., 2010a for PND 21 and PND 90 rats). The notable exception was that the concentrations of BPA-G observed in vehicle and naïve control groups were similar to the levels of conjugated BPA observed in the 2.5 μg/kg bw/day groups at all ages tested. These findings are best interpreted as resulting from similar levels of aggregate exposure to BPA, which suggests some unidentified source(s) of BPA exposure in these rats. This finding may represent a technical limitation of using such a comprehensive dose range in toxicology studies despite checking all input media (i.e., diet, water, vehicle, cages, bedding), which were tested a priori for background levels of BPA and found to be negligible. Although the source of the unintended exposure leading to BPA-G in serum from vehicle and naïve control groups was not ultimately identified, this did not affect interpretation of the companion toxicology study (Delclos et al., forthcoming) because no biologically significant effects of BPA were observed below the 100,000 μg/kg bw/day dose. It is similarly important that interpretation of other “low dose” effects from BPA studies could be affected, particularly in studies that do not include thorough evaluations of internal dosimetry and background contamination of input media. Indeed, some recent toxicity studies include dosing with isotopically labeled BPA, which obviates postexposure contamination problems; however, unless native BPA in untreated animals is also critically evaluated (i.e., BPA-G measurements), unintended exposure at low levels cannot be excluded. Other studies continue to dose with native BPA despite potential background problems. For example, a recent study on the carcinogenic potential of BPA (Acevedo et al., 2013) reported detectable levels of native total BPA from untreated rats (range 0.1–0.9 ng/ml, 0.4–3.6nM) along with detectable aglycone BPA (range 0.05—0.3 ng/ml, 0.2–1.2nM). These observations, which could be consistent with either unintended internal exposure (total BPA) or postexposure sample contamination (aglycone BPA), were not considered by the authors as influencing the reported study outcomes. Similarly, the native BPA dosimetry reported by Prins et al. (2011) in PND 3 Sprague Dawley pups could be compromised by contamination by the following criteria: (1) the aglycone fraction of total BPA reported at Cmax after oral administration was 25%, a value far higher than the 7% reported in an independent investigation of d6-BPA pharmacokinetics in Sprague Dawley pups (PND 3, Doerge et al., 2010a) or 4% in PND 4 pups (Fig. 1); (2) the levels of native BPA in serum did not change between 0.5 and 2 h [the last time point reported by Prins et al. (2011)] as opposed to a 10-fold decrease during the same time period when d6-BPA was measured (Doerge et al., 2010a; Fig. 1). However, there is always uncertainty inherent in comparing studies using quite different protocols (i.e., oil vs. aqueous vehicles, individual pup vs. pooled serum samples, different levels of analytical sensitivity and volumes analyzed). Although both these publications did report important measurements of aglycone and total BPA, the absence of a critical evaluation of the totality of pharmacokinetic evidence on BPA did not permit an adequate assessment of the potential impact of either postexposure sample contamination or unintentional exposure in vivo.
The contamination by native aglycone BPA observed in the current study is consistent with a substantial body of experimental evidence available in the literature that documents the pervasive influence of environmentally derived BPA on analysis of blood, urine, and tissue samples (Calafat et al., 2013; Markham et al., 2010; Patterson et al., 2013; Teeguarden et al., 2011; Twaddle et al., 2010; Vandentorren et al., 2011; Ye et al., 2013). Indeed, analytical chemists responsible for nation-level BPA biomonitoring programs have repeatedly emphasized the unsuitability of blood as a matrix because of its particular propensity for contamination (Calafat et al., 2008; Koch and Calafat, 2009; Koch et al., 2012; Ye et al., 2013). This analytical challenge is well documented for many other ubiquitous environmental chemicals, including phthalate diesters (Kessler et al., 2001), polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), triclosan, benzophenone-3, and parabens (Ye et al., 2013). Nonetheless, a less extensive set of papers reporting aglycone BPA levels in human serum in the ng/ml range has been interpreted as reflecting either a large unknown source of exposure (Vandenberg et al., 2010), release from depot tissues (Stahlhut et al., 2009), or extensive dermal and sublingual exposure (Vandenberg et al., 2013). A recent meta-analysis of BPA levels in human serum from 93 studies comprised of >30,000 subjects from 19 countries concluded that BPA aglycone levels in individuals exposed to upper bound aggregate daily doses (<1 μg/kg bw) are in the pM range, and orders of magnitude below levels detectable using current analytical techniques (Teeguarden et al., 2013). This conclusion was based on comparison of blood concentration estimates made from urinary and blood biomonitoring measurements, controlled pharmacokinetic studies using stable isotope-labeled BPA in animals and humans, and simulations from PBPK modeling. A consistent theme from such investigations is that although thorough a priori evaluation of blood collection materials and field blanks is essential to minimize the likelihood of sample contamination, it is insufficient to preclude it. An emerging corollary is that pharmacokinetic and biomonitoring data from humans provide objective post hoc criteria to evaluate the plausibility of true exposures versus unintended contamination events. Specifically, (1) the systemic fraction of total BPA present as aglycone, which is <1% for oral exposures and ∼10% for parenteral; (2) concurrent measurements of total BPA concentrations in urine and blood samples (urine ∼40-fold greater than blood); and (3) evaluation of total estimated intake and its relationship with oral bolus Cmax in blood for total BPA (∼20nM per μg/kg bw dose; Teeguarden et al., 2011, 2013; Völkel et al., 2002).
In light of this body of information regarding the impact of BPA contamination during sampling, it was not surprising that postexposure contamination of rat serum was observed in the current study despite extensive evaluation a priori of collection materials. However, the finding of BPA-G, a metabolite that cannot be derived from contamination, in vehicle and naïve control rat serum was unexpected. The source for this unintended internal exposure was never determined despite rigorous evaluation of diet, water, cages, bedding, vehicle, and careful attention to dose certification and delivery (see Delclos et al., 2014) but a seemingly likely source is within the animal room itself. This possibility arises because a majority of BPA is excreted in feces in the aglycone form by the rat (Twaddle et al., 2010). Therefore, the feces contained in cages of dosed rats represents a large reservoir for potential exchange to untreated controls housed alongside them. This hypothesis was evaluated by swabbing the inside and outside of cages from control, medium and high doses from a separate ongoing BPA study (see Experimental). The low ng levels found were insufficient to establish appreciable transmission through particulates. Although the low volatility of BPA would predict minimal exposure via inhalation, air sampling was not undertaken because of numerous plastic components in the air sample monitoring apparatus and the propensity to introduce background contamination during sample collection.
Life-Stage Dependence of Pharmacokinetics for the Reference Estrogen, EE2
Similar treatment of rats with EE2 showed evidence for dose-dependent and age-dependent changes in serum concentrations of EE2 (Fig. 5). These measurements show that the unconjugated EE2 levels and the unconjugated fraction were age-dependent, similar to BPA, presumably because of the development of phase II metabolic capabilities. Enzymatic hydrolysis procedures indicated that the aglycone fraction of EE2 was markedly higher than that seen for BPA in age-matched rats. This metabolic immaturity confers extra susceptibility to both EE2 and BPA exposures during the neonatal period because aberrant estrogenic signaling during this critical developmental window in rodents could produce irreversible effects that manifest in adulthood.
Methodological Strategy for Concurrent Evaluation of Endogenous E2 Levels Across Life Stages
There is a consistent body of evidence in the literature for overestimation of low pg/ml levels of E2 by antibody-based measurements, particularly in men (∼20 pg/ml), boys (<0.1 pg/ml), and postmenopausal women (<3 pg/ml) (Faupel-Badger et al., 2010; Haisenleder et al., 2011; Hsing et al., 2007; Huhtaniemi et al., 2012; Klein et al., 1994). This discrepancy prompted the development of a sensitive LC/MS/MS-based method for small volumes of serum that is much less susceptible to the cross-reactivity problems that can affect the accuracy of RIA and ELISA with which to evaluate the rats in this study, particularly the males. Figure 6 shows the levels of E2 determined in naïve and vehicle control male and female rats at the same life stages evaluated for BPA and EE2. These data were not generated to evaluate comprehensively the complexity associated with all life-stage-specific and daily fluctuations in endogenous hormone levels, but to provide a direct comparison with contemporaneous measurements of BPA and EE2. Further, the reported values represent total serum aglycone E2 and do not incorporate the potentially modulating effect of serum protein binding on tissue availability. The higher levels of E2 observed in PND 4 males versus females was interpreted as incomplete development of ovarian function by this age in females as opposed to more complete development of testicular synthesis of testosterone (∼0.2 ng/ml or 1nM in male pups as young as PND 1, Supplementary fig. 4) that would be available for aromatization to E2. The consistent finding of undetectable E2 levels (<0.5 pg/ml) in adult male Sprague Dawley rats by LC-MS/MS is not consistent with reports in the literature of much higher values derived from antibody-based methods (e.g., 50, 90, and 10 pg/ml in 3-, 12-, and 20-month-old Sprague Dawley male rats, respectively; Wu et al., 2009). This methodological discrepancy is critical to interpreting the reliability of animal models for hormonal carcinogenesis in which endogenous hormone levels in adult male rats (PND 90) are manipulated through subcutaneous silastic implants of E2 and testosterone to achieve E2 levels of 50–125 pg/ml (Lane et al., 1997; Ofner et al., 1992).
Relationship Between Estrogen Receptor-Based Dosimetry and Developmental Effects of BPA and EE2
It was important to evaluate contributions to the dose-response relationship between potential estrogenic effects reported in the companion BPA/EE2 toxicology study and internal dosimetry measurements reported here. This goal was accomplished by comparing the E2-equivalent molar concentrations of EE2 and BPA using relative ERα binding affinities. Receptor affinities have been reported for these three estrogens with several estrogen receptor types, including the classical nuclear receptors, ERα and ERβ (Gutendorf and Westendorf, 2001), and membrane-bound GPR30 (Thomas and Dong, 2006). Because well-recognized biological activities (e.g., uterine weight gain) are associated with its activation, ERα was chosen as an example, although the same relative affinity approach could be applied to any other receptor type. The ERα binding affinities have been reported using identical methodology for EE2 and BPA, relative to E2 = 1, as 1.2 and 0.0002, respectively (Gutendorf and Westendorf, 2001). Although the focus on relative binding affinity does not include other important factors that can serve to modulate overall potency (e.g., coactivator recruitment and receptor density), ligand binding affinity often correlates with cellular responses to estrogens (Jeyakumar et al., 2011). As a point of reference, a representative value for the KD for E2 binding to ERα is 200pM (Jeyakumar et al., 2011). Accordingly, 10% receptor occupancy occurs at E2 concentration at approximately 20pM and 90% occupancy occurs at respective concentrations of 2000pM. The dosimetry comparison presented here is derived from measured levels of total serum ligands, but it is the free (unbound) tissue levels in equilibrium with receptors that determine toxicodynamic responses (Mendel, 1989). Although these ligands could be anticipated to interact differentially with rat serum binding proteins, the free fractions for serum albumin are similar for BPA (6%, Teeguarden et al., 2005), EE2 (2%, Kuhnz et al., 1990), and E2 (5%, Plowchalk and Teeguarden, 2002). Unraveling the complex relationships between ligand-protein binding kinetics and the dependence of tissue responses on either total or free ligand concentrations (Mendel, 1989) is beyond the scope of this paper; however, such an analysis could be made across life stages and between species using computation approaches previously described (Teeguarden and Barton, 2004).
Figure 7 shows the serum estrogenic dosimetry relationships in male and female rats at different ages for the two highest BPA doses (100,000 and 300,000 μg/kg bw) and both EE2 doses (0.5 and 5 μg/kg bw) compared with the contemporaneous endogenous levels of E2. When plotted on a log scale, it is clear that on PND 4, EE2 levels exceed those of endogenous E2 and the respective values are similar at PND 21 and PND 80. Therefore, based on internal dosimetry and receptor occupancy, ERα-mediated signaling, especially at the high dose of EE2, could be possible in rats of both sexes in which daily dosing started the day after birth. The two high doses of BPA produced internal estrogenic exposures in males and females greater than either endogenous E2 levels or exogenously administered EE2 at all ages examined.
The relevance of these ERα-dosimetry relationships during rodent development to predicting changes in estrogen-responsive tissues is supported by the results presented in the companion paper where significant treatment effects were observed in both sexes of rats at the 300,000 μg BPA/kg bw/day dose levels and only in females at the 100,000 μg/kg bw/day (see Delclos et al., forthcoming). In females, the effects partially overlapped those of EE2, in terms of a consistent pattern of changes in the PND 90 ovary (increased cystic follicles, depleted corpora lutea and antral follicles), PND 80 serum hormone levels (increased E2 and prolactin, decreased progesterone), and disruption of the estrous cycle. The degree of overlap was less prominent in males where both doses of EE2 stimulated mammary gland hyperplasia at PND 21 and PND 90, whereas the high dose delayed the time of testicular descent and preputial separation, depressed male reproductive organ weights, and caudal epididymal sperm counts, and increased degeneration of the germinal epithelium. The highest dose of BPA (300,000 μg/kg bw/day) did not affect these endpoints, except for the delay in the time of testicular descent, and the 100,000 μg/kg bw/day dose had no treatment-related effects in males. Based on the internal dosimetry and ERα occupancies achieved discussed above, even more estrogenic effects might have been expected at the two highest doses of BPA.
CONCLUSIONS
This investigation is the first to evaluate thoroughly internal dosimetry for orally administered BPA throughout perinatal and adult life stages in rats over a range of doses spanning approximately 100- to 10,000,000-fold above median aggregate human daily intake, from which best estimates of steady state blood levels of aglycone BPA are in the low picomolar range (Teeguarden et al., 2013). The concurrent evaluation of two doses of a reference estrogen, EE2, and endogenous levels of E2 throughout the life stages facilitates critical evaluation of estrogenic hypotheses for effects observed in histological and clinical chemistry endpoints evaluated in parallel. Only the two highest doses of BPA produced a similar pattern of internal dosimetry and ERα occupancy, compared with E2 and EE2, and a similar pattern of estrogenic effects in vivo as shown in the companion paper (Delclos et al., 2014). Although alternative hypotheses for cellular targets of BPA have been promulgated, the data presented here for clearly adverse effects support an estrogenic mode of action in vivo. Despite care taken a priori to minimize contamination of serum samples during collection and storage (i.e., postexposure), trace level measurements of aglycone BPA were clearly compromised at the lower doses. This finding adds to a large body of evidence suggesting that the propensity for sample contamination will continue to make measurements of native aglycone BPA in blood difficult to interpret. Evidence was also presented to suggest that some unidentified source of BPA in the animal rooms led to levels of BPA-G in vehicle and naïve control rats similar to those in the 2.5 μg BPA/kg bw/day dose despite extensive screening of possible contamination in input media (feed, water, cage material, bedding, room dust) and strict attention to dose certification and delivery. This finding could reflect a technical limitation in conducting studies of this complexity and suggests that it may be difficult to interpret studies reporting adverse effects from “low dose” BPA that do not similarly document and interpret internal dosimetry in the context of the totality of pharmacokinetic evidence for BPA.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Study conducted under the auspices of the National Toxicology Program and funded by an Interagency agreement (IAG) between the Food and Drug Administration and the National Institute of Environmental Health Sciences/National Institutes of Health (FDA IAG: 224-12-0003; NIEHS IAG: AES12013).
Supplementary Material
Acknowledgments
The authors gratefully acknowledge helpful discussions with Dr Sherry Ye, Centers for Disease Control and Prevention, and the NTP for providing the native and isotopically labeled BPA-G. E.S. acknowledges support of a fellowship from the Oak Ridge Institute for Science and Education, administered through an interagency agreement between the U.S. Department of Energy and the U.S. Food and Drug Administration.
Footnotes
Disclaimer: The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration or National Toxicology Program.
REFERENCES
- Acevedo N., Davis B., Schaeberle C. M., Sonnenschein C., Soto A. M. Perinatally administered bisphenol A as a potential mammary gland carcinogen in rats. Environ. Health Perspect. 2013;121:1040–1046. doi: 10.1289/ehp.1306734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum L. S., Aungst J., Schug T. T., Goodman J. L. Working together: Research- and science-based regulation of BPA. Environ. Health Perspect. 2013;121:A206–A207. doi: 10.1289/ehp.1306963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M., Koch H. M., Swan S. H., Hauser R., Goldman L. R., Lanphear B. P., Longnecker M. P., Rudel R. A., Teitelbaum S. L., Whyatt R. M., et al. Misuse of blood serum to assess exposure to bisphenol A and phthalates. Breast Cancer Res. 2013;15:403. doi: 10.1186/bcr3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M., Ye X., Wong L.-Y., Reidy J. A., Needham L. L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos K. B., Camacho L., Lewis S. M., Vanlandingham M. M., Latendresse J. R., Olson G. R., Davis K. J., Patton R. E., Gamboa da Costa G., Woodling K. A., et al. Toxicity evaluation of bisphenol A administered by gavage to Sprague-Dawley rats from gestation day 6 through postnatal day 90. Toxicol. Sci. 2014 doi: 10.1093/toxsci/kfu022. 10.1093/toxsci/kfu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Brown R.P. , Fisher J. W. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2011a;255:261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Fisher J. W. Pharmacokinetics of bisphenol A in adult and neonatal Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2010a;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Fisher J. W. Pharmacokinetics of bisphenol A in adult and neonatal rhesus monkeys. Toxicol. Appl. Pharmacol. 2010b;248:1–11. doi: 10.1016/j.taap.2010.07.009. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Fisher J. W. Pharmacokinetics of bisphenol A in adult and neonatal CD-1 mice: Inter-species comparisons with Sprague-Dawley rats and rhesus monkeys. Toxicol. Lett. 2011b;207:298–305. doi: 10.1016/j.toxlet.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Faupel-Badger J. M., Fuhrman B. J., Xu X., Falk R. T., Keefer L. K., Veenstra T. D., Hoover R. N., Zeigler R. G. Comparison of liquid chromatography-tandem mass spectrometry, RIA, and ELISA methods for measurement of urinary estrogens. Cancer Epidemiol. Biomarkers Prev. 2010;19:292–300. doi: 10.1158/1055-9965.EPI-09-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher J. W., Twaddle N. C., Vanlandingham M., Doerge D. R. Pharmacokinetic modeling: Prediction and evaluation of route-dependent dosimetry of bisphenol A in monkeys with extrapolation to humans. Toxicol. Appl. Pharmacol. 2011;257:122–136. doi: 10.1016/j.taap.2011.08.026. [DOI] [PubMed] [Google Scholar]
- Gutendorf B., Westendorf J. Comparison of an array of in vitro assays for thre assessment of the estrogenic potential of natural and synthetic estrogens, phytoestrogens and xenoestrogens. Toxicology. 2001;166:79–89. doi: 10.1016/s0300-483x(01)00437-1. [DOI] [PubMed] [Google Scholar]
- Haisenleder D. J., Schoenfelder A. H., Marcinko E. S., Geddis L. M., Marshall J. C. Estimation of estradiol in mouse serum samples: Evaluation of commercial estradiol immunoassays. Endocrinology. 2011;152:4443–4447. doi: 10.1210/en.2011-1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing A. W., Stanczyk F. Z., Belanger A., Schroeder P., Chang L., Falk R. T., Fears T. R. Reproducibility of serum sex steroid assays in men by RIA and mass spectrometry. Cancer Epidemiol. Biomarkers Prev. 2007;16:1004–1008. doi: 10.1158/1055-9965.EPI-06-0792. [DOI] [PubMed] [Google Scholar]
- Huhtaniemi I. T., Tajar A., Lee D. M., O’Neill T. W., Finn J. D., Bartfai G., Boonen S., Casanueva F. F., Giwercman A., Han T. S., et al. Comparison of serum testosterone and estradiol measurements in 3174 European men using platform immunoassay and mass spectrometry; relevance for the diagnostics in aging men. Eur. J. Endocrinol. 2012;166:983–991. doi: 10.1530/EJE-11-1051. [DOI] [PubMed] [Google Scholar]
- Jeyakumar M., Carlson K. E., Gunther J. R., Katzenellenbogen J. A. Exploration of of dimensions of estrogen potency. Parsing ligand binding and coactivator binding affinities. J. Biol. Chem. 2011;286:12971–12982. doi: 10.1074/jbc.M110.205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler W., Phokha W., Csanády G. A., Filser J. G. No background concentrations of di(2-ethylhexyl) phthalate in blood of rats. Arch. Toxicol. 2001;75:62–64. doi: 10.1007/s002040000198. [DOI] [PubMed] [Google Scholar]
- Klein K. O., Baron J., Colli M. J., McDonnell D. P., Cutler G. B., Jr Estrogen levels in childhood determined by an ultrasensitive recombinant cell assay. J. Clin. Invest. 1994;94:2475–2480. doi: 10.1172/JCI117616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M., Calafat A. M. Human body burdens of chemnicals used in plastic manufacture. Philos. Trans. R. Soc. B. 2009;364:2063–2078. doi: 10.1098/rstb.2008.0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch H. M., Kolossa-Gehring M., Schröter-Kermani C., Angerer J., Brüning T. Bisphenol A in 24 h urine and plasma samples of the German Environmental Specimen Bank from 1995 to 2009: A retrospective exposure evaluation. J. Expo. Sci. Environ. Epidemiol. 2012;22:610–616. doi: 10.1038/jes.2012.39. [DOI] [PubMed] [Google Scholar]
- Kuhnz W. K., Pfeffer M., Al-Yacoub G. Protein binding of the contraceptive steroids gestodene, 3-keto-desogestrel, and ethinylestradiol in human serum. J. Steroid Biochem. 1990;35:313–318. doi: 10.1016/0022-4731(90)90290-9. [DOI] [PubMed] [Google Scholar]
- Kushnir M., Rockwood A., Bergquist J., Varshavsky M., Roberts W., Yue B., Bunker A., Meikle A. High-sensitivity tandem mass spectrometry assay for serum estrone and estradiol. Am. J. Clin. Pathol. 2008;129:530–539. doi: 10.1309/LC03BHQ5XJPJYEKG. [DOI] [PubMed] [Google Scholar]
- Lakind J. S., Levesque J., Dumas P., Bryan S., Clarke J., Naiman D. Q. Comparing United States and Canadian population exposures from national biomonitoring surveys: Bisphenol A intake as a case study: 2005–2006 National Health and Nutrition Examination Survey. J. Expo. Sci. Environ. Epidemiol. 2012:219–226. doi: 10.1038/jes.2012.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane K. E., Leav I., Ziar J., Bridges R. S., Rand W. M., Ho S.-M. Suppression of testosterone and estradiol-17β-induced dysplasia in the dorsolateral prostate of noble rats by bromocryptine. Carcinogenesis. 1997;18:1505–1510. doi: 10.1093/carcin/18.8.1505. [DOI] [PubMed] [Google Scholar]
- Markham D. A., Waechter J. M., Jr, Wimber M., Rao N., Connolly P., Chuang J. C., Hentges S., Shiotsuka R. N., Dimond S., Chappelle A. H. Development of a method for the determination of bisphenol A at trace concentrations in human blood and urine and elucidation of factors influencing method accuracy and sensitivity. J. Anal. Toxicol. 2010;34:293–303. doi: 10.1093/jat/34.6.293. [DOI] [PubMed] [Google Scholar]
- Mendel C. M. The free hormone hypothesis: A physiologically based mathematical model. Endocrinol. Rev. 1989;10:232–274. doi: 10.1210/edrv-10-3-232. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. 2008 [Google Scholar]
- Ofner P., Bosland M. C., Vena R. L. Differential effects of diethylstilbestrol and estradiol-17β in combination with testosterone on rat prostate lobes. Toxicol. Appl. Pharmacol. 1992;112:300–309. doi: 10.1016/0041-008x(92)90200-c. [DOI] [PubMed] [Google Scholar]
- Patterson T. A., Twaddle N. C., Roegge C. S., Callicott R. J., Fisher J. W., Doerge D. R. Concurrent determination of bisphenol A pharmacokinetics in maternal and fetal rhesus monkeys. Toxicol. Appl. Pharmacol. 2013;267:41–48. doi: 10.1016/j.taap.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Plowchalk D. R., Teeguarden J. G. Development of a physiologically based pharmacokinetic model for estradiol in rats and humans: A biologically motivated quantitative framework for evaluating responses to estradiol and other endocrine-active compounds. Toxicol. Sci. 2002;69:60–78. doi: 10.1093/toxsci/69.1.60. [DOI] [PubMed] [Google Scholar]
- Prins G. S., Ye S.-H., Birch L., Ho S. M., Kannan K. Serum bisphenol A pharmacokinetics and prostate neoplastic responses following oral and subcutaneous exposures in neonatal Sprague-Dawley rats. Reprod. Toxicol. 2011;31:1–9. doi: 10.1016/j.reprotox.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut R. W., Welshons W. V., Swan S. H. Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both. Environ. Health Perspect. 2009;117:784–789. doi: 10.1289/ehp.0800376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump D. G., Beck M. J., Radovsky A., Garman R. H., Freshwater L. L., Sheets L. P., Marty M. S., Waechter J. M., Jr, Dimond S. S., Van Miller J. P., et al. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 2010;115:167–182. doi: 10.1093/toxsci/kfq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeguarden J. G., Barton H. A. Computational modeling of serum-binding proteins and clearance in extrapolations across lifestages and species for endocrine active compounds. Risk Anal. 2004;24:751–770. doi: 10.1111/j.0272-4332.2004.00473.x. [DOI] [PubMed] [Google Scholar]
- Teeguarden J. G., Calafat A. M., Ye X., Doerge D. R., Churchwell M. I., Gunawan R., Graham M. Twenty-four hour human urine and serum profiles of bisphenol A during high-dietary exposure. Toxicol. Sci. 2011;123:48–57. doi: 10.1093/toxsci/kfr160. [DOI] [PubMed] [Google Scholar]
- Teeguarden J. G., Hanson-Drury S. A systematic review of bisphenol A “low dose” studies in the context of human exposure: A case for establishing standards for reporting “low-dose” effects of chemicals. Food Chem. Toxicol. 2013;62:935–948. doi: 10.1016/j.fct.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Teeguarden J. G., Hanson-Drury S., Fisher J. W., Doerge D. R. Typical human serum BPA concentrations measurable and sufficient to be estrogenic in the general population? Food Chem. Toxicol. 2013;62:949–963. doi: 10.1016/j.fct.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Teeguarden J. G., Waechter J. M., Jr, Clewell H. J., III, Covington T. R., Barton H. A. Evaluation of oral and intravenous route pharmacokinetics, plasma protein binding, and uterine tissue dose metrics of bisphenol A: A physiologically based pharmacokinetic approach. Toxicol. Sci. 2005;85:823–838. doi: 10.1093/toxsci/kfi135. [DOI] [PubMed] [Google Scholar]
- Thomas P., Dong J. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. J. Steroid Biochem. Mol. Biol. 2006;102:175–179. doi: 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Twaddle N. C., Churchwell M. I., Delclos K. B., Newbold R. R., Doerge D. R. Determination using LC-ES/MS/MS of ethinylestradiol serum pharmacokinetics in adult Sprague-Dawley rats. J. Chromatogr. B. 2003;793:309–315. doi: 10.1016/s1570-0232(03)00331-3. [DOI] [PubMed] [Google Scholar]
- Twaddle N. C., Churchwell M. I., Vanlandingham M., Doerge D. R. Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:3011–3020. doi: 10.1002/rcm.4733. [DOI] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Thomas B. F., Keimowitz A. R., Brine D. R., Vesilica M. M., Fail P. A., Chang T. Y., Seely J. C., et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- Vandenberg L. N., Chahoud I., Padmanabhan V., Paumgartten F. J. R., Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environ. Health Perspect. 2010;118:1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L. N., Hunt P. A., Myers J. P., vom Saal F. S. Human exposures to bisphenol A: Mismatches between data and assumptions. Rev. Environ. Health. 2013;28:37–58. doi: 10.1515/reveh-2012-0034. [DOI] [PubMed] [Google Scholar]
- Vandentorren S., Zeman F., Morin L., Sarter H., Bidondo M.-L., Oleko A., Leridon H. Bisphenol-A and phthalates contamination of urine samples by catheters in the Elfe pilot study: Implications for large-scale biomonitoring studies. Environ. Res. 2011;111:761–764. doi: 10.1016/j.envres.2011.05.018. [DOI] [PubMed] [Google Scholar]
- Völkel W., Colnot T., Csanady G. A., Filser J. G., Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chem. Res. Toxicol. 2002;15:1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- Wu D., Lin G., Gore A. C. Age-related changes in hypothalamic androgen receptor and estrogen receptor α in male rats. J. Comp. Neurol. 2009;512:688–701. doi: 10.1002/cne.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z., Sun X., Huo T., Li N., Zheng Y., Sun Y. Development and validation of UPLC-MS/MS method for simultaneous determination of gestodene and ethinyl estradiol in rat plasma. Biomed. Chromatogr. 2010;24:160–168. doi: 10.1002/bmc.1265. [DOI] [PubMed] [Google Scholar]
- Xu X., Roman J., Issaq H., Keefer L., Veenstra T., Ziegler R. Quantitative measurement of endogenous estrogens and estrogen metabolites in human serum by liquid chromatography-tandem mass spectrometry. Anal. Chem. 2007;79:7813–7821. doi: 10.1021/ac070494j. [DOI] [PubMed] [Google Scholar]
- Yang X., Doerge D. R., Fisher J. W. Prediction and evaluation of route dependent dosimetry of BPA in rats at different life stages using a physiologically based pharmacokinetic model. Toxicol. Appl. Pharmacol. 2013;270:45–59. doi: 10.1016/j.taap.2013.03.022. [DOI] [PubMed] [Google Scholar]
- Ye X., Zhou X., Hennings R., Kramer J., Calafat A. M. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring analysis: An elusive laboratory challenge. Environ. Health Perspect. 2013;121:283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.