Abstract
Bisphenol A (BPA) is a high production volume industrial chemical to which there is widespread human oral exposure. Guideline studies used to set regulatory limits detected adverse effects only at doses well above human exposures and established a no-observed-adverse-effect level (NOAEL) of 5 mg/kg body weight (bw)/day. However, many reported animal studies link BPA to potentially adverse effects on multiple organ systems at doses below the NOAEL. The primary goals of the subchronic study reported here were to identify adverse effects induced by orally (gavage) administered BPA below the NOAEL, to characterize the dose response for such effects and to determine doses for a subsequent chronic study. Sprague Dawley rat dams were dosed daily from gestation day 6 until the start of labor, and their pups were directly dosed from day 1 after birth to termination. The primary focus was on seven equally spaced BPA doses (2.5–2700 μg/kg bw/day). Also included were a naïve control, two doses of ethinyl estradiol (EE2) to demonstrate the estrogen responsiveness of the animal model, and two high BPA doses (100,000 and 300,000 μg/kg bw/day) expected from guideline studies to produce adverse effects. Clear adverse effects of BPA, including depressed gestational and postnatal body weight gain, effects on the ovary (increased cystic follicles, depleted corpora lutea, and antral follicles), and serum hormones (increased serum estradiol and prolactin and decreased progesterone), were observed only at the two high doses of BPA. BPA-induced effects partially overlapped those induced by EE2, consistent with the known weak estrogenic activity of BPA.
Keywords: Bisphenol A, ethinyl estradiol, Sprague Dawley rat, 90-day study
Bisphenol A (BPA) is a high production volume industrial chemical used as a monomer for polycarbonate plastics and epoxy resins that have broad applications in consumer products, including medical devices and storage containers for foods and beverages (Shelby, 2008; Willhite et al., 2008). Biomonitoring studies support widespread exposure to BPA (Calafat et al., 2005, 2008). Regulatory agencies and expert panels have concluded that the primary route of exposure of humans to BPA is through the diet and that, whereas levels of exposure vary somewhat across age groups, they are generally below 1 μg/kg body weight (bw)/day (Health Canada, 2012; USFDA, 2010; WHO, 2010). The toxicity of BPA has been debated in recent years, particularly with regard to effects in rodents that have been reported at doses claimed to produce internal exposures in the range reported in humans and/or exhibit nonmonotonic dose-response curves (reviewed in Richter et al., 2007; NTP, 2008a; Vandenberg et al., 2012). These reported effects include central nervous system and behavioral alterations, preneoplastic lesions in prostate, mammary gland, and uterus, altered development of the prostate, male urinary tract, and female mammary gland, obesity and insulin resistance. Studies have similarly shown epidemiological associations of urinary BPA levels with a variety of adverse human health effects (e.g., Carwile and Michels, 2011; LaKind et al., 2012; Lang et al., 2008; Melzer et al., 2010, 2012; Silver et al., 2011; Spanier et al., 2012; Teppala et al., 2012; Trasande et al., 2012; Wang et al., 2012, 2013). The reports of “low dose” effects in animal studies have provided support for the concerns raised by these association studies; however, other large rodent studies, including those conducted according to currently accepted regulatory guidelines, have failed to demonstrate such effects (e.g., Ema et al., 2001; Howdeshell et al., 2008; Ryan et al., 2010; Stump et al., 2010; Tyl et al., 2002, 2008).
Many of the studies that report low dose effects of BPA utilized an insufficient number of dose groups to establish a clear dose-response relationship, used small numbers of animals, and/or failed to appropriately account for possible litter effects in the analysis of the data (Shelby, 2008). Also there is a general lack of information, even from guideline studies, on potential background exposures and internal dosimetry of BPA. Altogether, these issues have led to difficulties in coming to a consensus on the interpretation of the large body of data in experimental animals with regard to the risk of BPA in humans. The present study was designed to address some of these uncertainties. Although the focus of the present study was on the reproductive toxicity of BPA, other endpoints that have been associated with BPA in animal and/or human studies (see above) were also evaluated, including fat pad weights, serum levels of glucose, triglycerides, insulin, cardiac troponin, and histopathology of the heart and blood vessels.
The “low” [below the current no-observed-adverse-effect level (NOAEL)] BPA doses in this study (2.5–2700 μg/kg bw/day) were selected to cover a range where effects of BPA have been reported in various research studies (Shelby, 2008) and were spaced at an interval (approximately a half-log dose progression) such that a dose response could be established. This is the BPA dose range of regulatory concern. The two high doses of BPA (100,000 and 300,000 μg/kg bw/day) were selected as doses in the range where the “high dose” effects of BPA have been observed, such as delayed puberty in both sexes in rats and systemic toxicity (Tan et al., 2003; Tyl et al., 2002). Because many of the reported effects of BPA involve estrogen signaling pathways, the use of a reference estrogen control has been recommended in BPA toxicity studies (e.g., Richter et al., 2007). Thus, two doses of ethinyl estradiol (EE2) were included in the study. The higher dose (5.0 μg EE2/kg bw/day) was selected based on our earlier dietary studies (Delclos et al., 2009; Latendresse et al., 2009) and a 10-fold lower dose of EE2 was also used, based on the differing exposure of the neonates in the present study.
MATERIALS AND METHODS
BPA, EE2, and Vehicle
BPA [CAS no. 80-05-7, TCI America Lot no. 111909/AOHOK (air-milled)] and EE2 (CAS no. 57-63-6, Sigma Lot no. 028K1411) were used. An extensive chemical analysis of the BPA was conducted at Battelle Laboratory (Columbus, OH). The Battelle analysis concluded that the BPA purity was consistent with the manufacturer's Certificate of Analysis value of 99.9%. The identities and purities of both BPA and EE2 were confirmed to be > 99% pure by the Chemistry Support Group and Mass Spectrometry Laboratory of the Division of Biochemical Toxicology, NCTR, prior to dosing of animals and at the end of the period of animal dosing.
The vehicle used to deliver the BPA and EE2 was 0.3% aqueous carboxymethylcellulose (CMC). CMC was obtained from Sigma-Aldrich (St. Louis, MO; catalog no. C5013, lot no. 048K0023).
Diet and Assessment of Background Levels of BPA in Diet and Other Study Materials
The study diet was soy- and alfalfa-free to minimize phytoestrogen content [5K96 verified casein diet 10 IF, round pellets, γ-irradiated (catalog no. 1810069), Test Diets, Purina Mills, Richmond, IN]. Extracts of each lot of diet were monitored for daidzein, genistein, and BPA content by liquid chromatography/mass spectrometry. The mean levels of daidzein and genistein in the six diet lots utilized in the study were 0.249 ± 0.064 (SD) ppm and 0.374 ± 0.118 ppm, respectively. The mean level of BPA measured in the diet was 2.6 ± 0.8 ppb. Other study materials screened for BPA levels included animal bedding, polysulfone cage leachates, silicone water bottle stoppers, and drinking water. None of these materials had BPA levels detectable above the average analytical blanks.
Preparation and Analysis of Dose Formulations
BPA and EE2 doses were prepared in the vehicle, 0.3% CMC in water, and administered to the animals seven days a week at a rate of 5 ml/kg body weight. Homogeneity was established for the highest BPA dose level suspension. Stability was determined to be acceptable (100 ± 10%) for at least 35 days for the lowest BPA dose solution (0.5 μg/ml) and both EE2 doses. A suspension of 100 mg/ml BPA, higher than the actual dose used, was determined to be stable for at least 47 days.
The vehicle solutions were certified to have undetectable (below the analytical blank) levels of BPA. Every batch of every dosing solution/suspension was assayed for BPA or EE2 concentration prior to delivery to the animal rooms and certified to be within ± 10% of the target dose with a coefficient of variation of 10% or less. Doses were administered by oral gavage with a modified Hamilton Microlab 500 series pump (Lewis et al., 2010). Separate dosing stations were used for vehicle control, low dose BPA (2.5–2700 μg/kg bw/day), high dose BPA (100,000 and 300,000 μg/kg bw/day), and EE2 doses (0.5 and 5.0 μg/kg bw/day). Dosing was always conducted from low dose to high dose on a given pump, with flushing of the Teflon tubing between dose levels. At the end of each day, the dosing pump, tubing, and syringes were flushed with Millipore-filtered water, followed by 70% ethanol, and again with filtered water; this flushing procedure was certified to reduce BPA to less than 10 ng/ml following use of a 100 mg/ml BPA suspension in 0.3% CMC.
Source and Specification of Animals
All animal use and procedures for this study were approved by the NCTR Laboratory Animal Care and Use Committee and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility. Throughout the study, animal rooms were maintained at 23 ± 3ºC with a relative humidity of 50 ± 20% and a 12 h light/dark cycle, with lights on at 6 a.m., and food and water were available ad libitum. There were at least 10 room air changes per hour. Four hundred male and 400 female weanling [circa postnatal day (PND 21)] NCTR Sprague Dawley cesarean-derived (CD) rats (strain code 23) were obtained from the NCTR breeding colony in four loads of 100 of each sex spaced three weeks apart. Although in the breeding colony, these F0 breeders were maintained with their dams on NIH-41-irradiated feed pellets (IRR. NIH-41, catalog no. 7919C, Harlan Laboratories, Madison, WI) and housed in polycarbonate cages with hardwood chip bedding (P.J. Murphy, Montville, NJ) and polycarbonate water bottles. Once assigned to the study at weaning, they were fed pelleted-irradiated Purina 5K96 feed (Test Diets, Purina Mills), housed in polysulfone cages fitted with microisolator tops (Ancare, Corp, Bellmore, NY) with hardwood chip bedding, and given Millipore-filtered water from glass water bottles with silicone stoppers. The rats were held under these conditions until they were mated (at 10–14 weeks of age for females and 11–15 weeks of age for males). The health of the animals was monitored during the study in accordance with NCTR's Sentinel Animal Program and the sentinel animals were determined to be free of pathogenic organisms.
Animal Breeding and Maintenance
A summary of the experimental design is presented in Figure 1. Approximately two weeks prior to mating, female breeders were randomized to treatment groups stratified by body weight to give approximately equivalent mean body weights in each group. The premating assignment of dams to dose groups was necessitated by the use of randomly cycling females and a 14-day mating period described below. Male breeders were assigned to breeding pairs with the stipulation that no sibling or first cousin mating was permitted. Animals were mated in four loads spaced three weeks apart. The number of dams assigned to each treatment group and the number of animals (up to one per sex per litter) evaluated at necropsy are shown in Supplementary table 1. The number of pairs assigned to treatment groups was adjusted in later loads based on the number of litters enrolled in the study from prior loads. Throughout the study, treatment groups were randomized to racks within each room. Racks and cages within racks were rotated every three weeks to minimize any environmental/room effects.
FIG. 1.
Schematic representation of the study experimental design. A detailed description of the protocol is found in the Materials and Methods section. Blood draws and terminal necropsies were conducted in PND 80 ± 3 and PND 90 ± 5 windows, respectively. The indicated measurements of serum BPA levels are reported (Churchwell et al., 2014 in addition to the animals indicated here, male and female pups from each litter were maintained until termination at PND 90 ± 5 and tissues were transferred to other studies (results not reported here). Data obtained from those animals were included in the analyses of AGD and pubertal landmarks.
Males were placed in wire-bottomed breeding cages for acclimation approximately 48 h prior to introduction of the female. The breeding pairs were housed together and the females were examined daily until evidence of mating had occurred or for 14 days, whichever occurred first. Either the presence of an in situ copulation plug or sperm in the vaginal smear was considered evidence of mating [gestation day (GD) 0] and triggered separation of the breeding pair. The males were removed from the study and euthanized after a successful mating and the female was placed in a separate solid-bottomed polysulfone cage with hardwood chip bedding. Except for the naïve control group, daily gavage dosing of the dams started on GD 6 and continued until the initiation of labor. The dose volume was determined by the daily body weight. Neither dams nor pups were dosed on the day of birth (PND 0). Litters were randomly culled to a maximum of five males and five females on PND 1. No fostering was conducted, and a minimum of three males and three females in the litter was required to keep the litter on study. Direct oral gavage dosing of the pups started on PND 1, after the litter was culled. Pups were weighed and dosed daily until the scheduled day of removal (PND 15 or PND 21) or until the day prior to their scheduled removal (PND 90 ± 5). For pups younger than PND 5, the gavage needle did not enter the esophagus. Naïve control dams and F1 pups were weighed daily and returned to their cages after any scheduled observations were conducted. Pups were housed individually after weaning, consistent with the Food and Drug Administration (FDA) guidance in place at the time the experiment was initiated (USFDA, 2007).
In-Life Data Collection
Morbidity/mortality checks were performed twice daily and clinical observations once daily. Daily body weights were obtained prior to dosing for dams from GD 6 through parturition and similarly for pups from PND 1. Naïve control animals were similarly weighed but not dosed. Feed consumption was measured weekly. On the day of birth (PND 0), the numbers of pups alive and dead were recorded, but there were no other manipulations of the dam or litter. On PND 1, the number of pups alive and dead, sex ratio, and live litter weight by sex were determined and litters were randomly culled as described above. Anogenital distance (AGD), the distance from the anterior edge of the anal opening (closest to genitalia) to the midpoint of the genital tubercle, was measured on PND 1 pups retained after culling. The pups were manually restrained, with care taken not to stretch the pup, and the measurement was made in triplicate using a dissecting microscope fitted with an ocular micrometer. The mean of the three determinations for each pup was used in the statistical analysis of AGD and was divided by the cube root of body weight for the analysis of AGD index (AGDI).
Males were evaluated for nipple retention on PND 13, and daily for testicular descent and for preputial separation from PND 15 and 30, respectively, until these events were noted. Females were monitored daily for vaginal opening from PND 22. Females (one per litter) designated for histopathology evaluation were monitored by daily vaginal smears from the day of vaginal opening up to 10 days, to determine the day of first estrus, and again daily from PND 69 until the day prior to termination at PND 90 ± 5, to evaluate treatment effects on estrous cyclicity and to attempt to stage animals for necropsy in estrus. A subset of females (one per litter, from litters with three females available at the time of weaning) was removed from dose at PND 90 ± 5 and maintained in a separate room to evaluate delayed treatment effects on estrous cyclicity by daily vaginal cytology from PND 150 to PND 170.
At PND 80 ± 3, a blood sample was taken from the lateral tail vein of animals designated for histopathology for measurement of luteinizing hormone (LH), follicle-stimulating hormone (FSH), prolactin, estradiol, and progesterone in females and LH, FSH, prolactin, and testosterone in males. This sampling occurred after dosing, between 7:30 and 11:00 a.m., and on days when females were predicted to be in estrus based on the vaginal smear of the previous day or on PND 83 for animals not cycling normally. Serum was aliquoted and frozen in a −80ºC freezer until batch testing. Rat-specific LH (IDS, Liege, Belgium), FSH (TSZ ELISA, Framingham, MA), estradiol, and prolactin (Calbiotech, Spring Valley, CA) assays were performed using ELISA methods according to the manufacturers’ instructions. Cross-reactivity of the test agents, BPA and EE2, in the estradiol assay was not evaluated. The plates were read on an ELx800 Universal Microplate Reader (Bio-Tek, Winooski, VT). Testosterone and progesterone quantification assays (“Coat-a-Count,” Siemens, Los Angeles, CA) were performed using radioimmunoassay (RIA) methods according to the manufacturer's instructions. The tubes were counted on a Wizard2 gamma counter (Perkin Elmer, Shelton, CT).
Data Collected at Termination
F0 dams
F0 dams that were observed to be sperm positive or had an in situ vaginal plug observed during mating were removed after litters were weaned, after delivery of litters that did not meet study criteria (i.e., less than three males and three females born or litter born before GD 20), or after GD 26 if no litter was delivered. The animals were euthanized with gaseous carbon dioxide, the uterus was removed, examined for visible implantation sites, and submerged in 10% ammonium sulfide for a minimum of 15 min to reveal early implantation sites. The total number of implantation sites (i.e., visible plus early) was used for statistical analysis. The number of resorptions was calculated by subtracting the number of pups born from the total number of implantation sites.
PND 15
On PND 15, in litters with four male pups, one male per litter was removed, decapitated, and trunk blood collected for measurement of serum T3, T4, and thyroid stimulating hormone (TSH). Euthanasia occurred after dosing and was completed between 7 and 11 a.m.. Serum was aliquoted and frozen in a −80ºC freezer until batch testing. Total T3, total T4 (both “Coat-a-Count” Siemens), and TSH (IDS) were measured using RIA according to the manufacturers’ instructions.
PND 21
On PND 21, in litters with three pups of a given sex, one animal per sex per litter was euthanized, blood collected by cardiac puncture for preparation of serum, and inguinal mammary glands, fat pads (retroperitoneal, epididymal, ovarian/parametrial), prostate, brain, ovaries, and uterus were collected. Tissues were weighed and flash frozen [except for the right ovary and fifth mammary glands that were fixed in 10% NBF (neutral buffered formalin)] for transfer to other studies. The fifth left mammary glands were processed for histopathology and the right for whole mount. Only the histopathology results from the fifth left mammary gland are reported.
PND 90
The main focus of this experiment was on the animals maintained until PND 90 ± 5 for necropsy and histopathology (designated the “histopathology study arm”). A littermate of each sex was also maintained after weaning. These animals were similarly treated in the same animal room as the histopathology arm animals and were necropsied at PND 90 ± 5 for harvest of tissues that were provided to other studies (designated the “frozen tissue study arm”). Only limited data from these littermates will be reported here. However, in cases where an animal that had been designated for histopathology was lost to the study for any reason, the same sex littermate was reassigned to be utilized for histopathology at PND 90 ± 5. This occurred in only five cases.
On PND 90 ± 5, one animal per sex per litter was euthanized after an overnight fast. Females were euthanized when predicted to be in estrus, based on daily vaginal smears collected up to the day before sacrifice, or on the last day of the specified time period that was not a holiday or weekend, if not cycling normally. Blood was collected from the retro-orbital sinus and serum was prepared. AGD, the distance between the inner edge of the anal opening closest to the genitalia and the inner edge of the prepuce or vaginal opening closest to the anal opening, was measured on the carcass with a caliper. At sacrifice, all tissues of interest (see below) were examined grossly and removed. Gross findings on all tissues were captured electronically and gross lesions were removed for histological evaluation. The following organs were dissected and weighed: adrenals, brain, dorsolateral and ventral prostate (dissected and weighed after fixation), epididymides, heart, kidneys, liver, ovaries, pituitary (after fixation), seminal vesicles with coagulating gland, spleen, testes, thymus, thyroid (after fixation), uterus (blotted), and fat pads (retroperitoneal, epididymal, and ovarian/parametrial). The left epididymis was dissected from the left testis and both processed for sperm evaluation (see below). If gross lesions or abnormalities were noted at necropsy with either the left testis or epididymis, the right organ was used for sperm motility and count studies and the left was used for histopathology. The following organs were evaluated microscopically: adrenals, aorta (thoracic), bone marrow (femur), brain, right epididymis, heart, kidneys, liver, fifth left mammary gland (inguinal, both sexes), ovaries, oviduct, pancreas, pituitary, prostate (dorsolateral and ventral), seminal vesicles with coagulating gland, spleen, right testis, thymus, thyroid, uterus, and vagina. The left fifth (inguinal) mammary gland and fat pad were removed as a unit to include the inguinal lymph node. It was placed in a cassette in a “flattened” dorsoventral orientation, similar to the orientation of the unit before it was removed from the carcass. This orientation provided a histological section with a similar profile to that of a routine mammary whole mount. All tissues except testes were fixed in 10% NBF; testes were fixed in modified Davidson's fixative (Latendresse et al., 2002). Fixation time for all tissues except brain was limited to 48 h. Brain remained in fixative until processing. Tissues were trimmed, processed, and embedded in infiltrating media (Formula R, Leica, Buffalo Grove, IL), sectioned at 5 µm, stained with hematoxylin and eosin (except for testes), and examined by light microscopy by a Study Pathologist (one for female tissues and one for male tissues). The testes were stained with periodic acid-Schiff stain. When applicable, non-neoplastic lesions were graded for severity as minimal (grade 1), mild (grade 2), moderate (grade 3), or marked (grade 4).
Serum clinical chemistry analyses were conducted on an Alfa Wassermann ALERA (West Caldwell, NJ). Alfa Wassermann reagents were used for glucose, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transpeptidase, total protein, albumin, cholesterol, triglycerides, blood urea nitrogen, and creatinine. Catachem (Bridgeport, CT) reagents were used for sorbitol dehydrogenase and total bile acids. All testing for these endpoints was completed on the day of collection. Rat-specific troponin I, troponin T (both Biotang, Waltham, MA), and leptin (Millipore, St. Charles, MO) were measured by ELISA in serum that had been frozen and stored in a −80ºC freezer. The plates were read on an ELx800 Universal Plate Reader. Total T3, total T4, and TSH were measured by RIA as described above. Insulin was also measured by RIA (“Coat-a-Count,” Siemens).
Epididymal (left) sperm evaluations were conducted using an integrated visual optical system (IVOS)-automated sperm analyzer (Hamilton Thorne Inc., Beverly, MA). The cauda section was dissected and immediately placed in 40 ml of a prewarmed (37ºC) solution consisting of 1% bovine serum albumin dissolved in phosphate buffered saline. A minimum of 2 min was allowed for sperm to swim out of the cauda. Five fields on an 80 μm deep slide were automatically selected by the analyzer to calculate the percent motility for each animal. After the motility sample was acquired, the left cauda was minced and approximately 1 ml of the suspension was frozen on dry ice and stored in a −80ºC freezer for later determination of the total cauda sperm count. The left testis was weighed, placed on dry ice, and stored in a −80ºC freezer until determination of testicular spermatid head counts. For sperm counting, the tunic was removed from the thawed testis, and the testis was weighed and homogenized in a solution of 1% bovine serum albumin in phosphate buffered saline. The suspensions were transferred to a vial containing a fluorescent DNA-specific stain (IDENT, Hamilton Thorne Inc.) to stain the sperm heads. The sperm counts were calculated from 20 automatically selected fields. For sperm morphology evaluation, two eosin-stained slides were prepared from the caudal epididymis suspension and a minimum of 200 sperm/animal was examined for morphological abnormalities (amorphous, small head, enlarged head, double head, and tail coiled or bent).
Statistical Methods
The statistical methods used varied by endpoint and are described in Supplementary materials.
The dose range of primary interest for the present study was between 2.5 and 2700 μg BPA/kg bw/day and a major purpose of the study was to provide information for a subsequent chronic study. To minimize false negatives within the dose range of interest due to over correction, comparisons of each dose group to vehicle controls were made in four analysis subgroups (naïve controls, low dose BPA, high dose BPA, and EE2) for each sex. Trends were also examined within the low BPA (2.5–2700 μg/kg bw/day) subgroup.
The tests of interest in this report are pairwise comparisons to vehicle control. Unless otherwise noted, pairwise tests are corrected for multiple comparisons using Dunnett's method (Dunnett, 1955) for those doses within the analysis subgroup. Any reported linear trends were performed using orthogonal linear contrasts. Simple means and standard errors are reported in tables.
Reported tests are two-sided at the 0.05 significance level with the exception of comparisons to vehicle control for histopathology data, which were conducted as one-sided tests at the 0.05 significance level per NTP convention.
Quality Assurance Procedures
This study was conducted in compliance with the FDA Good Laboratory Practice Regulations (21 CFR, Part 58).
For the PND 90 ± 5 pathology results, a Quality Assessment (QAS) Review and a Pathology Working Group (PWG) Review of the data and pathology slides were conducted by a group of external veterinary pathologists not associated with the original reading of the study slides. These reviews thoroughly evaluated the PND 90 ± 5 naïve and vehicle control groups and all BPA dose groups, but not the two EE2 dose groups. The final pathology report and the data reported here reflect the resulting consensus.
RESULTS
Selected data are presented in tables and figures or as Supplementary data, whereas all data collected on the study are available in the NCTR Study Report (available upon request).
Treatment Effects on Dams During Gestation and Litter Endpoints
BPA doses ≤ 2700 μg/kg bw/day did not affect gestational body weight gain, but the 100,000 and 300,000 μg BPA/kg bw/day doses reduced body weight gain over the period from GD 6 to parturition by 16% and 11%, respectively (Table 1). Similarly, both 0.5 and 5.0 μg EE2/kg bw/day reduced gestational body weight gain by 7% and 14%, respectively (Table 1). No treatment significantly affected gestation length or gestational metabolic efficiency (i.e., the change in body weight divided by food consumption for a given time interval) determined from the time that dosing started on GD 6 (data not shown).
TABLE 1. Gestational Body Weight Gain (from GD 6)a.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| n | 25 | 23 | 26 | 18 | 24 | 24 | 23 | 22 | 25 | 24 | 25 | 20 | 21 |
| Body weight gain (g) from GD6 – parturition | 163.6 ± 3.7 | 164.9 ± 4.2 | 162.0 ± 3.8 | 175.3 ± 6.1 | 164.2 ± 4.2 | 164.1 ± 3.5 | 172.3 ± 4.0 | 168.0 ± 5.8 | 164.3 ± 3.5 | 138.7 ± 4.0 *** | 147.2 ± 3.9 *** | 153.3 ± 3.6 * | 141.4 ± 3.8 *** |
aResults are presented as simple means ± SEM. Results presented for dams with pups on study. The statistical significance of the results did not change when all dams producing litters were analyzed. Significant differences from the vehicle control are indicated: *p < 0.05 and ***p < 0.001.
Neither BPA nor EE2 at any dose tested affected the number of implantation sites measured in the uterus of the F0 dams nor any other endpoints evaluated for the litters, including the AGD and AGDI (data not shown). At PND 90, the mean AGD and AGDI values of the male naïve control were 6–7% greater than those of the vehicle control. The AGDI of males in the 300,000 μg BPA/kg bw/day group was also approximately 6.5% greater than that of the vehicle control group (data not shown).
F1 Pup Preweaning Survival
There was a significant difference in survival between the naïve and the vehicle males (100% vs. 88.3%, respectively). The only significant BPA effect on pup preweaning survival was a decrease by 35%, both in males and females, in the 300,000 μg/kg bw/day dose group. EE2 did not significantly affect this endpoint (data not shown).
Body Weights
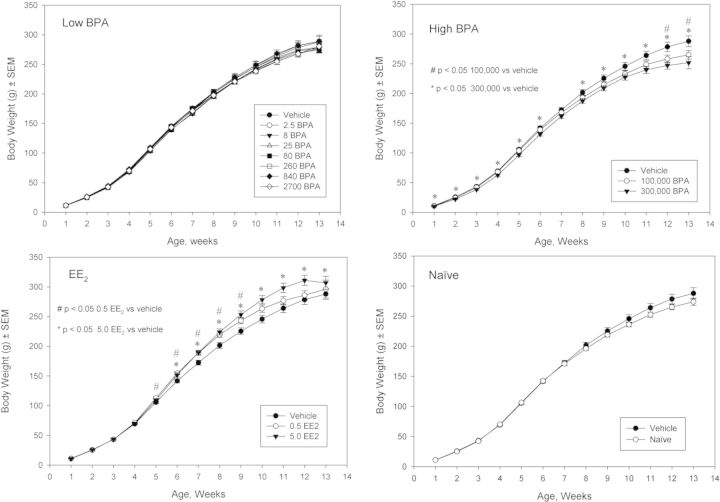
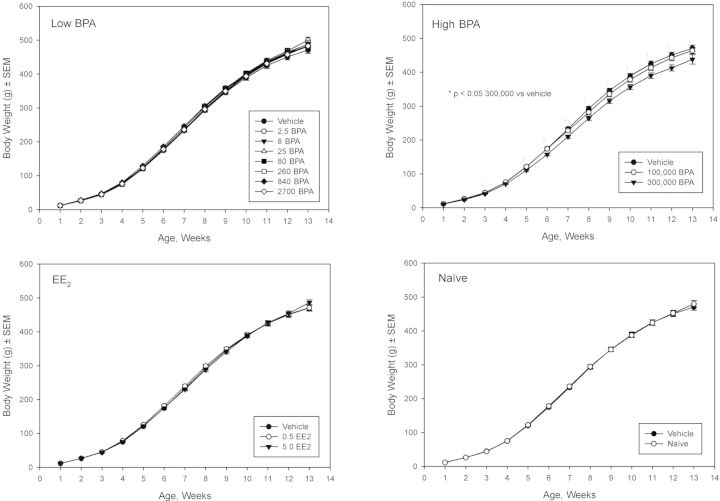
Growth curves over the entire study period for female and male animals are shown in Figures 2 and 3, respectively. There were no differences in body weights between the naïve and vehicle controls or the low dose BPA groups and vehicle control in either sex. Body weights of females and males in the highest BPA dose group (300,000 μg/kg bw/day) of females and males were consistently lower than vehicle control throughout the study, varying from approximately 6–13% (average across weeks approximately 10%) lower. For the 100,000 μg BPA/kg bw/day group, body weights were significantly lower by approximately 8–9% than vehicle control in the last two weeks (weeks 12 and 13) in females only. However, females used for the delayed vaginal cytology assessment did not show a high dose BPA body weight effect (Supplementary fig. 1). In females, low dose EE2 significantly increased body weight (approximately 6–9%) on weeks 5–9 and the high dose EE2 significantly increased (average across weeks approximately 10%) body weights from week 6 (Fig. 2). In the delayed vaginal cytology arm, the significant body weight difference in the high EE2 dose group persisted, whereas the low dose of EE2 did not show a significant body weight effect versus vehicle control (Supplementary fig. 1). Male body weight was not affected by EE2 (Fig. 3).
FIG. 2.
F1 female body weights. Mean weekly body weights (g) are shown for the study-defined subgroups (low BPA, high BPA, EE2, and naïve) versus vehicle.
FIG. 3.
F1 male body weights. Mean weekly body weights (g) are shown for the study-defined subgroups (low BPA, high BPA, EE2, and naïve) versus vehicle.
PND 15 Clinical Chemistry Measurements
In PND 15 males, total T3 was increased by 13% and 30%, respectively, at 100,000 and 300,000 μg BPA/kg bw/day, whereas TSH was decreased by approximately 28% at 300,000 μg BPA/kg bw/day (Table 2). There were no other significant effects among the low BPA doses, the EE2 doses, or naïve versus vehicle controls.
TABLE 2. Male PND 15 Clinical Chemistry: Serum T3, T4, and TSH Levelsa.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| n | 21 | 15 | 23 | 16 | 21 | 24 | 20 | 20 | 20 | 16 | 7 | 11 | 12 |
| T3, ng/dL | 86.88 ± 3.01 | 88.70 ± 5.52 | 90.05 ± 3.34 | 96.63 ± 3.88 | 89.79 ± 3.21 | 80.34 ± 2.26 | 88.46 ± 3.90 | 87.25 ± 3.27 | 85.80 ± 2.60 | 100.09 ± 4.45** | 115.26 ± 7.75*** | 92.74 ± 3.06 | 89.06 ± 4.53 |
| n | 21 | 15 | 23 | 16 | 21 | 24 | 20 | 20 | 20 | 16 | 7 | 11 | 13 |
| T4, μg/dL | 4.97 ± 0.22 | 5.31 ± 0.21 | 5.26 ± 0.18 | 4.92 ± 0.21 | 4.77 ± 0.22 | 4.81 ± 0.19 | 5.07 ± 0.19 | 4.87 ± 0.14 | 5.26 ± 0.25 | 5.31 ± 0.22 | 5.89 ± 0.62 | 4.93 ± 0.36 | 5.37 ± 0.32 |
| n | 21 | 15 | 21 | 16 | 19 | 24 | 20 | 19 | 20 | 15 | 6 | 10 | 13 |
| TSH, ng/ml | 5.84 ± 0.54 | 5.93 ± 0.61 | 5.22 ± 0.32 | 6.17 ± 0.59 | 4.85 ± 0.36 | 7.17 ± 1.01 | 5.50 ± 0.44 | 5.36 ± 0.31 | 4.68 ± 0.52 | 5.13 ± 0.44 | 4.24 ± 0.28* | 7.63 ± 0.62 | 6.17 ± 0.82 |
aResults are given as simple means ± SEM. Significant differences from the vehicle control are indicated: *p < 0.05, **p < 0.01, and ***p < 0.001.
Markers of Sexual Development
Table 3 summarizes the results for age and body weights at the time of vaginal opening, testicular descent, and preputial separation. None of the BPA doses affected the time of vaginal opening or the body weight at which the landmark was achieved. Both doses of EE2 affected the time of vaginal opening, with average delays of approximately 6.1 and 23.2 days at 0.5 and 5.0 μg EE2/kg bw/day, respectively. Consistent with this timing delay, the body weights at which the landmark was attained were 32% and 108% higher than vehicle controls at the low and high EE2 doses, respectively. Time to first estrus was significantly delayed by 5.0 μg EE2/kg bw/day, but neither the lower dose of EE2 nor any dose level of BPA affected this parameter. Naïve controls did not differ from vehicle controls for these endpoints.
TABLE 3. Markers of Sexual Developmenta.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| Vaginal opening | |||||||||||||
| No. of litters | 21 | 22 | 26 | 18 | 24 | 23 | 23 | 22 | 24 | 23 | 19 | 20 | 21 |
| Age, days | 34.0 ± 0.8 | 32.5 ± 0.5 | 33.1 ± 0.5 | 32.2 ± 0.5 | 32.6 ± 0.6 | 32.9 ± 0.4 | 33.1 ± 1.2 | 32.8 ± 0.6 | 32.4 ± 0.4 | 33.2 ± 0.8 | 35.2 ± 1.8 | 38.6 ± 1.7** | 56.2 ± 2.7*** |
| Body weight, g | 118.9 ± 4.2 | 108.8 ± 3.0 | 113.3 ± 2.6 | 106.3 ± 2.0 | 108.3 ± 2.7 | 110.8 ± 2.9 | 113.4 ± 8.3 | 112.1 ± 3.4 | 111.4 ± 3.1 | 111.6 ± 3.4 | 109.6 ± 7.1 | 142.5± 7.1*** | 227.4 ± 9.8*** |
| Testicular descent | |||||||||||||
| No. of litters | 20 | 20 | 23 | 18 | 21 | 20 | 20 | 21 | 21 | 23 | 18 | 20 | 20 |
| Age, days | 23.5 ± 0.3 | 23.6 ± 0.3 | 23.9 ± 0.2 | 23.7 ± 0.3 | 23.8 ± 0.3 | 24.0 ± 0.3 | 24.7 ± 0.3* | 23.8 ± 0.3 | 23.4 ± 0.2 | 24.3 ± 0.4 | 25.8 ± 0.6** | 24.5 ± 0.3 | 29.4 ± 0.8*** |
| Body weight, g | 67.7 ± 2.1 | 67.5 ± 2.0 | 70.6 ± 1.5 | 69.8 ± 1.9 | 70.2 ± 1.6 | 68.1 ± 1.7 | 72.5 ± 1.6 | 71.2 ± 1.9 | 68.0 ± 1.4 | 72.0 ± 1.9 | 74.5 ± 3.5 | 74.8 ± 2.1* | 102.9 ± 3.8*** |
| Preputial separation | |||||||||||||
| No. of litters | 20 | 20 | 23 | 18 | 21 | 20 | 20 | 21 | 21 | 23 | 18 | 19b | 20 |
| Age, days | 43.6 ± 0.5 | 44.0 ± 0.6 | 44.8 ± 0.8 | 43.3 ± 0.8 | 42.9 ± 0.8 | 45.4 ± 1.2 | 44.7 ± 1.1 | 42.9 ± 0.9 | 44.7 ± 0.6 | 44.6 ± 0.6 | 47.8 ± 1.7 | 45.8 ± 1.0 | 56.6 ± 2.3*** |
| Body weight, g | 220.0 ± 6.1 | 214.9 ± 4.7 | 230.4 ± 5.8 | 215.9 ± 5.4 | 207.1 ± 7.2 | 230.2 ± 12.5 | 221.8 ± 9.0 | 215.7 ± 8.7 | 228.8 ± 6.1 | 217.0 ± 4.7 | 217.9 ± 10.0 | 240.3 ± 9.0 | 309.0 ± 14.7*** |
aResults are presented as simple litter means ± SEM. Significant differences from the vehicle control are indicated: *p < 0.05, **p < 0.01, and ***p < 0.001.
bIn one litter in the 0.5 μg EE2/kg bw/day dose group, neither male pup was observed to have preputial separation by the time of termination.
No male in any dose group had retained nipples when examined at PND 13. Testicular descent was significantly delayed by approximately 1 and 2 days, respectively, in the 260 and 300,000 μg BPA/kg bw/day dose groups. There were no differences in body weights at the time of testicular descent in these groups. EE2 at 5.0 μg/kg bw/day delayed testicular descent by 5.8 days, and the body weights at the time of testicular descent were approximately 11% and 52% higher than that of vehicle controls in the 0.5 and 5.0 μg EE2/kg bw/day dose groups, respectively. None of the BPA doses differed significantly from the vehicle control in the time of preputial separation or body weight at which preputial separation was achieved. The high dose of EE2 significantly delayed the average time of preputial separation (approximately 11.5 days) and, accordingly, the mean body weight at which this landmark was achieved was 42% greater for this dose group than for the vehicle control. The naïve and vehicle control groups did not differ in the timing of testicular descent or preputial separation.
Vaginal Cytology
Vaginal smears were taken daily from PND 69 to termination at PND 90 ± 5 for evaluation of the estrous cycle. A summary of the occurrence of animals with abnormal cycles across treatment groups is shown in Table 4. Between 20% and 35% of vehicle control animals showed at least one abnormal cycle over the evaluation period, and there was no significant difference between naïve and vehicle controls. The majority of the abnormal cycles were due to extended diestrus. There was a clear effect of both doses of EE2 on estrous cyclicity, with 95–100% of the animals showing abnormal cycles, primarily due to extended estrus (strings of estrus alone or proestrus, proestrus/estrus, and estrus combined) and extended estrus/diestrus (Table 4). Low BPA doses had no effect on the percentage of animals showing abnormal cycles versus vehicle control. Similarly, 100,000 μg BPA/kg bw/day did not affect the proportion of animals showing abnormal cycles, whereas 300,000 μg BPA/kg bw/day significantly increased the proportion of animals showing abnormal cycles in a manner similar to that of EE2.
TABLE 4. Estrous Cycle Evaluations Summary, PND 69 to PND 90 ± 5.
| Patterna | Estrous cycle | Treatment | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | ||||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | ||
| Extended estrus | Total examined | 20 | 20 | 23 | 18 | 21 | 20 | 21 | 20 | 20 | 21b | 19 | 19c | 19c |
| Present | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 6**d | 10*** | 10*** | |
| Extended combined estrus | Total examined | 20 | 20 | 23 | 18 | 21 | 20 | 21 | 20 | 20 | 21 | 19 | 19 | 19 |
| Present | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5* | 9*** | 11*** | |
| Extended diestrus | Total examined | 20 | 20 | 23 | 18 | 21 | 20 | 21 | 20 | 20 | 21 | 19 | 19 | 19 |
| Present | 5 | 7 | 5 | 7 | 9 | 5 | 8 | 14 | 10 | 7 | 7 | 3 | 8 | |
| Extended E/D | Total examined | 20 | 20 | 23 | 18 | 21 | 20 | 21 | 20 | 20 | 21 | 19 | 19 | 19 |
| Present | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 5* | 11*** | 6** | |
| Any abnormal | Total examined | 20 | 20 | 23 | 18 | 21 | 20 | 21 | 20 | 20 | 21 | 19 | 19 | 19 |
| Present | 5 | 7 | 5 | 8 | 10 | 5 | 8 | 14 | 11 | 7 | 17** | 18*** | 19*** | |
aThe responses that were evaluated were: extended estrus (three or more consecutive days of E), extended combined estrus (four or more consecutive days of P, E, or P/E), extended diestrus (four or more consecutive days of D), extended E/D (two or more consecutive days of E/D), and any abnormal cycling (including patterns other than those listed, such as extended P (two or more in a row), extended P/E (three or more in a row), extended D/P (two or more in a row)). P/E, E/D, and D/P are transitional stages.
bIn the 100,000 μg BPA/kg bw/day, data from two littermates were inadvertently included in the analysis. An animal in the histopathology study arm died early and smears from its littermate in the frozen tissue arm were collected and included.
cFor one animal in each of the EE2 dose groups, vaginal opening was not recorded by the time of study termination and no vaginal cytology data were available.
dSignificant differences from the vehicle control are indicated: *p < 0.05, **p < 0.01, and ***p < 0.001.
A set of females was also maintained after PND 95 without further dosing and vaginal smears were monitored from PND 150 through PND 170. Results were similar to those observed in the younger animals in that only the two EE2 groups and the BPA doses above 2700 μg/kg bw/day increased abnormal cycling relative to the vehicle controls (data not shown).
PND 80 Clinical Chemistry (Serum Hormones)
The data for hormones analyzed in PND 80 ± 3 female and male serum are summarized in Supplementary tables 2 and 3, respectively. The vaginal cytologies collected on the day of the blood draws indicated that 60–90% of the females in any given dose group had profiles consistent with estrus or proestrus. The vaginal cytology data discussed above, as well as the PND 90 ± 5 histopathology data discussed below, indicated that the high dose BPA and EE2 groups were not cycling normally. There were no significant treatment-related effects in the low BPA dose range in females, but mean serum estradiol was significantly increased by 67% and 113%, respectively, in the 100,000 and 300,000 μg BPA/kg bw/day groups. Progesterone was significantly decreased by 51% in the highest BPA dose group. In the EE2 groups, estradiol was significantly increased by 34% and 29% and progesterone significantly decreased by 70% and 80% in the 0.5 and 5.0 μg EE2/kg bw/day groups, respectively. Both doses of EE2 and the highest dose of BPA had significantly elevated prolactin levels, although prolactin did not differ significantly from vehicle control in a two-factor analysis of treatment and cycle stage with interaction (Supplementary table 2). The observed differences in hormone levels are consistent with the ovarian histological changes (see below). There were no significant differences from vehicle controls for FSH and LH (data not shown) for any BPA or EE2 dose group, and there were no significant differences between vehicle and naïve controls for any serum hormone examined.
In males, there were no treatment-related differences between any treatment group and vehicle control for the measured hormones FSH, LH, testosterone, and prolactin (Supplementary table 3). Both doses of EE2 and the highest BPA dose had mean serum testosterone levels that were 16–29% lower than vehicle control means, but these differences were not statistically significant. The high variability of the testosterone measurements and insufficient group size may have reduced the power to detect potential changes in this endpoint.
Organ Weights
In PND 21 females, both doses of EE2 increased uterine weights and decreased ovarian weights (Supplementary table 4). These effects were observed for absolute organ weights as well as organ weights adjusted for brain or body weights. The highest BPA dose (300,000 μg/kg bw/day) also decreased ovarian weights (absolute and adjusted for brain weight).
The female organ weights measured at PND 90 ± 5 are shown in Supplementary table 5. Absolute weights and weights adjusted for brain and body weights were analyzed with the results of the body and brain weight adjustments in general agreement. The only significant difference between the naïve and vehicle controls was for the heart adjusted for body weight, with the naïve weight 7% higher than the vehicle (p = 0.047). There were no significant treatment effects of low dose BPA (2.5–2700 μg/kg bw/day) on any organ weighed from the females. At 100,000 μg BPA/kg bw/day, absolute spleen weight was approximately 10% lower than the vehicle control weight. All other statistically significant effects of BPA on organ weights were confined to the 300,000 μg BPA/kg bw/day group. Brain, heart, fat pads, and ovary weights, either absolute and/or adjusted to body or brain weights, were reduced by the highest dose of BPA tested. Liver weight adjusted for body weight was increased by 10%. EE2 significantly affected multiple organs, in most cases whether absolute weights or weights adjusted for brain or body weight were analyzed, at one or both doses. Both doses of EE2 increased the weight of the adrenal gland, heart, kidney, and liver. The low dose, but not the high dose, EE2 increased pituitary gland weight, whereas the spleen and thymus weights were significantly elevated only by the high dose EE2. Ovarian/parametrial fat and ovaries were significantly lighter than vehicle controls in both EE2 dose groups, whereas uterine weight was significantly lower only at the high dose. Retroperitoneal fat was significantly reduced in weight relative to vehicle control at the low EE2 dose (when analyzed with brain or body weight as covariates) and at the high EE2 dose (when analyzed with body weight as covariate).
The organ weight data for males at PND 90 ± 5 are summarized in Supplementary table 6. There were no statistically significant differences between the vehicle and naïve controls for any organ. The sole significant effect in the low dose BPA groups was a decreased (approximately 10%) heart weight adjusted for body weight relative to vehicle control at 2700 μg BPA/kg bw/day. Significant effects of high dose BPA were restricted to the 300,000 μg/kg bw/day group. Absolute brain weight was approximately 5% lower than vehicle. Absolute and brain weight-adjusted epididymal fat pad, heart, kidney, liver, and spleen weights were all significantly less than vehicle control weights. None of these effects were significant when the body weight-adjusted weights were analyzed, suggesting that the effects were secondary to the body weight reduction observed in the highest BPA dose group. All significant effects of EE2 were confined to the high dose group (5.0 μg/kg bw/day). Adrenal gland, pituitary gland, and spleen weights were significantly increased by the high EE2 dose over vehicle controls whether absolute or brain- or body-adjusted weights were analyzed. Epididymis, epididymal fat pad, dorsolateral and ventral prostate, seminal vesicles with coagulating gland, and testis were significantly decreased by the high EE2 dose. Liver weight adjusted for body weight was increased by approximately 9% in the high EE2 dose group.
PND 90 Clinical Chemistry
Females
Table 5 summarizes selected clinical chemistry parameters measured in serum collected at necropsy from PND 90 ± 5 females after an overnight fast. There were no significant differences between the naïve and vehicle control groups for any of the analytes. In the low BPA dose range, the only statistically significant effect was an increase (10%) in aspartate aminotransferase at 2700 μg BPA/kg bw/day. The 100,000 μg BPA/kg bw/day dose significantly depressed cholesterol and triglycerides by 16% and 30%, respectively, relative to vehicle controls, but there was no effect on these endpoints at 300,000 μg BPA/kg bw/day. TSH was significantly increased by 43% and 62% at 100,000 and 300,000 μg BPA/kg bw/day, respectively. Leptin was significantly decreased (56%) in the highest BPA dose group. Both doses of EE2 significantly affected cholesterol, with a 19% increase at the 0.5 μg/kg bw/day dose and a 17% decrease at the 5.0 μg/kg bw/day dose. TSH was also significantly increased in both EE2 dose groups, with elevations of 66% and 62% at 0.5 and 5.0 μg EE2/kg bw/day, respectively. T4 was significantly depressed (22%) in the low, but not the high, EE2 dose group. Total protein was significantly higher (7%) in the low EE2 dose group. In the high EE2 dose group, alkaline phosphatase, alanine aminotransferase, blood urea nitrogen, and sorbitol dehydrogenase were significantly elevated.
TABLE 5. Clinical Chemistry, Females PND 90 ± 5a.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| n | 20 | 20 | 23 | 18 | 21 | 20 | 20 | 20 | 20 | 20 | 19 | 20 | 20 |
| Aspartate aminotransferase, U/L | 86.6 ± 2.5 | 81.2 ± 2.3 | 81.9 ± 2.7 | 88.2 ± 3.8 | 85.5 ± 2.9 | 88.6 ± 2.2 | 89.6 ± 2.5 | 79.2 ± 2.6 | 89.0 ± 2.4* | 81.1 ± 2.8 | 87.3 ± 7.7 | 79.3 ± 3.6 | 91.9 ± 6.4 |
| Blood urea nitrogen, mg/dL | 17.5 ± 0.5 | 16.5 ± 0.8 | 16.6 ± 0.7 | 17.1 ± 0.7 | 16.7 ± 0.6 | 16.8 ± 0.7 | 15.8 ± 0.4 | 15.4 ± 0.6 | 16.0 ± 0.8 | 16.1 ± 0.8 | 17.3 ± 0.6 | 17.6 ± 1.0 | 19.6 ± 0.7** |
| Cholesterol, mg/dL | 104.9 ± 6.0 | 99.0 ± 2.5 | 106.3 ± 4.0 | 100.3 ± 4.2 | 98.9 ± 2.1 | 103.1 ± 3.6 | 107.1 ± 4.5 | 101.0 ± 4.3 | 97.3 ± 4.5 | 83.2 ± 3.9* | 94.3 ± 4.8 | 117.5 ± 6.3* | 82.3 ± 5.0** |
| Glucose, mg/dL | 120.0 ± 6.3 | 122.2 ± 5.3 | 116.0 ± 3.5 | 125.4 ± 7.5 | 115.9 ± 4.7 | 124.7 ± 6.3 | 120.0 ± 6.3 | 115.1 ± 4.3 | 108.4 ± 4.3 | 112.5 ± 3.9 | 110.9 ± 4.6 | 121.4 ± 6.2 | 122.5 ± 7.3 |
| Insulin, mIU/ml | 7.7 ± 1.7 | 8.9 ± 1.8 | 7.1 ± 1.0 | 9.2 ± 1.9 | 7.4 ± 1.0 | 7.7 ± 1.2 | 5.7 ± 0.6 | 5.8 ± 0.6 | 7.2 ± 1.5 | 5.4 ± 0.8 | 4.9 ± 0.6 | 7.8 ± 1.4 | 9.4 ± 1.6 |
| Leptin, ng/ml | 2.5 ± 0.3 | 2.7 ± 0.4 | 2.6 ± 0.3 | 3.0 ± 0.3 | 2.1 ± 0.2 | 2.3 ± 0.3 | 2.6 ± 0.4 | 2.3 ± 0.2 | 2.1 ± 0.3 | 1.8 ± 0.2 | 1.2 ± 0.1*** | 1.9 ± 0.3 | 2.4 ± 0.4 |
| Sorbitol dehydrogenase, U/L | 43.2 ± 4.8 | 39.8 ± 4.0 | 37.3 ± 2.4 | 43.4 ± 3.9 | 46.6 ± 4.5 | 52.2 ± 5.7 | 40.3 ± 4.2 | 43.1 ± 6.0 | 44.6 ± 4.9 | 50.3 ± 5.8 | 31.3 ± 2.7 | 36.4 ± 2.9 | 60.8 ± 6.4* |
| T3, ng/dL | 68.7 ± 2.7 | 72.2 ± 3.1 | 76.4 ± 3.0 | 63.9 ± 3.0 | 68.2 ± 1.7 | 71.9 ± 3.6 | 72.3 ± 3.0 | 74.9 ± 4.2 | 67.3 ± 3.1 | 65.8 ± 2.7 | 74.4 ± 4.2 | 73.3 ± 4.8 | 73.9 ± 4.0 |
| T4, μg/dL | 4.8 ± 0.2 | 4.9 ± 0.3 | 5.0 ± 0.3 | 4.7 ± 0.2 | 4.5 ± 0.2 | 4.7 ± 0.3 | 4.7 ± 0.3 | 5.0 ± 0.4 | 4.6 ± 0.3 | 5.2 ± 0.3 | 5.8 ± 0.5 | 3.8 ± 0.2** | 5.4 ± 0.2 |
| TSH, ng/ml | 6.4 ± 0.5 | 6.1 ± 0.5 | 7.4 ± 0.6 | 6.0 ± 0.6 | 6.3 ± 0.6 | 7.9 ± 0.9 | 7.6 ± 1.0 | 6.5 ± 0.6 | 6.4 ± 0.8 | 8.7 ± 0.9* | 9.9 ± 1.4* | 10.1 ± 1.1** | 9.9 ± 0.7*** |
| Triglycerides, mg/dL | 81.9 ± 10.6 | 85.2 ± 7.6 | 81.2 ± 7.2 | 76.3 ± 5.1 | 93.7 ± 9.2 | 75.5 ± 5.2 | 83.7 ± 9.5 | 82.5 ± 7.4 | 75.8 ± 5.8 | 59.8 ± 2.6** | 68.9 ± 7.8 | 102.3 ± 7.9 | 147.8 ± 21.3 |
aResults are given as mean ± SEM. Significant differences from vehicle control are indicated: *p < 0.05, **p < 0.01, and ***p < 0.001.
Males
Table 6 summarizes selected clinical chemistry parameters measured in serum collected at necropsy from PND 90 ± 5 males after an overnight fast. There were no statistically significant differences between the naïve and vehicle control for any measured parameter. BPA had limited statistically significant effects on the clinical chemistry parameters measured in PND 90 ± 5 males. 100,000 and 300,000 μg BPA/kg bw/day reduced cholesterol by 16% and 21%, respectively. The 100,000 μg BPA/kg bw/day dose group had a glucose level 8% lower than vehicle controls, whereas the 300,000 μg BPA/kg bw/day dose reduced leptin and total bile acids by 33% and 25%, respectively, and elevated creatinine and T4 by 16% and 18%, respectively. There were no statistically significant differences between the low EE2 dose group and vehicle control. For the high dose of EE2 (5.0 μg/kg bw/day), cholesterol was significantly lower (16%) than in the vehicle control and creatinine and T3 were elevated by 20% and 18%, respectively, over the vehicle control group.
TABLE 6. Clinical Chemistry, Males PND 90 ± 5a.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| n | 20 | 20 | 23 | 18 | 21 | 20 | 20 | 20 | 20 | 20 | 18 | 20 | 20 |
| Cholesterol, mg/dL | 83.5 ± 5.0 | 77.5 ± 3.8 | 81.0 ± 4.5 | 77.7 ± 3.4 | 86.1 ± 4.3 | 81.1 ± 2.6 | 76.9 ± 5.3 | 85.5 ± 4.2 | 84.1 ± 3.7 | 65.3 ± 2.9* | 61.2 ± 3.9** | 69.3 ± 4.4 | 65.1 ± 3.3* |
| Creatinine, mg/dL | 0.470 ± 0.016 | 0.455 ± 0.022 | 0.452 ± 0.020 | 0.489 ± 0.024 | 0.486 ± 0.021 | 0.480 ± 0.022 | 0.515 ± 0.017 | 0.485 ± 0.017 | 0.415 ± 0.020 | 0.490 ± 0.040 | 0.528 ± 0.027* | 0.510 ± 0.018 | 0.555 ± 0.020*** |
| Glucose, mg/dL | 110.8 ± 2.9 | 116.3 ± 3.1 | 123.5 ± 6.1 | 113.4 ± 4.3 | 126.9 ± 8.6 | 111.8 ± 4.0 | 117.6 ± 4.6 | 127.1 ± 6.4 | 118.3 ± 5.5 | 107.6 ± 4.0* | 111.4 ± 3.7 | 110.0 ± 3.3 | 120.9 ± 5.0 |
| Insulin, mIU/ml | 10.5 ± 1.0 | 12.5 ± 3.6 | 10.7 ± 2.0 | 10.4 ± 1.1 | 11.5 ± 0.9 | 9.3 ± 1.1 | 9.7 ± 1.4 | 12.7 ± 1.9 | 9.9 ± 1.2 | 7.3 ± 0.7 | 8.7 ± 0.8 | 7.9 ± 0.7 | 10.7 ± 2.0 |
| Leptin, ng/ml | 4.1 ± 0.4 | 3.9 ± 0.4 | 3.7 ± 0.3 | 4.3 ± 0.5 | 4.7 ± 0.4 | 4.5 ± 0.6 | 4.4 ± 0.5 | 4.4 ± 0.3 | 4.6 ± 0.5 | 3.2 ± 0.2 | 2.6 ± 0.3** | 3.6 ± 0.4 | 3.3 ± 0.3 |
| T3, ng/dL | 75.9 ± 3.7 | 68.1 ± 3.4 | 73.7 ± 2.9 | 75.4 ± 3.2 | 68.0 ± 3.6 | 69.7 ± 3.7 | 76.0 ± 3.4 | 66.7 ± 3.8 | 73.1 ± 3.6 | 73.1 ± 4.1 | 74.4 ± 3.9 | 71.9 ± 3.4 | 80.5 ± 3.0** |
| T4, μg/dL | 6.4 ± 0.2 | 6.6 ± 0.3 | 6.2 ± 0.3 | 6.4 ± 0.3 | 6.9 ± 0.3 | 6.4 ± 0.4 | 6.8 ± 0.4 | 6.5 ± 0.3 | 6.4 ± 0.2 | 7.2 ± 0.3 | 7.8 ± 0.3* | 6.7 ± 0.3 | 7.2 ± 0.3 |
| TSH, ng/ml | 8.0 ± 0.7 | 7.6 ± 0.9 | 8.2 ± 0.8 | 8.2 ± 1.0 | 7.8 ± 0.6 | 8.4 ± 0.6 | 7.0 ± 0.6 | 7.8 ± 0.5 | 7.9 ± 1.0 | 7.9 ± 0.6 | 6.9 ± 0.6 | 7.1 ± 0.5 | 9.2 ± 1.0 |
| Total bile acids, μmol/L | 49.1 ± 4.0 | 54.2 ± 4.8 | 58.7 ± 5.3 | 60.3 ± 5.7 | 54.4 ± 5.3 | 52.9 ± 5.9 | 63.8 ± 6.7 | 53.9 ± 4.1 | 49.9 ± 4.5 | 44.7 ± 4.8 | 40.5 ± 2.7* | 57.1 ± 5.2 | 55.3 ± 5.1 |
| Triglycerides, mg/dL | 130.7 ± 10.5 | 119.0 ± 8.5 | 105.7 ± 8.5 | 118.4 ± 6.3 | 137.2 ± 10.5 | 125.1 ± 14.4 | 125.4 ± 12.7 | 126.8 ± 10.4 | 126.2 ± 10.4 | 120.6 ± 7.9 | 138.2 ± 15.3 | 129.1 ± 11.5 | 142.1 ± 13.2 |
aResults are given as mean ± SEM. Significant differences from vehicle control are indicated: *p < 0.05, **p < 0.01, and ***p < 0.001.
PND 90 Sperm Parameters
There were no statistically significant differences between the naïve and vehicle control groups for any sperm parameter evaluated or between any treatment group and the vehicle controls for sperm motility or morphology (data not shown). The only treatment-related difference between a dosed group and the vehicle control was a reduction (33%) of the cauda sperm count in the 5.0 μg EE2/kg bw/day dose group.
Necropsy Observations and Histopathology
The lesions discussed below are selected lesions of interest based primarily on statistically and/or biologically significant effects. Statistical results from the Poly-3 test on lesion incidences are presented in the summary tables (Tables 7–9). For lesions for which subjective severity scores were assigned by the Study Pathologists, results of two additional statistical tests incorporating these scores, the Jonckheere-Terpstra/Shirley-Williams (JT/SW) and Relative Treatment Effect (RTE) tests, were also performed. The rationale for preferring the Poly-3 test is presented in the Supplementary material. In general, the three statistical tests applied gave similar results for strong effects (i.e., high incidence lesions), whereas low incidence lesions gave occasional positives in the alternate tests, but did not show a consistent dose dependency (e.g., positive only in one or two intermediate and nonadjacent doses). Such lesions were judged unlikely to be biologically significant treatment-related lesions.
TABLE 7. Histological Evaluation of Female and Male PND 21 Mammary Glandsa.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| Females | |||||||||||||
| Duct, hyperplasia | |||||||||||||
| Significance | - | - | - | - | - | - | - | * | ** | - | - | - | |
| Incidence | 2/17 | 0/16 | 2/19 | 1/13 | 4/19 | 1/20 | 1/13 | 2/18 | 5/17 | 6/17 | 3/12 | 0/20 | 1/20 |
| Severity profile | 2/0/0/0 | 0/0/0/0 | 2/0/0/0 | 1/0/0/0 | 4/0/0/0 | 1/0/0/0 | 1/0/0/0 | 2/0/0/0 | 5/0/0/0 | 6/0/0/0 | 3/0/0/0 | - | - |
| Males | |||||||||||||
| Duct, hyperplasia | |||||||||||||
| Significance | - | - | - | - | - | - | - | - | - | - | *** | *** | |
| Incidence | 2/20 | 1/19 | 0/22 | 0/17 | 0/21 | 0/20 | 1/20 | 0/20 | 3/20 | 2/20 | 2/10 | 11/19 | 14/17 |
| Severity profile | 1/1/0/0 | 0/1/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 1/0/0/0 | 0/0/0/0 | 2/1/0/0 | 2/0/0/0 | 2/0/0/0 | 9/2/0/0 | 1/8/5/0 |
aData are presented as incidence (the number of animals with lesion/the number of animals examined). The severity profile is also shown, i.e, the number of animals with lesions graded as minimal/mild/moderate/marked or grades 1/2/3/4, respectively. Statistical significance based on incidence, or lack thereof, is indicated in the row above the incidence row. A “-“ indicates no significance. *p < 0.05, **p < 0.01, and ***p < 0.001.
TABLE 9. Male Histopathology, PND 90 ± 5a, b, c.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| Mammary gland | |||||||||||||
| Duct, hyperplasia | - | - | - | - | - | - | - | - | - | - | * | *** | |
| Incidence | 1/20 | 0/20 | 3/23 | 0/18 | 1/21 | 0/20 | 0/20 | 2/21 | 1/20 | 1/23 | 1/18 | 5/20 | 14/20 |
| Severity profile | 1/0/0/0 | 0/0/0/0 | 3/0/0/0 | 0/0/0/0 | 1/0/0/0 | 0/0/0/0 | 0/0/0/0 | 1/1/0/0 | 0/1/0/0 | 1/0/0/0 | 1/0/0/0 | 3/2/0/0 | 3/4/5/2 |
| Alveolus, hyperplasia | - | - | - | - | - | - | - | - | - | - | ** | ** | |
| Incidence | 6/20 | 5/20 | 3/23 | 7/18 | 5/21 | 7/20 | 7/20 | 4/21 | 8/20 | 10/23 | 6/18 | 14/20 | 13/20 |
| Severity profile | 3/3/0/0 | 4/1/0/0 | 2/1/0/0 | 7/0/0/0 | 5/0/0/0 | 3/4/0/0 | 6/0/1/0 | 3/1/0/0 | 6/2/0/0 | 7/2/1/0 | 3/3/0/0 | 5/5/3/1 | 1/3/4/5 |
| Duct or alveolus, hyperplasia | - | - | - | - | - | - | - | - | - | - | *** | *** | |
| Incidence | 6/20 | 5/20 | 6/23 | 7/18 | 6/21 | 7/20 | 7/20 | 6/21 | 9/20 | 11/23 | 7/18 | 16/20 | 20/20 |
| Severity profile | 3/3/0/0 | 4/1/0/0 | 5/1/0/0 | 7/0/0/0 | 6/0/0/0 | 3/4/0/0 | 6/0/1/0 | 4/2/0/0 | 6/3/0/0 | 8/2/1/0 | 4/3/0/0 | 5/7/3/1 | 1/3/9/7 |
| Coagulating gland | |||||||||||||
| Epithelium, hyperplasia | - | - | - | - | - | - | - | - | - | - | - | ** | |
| Incidence | 0/20 | 0/20 | 0/23 | 0/18 | 0/21 | 0/20 | 0/19 | 0/21 | 0/20 | 0/20 | 0/18 | 0/20 | 6/20 |
| Severity profile | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/5/1/0 |
| Testis | |||||||||||||
| Germinal epithelium, degeneration | - | - | - | - | - | - | - | - | - | - | - | ** | |
| Incidence | 1/20 | 1/20 | 0/23 | 0/18 | 0/21 | 0/20 | 2/20 | 0/21 | 0/20 | 0/20 | 0/18 | 2/20 | 9/20 |
| Severity profile | 1/0/0/0 | 0/0/0/1 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/1/1 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/2 | 2/2/1/4 |
| Seminiferous tubule, giant cell | − | * | - | - | - | - | - | - | - | - | 0/20 | 2/20 | |
| Incidence | 0/20 | 0/20 | 5/23 | 1/18 | 4/21 | 2/20 | 2/20 | 1/21 | 3/20 | 0/20 | 2/18 | - | - |
| Severity profile | 0/0/0/0 | 0/0/0/0 | 5/0/0/0 | 1/0/0/0 | 4/0/0/0 | 2/0/0/0 | 2/0/0/0 | 1/0/0/0 | 3/0/0/0 | 0/0/0/0 | 2/0/0/0 | 0/0/0/0 | 2/0/0/0 |
| Epididymis | |||||||||||||
| Hypospermia | - | - | - | - | - | - | - | - | - | - | - | * | |
| Incidence | 0/20 | 1/20 | 0/23 | 0/18 | 0/21 | 0/20 | 2/20 | 0/21 | 0/20 | 0/20 | 0/18 | 2/20 | 7/20 |
| Severity profile | 0/0/0/0 | 0/0/0/1 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/1/0/1 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/2 | 0/4/0/3 |
| Exfoliated germ cell | - | - | - | - | - | - | - | - | - | - | - | * | |
| Incidence | 0/20 | 1/20 | 0/23 | 0/18 | 0/21 | 0/20 | 3/20 | 0/20 | 0/20 | 0/20 | 0/18 | 2/20 | 6/20 |
| Severity profile | 0/0/0/0 | 1/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 1/2/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 0/0/0/0 | 2/0/0/0 | 3/3/0/0 |
aData are presented as incidence (the number of animals with lesion/the number of animals examined). The severity profile is also shown, i.e, the number of animals with lesions graded as minimal/mild/moderate/marked, or grades 1/2/3/4, respectively.
bThe number of animals examined includes early death animals. One male in each of the 840 and 100,000 μg BPA/kg bw/day dose group was sacrificed early in moribund condition and both were diagnosed with nephropathy associated with polyarteritis. Two males in the 100,000 μg BPA/kg bw/day dose group were removed early due to dosing accidents. Cases where the total number of tissues examined differs among tissues reflect insufficient or missing tissue.
cStatistical significance, or lack thereof, is indicated in the row above the incidence row. A “-“ indicates no significance. *p < 0.05, **p < 0.01, and ***p < 0.001.
PND 21
Results of the microscopic evaluation of the PND 21 mammary glands in both sexes are summarized in Table 7. There were no significant differences between naïve and vehicle controls in either sex. No significant effects were observed in females for either dose of EE2. Intraductal hyperplasia, i.e, focal, segmental, or diffuse proliferation (thickening or piling up) of epithelial cells lining the mammary ducts, was not observed as a treatment-related lesion. Ductal hyperplasia of the mammary gland in the context of this study indicates a relative increase in the number (density) of branching ducts and alveolar buds per unit area of mammary gland. There was a statistically significant trend in mammary gland ductal hyperplasia in the low dose BPA subgroup and the 2700 and 100,000 μg BPA/kg bw/day dose group differed significantly from the vehicle (Table 7). The incidence (4/19, or 21%) at 25 μg BPA/kg bw/day was significant by the RTE test. In all cases, the severity grade for the hyperplasia was minimal. The vehicle control incidence of mammary gland ductal hyperplasia was 0/16, whereas the naïve control incidence was 2/17, which diminishes the likelihood of the biological significance of the statistical findings in the dosed groups.
There was a significantly increased incidence of ductal hyperplasia in the male mammary glands of PND 21 pups treated with 0.5 and 5.0 μg EE2/kg bw/day, with severity as well as incidence increased at the highest EE2 dose level (Table 7). There were no statistically significant effects of BPA on the male mammary gland ductal hyperplasia at any dose level.
PND 90
Naïve versus vehicle control
Significant differences in non-neoplastic lesions between the naïve and vehicle control groups for both sexes at PND 90 were few. In females, renal cysts were higher in naïve controls (5/20) than in the vehicle controls (1/20). As discussed below, the low incidence of renal cysts in the vehicle control females relative to females in other dose groups affected the interpretation of apparent treatment effects. In males, degeneration of acinar cells (12/20 in naïve and 4/20 in vehicle) and infiltration of lymphocytic cells (8/20 in naïve and 2/20 in vehicle) in the pancreas were significantly different, but no other group differed significantly from the vehicle control (data not shown).
Females
Females were sacrificed on a day they were predicted to be in estrus based on the vaginal cytology of the previous day. Histological evaluation of the female reproductive tract (ovary, uterus, and vagina) indicated that the percentage of females in proestrus, estrus, or transition from estrus to metestrus at the time of necropsy ranged from 75% to 100% across dose groups, except for animals in both EE2 groups and the 300,000 μg BPA/kg bw/day group, which had high numbers of anestrus animals (see below).
Bilaterally small ovaries and abnormal genitalia and thick vagina were common treatment-related gross observations. Small ovaries were noted in both EE2 groups and the 300,000 μg BPA/kg bw/day group, with incidences of 8/20, 16/20, and 10/19 in the 0.5 EE2, 5.0 EE2, and 300,000 BPA groups, respectively. The small ovaries phenotype correlated microscopically with depletion of corpora lutea and antral follicles. Abnormal genitalia and gross thickening of the vaginal wall were observed in the 5.0 μg EE2/kg bw/day treatment group (14/20) and were manifested microscopically as hypertrophy of the vaginal muscular wall (muscularis) and mucosal hyperplasia.
A mammary gland ductal adenocarcinoma was observed in the 2.5 μg BPA/kg bw/day group and this was the only neoplasm observed in the PND 90 ± 5 histopathology animals. Although there are limited historical data for this lesion in the NCTR Sprague Dawley rat at this age, a similar neoplasm was recently diagnosed in a PND 90 female NCTR Sprague Dawley rat on an unrelated NCTR study on silver nanoparticles (Boudreau, personal communication).
Non-neoplastic lesions in the mammary gland, ovary, uterus, and kidney are summarized in Table 8. The incidence of mammary gland ductal hyperplasia was variable across the dose range, including naïve and vehicle controls, but was significantly increased in the 300,000 μg BPA/kg bw/day group relative to vehicle control. There were no other BPA-induced significant effects on the mammary gland in females as determined by the Poly-3 test, although the RTE and JT/SW analyses, which incorporate severity information, indicated significant increases of mammary gland ductal hyperplasia at 2700 and 100,000 μg BPA/kg bw/day. Non-neoplastic lesions in females in the low BPA dose range that were significantly different from those in vehicle controls were few and limited to the kidney (Table 8). Renal tubular cysts were elevated above vehicle control in multiple low dose BPA groups, in the naïve controls, in both high BPA groups, and in the low EE2 group. Unlike with the other groups, the higher incidence and severity of renal cysts at 300,000 μg BPA/kg bw/day suggest a possible treatment effect.
TABLE 8. Female Histopathology, PND 90 ± 5a,b.
| Treatment | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative controls | BPA (μg/kg bw/day) | EE2 (μg/kg bw/day) | |||||||||||
| Naïve | Vehicle | 2.5 | 8 | 25 | 80 | 260 | 840 | 2700 | 100,000 | 300,000 | 0.5 | 5.0 | |
| Ovary | |||||||||||||
| Cyst, follicle | − | - | - | - | - | - | - | - | - | *** | *** | *** | |
| Incidence | 2/20 | 4/20 | 3/23 | 1/18 | 7/21 | 5/20 | 2/20 | 5/20 | 7/20 | 0/21 | 14/19 | 16/19 | 19/20 |
| Severity profile | 0/2/0/0 | 0/4/0/0 | 1/2/0/0 | 1/0/0/0 | 3/4/0/0 | 1/4/0/0 | 0/2/0/0 | 3/2/0/0 | 3/4/0/0 | 0/0/0/0 | 1/5/4/4 | 0/2/4/10 | 1/1/5/12 |
| Corpora lutea, depletion | - | - | - | - | - | - | - | - | - | *** | *** | *** | |
| Incidence | 0/20 | 0/20 | 2/23 | 2/18 | 0/21 | 1/20 | 1/20 | 2/20 | 2/20 | 1/21 | 14/19 | 17/19 | 18/20 |
| Severity profile | 0/0/0/0 | 0/0/0/0 | 0/2/0/0 | 2/0/0/0 | 0/0/0/0 | 0/2/0/0 | 1/0/0/0 | 0/2/0/0 | 1/1/0/0 | 0/1/0/0 | 3/6/4/1 | 0/5/5/7 | 0/0/1/17 |
| Antral follicles, depletion | - | - | - | - | - | - | - | - | - | * | *** | *** | |
| Incidence | 1/20 | 3/20 | 1/23 | 1/18 | 0/21 | 2/20 | 0/20 | 1/20 | 2/20 | 2/21 | 10/19 | 16/19 | 19/20 |
| Anestrusc | |||||||||||||
| Incidence | 2/20 | 3/20 | 2/23 | 0/18 | 1/21 | 1/20 | 1/20 | 3/20 | 3/20 | 1/21 | 13/19 | 18/19 | 19/20 |
| Uterus, endometrium | |||||||||||||
| Hyperplasia | - | - | - | - | - | - | - | - | - | * | - | - | |
| Incidence | 1/20 | 0/20 | 0/23 | 4/18 | 2/21 | 0/20 | 0/21 | 2/20 | 1/20 | 3/21 | 7/19 | 5/20 | 5/20 |
| Severity | 0/1/0/0 | 0/0/0/0 | 0/0/0/0 | 4/0/0/0 | 2/0/0/0 | 0/0/0/0 | 0/0/0/0 | 2/0/0/0 | 1/0/0/0 | 3/0/0/0 | 3/4/0/0 | 4/1/0/0 | 2/3/0/0 |
| Uterus, endometrium (continued) | |||||||||||||
| Metaplasia | - | - | - | - | - | - | - | - | - | - | *** | *** | |
| Incidence | 2/20 | 1/20 | 3/23 | 2/18 | 3/21 | 3/20 | 1/21 | 0/20 | 3/20 | 4/21 | 2/19 | 11/20 | 13/20 |
| Severity | 2/0/0/0 | 1/0/0/0 | 3/0/0/0 | 2/0/0/0 | 3/0/0/0 | 3/0/0/0 | 1/0/0/0 | 0/0/0/0 | 3/0/0/0 | 4/0/0/0 | 2/0/0/0 | 6/3/2/0 | 5/7/0/1 |
| Mammary gland | |||||||||||||
| Duct, hyperplasia | - | - | - | - | - | - | - | - | - | ** | - | - | |
| Incidence | 9/20 | 7/20 | 11/23 | 6/18 | 11/21 | 8/20 | 8/20 | 9/20 | 11/20 | 13/20 | 14/19 | 13/20 | 7/20 |
| Severity profile | 7/2/0/0 | 7/0/0/0 | 7/4/0/0 | 4/2/0/0 | 10/1/0/0 | 1/7/0/0 | 3/5/0/0 | 5/4/0/0 | 4/7/0/0 | 8/5/0/0 | 13/1/0/0 | 8/5/0/0 | 4/3/0/0 |
| Kidney | |||||||||||||
| Renal tubule, cyst | - | - | - | * | * | * | - | * | ** | *** | * | - | |
| Incidence | 5/20 | 1/20 | 5/23 | 5/18 | 8/21 | 6/20 | 6/21 | 5/20 | 6/20 | 8/21 | 12/19 | 7/20 | 2/20 |
| Severity profile | 5/0/0/0 | 1/0/0/0 | 2/2/0/1 | 4/0/1/0 | 5/3/0/0 | 4/0/2/0 | 5/1/0/0 | 3/0/2/0 | 5/1/0/0 | 7/1/0/0 | 5/3/3/1 | 5/2/0/0 | 2/0/0/0 |
aData are presented as incidence (the number of animals with lesion/the number of animals examined). The severity profile is also shown, i.e, the number of animals with lesions graded as minimal/mild/moderate/marked or grades 1/2/3/4, respectively. Statistical significance based on incidence, or lack thereof, is indicated in the row above the incidence row. A “-“ indicates no significance. *p < 0.05, **p < 0.01, and ***p < 0.001.
bThe number of animals examined includes early death animals. One female in the 260 μg BPA/kg bw/day dose group was accidentally killed and one in the 100,000 μg BPA/kg bw/day dose group was sacrificed early in a moribund condition (diagnosed with nephropathy associated with polyarteritis). Cases where the total number of tissues examined differs among tissues reflect insufficient or missing tissue.
cFor this study, the term “anestrus” designates a likely abnormal state of the estrous cycle, either arrested or prolonged based on the accompanying histopathological changes in the ovary that are consistent with inactivity or abnormal activity.
There was a low incidence of cystic endometrial hyperplasia in the uterus of the naïve control (5%) and several BPA dose groups (Table 8). The Poly-3 test indicated a significant increase in the incidence of cystic endometrial hyperplasia in the uterus of animals dosed with 300,000 μg BPA/kg bw/day versus vehicle control. The RTE test further indicated a significant increase in the 8 μg BPA/kg bw/day dose group. In the 300,000 μg BPA/kg bw/day group, there were high and statistically significant incidences of corpora lutea depletion, cystic follicles, and antral follicle depletion (Table 8 and Fig. 4).
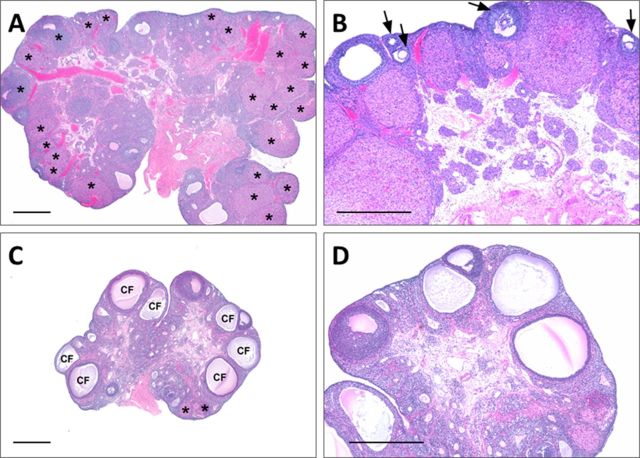
FIG. 4.
Ovary histology of animals dosed with the vehicle [(A) and (B)] or 300,000 μg BPA/kg bw/day [(C) and (D)]; tissue sections stained with hematoxylin and eosin. (A) Ovary from a normal cycling rat. A minimum of three generations (sets) of corpora lutea (*) from current and previous consecutive estrous cycles are present. The sets were determined morphologically by the relative size, cellularity, and lipochrome pigment content typically present in various stages of corpora luteal growth, degeneration, and regression. (B) Normal appearing antral follicles (arrows) in various stages of maturation from early to late. (C) Numerous cystic follicles (CF) and depletion of corpora lutea [*, compare with panel (A)]. Follicular cysts and fewer sets of corpora lutea than normal imply that either the estrous cycle has been arrested or prolonged. (D) Lack of normal appearing antral follicles [compare with panel (B)]. Depletion of antral follicles was defined as less than two to three normal appearing antral follicles and/or a relative increase in atretic ones. Severity for cystic follicles was subjectively based on the relative number of cysts present in sections; severity for corpora lutea depletion was based on the number of sets present (three sets only = grade 1; two sets = grade 2; one set = grade 3; and no sets = grade 4); depletion of antral follicles coded as present or absent. Bar, 50 μm.
Asynchrony, defined as a difference of at least two stages of the estrous cycle in either the uterus or vagina compared with the ovary or when the ovary was designated as anestrus, was prevalent in the 300,000 μg BPA/kg bw/day group compared with the vehicle control (63% vs. 12%). All other BPA dose groups had a percentage of asynchronous animals lower than the vehicle controls.
Lesions in the EE2 dose groups in the ovary, uterus, mammary gland, and kidney are also shown in Table 8. As indicated in the Materials and Methods section, the histopathology data of the EE2 groups were not evaluated in the QAS and PWG reviews and therefore cannot be directly compared with the BPA groups.
Animals in both EE2 dose groups had increased follicular cysts, depletion of corpora lutea, and depletion of antral follicles (Table 8) consistent with the altered cycling observed (see above). In addition, high incidences of uterine endometrial metaplasia were diagnosed in both EE2 dose groups that, in contrast to the low incidence endometrial metaplasia observed in control and BPA groups, was luminal rather than glandular. In the low and high EE2 dose groups, 94% and 100% of the animals, respectively, were determined to be asynchronous versus 12% in the vehicle control group.
Males
The most commonly recorded gross pathology observation in males was decreased testicular size. This was noted in the 0.5 and 5.0 μg EE2/kg bw/day groups, with incidences of 4/20 and 16/20, respectively, and in the 260 and 300,000 μg BPA/kg bw/day groups, with incidences of 5/20 in each group. Less than half of the animals with small testis showed degeneration of the seminiferous tubular epithelium at a microscopic level (2/5, 0/5, 2/4, and 7/16 in the 260 BPA, 300,000 BPA, 0.5 EE2, and 5.0 EE2, respectively).
The incidences and severities of histopathological lesions observed in the male reproductive tract, mammary glands, and kidney are shown in Table 9. The Poly-3 test indicated a significantly elevated incidence of seminiferous tubule giant cells in the 2.5 μg BPA/kg bw/day group. The RTE test also indicated the incidence at 25 μg BPA/kg bw/day (4/21, 19%) was significant (p = 0.022). The incidence of seminiferous cell giant cells was variable (ranging from 5% to 22%) across the low BPA dose groups, was present at 2/18 (11%) in the 300,000 μg BPA/kg bw/day group, and was not observed in the vehicle or naïve control groups. This diagnosis was characterized by a multinucleated syncytial cell present along the apical epithelial border, and while this is often associated with degeneration, most of these observations featured only one or two syncytial cells and were thus not coded as degeneration. Unlike the situation in the 5 μg EE2/kg bw/day group, where degeneration of the seminiferous epithelium was diagnosed and associated with hypospermia and exfoliated germ cells in the epididymides along with reduced epididymal sperm counts (Table 9), there were no such related lesions in the BPA groups. There was a slightly elevated incidence of epididymal exfoliated germ cells in the 260 μg BPA/kg bw/day group (Table 9) that was not significant by the Poly-3 test, but was significant by the RTE test.
The high dose of EE2 significantly increased the incidence of germinal epithelium degeneration in testes with hypospermia and exfoliated germ cells noted in the epididymides. Epithelial hyperplasia was induced in the coagulating gland in this same group.
Histopathologic assessment of the male mammary gland indicated that ductal hyperplasia occurred at low incidence (ranging from 0% to 13%) across the BPA doses. There were no significant treatment effects by the Poly-3 method, but the RTE test indicated significance at 2.5 and 840 μg BPA/kg bw/day. Ductal hyperplasia showed a clear EE2 dose-dependent increase in both incidence and severity (Table 9). Alveolar hyperplasia was also elevated by both EE2 doses, with an apparent increased severity in the high dose group.
DISCUSSION
Under the conditions of this study, BPA had clear adverse effects at doses of 100,000 and 300,000 μg/kg bw/day, with the majority of these effects observed in females. In the study-defined “low BPA” dose range of 2.5–2700 μg/kg bw/day, which was the primary focus of this study, potential effects could not be clearly linked to treatment as they were observed sporadically across the dose groups and did not occur in a consistent grouping across organs as did effects of EE2 (0.5 and 5.0 μg/kg bw/day) or “high BPA” (100,000 and 300,000 μg/kg bw/day).
Differences in sensitivity of animal strains to BPA and other agents that may act to disrupt estrogen signaling pathways have been much debated (e.g., Beronius et al., 2013; EFSA, 2010; Richter et al., 2007). The animal model used in the current study, the Sprague Dawley rat from the NCTR colony, has been used previously for assessments of dietary EE2 and genistein (Delclos et al., 2009; Latendresse et al., 2009; NTP, 2008a,b, 2010a,b), and shown to be sensitive to estrogenic compounds. In addition, a comprehensive evaluation of the pharmacokinetics of BPA across life stages has been reported in this strain (Doerge et al., 2010a,b, 2011; Twaddle et al., 2010).
BPA is a ubiquitous environmental contaminant, making the assessment of its toxicity at low levels problematic. Safety assessments of other ubiquitous environmental contaminants or common dietary components [e.g., phthalates, 2,3,7,8-tetrachlorodibenzodioxin (TCDD), acrylamide, and soy isoflavones] present common issues in study conduct and interpretation, given the potential presence of these agents in study materials that may lead to exposures in negative control animals (Kondo et al., 2010; Vanden Heuvel et al., 1994; Twaddle et al., 2004; NTP, 2008a,b). The presence of additional environmental or dietary components that might affect pathways common to the BPA also needs to be taken into account (e.g., Thigpen et al., 2013). In the current study, low-BPA study materials (including polysulfone cages, glass water bottles, and hardwood chip bedding) were used and the levels of BPA in these materials were monitored regularly. Because many of the effects of BPA are thought to occur through interference with estrogen signaling pathways, a soy- and alfalfa-free diet was used and the dietary levels of the phytoestrogens genistein and daidzein were monitored, in addition to BPA. All study materials, except the feed, had BPA levels below the average analytical blanks. A cutoff of 5 ppb BPA was established for the diet, because ingestion of feed containing 5 ppb or more of BPA would have resulted in a BPA dietary exposure of approximately 0.25 μg/kg bw/day, a 10-fold lower dose than the lowest BPA dose used in the study; a single lot of feed was rejected based on this criterion. Blood samples were collected from study animals at various time points to evaluate systemic exposures to aglycone (bioactive) and conjugated BPA (Churchwell et al., 2014). Despite our efforts to minimize the unintentional exposure of animals to BPA and the precautions taken to physically separate vehicle dosing from the BPA and EE2 doses (see the Materials and Methods section), BPA-glucuronide was detected in the serum of vehicle and naïve control animals at levels similar to those detected in animals dosed with 2.5 μg BPA/kg bw/day (Churchwell et al., 2014). The presence of BPA-glucuronide, which is formed by metabolism of aglycone BPA in the body, in the serum of control animals implies unintentional exposure of the animals to BPA rather than postsampling contamination of the serum samples. The source of this exposure has not been definitively identified (Churchwell et al., submitted). Thus, the 2.5 μg BPA/bw/day dose group is not distinguishable from the two negative control groups.
The current study involved daily oral gavage dosing of pregnant dams from GD 6 until the start of labor followed by direct dosing of their pups from PND 1 until PND 90 ± 5. This exposure regimen complements many of the existing studies of BPA that have been conducted for regulatory purposes, in which BPA was administered by diet or gavage to lactating dams (Ema et al., 2001; Stump et al., 2010; Tyl et al., 2002, 2008). Pharmacokinetic data from the animal model used in the current study have demonstrated marked differences in BPA metabolism with age (Doerge et al., 2010a) and also indicated limited lactational transfer of BPA to pups (Doerge et al., 2010b). Thus, it is important to recognize that, whereas the BPA dose rate in terms of μg/kg bw/day was constant across our study, the internal dose of BPA varied markedly with age. A very similar pattern of age-dependent serum concentrations was obtained for EE2 (Churchwell et al., submitted). The direct dosing of pups better mimics the exposure of concern in infants and young children than does exposure through the dam's milk and bypasses the low lactational transfer of BPA, allowing a more substantial and controlled neonatal exposure. The magnitude of exposure during the early postnatal period is of particular concern because this is a sensitive age for rodent reproductive tract development (e.g., Barraclough, 1961; Foster et al., 2004; Gorski, 1963; McPherson et al. 2008; Newbold et al., 2004) and that of other organs (e.g., Burri, 2006; Eriksson, 1997; Schreuder et al., 2011).
Although evidence exists to suggest that the low dose effects of BPA reported in the literature would not be mediated through classical estrogen receptors alone (Hugo et al., 2008; Alonso-Magdalena et al., 2008; NTP, 2008a; Thayer and Belcher, 2010), many of the reported BPA effects do involve estrogen-related pathways and inclusion of a reference estrogen control has been recommended for low dose BPA studies (e.g., Richter et al., 2007). Both dose levels of EE2 tested in the current study induced multiple adverse effects in females that are consistent with other studies on EE2 (Andrews et al., 2002; Ferguson et al., 2011; Howdeshell et al., 2008; Sawaki et al., 2003a,b; Shiorta et al., 2012), including the depression of gestational body weight gain, disruption of estrous cyclicity, induction of cystic ovarian follicles, and depletion of corpora lutea and antral follicles. The effects on the estrous cycle and the ovary are consistent with the anovulatory syndrome induced by neonatal treatment with estrogens or androgens (Barraclough, 1961; Gorski, 1963). The masculinizing organizational effect of perinatal estrogen exposure has also been reported for body weight gain in females (e.g., Bell and Zucker, 1971; Donohoe and Stevens, 1983), an effect induced by the high EE2 dose used here. Partially overlapping effects were produced by one or both high doses of BPA used in this study, with depression of gestational body weight gain at 100,000 and 300,000 μg/kg bw/day, and ovarian and estrous cycle effects only at the highest BPA dose. The depression of gestational body weight gain by BPA is consistent with other reports of BPA in rats (Tyl et al., 2002; Zoeller et al., 2005). The dosing method used in the current study, with high exposure to young neonates, likely accounts for the observed histopathological effects in the reproductive tract of females dosed with 100,000 and 300,000 μg BPA/kg bw/day, in contrast to the lack of such effects in the dietary study of Tyl et al. (2002), in which doses near 500,000 μg BPA/kg bw/day were administered in the diet without notable reproductive tract lesions in either sex.
Unexpectedly, the EE2 dosing regimen used in the present study produced a significant dose-dependent delay in the time of vaginal opening relative to vehicle controls. The delay in vaginal opening was also noted in a parallel study conducted at NCTR under identical conditions, in which animals were orally dosed by gavage with 5 or 10 μg EE2/kg bw/day from GD 6 through PND 21 (Ferguson, in preparation). Although exposure to neonatal androgen has been reported to delay or accelerate the timing of vaginal opening depending on timing of neonatal exposure and dose (Tramezzani et al., 1963; Rossano et al., 1973), oral administration (diet, water, or gavage) of diethylstilbestrol (DES) or EE2 to the dam during gestation and/or lactation has been reported mostly to accelerate the time of vaginal opening or to have no effect, depending on timing and dose level (e.g., Delclos et al., 2009; Odum et al., 2002; Rasier et al., 2006; Ryan et al., 2010). Sawaki et al. (2003a,b), evaluated the effects of gavage doses of 0.5, 5, and 50 μg EE2/kg bw/day to dams from GD 7 through PND 18 on the development of female pups and reported a delay of vaginal opening at the lower doses, but not the high dose (Sawaki, 2003a), but this was not reproducible (Sawaki, 2003b). Hines and Goy (1985) and Hines et al. (1987) reported that subcutaneous or intramuscular administration of 1–3 μg DES/day to guinea pigs during gestation significantly delayed vaginal opening. Although vaginal opening in rodents is known to occur with the rise of estrogen levels near puberty, the precise mechanism whereby estrogens and androgens affect the timing of vaginal opening has not been established, although direct actions on vaginal epithelial cells to open the vaginal canal are likely (Silbergeld et al., 2002). In discussing the exposure time-dependent delay of vaginal opening resulting from neonatal treatment of female mice with the estrogenic fungal product zearalenone, Ito and Ohtsubo (1994) suggested the potential involvement of inhibition of mitosis resulting from multiple neonatal doses of estradiol prior to PND 5 in vaginal epithelial cells as described by Forsberg (1969). Further investigation would be required to examine the molecular changes induced in the vaginal epithelium that might explain the EE2-induced delay in vaginal opening observed in the current study. In any case, BPA did not alter the timing of puberty in females at any dose.
The effects of the highest BPA dose on the ovary and estrous cycles were similar to the effects of EE2, suggesting an estrogenic effect at 300,000 μg BPA/kg bw/day. The observation of estrogenic effects of BPA only at high oral dose levels is consistent with observations made in several uterotrophic assays (e.g., Ashby and Tinwell, 1998; Diel et al., 2004; Laws et al., 2000; Yamasaki et al., 2000). On the other hand, the interpretation of the high dose BPA effects are confounded by apparent systemic toxicity indicated by the significant body weight depression observed at this dose. This is also true for the organ weight effects of BPA, which appeared to be secondary to the treatment-induced body weight depression, particularly in males. Body weight decrements have been reported previously with high doses of BPA in rats and mice (Tyl et al., 2002, 2008).
High EE2 adversely affected PND 90 male reproductive organ weights and sperm production, whereas none of the BPA doses induced these effects. Induction of male mammary hyperplasia by EE2 and other estrogenic agents has been reported previously by us and others to be a very sensitive indicator of estrogen exposure (Latendresse et al., 2009 and references therein). In the current study, both doses of EE2 increased the incidence of mammary gland hyperplasia in males; however, BPA did not induce a similar effect at any tested dose.
F1 females and males had body weights that differed from vehicle controls across the study period only at the highest BPA dose tested (300,000 μg /kg bw/day), with a treatment-induced depression of body weight in both sexes. Effects of BPA on body weight and fat deposition at much lower doses (<5000 μg/kg bw/day) have been investigated in multiple experimental models with mixed results including increases, decreases, or no effect (discussed in Thayer et al., 2012; Harley et al., 2013). In the present study, the low dose range of BPA did not significantly affect body or fat pad weights nor the serum levels of glucose, triglycerides, insulin, or leptin. Likewise, no effects on thyroid hormones, thyroid weight, or thyroid histology were observed in the low BPA dose region. At PND 15, elevated T3 was observed at both 100,000 and 300,000 μg BPA/kg bw/day with depressed TSH observed only at the highest BPA dose; both high doses of BPA depressed the TSH levels in PND 90 females. Zoeller et al. (2005) had reported hyperthyroid effects in PND 15 offspring of dams dosed orally (dosed wafer) daily from GD 6 at 1000 μg BPA/kg bw/day, although later consequences of these effects were not evaluated in that study.
Our interpretation of the results of the present study is that BPA in the “low dose” region from 2.5 to 2700 μg/kg bw/day did not produce effects in the evaluated endpoints that differ from normal background biological variation. The experimental model was sufficiently sensitive to detect clear effects of EE2 at both dose levels tested, as well as of the high BPA doses. Some endpoints, such as the PND 80 hormone assessments, although showing effects in females dosed with high BPA and EE2 that were consistent with the histopathology data, were not performed under optimal conditions and may have been underpowered to detect subtle effects in some cases (e.g., testosterone; Chapin and Creasy, 2012). As a follow-up to this study, we are currently pursuing a collaborative research project under the auspices of the NTP involving multiple academic investigators funded by NIEHS extramural grants (Schug et al., 2013). This project combines a chronic guideline-compliant oral BPA toxicity study, conducted in the animal model and with the dosing regimen described here, with in-depth evaluations of multiple functional, morphological, and molecular endpoints previously reported to be affected by BPA doses below 5 mg/kg bw/day (the current NOAEL). This study is an attempt to initiate a more comprehensive interagency exchange in order to resolve ultimately issues surrounding the toxicity of BPA and other potential hormonally active agents.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Study conducted under theauspices of the National Toxicology Program and funded by an Interagency agreement (IAG) between the Food and Drug Administration and the National Institute of Environmental Health Sciences/National Institutes of Health (FDA IAG: 224-12-0003; NIEHS IAG: AES12013).
Supplementary Material
Acknowledgments
We are grateful for the extraordinary efforts of the NCTR Animal Care, Diet Preparation, Information Technology, Microbiology, Pathology, Quality Assurance, and Veterinary Services staffs, as well as Ms Kathy Carroll of the Office of Scientific Coordination in the planning, conduct, and reporting of this study. Mr Steven J. Moon (deceased) of the Division of Biochemical Toxicology contributed to the conduct of the study. Messrs Paul H. Siitonen, Thomas Heinze, and Donald Paine also contributed to this study as members of the Chemistry, Mass Spectrometry, and Microbiology groups, respectively, prior to their retirement. Helpful comments of Drs Daniel Doerge and Jeffrey Fisher of NCTR on the manuscript are appreciated. We thank Drs Sherry Ferguson and Mary Boudreau of NCTR for sharing unpublished data. This manuscript was reviewed in accordance with USFDA and NIEHS procedures prior to submission. The opinions expressed in this paper do not necessarily reflect those of the USFDA or the NIEHS/NTP.
REFERENCES
- Alonso-Magdalena P., Ropero A. B., Carrera M. P., Cederroth C. R., Baquie M., Gauthier B. R., Nef S., Stefani E., Nadal A. Pancreatic insulin content regulation by the estrogen receptor ER alpha. PLoS ONE. 2008;3:e2069. doi: 10.1371/journal.pone.0002069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P., Freyberger A., Hartmann E., Eiben R., Loof I., Schmidt U., Temerowski M., Folkerts A., Stahl B., Kayser M. Sensitive detection of the endocrine effects of the estrogen analogue ethinylestradiol using a modified enhanced subacute rat study protocol (OECD Test Guideline no. 407) Arch. Toxicol. 2002;76:194–202. doi: 10.1007/s00204-002-0337-7. [DOI] [PubMed] [Google Scholar]
- Ashby J., Tinwell H. Uterotrophic activity of bisphenol A in the immature rat. Environ. Health Perspect. 1998;106:719–720. doi: 10.1289/ehp.98106719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barraclough C. A. Production of anovulatory, sterile rats by single injections of testosterone propionate. Endocrinology. 1961;68:62–67. doi: 10.1210/endo-68-1-62. [DOI] [PubMed] [Google Scholar]
- Bell D. D., Zucker I. Sex differences in body weight and eating: Organization and activation by gonadal hormones in the rat. Physiol. Behav. 1971;7:27–34. doi: 10.1016/0031-9384(71)90231-9. [DOI] [PubMed] [Google Scholar]
- Beronius A., Johansson N., Ruden C., Hanberg A. The influence of study design and sex-differences on results from developmental neurotoxicity studies of bisphenol A, implications for toxicity testing. Toxicology. 2013;311:13–26. doi: 10.1016/j.tox.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Burri P. H. Structural aspects of postnatal lung development: Alveolar formation and growth. Biol. Neonate. 2006;89:313–322. doi: 10.1159/000092868. [DOI] [PubMed] [Google Scholar]
- Calafat A. M., Kuklenyik Z., Reidy J. A., Caudill S. P., Ekong J., Needham L. L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat A. M., Ye X., Wong L. Y., Reidy J. A., Needham L. L. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carwile J. L., Michels K. B. Urinary bisphenol A and obesity: NHANES 2003–2006. Environ. Res. 2011;111:825–830. doi: 10.1016/j.envres.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin R. E., Creasy D. M. Assessment of circulating hormones in regulatory toxicology studies II. Male reproductive hormones. Toxicol. Pathol. 2012;40:1063–1078. doi: 10.1177/0192623312443321. [DOI] [PubMed] [Google Scholar]
- Churchwell M. I., Camacho L., Vanlandingham M. M., Twaddle N. C., Sepehr E., Delclos K. B., Fisher J. W., Doerge D. R. Comparison of Lifestage-Dependent Internal Dosimetry for Bisphenol A, Ethinyl Estradiol, a Reference Estrogen, and Endogenous Estradiol to Test an Estrogenic Mode of Action in Sprague-Dawley Rats. Toxicol. Sci. 2014 doi: 10.1093/toxsci/kfu021. doi: 10.1093/toxsci/kfu021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delclos K. B., Weis C. C., Bucci T. J., Olson G., Mellick P., Sadovova N., Latendresse J. R., Thorn B., Newbold R. R. Overlapping but distinct effects of genistein and ethinyl estradiol (EE(2)) in female Sprague-Dawley rats in multigenerational reproductive and chronic toxicity studies. Reprod. Toxicol. 2009;27:117–132. doi: 10.1016/j.reprotox.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diel P., Schmidt S., Vollmer G., Janning P., Upmeier A., Michna H., Bolt H. M., Degen G. H. Comparative responses of three rat strains (DA/Han, Sprague-Dawley and Wistar) to treatment with environmental estrogens. Arch. Toxicol. 2004;78:183–193. doi: 10.1007/s00204-003-0535-y. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Brown R. P., Fisher J. W. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2011;255:261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Twaddle N. C., Vanlandingham M., Fisher J. W. Pharmacokinetics of bisphenol A in neonatal and adult Sprague-Dawley rats. Toxicol. Appl. Pharmacol. 2010a;247:158–165. doi: 10.1016/j.taap.2010.06.008. [DOI] [PubMed] [Google Scholar]
- Doerge D. R., Vanlandingham M., Twaddle N. C., Delclos K. B. Lactational transfer of bisphenol A in Sprague-Dawley rats. Toxicol. Lett. 2010b;199:372–376. doi: 10.1016/j.toxlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- Donohoe T. P., Stevens R. Effects of ovariectomy, estrogen treatment and CI-628 on food intake and body weight in female rats treated neonatally with gonadal hormones. Physiol. Behav. 1983;31:325–329. doi: 10.1016/0031-9384(83)90196-8. [DOI] [PubMed] [Google Scholar]
- Dunnett C. W. A multiple comparison procedure for comparing several treatments with a control. J. Am. Stat. Assoc. 1955;50:1096–1121. [Google Scholar]
- EFSA Panel on food contact materials, enzymes, flavourings, and processing aids (CEF) Scientific opinion on bisphenol A: Evaluation of a study investigating its neurodevelopmental toxicity, review of recent scientific literature on its toxicity and advice on the Danish risk assessment of bisphenol A. EFSA Journal. 2010;8:1829. Available at: www.efsa.europa.eu/efsajournal.htm. Accessed, February 20, 2014. [Google Scholar]
- Ema M., Fujii S., Furukawa M., Kiguchi M., Ikka T., Harazono A. Rat two-generation reproductive toxicity study of bisphenol A. Reprod. Toxicol. 2001;15:505–523. doi: 10.1016/s0890-6238(01)00160-5. [DOI] [PubMed] [Google Scholar]
- Eriksson P. Developmental neurotoxicity of environmental agents in the neonate. Neurotoxicology. 1997;18:719–726. [PubMed] [Google Scholar]
- Ferguson S. A., Law C. D., Jr, Abshire J. S. Developmental treatment with bisphenol A or ethinyl estradiol causes few alterations on early preweaning measures. Toxicol. Sci. 2011;124:149–160. doi: 10.1093/toxsci/kfr201. [DOI] [PubMed] [Google Scholar]
- Forsberg J.-G. The development of atypical epithelium in the mouse uterine cervix and vaginal fornix after neonatal oestradiol treatment. Br. J. Exp. Pathol. 1969;50:187–195. [PMC free article] [PubMed] [Google Scholar]
- Foster W. G., Younglai E. V., Boutross-Tadross O., Hughes C. L., Wade M. G. Mammary gland morphology in Sprague-Dawley rats following treatment with an organochlorine mixture in utero and neonatal genistein. Toxicol. Sci. 2004;77:91–100. doi: 10.1093/toxsci/kfg247. [DOI] [PubMed] [Google Scholar]
- Gorski R. A. Modification of ovulatory mechanisms by postnatal administration of estrogen to the rat. Am. J. Physiol. 1963;205:842–844. doi: 10.1152/ajplegacy.1963.205.5.842. [DOI] [PubMed] [Google Scholar]
- Harley K. G., Schall R. A., Chevrier J., Tyler K., Aguirre H., Bradman A., Holland N. T., Lustig R. H., Calafat A. M., Eskenazi B. Prenatal and postnatal bisphenol A exposure and body mass index in childhood in the CHAMACOS cohort. Environ. Health Perspect. 2013;121:514–520. doi: 10.1289/ehp.1205548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Canada. F. D. Bureau of Chemical Safety, Health Products and Food Branch. Health Canada; 2012. Health Canada's updated assessment of bisphenol A (BPA) exposure from food sources. Available at: http://www.hc-sc.gc.ca/fn-an/securit/packag-emball/bpa/bpa_hra-ers-2012-09-eng.php. Accessed February 20, 2014. [Google Scholar]
- Hines M., Alsum P., Roy M., Gorski R. A., Goy R. W. Estrogenic contributions to sexual differentiation in the female guinea pig: Influences of diethylstilbestrol and tamoxifen on neural, behavioral, and ovarian development. Horm. Behav. 1987;21:402–417. doi: 10.1016/0018-506x(87)90024-9. [DOI] [PubMed] [Google Scholar]
- Hines M., Goy R. W. Estrogens before birth and development of sex-related reproductive traits in the female guinea pig. Horm. Behav. 1985;19:331–347. doi: 10.1016/0018-506x(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Howdeshell K. L., Furr J., Lambright C. R., Wilson V. S., Ryan B. C., Gray L. E., Jr. Gestational and lactational exposure to ethinyl estradiol, but not bisphenol A, decreases androgen-dependent reproductive organ weights and epididymal sperm abundance in the male long evans hooded rat. Toxicol. Sci. 2008;102:371–382. doi: 10.1093/toxsci/kfm306. [DOI] [PubMed] [Google Scholar]
- Hugo E. R., Brandebourg T. D., Woo J. G., Loftus J., Alexander J. W., Ben-Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ. Health. Perspect. 2008;116:1642–1647. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Ohtsubo K. Effects on neonatal administration of zearalenone on the reproductive physiology of female mice. J. Vet. Med. Sci. 1994;56:1155–1159. doi: 10.1292/jvms.56.1155. [DOI] [PubMed] [Google Scholar]
- Kondo F., Okumura M., Oka H., Nakazawa H., Izumi S., Makino T. Determination of phthalates in diet and bedding for experimental animals using gas chromatography-mass spectrometry. Bull. Environ. Contam. Toxicol. 2010;84:212–216. doi: 10.1007/s00128-009-9919-x. [DOI] [PubMed] [Google Scholar]
- LaKind J. S., Goodman M., Naiman D. Q. Use of NHANES data to link chemical exposures to chronic diseases: A cautionary tale. PLoS One. 2012;7:e51086. doi: 10.1371/journal.pone.0051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang I. A., Galloway T. S., Scarlett A., Henley W. E., Depledge M., Wallace R. B., Melzer D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Latendresse J. R., Bucci T. J., Olson G., Mellick P., Weis C. C., Thorn B., Newbold R. R., Delclos K. B. Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats. Reprod. Toxicol. 2009;28:342–353. doi: 10.1016/j.reprotox.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Latendresse J. R., Warbritton A. R., Jonassen H., Creasy D. M. Fixation of testes and eyes using a modified Davidson's fluid: Comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol. Pathol. 2002;30:524–533. doi: 10.1080/01926230290105721. [DOI] [PubMed] [Google Scholar]
- Laws S. C., Carey S. A., Ferrell J. M., Bodman G. J., Cooper R. L. Estrogenic activity of octylphenol, nonylphenol, bisphenol A and methoxychlor in rats. Toxicol. Sci. 2000;54:154–167. doi: 10.1093/toxsci/54.1.154. [DOI] [PubMed] [Google Scholar]
- Lewis S. M., Lee F. W., Ali A. A., Allaben W. T., Weis C. C., Leakey J. E. Modifying a displacement pump for oral gavage dosing of solution and suspension preparations to adult and neonatal mice. Lab Anim. (N.Y.) 2010;39:149–154. doi: 10.1038/laban0510-149. [DOI] [PubMed] [Google Scholar]
- McPherson S. J., Ellem S. J., Risbridger G. P. Estrogen-regulated development and differentiation of the prostate. Differentiation. 2008;76:660–670. doi: 10.1111/j.1432-0436.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- Melzer D., Osborne N. J., Henley W. E., Cipelli R., Young A., Money C., McCormack P., Luben R., Khaw K. T., Wareham N. J., et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation. 2012;125:1482–1490. doi: 10.1161/CIRCULATIONAHA.111.069153. [DOI] [PubMed] [Google Scholar]
- Melzer D., Rice N. E., Lewis C., Henley W. E., Galloway T. S. Association of urinary bisphenol a concentration with heart disease: Evidence from NHANES 2003/06. PLoS One. 2010;5:e8673. doi: 10.1371/journal.pone.0008673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold R. R., Jefferson W. N., Padilla-Banks E., Haseman J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: Low versus high dose effects. Reprod. Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- NTP. Multigenerational reproductive study of genistein (Cas No. 446–72–0) in Sprague-Dawley rats (feed study) Natl. Toxicol. Program Tech. Rep. Ser. 2008a;539:1–266. [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis studies of genistein (Cas No. 446–72–0) in Sprague-Dawley rats (feed study) Natl. Toxicol. Program Tech. Rep. Ser. 2008b;545:1–240. [PubMed] [Google Scholar]
- NTP. Multigenerational reproductive toxicology study of ethinyl estradiol (CAS No. 57-63-6) in Sprague-Dawley rats. Natl. Toxicol. Program Tech. Rep. Ser. 2010a;547:1–312. [PubMed] [Google Scholar]
- NTP. Toxicology and carcinogenesis study of ethinyl estradiol (CAS No. 57–63–6) in Sprague-Dawley rats (feed study) Natl. Toxicol. Program Tech. Rep. Ser. 2010b;548:1–210. [PubMed] [Google Scholar]
- Odum J., Lefevre P. A., Tinwell H., Van Miller J. P., Joiner R. L., Chapin R. E., Wallis N. T., Ashby J. Comparison of the developmental and reproductive toxicity of diethylstilbestrol administered to rats in utero, lactationally, preweaning, or postweaning. Toxicol. Sci. 2002;68:147–163. doi: 10.1093/toxsci/68.1.147. [DOI] [PubMed] [Google Scholar]
- Rasier G., Toppari J., Parent A.-S., Bourguignon J.-P. Female sexual maturation and reproduction after prepubertal exposure to estrogens and endocrine disrupting chemicals: A review of rodent and human data. Mol. Cell. Endocrinol. 2006;254–255:187–201. doi: 10.1016/j.mce.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Richter C. A., Birnbaum L. S., Farabollini F., Newbold R. R., Rubin B. S., Talsness C. E., Vandenbergh J. G., Walser-Kuntz D. R., vom Saal F. S. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossano G. L., Justo S. N., Tramezzani J. H. Response of the vagina of newborn rats to sequential treatment with sexual hormones. Acta Anat. 1973;84:492–497. doi: 10.1159/000143957. [DOI] [PubMed] [Google Scholar]
- Ryan B. C., Hotchkiss A. K., Crofton K. M., Gray L. E., Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 2010;114:133–148. doi: 10.1093/toxsci/kfp266. [DOI] [PubMed] [Google Scholar]
- Sawaki M., Noda S., Muroi T., Mitoma H., Takakura S., Sakamoto S., Yamasaki K. Evaluation of an in utero through lactational exposure protocol for detection of estrogenic effects of ethinyl estradiol on the offspring of rats: Preliminary trial. Reprod. Toxicol. 2003a;17:335–343. doi: 10.1016/s0890-6238(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Sawaki M., Noda S., Muroi T., Mitoma H., Takakura S., Sakamoto S., Yamasaki K. In utero through lactational exposure to ethinyl estradiol induces cleft phallus and delayed ovarian dysfunction in the offspring. Toxicol. Sci. 2003b;75:402–411. doi: 10.1093/toxsci/kfg180. [DOI] [PubMed] [Google Scholar]
- Schreuder M. F., Bueters R. R., Huigen M. C., Russel F. G. M., Masereeuw R., van den Heuvel L. P. Effect of drugs on renal development. Clin. J. Am. Soc. Nephrol. 2011;6:212–217. doi: 10.2215/CJN.04740510. [DOI] [PubMed] [Google Scholar]
- Schug T. T., Heindel J. H., Camacho L., Delclos K. B., Howard P., Johnson A. F., Aungst J., Keefe D., Newbold R., Walker N. J., et al. A new approach to synergize academic and regulatory-compliant research: The CLARITY-BPA research program. Reprod. Toxicol. 2013;40:35–40. doi: 10.1016/j.reprotox.2013.05.010. [DOI] [PubMed] [Google Scholar]
- Shelby M. D. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NTP CERHR MON. 2008 v, vii-ix, 1–64 passim. [PubMed] [Google Scholar]
- Shiorta M., Kawashima J., Nakamura T., Ogawa Y., Kamiie J., Yasuno K., Shirota K., Yoshida M. Delayed effects of single neonatal subcutaneous exposure of low-dose 17alpha-ethynylestradiol on reproductive function in female rats. J. Toxicol. Sci. 2012;37:681–690. doi: 10.2131/jts.37.681. [DOI] [PubMed] [Google Scholar]
- Silbergeld E. K., Flaws J. A., Brown K. M. Organizational and activational effects of estrogenic endocrine disrupting chemicals. Cad. Saúde Pública, Rio de Janeiro. 2002;18:495–504. doi: 10.1590/s0102-311x2002000200014. [DOI] [PubMed] [Google Scholar]
- Silver M. K., O'Neill M. S., Sowers M. R., Park S. K. Urinary bisphenol A and type-2 diabetes in U.S. adults: Data from NHANES 2003–2008. PLoS One. 2011;6:e26868. doi: 10.1371/journal.pone.0026868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanier A. J., Kahn R. S., Kunselman A. R., Hornung R., Xu Y., Calafat A. M., Lanphear B. P. Prenatal exposure to bisphenol A and child wheeze from birth to 3 years of age. Environ. Health. Perspect. 2012;120:916–920. doi: 10.1289/ehp.1104175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stump D. G., Beck M. J., Radovsky A., Garman R. H., Freshwater L. L., Sheets L. P., Marty M. S., Waechter J. M., Jr., Dimond S. S., Van Miller J. P., et al. Developmental neurotoxicity study of dietary bisphenol A in Sprague-Dawley rats. Toxicol. Sci. 2010;115:167–182. doi: 10.1093/toxsci/kfq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B. L., Kassim N. M., Mohd M. A. Assessment of pubertal development in juvenile male rats after sub-acute exposure to bisphenol A and nonylphenol. Toxicol. Lett. 2003;143:261–270. doi: 10.1016/s0378-4274(03)00172-3. [DOI] [PubMed] [Google Scholar]
- Teppala S., Madhavan S., Shankar A. Bisphenol A and metabolic syndrome: Results from NHANES. Int. J. Endocrinol. 2012;2012:598180. doi: 10.1155/2012/598180. doi: doi: 10.1155/2012/598180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer K. A., Belcher S.M. Mechanisms of Action of Bisphenol A and Other Biochemical/Molecular Interactions. FAO/WHO Expert Meeting on Bisphenol A (BPA) Ottawa, Canada: 2010. Available at: http://www.who.int/foodsafety/chem/chemicals/5_biological_activities_of_bpa.pdf. [Google Scholar]
- Thayer K. A., Heindel J. J., Bucher J. R., Gallo M. A. Role of environmental chemicals in diabetes and obesity: A National Toxicology Program workshop review. Environ. Health. Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thigpen J. E., Setchell K. D., Kissling G. E., Locklear J., Caviness G. F., Whiteside T., Belcher S. M., Brown N. M., Collins B. J., Lih F. B., et al. The estrogenic content of rodent diets, bedding, cages, and water bottles and its effect on bisphenol a studies. J. Am. Assoc. Lab. Anim. Sci. 2013;52:30–41. [PMC free article] [PubMed] [Google Scholar]
- Tramezzani J. H., Voloschin L. M., Nallar R. Effect of a single dose of testosterone propionate on vaginal opening in the rat. Acta Anat. 1963;52:244–251. doi: 10.1159/000142355. [DOI] [PubMed] [Google Scholar]
- Trasande L., Attina T. M., Blustein J. Association between urinary bisphenol A concentration and obesity prevalence in children and adolescents. JAMA. 2012;308:1113–1121. doi: 10.1001/2012.jama.11461. [DOI] [PubMed] [Google Scholar]
- Twaddle N. C., Churchwell M. I., McDaniel L. P., Doerge D. R. Autoclave sterilization produces acrylamide in rodent diets: Implications for toxicity testing. J. Agric. Food Chem. 2004;52:4344–4349. doi: 10.1021/jf0497657. [DOI] [PubMed] [Google Scholar]
- Twaddle N. C., Churchwell M. I., Vanlandingham M., Doerge D. R. Quantification of deuterated bisphenol A in serum, tissues, and excreta from adult Sprague-Dawley rats using liquid chromatography with tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2010;24:3011–3020. doi: 10.1002/rcm.4733. [DOI] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Sloan C. S., Castillo N. P., Veselica M. M., Seely J. C., Dimond S. S., Van Miller J. P., Shiotsuka R. N., et al. Two-generation reproductive toxicity study of dietary bisphenol A in CD-1 (Swiss) mice. Toxicol. Sci. 2008;104:362–384. doi: 10.1093/toxsci/kfn084. [DOI] [PubMed] [Google Scholar]
- Tyl R. W., Myers C. B., Marr M. C., Thomas B. F., Keimowitz A. R., Brine D. R., Veselica M. M., Fail P. A., Chang T. Y., Seely J. C., et al. Three-generation reproductive toxicity study of dietary bisphenol A in CD Sprague-Dawley rats. Toxicol. Sci. 2002;68:121–146. doi: 10.1093/toxsci/68.1.121. [DOI] [PubMed] [Google Scholar]
- USFDA. Toxicological Principles for the Safety Assessment of Food Ingredients. Redbook 2000 (Revised July, 2007) 2007 Available at: http://www.fda.gov/downloads/Food/GuidanceRegulation/UCM222779.pdf. [Google Scholar]
- USFDA, Center for Food Safety and Applied Nutrition. Exposure to Bisphenol A for Infants, Toddlers and Adults from the Consumption of Infant Formula. Toddler Food and Adult (Canned) Food. 2010 Available at: http://www.regulations.gov/#!documentDetail;D=FDA-2010-N-0100-0009;oldLink=false. [Google Scholar]
- Vandenberg L. N., Colborn T., Hayes T. B., Heindel J. J., Jacobs D. R., Jr., Lee D. H., Shioda T., Soto A. M., vom Saal F. S., Welshons W. V., et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012;33:378–455. doi: 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Heuvel J. P., Clark G. C., Tritscher A. M., Lucier G. W. Accumulation of polychlorinated dibenzo-p-dioxins and dibenzofurans in liver of control laboratory rats. Fundam. Appl. Toxicol. 1994;23:465–469. doi: 10.1006/faat.1994.1128. [DOI] [PubMed] [Google Scholar]
- Wang T., Lu J., Xu M., Xu Y., Li M., Liu Y., Tian X., Chen Y., Dai M., Wang W., et al. Urinary bisphenol A concentration and thyroid function in Chinese adults. Epidemiology. 2013;24:295–302. doi: 10.1097/EDE.0b013e318280e02f. [DOI] [PubMed] [Google Scholar]
- Wang H. X., Zhou Y., Tang C. X., Wu J. G., Chen Y., Jiang Q. W. Association between bisphenol A exposure and body mass index in Chinese school children: A cross-sectional study. Environ. Health. 2012;11:79. doi: 10.1186/1476-069X-11-79. doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willhite C. C., Ball G. L., McLellan C. J. Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration. J. Toxicol. Environ. Health B Crit. Rev. 2008;11:69–146. doi: 10.1080/10937400701724303. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Joint FAO/WHO Expert Meeting to Review Toxicological and Health Aspects of Bisphenol A: Summary Report. Ottawa, CA: World Health Organization; 2010. Available at: http://www.who.int/foodsafety/chem/chemicals/BPA_Summary2010.pdf. [Google Scholar]
- Yamasaki K., Sawaki M., Takatsuki M. Immature rat uterotrophic assay of bisphenol A. Environ. Health Perspect. 2000;108:1147–1150. doi: 10.1289/ehp.001081147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller R. T., Bansal R., Parris C. Bisphenol-A, an environmental contaminant that acts as a thyroid hormone receptor antagonist in vitro, increases serum thyroxine, and alters RC3/neurogranin expression in the developing rat brain. Endocrinology. 2005;146:607–612. doi: 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.