Abstract
Understanding pathogen/mosquito interactions is essential for developing novel strategies to control mosquito-borne diseases. Technical advances in reverse-genetics, such as RNA interference (RNAi), have facilitated elucidation of components of the mosquito immune system that are antagonistic to pathogen development, and host proteins essential for parasite development. Forward genetic approaches, however, are limited to generation of transgenic insects, and while powerful, mosquito transgenesis is a resource- and time-intensive technique that is not broadly available to most laboratories. The ability to easily “over-express” genes would enhance molecular studies in vector biology and expedite elucidation of pathogen-refractory genes without the need to make transgenic insects. We developed and characterized an efficient Anopheles gambiae densovirus (AgDNV) over-expression system for the major malaria vector Anopheles gambiae. High-levels of gene expression were detected at 3 days post-infection and increased over time, suggesting this is an effective system for gene induction. Strong expression was observed in the fat body and ovaries. We validated multiple short promoters for gene induction studies. Finally, we developed a polycistronic system to simultaneously express multiple genes of interest. This AgDNV-based toolset allows for consistent transduction of genes of interest and will be a powerful molecular tool for research in Anopheles gambiae mosquitoes.
Anopheles sp. are the only mosquitoes that transmit Plasmodium parasites to humans, and as such, are a major concern for public health1. Anopheles gambiae is the major vector of Plasmodium falciparum, the major cause of malaria in sub-Saharan Africa2. Issues with current control strategies such as the lack of effective vaccines and emergence of insecticide resistance1,3 necessitate the need for novel disease prevention measures. One such strategy is to control pathogen transmission by mosquitoes using transgenic technology. However, generation of transgenic Anopheles mosquitoes is technically challenging, and limited success has been reported in An. gambiae4,5,6. Alternative strategies such as the use of microbes to control pathogens are gaining considerable attention (reviewed in refs)7,8,9.
Paratransgenesis (genetic manipulation of symbiotic microorganisms to interfere with pathogen development in the host) has been proposed as a control strategy for vector-borne diseases10. Paratransgenic control approaches have several advantages over strategies based on insect transgenesis. Microorganisms help maintain host homeostasis can be tightly associated with their insect host. These microbes are often more straightforward to transform compared to the insect11, and microbes can often spread through insect populations12,13,14,15. As such, paratransgenic control strategies are being considered for a wide range of viral, bacterial and fungal microbes16,17,18,19,20,21,22,23,24,25. Paratransgenic approaches using bacteria and fungi have been shown to significantly reduce Plasmodium levels in An. gambiae18,20.
Pathogens within mosquitoes can also be inhibited by manipulating host genes essential for pathogen development26,27,28. To explore such gene functions, effective tools to manipulate the host are essential. However manipulating gene expression in Anopheles mosquitoes has been constrained because only a few techniques are available, mainly RNA interference (RNAi) and transgenic manipulation10,29,30,31. As such, the development of efficient and simple transient over-expression systems in mosquitoes will facilitate investigations on mosquito biology and applied mosquito-borne diseases control strategies.
Viral vector transduction is a common approach to over-express genes in many host systems (reviewed in refs)32,33,34,35. Viruses actively enter target cells, are effective in vitro and in vivo and can be modified for specific aims such as tissue tropism36,37. Densoviruses (DNVs) are non-enveloped single-stranded parvoviruses that are widely distributed among arthropods including multiple mosquito species38,39,40,41,42,43. Aedes aegypti densovirus (AeDNV) has been intensively studied as a transducing vector for Aedes mosquitoes44,45,46,47. DNVs are often pathogenic to mosquitoes45,48, and their pathogenicity can be improved by engineering46. Previously, we isolated a DNV from Anopheles gambiae (AgDNV), showed that it replicates preferentially in adult mosquitoes, imparts minimal impact on host genes expression and is avirulent19,49,50. Here we report on the development of an improved viral transduction system, which can efficiently overexpress multiple genes of interest in An. gambiae mosquitoes at high frequency.
Results
Generation and evaluation of a new recombinant AgDNV vector
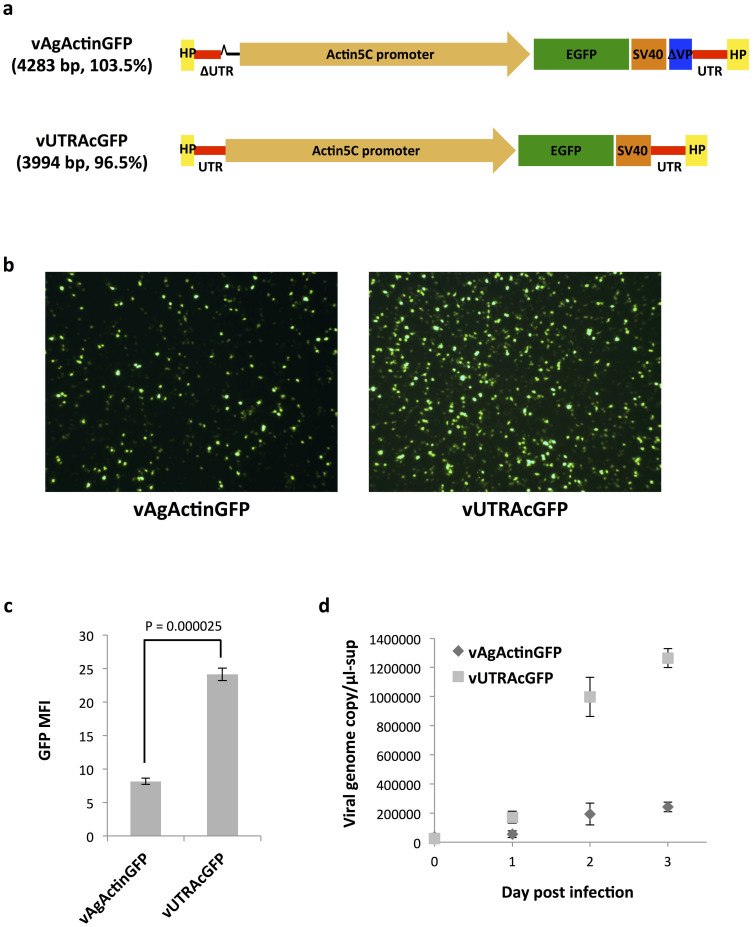
We use the prefix “v” to denote viral vectors and “p” to indicate their infectious plasmids. Our previous recombinant AgDNV harboring the EGFP gene, vAgActinGFP (derived from pAgActinGFP) is 4283 base pairs (bp) in length and is approximately 3.5% longer than the wild-type AgDNV genome19. In the course of working with this virus for several years, we noted that EGFP expression in An. gambiae adults infected with vAgActinGFP is highly variable (unpublished observation). The variation is possibly due to the large size of the viral genome. For other DNVs, efficient packaging of the viral genome is inhibited when the transducing genome was larger than the wild-type genome51. To shorten our recombinant AgDNV vector, we generated a new DNV vector plasmid (pUTR) which contained both hairpins and the entire AgDNV 5′ and 3′ untranslated regions without any ORFs from the wild-type AgDNV plasmid pBAgα19. The Actin5C promoter-EGFP-SV40 terminator cassette was inserted into pUTR and a new transducing construct developed (pUTRAcGFP). vUTRAcGFP has a genome length of 4011 bp, which is 128 bp shorter than wild-type AgDNV genome (4139 bp). We compared transduction and replication efficiency between vUTRAcGFP and vAgActinGFP (Fig. 1a). To provide viral proteins for replication of recombinant viruses, all recombinant virus samples were prepared by co-transfection of pBAgα (wild-type AgDNV plasmid) and recombinant virus plasmids. MOS55 cells were infected with equal titers of the vAgAcGFP or vUTRAcGFP. vUTRAcGFP-infected cells showed 3-fold higher intensity of EGFP than cells infected with vAgAcGFP (Fig. 1b and c). To compare the replication kinetics of each viral vector, supernatant was collected from the DNV-infected MOS55 cells at 0–3 days post infection and the encapsulated recombinant viral genome DNA enumerated by quantitative PCR (qPCR). The recombinant viral DNA of vAgActinGFP plateaued at approximately 2–2.5 × 105 genome copies per μl after two days. In comparison, vUTRAcGFP showed over a 5-fold increase of 1.2 × 106 copies per μl at the same time point and had not yet plateaued (Fig. 1d).
Figure 1. New AgDNV transducing vector.
(a) Schematic representation of recombinant AgDNV vectors. Both vectors consist of the viral terminal hairpins, UTR's and EGFP gene driven by Actin5C promoter and SV40 terminator. vAgActinGFP lacks 27 bp of the 5′ UTR (referred to as ΔUTR) and contains a portion of the viral capsid gene (ΔVP). vUTRAcGFP has the intact 5′ UTR and no viral capsid gene sequence. Each vector genome size relative to wild-type virus is indicated. (b) MOS55 cells were infected with equivalent titers of vAgActinGFP or vUTRAcGFP. EGFP expression was visualized by fluorescence microscopy. (c) The mean fluorescence intensity (MFI) of EGFP was determined by flow cytometry. (d) Recombinant AgDNV replication in MOS55 cells. Supernatants from infected cells were collected from Days 0–3 post infection. Viral DNA was quantified by qPCR. Mean and standard deviation (S.D.) were calculated from three independent infections in (c) and (d). Treatments are significantly different (Student's T test).
High level and consistent transduction of AgDNV in An. gambiae mosquitoes
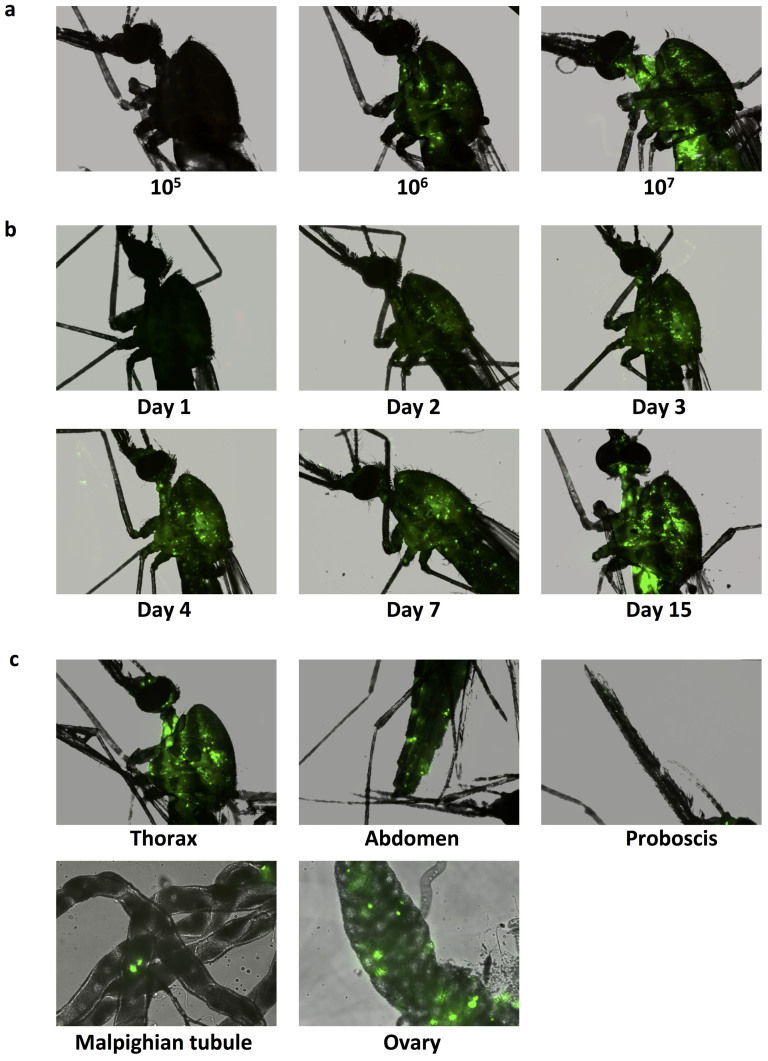
Next, we assessed the infectivity of vUTRAcGFP in vivo. Recombinant virus and helper virus was intrathoracically injected into the thorax of adult female mosquitoes, with 10–20 mosquitoes per treatment. Adult mosquitoes were injected with 1 × 105, 106 or 107 vUTRAcGFP and collected 7 days post injection to examine EGFP expression. Fluorescence was observed in all mosquitoes in a dose-dependent manner (Fig. 2a). We next conducted a virus replication time course experiment, repeatedly non-destructively examining the same mosquito at each time point. EGFP was visible beginning day two post-injection. Fluorescence increased throughout the experiment to 15 days post-injection (Fig. 2b). To determine the tissue tropism of vUTRAcGFP, we dissected infected mosquitoes. The majority of EGFP expression was observed in the fat body and the ovary (Fig. 2c), but was also visible in other tissues including muscle, malpighian tubules and the proboscis (Fig. 2c). No significant fluorescence was observed in midgut or salivary glands (data not shown).
Figure 2. Transduction of An. gambiae with vUTRAcGFP by intrathoracic microinjection.
EGFP expression was visualized by fluorescence microscopy. (a) An. gambiae adults were injected with 1 × 105, 106 or 107 particles of vUTRAcGFP and visualized at seven days post-injection. (b) Non-destructive time course of EGFP expression in the mosquitoes injected with 1 × 107 of vUTRAcGFP. (c) Tissue tropism of recombinant AgDNV. Fluorescence was observed in the fat body, ovaries, malpigian tubules and proboscis.
Comparison of 6 different promoter cassettes for recombinant AgDNV gene transduction
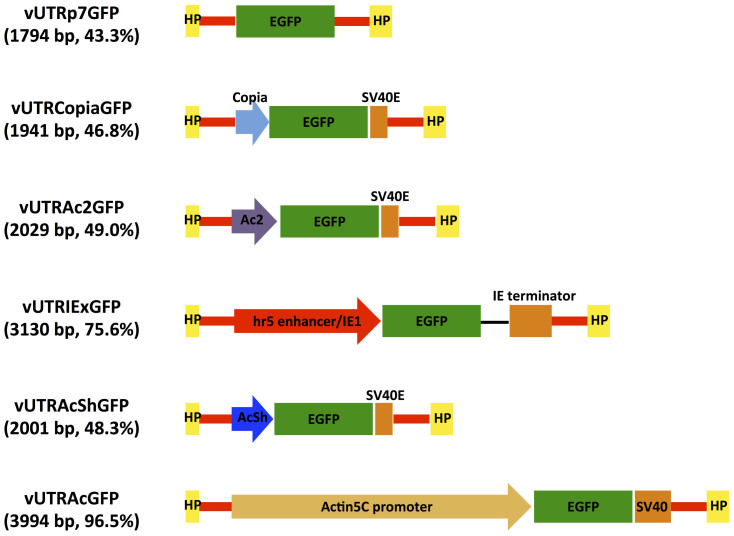
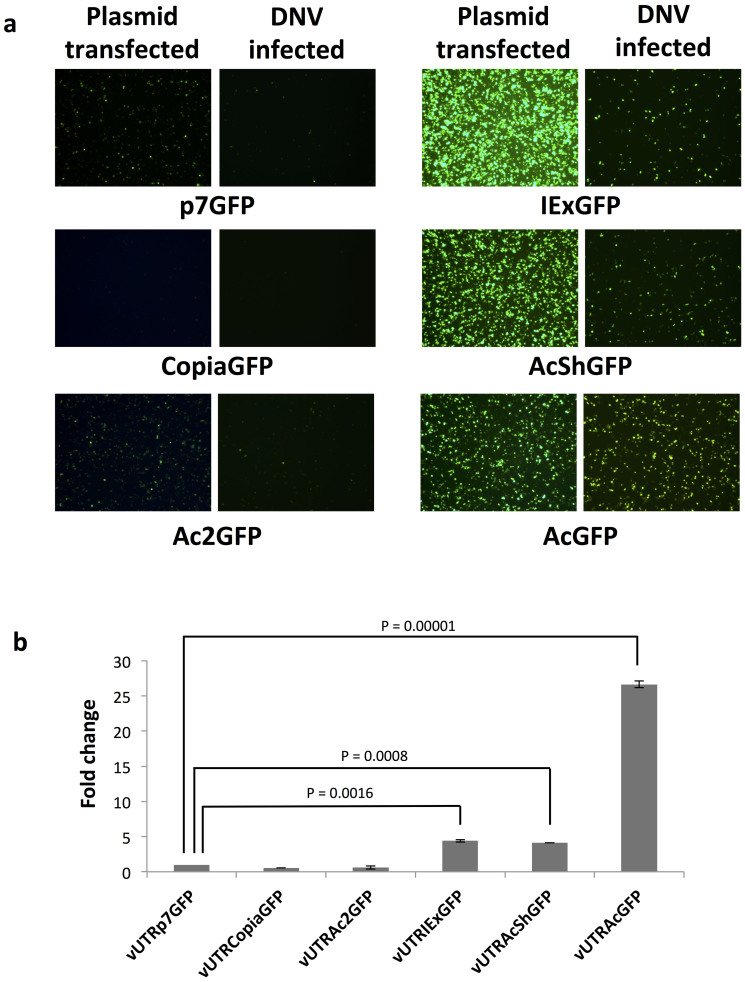
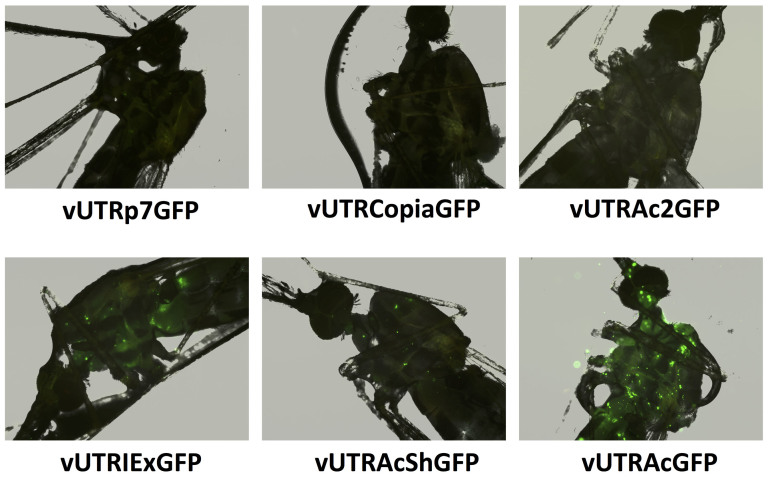
Parvoviral vectors have a size limitation for efficient genome packaging. In Aedes aegypti DNV-based vectors, genomes that exceed the wild-type size by more than 10% cannot be efficiently packaged51. pUTR with the Actin5C promoter cassette is 3291 bp in length, allowing for the insertion of genes less than 1000 bp. It would be desirable to shorten the promoter so that larger genes of interest can be expressed in this system. We assessed several shorter promoters by exchanging the Actin5C promoter in pUTRAcGFP. All viruses were compared for their ability to transduce Anopheles gambiae both in vitro and in vivo. All recombinant DNV genomes contained the EGFP gene under the control of different promoters: vUTRp7, the native AgDNV non-structural promoter and polyadenylation sequence that exist in the UTR sequences; vUTRCopia, 247 bp copia promoter and 134 bp SV40 early polyadenylation signal (SV40E polyA); vUTRAc2, 335 bp Actin5C promoter for exon2 and exon352 and SV40E polyA; vUTRIEx, 1082 bp Autographa californica nuclear polyhedrosis virus (AcNPV)-derived hr5 enhancer and immediate early promoter IE1 and 308 bp IE terminator; vUTRAcSh a 307 bp truncated version of the Actin5C promoter53 and SV40E polyA (Fig. 3). We evaluated activity of each promoter cassette in plasmid-transfected and virus-infected MOS55 cells (Fig. 4a). In plasmid-transfected cells, the IEx promoter showed the highest level of EGFP expression (Fig 4a). In pUTRAcShGFP-transfected MOS55 cells, relatively high expression was observed (Fig 4a). Both of IEx and AcSh promoter cassettes showed higher expression of EGFP than the longest Actin5C promoter. In comparison, transduction experiments with virus demonstrated that vUTRAcGFP had higher transduction efficiency than vUTRIExGFP or vUTRAcShGFP (Fig. 4a). We quantified viral promoter efficiency using flow cytometry. Viruses driving EGFP from p7, copia or Ac2 showed very little fluorescence. In contrast, viruses containing the IEx, short actin or long actin promoters showed strong fluorescence. The Actin5C promoter had 21 times higher EGFP intensity than the p7 promoter (P < 0.00005) (Fig. 4b). IEx and AcSh promoters showed 4.4 and 4.1 times higher activity than p7 respectively (P < 0.005) (Fig. 4b). Next, we compared EGFP transduction in vivo. An. gambiae adults were injected with 5 × 106 of each of the recombinant DNVs and EGFP expression was visualized at day 7 post-injection. Similar to results in vitro, vUTRAcGFP-transduced mosquitoes showed the highest EGFP expression (Fig. 5). Although the intensities were lower than Actin5C, intermediate EGFP expression was observed in vUTRIExGFP- and vUTRAcShGFP-infected mosquitoes. No visible fluorescence was observed in the mosquitoes infected with vUTRP7GFP, vUTRCopiaGFP or vUTRAc2GFP.
Figure 3. Schematic representation of constructed recombinant AgDNV vectors harboring different promoter sequences.
Each vector genome size ratio relative to wild-type virus is indicated.
Figure 4. Comparison of expression promoter constructs in vitro.
(a) EGFP expression visualized by microscopy in plasmid-transfected and DNV-infected MOS55 cells. 2.5 μg of plasmids and 5 × 108 of recombinant DNVs were used for transfection and infection experiments respectively. (b) EGFP intensity was quantified by a flow cytometry in each of the DNV-infected MOS55 cell cultures. Fold-changes in EGFP expression was compared to vUTRp7GFP-infected cells. Mean and S.D. were calculated from three independent infections. P-values were calculated by Student's T test.
Figure 5. Comparison of different promoter efficiency for AgDNV transduction in vivo.
An. gambiae adults were injected with 5 × 106 of each recombinant DNV. EGFP expression in mosquitoes was examined at seven days post-injection.
Development of a polycistronic AgDNV-based expression vector
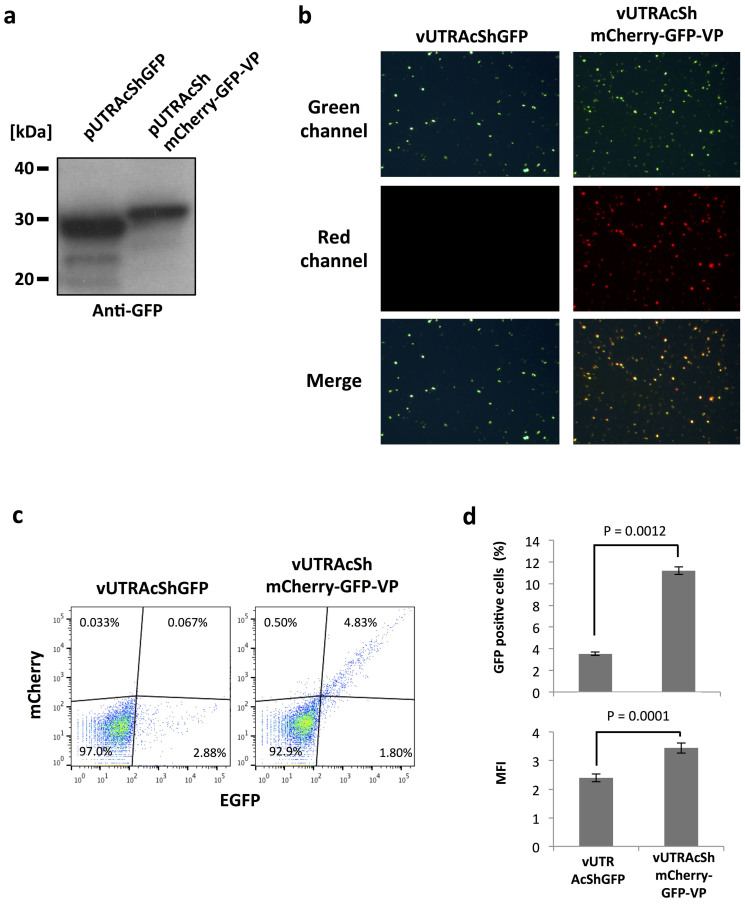
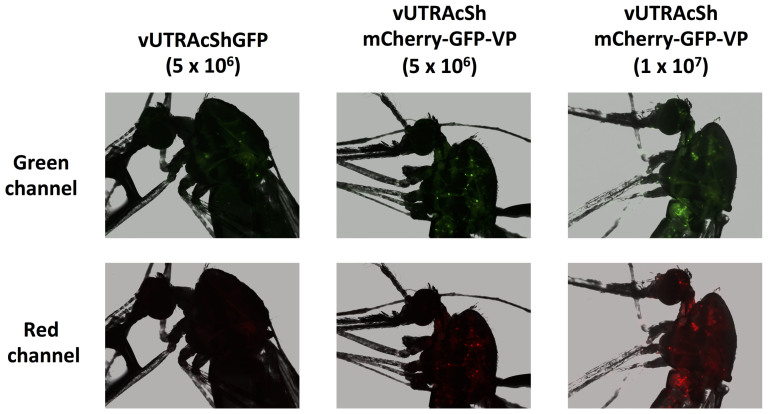
vUTRAcGFP harbors the longest promoter-cassette and showed the highest expression of EGFP among the investigated AgDNV vectors. However, the smaller vUTRIExGFP and vUTRAcShGFP had intermediate expression activity in vitro and in vivo (Fig. 4 and 5). We chose the recombinant AgDNV containing the AcSh promoter as a backbone as it could accommodate up to 2.7 kb of cargo. To polycistronically express multiple open reading frames we used the Thosea asigna virus-derived 2A-like sequence54,55 to simultaneously express 3 genes; mCherry, EGFP and the AgDNV viral capsid protein (Fig. 6, vUTRAcShmCherry-GFP-VP). Western blot for EGFP confirmed that the 2A-like sequences were cleaved in mosquito cells (Fig. 7a, Supplementary Fig. 1). mCherry and EGFP fluorescence was visually observed in vUTRAcShmCherry-GFP-VP-infected MOS55 cells (Fig. 7b) and quantified using flow cytometry (Fig. 7c). The percentage and intensity of EGFP-positive cells were significantly higher than those infected with vUTRAcShGFP-infected cells (P < 0.005 and P < 0.0005 respectively) (Fig. 7d). An. gambiae adults were injected with 5 × 106 of each recombinant DNV. Both mCherry and EGFP fluorescence was detected in vUTRAcShmCherry-GFP-VP-infected mosquitoes (Fig. 8). Again, EGFP expression was higher in vUTRAcShmCherry-GFP-VP-infected compared to vUTRAcShGFP mosquitoes (Fig. 8) even though mosquitoes in both treatments were infected with the same amount of virus. Levels of expression could also be boosted in a dose-dependent manner (Fig. 8).
Figure 6. Schematic representation of polycistronic AgDNV vector.
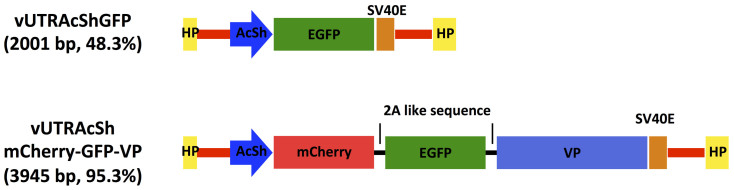
vUTRAcShmCherry-GFP-VP and its backbone vector vUTRAcShGFP genomes are illustrated. Each vector genome size ratio relative to wild-type virus is indicated.
Figure 7. Multiple gene transduction by a polycistronic AgDNV vector in vitro.
(a) Western blot analysis of pUTRAcShGFP- and pUTRAcShmCherry-GFP-VP-transfected MOS55 cells with anti-GFP antibody. Samples were run on the same gel under identical conditions. Masses of protein size standards were indicated at left. Blot has been cropped; full uncropped blot with duplicates available as Supplementary Figure 1. MOS55 cells were infected with 5 × 108 of each vUTRAcShGFP and vUTRAcShmCherry-GFP-VP. EGFP and mCherry expression was (b) visualized by fluorescence microscopy and (c) quantified by flow cytometry analysis. (d) The rate of EGFP positive cells (upper panel) and MFI (lower panel) was quantified by flow cytometry. Mean and S.D. were calculated from three independent infections. P-values were determined by Student's T test.
Figure 8. Multiple gene transduction by a polycistronic AgDNV vector in vivo.
An. gambiae adults were injected with 5 × 106 or 1 × 107 of each recombinant DNV. EGFP and mCherry expression in the mosquitoes was visualized at seven days post-injection.
Discussion
Molecular tools to manipulate gene expression have drastically changed the scientific landscape of vector biology56,57,58. Engineering mosquitoes with enhanced immunity is a potential approach for the control of mosquito-borne diseases59,60. However, over-expression of host genes has not been widely employed due to the lack of efficient and convenient approaches61. Our previous recombinant AgDNV vector could transduce An. gambiae adults, but transduction efficiency was highly variable. A new smaller vector containing the complete viral UTR sequence, (vUTRAcGFP) had 3-fold higher transduction efficiency in vitro compared to our previous vector (vAgActinGFP). In infected cells, vUTRAcGFP showed more efficient replication than vAgActinGFP. Within parvoviruses, the viral genome size significantly affects packaging and replication51,62. Afanasiev et al. suggested that longer DNV genomes were packaged less efficiently than shorter ones51. Other studies demonstrated that a specific region of viral DNA including the 5′ UTR was important for efficient AAV production63. Taken together, these studies suggest shortening the recombinant viral genome and complementing the complete 5′ UTR sequence may synergistically increase transduction efficiency of vUTRAcGFP. In the mosquito, high-levels of GFP expression lasted over fifteen days indicating that AgDNV-based transduction system can efficiently over-express genes for the entire lifespan of the mosquito. By visual observation, there was minimal variation in the intensity detected among individuals.
The most prolific vector in terms of EGFP expression had a relatively long promoter (Actin5C) allowing the insertion of genes approximately 800 bp. We compared the activities of several shorter promoters. In plasmid-transfected cells, the IEx and AcSh promoters showed higher expression than Actin5C (Fig. 4a). Conversely, vUTRAcGFP transduced both the cell line and the mosquito more efficiently than other transducing viruses. The fluorescence intensity in transduced cells and mosquitoes should reflect not only promoter activity but also viral replication. Tullus et al. demonstrated that efficient production of single-stranded AAV DNA that can be packaged into the virion needs a certain minimal size63. AgDNV may also have a limitation on the minimum genome DNA size required for the efficient replication. The polycistronic DNV vector under the control of AcSh promoter, which has a similar size to the wild-type AgDNV, showed higher level of EGFP expression than the much shorter vUTRAcShGFP in cell cultures and mosquitoes. Taken together, recombinant AgDNV genome size may be involved in replication efficiency. Future analyses will address the detailed requirements and mechanisms for generation of efficient AgDNV vectors.
By EGFP expression, AgDNV infection was primarily visible in the fat body. The mosquito fat body plays crucial roles in mosquito physiology, including energy storage, metabolism and immunity. This tissue is also proximal to Plasmodium parasites which migrate throughout the hemocoel to invade the salivary glands64,65.
The other main target tissue of AgDNV was the ovary. Infection of the ovary may be involved in potential vertical transmission of the virus or inoculation of virus into the larval habitat during oviposition. Although significant transduction was not observed in midgut and salivary gland (which are important tissues for Plasmodium infection) transduction of the fat body and ovary suggest promising strategies to use AgDNV to control Anopheles-transmitted parasites. For example, anti-Plasmodium peptides such as SM1 or scorpine would be desirable molecules to express as they are short enough to insert into the current AgDNV vector. High expression in the fat body and secretion into the hemolymph has the potential to dramatically inhibit Plasmodium18,20,66,67. Using the polycistronic AgDNV vector multiple anti-Plasmodium genes could be expressed simultaneously, leading to a synergistic effect on the parasite. In addition, transduction in the ovaries opens the possibility of using AgDNV to manipulate Anopheles reproduction.
In conclusion, we have developed an efficient AgDNV-based over-expression system for Anopheles gambiae mosquitoes. Our data suggested that viral genome size and the 5′ UTR sequence are important elements for efficient AgDNV infection and replication. These insights may shed light upon other insect parvovirus life cycles, which are less well known. Further studies will expand applications to investigation of mosquito biology and paratransgenesis for malaria control.
Methods
Cells culture and mosquito rearing
An. gambiae MOS55 cells were maintained in Schneider's medium supplemented with 10% fetal bovine serum at 28°C. An. gambiae mosquitoes (Keele strain) were maintained on 10% sugar solution at 28°C and 80% humidity with 12/12 h light/dark cycle. The larvae were fed with tetramin fish food. Adults were offered expired human blood through a membrane feeder for reproduction.
Plasmid construction
The pUTR vector was generated based on pBAgα19 (infectious clone of AgDNV) by PCR using the forward primer (5′-ATA-TTT-TAA-TCA-ACA-TGT-ATC-AAC-TAT-A-3′) and reverse primer containing an EcoRV site (5′ GAT-ATC-CAC-TCA-ATT-CGC-CTC-TCC-TTT-TG 3′). The Actin5C promoter-EGFP-SV40 terminator cassette from pAgActinGFP19 was inserted into the EcoRV site in pUTR to make pUTRAcGFP. pUTRp7GFP was constructed by inserting the EGFP ORF into pUTR. The copia-SV40E, AcSh-SV40E and Ac2-SV40E cassettes were commercially synthesized (Integrated DNA Technologies) and inserted into the pUTR vector with the EGFP gene. The resulting vectors were referred to pUTRCopiaGFP, pUTRAcShGFP and pUTRAc2GFP respectively. To clone the AcNPVhr5 enhancer-IE1 promoter and IE1 terminator, pIEx4 (Novagen) was digested with SmaI/ZraI. pUTRIExGFP was made by ligating pUTR and the resulting fragment with the EGFP gene. The polycistronic vector pUTRAcShmCherry-GFP-VP was constructed based on pUTRAcSh, which contains the short actin promoter and multiple cloning site. The AgDNV VP gene was amplified from pBAgα and cloned into the BglII/MluI site in pUTRAcSh. The mCherry-T2A-GFP-dT2A sequence was obtained by PCR from pAc5-STABLE1-Neo (Addgene)54 and inserted into the BglII site in pUTRAcShVP. SURE2 Competent cells (Stratagene) were used for all cloning strategies, and all plasmids confirmed by direct sequencing.
Densovirus production
MOS55 cells at 70% at confluence in a 6-well plate were transfected with each recombinant AgDNV plasmid and the helper plasmid pBAgα at a ratio of 2:1 (1.67 μg and 0.83 μg respectively) using Lipofectamine LTX reagent (Life Technologies). At 3 days post transfection, cells were harvested and suspended in PBS. The cell suspension was subjected to freezing-thawing three times and cell debris was removed by centrifugation. The supernatant was used as densovirus samples for DNA extractions.
Quantification of Densovirus DNA by real-time PCR
Recombinant DNV samples were treated with TURBO DNase (Ambion) to digest plasmid DNAs. The DNase-resistant viral DNA number was considered as the number of recombinant virions. Total DNA from the densovirus samples or densovirus-infected cell supernatants was extracted using DNEasy kits (Qiagen) according to the manufacturer's suggested protocol. Quantitative PCR was performed using Quantitect SYBR Green Kit (Qiagen) using a Rotor-Gene Q (Qiagen). The following primer pairs were used for amplification of EGFP gene: EGFP forward: 5′ TCA-AGA-TCC-GCC-ACA-ACA-TC 3′, EGFP reverse: 5′ TTC-TCG-TTG-GGG-TCT-TTG-CT 3′. To quantify recombinant DNV genome copy number, standard curve was created from a dilution series of pUTRAcGFP ranging from 103 to 108 copies.
Transduction of Anopheles gambiae cell lines and mosquitoes
MOS55 cells were infected with recombinant DNV sample corresponding to 5 × 108 of recombinant virus. For quantification of the viral DNA genome, the supernatant of infected cells was collected at indicated days after infection. EGFP expression in the cell line was observed using an Axiovert S100 fluorescence microscope (Zeiss) and images captured using a ProgRes CF camera (Jenoptik). EGFP signal was quantified by flow cytometry on a Cytomics FC500 (Beckman Coulter). Data were analyzed using BD LSRFortessa (BD Biosciences) and FlowJo software. Anopheles gambiae adults were intrathoracically injected according to Hughes et al. (2011)68 with the indicated viral copy number of recombinant DNV virions. Fluorescence in mosquitoes and dissected tissues was monitored at indicated days post-injection using an Olympus BX-41 microscope. Images were processed using PictureFrame software (Olympus).
Western Blotting
Plasmid-transfected MOS55 cells were lysed in Laemmli Sample Buffer (BioRad) containing 2.5% of β-mercaptoethanol. Protein samples were separated by SDS-PAGE on 4–15% Mini-PROTEAN® TGX™ Gel (BioRad). EGFP was detected with anti-GFP polyclonal antibody (Santa Cruz Biotechnology) and anti-rabbit IgG HRP-liked antibody (Cell Signaling). Signals were developed on Amersham Hyperfilm ECL (GE healthcare) with Amersham ECL Plus Western Blotting Detection Reagents (GE healthcare).
Author Contributions
Y.S. designed and performed the experiments, analyzed the data and contributed to drafting the manuscript. G.N. provided technical support for experiments. G.L.H. assisted in data analysis and contributed to drafting the manuscript. J.L.R. conceived the project, designed the experiments, assisted in data analysis and contributed to drafting the manuscript.
Supplementary Material
Supplementary Figure 1 and Figure Legend
Acknowledgments
We thank Rhiannon Barry for insectary support. We appreciate assistance from the Microscopy and Flow cytometry core facilities at the Huck institutes of the Life Sciences at Pennsylvania State University. This research was supported by NIH grants R21AI088311 and R21AI111175 to J.L.R.
References
- Enayati a. & Hemingway J. Malaria management: past, present, and future. Annu. Rev. Entomol. 55, 569–591 (2010). [DOI] [PubMed] [Google Scholar]
- Mendes A. M. et al. Conserved mosquito/parasite interactions affect development of Plasmodium falciparum in Africa. PLoS Pathog. 4, e1000069 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemingway J. & Ranson H. Insecticide resistance in insect vectors of human disease. Annu. Rev. Entomol. 45, 371–391 (2000). [DOI] [PubMed] [Google Scholar]
- Grossman G. L. et al. Germline transformation of the malaria vector, Anopheles gambiae, with the piggyBac transposable element. Insect Mol. Biol. 10, 597–604 (2001). [DOI] [PubMed] [Google Scholar]
- Kim W., Koo H., Richman A. & Seeley D. Ectopic expression of a cecropin transgene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): Effects on susceptibility to Plasmodium. J. Med. Entomol. 41, 447–455 (2004). [DOI] [PubMed] [Google Scholar]
- Windbichler N. et al. A synthetic homing endonuclease-based gene drive system in the human malaria mosquito. Nature 473, 212–215 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis K. et al. Harnessing mosquito-Wolbachia symbiosis for vector and disease control. Acta Trop. 132 Suppl, S150–163 (2014). [DOI] [PubMed] [Google Scholar]
- McGraw E. & O'Neill S. Beyond insecticides: new thinking on an ancient problem. Nat. Rev. Microbiol. 11, 181–193 (2013). [DOI] [PubMed] [Google Scholar]
- Ricci I. et al. Symbiotic control of mosquito borne disease. Pathog. Glob. Health 106, 380–385 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-abreu I. V., Yan K. & Ramalho-ortigao M. Transgenesis and paratransgenesis to control insect-borne diseases: Current status and future challenges. Parasitol. Int. 59, 1–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minard G., Mavingui P. & Moro C. V. Diversity and function of bacterial microbiota in the mosquito holobiont. Parasit. Vectors 6, 146 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Evans J. & Feldlaufer M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 92, 152–159 (2006). [DOI] [PubMed] [Google Scholar]
- Maniania N. K. & Ekesi S. The use of entomopathogenic fungi in the control of tsetse flies. J. Invertebr. Pathol. 112 Suppl, S83–88 (2013). [DOI] [PubMed] [Google Scholar]
- Walker T. et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476, 450–453 (2011). [DOI] [PubMed] [Google Scholar]
- Morick D. et al. Vertical nontransovarial transmission of Bartonella in fleas. Mol. Ecol. 22, 4747–4752 (2013). [DOI] [PubMed] [Google Scholar]
- Hughes G. L. et al. Identification of yeast associated with the planthopper, Perkinsiella saccharicida: potential applications for Fiji leaf gall control. Curr. Microbiol. 63, 392–401 (2011). [DOI] [PubMed] [Google Scholar]
- Ricci I. et al. Different mosquito species host Wickerhamomyces anomalus (Pichia anomala): perspectives on vector-borne diseases symbiotic control. Antonie Van Leeuwenhoek 99, 43–50 (2011). [DOI] [PubMed] [Google Scholar]
- Wang S. et al. Fighting malaria with engineered symbiotic bacteria from vector mosquitoes. Proc. Natl. Acad. Sci. USA 109, 12734–12739 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Hoiczyk E. & Rasgon J. L. Viral paratransgenesis in the malaria vector Anopheles gambiae. PLoS Pathog. 4, e1000135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang W. et al. Development of transgenic fungi that kill human malaria parasites in mosquitoes. Science 331, 1074–1047 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula R. V. et al. Genetic transformation of a Corynebacterial symbiont from the Chagas disease vector Triatoma infestans. Exp. Parasitol. 119, 94–98 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durvasula R. V. et al. Expression of a functional antibody fragment in the gut of Rhodnius prolixus via transgenic bacterial symbiont Rhodococcus rhodnii. Med. Vet. Entomol. 13, 11511–9 (1999). [DOI] [PubMed] [Google Scholar]
- Durvasula R. & Gumbs A. Prevention of insect-borne disease: An approach using transgenic symbiotic bacteria. Proc. Natl. Acad. Sci. 94, 3274–3278 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia G. et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proc. Natl. Acad. Sci. U. S. A. 104, 9047–9051 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C., Ren X. & Rasgon J. L. The virulent Wolbachia strain wMelPop efficiently establishes somatic infections in the malaria vector Anopheles gambiae. Appl. Environ. Microbiol. 75, 3373–3376 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K. & Kafatos F. C. Mosquito immunity against Plasmodium. Insect Biochem. Mol. Biol. 35, 677–689 (2005). [DOI] [PubMed] [Google Scholar]
- Blandin S. a., Marois E. & Levashina E. A. Antimalarial responses in Anopheles gambiae: from a complement-like protein to a complement-like pathway. Cell Host Microbe 3, 364–374 (2008). [DOI] [PubMed] [Google Scholar]
- Merkling S. H. & van Rij R. P. Beyond RNAi: antiviral defense strategies in Drosophila and mosquito. J. Insect Physiol. 59, 159–170 (2013). [DOI] [PubMed] [Google Scholar]
- Shin S. W. Transgenesis and reverse genetics of mosquito innate immunity. J. Exp. Biol. 206, 3835–3843 (2003). [DOI] [PubMed] [Google Scholar]
- Fraser M. J. Insect transgenesis: current applications and future prospects. Annu. Rev. Entomol. 57, 267–289 (2012). [DOI] [PubMed] [Google Scholar]
- Chen X.-G., Mathur G. & James A. A. Gene expression studies in mosquitoes. Adv. Genet. 64, 19–50 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Airenne K. J. et al. Baculovirus: an insect-derived vector for diverse gene transfer applications. Mol. Ther. 21, 739–749 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waehler R., Russell S. J. & Curiel D. T. Engineering targeted viral vectors for gene therapy. Nat. Rev. Genet. 8, 573–587 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amsterdam A., Varshney G. K. & Burgess S. M. Retroviral-mediated Insertional Mutagenesis in Zebrafish. Methods Cell Biol. 104, 59–82 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson W. O. & Folimonova S. Y. Virus-based transient expression vectors for woody crops: a new frontier for vector design and use. Annu. Rev. Phytopathol. 51, 321–337 (2013). [DOI] [PubMed] [Google Scholar]
- Bartel M. A., Weinstein J. R. & Schaffer D. V. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 19, 694–700 (2012). [DOI] [PubMed] [Google Scholar]
- Maheshri N., Koerber J. T., Kaspar B. K. & Schaffer D. V. Directed evolution of adeno-associated virus yields enhanced gene delivery vectors. Nat. Biotechnol. 24, 198–204 (2006). [DOI] [PubMed] [Google Scholar]
- Carlson J., Suchman E. & Buchatsky L. Densoviruses for control and genetic manipulation of mosquitoes. Adv. Virus Res. 68, 368–392 (2006). [DOI] [PubMed] [Google Scholar]
- Li Y. et al. Genome organization of the densovirus from Bombyx mori (BmDNV-1) and enzyme activity of its capsid. J. Gen. Virol. 82, 2821–2825 (2001). [DOI] [PubMed] [Google Scholar]
- El-Far M. et al. Organization of the ambisense genome of the Helicoverpa armigera Densovirus. J. Virol. 86, 7024 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fédière G., Li Y., Zádori Z., Szelei J. & Tijssen P. Genome organization of Casphalia extranea densovirus, a new iteravirus. Virology 292, 299–308 (2002). [DOI] [PubMed] [Google Scholar]
- Dumas B., Jourdan M., Pascaud A. & Bergoin M. Complete nucleotide sequence of the cloned infectious genome of Junonia coenia densovirus reveals an organization unique among parvoviruses. Virology 191, 202–222 (1992). [DOI] [PubMed] [Google Scholar]
- Amargier A., Vago C. & Meynadier G. Etude histopathologique d'un nouveau type de virose mis en évidence chez le lépidoptère Galleria mellonella. Arch. Gesamte Virusforsch. 15, 659–667 (1965). [PubMed] [Google Scholar]
- Afanasiev B. N., Ward T. W., Beaty B. J. & Carlson J. O. Transduction of Aedes aegypti mosquitoes with vectors derived from Aedes densovirus. Virology 257, 62–72 (1999). [DOI] [PubMed] [Google Scholar]
- Ward T. W. et al. Aedes aegypti transducing densovirus pathogenesis and expression in Aedes aegypti and Anopheles gambiae larvae. Insect Mol. Biol. 10, 397–405 (2001). [DOI] [PubMed] [Google Scholar]
- Gu J.-B., Dong Y.-Q., Peng H.-J. & Chen X.-G. A recombinant AeDNA containing the insect-specific toxin, BmK IT1, displayed an increasing pathogenicity on Aedes albopictus. Am. J. Trop. Med. Hyg. 83, 614–23 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Liu M., Deng Y., Peng H. & Chen X. Development of an efficient recombinant mosquito densovirus-mediated RNA interference system and its preliminary application in mosquito control. PLoS One 6, e21329 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledermann J. & IV E. S. Infection and pathogenicity of the mosquito densoviruses AeDNV, HeDNV, and APeDNV in Aedes aegypti mosquitoes (Diptera: Culicidae). J. Econ. Entomol. 97, 1828–1835 (2004). [DOI] [PubMed] [Google Scholar]
- Ren X. & Rasgon J. L. Potential for the Anopheles gambiae densonucleosis virus to act as an “evolution-proof” biopesticide. J. Virol. 84, 7726–7729 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X., Hughes G. L., Niu G., Suzuki Y. & Rasgon J. L. Anopheles gambiae densovirus (AgDNV) is avirulent in its mosquito host. In review. [DOI] [PMC free article] [PubMed]
- Afanasiev B. & Kozlov Y. Densovirus of Aedes aegypti as an expression vector in mosquito cells. Exp. Parasitol. 79, 322–329 (1994). [DOI] [PubMed] [Google Scholar]
- Chung Y. T. & Keller E. B. Regulatory elements mediating transcription from the Drosophila melanogaster actin 5C proximal promoter. Mol. Cell. Biol. 10, 206–216 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J. Y. et al. Systematic comparison of constitutive promoters and the doxycycline-inducible promoter. PLoS One 5, e10611 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M. et al. Generation of stable Drosophila cell lines using multicistronic vectors. Sci. Rep. 1, 75 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly M. L. et al. The “cleavage” activities of foot-and-mouth disease virus 2A site-directed mutants and naturally occurring “2A-like” sequences. J. Gen. Virol. 82, 1027–1041 (2001). [DOI] [PubMed] [Google Scholar]
- Blandin S. et al. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 3, 852–856 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S. et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell 116, 661–670 (2004). [DOI] [PubMed] [Google Scholar]
- Dong Y. et al. Anopheles gambiae immune responses to human and rodent Plasmodium parasite species. PLoS Pathog. 2, e52 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides G. K. Transgenic mosquitoes and malaria transmission. Cell. Microbiol. 7, 325–333 (2005). [DOI] [PubMed] [Google Scholar]
- Dong Y. et al. Engineered Anopheles immunity to Plasmodium infection. PLoS Pathog. 7, e1002458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G. et al. An in vivo transfection approach elucidates a role for Aedes aegypti thioester-containing proteins in flaviviral infection. PLoS One 6, e22786 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., Yang H. & Colosi P. Effect of genome size on AAV vector packaging. Mol. Ther. 18, 80–86 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullis G. & Shenk T. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J. Virol. 74, 11511–11521 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrese E. L. & Soulages J. L. Insect fat body: energy, metabolism, and regulation. Annu. Rev. Entomol. 55, 207–225 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barillas-Mury C., Han Y. S., Seeley D. & Kafatos F. C. Anopheles gambiae Ag-STAT, a new insect member of the STAT family, is activated in response to bacterial infection. EMBO J. 18, 959–967 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde R., Zamudio F. Z. & Rodr M. H. Scorpine, an anti-malaria and anti-bacterial agent purified from scorpion venom. FEBS Lett. 471, 165–168 (2001). [DOI] [PubMed] [Google Scholar]
- Ghosh A. K., Ribolla P. E. & Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proc. Natl. Acad. Sci. U. S. A. 98, 13278–13281 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes G. L., Koga R., Xue P., Fukatsu T. & Rasgon J. L. Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7, e1002043 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 and Figure Legend