Abstract
Melanocytomas are present in leptomeninges and arise from neural crest during early embryonic development. They are a rare entity and usually occur in the thoracic spine and infratentorial region. We report a 32-year-old female with meningeal melanocytoma of D9-10. Magnetic resonance imaging revealed an intramedullary spinal tumor at D9-D10. Intraoperatively, the tumor was greyish-black in color with moderate vascularity, and was adherent to the cord. The clinical differential diagnoses included cavernoma and melanocytoma. On microscopic examination, the lesion showed sheets of cells with marked pigment deposition, which was obscuring the cellular morphology. The pigment was confirmed to be melanin by Masson's Fontana stain. Immunohistochemistry was performed, which showed positivity for HMB-45, S-100, Vimentin and Melan-A. The cells were negative for cytokeratin, epithelial membrane antigen, Glial fibrillary acidic protein and neuron-specific enolase. Mib-1 labeling index was less than 1%. In view of the lack of nuclear atypia, mitoses, necrosis and low Mib-1-labeling index along with immunohistochemistry profile, the diagnosis of Melanocytoma was made. Melanocytomas are rare pigmented tumors of the spinal cord and posterior cranial fossa. They are benign in nature, but can also be locally aggressive. Melanocytic lesions of the nervous system are to be differentiated from metastatic melanomas and also tumors showing melanin pigment deposition like schwanomma, paraganglioma, medulloblastoma and various gliomas.
Keywords: Intramedullary, melanocytoma, thoracic spine
Introduction
Melanocytoma is a pigmented neoplasm of the meninges, which arises from melanocytes derived from the neural crest.[1] It is a rare tumor and was first described in 1972 by Limas and Tito.[2] It usually occurs in the spine and posterior fossa.[3] The tumor is usually seen in adults in the fourth to fifth decades, with a slight female preponderance.[4] A minority of the cases occur in the pediatric population. The histological differential diagnoses include melanotic schwanomma, malignant melanoma, melanotic meningioma and melanoblastosis. Immunohistochemical panel along with Mib-1 labeling index is helpful in differentiating these conditions as, morphologically, they may look similar. Radiologically, magnetic resonance imaging (MRI) shows a characteristic appearance due to the paramagnetic properties of melanin.
Case Description
A 32-year-old female presented with a history of backache for 4 years and heaviness and numbness over both lower limbs for 1 year. On examination, she had mild numbness of the left leg with no motor weakness. The patient was radiologically evaluated and the MRI scan revealed an intramedullary well-circumscribed hemorrhagic lesion involving the spinal cord at the D9-D10 level, showing no significant postcontrast enhancement. Associated cord edema is seen from the D5 to the D12 level [Figure 1]. Radiological differential diagnoses included cavernoma, primary melanoytic tumor or hemorrhagic neoplasm. The patient was operated upon and, intraoperatively, the tumor was greyish-black in color. It had moderate vascularity and was adherent to the cord. The clinical differential diagnoses included cavernoma and melanocytoma. The tumor was excised and sent to the hisopathology lab.
Figure 1.
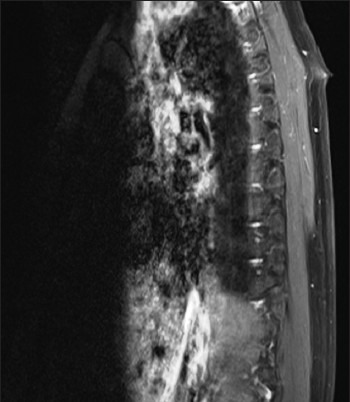
Magnetic resonance imaging scan showing an intramedullary well-circumscribed hemorrhagic lesion involving the spinal cord at the D9-D10 level, showing no significant postcontrast enhancement
Gross examination revealed tumor tissue pieces altogether measuring 2.2 × 2 cm. The entire tissue was processed routinely.
On microscopic examination of the hematoxylin and eosin-stained slides, a lesion was seen showing sheets and nests of cells with marked pigment deposition [Figure 2]. This pigment was obscuring the cellular morphology. Masson’ Fontana was used to confirm the pigment to be melanin. The sections were bleached in order to better appreciate the cellular morphology. It showed cells with moderate cytoplasm and oval to elongated nuclei [Figure 3]. No significant nuclear atypia or mitotic activity was seen. A provisional diagnosis of melanocytoma was made. Perl's stain for iron was negative [Figure 4]. However, immunohistochemistry was performed to confirm the findings.
Figure 2.
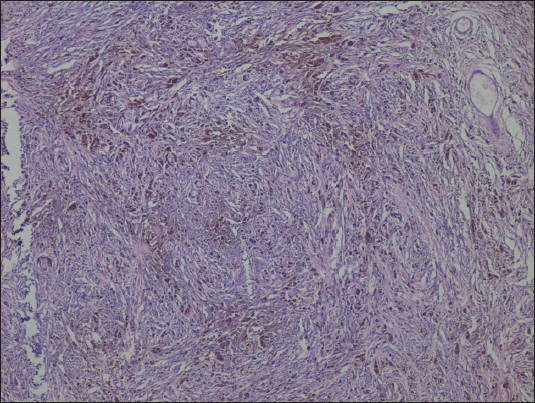
H and E, ×40: Sheets and nests of cells with marked pigment deposition
Figure 3.
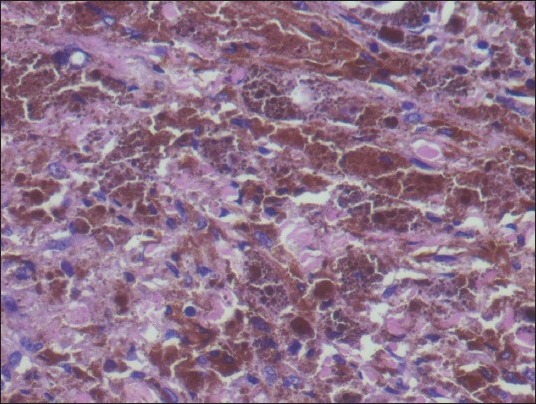
H and E, ×400: Cells with moderate cytoplasm and oval to elongated nuclei
Figure 4.
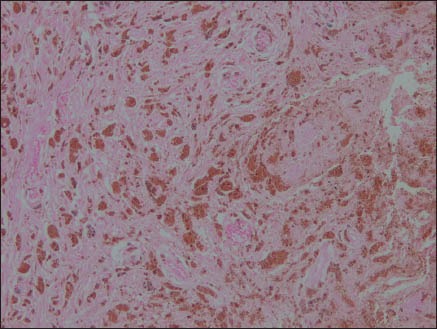
Perl's stain ×100: Negative for iron
Immunohistochemical markers used were: Cytokeratin (Biogenix San Ramon, CA, USA; AE1), Vimentin (Dako Corporation, Carpinteria, CA, USA; V9), HMB-45 (Dako Corporation, Carpinteria, CA, USA; HMB-45), S-100 (Dako Corporation, Carpinteria, CA, USA; 5Y38), Melan A (Dako Corporation, Carpinteria, CA, USA; A103), epithelial membrane antigen (Dako Corporation, Carpinteria, CA, USA, NCH-38), GFAP (Dako Corporation, Carpinteria, CA, USA, 1S524), NSE (Dako Corporation, Carpinteria, CA, USA, BBS/NC/V1-HI4) and Ki-67 (Dako Corporation, Carpinteria, CA, USA, Mib-1).
The neoplastic cells were positive for HMB-45 [Figure 5], S-100 [Figure 6], Vimentin and Melan-A, while they were negative for CK, EMA [Figure 7], CD34 [Figure 8], GFAP and NSE. Mib-1 labeling index was less than 1% [Figure 9]. In view of the presence of melanocytes with no nuclear atypia or mitoses, less than 1% Mib-1 and immunohistochemical profile, the diagnosis of melanocytoma was made.
Figure 5.
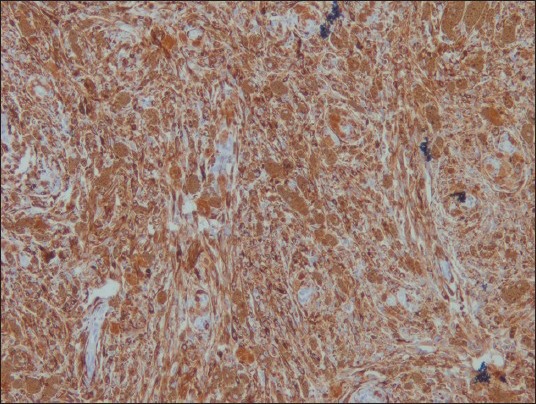
HMB 45 ×100: Positive
Figure 6.
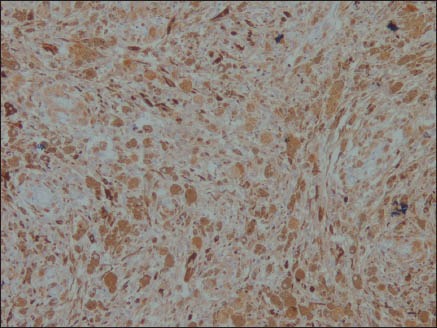
S-100 ×100: Positive
Figure 7.
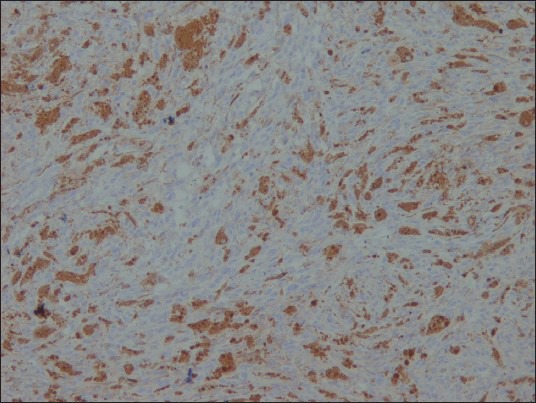
EMA ×100: Negative
Figure 8.
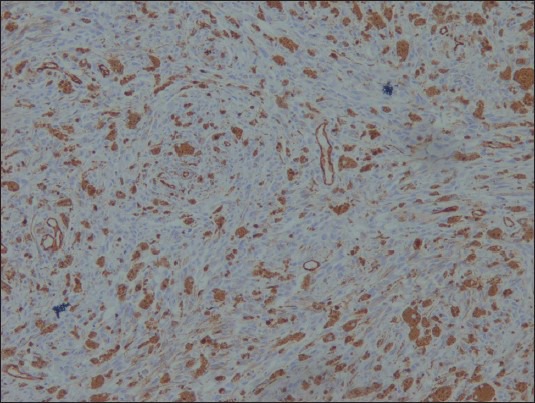
CD34 ×100: Negative
Figure 9.
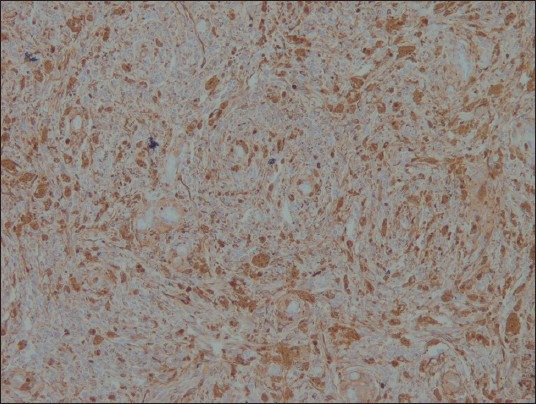
Ki-67 ×100: Very low
Postoperative MRI showed no residual tumor and reduced edema. On follow-up, the patient is asymptomatic 1 year post surgery.
Discussion
Melanocytoma is a rare tumor that arises from leptomeninges, with an annual incidence of 1 per 10 million population and count for less than 0.1% of all central nervous system (CNS) tumors.[5] Most of the melanocytic tumors of the meninges are malignant melanomas. The World Health Organization has classified melanotic lesions of CNS into (1) diffuse melanocytosis and melanomatosis, (2) melanocytoma and (3) malignant melanoma. The melanocytic lesions of CNS have been classified as low grade, intermediate grade and high grade by Brat et al.[6]. Normally, the melanocytes are localized at the base of the brain, ventral medulla and cervical spinal cord. Most melanocytomas arise from the cevical and thoracic spine and are extramedullary – intradural. On the contrary, this case was intramedullary in location. The symptoms related to melanocytomas depend on their location.[7] The tumor in our case was present at the lower dorsal level and hence the patient presented with symptoms in the lower limb. The melanin pigment of melanocytomas give a characteristic MRI appearance due to its paramagnetic effect.
Melanocytomas are usually solitary and no invasion is seen. The tumor cells are oval to spindly and present in nests, and may also show whorling. Our case too showed a similar morphology.
Lesions without melanin pigment are rare and require special techniques like immunohistochemistry and electron microscopy for confirmation.
The immunohistochemistry profile of melanotic lesions (i.e., melanocytoma, malignant melanoma and melanocytosis) are similar, i.e., they are positive for HMB-45, Melan-A, S-100 and Vimentin. These markers help to differentiate these lesions from melanotic schwanomma and melanotic meningioma. Mib-1 labeling index and mitotic count helps to differentiate melanocytoma from malignant melanoma, which is <1-2% in melanocytoma and around 8% in malignant melanoma.[6] Electron microscopy can also be used for identifying melanosomes in these lesions; however, they are rarely required for diagnosis.
The management of melanocytoma has no definite guidelines. However, surgery is considered best for treatment. If the surgery leads to sub-total resection, postoperative radiotherapy may be undertaken.[8]
They are benign in nature, but can be locally aggressive.[9] Melanocytomas rarely recur; however, the data on this entity is too little to state its exact prognosis.
Acknowledgment
The authors would like to profusely thank Dr. Moushumi Suryavanshi for her role in diagnosing the case. The authors would also like to thank Dr. Vimarsh Raina for his guidance along with Dr. Ritesh Sachdev, Dr. Rajan Duggal, Dr. Ruchika Goel, Dr. Lipika Lipi and Dr. Tushar Sahni for their valuable inputs in manuscript checking. Also authors would like to acknowledge Mr. D.R. Singh, Mr. Deepak Sharma and Mr. Nitesh Pandey for helping in performing the histopathological techniques, including immunohistochemistry.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Sahin S, Simsek M, Yilmaz MS, Kir G, Naderi S. World Spinal Column Journal. 2011;2:37–41. [Google Scholar]
- 2.Park SH, Park HR, Ko Y. Spinal meningeal melanocytoma. J Korean Med Sci. 1992;7:364–8. doi: 10.3346/jkms.1992.7.4.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanthi V, Ramakrishna BA, Bheemaraju VV, Rao NM, Athota VR. Spinal meningeal melanocytoma: A rare meningeal tumor. Ann Indian Acad Neurol. 2010;13:308–10. doi: 10.4103/0972-2327.74192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clarke DB, Leblanc R, Bertrand G, Quartey GR, Snipes GJ. Meningeal melanocytoma. Report of a case and a historical comparison. J Neurosurg. 1998;88:116–21. doi: 10.3171/jns.1998.88.1.0116. [DOI] [PubMed] [Google Scholar]
- 5.Jaenisch W, Schreiber D, Guthert H. Neuropathologie. Tumoren des Nervensystems. Neuropathologie. Tumoren des Nervensystems. Stuttgart: Gustav Fischer; 1988. Primaremelanome der ZNS; pp. 347–53. [Google Scholar]
- 6.Brat DJ, Giannini C, Scheithauer BW, Burger PC. Primary melanocytic neoplasms of CNS. Am J Surg Pathol. 1999;23:745–54. doi: 10.1097/00000478-199907000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Offiah CJ, Laitt RD. Case report: Intracranial meningeal melanocytoma: A cause for high signal on T1 and low signal on T2-weighed MRI. Clin Radiol. 2006;61:294–8. doi: 10.1016/j.crad.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Rades D, Heidenreich F, Tatagiba M, Brandis A, Karstens JH. Therapeutic options for meningeal melanocytoma. Case report. J Neurosurg. 2001;95:225–31. doi: 10.3171/spi.2001.95.2.0225. [DOI] [PubMed] [Google Scholar]
- 9.El-Khashab M, Koral K, Bowers DC, Johnson-Welch S, Swift D, Nejat F. Intermediate grade meningeal melanocytoma of cervical spine. Childs Nerv Syst. 2009;25:407–10. doi: 10.1007/s00381-008-0782-6. [DOI] [PubMed] [Google Scholar]


