Abstract
The pathogenesis underlying communicating hydrocephalus has been centered on impaired cerebrospinal fluid (CSF) outflow secondary to abnormal CSF pulsation and venous hypertension. Hydrodynamic theory of hydrocephalus fares better than traditional theory in explaining the possible mechanisms underlying communicating hydrocephalus. Nonetheless, hydrodynamic theory alone could not fully explain some conditions that have ventriculomegaly but without hydrocephalus. By revisiting brain buoyancy from a fresher perspective, called microgravity environment of the brain, introducing wider concepts of anatomical and physiological compensatory–decompensatory phases for a persistent raise in intracranial pressure, and along with combining these two concepts with the previously well-accepted concepts of Monro–Kellie doctrine, intracranial hypertension, cerebral blood flow, cerebral perfusion pressure, brain compliance and elasticity, cerebral autoregulation, blood–brain and blood–CSF barriers, venous and cardiopulmonary hypertension, Windkessel phenomenon, and cerebral pulsation, we provide plausible explanations to the pathogenesis for communicating hydrocephalus and its related disorders.
Keywords: Brain compliance, brain pulsation, buoyancy, communicating hydrocephalus, microgravity, Monro – Kellie doctrine, Windkessel effect
Introduction
Communicating hydrocephalus has been traditionally viewed as an obstructive pathology at the levels of superior sagittal sinus (SSS) or Virchow-Robin spaces. This notion is increasingly challenged by the new hydrodynamic theory of cerebrospinal fluid (CSF).[1] However, even the latest hydrodynamic theory fails to fully explain the pathophysiology underlying communicating hydrocephalus such as post-trauma, post-meningitis or post-subarachnoid hemorrhage ventriculomegaly with and without hydrocephalus, and normal pressure hydrocephalus (NPH) and its related disorders. For this reason, we revisit the old concept of brain buoyancy from the perspective of “microgravity or weightlessness intracranial space” and propose a wider concept called “anatomical and physiological compensatory-decompensatory phases for persistent raise in intracranial pressure (ICP).” Deduce together with known and accepted concepts of brain pulsation,[1,2] brain elasticity and compliance,[3,4,5] cerebral autoregulation,[6,7] blood-brain barrier,[7] cerebral perfusion pressure (CPP) and ischemia,[8,9] cerebral blood flow (CBF),[10,11] ICP, and venous hypertension,[12,13] we motion plausible mechanisms that underlie ventriculomegaly with and without hydrocephalus. In addition, we also highlight the concept of “microgravity–gravity interface” in human body to relate the importance of this interaction to maintain brain and body homeostasis. Finally, we briefly equate on comparable environment for the development of fetal brain that occurs within another or “temporary microgravity or buoyant space” in-utero, made possible by CSF-mimic, the uterine amniotic fluid.
New Perspectives
The CSF baths the brain and spinal cord, and occupies the ventricular system as well as the subarachnoid spaces or cisterns. The average brain weights 50 g in CSF and 1400 g without CSF (actual weight).[14,15] The reduction in brain weight is believed to have resulted from the effect of CSF buoyancy or microgravity environment created by CSF. The new perspective is therefore, “the intracranial space is a microgravity or weightlessness space and interacts normally with earth gravity spaces to ensure normal brain homeostasis.” A concurrent new perspective is the existence of “anatomical and physiological compensatory–decompensatory phases for persistent raise in ICP.” Combination of these two new perspectives with well-accepted concepts of brain pulsation for hydrocephalus, cerebral autoregulation, blood–brain barrier, brain elastance and compliance, ICP, venous hypertension, CPP, and CBF lead us to the possible mechanistic explanations for ventriculomegaly with and without hydrocephalus and its related disorders.
Evaluation of these New Perspectives
Earth gravity is defined scientifically as the attraction between an object and the center of the earth. This entails that such an object tends to fall toward earth owing to earth's greater size and density, and hence larger gravity force than the object. There are three ways to overcome gravity: (a) Acceleration or aerodynamic force, (b) buoyant force, and (c) object with no (or negative) mass or time. Speeding rocket or aeroplane, with their force of acceleration, is the obvious example of resisting the earth gravity. In contrast, buoyant force achieves the same effect by reducing the weight of an object within a buoyant setting, say in a fluid or water environment. Archimedes in 212 BC had first coined the buoyant force as “any submerged object is subject to a greater pressure force on its lower surface than on its upper surface, creating a tendency for the object to rise. This tendency is counteracted by the weight of the object, which will sink if it is heavier than the surrounding fluid and will rise if it is lighter. If the object weights the same as an equivalent volume of the fluid, it will be in equilibrium and remain motionless.” Since we know that the average brain's weight is only 50 g in CSF (and actual weight is 1400 g), buoyant force created by CSF has succeeded to overcome the gravity force. In this context, buoyant force exerts a lifting effect against gravity and creates a “floating brain” [Figure 1].
Figure 1.
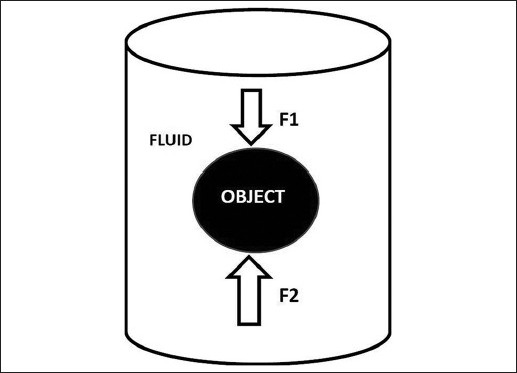
The Archimedes's principle: Any submerged object is in equilibrium and remains motionless whenever weight of the object (F1) equals to object lower surface fluid pressure (F2)
A rigid skull in normal healthy adult that houses the intracranial microgravity space created by CSF confers structural protection from the earth gravity force. Removing part of the skull, as in decompressive craniectomy for malignant or refractory intracranial hypertension would mostly result in sinking skin flap syndrome after resolution of intracranial hypertension [Figure 2]. Monro–Kellie doctrine stated that the intracranial volume of CSF, blood, and brain is constant; any alteration to the volume will initially displace either three out from the cranium.[16] The first, likely to be displaced is CSF during episode of intracranial hypertension (manifested as slit ventricle, obliteration of sulci), followed by blood (manifested as ischemia), and brain parenchyma (manifested as infarction and atrophy). Arguably, the displacement of CSF together with intracranial hypertension would disturb the microgravity environment of the brain and altered its homeostasis. By opening the skull (decompressive craniectomy), more space is created for the brain to accommodate rising ICP; however, once the insult is resolved, the external or earth gravity force (via atmospheric pressure) would normally exert its force internally toward the area of the brain, where a hiatus exists and causes a sinking skin flap syndrome. Hence, iatrogenic communication between gravity (extracranial) and microgravity (intracranial) spaces as in this case converts a “floating” to “non-floating” state of the brain, leading to various symptoms or function alterations, secondary to the corresponding changes in anatomy and cerebral blood flow (CBF) of the brain.[17,18]
Figure 2.
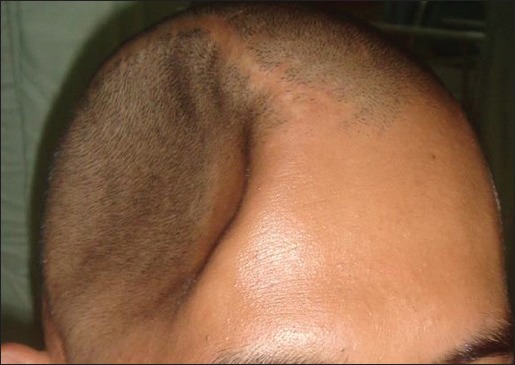
Sinking skin flap syndrome after right frontotemporoparietal decompressive craniectomy
Besides phenomenon of sunken skin flap syndrome, loss of CSF through clinical procedure or surgery may result in a non-floating brain. Over-drainage of CSF via the shunt system would lead to non-floating brain (loss of microgravity environment), and therefore traction on the blood vessels, especially the bridging veins that can cause subdural hemorrhage.[19,20] Leaking of CSF or excessive removal of CSF after lumbar puncture procedure may lead to intracranial hypotensive symptoms and sagging brain syndrome.[21,22,23] Alternatively, this can be viewed as combination of two effects: (a) Removal of buoyant force which leads to non-floating brain and (b) compensatory mechanism to maintain Monro–Kellie doctrine, by augmenting the CBF after excessive removal of CSF which is typically shown as dura enhancement on cranial imaging.[24] Augmentation of CBF can also be in the form of vasodilatation at microcirculatory levels (arterioles) acting via principle of cerebral autoregulation,[6] and at macrocirculatory levels (arteries) via principle of vascular pulsation.[25] Dry brain, vasodilatation, and highly pulsating brain form the possible etiology of the headache symptom in intracranial hypotensive syndrome. As commonly practiced, releasing CSF during brain surgery would lax the brain and facilitate traction onto the non-floating brain. A word of prognostic caution here since creating non-floating or dry brain and if added with traction may induce direct injury to the brain parenchyma, or indirect injury of falling or retracted brain onto adjacent atherosclerotic artery, both of which may result in poor outcomes. Evidently, splitting of sylvian fissure, draining of CSF, and applying traction onto the temporal lobe in elderly patients may cause compression onto the ipsilateral atherosclerotic posterior cerebral artery and risking cerebral infarct.[26,27] Irrigation of surgical field with physiological solution will not re-create the microgravity environment of the brain. Therefore, long duration brain surgery is known to be associated with delayed emergence from anesthesia (altered brain functions) and cognitive changes if extubation undertook immediately.[28] Even with burr hole endoscopic technique, loss of CSF may well occur. Therefore, the future greatest challenge for neurosurgeon is to maintain and operate in a microgravity space for a corresponding cranial pathology. Shift in brain structures is also secondary to the loss of CSF,[29,30] as a result of the changes from floating to non-floating brain. Despite patient's positional change during imaging and during surgery, extraoperative image-guided system remains valid, provided no CSF leak occurs during the actual surgery. For instance, minimal loss in CSF should be assured for posterior fossa neuronavigated endoscopic surgery by positioning patient in prone position to maintain buoyant environment of the brain and therefore validation of extraoperative images.
Moving on from first new perspective, the subsequent new perspective is on the existence of anatomical and physiological compensatory–decompensatory phases for persistent raise in ICP. Since this is part of the possible mechanism underlying the communicating hydrocephalus and its related disorders, details of it is in the next section addressing the consequences of these new perspectives.
Consequences of these New Perspectives and Literature Review
The consequences of these new perspectives are divided into two subtopics.
Mechanism underlying communicating hydrocephalus and its related disorders
Unlike the obstruction-basis traditional theory of hydrocephalus, the hydrodynamic theory places vascular pulsation as the central essence and indirectly denotes closed relationship between the heart and the brain.[1] CSF is produced by choroid plexus lying in brain ventricles and flows out from the ventricular system to cisterns via foramina of Magendie and Luschka. Obstructive hydrocephalus develops because of anatomical obstruction at or proximal to those foramina. Although communicating hydrocephalus has been traditionally viewed as obstruction at the level of SSS,[31,32] the traditional theory of communicating hydrocephalus, however, has failed to answer many questions regarding the pathophysiology of communicating hydrocephalus as clearly pointed out by Egnor et al. for several reasons: (a) Lack of CSF accumulation in subarachnoid spaces and retrograde transmission of pressure to the ventricles would not occur until subarachnoid spaces expanded; (b) if there is pressure gradient from subarachnoid space to ventricles secondary to obstruction, the ventricular dilatation should begin distally at 4th ventricle and progress proximally to lateral ventricles. This pattern is not observed, instead temporal horns are the first to dilate; and (c) subarachnoid space pressure is normally higher than SSS pressure (ratio 1.7:1), but in communicating hydrocephalus, narrowing pressure gradient exists suggesting venous hypertension could play a role.[1]
Besides bulk flow, oscillation or pulsation is also required to mobilize CSF at the base of the brain to Virchow–Robin spaces.[1,32] Without macrocirculatory pulsation, CSF is unlikely be properly distributed to upper convexity and Virchow–Robin spaces. Distribution of CSF to cover the whole brain and spinal cord seems important to maintain the microgravity environment of the brain and hence creates weightlessness of the brain.[33] Unlike the spinal cord or brainstem, the cerebrum is not anchored by the peripheral or cranial nerves; therefore, buoyant force is needed to ensure little movement happens to the brain. Acceleration–deceleration force which could work against gravity is indeed capable of disturbing this buoyant environment of the brain and leads to contre-coup brain contusion. Therefore, buoyant environment of the brain created by constant flow of CSF and maintained by macro circulatory pulsation via Windkessel phenomenon is beneficial to the brain. Windkessel phenomenon arises from arterial distension during systolic phase of the blood pressure, and arterial recoiling in the diastolic phase providing constant blood flow to arterioles and capillaries, and also reducing cardiac and arterial after loading by lowering the arterial pulse pressure.[25]
Besides hydrodynamic theory of CSF flow and buoyancy of the brain, persistent or refractory intracranial hypertension is thought to play a significant role in communicating hydrocephalus. From an initial non-refractory state, intracranial hypertension turns refractory due to failing compensatory responses. At the beginning of raised ICP, the “anatomical compensation” in the form of CSF diversion occurs first. In fact, this anatomical compensatory mechanism is contributed partly by an initial and slight increment in vascular pulsation. This can be viewed as an anatomical effort to reduce the ICP and seems to be in agreement with Monro–Kellie doctrine.[24] Either persistent increment in ICP or persistent reduction in CPP would subsequently reduce CBF and acts as an obvious threat to the brain through ischemia, brain edema, brain swelling, and finally infarction and/or herniation.[8,9] However, persistent reduction in CBF or decompensatory phase is thought not to occur until physiological compensatory mechanism via further increase in macrocirculatory pulsation and microcirculatory vasodilatation (if intact autoregulation) becomes exhausted. During “physiological compensation,” the vascular pulsation and CBF are increased. In patients with non-acute or slow rise in ICP with “intact autoregulation,” increased CBF (via increased pulsation) would finally lead to vasoconstriction despite an early vasodilatory response at microcirculatory level with consequential impending infarct at “those areas” in some patients. In the brain, a rise in CBF is distributed unevenly with grey matter “total blood flow” is higher than white matter and over the surface of the brain, the macrocirculation (pulsating arteries) are mostly located in the sulci adjacent to the gray matter, therefore pulsation should be greater at grey matter than white matter.[34] In summary, the pulsation therefore seems stronger in the grey matter and in the ventricle (choroid vessels pulsation), creating compressive force (transmantle pressure) toward the white matter and later development of ventriculomegaly. The dual effects of increased pulsation and subsequent delay effect of microcirculatory ischemia with disruption in blood–brain and blood–CSF barriers would finally lead to marked ventriculomegaly, periventricular edema, and subcortical–cortical ischemia which can also cause cortical spreading depression;[9] (note: White matter ischemia tends to happen earlier than grey matter because of less total blood flow) and if it is shifted to decompensatory phase, the uncompensated compromise in pressure and blood flow would finally manifest as hydrocephalus (increase pressure) and infarct (decrease blood flow). On the other hand, patients with “impaired cerebral autoregulation” such as after acute and severe head trauma, during physiological compensatory phase, brain swelling is the end-result because of an increase in vascular pulsation (vascular congestion) and hyperemia (impaired vasoconstrictive autoregulation).[10,35] The shift to decompensatory phase would further cause brain swelling via venous hypertension (discussed later) and commonly manifest radiologically as slit ventricles, obliteration of sulci, and effacement of basal cisterns. Once recovery occurs, patients with recovered Windkessel phenomenon and normal cisterns would re-establish CSF outflow pathways to Virchow–Robin spaces and manifested radiologically as ventriculomegaly secondary to brain atrophy or infarct. Conversely, patients with either impaired Windkessel phenomenon or/and distorted cisterns (secondary to blood, inflammation, or infection) would not be able to optimize his CSF outflow, therefore manifest radiologically as hydrocephalus which requires treatment.
Decompensatory phase normally happens when ICP becomes refractory or malignant. Persistent brain ischemia during decompensatory phase would lead to brain edema and swelling, therefore reducing compliance of the brain parenchyma (stiff brain) and imposes counteracting force toward vascular pulsation.[10] The vicious circle sets in whenever a shift from compensatory to decompensatory phase occurs. Two main factors that may contribute to this vicious circle (further increase in ICP) during decompensatory phase are: (a) Raise ICP causes uncompensated ischemic processes that lead to disruption of blood–brain and blood–CSF barriers, brain edema, and swelling and (b) vascular congestion due to decrease in “macrocirculatory” pulsation (macrocirculatory stagnant blood flow or loss of Windkessel effect) and “microcirculatory” stagnant blood flow secondary to diminish force of blood transmission from macrocirculation and also due to venous hypertension (raised ICP acts through cardio-pulmonary system to cause pulmonary hypertension and raises SSS pressure or venous hypertension). These changes correspond to the ICP wave studies. The CSF pulse wave resembles the arterial pulse (peak P1) at the beginning of intracranial hypertension (compensatory phase). The wave becomes venous in shape (peak P2) when ICP is persistently raised and the cranium becomes non-compliant system (decompensatory phase).[36] This description is depicted in Figures 3 and 4.
Figure 3.
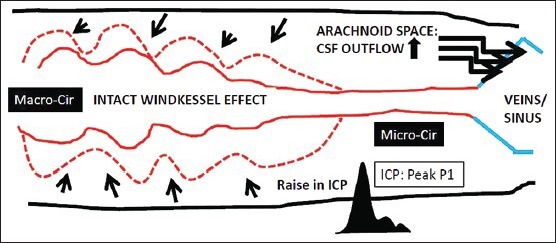
During “compensatory phase,” the macrocirculatory arterial (Macro-cir) pulsation is increased to augment cerebral blood flow, more cerebrospinal fluid in the arachnoid spaces is absorbed to the venous sinuses; and at microcirculatory arterioles (Micro-cir) and in intact autoregulation, the vasoconstrictive response may occur toward the end of the compensatory phase. Cases with impaired autoregulation, hyperemia, and brain edema can cause brain swelling during this phase. During this compensatory phase, the intracranial pressure waveform shows peak P1 wave
Figure 4.
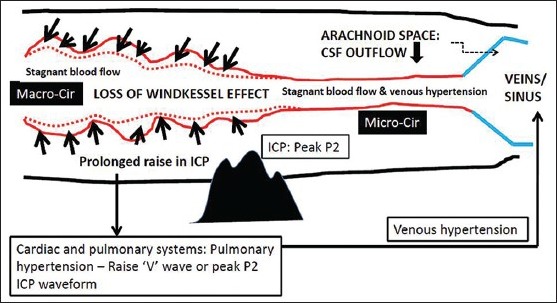
During “decompensatory phase,” presence of loss of Windkessel effect or pulsation in macrocirculation (Macro-cir) secondary to a persistent rise in intracranial pressure that would result in stagnation of blood in both macro- and microcirculation (Micro-cir) causing hyperemia and venous hypertension. Venous hypertension (raise V jugular wave or peak P2 intracranial pressure wave) is also aggravated by secondary pulmonary hypertension and all these may contribute toward brain swelling and low brain compliance or stiff brain. Cerebrospinal fluid outflow to venous sinuses is reduced by lack of pulsation, venous hypertension, and swollen brain
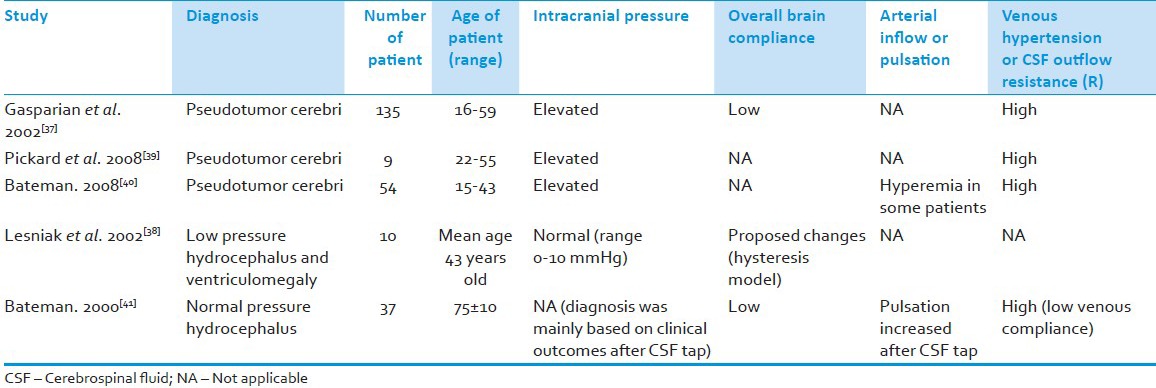
Concerning NPH and pseudotumor cerebri, venous hypertension secondary to persistent slow rise in ICP thought to occur late during decompensatory phase. As discussed above, similar mechanisms, would initially result in ventriculomegaly (increase transmantle pressure secondary to increase pulsation during compensatory phase) and later subcortical–cortical ischemia with “subsequent” disruption of blood–brain and blood–CSF barriers manifested as more marked ventriculomegaly, periventricular edema or lucency, signs and symptoms of subcortical–cortical ischemia (note: Autoregulation is initially thought to be intact but in the presence of common intraluminal vascular pathology in elderly, microcirculatory ischemia is unavoidable at some brain areas and finally could lead to impairment in cerebral autoregulation, disruption of blood–brain, and blood–CSF barriers) in the former (NPH), but without ventriculomegaly in the latter condition (pseudotumor cerebri). The possible explanation for this discrepancy is the fact that in a typical elderly with NPH, the elasticity of brain parenchyma is not at its best (same as in neonate) as compared to middle-aged person with pseudotumor cerebri who is likely to possess greater reserves of brain elasticity.[37] Furthermore, intraluminal vascular pathology is likely to occur in the elderly compared to younger patients which can aggravate the degree of ischemia or infarction. Correspondingly, the ICP values for patient suffering from NPH may be within the normal range. The reason for this can possibly be explained by the concept of cranial compliance. Given that the whole cranial compliance system in NPH has changed (decompensatory phase with new brain and vascular compliance, and physiologically altered elastance of the brain parenchyma), the definition of a raised ICP should also be revised.[38] Since compliance is defined as a change in volume per unit change in pressure (dV/dP), a change in cranial compliance would certainly change its normal ICP. This means, even at low ICP, the brain is vulnerable to injury in a poor compliance system.[38] By grasping these concepts, arrested hydrocephalus may occur at new brain compliance level whenever there is a balance between recovering pulsation profiles with recovering CSF outflow pathways. Table 1 lists out recent clinical studies on NPH and pseudotumor cerebri focusing on ICP, brain compliance, pulsation, and CSF outflow resistance. In general, their findings are in agreement with our views on pathogenesis of pseudotumor cerebri (high ICP, low whole brain compliance, hyperemia, and venous hypertension) and NPH (change in whole brain compliance, normal or low ICP, low venous compliance, and decrease pulsation).[37,38,39,40,41]
Table 1.
Recent clinical studies on normal pressure hydrocephalus and pseudotumor cerebri focusing on intracranial pressure, brain compliance, pulsation, and cerebrospinal fluid outflow resistance
Interaction between microgravity and gravity environment
Though protective, the rigid skull does not cover the brain as a whole since there are communicative holes between intracranial and extracranial spaces. These include foramina for the nerves and blood vessels. Nerve communication is in the form of action potential which creates physiological functions. Blood communication can either be venous or arterial and both play significant role in controlling the ICP. In physiological conditions such as upstanding position, the amount of CSF outflow into SSS is limited by reduction in neck jugular veins diameter secondary to atmospheric pressure. Jugular veins at the neck can be viewed as an anti-siphon device.[42] On the other hand, in pathological conditions such as venous hypertension, a rise in venous pressure is one of the mechanisms that lead to a vicious circle of intracranial hypertension, and similarly, a rise in arterial pressure (or pulsation) is one of the mechanisms to compensate for low CPP or raise ICP. Therefore, existence of two different gravity compartments (intracranial and extracranial) seems critical in order to maintain normal ICP and brain functions. Normal functions of the brain might be disturbed if changes happen between these two compartments. This can be in the forms of: (a) Input via nerves. For instance, by rotating the head, the function of the brain get altered;[43,44] (b) venous hypertension and abnormal arterial pulsation as discussed in great length to cause communicating hydrocephalus and its related disorders; (c) blockage of the vessels as found in syndromic craniosynostosis;[12] and (d) altered atmospheric pressure. Atmospheric pressure is related to the gravity, air, and temperature.[45] Change in atmospheric pressure would certainly cause alteration in hemodynamic pressure at our neck, cardiopulmonary, limbs, or other body systems. Therefore, outer space travelers should wear protective gear that encapsulates the whole body, including the head (to get proper protection over the orbit, ear canal, oral, and nasal airways - communicative holes for nerves and blood to/from the brain). Finally, the brain is not the only compartment in our body that might have microgravity/0 gravity/free fall/buoyant compartment; a pregnant mother with a fetus surrounded by the amniotic fluid (with different amount of water and proteins) acts also in similar way. Therefore, development of the brain and other organ systems can proceed normally and will not be disturbed by earth gravity.
Conclusion
By combining various concepts or theories-pulsation theory of hydrocephalus, buoyancy of CSF compartment which viewed as microgravity compartment, Monro–Kellie doctrine, anatomical and physiological compensatory–decompensatory phases of our body system added with well-known concepts such as ICP, venous hypertension, constriction of jugular veins according to our body position, elastance and compliance of the intracranial system, Windkessel phenomenon, CBF, CPP, effect of atmospheric pressure onto our body, cerebral autoregulation, and blood–brain and blood–CSF barriers - this paper puts together integrative views for plausible mechanisms involving the communicating hydrocephalus and its related disorders. Further research, however, is still needed to prove some of the points highlighted in these new perspectives.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Egnor M, Zheng L, Rosiello A, Gutman F, Davis R. A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg. 2002;36:281–303. doi: 10.1159/000063533. [DOI] [PubMed] [Google Scholar]
- 2.Greitz D. Radiological assessment of hydrocephalus: New theories and implications for therapy. Neurosurg Rev. 2004;27:145–65. doi: 10.1007/s10143-004-0326-9. [DOI] [PubMed] [Google Scholar]
- 3.Czosnyka M, Smielewski P, Kirkpatrick P, Laing RJ, Menon D, Pickard JD. Continuous assessment of the cerebral vasomotor reactivity in head injury. Neurosurgery. 1997;41:11–7. doi: 10.1097/00006123-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Marshall I, MacCormick I, Sellar R, Whittle I. Assessment of factors affecting MRI measurement of intracranial volume changes and elastance index. Br J Neurosurg. 2008;22:389–97. doi: 10.1080/02688690801911598. [DOI] [PubMed] [Google Scholar]
- 5.Portella G, Cormio M, Citerio G, Contant C, Kiening K, Enblad P, et al. Continuous cerebral compliance monitoring in severe head injury: Its relationship with intracranial pressure and cerebral perfusion pressure. Acta Neurochir (Wien) 2005;147:707–13. doi: 10.1007/s00701-005-0537-z. [DOI] [PubMed] [Google Scholar]
- 6.Eide PK, Czosnyka M, Sorteberg W, Pickard JD, Smielewski P. Association between intracranial, arterial pulse pressure amplitudes and cerebral autoregulation in head injury patients. Neurol Res. 2007;29:578–82. doi: 10.1179/016164107X172167. [DOI] [PubMed] [Google Scholar]
- 7.Lang EW, Lagopoulos J, Griffith J, Yip K, Mudaliar Y, Mehdorn HM, et al. Noninvasive cerebrovascular autoregulation assessment in traumatic brain injury: Validation and utility. J Neurotrauma. 2003;20:69–75. doi: 10.1089/08977150360517191. [DOI] [PubMed] [Google Scholar]
- 8.Idris Z, Ghani RI, Musa KI, Ibrahim MI, Abdullah M, Nyi NN, et al. Prognostic study of using different monitoring modalities in treating severe traumatic brain injury. Asian J Surg. 2007;30:200–8. doi: 10.1016/S1015-9584(08)60023-8. [DOI] [PubMed] [Google Scholar]
- 9.Tully B, Ventikos Y. Cerebral water transport using multiple-network poroelastic theory: Application to normal pressure hydrocephalus. J Fluid Mech. 2011;667:188–215. [Google Scholar]
- 10.Anile C, De Bonis P, Di Chirico A, Ficola A, Mangiola A, Petrella G. Cerebral blood flow autoregulation during intracranial hypertension: A simple, purely hydraulic mechanism? Childs Nerv Syst. 2009;25:325–35. doi: 10.1007/s00381-008-0749-7. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson PJ, Gupta AK, Fryer TF, Al-Rawi PG, Chatfield DA, Coles JP, et al. Correlation between cerebral blood flow, substrate delivery, and metabolism in head injury: A combined microdialysis and triple oxygen positron emission tomography study. J Cereb Blood Flow Metab. 2002;22:735–45. doi: 10.1097/00004647-200206000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Rich PM, Cox TC, Hayward RD. The jugular foramen in complex and syndromic craniosynostosis and its relationship to raised intracranial pressure. AJNR Am J Neuroradiol. 2003;24:45–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Portnoy HD, Branch C, Castro ME. The relationship of intracranial venous pressure to hydrocephalus. Childs Nerv Syst. 1994;10:29–35. doi: 10.1007/BF00313582. [DOI] [PubMed] [Google Scholar]
- 14.Noback C, Norman LS, Robert JD, David AR. Totowa, N.J: Humana Press; 2005. Meninges, Ventricles and Cerebrospinal Fluid in The Human Nervous System: Structure and function; p. 93. [Google Scholar]
- 15.Hartmann P, Ramseier A, Gudat F, Mihatsch MJ, Polasek W. Normal weight of the brain in adults in relation to age, sex, body height and weight. Pathologe. 1994;15:165–70. doi: 10.1007/s002920050040. [DOI] [PubMed] [Google Scholar]
- 16.Neff S, Subramaniam RP. Monro-Kellie doctrine. J Neurosurg. 1996;85:1195. [PubMed] [Google Scholar]
- 17.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: A case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–92. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 18.Kemmling A, Duning T, Lemcke L, Niederstadt T, Minnerup J, Wersching H, et al. Case report of MR perfusion imaging in sinking skin flap syndrome: Growing evidence for hemodynamic impairment. BMC Neurol. 2010;10:80. doi: 10.1186/1471-2377-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCullough DC, Fox JL. Negative intracranial pressure hydrocephalus in adults with shunts and its relationship to the production of subdural hematoma. J Neurosurg. 1974;40:372–5. doi: 10.3171/jns.1974.40.3.0372. [DOI] [PubMed] [Google Scholar]
- 20.Pudenz RH, Foltz EL. Hydrocephalus: Overdrainage by ventricular shunts. A review and recommendations. Surg Neurol. 1991;35:200–12. doi: 10.1016/0090-3019(91)90072-h. [DOI] [PubMed] [Google Scholar]
- 21.Schievink WI, Dodick DW, Mokri B, Silberstein S, Bousser MG, Goadsby PJ. Diagnostic criteria for headache due to spontaneous intracranial hypotension: A perspective. Headache. 2011;51:1442–4. doi: 10.1111/j.1526-4610.2011.01911.x. [DOI] [PubMed] [Google Scholar]
- 22.Zaatreh M, Finkel A. Spontaneous intracranial hypotension. South Med J. 2002;95:1342–6. [PubMed] [Google Scholar]
- 23.Hwang TN, Rofagha S, McDermott MW, Hoyt WF, Horton JC, McCulley TJ. Sunken eyes, sagging brain syndrome: Bilateral enophthalmos from chronic intracranial hypotension. Ophthalmology. 2011;118:2286–95. doi: 10.1016/j.ophtha.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 24.Mokri B. The Monro-Kellie hypothesis: Applications in CSF volume depletion. Neurology. 2001;56:1746–8. doi: 10.1212/wnl.56.12.1746. [DOI] [PubMed] [Google Scholar]
- 25.Chan GS, Ainslie PN, Willie CK, Taylor CE, Atkinson G, Jones H, et al. Contribution of arterial Windkessel in low-frequency cerebral hemodynamics during transient changes in blood pressure. J Appl Physiol. 2011;110:917–25. doi: 10.1152/japplphysiol.01407.2010. [DOI] [PubMed] [Google Scholar]
- 26.Umredkar A, Gupta SK, Khandelwal N, Chhabra R, Mathuriya SN, Pathak A, et al. Intracerebral infarcts following clipping of intracranial aneurysms: Incidence, clinical correlation and outcome. Br J Neurosurg. 2010;24:156–62. doi: 10.3109/02688690903513412. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki T, Kodama N, Matsumoto M, Suzuki K, Konno Y, Sakuma J, et al. Blood flow disturbance in perforating arteries attributable to aneurysm surgery. J Neurosurg. 2007;107:60–7. doi: 10.3171/JNS-07/07/0060. [DOI] [PubMed] [Google Scholar]
- 28.Rhona CF, Richard JF. Delayed recovery of consciousness after anaesthesia. Contin Educ Anaesth Crit Care Pain. 2006;6:114–8. [Google Scholar]
- 29.Coenen VA, Abdel-Rahman A, McMaster J, Bogod N, Honey CR. Minimizing brain shift during functional neurosurgical procedures: A simple burr hole technique that can decrease CSF loss and intracranial air. Cent Eur Neurosurg. 2011;72:181–5. doi: 10.1055/s-0031-1279748. [DOI] [PubMed] [Google Scholar]
- 30.Etame AB, Fox WC, Sagher O. Osmotic diuresis paradoxically worsens brain shift after subdural grid placement. Acta Neurochir (Wien) 2011;153:633–7. doi: 10.1007/s00701-010-0856-6. [DOI] [PubMed] [Google Scholar]
- 31.Welch K, Friedman V. The cerebrospinal fluid valves. Brain. 1960;83:454–69. doi: 10.1093/brain/83.3.454. [DOI] [PubMed] [Google Scholar]
- 32.McComb JG. Recent research into the nature of cerebrospinal fluid formation and absorption. J Neurosurg. 1983;59:369–83. doi: 10.3171/jns.1983.59.3.0369. [DOI] [PubMed] [Google Scholar]
- 33.Tsutsumi S, Ito M, Yasumoto Y, Tabuchi T, Ogino I. The Virchow-Robin spaces: Delineation by magnetic resonance imaging with considerations on anatomofunctional implications. Childs Nerv Syst. 2011;27:2057–66. doi: 10.1007/s00381-011-1574-y. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson IM, Bull JW, Duboulay GH, Marshall J, Russell RW, Symon L. Regional blood flow in the normal cerebral hemisphere. J Neurol Neurosurg Psychiatry. 1969;32:367–78. doi: 10.1136/jnnp.32.5.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carmelo A, Ficola A, Fravolini ML, La Cava M, Maira G, Mangiola A. ICP and CBF regulation: A new hypothesis to explain the “windkessel” phenomenon. Acta Neurochir Suppl. 2002;81:112–6. [PubMed] [Google Scholar]
- 36.Hamer J, Alberti E, Hoyer S, Wiedemann K. Influence of systemic and cerebral vascular factors on the cerebrospinal fluid pulse waves. J Neurosurg. 1977;46:36–45. doi: 10.3171/jns.1977.46.1.0036. [DOI] [PubMed] [Google Scholar]
- 37.Gasparian SS, Serova NK, Sherbakova EY, Belova TN. Compensatory mechanisms in patients with benign intracranial hypertension syndrome. Acta Neurochir Suppl. 2002;81:31–3. doi: 10.1007/978-3-7091-6738-0_8. [DOI] [PubMed] [Google Scholar]
- 38.Lesniak MS, Clatterbuck RE, Rigamonti D, Williams MA. Low pressure hydrocephalus and ventriculomegaly: Hysteresis, non-linear dynamics, and the benefits of CSF diversion. Br J Neurosurg. 2002;16:555–61. [PubMed] [Google Scholar]
- 39.Pickard JD, Czosnyka Z, Czosnyka M, Owler B, Higgins JN. Coupling of sagittal sinus pressure and cerebrospinal fluid pressure in idiopathic intracranial hypertension: A preliminary report. Acta Neurochir Suppl. 2008;102:283–5. doi: 10.1007/978-3-211-85578-2_53. [DOI] [PubMed] [Google Scholar]
- 40.Bateman GA. Arterial inflow and venous outflow in idiopathic intracranial hypertension associated with venous outflow stenoses. J Clin Neurosci. 2008;15:402–8. doi: 10.1016/j.jocn.2007.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Bateman GA. Vascular compliance in normal pressure hydrocephalus. AJNR Am J Neuroradiol. 2000;21:1574–85. [PMC free article] [PubMed] [Google Scholar]
- 42.el-Shafei IL, el-Rifaii MA. Ventriculojugular shunt against the direction of blood flow. I. Role of the internal jugular vein as an antisiphonage device. Childs Nerv Syst. 1987;3:282–4. doi: 10.1007/BF00271824. [DOI] [PubMed] [Google Scholar]
- 43.Schneider S, Guardiera S, Abel T, Carnahan H, Strüder HK. Artificial gravity results in changes in frontal lobe activity measured by EEG tomography. Brain Res. 2009;1285:119–26. doi: 10.1016/j.brainres.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 44.Brümmer V, Schneider S, Vogt T, Strüder H, Carnahan H, Askew CD, et al. Coherence between brain cortical function and neurocognitive performance during changed gravity conditions. J Vis Exp. 2011;51:e2670. doi: 10.3791/2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merriam JB. Atmospheric pressure and gravity. Geophys J Int. 1992;109:488–500. [Google Scholar]


