Abstract
Background
This study evaluated the effect of immediate postpartum depot medroxyprogesterone (DPMA) on breastfeeding cessation within 6 weeks postpartum.
Study design
At low income-serving obstetric and pediatric clinics, eligible mothers within one-year postpartum were recruited to participate in a retrospective cohort study. The 183 participants completed a self-administered survey. Surveys were merged with birth-certificate data and perinatal maternal/infant medical records. Kaplan-Meier distributions assessed the relationship between DPMA use and breastfeeding cessation. A multivariable Cox Proportional Hazards Model estimated hazard ratios (HR) and included five known risk factors (age, education, race, parity, and parental cohabitation) and identified potential confounders.
Results
Consistent with the biologic model, the Kaplan-Meier results raised the possibility of a detrimental effect of DPMA on duration of any breastfeeding, but differences in these distributions did not achieve statistical significance (p=0.24); after adjustment for potential confounders, this non-statistically significant association remained (HR:1.22; CI:0.75–1.98).
Conclusion
Given the state of the evidence, it is unclear whether a causal effect does or does not exist. However, if there is a causal effect of DPMA on breastfeeding duration, it is minimal. Additional well-designed research is warranted.
Keywords: breastfeeding, postpartum contraception, depot medroxyprogesterone
1. Introduction
Empiric evidence establishing an association between immediate postpartum DPMA receipt (prior to hospital discharge) and breastfeeding cessation is both limited and equivocal [1–3]. Inconsistent results are attributable to insufficient sample size, broad inclusion criteria, inadequate methods and lack of any stratified or multivariable analyses to account for potential confounding factors [4]. A systematic review concluded that existing evidence is of ‘low’ methodologic quality, and consequently, inferences drawn from these studies, may be invalid; the authors suggested the evidence is not adequate to either accept or reject a causal relationship between immediate postpartum DPMA use and early breastfeeding cessation [4]. A biologic model describing an alteration in the inverse homeostatic relationship between prolactin and progesterone in the presence of postpartum DPMA use has been discussed in detail elsewhere [4–6].
The Centers for Disease Control and Prevention (CDC) used these studies of “low’ methodologic rigor as the basis for their recommendation supporting the use of immediate postpartum DPMA use among lactating women [4,7,8]. As a result, in the US, administration of DPMA in the immediate postpartum period occurs in many settings. Employing a methodologically rigorous study design and multivariable analyses, we evaluated the effect of immediate postpartum DPMA on breastfeeding cessation within 6 weeks postpartum. We expected DPMA recipients to have an increased risk of breastfeeding cessation within 6 weeks postpartum relative to non-recipients when controlling for other factors.
2. Material and Methods
Between January 2010 and December 2012, we recruited eligible mothers from low income-serving obstetric and pediatric clinics in Rochester, New York, to participate in a retrospective cohort study. Eligible mothers were aged ≥18 years, spoke English, with singleton, healthy, term infants ≤1-year-old, gave birth at one of three participating hospitals and initiated breastfeeding. The University of Rochester Institutional Review Board approved the study that required written informed consent from all study participants. Participants completed a self-administered survey and authorized release of maternal/infant hospital medical records and hospital-supplied birth certificate data.
The exposure, immediate postpartum DPMA receipt (prior to hospital discharge), was collected via both the maternal self-report (survey) and medical records (considered the gold standard). The primary outcome was maternal self-reported breastfeeding cessation within 6 weeks postpartum. We selected this window to test the guidelines contained in the DPMA package insert that recommend administration of DPMA after 6 weeks. In addition to cessation of any breastfeeding, data obtained from the self-completed survey included planned breastfeeding goal, previous breastfeeding experience, maternal transience, social support, confidence in ability to breastfeed, history of DPMA use and maternal return to work. We acquired data regarding maternal age, race, education, parental cohabitation, parity, adequacy of prenatal care, intendedness of pregnancy and pre-pregnancy body mass index (BMI) from the hospital birth certificate data. When compared to the medical records, maternal characteristics abstracted from birth certificate data previously demonstrated satisfactory concordance (e.g., race/ethnicity ≥75% and previous live birth ≥92%) [9].
Parametric or non-parametric statistical testing (chi-square/Fisher’s exact test for categorical data and t-test/Wilcoxon Mann-Whitney test for continuous data) across relevant groups (exposure and outcome) evaluated differences in the distribution of each variable. Kaplan-Meier distributions measured the crude relationship between DPMA and breastfeeding cessation. The generalized Wilcoxon test gauged statistical significance between groups. We selected this test in favor of the log rank test since its weighting favors events occurring earlier in the follow-up period (consistent with the biologic model) [10]. Duration of breastfeeding was calculated from birth (time zero) until either the day of self-report breastfeeding cessation or until the 6th postpartum week; participants still breastfeeding a 6 weeks were administratively (right) censored.
Adjusted hazard ratios (HR) and 95% confidence intervals (CI) estimating cessation of any breastfeeding were calculated using the Cox Proportional Hazards Model; in addition to five known risk factors (age, education, race, parity, and parental cohabitation), we included identified potential confounders in the multivariable model. The latter were included in the multivariable model if they were associated with both DPMA receipt and breastfeeding cessation at 6 weeks and (p≤0.1).
Lastly, to pinpoint the timing of the potential effect and further evaluate the biologic model, we analyzed three pre-specified intervals: birth through two weeks, >2 through 6 weeks and >4 through 6 weeks. For each period, survival analysis techniques compared the effect of DPMA receipt on the cessation of any breastfeeding. Visual examination of Kaplan Meier distributions and corresponding Wilcoxon tests assessed the distribution of events between groups across intervals and interval censored Cox Proportional Hazard models to calculate interval specific HR. In each interval, all participants with follow-up time longer than the start of follow-up for that interval were included. Within the respective interval, participants’ time to event were calculated from the start of the interval to the day of the event (resetting ‘time-zero’ at the beginning of each interval). Those not having an event (self-reported cessation of any breastfeeding) were administratively censored at the end of the interval, assuming cessation occurs after that time point. Each of the three unadjusted interval models explains the effect of DPMA receipt on the cessation of any breastfeeding during the pre-specified intervals.
Using the exponential survival analysis of two independent groups (with censoring), assuming a clinically meaningful difference of 20% (75% versus 55%) in breastfeeding proportion between groups and a two-sided significance level of 0.05, 150 participants were needed to address this research question with 80% power. To compensate for potential missing medical records, misclassified inclusion criteria or partially completed survey data, we inflated the calculated sample size by 10%. Since the prevalence of DPMA use was unknown, we conducted a post hoc power analysis with consented participants to determine the actual power achieved. Analyses were conducted in Statistical Analysis Systems, version 9.2 (SAS Institute, Cary, NC). All p-values were two-sided, and significance was set at p<0.05.
3. Results
Waiting room recruitment required recruiters to approach potential participants if the infant visually appeared to be no more than 12 months old. Of the 941 potential participants approached, 683 (75.3%) did not satisfy the inclusion criteria (e.g., never breastfeed their infant, twins, mother not present at clinic, maternal or infant age not appropriate, unhealthy infant, no English, non-qualifying hospital of birth or repeat study participants); 79 (8.4%) refused to participate in the study (primarily refusing release of the medical records) and 179 (19.0%, 69.4% of eligible patients) consented to participate (Table 1). An additional 20 participants were recruited in the obstetric clinic (no statistically significant differences between participants recruited from the obstetric clinic and those recruited from the pediatric clinic were detected); 199 mothers consented to study participation. Of these, we excluded 16 (8.0%) individuals (n=7 gave birth at a non-qualifying hospital of birth and n=9 maternal medical records were missing or unobtainable), resulting in a final study sample of 183 participants.
Table 1.
Recruitment summary
| N | Excluded | % of total Screened | % of Total Eligible | |
|---|---|---|---|---|
| Total screened | 941 | -- | -- | -- |
| Ineligible | 683 | 683 | 72.6 | -- |
| Eligible to consent | 258 | -- | 27.4 | 100.0 |
| Refused | 79 | 79 | 8.4 | 30.6 |
| Consented | 179 | -- | 19.0 | 69.4 |
| Additional consenteda | 20 | -- | -- | -- |
| Total enrolled | 199 | -- | -- | -- |
| Excludedb | 16 | 16 | -- | -- |
| Final study sample | 183 |
Participants were recruited from the obstetric clinic through use of a dedicated on-site enroller who assessed clients’ eligibility for multiple studies. As a result, the number approached for this study cannot be determined. Equally, this process did not provide for tracking the number of individuals who were ineligible or refused. Therefore the n=20 consented in the obstetric clinic are not included in the overall recruitment numbers and process until the point at which they are consented.
Ineligible hospital of birth n=7 (28.0%); unobtainable medical record n=9 (72.0%); total excluded=8.0% of those enrolled.
Study participants were classified into an exposed group of 68 mothers (37.2%) who received DPMA prior to hospital discharge (3.3–123 hours postpartum, mean 37.0; SD 24.1; Fig 1) and an unexposed group of 115 mothers (68.2%) who did not receive DPMA (Table 2). The distribution of age, race, education, parity, adequacy of prenatal care and cohabitation with the infant’s father were not statistically different between DPMA recipients and non-recipients. Those who received DPMA (versus those who did not) were significantly less likely to have a timed breastfeeding goal (e.g., plan to breastfeed the infant for 6 months) or a breastfeeding milestone (until a specific event occurs, e.g., ‘until the infant gets teeth’) and to have social support of breastfeeding (e.g. breastfeeding supported by father of the baby, family or friends). No statistically significant differences were observed across other demographic or lifestyle factors.
Fig. 1.
Distribution of postpartum hour of DPMA receipt (n=47)
Table 2.
Demographics and potential confounders by DPMA status and by breastfeeding cessation status at 6 weeks postpartum
| Variables | DPMA: No | DPMA: Yes | p-valuea | Any BF: No | Any BF: Yes | p-valuea | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| n=115 | n=68 | n=119 | n=64 | |||||||
| Demographics | ||||||||||
| Age b,c (Mean, SD) | 24.96 | 5.27 | 24.65 | 5.10 | 0.70 | 24.27 | 5.08 | 25.92 | 5.28 | 0.04 |
| n | % | n | % | n | % | n | % | |||
| Racec | 0.33 | 0.21 | ||||||||
| White | 44 | 40.37 | 19 | 29.23 | 45 | 39.82 | 18 | 29.51 | ||
| Black | 49 | 44.95 | 35 | 53.85 | 49 | 43.36 | 35 | 57.38 | ||
| Other | 16 | 14.68 | 11 | 16.92 | 19 | 16.81 | 8 | 13.11 | ||
| Education | 0.05 | 0.40 | ||||||||
| ≤ High school | 63 | 57.80 | 47 | 72.31 | 74 | 65.49 | 36 | 59.02 | ||
| > High school | 46 | 42.20 | 18 | 27.69 | 39 | 34.51 | 25 | 40.98 | ||
| Parity b | 0.12 | 0.98 | ||||||||
| Primiparous | 55 | 50.46 | 25 | 38.46 | 52 | 46.02 | 28 | 45.90 | ||
| Multiparous | 54 | 49.54 | 40 | 61.54 | 61 | 53.98 | 33 | 54.10 | ||
| Adequacy of prenatal carec,d | 0.17 | 0.09 | ||||||||
| Adequate/adequate plus | 62 | 64.58 | 45 | 78.95 | 74 | 75.51 | 33 | 60.00 | ||
| Intermediate | 18 | 18.75 | 6 | 10.53 | 11 | 11.22 | 13 | 23.64 | ||
| Inadequate | 16 | 16.67 | 6 | 10.53 | 13 | 13.27 | 9 | 16.36 | ||
| Parental cohabitation | 0.31 | 0.66 | ||||||||
| Yes | 54 | 49.54 | 27 | 41.54 | 54 | 47.79 | 27 | 44.26 | ||
| No | 55 | 50.46 | 38 | 58.46 | 59 | 52.21 | 34 | 55.74 | ||
| Parity/previous BF experiencec | 0.17 | 0.06 | ||||||||
| Primparous | 55 | 54.46 | 25 | 40.98 | 52 | 50.00 | 28 | 48.28 | ||
| Multiparous with BF experience | 38 | 37.62 | 27 | 44.26 | 37 | 35.58 | 28 | 48.28 | ||
| Multiparous without BF experience | 8 | 7.92 | 9 | 14.25 | 15 | 14.4 | 2 | 3.45 | ||
| BF planc | <0.01 | 0.43 | ||||||||
| BF goal/milestone | 62 | 66.67 | 25 | 44.64 | 52 | 55.91 | 35 | 62.50 | ||
| None | 31 | 33.33 | 31 | 55.36 | 41 | 44.09 | 21 | 37.50 | ||
| Pre-pregnancy BMIc | 0.93 | 0.53 | ||||||||
| Healthy | 39 | 36.79 | 24 | 37.50 | 40 | 35.40 | 23 | 40.35 | ||
| Other | 67 | 63.21 | 40 | 62.50 | 72 | 64.60 | 24 | 59.65 | ||
| Intendedness of pregnancyc | 0.11 | 0.60 | ||||||||
| Intended | 52 | 53.06 | 23 | 39.66 | 47 | 46.53 | 28 | 50.91 | ||
| Unintended | 46 | 46.94 | 35 | 60.34 | 54 | 53.47 | 27 | 49.09 | ||
| Maternal transience within 2 yearsc | 0.27 | 0.33 | ||||||||
| 0 Moves | 27 | 28.42 | 24 | 39.34 | 33 | 33.00 | 18 | 32.14 | ||
| 1 Move | 33 | 34.74 | 15 | 24.59 | 27 | 27.00 | 21 | 37.50 | ||
| > 1 Move (2–10) | 35 | 36.84 | 22 | 36.07 | 40 | 40.00 | 17 | 30.36 | ||
| Postpartum return to work/schoolc | 0.81 | 0.22 | ||||||||
| Yes | 60 | 65.22 | 38 | 63.33 | 66 | 68.04 | 32 | 41.82 | ||
| No | 32 | 34.78 | 22 | 36.67 | 31 | 31.96 | 32 | 58.18 | ||
| Maternal alcohol use during pregnancyc | 0.53 e | >0.99 e | ||||||||
| Yes | 2 | 1.83 | 0 | 0.0 | 1 | 0.89 | 1 | 1.64 | ||
| No | 107 | 98.17 | 64 | 100.0 | 111 | 99.11 | 60 | 98.36 | ||
| Maternal tobacco use during pregnancyc | 0.67 | 0.13 | ||||||||
| Yes | 30 | 27.78 | 20 | 30.77 | 37 | 32.74 | 13 | 21.67 | ||
| No | 78 | 72.22 | 45 | 69.23 | 76 | 67.26 | 47 | 78.33 | ||
| Social support of BFc,f | 0.03 | 0.87 | ||||||||
| Yes | 92 | 93.88 | 49 | 83.05 | 91 | 90.10 | 50 | 89.29 | ||
| No | 6 | 6.12 | 10 | 16.95 | 10 | 9.90 | 6 | 10.71 | ||
| Previous DP usec | 0.86 | 0.02 | ||||||||
| Yes | 49 | 47.12 | 32 | 48.48 | 59 | 54.63 | 22 | 35.48 | ||
| No | 55 | 52.88 | 34 | 51.52 | 49 | 45.37 | 40 | 64.52 | ||
| BF confidence at hospital dischargec | 0.34 | <0.01 | ||||||||
| Very | 48 | 46.60 | 33 | 56.90 | 39 | 38.61 | 42 | 70.00 | ||
| Fairly/somewhat | 42 | 40.78 | 17 | 29.31 | 42 | 41.58 | 17 | 28.33 | ||
| Not at all | 13 | 12.62 | 8 | 13.79 | 20 | 19.80 | 1 | 1.67 | ||
| Hospital of birthc | 0.52 | 0.03 | ||||||||
| Highland Hospital | 26 | 23.85 | 11 | 16.92 | 31 | 27.43 | 6 | 9.84 | ||
| Rochester General Hospital | 27 | 24.77 | 16 | 24.62 | 26 | 23.01 | 17 | 27.87 | ||
| Strong Memorial Hospital | 56 | 51.38 | 38 | 58.46 | 56 | 49.56 | 38 | 62.30 | ||
| WIC participation during pregnancyc | 0.09 | 0.23 | ||||||||
| Yes | 83 | 72.81 | 57 | 83.82 | 94 | 79.66 | 46 | 71.88 | ||
| No | 31 | 27.19 | 11 | 16.18 | 24 | 20.34 | 18 | 28.13 | ||
Abbreviations: WIC, Women Infant and Children; BF, breastfeeding; DPMA, depot medroxyprogesterone.
Missing data were excluded when calculating global p-values.
Age range 17–40 years; this represents age at the time of delivery, not age at the time of consent and data collection.
Observations <183.
Adequacy of prenatal care as calculated by the Kotelchuck Index (accounting for month of initial prenatal care visit, number of visits, and gestational age).
p-value generated from a Fisher’s exact test instead of a chi-square test.
Social Support of breastfeeding defined as a positive response regarding father, other family members or friend supporting a woman’s decision to breastfeed.
There were 41 individuals who were censored early (last recorded day of inhospital breastfeeding determined the day of early censoring), 80 women ceased breastfeeding prior to 6 weeks postpartum and 67 continued breastfeeding throughout the follow-up period. Early censored observations did not statistically differ from the remainder of the study population based on maternal age, education, race, parity, adequacy of prenatal care and cohabitation with infant’s father. Therefore, these data support the assumption that these groups are similar to each other regarding breastfeeding duration.
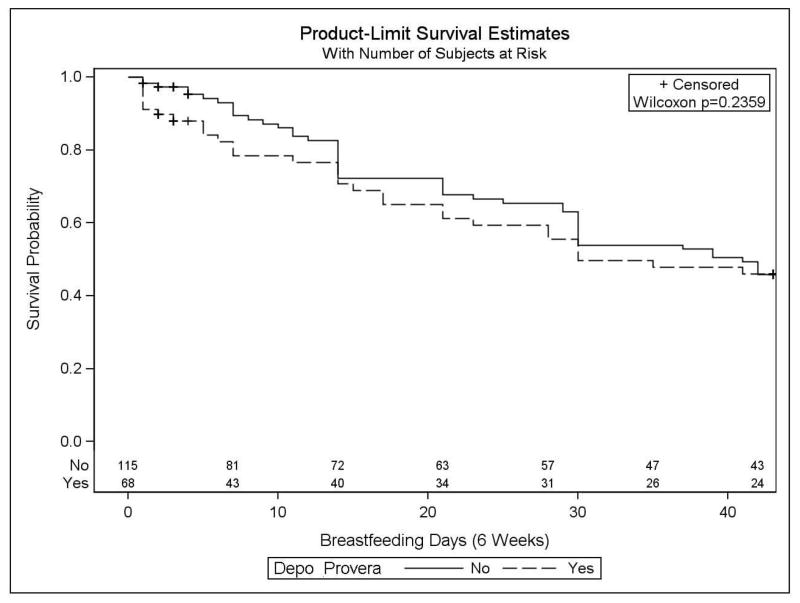
Kaplan-Meier distributions and the corresponding Wilcoxon test did not detect a statistically significant difference in breastfeeding cessation within 6 weeks between DPMA groups (p=0.24; Fig. 2). The distribution of time to breastfeeding cessation for subjects in the DPMA group is consistently lower compared to those who did not receive DPMA, but the two curves do meet at 2, 4 and 6 weeks. While the overall median time to breastfeeding cessation (survival) is 39 days, it is 41 days for women who did not receive DPMA and 30 days for women who did receive DPMA. Despite the lack of statistical significance, the directionality is consistent with both the crude hazard ratio (HR 1.14; CI 0.72–1.80) and the biologic model. Women receiving DPMA in the immediate postpartum period were 14% more likely to cease breastfeeding within 6 weeks postpartum than those not receiving DPMA (again, not statistically significant).
Figure 2.
Cessation of any breastfeeding within six weeks.
The proportional hazards assumption for the Cox proportional hazard model was satisfied; this suggests the probability of an event occurring, at any point of follow-up, was proportional between DPMA recipients and non-recipients. No additional potential confounders qualified for inclusion into the multivariable model (Table 2). After adjusting for known risk factors (age, education, race, parity, and parental cohabitation), in our sample, the increased risk of breastfeeding cessation within 6 weeks among DPMA recipients persisted, and in fact, increased slightly, but this result was not statistically significant (HR 1.22; CI 0.75–1.98, Table 3). In the multivariable model, parity and age were the only statistically significant risk factors for early breastfeeding cessation. Primiparous and older mothers were less likely to cease breastfeeding within 6 weeks postpartum relative to multiparous and younger women, respectively (HR 0.54; CI 0.31–0.95, HR 0.94 CI 0.86–0.98; Table 3).
Table 3.
Cessation of any breastfeeding within six weeks: Cox proportional hazard model
| Variables | HR (95%CI) N=174 |
p-valuea |
|---|---|---|
| DMPA | 0.42 | |
| No | 1 (reference) | |
| Yes | 1.22 (0.75–1.98) | |
| Age (continuous) | 0.94 (0.86–0.98) | <0.01 |
| Race | 0.15 | |
| White | 1 (reference) | |
| Black | 0.61 (0.36–1.04) | |
| Other | 0.96 (0.48–1.92) | |
| Education | 0.96 | |
| ≤High school | 0.99 (0.55–1.77) | |
| >High school | 1 (reference) | |
| Parity | 0.03 | |
| Primiparous | 0.54 (0.31–0.95) | |
| Multiparous | 1 (reference) | |
| Parental Cohabitation | 0.58 | |
| No | 1.15 (0.70–1.91) | |
| Yes | 1 (reference) |
Abbreviations: HR, hazard ratio; CI, confidence interval; DMPA depot medroxyprogesterone.
p-values represent the type 3 analysis of effects Wald chi-square p-value.
Fig. 3 depicts breastfeeding cessation by DPMA status in intervals across the follow-up period. In Fig. 3A (birth – 2 weeks postpartum), there are no statistically significant differences in breastfeeding cessation by DPMA status during 2 weeks postpartum (p=0.24). The distribution for subjects treated with DPMA is consistently lower than that for women who did not receive DPMA, and while not statistically significant, these curves reinforce the biologic model. Neither curve approached 50% survival, and so it is not possible to report the overall or curve-specific median survival times. The directionality of the point estimate is consistent with the Kaplan-Meier distributions; however, this result is not statistically significant (HR 1.23; CI 0.66–2.28). The results are similar for Fig. 3B (>2 – 6 weeks postpartum) and represents only the women still breastfeeding at 2 weeks (not the observations that ceased breastfeeding within the first 2 weeks postpartum). The distribution of time to breastfeeding cessation for subjects in the DPMA group is consistently lower than that for the non-DPMA group, but this difference is not statistically significant (p=0.73). Again, the median survival time was not reached in either group and the HR is not statistically significant (HR 2.43; CI 0.84–7.01). The last interval is presented in Fig. 3C (>4 – 6 weeks postpartum). In this interval, the distribution of time to breastfeeding cessation in the unexposed is lower than that for the DPMA group (inversed distributions as compared to earlier intervals); this result is not statistically significant (p=0.21). The Kaplan-Meier curves are consistent with the protective hazard ratio (HR 0.55; CI 0.20–1.49), which is not statistically significant. There are insufficient events during this interval to report the median survival time for either the overall population or individual groups.
Fig. 3.

Cessation of any breastfeeding within intervals
A – Interval 1 (birth – 2 weeks; n=183).
B – Interval 2 (>2 – 6 weeks; n=100).
C – Interval 3 (>4 – 6 weeks; n=86).
4. Discussion
This study evaluated the effect of the administration of immediate postpartum DPMA (administered prior to hospital discharge) on breastfeeding cessation within 6 weeks. Previous research is methodologically weak, and did not include any stratified or multivariable analyses to account for potential confounding factors [4]. In our study sample, these data did not demonstrate a statistically significant increased risk in early breastfeeding cessation with DPMA use (HR 1.22; CI 0.75–1.98). However, while these results did not achieve statistical significance, the directionality of the point estimate is consistent with the biologic model. Additionally, our demonstrated Kaplan-Meier distributions are inverted relative to the only previous published Kaplan-Meier analysis [2].
These findings are not entirely consistent with previous research. Fundamental differences in study design, study population, exposure classification and analytic methods threatened the internal validity of previous results and may account for differences between our study and the existing, methodologically flawed evidence [4].
Guiloff et al. [1] concluded that breastfeeding cessation occurred less frequently in DPMA recipients (as measured by median breastfeeding duration), thus indicating that DPMA protects against early breastfeeding cessation; this result was statistically significant. Discrepancies between our results and those of Guiloff et al. are likely related to study design and analytic methods; Guiloff et al. conducted a clinical trial in Chile (where breastfeeding practices in the 1970s may be very different from a low-income United States inner city population in the 2000s); they subjectively identified historic controls and did not conduct any stratified or multivariable analyses [1].
Hannon et al. [2] reported no significant differences in early breastfeeding cessation between DPMA recipients and non-recipients; however the authors observed and discussed a non-statistically significant trend toward favorable breastfeeding outcomes among the exposed. Similarly, our study did not observe a statistically significant difference between groups, but the point estimate (>1.0) suggests DPMA may impact lactation. Several potential alternate explanations for these opposing results exist. First, inclusion criteria into this cohort required women to intend to continue breastfeeding at home. Second, agreement to prospective follow-up and presence of a home-phone number may have introduced selection bias. Regardless of DPMA status, study participants in Hannon and colleagues’ cohort may have been more determined and/or motivated to continue breastfeeding.
Halderman and Nelson [3] also reported no statistically significant differences in breastfeeding frequency, duration or exclusivity between non-hormonal and a progestin-only contraceptive groups. The progestin-only contraceptive group received either DPMA prior to hospital discharge or progestin-only pills (to commence upon postpartum return home). The authors observed an increased risk in early breastfeeding cessation in the hormonal group relative to the non-hormonal group, but these estimates did not achieve statistical significance [4]. A heterogenous exposure classification and failure to adjust for baseline differences between groups may explain these results [4]. Our study observed a similar non-statistically significant effect, but we utilized a DPMA-only exposure classification and adjusted for known risk factors and identified confounders in the multivariable model.
4.1. Limitations
Regarding recruitment, we did not systematically collect data about the reason for refusal to consent, and so it is not possible to provide proportions of specific reasons. The length and design of the survey may have contributed to missing outcome data. Items 17–19 (of 47 total items) assessed the breastfeeding outcomes. Including these items in the middle of the survey, while possibly masking participants to the exact purpose of the study, may have increased the amount of missing outcome data. Item 1 collected contact information in the event a participant was not able to complete the survey, but due to the transient nature of this population (changing/disconnecting phone numbers or not wanting to utilize mobile minutes for a research study), we had minimal success following up with participants to collect missing data. In turn, missing outcome and limited follow-up resulted in a higher proportion of early censored observations than expected, and although these observations were equally distributed between the exposure groups, this does minimize power for statistical tests and produce imprecise (wide) confidence intervals.
No estimates of the true incidence of postpartum DPMA use exist (regardless of breastfeeding status). Since no previous studies detected a statistically significant effect of DPMA on early breastfeeding cessation, we utilized a 20% detectable difference between groups. Arguably, this 20% effect size was too large, and we should have attempted to detect a smaller effect size (thus requiring more participants). Our post hoc power analysis indicated we had 79.4% power to detect a 20% difference between groups. The post hoc power analysis indicates the analyses included adequate observations in each group to statistically detect a 20% difference in breastfeeding cessation rates between groups if such a difference actually exists. Since all of the Kaplan-Meier curves and point estimates are consistent with our biologic model, and the directionality of the point estimates were as expected (HR >1), perhaps smaller (<20%) differences may exist. While 20% was considered clinically important, we were limited in sample size for the scope of this study.
Regarding the outcome assessment, we collected cessation of breastfeeding via self-report. If reported within 3 years postpartum, maternal self-report is a validated measure for cessation of any breastfeeding; corresponding measures of performance are reasonable: sensitivity (71.4%) and specificity (79.4%) [11,12].
These data are susceptible to a potential social desirability bias; participants were masked to the research hypotheses, and so we would not expect differences between exposure groups. If anything, this would bias our results toward the null by diluting any observed effect.
Lastly, of course, there is potential to observe a spurious relationship (such as our unexpected observed protective effect of primiparity on early breastfeeding cessation) due to unmeasured confounding. Given we had data on many variables of interest and that this is the first study to account for any confounding factors, these point estimates are a better representation of the effect than others previously published.
4.2. Strengths
We improved upon the methodologic rigor of previous research. First, unlike Halderman and Nelson [3], we utilized a homogenous exposure classification. Second, we applied statistical methods not previously used to evaluate this research question (e.g., assessment of confounding variables, multivariable analysis and interval survival analyses to test the biologic plausibility of the model). Third, we employed conservative statistic methods to identify potential confounders to detect statistical differences between groups (e.g., the Wilcoxon test and a low p-value cutoff for confounders associated with both exposure and outcome). Fourth, all observed Kaplan-Meier distributions and the crude and adjusted point estimates occurred in the direction consistent with our biologic model. Lastly, the Centers for Disease Control and Prevention identified postpartum progestin-only contraceptive use in breastfeeding women as a research gap; these findings begin to fill this acknowledged research gap [13].
Decisions regarding postpartum contraception require weighing the cost of unintended pregnancy, potential postpartum lost-to-follow-up with the maternal/infant benefits attributable to breastfeeding. Women may benefit from potential interventions designed to support maintenance of lactation by encouraging frequent breastfeeding, pumping or breast stimulation to maintain increased prolactin levels. Given the state of the evidence, it is unclear whether a causal effect does or does not exist. This study was not powered to detect a 14% increased risk in breastfeeding cessation; however, given the prevalence of birth and breastfeeding, further confirmatory studies are warranted. Specifically, subsequent research studies should be designed to detect smaller differences (<20%) and replicate our findings.
Acknowledgments
This research study was conducted in partial fulfillment of the requirements for a degree of Doctor of Philosophy, Division of Epidemiology, Department of Community and Preventive Medicine, University of Rochester School of Medicine and Dentistry.
References
- 1.Guiloff E, Ibarra-Polo A, Zanartu J, Toscanini C, Mischler TW, Gomez-Rogers C. Effect of contraception on lactation. Am J Obstet Gynecol. 1974;118:42–45. doi: 10.1016/s0002-9378(16)33643-2. [DOI] [PubMed] [Google Scholar]
- 2.Hannon PR, Duggan AK, Serwint JR, Vogelhut JW, Witter F, DeAngelis C. The influence of medroxyprogesterone on the duration of breast-feeding in mothers in an urban community. Arch Pediatr Adolesc Med. 1997;151:490–496. doi: 10.1001/archpedi.1997.02170420060010. [DOI] [PubMed] [Google Scholar]
- 3.Halderman LD, Nelson AL. Impact of early postpartum administration of progestin-only hormonal contraceptives compared with nonhormonal contraceptives on short-term breast-feeding patterns. Am J Obstet Gynecol. 2002;186:1250–1256. doi: 10.1067/mob.2002.123738. [DOI] [PubMed] [Google Scholar]
- 4.Brownell EA, Fernandez ID, Howard CR, et al. A systematic review of early postpartum medroxyprogesterone receipt and early breastfeeding cessation: evaluating the methodological rigor of the evidence. Breastfeed Med. 2012;7:10–18. doi: 10.1089/bfm.2011.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy KI, Short RV, Tully MR. Premature introduction of progestin-only contraceptive methods during lactation. Contraception. 1997;55:347–350. doi: 10.1016/s0010-7824(97)00042-5. [DOI] [PubMed] [Google Scholar]
- 6.Queenan JT. Contraception and breastfeeding. Clin Obstet Gynecol. 2004;47:734–739. doi: 10.1097/01.grf.0000139710.63598.b1. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Medical eligibility criteria for contraceptive use. 2009. [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. U.S medical eligibility criteria for contraceptive use. Morb Mortal Wkly Rep. 2010;59:1–86. [Google Scholar]
- 9.Andrade SE, Scott PE, Davis RL, et al. Validity of health plan and birth certificate data for pregnancy research. LID. 20120703 (1099-1557 (Electronic)) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: Wiley, John & Sons, Incorporated; 1999. [Google Scholar]
- 11.Li R, Scanlon KS, Serdula MK. The validity and reliability of maternal recall of breastfeeding practice. Nutr Rev. 2005;63:103–110. doi: 10.1111/j.1753-4887.2005.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 12.Chapman DJ, Perez-Escamilla R. Maternal perception of the onset of lactation is a valid, public health indicator of lactogenesis stage II. J Nutr. 2000;130:2972–2980. doi: 10.1093/jn/130.12.2972. [DOI] [PubMed] [Google Scholar]
- 13.Folger SG, Curtis KM, Tepper NK, Gaffield ME, Marchbanks PA. Guidance on medical eligibility criteria for contraceptive use: identification of research gaps. Contraception. 2010;82:113–118. doi: 10.1016/j.contraception.2010.02.015. [DOI] [PubMed] [Google Scholar]