Abstract
Background
Stress-response biological systems are altered in alcohol-dependent individuals and are reported to predict future relapse. This study was designed to assess neural disruptions in alcohol-dependent participants when exposed to a conditioned stimulus (CS) warning of the impending onset of a universal, nonpersonalized stressor.
Methods
Fifteen alcohol-dependent men abstinent for 3 to 5 weeks and 15 age- and race-similar healthy controls were studied. Anticipatory anxiety was induced by a CS paired with an uncertain, physically painful unconditioned stressor. Neural response was assessed using functional magnetic resonance imaging.
Results
Both groups experienced significant, similar levels of anticipatory anxiety in response to the high-threat relative to the low-threat CS. Whereas control participants markedly increased the blood oxygen level-dependent (BOLD) amplitude in cortical–limbic–striatal regions during the high-threat, relative to low-threat, stimulus, alcohol-dependent participants decreased BOLD amplitude in the pregenual anterior cingulate cortex (pgACC), medial prefrontal cortex (mPFC), medial orbitofrontal cortex, posterior cingulate cortex (PCC), bilateral parietal/occipital cortex, and right hippocampus. Alcohol-dependent participants significantly deactivated pgACC/mPFC and PCC clusters, relative to controls, during the high- versus low-threat stimulus. This difference was due to a decrease in %BOLD amplitude during the high-threat stimulus in the alcohol-dependent, but not the control, participants.
Conclusions
Alcohol-dependent men show cortical–limbic–striatal deactivation during anticipatory anxiety, particularly in regions associated with emotional regulation. These findings suggest a lack of engagement of affective regulatory mechanisms during high-stress situations in alcohol-dependent men.
Keywords: Anticipatory Anxiety, Functional Magnetic Resonance Imaging, Emotional Stress, Alcoholism, Striatal–Limbic–Cortical System
Disrupted functioning of biological stress-response systems is thought to underlie stress-induced drinking. Peripherally, both basal reactivity (Sinha et al., 2011) and provoked pituitary–adrenal reactivity to pharmacologic and behavioral stressors (Adinoff et al., 2005b,c) are altered in alcohol-dependent individuals. Centrally, multiple neurotransmitters and neuropeptides are mutually involved in stress reactivity and drinking behaviors, including serotonin, norepinephrine, opioids, corticotropin-releasing factor, neuropeptide Y, and GABA (Brady and Sinha, 2005). Persistent and excessive alcohol use, repeated withdrawal episodes, and recurrent environmental stressors (Heilig et al., 2010), overlaid upon genetic predisposing factors and early life trauma (Schepis et al., 2011), all appear relevant to the induction of stress sensitivity and resultant alcohol intake. Clinically, disruptions in peripheral stress-response systems have been associated with poorer treatment retention (Daughters et al., 2009) and increased relapse rates (Adinoff et al., 2005a; Sinha et al., 2011).
The experience and response to stress requires an organism to simultaneously assess, regulate, predict, and respond to anxiety- and/or fear-inducing stimuli. The dorsal–caudal regions of the anterior cingulate cortex (ACC) and medial prefrontal cortex (mPFC) appear integral to the appraisal and expression of emotions generated during stress, whereas the ventral–rostral regions regulate these emotions (Etkin et al., 2011). The expression of fear, including conditioned fear, induces amygdalar activation (Sehlmeyer et al., 2009), and both the amygdala and striatum track fearpredictive stimuli (Schiller et al., 2008). Both hippocampal reactivity and amygdalar reactivity are highly correlated with the hypothalamic–pituitary–adrenal (HPA) stress response (Pruessner et al., 2010). Connectivity within the default mode network (DMN), which surveys and assesses internal and external environments (Gusnard et al., 2001), shows decreased coupling in post-traumatic stress disorder (PTSD), a disorder notable for abnormal stress responses (Sripada et al., 2012). Disruptions in these various brain regions have been identified in alcohol-dependent individuals, although whether these alterations are related to stress-response disruptions is uncertain.
To further clarify and isolate the neurobiological alterations triggering stress-related drinking, the central processes occurring during stress require real-time assessment. A number of paradigms have been developed to evoke stress while simultaneously monitoring the central nervous system activity in humans. These paradigms include computerized mental arithmetic tasks coupled with negative social comments (Pruessner et al., 2010), verbal serial subtraction with increasing time speed demands (Wang et al., 2005), negative pictures from the International Affective Pictures System (Sarinopoulos et al., 2010), and script-guided imagery (Sinha and Li, 2007). These paradigms utilize a block design, in which stressors are administered for several minutes and compared to a nonstressed condition administered for the same amount of time. Despite the relevance of stress to alcohol use, only Seo and colleagues (2013) have assessed stress reactivity using functional magnetic resonance imaging (fMRI) in alcohol-dependent patients. In that study, stress-induced script-guided imagery paradigm evoked a hyperactive response during neutral-relaxing trials in the patients relative to healthy controls.
In order to further clarify the central processes involved in stress reactivity in alcohol dependence, we assessed the blood oxygen level-dependent (BOLD) response with fMRI during anticipatory anxiety in a group of abstinent, alcohol-dependent men and age- and race-matched healthy controls. In an attempt to remove, at least in part, the personalized interpretation and demands of a particular stressor, anxiety was induced by a conditioned stimulus (CS) paired with a painful unconditioned stimulus (US). This approach, adapted from Ploghaus and colleagues (1999), has the advantage of (i) eliciting anxiety in association with an otherwise neutral stimulus, thus disassociating the stimulus from any intrinsic, personalized interpretation; (ii) eliciting anxiety in response to the threat of a universal noxious stimulus (pain); (iii) using a US that can be adjusted for individual differences in pain response; (iv) using a mixed event-related/block design, providing the relatively rapid alteration of a high-anxiety and a low-anxiety CS; and (v) providing real-time, participant anxiety ratings during the presentation of each CS. We have previously reported that this paradigm induces marked striatal–limbic–cortical activation in healthy controls during the high-threat CS relative to a low-threat CS (Yang et al., 2012). In addition, the amplitude of BOLD signal change generally paralleled the subjective rating of anticipatory anxiety (Yang et al., 2012). To avoid the fluctuation in stress-response systems during early withdrawal (Adinoff et al., 1991), alcohol-dependent subjects were studied during 3 to 5 weeks of abstinence.
Materials and Methods
Participants
Fifteen alcohol-dependent men were recruited from patients requesting treatment for alcohol dependence at the VA North Texas Health Care System (VANTHCS) and Homeward Bound, Inc., in Dallas, TX. Alcohol-dependent subjects reported heavy drinking for at least 30 days prior to admission and at least a 4-year history of problematic drinking. Patients with active DSM-IV (American Psychiatric Association, 1994) Axis I non-substance-abuse psychiatric disorders, significant medical disorders, or a history of major head trauma were excluded. Exclusion criteria also included active use of medications that interfered with stress-response functioning (e.g., psychotropics, antihypertensives other than thiazides, hypoglycemic agents). Fifteen healthy male controls did not meet criteria for lifetime diagnosis of a substance use disorder (except nicotine) or any other active Axis I disorder. Controls had no more than 1 first or 2 second-degree relatives with substance dependence disorders. Other psychiatric/medical inclusion/exclusion criteria were the same as for alcohol-dependent participants. All subjects were screened for exclusion criteria for fMRI. As significant gender differences are apparent in the subjective and neural responses to stress (Wang et al., 2007), the interaction between stress and alcohol use (Chaplin et al., 2008), the heritability of alcohol use disorders (Hardie et al., 2008), and the rates and patterns of alcohol use, comorbid psychiatric disorders, and physiological stress responses in those with alcohol dependence (Adinoff et al., 2010; Greenfield et al., 2003), this relatively small exploratory study was limited to male subjects.
Clinical Assessments
Informed consents for both the University of Texas Southwestern Medical Center (UTSW) and VANTHCS were obtained after the study was fully explained. Subjects were financially compensated for their participation. Psychiatric and substance use disorders were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID)-Lifetime (First et al., 1996). Alcohol-dependent patients were detoxified from alcohol and housed in a residential treatment unit until studied at 3 to 5 weeks of abstinence. The Drinker Inventory of Consequences-Lifetime Version (DrInC) (Miller et al., 1995) was used to assess lifetime severity of alcohol-related problems. A timeline follow-back interview (Sobell and Sobell, 1978) was used to assess 3-month and lifetime drinking history (i.e., days drinking). Depression and anxiety were assessed using the Beck Depression Inventory (BDI) (Beck et al., 1979) and State Trait Anxiety Inventory (STAI) (Speilberger, 1971), respectively. Smoking was assessed with cigarettes per day.
fMRI Task
This task has previously been described in Yang and colleagues (2012). Sensory threshold calibration and fMRI studies were performed at the Meadows Diagnostic Imaging Center at UTSW. A computerized thermal stimulator (Pathway Pain & Sensory Evaluation System; Medoc Ltd., Haifa, Israel) administered stimuli through a nonmagnetic contact heat-evoked potential stimulator thermode secured to participants' left ventral inner forearm. Thermode temperature was increased from 32°C by 1.5°C/s (Heldestad et al., 2010), and participants clicked a mouse button when they felt that if the thermode got any hotter, it would burn them. The mean threshold temperature of 4 measurements was calculated.
The anticipatory anxiety paradigm was designed to induce anticipatory anxiety using a combination of pain (the CS warned of an impending painful US) and unpredictability (the participant did not know either whether a painful US would occur following any given CS or how long following the CS onset the US would occur). Two CSs were therefore presented: a high-threat CS (square) denoting duration uncertainty and the possibility of a painful US and a low-threat CS (triangle) denoting a predictable, nonpainful US. Visual stimuli were generated using Presentation software (version 10.0; Neurobehavioral Systems, Albany, CA) and projected with an LCD projector (NEC LT260, Tokyo, Japan) via a back projection system. For each trial, a triangle or square was presented as a CS and followed by an US: low heat (5°C below threshold) or high heat (1°C above threshold). A triangle (low-threat CS) signaled the impending and certain application of a low-heat US; a square (high-threat CS) signaled the impending uncertain application of either a low- or high-heat US. The CS remained on screen throughout the trial. The trial was separated into 2 periods: anticipatory anxiety (10 to 18 seconds) and heat pulse (8 seconds). During the first 4 seconds of the anticipatory anxiety period, the CS was accompanied by the question “How anxious are you now?” and a rating scale (1 through 4) (see Fig. 1). Participants were instructed to rate their anxiety regarding the impending heat stimulus during this 4-second period. The CS (without the rating question) then remained on the screen for an additional 6 to 14 seconds (pseudorandomized 6, 8, 10, 12, and 14 seconds) for the duration of the anticipatory anxiety period. During the subsequent (and final) 8 seconds of each trial (heat pulse period), the heat stimulus increased from a baseline temperature of 32°C by 10°C/s to the low or high temperature (using a ramp-and-hold program), remained at the peak for 3 seconds, and then returned to baseline temperature. Forty trials, 20 squares (10 low heat and 10 high heat) and 20 triangles (all low heat), were presented over 23 minutes. Trial order was pseudorandomized with the stipulation that neither triangles nor squares were presented more than twice consecutively. A pseudorandomized interstimulus interval consisted of a circle shown between each trial for 9, 10, or 11 seconds. A 90-second rest period separated the paradigm into halves during which “break” was presented on the screen. Nicotine-dependent subjects were allowed a cigarette 1 hour before study session and abstained until after completing the session.
Fig. 1.
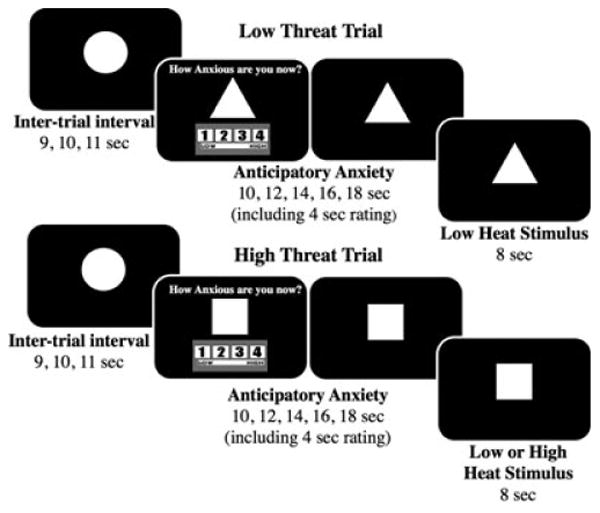
Anticipatory anxiety paradigm. Presentation of the triangle conditioned stimulus (CS) predicted a warm unconditioned stimulus (US), whereas presentation of a square CS predicted either a hot or a warm US. A circle was presented for each intertrial interval. From Yang and colleagues (2012).
To familiarize subjects with the paradigm and the MR scanner environment, a mock scan was performed on the same day as threshold testing. Participants were instructed that the triangle signaled the onset of a (predictable) warm stimulus and a square signaled the onset of either a warm or a hot (unpredictable) stimulus. The high-heat stimulus was set 2°C lower than threshold for the mock scan. The task was presented for 10 minutes. To avoid demand characteristics, there was no attempt to encourage or advise subjects to rate the square (high-threat) CS differently than triangle (low-threat) CS.
Scans were obtained on a Siemens 3T TIM Trio scanner (Siemens AG, Munich & Berlin, Germany), equipped with 12-channel receiver array head coil. fMRI scans were obtained using gradient echo planar imaging: TR = 2,000 ms, TE = 20 ms, flip angle = 90 degrees, base resolution = 64 × 64, voxel size = 3.3 × 3.3 × 3.0 mm, field of view (FOV) = 210 mm, A-P-phase encode, bandwidth = 2,442 Hz/pixel. After 3 discarded acquisitions to establish magnetization equilibrium, 690 image volumes of 40 interleaved sagittal slices with 3-mm slice thickness were obtained (whole brain). Anatomical scans using a T1-weighted multiplanar reformatted MPRAGE sequence (TR = 2,250 ms, TI = 900 ms, TE = 2.9 ms, flip angle = 9 degrees, base resolution = 256 × 256, FOV = 230 ms, voxel size = 0.9 × 0.9 × 1.1 mm) facilitated localization and coregistration of fMRI data.
Statistics
Descriptive statistics quantified drinking characteristics of the alcohol-dependent group, including years of problem drinking and drinks per day in the past 90 days and lifetime. Groups were compared using 2-sample t- or χ2 tests. Anticipatory anxiety ratings for high- and low-anxiety CSs were averaged across the 20 ratings. Between-group temperature threshold and anxiety ratings were compared by 2-sample unpaired t-tests.
The fMRI data were analyzed using FMRI Expert Analysis Tool, version 5.98, a tool of FMRIB's Software Library (FSL, www.fmrib.ox.ac.uk/fsl). Preprocessing included motion correction, slice-timing correction, spatial smoothing with a 5-mm full-width, half-maximum Gaussian filter, intensity normalization using a grand mean scaling factor, and high-pass temporal filtering (sigma = 30.0 seconds) to remove low-frequency drift. Brain extraction and registration to high-resolution and template (Montreal Neurological Institute [MNI] 152) images were carried out by Brain Extraction Tool (Smith, 2002) and FMRIB's Linear Image Registration Tool (Jenkinson and Smith, 2001; Jenkinson et al., 2002), respectively.
Time-series statistical analysis was performed by FMRIB's improved linear model, in which a general linear model framework of the stimulus-onset types as explanatory variables, convolved with a hemodynamic response function with time derivatives, was used to fit the time-series data of each voxel. Two regressors were defined and analyzed: low and high anticipatory anxiety (triangle CS and square CS). Individual contrast images were computed for the anticipatory anxiety contrast (square CS vs. triangle CS), and the group-by-condition interaction was assessed by group-level t-test of condition differences. Group-level inference was at the cluster level, prethresholded, and constrained by a gray matter mask of 40% probability based on the avg152T1 (average of the T1W anatomical images spatially normalized to the MNI1532 template), using a cluster-defining threshold of Z = 2.3 and an extent threshold corresponding to a p-value <0.05 based on Gaussian random field theory to correct for multiple comparisons (Worsley, 2001). Threshold temperature, smoking, BDI, education, and STAI were used as covariates in the group analysis to remove the effects of unmatched variables in alcohol-dependent and healthy control participants.
Relevant functionally derived cluster-level results were summarized by averaging %BOLD amplitudes over the voxels within the identified cluster using the Featquery tool of FSL. %BOLD amplitudes of a functionally derived cluster within specific anatomically identified regions of interest were summarized based on the FSL atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html for posterior cingulate cortex [PCC] and hippocampus) and an in-house template (for ACC, Bush et al., 2000; for striatum, Gopinath et al., 2011). Regression analyses were also conducted to explore whether abstinent days, past 90 days drinking, or lifetime drinks were correlated with the BOLD response in alcohol-dependent subjects.
Results
Participant Characteristics
Control and alcohol-dependent subjects did not significantly differ in race or age (Table 1). The alcohol-dependent subjects were more likely to smoke cigarettes and had higher BDI and STAI scores relative to controls and had lower education than control subjects. One patient met DSM-IV criteria for PTSD.
Table 1. Demographic Characteristics of Alcohol-Dependent Participants and Healthy Controls.
| Controls (n = 15) | Alcohol dependent (n = 15) | t or χ2 | df | |
|---|---|---|---|---|
| Age (years) | 45.5 ± 8.5 | 42.3 ± 7.1 | 1.12 | 28 |
| Race | 0.97 | 2 | ||
| White | 12 | 12 | ||
| Black | 3 | 3 | ||
| Hispanic | 0 | 1 | ||
| Handedness | 1.97 | 2 | ||
| Right | 12 | 12 | ||
| Left | 1 | 0 | ||
| Ambidextrous | 1 | 3 | ||
| Education (years) | 15.2 ±1.5 | 11.4 ± 1.2 | 7.64 | 28 |
| Cigarettes/d | 1.2 ± 4.6 | 14.1 ± 11.9 | 4.25 | 20 |
| BDI | 2.3 ± 2.9 | 7.3 ± 4.3 | 3.77 | 28 |
| STAI | 26.9 ± 7.7 | 34.7 ± 8.3 | 2.67 | 28 |
| DrInC | 8.6 ± 8.0 | 38.5 ± 4.7 | 12.34 | 28 |
| Years of problem drinking | 20.3 ± 7.2 | |||
| Drinks in 90 days | 1,312.9 ± 670.7 | |||
| Lifetime drinking | 102,410.5 ± 84,673.6 | |||
| Days abstinent | 25.3 ± 5.2 |
BDI, Beck Depression Inventory; STAI, State Trait Anxiety Inventory; DrInC, Drinker Inventory of Consequences-Lifetime Version.
Data are mean ± SD. Comparisons between groups were by t-test or χ2.
Temperature Threshold and Anxiety Ratings
Threshold temperatures were slightly higher in alcohol-dependent relative to control subjects (mean ± SD: 46.8 ± 2.3 vs. 45.4 ± 1.4°C, respectively; t = 2.10, df = 28, p = 0.05). High-threat ratings were significantly higher in both the control and alcohol-dependent participants relative to the low-threat ratings (controls: 2.7 ± 0.79 vs. 1.1 ± 0.20, alcohol-dependent participants: 2.8 ± 0.85 vs. 1.3 ± 0.41; t = 7.7, p < 0.001). Using repeated-measures ANOVA, there were not significant group differences across anxiety ratings during either the low- (p = 0.284) or high-threat stimulus (p = 0.492) (Fig. 2, left and middle panels). Anxiety ratings for both high and low heat remained stable in both groups throughout the task (Fig. 2, right panel).
Fig. 2.
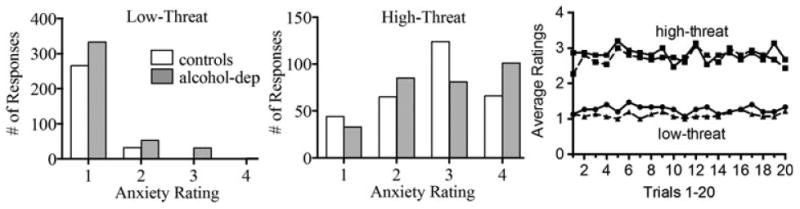
Distribution of anxiety ratings to low (left panel)- and high (middle panel)-threat conditioned stimulus in healthy control and alcohol-dependent participants. The right panel shows group mean high- and low-threat ratings over the 20 trials for control (dashed line) and alcohol-dependent (solid line) participants.
BOLD Response to High Versus Low Anticipatory Anxiety
Healthy Control Participants
The high- relative to low-threat CS elicited large differences in BOLD signal in bilateral insula, caudate, putamen, pallidum, thalamus, lateral orbital frontal vortex, and superior frontal cortex (SFC) as well as in the dorsal ACC (dACC), brainstem, occipital cortex, and cerebellum. Left precuneus and left lateral occipital cortex showed a decreased BOLD signal in controls (Table 2, Fig. 3A).
Table 2. MNI Coordinates of BOLD Response to High- Minus Low-Treat Conditioned Stimulus.
| Identified region | MNI coordinates (mm) | Ke (voxels) | Volume (mm3) | Z maximum* | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| X | Y | Z | |||||
| Healthy controls | |||||||
| Activation | |||||||
| Cluster 1 | L/R occipital cortex | 12 | −90 | −8 | 23,301 | 186,408 | 4.82 |
| L/R cerebellum | −6 | −78 | −22 | 3.76 | |||
| Cluster 2 | L/R superior frontal cortex | 4 | 32 | 52 | 4,763 | 38,104 | 4.63 |
| L/R dorsal anterior cingulate cortex | 0 | 38 | 36 | 4.12 | |||
| L/R pregenual anterior cingulate cortex | 4 | 40 | 22 | 3.48 | |||
| Cluster 3 | R caudate | 10 | 8 | 10 | 3,316 | 26,528 | 4.44 |
| L/R thalamus | 4 | −18 | 10 | 4.28 | |||
| L/R brain stem | −4 | −30 | −4 | 4.10 | |||
| L caudate | −10 | 6 | 12 | 4.04 | |||
| R pallidum | 18 | 4 | −2 | 3.93 | |||
| L pallidum | −12 | 4 | −2 | 2.74 | |||
| R putamen | 20 | 4 | −4 | 3.64 | |||
| L putamen | −24 | 12 | −8 | 3.01 | |||
| Cluster 4 | L lateral orbital frontal cortex | −36 | 30 | −2 | 1,353 | 10,824 | 4.43 |
| L insula | −44 | 6 | −4 | 3.36 | |||
| Cluster 5 | R lateral orbital frontal cortex | 40 | 24 | −8 | 784 | 6,272 | 4.30 |
| R insula | 40 | 8 | −10 | 2.95 | |||
| Deactivation | |||||||
| Cluster 1 | L precuneus cortex | −16 | 60 | 12 | 690 | 5,520 | 3.96 |
| Cluster 2 | L lateral occipital cortex | −38 | 84 | 28 | 517 | 4,136 | 3.41 |
| Alcohol dependent | |||||||
| Activation | |||||||
| Cluster 1 | L/R superior frontal cortex | 4 | 6 | 62 | 892 | 7,136 | 3.86 |
| Deactivation | |||||||
| Cluster 1 | L/R precuneus cortex | 10 | −54 | 16 | 3,115 | 24,920 | 4.68 |
| L/R posterior cingulate cortex | −2 | −50 | 28 | 4.38 | |||
| Cluster 2 | L lateral occipital cortex | −36 | −86 | −4 | 1,973 | 15,784 | 4.56 |
| Cluster 3 | L/R medial orbital frontal cortex | 2 | 6 | −14 | 1,912 | 15,296 | 4.09 |
| R pregenual anterior cingulate cortex | 4 | 52 | 8 | 4.00 | |||
| L/R medial prefrontal cortex | 0 | 56 | −18 | 3.58 | |||
| Cluster 4 | R lateral occipital cortex | 46 | −78 | −4 | 908 | 7,264 | 4.42 |
| Cluster 5 | R hippocampus | 32 | −18 | −22 | 773 | 6,184 | 3.82 |
| Cluster 6 | R lateral occipital cortex | 30 | −80 | 32 | 760 | 6,080 | 3.69 |
| Alcohol dependent < Healthy controls | |||||||
| Cluster 1 | L/R pregenual anterior cingulate cortex | 4 | 52 | 8 | 1,527 | 12,216 | 5.58 |
| R medial prefrontal cortex | 8 | 60 | 0 | 3.12 | |||
| Cluster 2 | L lateral occipital cortex | −38 | −76 | −10 | 676 | 5,408 | 2.51 |
| Cluster 3 | L/R posterior cingulate cortex | 6 | −44 | 32 | 529 | 4,232 | 3.42 |
MNI, Montreal Neurological Institute; BOLD, blood oxygen level-dependent; L, left; R, right.
Corrected p < 0.05.
Fig. 3.
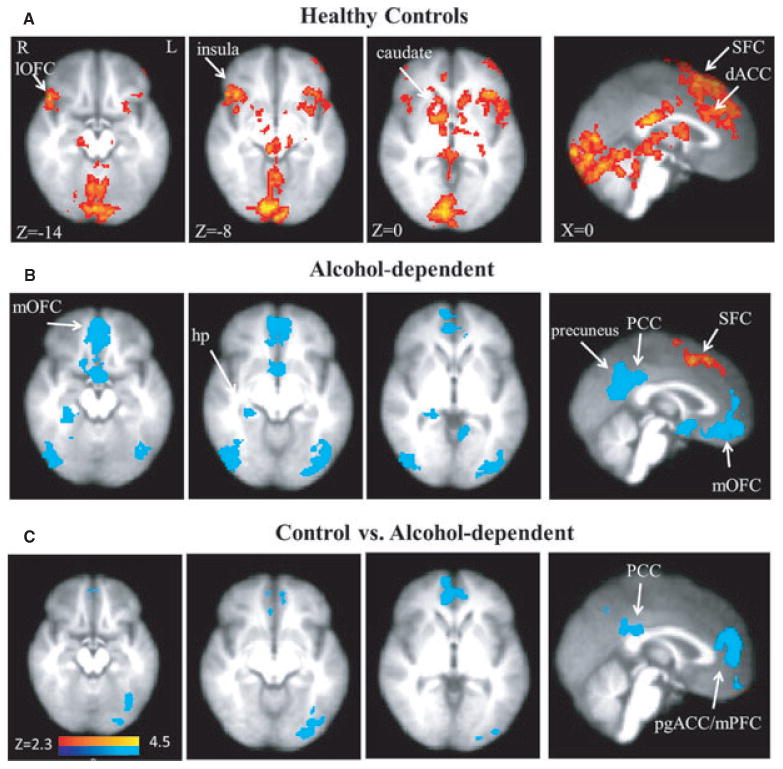
(A) Increased striatal–limbic–cortical BOLD activation (red clusters) induced by high- versus low-threat conditioned stimulus (CS) in healthy control participants (from Yang et al., 2012). (B) Increased BOLD activation induced by high- versus low-threat CS in alcohol-dependent participants was limited to the SFC. In contrast, widespread decreased BOLD amplitude (blue clusters) was observed. (C) BOLD amplitude was lower in the alcohol-dependent participants relative to the control participants during the high- versus low-threat CS. BOLD, blood oxygen level-dependent; R, right; L, left; pgACC, pregenual anterior cingulate cortex; dACC, dorsal anterior cingulate cortex; mPFC, medial prefrontal cortex; hp, hippocampus; SFC, superior frontal cortex; PCC, posterior cingulate cortex; lOFC, lateral orbitofrontal cortex; mOFC, medial orbitofrontal cortex.
Alcohol-Dependent Participants
Only bilateral SFC showed an increased BOLD activity during the high- relative to low-threat CS (Table 2, Fig. 3B). However, decreased BOLD responses in bilateral medial OFC (Brodmann areas [BA] 11 and 12), precuneus, PCC, right and left lateral occipital/parietal cortex, mPFC, and pregenual ACC (pgACC), and right hippocampus were observed (Table 2, Fig. 3B).
Control Versus Alcohol-Dependent Participants
Compared with controls, alcohol-dependent participants showed a decreased BOLD response in clusters containing the bilateral pgACC and right mPFC, bilateral PCC, and left lateral occipital cortex in the high- versus low-threat anxiety CS contrast (Table 2 and Fig. 3C). %BOLD amplitude in each cluster (Fig. 4) showed that the pgACC/mPFC cluster (mean ± SD, controls: 0.039 ± 0.055, alcohol-dependent participants: −0.074 ± 0.115; t = 3.42, df = 28, p = 0.002) and the PCC cluster (controls: 0.014 ± 0.047, alcohol-dependent participants: −0.102 ± 0.094; t = 4.25, df = 28, p = 0.0004) group differences were primarily driven by decreased responses in alcohol-dependent participants during high- versus low-threat CS. The %BOLD change was significantly correlated between the 2 regions (r = 0.66, df = 28, p < 0.001). %BOLD amplitude in these 2 regions did not significantly correlate with past 90 days drinking or lifetime drinking measures.
Fig. 4.
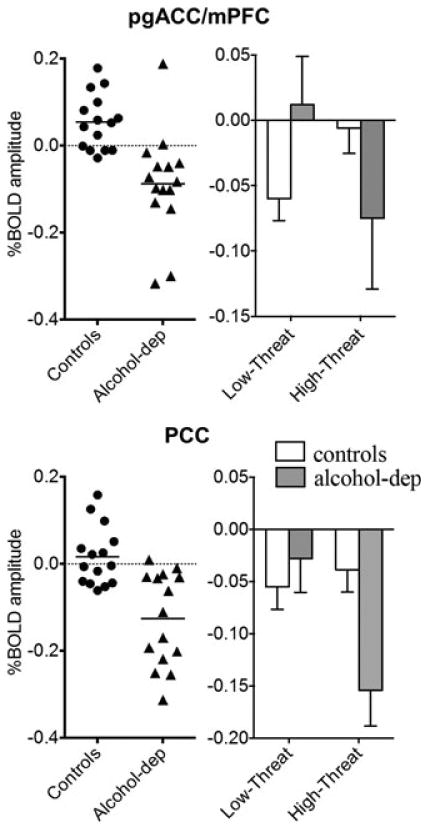
Left column:%BOLD amplitude in individual subjects during high-threat (square) minus low-threat (triangle) conditioned stimulus (CS) in the pregenual anterior cingulate cortex (pgACC)/medial prefrontal cortex (mPFC) and posterior cingulate cortex (PCC). Circles = controls. Triangles = alcohol-dependent group. Right column: %BOLD amplitude (mean ± SEM), relative to baseline (circle), during low-threat CS and high-threat CS. White bar = controls. Dark gray bar = alcohol-dependent group. BOLD, blood oxygen level-dependent.
While the decreased pgACC/mPFC and PCC BOLD amplitude in the alcohol-dependent participants during high-relative to low-threat was presumed to be the result of a decreased BOLD response to the high-threat stimulus, it may have been due to an increased activation in the patient group during the low-threat condition. To assist in determining the origin of this finding, the %BOLD amplitude in these 2 regions during both low- and high-threat was assessed in both groups relative to the baseline (circle) condition. During the low-threat CS, controls showed robust decreases in pgACC/mPFC and PCC regions relative to baseline (Fig. 4). In contrast, the alcohol-dependent subjects showed either a small increase (in the pgACC/mPFC) or small decrease (in the PCC) during the low-threat stimulus. During the high-threat CS, controls showed either no change (pgACC/mPFC) or small decrease (PCC), whereas the patient group experienced a dramatic decrease in both regions. Thus, group differences in the high- versus low-threat CS were primarily a result of a decrease in %BOLD amplitude in the control group during the low-threat stimulus and a decrease in % BOLD amplitude in the alcohol-dependent group during the high-threat stimulus.
Although subjective measures of anxiety were similar between groups and the temperature threshold was higher in alcohol-dependent subjects relative to controls, we nevertheless considered that the patient group may experience the high-threat US as less painful (and subsequently less threatening) than the control group. A contrast was therefore performed between the high-heat and low-heat US following high-threat stimulus. The only group difference was an increased BOLD activation in alcohol-dependent relative to the control participants in a cluster comprising the left posterior insula (x = −34, y = −10, z = 16, maximum Z = 3.22) and Heschl's gyrus (x = −54, y = −18, z = 8, maximum Z = 4.11) and a cluster in the postcentral gyrus (x = −52, y = −18, z = 38, maximum Z = 3.54) (Fig. 5).
Fig. 5.
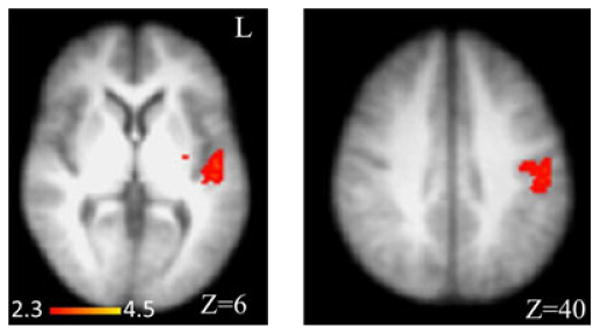
Increased blood oxygen level-dependent (BOLD) amplitude in alcohol-dependent compared to control participants during high- versus low-heat unconditioned stimulus (US) during the high-threat conditioned stimulus (square). Regions with increased BOLD amplitude for high- versus low-heat US in alcohol-dependent relative to control participants included the left posterior insula/Heschl's gyrus and postcentral gyrus. Montreal Neurological Institute coordinate (z-axis) is noted at the bottom of each image. L, left.
Discussion
During experimentally induced anticipatory anxiety, healthy control participants demonstrated a significant positive BOLD activation in cortical–striatal–limbic areas, whereas alcohol-dependent participants did not. Relative to controls, alcohol-dependent participants showed a significantly decreased activation in the pgACC/mPFC and PCC, in particular, during a high-threat compared to a low-threat stimulus. These differences were a result of significantly greater deactivation in the patient group than in controls during the high-threat stimulus, as well as more positive (less negative) BOLD amplitude in the patient group than in controls during the low-threat stimulus, indicating inappropriate disengagement during a stressful event and overengagement at rest or in low-stress conditions.
pgACC BOLD response is increased during emotional conflict resolution (Etkin et al., 2011), threat-related distractors (Bishop et al., 2004), and the anticipation of electrical shocks (Straube et al., 2009). Similar deficits in the pgACC response to emotional stimuli and/or conflict observed in the present study also have been reported in patients with PTSD (Kim et al., 2008; Shin et al., 2001) and generalized anxiety disorder (Etkin et al., 2011). Seo and colleagues (2013) have recently reported that a similar prefrontal region shows an increased activation in alcohol-dependent participants, relative to controls, during a relaxing task and a decreased activation during a stressful task. Hyper- and hypoactivation, respectively, were predictive of subsequent relapse severity. This region, coupled with other dorsal–caudal regions of the ACC and mPFC, has been posited to contribute to the appraisal and expression of negative emotion (Etkin et al., 2011). pgACC/mPFC deactivation in response to high-threat stimuli, as observed in our alcohol-dependent group, might therefore portend difficulty in regulating—and inhibiting— stimulus-induced anxiety and/or craving. Our findings showing that pgACC/mPFC is deactivated during low-threat in controls but deactivated during high-threat in alcohol-dependent participants, coupled with the work of Seo and colleagues (2013) noted above, suggest that the pgACC/mPFC may be overengaged at rest in alcohol-dependent individuals and then inappropriately disengaged during a stressful event. These findings are consistent with those observed in HPA axis responsivity, where heightened basal cortisol concentrations (Sinha et al., 2011) and suppressed stress-induced ACTH or cortisol concentrations are associated with increased relapse following the treatment (Adinoff et al., 2005a).
During a psychosocial stressor, Pruessner and colleagues (2008) observed a decrease in pgACC amplitude in nonstress responders (assessed by cortisol reactivity) relative to responders. In our 2 groups (controls and alcohol-dependent participants), however, subjective (anxiety ratings) and other neural responses of stress reactivity did not differ. For example, the SFC response was evident in both the alcohol-dependent and controls participants and did not significantly differ between the 2 groups. The SFC has been posited as important for the appraisal and expression of negative emotions, in contrast to the relevance of the dACC/mPFC for affect regulation (Etkin et al., 2011). Thus, alcohol-dependent subjects show decreased pgACC/mPFC activation during the high-versus low-threat CS despite a preservation of subjective anxiety and neural SFC responses to threat. Whereas appropriate SFC activation may have enabled subjective anxiety to be appropriately experienced, the mismatch between pgACC/mPFC activation and subjective anxiety ratings may reflect a maladaptive mechanism, either as a pre-existing vulnerability or in response to chronic alcohol use, for regulating or reducing anxiety. This dysregulation may, at least in part, portend difficulties in alcohol-dependent patients responding to high-stress situations, often resulting in relapse (Schepis et al., 2011; Sinha et al., 2011).
In contrast to the muted pgACC/mPFC response to high-relative to low-threat CS in the alcohol-dependent group, left posterior insular activity was increased in the alcohol-dependent group during the high-heat US. Posterior insula activation reflects the neural response to physical pain (Craig, 2003). This finding suggests that the neural experience of pain was heightened in the patient population, possibly due to decreased neural affect regulation (mediated through the pgACC/mPFC), or simply due to the higher temperature administered to this group as a result of the higher threshold for pain that they endorsed. Increased neural response to pain could also have been a consequence of subclinical alcohol-induced peripheral neuropathy (Chopra and Tiwari, 2012), although this explanation is inconsistent with the attenuated pain reactivity observed in our patient population during threshold testing. An alternate explanation, accounting for both the higher temperature threshold and higher posterior insular response, is informed by a study assessing pain sensitivity following fear (induced by 3 brief shocks) and anxiety (induced by the threat of shock) (Rhudy and Meagher, 2000). The fear condition used in Rhudy and Meagher (2000) shares similarities with our threshold testing, where threshold was assessed in 4 consecutive trials, and the anxiety condition was similar to our high-threat condition. Due to putative dysregulation between the insular cortex and mPFC (Verdejo-Garcia et al., 2012), both fear and anxiety responses may have been exaggerated in our patient population: fear resulting in a greater decrease in peripheral pain responsiveness (consistent with our findings of increased temperature threshold in the alcohol-dependent group) and anxiety (high-threat) producing an increase in the neural response to pain (consistent with our findings of accentuated posterior insular activation during the high-heat stimulus in the alcohol-dependent group).
The decrease in PCC BOLD response in the alcohol-dependent group, relative to the controls, was unexpected. The PCC (as well as the mPFC) is a key region of the DMN. The DMN is active when an individual is at rest and alert (Raichle et al., 2001). The PCC BOLD decrease during high-, relative to low-, threat indicates that alcohol-dependent patients markedly suppress DMN activity in the presence of affectively powerful (e.g., high-threat) stimuli compared to controls. As noted earlier, this finding may also suggest that the DMN is hypervigilant during rest in the patient group and inappropriately lessens vigilance when threatened.
Although amygdalar activation is often observed during high-threat stimuli, this is not always the case. A review by Sehlmeyer and colleagues (2009) reported that amygdalar activation was not evident in 19 of 44 studies utilizing a “delay conditioning” of fear (where the CS overlaps or is immediately followed by the US, as was utilized in our paradigm). As habituation of amygdalar activation often occurs, amygdala activation may not be detected in a paradigm such as ours, where the CS is repeatedly administered. Thus, a condition-by-time interaction may be required to determine amygdala involvement. In delay conditioning paradigms, for example, Morris and colleagues (2001) found that amygdalar activity lessened across session in parallel with skin conductance and LaBar and colleagues (1998) observed amygdalar activation during early, but not late, stimulus presentation. Although signal loss from magnetic susceptibility gradients can cause signal loss in amygdala and compromise ability to detect the activation of amygdala with BOLD fMRI, we have detected robust amygdalar activation in other paradigms with the same or very similar MR parameters (Gopinath et al., 2012).
Strengths of this study included the elimination of potential confounds of comorbid nonsubstance use disorders and psychotropic medications and the assessment of alcohol-dependent subjects followed at least 3 weeks of abstinence. The task was successful in inducing a strong (and similar magnitude) anticipatory anxiety response in both groups. Among the weaknesses, groups were not matched for threshold temperature, smoking, BDI, and STAI. However, activation maps of intra- and intergroup differences did not differ when these covariates were considered. The lack of women in the study limits the interpretation of our findings to men.
With respect to the fMRI paradigm, we utilized an uncertain onset as a key attribute of our high-threat CS. Thus, our paradigm was unable to assign possible contributions of distal versus proximal threats upon brain activity (Mobbs et al., 2007). Second, only subjective anxiety was recorded during the anticipatory anxiety period. Objective indicators of anxiety previously used to model anxiety and fMRI response, such as heart rate or galvanic skin conductance (Somerville et al., 2010), were not obtained. Third, a combined epoch, the rating and anticipatory anxiety period, was used to assess the neural response during anticipatory anxiety. As the anticipatory anxiety period always immediately followed the rating period and subjects were considered to be anticipating the upcoming stimulus during the entire epoch, these events were combined into a single epoch variable to avoid the problem of colinearity and improve the paradigm's design. Although the rating period also included a brief cognitive appraisal of anxiety accompanied by motor movement, these neural signals would be expected to be similar on every trial and would thereby be excluded from the contrasts. The shapes (triangle, square) were not counterbalanced between the high- and low-stress CS. Although previous research suggests that a triangle might be interpreted as more threatening than a square (Larson et al., 2009), this was not found to be the case in our study. Finally, although subjects were trained in the paradigm prior to the fMRI session, the endorsement of variable levels of anxiety during the high-threat CS supports the absence of training-induced demand characteristics (i.e., subjects did not recognize or learn that they were expected to endorse high subjective ratings for the high-threat CS).
In summary, the deactivation of pgACC/mPFC in response to anticipatory anxiety in alcohol-dependent participants may indicate a disruption of affect regulation. Further research should explore whether these disturbances reflect hypervigilance of the DMN during rest and disengagement during stressful events, whether these findings are specific to alcohol dependence or are evident in other psychiatric disorders (e.g., other substance use disorders, depression, PTSD), and the long-term implications upon disease severity and relapse.
Acknowledgments
This work was funded by INIAStress U01AA013641, U01AA16668, UL1TR000451, and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences Research and Development. We are grateful to Larry Steier and Victoria Vescovo for their skilled assistance of fMRI scanning and the staff of the Substance Abuse Team at the VA North Texas Health Care System and Homeward Bound, Inc., for their support in the screening and recruitment of study subjects.
References
- Adinoff B, Best SE, Ye W, Williams MJ, Iranmenesh A. Adrenocortical and pituitary glucocorticoid feedback in abstinent alcohol-dependent women. Alcohol Clin Exp Res. 2010;34:915–924. doi: 10.1111/j.1530-0277.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Junghanns K, Kiefer F, Krishnan-Sarin S. Suppression of the HPA axis stress-response: implications for relapse. Alcohol Clin Exp Res. 2005a;29:1351–1355. doi: 10.1097/01.ALC.0000176356.97620.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 1: adrenocortical and pituitary glucocorticoid responsiveness. Alcohol Clin Exp Res. 2005b;29:517–527. doi: 10.1097/01.ALC.0000158940.05529.0A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Krebaum SR, Chandler PA, Ye W, Brown MB, Williams MJ. Dissection of hypothalamic-pituitary-adrenal axis pathology in 1-month-abstinent alcohol-dependent men, part 2: response to ovine corticotropin-releasing factor and naloxone. Alcohol Clin Exp Res. 2005c;29:528–537. doi: 10.1097/01.ALC.0000158939.25531.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adinoff B, Risher-Flowers D, De Jong J, Ravitz B, Bone GHA, Nutt DJ, Roehrich L, Martin PR, Linnoila M. Disturbances of hypothalamic-pituitary-adrenal axis functioning during ethanol withdrawal in six men. Am J Psychiatry. 1991;148:1023–1025. doi: 10.1176/ajp.148.8.1023. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1979;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nat Neurosci. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Hong K, Bergquist K, Sinha R. Gender differences in response to emotional stress: an assessment across subjective, behavioral, and physiological domains and relations to alcohol craving. Alcohol Clin Exp Res. 2008;32:1242–1250. doi: 10.1111/j.1530-0277.2008.00679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. 2012;73:348–362. doi: 10.1111/j.1365-2125.2011.04111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–505. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Daughters SB, Richards JM, Gorka SM, Sinha R. HPA axis response to psychological stress and treatment retention in residential substance abuse treatment: a prospective study. Drug Alcohol Depend. 2009;105:202–208. doi: 10.1016/j.drugalcdep.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MH, Spitzer RL, Gibbon M, Williams JBW. Biometrics Research Department. New York State Psychiatric Institute; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient ed (SCID-I/P, Version 2.0) [Google Scholar]
- Gopinath K, Gandhi P, Goyal A, Jiang L, Fang Y, Ouyang L, Ganji S, Buhner D, Ringe W, Spence J, Biggs M, Briggs R, Haley R. FMRI reveals abnormal central processing of sensory and pain stimuli in ill Gulf War veterans. Neurotoxicology. 2012;33:261–271. doi: 10.1016/j.neuro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath K, Ringe W, Goyal A, Carter K, Dinse HR, Haley R, Briggs R. Striatal functional connectivity networks are modulated by fMRI resting state conditions. Neuroimage. 2011;54:380–388. doi: 10.1016/j.neuroimage.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Greenfield SF, Manwani SG, Nargiso JE. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie TL, Moss HB, Lynch KG. Sex differences in the heritability of alcohol problems. Am J Addict. 2008;17:319–327. doi: 10.1080/10550490802139010. [DOI] [PubMed] [Google Scholar]
- Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldestad V, Linder J, Sellersjo L, Nordh E. Reproducibility and influence of test modality order on thermal perception and thermal pain thresholds in quantitative sensory testing. Clin Neurophysiol. 2010;121:1878–1885. doi: 10.1016/j.clinph.2010.03.055. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Chey J, Chung A, Bae S, Khang H, Ham B, Yoon SJ, Jeong DU, Lyoo IK. Diminished rostral anterior cingulate activity in response to threat-related events in posttraumatic stress disorder. J Psychiatr Res. 2008;42:268–277. doi: 10.1016/j.jpsychires.2007.02.003. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Larson CL, Aronoff J, Sarinopoulos IC, Zhu DC. Recognizing threat: a simple geometric shape activates neural circuitry for threat detection. J Cogn Neurosci. 2009;21:1523–1535. doi: 10.1162/jocn.2009.21111. [DOI] [PubMed] [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse. National Institutes of Health; Rockville, MD: 1995. [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, Dolan RJ, Frith CD. When fear is near: threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13:1044–1052. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Ploghaus A, Tracey I, Gati JS, Clare S, Menon RS, Matthews PM, Rawlins JN. Dissociating pain from its anticipation in the human brain. Science. 1999;284:1979–1981. doi: 10.1126/science.284.5422.1979. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Khalili-Mahani N, Engert V, Pruessner M, Buss C, Renwick R, Dagher A, Meaney MJ, Lupien S. Deactivation of the limbic system during acute psychosocial stress: evidence from positron emission tomography and functional magnetic resonance imaging studies. Biol Psychiatry. 2008;63:234–240. doi: 10.1016/j.biopsych.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Dedovic K, Pruessner M, Lord C, Buss C, Collins L, Dagher A, Lupien SJ. Stress regulation in the central nervous system: evidence from structural and functional neuroimaging studies in human populations—2008 Curt Richter Award Winner. Psychoneuroendocrinology. 2010;35:179–191. doi: 10.1016/j.psyneuen.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84:65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Sarinopoulos I, Grupe DW, Mackiewicz KL, Herrington JD, Lor M, Steege EE, Nitschke JB. Uncertainty during anticipation modulates neural responses to aversion in human insula and amygdala. Cereb Cortex. 2010;20:929–940. doi: 10.1093/cercor/bhp155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepis TS, Rao U, Yadav H, Adinoff B. The limbic-hypothalamic-pituitary-adrenal axis and the development of alcohol use disorders in youth. Alcohol Clin Exp Res. 2011;35:595–605. doi: 10.1111/j.1530-0277.2010.01380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. J Neurosci. 2008;28:11517–11525. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS ONE. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, Orr SP, McInerney SC, Rauch SL. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry. 2001;50:932–942. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KI, Hansen J, Tuit K, Kreek MJ. Effects of adrenal sensitivity, stress- and cue-induced craving, and anxiety on subsequent alcohol relapse and treatment outcomes. Arch Gen Psychiatry. 2011;68:942–952. doi: 10.1001/archgenpsychiatry.2011.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Li CS. Imaging stress- and cue-induced drug and alcohol craving: association with relapse and clinical implications. Drug Alcohol Rev. 2007;26:25–31. doi: 10.1080/09595230601036960. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC. Behavioral Treatment of Alcohol Problems. Plenum Press; New York, NY: 1978. [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speilberger CD. Trait-state anxiety and motor behavior. J Mot Behav. 1971;3:265–279. doi: 10.1080/00222895.1971.10734907. [DOI] [PubMed] [Google Scholar]
- Sripada RK, King AP, Welsh RC, Garfinkel SN, Wang X, Sripada CS, Liberzon I. Neural dysregulation in posttraumatic stress disorder: evidence for disrupted equilibrium between salience and default mode brain networks. Psychosom Med. 2012;74:904–911. doi: 10.1097/PSY.0b013e318273bf33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straube T, Schmidt S, Weiss T, Mentzel HJ, Miltner WH. Dynamic activation of the anterior cingulate cortex during anticipatory anxiety. Neuroimage. 2009;44:975–981. doi: 10.1016/j.neuroimage.2008.10.022. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Clark L, Dunn BD. The role of interoception in addiction: a critical review. Neurosci Biobehav Rev. 2012;36:1857–1869. doi: 10.1016/j.neubiorev.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Wang J, Korczykowski M, Rao H, Fan Y, Pluta J, Gur RC, McEwen BS, Detre JA. Gender difference in neural response to psychological stress. Soc Cogn Affect Neurosci. 2007;2:227–239. doi: 10.1093/scan/nsm018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Rao H, Wetmore GS, Furlan PM, Korczykowski M, Dinges DF, Detre JA. Perfusion functional MRI reveals cerebral blood flow pattern under psychological stress. Proc Natl Acad Sci USA. 2005;102:17804–17809. doi: 10.1073/pnas.0503082102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Statistical analysis of activation image. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods, Functional MRI: An Introduction to Methods. Oxford University Press; Oxford: 2001. pp. 251–270. [Google Scholar]
- Yang H, Spence JS, Devous MD, Sr, Briggs RW, Goyal A, Xiao H, Yadav H, Adinoff B. Striatal-limbic activation is associated with intensity of anticipatory anxiety. Psychiatry Res. 2012;204:123–131. doi: 10.1016/j.pscychresns.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


